Abstract
We demonstrate the first application of synthetic RNA gene silencers in Streptomyces coelicolor A3(2). Peptide nucleic acid and expressed antisense RNA silencers successfully inhibited actinorhodin production. Synthetic RNA silencing was target-specific and is a new tool for gene regulation and metabolic engineering studies in Streptomyces.
Introduction
Bacteria use endogenous transcripts to help regulate a diverse range of cellular processes. These RNAs include riboswitches (mRNA leaders that affect transcription in cis), protein-binding small RNAs, CRISPR RNAs (involved in the targeting and degradation of foreign DNA) and both cis- and trans-encoded mRNA-binding sRNAs [1]. The application of computational searches in combination with RNA sequencing has enabled prediction of hundreds of putative regulatory RNAs in multiple species; the verified sRNAs of Escherichia coli account for ≈ 2% of its identified genes [2]. Regulatory RNAs can act as repressors or activators of transcription, or as stabilizers of target transcripts, and are involved in a wide variety of cellular processes including virulence regulation, quorum sensing, stress responses and secretion [3].
The genus Streptomyces includes species that have complex genetic regulatory pathways, in part due to a need for morphological differentiation and the production of diverse bioactive secondary metabolites, including the majority of known antibiotics [4]. The biotechnological importance of this genus makes advances in the understanding and manipulation of regulatory RNAs of interest. Accordingly, bioinformatics [5], [6], [7] and deep-sequencing [8] have been applied to identify putative small RNAs in the genomes of Streptomyces species. In a recent study, D’Alia et al. [9] were the first to elucidate the regulatory effect of a cis-encoded antisense sRNA, cnc2198.1 in glutamine synthetase I. Expression of cnc2198.1 in Streptomyces coelicolor A3(2) resulted in decreased growth, reduced protein production and synthesis of the red-pigmented antibiotic, undecylprodigiosin. A second example of a cis-encoded sRNA α-abeA was identified in S. coelicolor as part of a four-gene cluster involved in the enhanced production of the blue-pigmented antibiotic, actinorhodin, although the exact role of α-abeA has yet to be elucidated [4], [6]. Trans-encoded sRNAs are analogous to eukaryotic miRNAs, and, in many cases, require an RNA chaperone to mediate regulation [1]. In vitro and in silico evidence of a trans-encoded antisense sRNA micX, a putative activator of actinorhodin biosynthesis, has been reported [10], [11] with a recent study providing in vivo evidence for a S. coelicolor trans-encoded sRNA (scr5239) acting as a repressor of extracellular agarase expression [12]. Both cis- and trans-encoded sRNAs are thought to regulate gene expression as antisense RNA silencing molecules (asRNAs) that bind to their mRNA target, inhibit ribosome access and/or trigger mRNA degradation [13].
The studies cited above demonstrate that Streptomyces species use sRNAs to regulate gene expression; as such it is attractive to consider ways to exploit these molecules in practical applications. The use of conditional antisense RNA silencing may be of use not only in the elucidation of secondary metabolite regulation, but also in studies where gene knockouts are unsuitable e.g. when monitoring the affect of transcript abundance on gene expression; determining the minimally required levels of expression of essential genes [14]; and where the physical structure of the chromosome is related to transcriptional activity [15]. Synthetic RNA silencing, here defined as the use of antisense sequences that are either non-biological in origin or trans-acting RNAs that are not naturally expressed by the host, has yet to be demonstrated in the genus Streptomyces. Oligo-nucleobase antisense RNA silencers, such as peptide nucleic acid (PNA) and phosphorodiamidate morpholino oligomers (PMO), are DNA mimics that maintain naturally occurring DNA bases but have non-natural linkages between bases. PNAs and PMOs, conjugated to peptide carrier molecules, have been used to silence gene expression in a number of Gram-positive and Gram-negative species [16], [17], [18], and have the advantage of being resistant to biological degradation and do not require genetic transformation for delivery; carrier peptides attached to the PNA mediate delivery across the cell wall/membrane. Expressed RNAs designed to hybridize to target mRNA have been applied successfully in both Gram-positive and Gram-negative species, with Gram-positive species initially yielding more consistent gene silencing [19]. Recently, the addition of paired-termini (antisense RNA flanked by inverted repeats) proved successful in improving the efficacy of antisense RNA gene silencing in E. coli, most likely through the stabilization, and thereby increased abundance, of the asRNA [20].
In this study, we demonstrate, for the first time, the successful application of synthetic RNA silencing in Streptomyces. Both peptide-PNA conjugants and paired-termini antisense RNA were used to silence the expression of a gene required for actinorhodin biosynthesis. The development of antisense RNA gene silencers for Streptomyces species provides complementary tools to conventional genetics for the elucidation of regulatory pathways and gene function and will be a valuable tool in metabolic engineering.
Results and Discussion
Peptide-PNA Gene Silencing of actI-ORF1
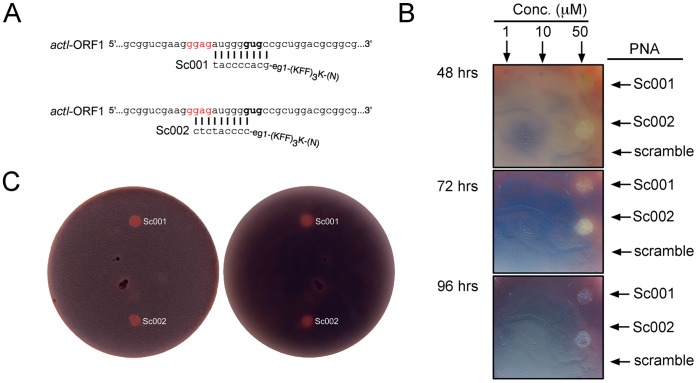
To investigate the use of synthetic RNA gene silencers, we targeted the actinorhodin polyketide beta-ketoacyl synthase subunit gene (actI-ORF1). Two peptide-PNAs (Sc001 and Sc002) were designed and applied as antisense agents targeting the translation initiation region of actI-ORF1 (Fig. 1A). Guidelines for the design of peptide-PNA antisense gene silencers have been reported elsewhere [21], [22]. In this study we used 9 bp PNAs as they are reported to have improved uptake properties; longer PNAs also have an increased risk for non-target binding and self-complementarity [35]. In PNAs of 10–15 bp, mismatches with the target of >2 bp abolish activity, while mismatches of 1 bp abolish or significantly reduce activity. Thus, for the PNAs used in this study, it is likely that only genes with perfect matches in the target region will be significantly repressed. We identified all potential off-target genes for Sc001 and Sc002 (19 and 33 targets, respectively [Table S1 information]), none of which are predicted to have a role in antibiotic synthesis (many are annotated as possible or hypothetical proteins). For Sc001 only the intended target has a perfect match with the PNA, while Sc002 matches the target and SCO6703, a gene predicted to encode a putative 3-oxoacid CoA-transferase that is possibly involved in aromatic acid degradation. While a role for SCO6703 in actinorhodin production cannot be ruled out, it is unlikely that repression of this gene would play a significant role in the peptide-PNA mediated silencing of actinorhodin biosynthesis (Sc001 has 4 bp mismatches with the TIR of SCO6703 and results in a similar phenotype to Sc002). Actinorhodin production was clearly inhibited at 50 µM for both Sc001 and Sc002, but not by a scrambled peptide-PNA (Fig. 1B). The viability of subcultures taken from the area of peptide-PNA application and the lack of inhibition by the scrambled peptide-PNA indicates that the silencing of actinorhodin production was due to gene silencing and not growth inhibition. Experiments using ISP-4 agar medium indicated that neither PNA affected production of undecylprodigiosin (Fig. 1C). These results show that S. coelicolor is accessible to synthetic RNA silencing. Furthermore, susceptibility to PNA mediated gene silencing suggests that other RNA silencing strategies may also be effective in this genus. As PNA synthesis is relatively expensive, we also evaluated synthetic RNA silencing in S. coelicolor using expressed antisense RNA.
Figure 1. RNA silencing using peptide-PNA.
(A) Schematic diagram representing the binding site of antisense peptide-PNAs designed to prevent transcription of actI-ORF1. The start codon of actI-ORF1 is shown in bold, the putative Shine-Dalgarno site in red. Sc001 and Sc002 differ in the degree of coverage of the start codon. (B) Demonstration of the ability of peptide-PNAs to silence production of actinorhodin in S. coelicolor. Peptide-PNA solutions were applied to a lawn of S. coelicolor (after 24 hrs) on MPCA agar and incubation continued for a further 72 hrs. Repression of actinorhodin production is clearly visible with 50 µM treatment using either Sc001 or Sc002; no reduction in actinorhodin production was evident when a scramble-PNA with limited complementarity to the S. coelicolor genome was used at the same concentration. (C) Peptide-PNA solutions were applied directly to a lawn of S. coelicolor MT1110 on ISP-4 agar and were photographed from on top (left) and from bottom (right) after 96 hrs incubation at 28°C.
Expressed Antisense RNA Silencing of actI-ORF1
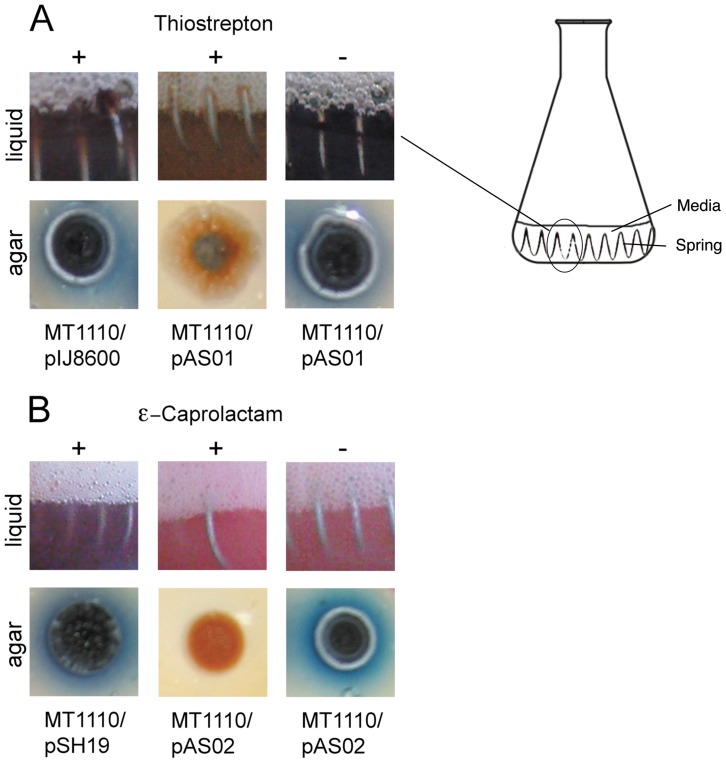
DNA sequences (120–160 bp) covering the 5′UTR, RBS and >50 bp of the coding region of actI-ORF1 were examined as potential antisense RNAs. Sequences with low secondary structure, and high positional entropy in the mRNA binding portion of the asRNA, were identified using RNAfold [23] (Fig. 2). A 155 bp region (-92 to +63) with the desired features was PCR-amplified from S. coelicolor MT1110 gDNA using primers with 21 bp inverted repeat overhangs, designed to generate antisense RNA transcripts with paired termini (PTasRNA) [20]. This amplicon was cloned into integrative pIJ8600 [24] and replicative pSH19 [25] vectors to form pAS01 and pAS02, respectively. Both pAS01 and pAS02 were used to transform S. coelicolor MT1110 and actinorhodin production was monitored in a number of media. For liquid and agar R5 media, both S. coelicolor MT1110/pAS01 and pAS02 showed visible reduction of actinorhodin production when induced with thiostrepton and ε–caprolactam, respectively (Fig. 3). In liquid culture, actinorhodin production was clearly reduced in non-induced MT1110/pAS02 indicating that, in our hands, expression of antisense RNA from vector pSH19 was likely to occur in the absence of induction. For this reason, pAS02 was excluded from further analysis.
Figure 2. Predicted secondary structures of antisense RNAs.
Secondary structure of (A) the paired-termini antisense actI-ORF1 asRNA (−92 to +63 bp) designed in this study for silencing of actinorhodin production, and (B) micX, a naturally occurring trans-encoded antisense RNA from Streptomyces lividans involved in the activation of actinorhodin biosynthesis. The micX asRNA has a predicted paired-end structure which suggests that the synthetic strategy followed in this study (addition of paired termini to antisense RNA) may be analogous to natural antisense mechanisms in Streptomyces. micX also contains non-complementary nucleotide sequences in the paired region which may serve to protect antisense RNAs from RNase III cleavage.
Figure 3. Actinorhodin production in S. coelicolor MT1110 and effects of RNA silencing.
(A) MT1110/pAS01 after 6 days of growth in R5 liquid medium (top panels, the region shown is represented by the flask on the right) and R5 agar (bottom panels) containing thiostrepton (+ induction) or DMSO (- induction). MT1110/pIJ8600 served as a control. (B) As above with MT1110/pAS02, ε-caprolactam as the inducer of expression and MT1110/pSH19 as the control.
Quantification of the Effects on Antibiotic Production
To determine whether RNA silencing using PTasRNA was specific to actI-ORF1, growth and antibiotic production were monitored for MT1110/pAS01 and control cultures in R5 liquid medium (Fig. 4). In all cases, growth and the production of undecylprodigiosin was equivalent to that of uninduced S. coelicolor MT1110, indicating that expression of the antisense RNA was not growth inhibitory, and that reduced antibiotic production was restricted to actinorhodin biosynthesis (production of the calcium-dependent antibiotic was also unaffected by antisense RNA production, Figure S2). In contrast, induced MT1110/pAS01 displayed unaltered growth but ≈ 4-fold reduced actinorhodin production; 10 µM for induced cultures, compared to >40 µM for uninduced MT1110/pAS01 and other controls after 7 days incubation (Fig. 4).
Figure 4. Antisense mediated decrease in actinorhodin production.

S. coelicolor MT1110 strains were grown in R5 liquid medium containing thiostrepton (+ induction) or DMSO (- induction) for 5 days. (A) Cell growth, and (B) undecylprodigiosin production were similar in all cases. (C) Actinorhodin production was reduced when antisense RNA was induced (MT1110/pAS01+). The data represent the average of three independent determinations.
Quantification of the Effect on actI-ORF1 Transcription
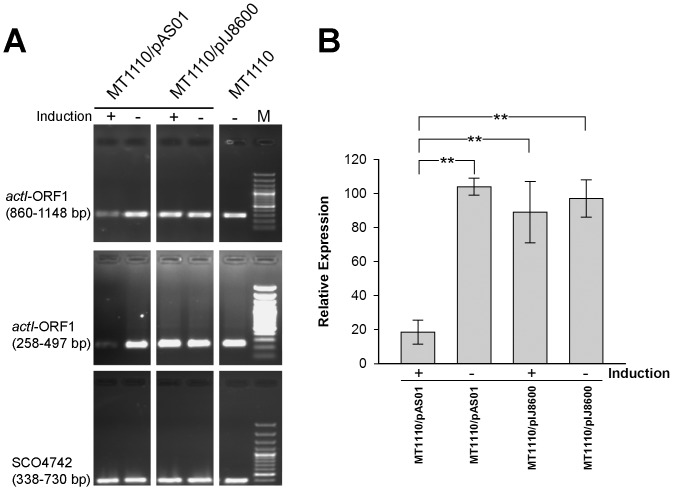
To confirm that the asRNA was reducing actinorhodin biosynthesis through a transcript silencing mechanism, RT-PCR and qRT-PCR were employed to monitor expression of actI-ORF1 mRNA (Fig. 5). RT-PCR analysis confirmed earlier experiments, with only induced MT1110/pAS01 showing a decrease in the abundance of actI-ORF1 mRNA. Primers targeting the 5′ end of the transcript yielded less amplification product than those hybridising towards the 3′ end (Fig. 5A). Assuming equal primer access and amplification kinetics, this is empirical evidence for the mRNA/asRNA hybrid being degraded in the 5′ –3′ direction triggered by hybridization of the asRNA. qRT-PCR analysis of replicate RNA extracts from 3-d-old cultures was carried out with SCO4742 as the internal reference and values were calculated relative to the expression levels of uninduced MT1110. Consistent with antibiotic assays and RT-PCR data, only induced MT1110/pAS01 showed a significant difference (≈ 5-fold decrease) in the level of actI-ORF1 expression (Fig. 5B).
Figure 5. Effect of expressed antisense RNA on actI-ORF1 mRNA levels.
RT-PCR of actI-ORF1 from RNA extracted from MT1110 strains grown for 3 days in R5 liquid medium that contained thiostrepton (+) or DMSO (-) as a control. Two primers sets (top and middle panel) designed to amplify different regions of the actI-ORF1 mRNA were used, with SCO4742 (bottom panel) serving as an internal control. M = 100 bp DNA ladder. (B) qRT-PCR of actI-ORF1; SCO4742 served as the internal reference and expression was calculated relative to uninduced MT1110. Error bars represent standard error for biological replicates (n = 3 ** highly significant difference, one way ANOVA, F = 11.96, p = <0.01).
Conclusions/Concluding Remarks
In this study we have demonstrated that synthetic asRNA can be designed and used to regulate gene expression in S. coelicolor. Furthermore, RNA silencing was effective when expressed from either the chromosome or from a replicative plasmid. The application of antisense RNA silencing in the genus Streptomyces provides a complimentary tool to those of standard genetics and is applicable in instances where gene knockouts are not suitable (see above). This genus is responsible for the production of a large number of high value compounds and the development of techniques for improving titre through metabolic engineering are industrially significant [26]. Antisense RNA silencing is of particular value in the field of metabolic engineering since it can avoid problems associated with lethal mutations caused by gene knockouts; the level of silencing is variable and complete inhibition of protein production can be avoided [27], [28]. We applied RNA silencing to inhibit production of an antibiotic, however as earlier studies with natural asRNAs have shown [10], [11], it is possible that these methods could be used to induce expression of silent biosynthetic gene clusters by silencing repressors, or by interfering with mRNA secondary structures that sequester ribosome binding sites [1]. Comparison of the natural micX asRNA with that of the synthetic asRNA designed for this study revealed possible areas for the future development of asRNAs for use in Streptomyces: The micX asRNA has a predicted paired-end structure that contains regions of non-complementary nucleotides (Fig. 2); in E. coli these regions have been shown to protect antisense RNAs from RNase III cleavage [29]. Furthermore, as with other natural trans-encoded antisense RNAs, the mRNA binding region of micX is ≈ 25 bp. The paired-termini approach used in this study has been applied successfully using mRNA binding regions <30 bp (Nobutaka Nakashima, AIST Sapporo, personal communication) and thus the design of synthetic asRNAs based on naturally occurring trans-encoded asRNAs may yield more effective gene silencers. It is also likely that a better understanding of the mechanism of antisense RNA gene silencing in Streptomyces will lead to improvements in asRNA design. For example, in many species, limited complementarity between natural trans-encoded asRNAs and the corresponding mRNA requires the RNA chaperone Hfq to facilitate RNA-RNA interactions [30]. Hfq is believed to protect asRNAs from degradation when they are not bound to their target mRNAs, and to recruit the RNA degradation machinery once the asRNA is paired to the target mRNA. [1] There are no obvious homologues of Hfq in the genome of S. coelicolor [9] and it remains to be determined whether an RNA chaperone is required for asRNA gene silencing in Streptomyces. Long stretches of base pairing, or high concentrations of the asRNA, as in the case of the synthetic asRNAs used in this study, may obviate a chaperone requirement [1]. However, scr5239, a trans-encoded sRNA repressor of extracellular agarase expression, has five stem-loop structures that presumably require facilitated opening in order for annealing to occur, leading the authors to speculate that Streptomyces species may require proteins to carry out the functions of Hfq [12]. Consequently, the identification of such an RNA chaperone in Streptomyces could aid in the development of synthetic asRNAs since synthetic RNAs could then be designed to mimic naturally occurring trans-acting sRNAs. The use of peptide-PNAs in Streptomyces is of particular interest as genetic transformation is not required for gene silencing, thus they can be used in species in which genetic tools have not been established or to simply accelerate the process of genetic analysis. The current synthesis costs and high concentrations required when using these molecules in agar-based assays makes optimization studies prohibitive. However, improvements in micro-culture of Streptomyces [31] and reduced synthesis costs will enable different peptide-PNA designs to be evaluated for this genus. In summary, recent reports reveal that Streptomyces use endogenous transcripts to regulate gene expression, and here we show for the first time that synthetic strategies using expressed RNA or a delivered DNA mimic can provide useful levels of RNA silencing. As a method, RNA silencing can be used to improve our understanding of Streptomyces biology and possibly alter metabolic flux in industrial applications.
Materials and Methods
Bacterial Strains, Plasmids and Culture Conditions
The strains and plasmids used in this study are listed in Table 1. Standard media and conditions for growth were as previously described [24], [32]. The media were supplemented with the following chemicals when required: 100 µg/ml ampicillin (Amp), 50 µg/ml kanamycin (Kan), 25 µg/ml chloramphenicol (Chl), 25 µg/ml nalidixic acid (Nal), 50 µg/ml apramycin (Apr), 25 (or 50) µg/ml thiostrepton (Tsr) and 0.1% (wt/vol) ε-caprolactam. Growth in liquid R5 was monitored by optical density (A430).
Table 1. Bacterial Strains and plasmids used in this study.
| Strain or plasmid | Description | Source or reference |
| Streptomyces coelicolor MT1110 | Wild-type prototrophic; SCP1−, SCP2− derivative of Streptomyces coelicolor A3(2) | [24] |
| Escherichia coli DH5α | For cloning and propagation of host strain, recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F’ proAB lacI q ZΔM15 Tn10 (Tetr)] | Stratagene |
| E. coli ET12567 | dam dcm hsdS hsdR Chlr Kanr; carrying plasmid pUB307 | [39] |
| Bacillus mycoides ATCC 6462 | As an indicator organism for bioassay of calcium-dependent antibiotic | American Type Culture Collection |
| pGEMT-Easy | Ampr; E. coli vector for cloning PCR products | Promega |
| pUB307 | RP1 derivative contains oriT from IncP-group plasmid RK2 | [39] |
| pIJ8600 | Aprr Tsr tipAp; thiostrepton inducible integrative expression vector | [24] |
| pSH19 | Tsrr PnitA; replicative expression vector | [25] |
| pAS01 | Aprr Tsrr; 155 bp (-92 to +63) of actI-ORF1. Cloned in antisense orientationinto pIJ8600 | This study |
| pAS02 | Aprr Tsrr; 155 bp (-92 to +63) of actI-ORF1. Cloned in antisense orientationinto pSH19 | This study |
Antibiotic Assays
Spores (3×106 colony forming units [CFU]) of the experimental and control strains were inoculated into 20 ml of R5 liquid medium containing the appropriate antibiotics to an OD450 of ≈ 0.05. Cultures were grown for 18 h before 1 ml was transferred to 50 ml of R5 liquid medium containing appropriate antibiotics and inducers (for induction samples). Actinorhodin and undecylprodigiosin samples from three biological replicates were quantified according to standard protocols [24], [33]. Production of the calcium-dependent antibiotic was assayed using Bacillus mycoides ATCC 6462 as an indicator strain [34].
Peptide Nucleic Acid (PNA) RNA Silencing of actI-ORF1
Sc001 and Sc002, antisense PNAs specific for actI-ORF1, were designed using previously described parameters [16], [35] and differed in their degree of coverage of the start codon of actI-ORF1. A scrambled PNA that had no sites of perfect complementarity in the genome of S. coelicolor MT1110 was used as a negative control (see Table 2 for PNA sequences). The cell-wall permeating peptide (KFF)3K, was attached to the N-terminus of each PNA (which corresponds to a 5′ nucleic acid terminus) via a flexible ethylene glycol linker [16]. Peptide-PNAs were synthesized as previously described [14]. Fifty microlitres of a S. coelicolor MT1110 spore suspension (1.5×108 CFU/mL) were used to prepare a confluent lawn of growth (24 h incubation at 28°C) on MPCA agar plates (yeast extract, 0.2%, meat extract 0.2%, Bacto-peptone 0.4%, NaCl 0.5%, MgSO4.7H2O 0.2%, glycine 0.5%, glucose 1.0%, sucrose 10%, casamino acid 0.01%, TES buffer 0.573% and agar 2%). Peptide-PNAs were applied directly to the lawn at concentrations of 1, 10 and 50 µM and incubation continued at 28°C for 72 hrs. To determine whether inhibition of actinorhodin production was due to RNA silencing of actI-ORF1 or to non-specific toxicity of the PNAs, subcultures from the zones of application were inoculated onto MPCA agar plates. ISP-4 agar (inorganic salts-starch; Difco) that supports both undecylprodigiosin and actinorhodin production in S. coelicolor MT1110 was used to evaluated the specificity of gene silencing: agar plates were overlaid with 0.5% ISP-4 agar containing 2.3×106 CFU/mL S. coelicolor MT1110 spores, 10 µL of PNAs (50 µM) were applied to antibiotic assay discs (Whatman) and placed onto the soft agar. After 96 hours of incubation, the disks were removed and the agar plates were photographed.
Table 2. Primers and peptide-PNAs used in this study.
| Oligomera | Sequence 5′–3′ | Description |
| actIORF1258f | gccctaccgttcacaggtc | RT-PCR of actI-ORF1 |
| actIORF1497r | tccgacagcagcagatactc | RT-PCR of actI-ORF1 |
| actIORf1860f | tcgtcctggaggactacgac | RT-PCR and qRT-PCR of actI-ORF1 |
| actIORF11148r | atcgagctgaccggagtg | RT-PCR and qRT-PCR of actI-ORF1 |
| SCO4742_338f | gttccgccgaggagttgat | RT-PCR and qRT-PCR of SC04742 (internal control) |
| SCO4742_730r | gccggtacttgtcgctctc | RT-PCR and qRT-PCR of SC04742 (internal control) |
| actIPT1HindSph-F | gcgcaagcttCAGTGGTGGTGGTGGTGGTGCgcatgcaggctcgaaggccgatac | PCR of paired-termini antisense actI-ORF1 for cloning into pIJ8600 |
| actIPT1EcoRPst-R | cgggaattcCAGTGGTGGTGGTGGTGGTGCctgcagctgcgtggccatgtgttc | PCR of paired-termini antisense actI-ORF1 for cloning into pIJ8600 |
| actIPT1NdeSph-F | gcgccatatgCAGTGGTGGTGGTGGTGGTGCgcatgcaggctcgaaggccgatac | PCR of paired-termini antisense actI-ORF1 for cloning into pSH19 |
| actIPT1XbaPst-R | cggtctagaCAGTGGTGGTGGTGGTGGTGCctgcagctgcgtggccatgtgttc | PCR of paired-termini antisense actI-ORF1 for cloning into pSH19 |
| Peptide-PNAb | ||
| Sc001 | (KFF)3K-eg1-gcaccccat | Antisense RNA silencing of actI-ORF1– binds -5 to +4 relative to start codon |
| Sc002 | (KFF)3K-eg1-ccccatctc | Antisense RNA silencing of actI-ORF1– binds −8 to +1 relative to start codon |
| Scramble | (KFF)3K-eg1-ccatttagtt | Control PNA |
Restriction sites are in bold, paired termini sequences are in capitals.
eg1 = ethylene glycol linker.
Construction of Antisense RNA Expressing Vectors
A 155 bp fragment (−92 to +63 bp of the actI-ORF1 gene) was amplified from S. coelicolor MT1110 gDNA using primers modified to include paired-termini sequences [20]. Primers actIPT1HindSph-F and actIPT1EcoRPst-R were used for cloning into pSH19. Primers actIPT1NdeSph-F and actIPT1XbaPst-R were used for cloning into pIJ8600 (see Table 2 and Figure S1). Primers contained SphI and PstI restriction sites for convenient excision and replacement of the antisense RNA expressing fragment of the vectors.
DNA Cloning and Bacterial Transformation and Streptomyces Conjugation
DNA manipulations, E. coli transformations and Streptomyces conjugations were carried out according to standard protocols [24], [32].
RNA Extraction from Streptomyces Strains
RNA was extracted from Streptomyces strains grown in liquid R5 medium (as above) using a modified Kirby mix, phenol/chloroform extraction and DNAse I treatment [24]. RNA samples were further purified using RNeasy columns according to the manufacturer’s instructions (Qiagen, UK).
RT-PCR and qRT-PCR
RT-PCR was performed using the Promega Access RT-PCR system with 400 ng RNA per sample. The RT-PCR conditions were as follows: for cDNA synthesis, 45°C for 45 min, followed by 94°C for 2 min. For PCR amplification, 20 cycles at 94°C for 30 s, 55°C for 1 min, and 68°C for 2 min and 1 cycle at 68°C for 10 min. Two primer pairs (actIORF1258f/actIORF1497r and actIORf1860f/actIORF11148r) were used to amplify distinct regions of the actI-ORF1 mRNA. SCO4742 a hypothetical gene that shows little variation in expression [36], [37] was used as an internal reference (see Table 2 for primer sequences). Negative control experiments were run for each pair of primers in the absence of reverse transcriptase (but with DNA polymerase present). qRT-PCR was done using a Brilliant II SYBR® Green QRT-PCR Master Mix 1-step kit (Agilent). Amplification and analysis were performed on a Bio-Rad iCycler. Each 20-µl reaction contained 500 ng RNA template, 1 µM each of actIORf1860f and actIORF11148r, 10 µl 2x Master mix and 1 µl of reverse transcriptase enzyme mix (containing RNase inhibitor). The qRT-PCR conditions were as follows: 1 cycle of 50°C for 30 min (reverse transcription), followed by another 1 cycle of 95°C for 10 min (to activate the DNA polymerase), then 40 cycles of 95°C for 30 s, 50°C for 30 s, 72°C for 1 min. An additional 1 cycle at 72°C for 5 min was performed. SCO4742 was used as the internal standard. Estimation of the relative amounts of transcript in each sample and the statistical calculations were as described [34] with the following modifications: a standard curve was obtained with a set of sonicated serially diluted genomic DNA positive controls. The amplification efficiency was calculated by taking the gradient of the line of best fit through plots of log10 dilution factors against the Ct values. The efficiency was corrected based on multiple samples [38]. Negative controls were as above.
Supporting Information
Schematic representation of the cloning strategy used to construct antisense RNA expressing vector pAS02.
(TIFF)
Calcium-dependent antibiotic assay. The assay was performed as previously reported [34] using B. mycoides as the indicator strain (A) wild-type S. coelicolor MT1110. (B) MT1110/pAS01 induced by adding thiostrepton to the agar. Plates were photographed and half images from each strain were aligned to compare the size of the zone of inhibition.
(PNG)
Off-target matches in S. coelicolor for PNAs used in this study.
(DOCX)
Acknowledgments
We are grateful to Professor Michihiko Kobayashi for the gift of pSH19.
Funding Statement
This work was funded by the Biotechnology and Biological Sciences Research Council (BBSRC) grant BB/E017053/1 (http://www.bbsrc.ac.uk/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Waters LS, Storz G (2009) Regulatory RNAs in bacteria. Cell 136: 615–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Livny J, Teonadi H, Livny M, Waldor MK (2008) High-throughput, kingdom-wide prediction and annotation of bacterial non-coding RNAs. PLoS ONE 3: e3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Livny J, Waldor MK (2007) Identification of small RNAs in diverse bacterial species. Curr Opin Microbiol 10: 96–101. [DOI] [PubMed] [Google Scholar]
- 4. Hindra P, Elliot M (2010) Regulation of a novel gene cluster involved in secondary metabolite production in Streptomyces coelicolor . J Bacteriol 192: 4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pánek J, Bobek J, Mikulík K, Basler M, Vohradský J (2008) Biocomputational prediction of small non-coding RNAs in Streptomyces . BMC Genomics 9: 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Swiercz JP, Hindra, Bobek J, Haiser HJ, Di Berardo C, et al. (2008) Small non-coding RNAs in Streptomyces coelicolor . Nucleic Acids Res 36: 7240–7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tezuka T, Hara H, Ohnishi Y, Horinouchi S (2009) Identification and gene disruption of small noncoding RNAs in Streptomyces griseus . J Bacteriol 191: 4896–4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vockenhuber MP, Sharma CM, Statt MG, Schmidt D, Xu Z, et al. (2011) Deep sequencing-based identification of small non-coding RNAs in Streptomyces coelicolor . RNA biology 8: 468–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. D'alia D, Nieselt K, Steigele S, Müller J, Verburg I, et al. (2010) Noncoding RNA of glutamine synthetase I modulates antibiotic production in Streptomyces coelicolor A3(2). J Bacteriol 192: 1160–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Romero NM, Mellado RP (1995) Activation of the actinorhodin biosynthetic pathway in Streptomyces lividans . FEMS Microbiol Lett 127: 79–84. [DOI] [PubMed] [Google Scholar]
- 11. Romero NM, Parro V, Malpartida F, Mellado RP (1992) Heterologous activation of the actinorhodin biosynthetic pathway in Streptomyces lividans . Nucleic Acids Res 20: 2767–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vockenhuber MP, Suess B (2012) Streptomyces coelicolor sRNA scr5239 inhibits agarase expression by direct base pairing to the dagA coding region. Microbiology 158: 424–435. [DOI] [PubMed] [Google Scholar]
- 13. Morita T, Mochizuki Y, Aiba H (2006) Translational repression is sufficient for gene silencing by bacterial small noncoding RNAs in the absence of mRNA destruction. Proc Natl Acad Sci USA 103: 4858–4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goh S, Boberek JM, Nakashima N, Stach J, Good L (2009) Concurrent growth rate and transcript analyses reveal essential gene stringency in Escherichia coli . PLoS ONE 4: e6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McArthur M, Bibb M (2006) In vivo DNase I sensitivity of the Streptomyces coelicolor chromosome correlates with gene expression: implications for bacterial chromosome structure. Nucleic Acids Res 34: 5395–5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Good L, Awasthi SK, Dryselius R, Larsson O, Nielsen PE (2001) Bactericidal antisense effects of peptide-PNA conjugates. Nat Biotechnol 19: 360–364. [DOI] [PubMed] [Google Scholar]
- 17. Nekhotiaeva N, Awasthi SK, Nielsen PE, Good L (2004) Inhibition of Staphylococcus aureus gene expression and growth using antisense peptide nucleic acids. Mol Ther 10: 652–659. [DOI] [PubMed] [Google Scholar]
- 18. Shen N, Ko JH, Xiao G, Wesolowski D, Shan G, et al. (2009) Inactivation of expression of several genes in a variety of bacterial species by EGS technology. Proc Natl Acad Sci USA 106: 8163–8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wagner E, Flardh K (2002) Antisense RNAs everywhere? Trends Genet 18: 223–226. [DOI] [PubMed] [Google Scholar]
- 20. Nakashima N, Tamura T, Good L (2006) Paired termini stabilize antisense RNAs and enhance conditional gene silencing in Escherichia coli . Nucleic Acids Res 34: e138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Good L (2002) Antisense Inhibition of Bacterial Gene Expression and Cell Growth. In: Nielsen P, editor. Peptide Nucleic Acids: Protocols and Applications (Methods in Molecular Biology). New York: Springer. 237–248. [DOI] [PubMed]
- 22. Good L, Awasthi SK, Dryselius R, Larsson O, Nielsen PE (2001) Bactericidal antisense effects of peptide-PNA conjugates. Nat Biotechnol 19: 360–364. [DOI] [PubMed] [Google Scholar]
- 23. Hofacker IL (2003) Vienna RNA secondary structure server. Nucleic Acids Res 31: 3429–3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kieser T, Bibb MJ, Buttner MJ, Chater KF, Hopwood DA (2000) Practical Streptomyces genetics: John Innes Foundation, Norwich, United Kingdom.
- 25. Herai S, Hashimoto Y, Higashibata H, Maseda H, Ikeda H, et al. (2004) Hyper-inducible expression system for streptomycetes. Proc Natl Acad Sci USA 101: 14031–14035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen Y, Smanski MJ, Shen B (2010) Improvement of secondary metabolite production in Streptomyces by manipulating pathway regulation. Appl Microbiol Biotechnol 86: 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Desai RP, Papoutsakis ET (1999) Antisense RNA strategies for metabolic engineering of Clostridium acetobutylicum . Appl Environ Microbiol 65: 936–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hebert CG, Valdes JJ, Bentley WE (2008) Beyond silencing - engineering applications of RNA interference and antisense technology for altering cellular phenotype. Curr Opin Biotechnol 19: 500–505. [DOI] [PubMed] [Google Scholar]
- 29. Hjalt TA, Wagner EG (1995) Bulged-out nucleotides protect an antisense RNA from RNase III cleavage. Nucleic Acids Res 23: 571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aiba H (2007) Mechanism of RNA silencing by Hfq-binding small RNAs. Curr Opin Microbiol 10: 134–139. [DOI] [PubMed] [Google Scholar]
- 31. Minas W, Bailey JE, Duetz W (2000) Streptomycetes in micro-cultures: growth, production of secondary metabolites, and storage and retrieval in the 96-well format. Antonie Van Leeuwenhoek 78: 297–305. [DOI] [PubMed] [Google Scholar]
- 32.Ausable FM, Brent R, Kingston RE (1996) Current protocols in molecular biology; Kingston RE, editor. New York, N.Y.: John Wiley and Sons.
- 33. Kang SG, Jin W, Bibb MJ, Lee KJ (1998) Actinorhodin and undecylprodigiosin production in wild-type and relA mutant strains of Streptomyces coelicolor A3(2) grown in continuous culture. FEMS Microbiol Lett 168: 221–226. [DOI] [PubMed] [Google Scholar]
- 34. Uguru GC, Stephens KE, Stead JA, Towle JE, Baumberg S, et al. (2005) Transcriptional activation of the pathway-specific regulator of the actinorhodin biosynthetic genes in Streptomyces coelicolor . MolMicrobiol 58: 131–150. [DOI] [PubMed] [Google Scholar]
- 35. Dryselius R, Aswasti SK, Rajarao GK, Nielsen PE, Good L (2003) The translation start codon region is sensitive to antisense PNA inhibition in Escherichia coli . Oligonucleotides 13: 427–433. [DOI] [PubMed] [Google Scholar]
- 36. Hesketh A, Chen WJ, Ryding J, Chang S, Bibb M (2007) The global role of ppGpp synthesis in morphological differentiation and antibiotic production in Streptomyces coelicolor A3(2). Genome biology 8: R161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McArthur M, Bibb MJ (2008) Manipulating and understanding antibiotic production in Streptomyces coelicolor A3(2) with decoy oligonucleotides. Proc Natl Acad Sci USA 105: 1020–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Flett F, Mersinias V, Smith CP (1997) High efficiency intergeneric conjugal transfer of plasmid DNA from Escherichia coli to methyl DNA-restricting streptomycetes. FEMS Microbiol Lett 48: 223–229. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Schematic representation of the cloning strategy used to construct antisense RNA expressing vector pAS02.
(TIFF)
Calcium-dependent antibiotic assay. The assay was performed as previously reported [34] using B. mycoides as the indicator strain (A) wild-type S. coelicolor MT1110. (B) MT1110/pAS01 induced by adding thiostrepton to the agar. Plates were photographed and half images from each strain were aligned to compare the size of the zone of inhibition.
(PNG)
Off-target matches in S. coelicolor for PNAs used in this study.
(DOCX)