Abstract
The ecology of small rodent food selection is poorly understood, as mammalian herbivore food selection theory has mainly been developed by studying ungulates. Especially, the effect of food availability on food selection in natural habitats where a range of food items are available is unknown. We studied diets and selectivity of grey-sided voles (Myodes rufocanus) and tundra voles (Microtus oeconomus), key herbivores in European tundra ecosystems, using DNA metabarcoding, a novel method enabling taxonomically detailed diet studies. In order to cover the range of food availabilities present in the wild, we employed a large-scale study design for sampling data on food availability and vole diets. Both vole species had ingested a range of plant species and selected particularly forbs and grasses. Grey-sided voles also selected ericoid shrubs and tundra voles willows. Availability of a food item rarely affected its utilization directly, although seasonal changes of diets and selection suggest that these are positively correlated with availability. Moreover, diets and selectivity were affected by availability of alternative food items. These results show that the focal sub-arctic voles have diverse diets and flexible food preferences and rarely compensate low availability of a food item with increased searching effort. Diet diversity itself is likely to be an important trait and has previously been underrated owing to methodological constraints. We suggest that the roles of alternative food item availability and search time limitations for small rodent feeding ecology should be investigated.
Nomenclature
Annotated Checklist of the Panarctic Flora (PAF), Vascular plants. Available at: http://nhm2.uio.no/paf/, accessed 15.6.2012.
Introduction
Current understanding of mammalian herbivore foraging ecology is mainly based on studies focusing on ungulates; see for example [1], [2] and [3]. Other herbivores with a central role in many ecosystems, such as small rodents, have been less studied. Small rodents are non-ruminant herbivores with fast digestion, invest greatly in reproduction and little in growth, generally have a high risk of predation and are often territorial [4]–[6]. They can therefore be expected to have different nutritional needs and face different trade-offs both physiologically and behaviorally than ungulates. Due to such differences in trade-offs, small rodent functional responses, i.e. the relationship between food intake and food availability [7], are also likely to differ from those developed using ungulates as empirical models [8]. Functional response models can improve the understanding of how herbivores select their food and thus aid in predicting how they may cope with current vegetation changes, as well as how they may themselves affect vegetation. A range of parameters have been suggested to be incorporated into functional response models for herbivores [2], [9], [10]. However, to target those parameters that are important determinants of small rodent functional responses, more exploratory empirical work is required to assess which processes shape their food selection in the wild.
Within a food item category such as plant species or genus, small rodent functional responses to food availability have been studied experimentally [8], [9], [11], [12]. In these studies, food availability has, unavoidably, been found to increase food intake. However, various processes such as handling time, bite size or plant spacing have been shown to have the potential to regulate this relationship [9], [11], [12]. Even though feeding trials using single food items may identify mechanisms that operate in the wild, the value of a food item to an animal is relative to what else is available [13]–[15]. Studies investigating how the availability of alternative food items impacts on consumption of other food items by small rodents are, however, scarce [16]–[18]. These studies experimentally demonstrate that availability of a high-quality food item can reduce the consumption of a low-quality food item. In natural environments, a range of food items of different quality are available and the composition of vegetation may vary greatly. However, small rodent functional responses to the spatially and temporally variable food availability in the setting of complex natural plant communities remain unexplored.
Grey-sided voles (Myodes rufocanus) and tundra voles (Microtus oeconomus) are among the key herbivores of subarctic tundra ecosystems [19] where they greatly modify tundra vegetation during their cyclic population density peaks [20]–[23]. During recent decades, their cyclic population dynamics have dampened in many areas [24]. While changes in winter climate have mainly been suggested to cause these changes in population dynamics, the role of concurrent vegetation changes is unclear [19], [24]–[26]. However, any evaluation of such bottom-up effects in tundra food webs is severely hampered by the current gaps in knowledge of vole diets and how diet is affected by food availability.
Grey-sided voles are considered to prefer Vaccinium myrtillus but to also feed on forbs during summer [27], [28], while tundra voles are considered to feed primarily on monocotyledons, with an increased proportion of Equisetum and forbs during the summer [29]–[31]. These generalizations are mostly based on microhistological analysis of ingested material [28]–[31] and observations of feeding signs on vegetation [27]. However, taxonomic resolution of microhistological studies of small rodent diets is limited [32], whereas feeding signs of vegetation give limited information on proportional abundance of different food items in diets.
We analyzed stomach contents of grey-sided voles and tundra voles using DNA metabarcoding [33], [34]. This novel methodology has lately opened new avenues of herbivore diet studies, as it enables analysis of large numbers of samples and identification of the ingested plants at a detailed taxonomic level [32], [35]–[39]. We used a spatially extensive study design, spanning across two river catchment areas and two habitats and sampled vole diets and vegetation composition in common locations over two seasons. Thus, we were able to study the impact of food availability on diets and selectivity both at a taxonomically more detailed level than previous studies and across the range of food availability variation present in natural habitats of voles.
We first compared vole diets to vegetation in order to determine which food plants were selected for. We then investigated how vole diets and selectivity were related to availability of food plants. We analyzed these relationships using both plant families and plant functional groups, and use hereafter the term “food item” to describe any plant group. We predicted that the proportion of any preferred food item in diets would increase with its availability but that it also would be affected by availability of alternative food items. We further predicted that selectivity for a food item would also be affected by availabilities of both the food item in question and alternative food items.
Plant families allowed the most precise taxonomic units for diet and vegetation comparison. Plant functional groups coarsely reflect plant nutrient content and digestibility and allowed grouping of plants according to their presumed nutritional value for herbivores [40], [41]. By analyzing vole feeding habits using both taxonomic and ecological groupings we aimed to both perform a taxonomically detailed analysis of vole diet and to evaluate how the different food item units reflect vole feeding ecology.
Materials and Methods
Study Area
This study took place at Varanger peninsula, (70°N, 31°E), Finnmark, North-Eastern Norway (Figure 1). Two prominent habitat types of the area, dwarf-shrub heaths and meadows with scattered willow (Salix spp.) thickets harbor different vegetation and small rodent communities. Vegetation in the heath is mostly dominated by Empetrum nigrum s. lat. but also Betula nana and Vaccinium myrtillus are frequent. Field layer of the meadow vegetation is more diverse and dominated by grasses (e.g. Avenella flexuosa, Deschampsia cespitosa), forbs (e.g. Rumex acetosa, Trollius europaeus, Viola spp.), vascular cryptogams (mainly Equisetum spp.), deciduous shrubs (mainly Salix spp.), sedges and rushes (e.g. Carex bigelowii, Carex aquatilis coll., Juncus filiformis) and mosses. Average (and range) total plant biomass during this study was 525 g/m (280–1056 g/m) in the heath habitat and 206 g/m (82–439 g/m) in the meadow habitat (see details on biomass measurements below). Biomass ranges for plant functional groups are shown in Figure 2.
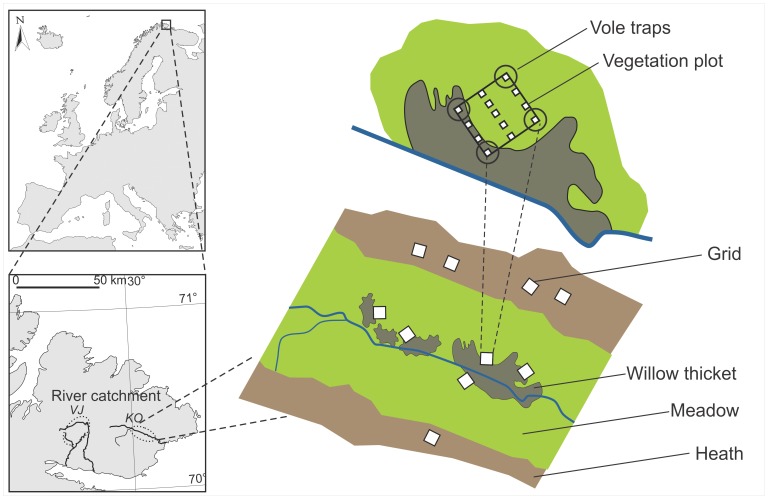
Figure 1. Study location and design.
The study was conducted in two river catchment areas; Vestre Jakobselva (VJ) and Komagelva (KO), in low-arctic tundra zone of north-eastern Norway. We established 26 (VJ) and 24 (KO) sampling grids (15 m×15 m), distributed in pairs in heath and meadow habitat throughout major parts of each catchment. In each sampling grid, we estimated plant biomass in 13 plots and small rodent density with 12 traps, 3 per grid corner.
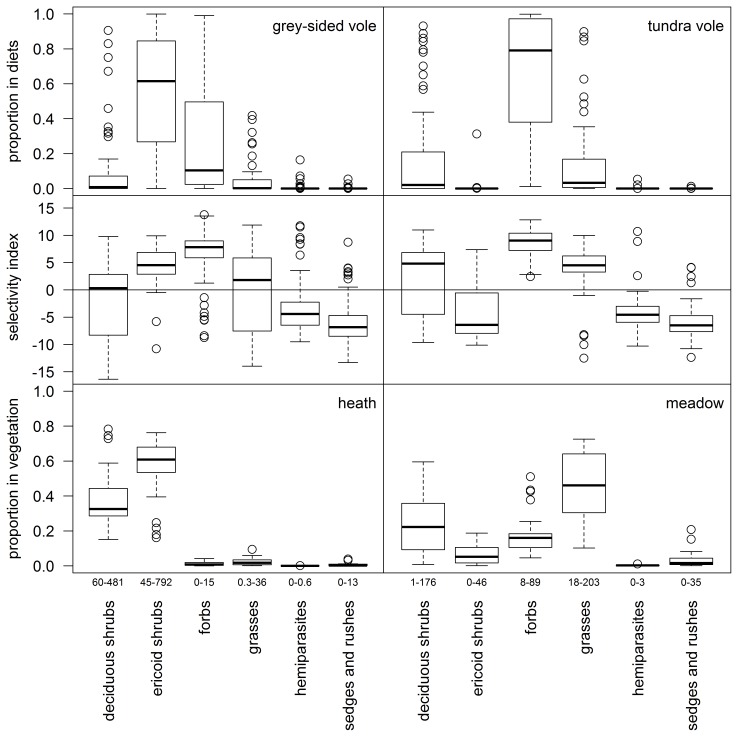
Figure 2. Vole diets, selectivity and food availability based on plant functional groups.
To the left grey-sided voles (Myodes rufocanus, n = 81) and heath vegetation, to the right tundra voles (Microtus oeconomus, n = 66) and meadow vegetation. Upper panels show proportions in diets, middle panels selectivity index and lower panels proportions in vegetation biomass. Selectivity index has been calculated as ratio between diet and vegetation proportions using compositional analysis; see methods for details. Index values above zero indicate preference whereas values below zero avoidance. Black line represents median, boxes first and third quartiles, whiskers either maximum values or 1.5 times interquartile range (whichever is smaller) and points outliers. Numbers below vegetation proportions represent the actual range of biomass (g/m) per group.
In heath habitats, grey-sided voles (Myodes rufocanus) are the most common small rodent species, whereas in the meadow habitats tundra voles (Microtus oeconomus) dominate the small rodent community [26], [42]. In addition to voles, Norwegian lemmings (Lemmus lemmus) are found in the area during their outbreak years. Small rodent populations in the region have cyclic population dynamics with high-amplitude peaks every 4–5 years [43], [44] and this study included a summer season peak in 2007. In addition to small rodents, semi-domesticated reindeer are abundant in the study area, whereas other mammalian herbivores are scarce. More detailed descriptions of the study area can be found in [22], [42], [45] and [45].
Study Design
In order to cover the range of variation in vegetation composition present at Varanger peninsula, we used a large-scale study design encompassing two river catchment areas; Komagelva (KO) and Vestre Jakobselva (VJ). In both river catchments, we established sampling grids (15×15 m) in equal numbers in both meadows and heaths (Figure 1). The sampling grids were selected to represent the range of variable field layer species compositions of both habitats. In total, KO had 12 sampling grids per habitat and VJ had 13. The distance between neighboring grids had a range of 160–2200 m, while the two most distant grids were 40 km apart. In order to measure food availability in the immediate habitat of each vole individual, we used the same study design for both vole trapping and plant biomass analysis. Population dynamics of voles differ between the focal river catchments [22], [42] and both vole species had lower densities in VJ compared to KO (Table 1).
Table 1. Vole density index during 2007 at Varanger peninsula.
| Grey-sided vole | Tundra vole | |||
| summer | autumn | summer | autumn | |
| Vestre Jakobselva | 3.21 (1.95) | 8.97 (1.88) | 2.56 (0.89) | 3.53 (1.14) |
| Komagelva | 7.64 (2.61) | 14.58 (2.32) | 14.24 (2.92) | 27.43 (4.34) |
Vole density index during 2007 in two river catchments at Varanger peninsula, based on snap-trapping, measured as individuals per 100 trapnights per sampling grid (mean and SE). Data for grey-sided voles is from heath grids only, for tundra voles meadow grids only. Vestre Jakobselva had 13 sampling grids per habitat, Komagelva 12.
Vole Trapping; Samples for Diet Analysis
In order to obtain samples for diet analysis, we conducted snap-trapping of voles in each sampling grid according to [46]. To estimate changes in diet during the growing season, the trapping was done twice, with the first period occurring between 22nd and 24th July and the second period occurring between 3rd and 5th September. Each trapping event consisted of 600 trap nights per habitat, with 12 traps in each grid, 25 grids and trapping over 2 nights. The traps were baited with raisins (Vitis vinifera) and oat flakes (Avena sativa). Voles were dissected and their stomachs stored in 70% ethanol until diet analysis.
Snap-traps were required, as the rodent trapping was part of a project where also the Norwegian lemmings were studied. Norwegian lemmings are hard to trap with live-traps, a phenomenon which has been repeatedly observed by different research groups [47]. In another study using live-traps, only one lemming was caught despite a large trapping effort (ca 6,000 trap-nights every year) and the occurrence of two small rodents peaks (2007 and 2011, resulting >10, 000 trapped voles) (Ims and Yoccoz unpublished data).
Vegetation Composition; Food Availability Data
Vegetation of each grid was sampled during the peak of the growing season, i.e. between 22nd July and 8th August. We established 13 vegetation sampling plots (0.5×0.5 m) in each grid (Figure 1) and estimated the biomass of all vascular plant species present in the plots using a non-destructive point intercept method [48], [49] with 20 pins in each plot. We then converted the point intercept counts to biomass estimates (g/m) for each grid, by first converting the hits to biomass per plot using calibration described in [50]. In another study across northern Norwegian landscapes, encompassing similar habitats as the current study, plant growth form was found to be the most important predictor for both vegetative and flowering phenology of plants [51]. Hence, the phenology of biomass in our study area could be expected to fairly similar in both river catchements. To account for the temporal changes in biomass we included the effect of season to our analyses.
Ethics Statements
The study area is part of Varangerhalvøya National Park. No permit was required for the non-destructive vegetation sampling, as only destructive use of vegetation is prohibited in the national park (FOR-2006-12-08-1384, Regulation of Varangerhalvøya nationalpark protection plan). Vole trapping was conducted as part of the “Arctic fox in Finnmark” project (http://www.fjellrev-finnmark.uit.no/), which was initiated, financed and approved by The Norwegian Directorate of Nature Management (DN). The DN is the legal Norwegian authority that licenses sampling of all vertebrate wild life species for scientific purposes (LOV 1981-05-29 nr 38: Lov om jakt og fangst av vilt (viltloven) http://www.lovdata.no/cgi-wift/ldles?doc=/all/nl-19810529-038.html&26) and regulation about sampling wildlife for scientific or other specific purposes (FOR-2003-03-14-349 Forskrift om innfanging og innsamling av vilt for vitenskapelige eller andre særlige formal http://www.lovdata.no/for/sf/md/md-20030314-0349.html). No specific permit was issued for this project, but sampling protocol was approved by the DN. No protected species were sampled.
Diet Analysis
Stomach contents of grey-sided voles trapped from heath habitat (n = 82) and tundra voles trapped from meadow habitat (n = 67) were analyzed for spermatophyte (i.e. seed plant) content. Part of the dataset is published by [32], who described in detail the DNA metabarcoding methods used (see Table S1 for additional details on the datasets). In summary, spermatophyte plant DNA was amplified from a sample of each voles stomach content using primer pair g-h, which targets the P6-loop of chloroplast trnL (UAA) intron [52]. Samples from different individuals were thereafter individually tagged, pooled to one sample and pyrosequenced. The resulting sequences were sorted to individual voles based on the tags and compared to two taxonomic reference libraries to identify which taxon they belonged to. We first used a library containing sequences of 842 arctic vascular plants [53] (accession numbers GQ244527 - GQ245667 in GenBank). Thereafter, we compared sequences which could not be satisfactorily identified to sequences retrieved from GenBank (available at http://www.ncbi.nlm.nih.gov/genbank/). For each vole individual, we thus achieved a count of sequences belonging to different taxa. To make data from different vole individuals comparable, we transformed these counts to proportions of different taxa in an individuals’ stomach content, hereafter termed as “diet proportions”.
Quantitative use of DNA metabarcoding data is potentially hampered by several technical issues [38]. However, based on a comparison with traditionally used microhistological method [32], DNA metabarcoding reflects well the actual proportions of spermatophytes in vole diets. We also verified that diet at the vole population level, measured as food item proportions, did not differ greatly from diets determined by frequency of occurrence (i.e. percentage of vole individuals which had ingested the taxa in question), as recommended by [37] (Tables S2 and S3). Moreover, a taxon may be over-represented in a DNA metabarcoding dataset if it has a short target DNA-region in comparison to other simultaneously analyzed taxa [54]. We therefore also confirmed that the most abundant taxa did not have clearly shorter target-DNA regions than other taxa. Both frequencies of occurrence and lengths of the targeted DNA region are given in Tables S2 and S3. We removed two vole individuals from the dataset prior to the analyses. One of these was a grey-sided vole that had seemingly eaten only one plant species, an unlikely result which could easily be due to low DNA quality of the sample. The other was a tundra vole whose diet was composed 99% of Pinus sylvestris, a species not present in the study area. Rather than representing a new species in the region’s flora, such a result is probably caused by errors during the analyses [38].
The reference libraries we used included a different range of species than those present in the study area. We therefore checked for potential mis-identifications and adjusted the sequence assignments based on taxa present in Northern Fennoscandia [55]. Taxa which are not found in the region were assigned to their next higher taxonomic level (e.g. Cerastium maximum was assigned to Cerastium sp. and Gaylussacia sp. to Ericaceae). Adjustments were also made to more specific taxa, i.e. when a genus (or family) was represented by only one species (or genus), it was assigned to this representative (e.g. Bistorta sp. was assigned to Bistorta vivipara and Betulaceae were assigned to Betula spp.). Sequences originally assigned to Vaccinium alaskense, which is not found in the region were grouped together with those assigned to Vaccinium myrtillus. These species are almost identical at the DNA region we used for identification but differ from other Vaccinium species of Northern Fennoscandia, namely Vaccinium uliginosum and Vaccinium vitis-ideae (accession numbers GQ245635-GQ245641 in GenBank).
Definitions of Food Item Groups
For analysis at plant functional group level we classified plants as forbs, grasses, sedges and rushes, deciduous shrubs, ericoid shrubs, or hemiparasites. The grouping was primarily based on nutritional characteristics [40], [56] as well as responses to herbivory in the focal ecosystem [22], [57]. However, we grouped all ericoid shrubs together as less than half of the sequences within Ericaceae were identified at a detailed enough level to allow distinguishing between deciduous and evergreen shrubs. The deciduous shrubs -group was thus composed of Betulaceae and Salicaceae. Only a few non-ericoid evergreens (Pyrolaceae, Pinaceae, Cupressaceae) were recorded in the diets and each of them occurred only in one vole individual (Tables S2 and S3). These taxa compose a very small fraction of the biomass (on average 0.003% and 0.4% in heaths and meadows respectively) and we therefore excluded them from all analyses. We also excluded data on vascular spore plants (i.e. Equisetum and ferns) from the analyses, as the primer pair g-h is designed particularly for spermatophytes and does not reflect well the abundance of other plant groups.
For analyses based on taxonomic units, we grouped the plants at family level in order to be able to include majority of the data. For example, 36% of sequences identified to Ericaceae in grey-sided voles diets could not be identified to genera (Tables S2 and S3). However, for two families we had sufficient data to refine the analyses to species level. One of these, Cornaceae is represented in Northern Fennoscandia by only one species (Chamaepericlymenum suecica). The other family for which we achieved species level resolution was Ranunculaceae for tundra voles. Ranunculus acris coll. was the only representative in the meadow grids and Ranunculus sp. constituted 99% of Ranunculaceae in tundra vole diet. We therefore used data on Ranunculus acris coll. in all analyses of Ranunculaceae in tundra vole diets and selectivity.
Statistical Analysis
Food selection: compositional analysis
To determine selectivity we used compositional analysis of centered log-ratio transformed proportions [58] of food items in individual diets and available vegetation, at both plant family and functional group level. The centered log-ratio transformation was implemented by function named “clr” in in the R library compositions [59]. As food availability data we used for each vole individual, the biomass proportions from the grid in which it was trapped. The selectivity index was calculated as clr(diet proportions) -clr(available proportions) [58]. To test whether selectivity for different food items was significantly different, we used compana -function in adehabitat-library of R, which computes pairwise significances in preference among food items using Wilks lambda [60]. Results of these significance tests are presented in Tables S4, S5, S6, S7, while diets, food availability and selectivity index values are shown in Figures 2 and 3.
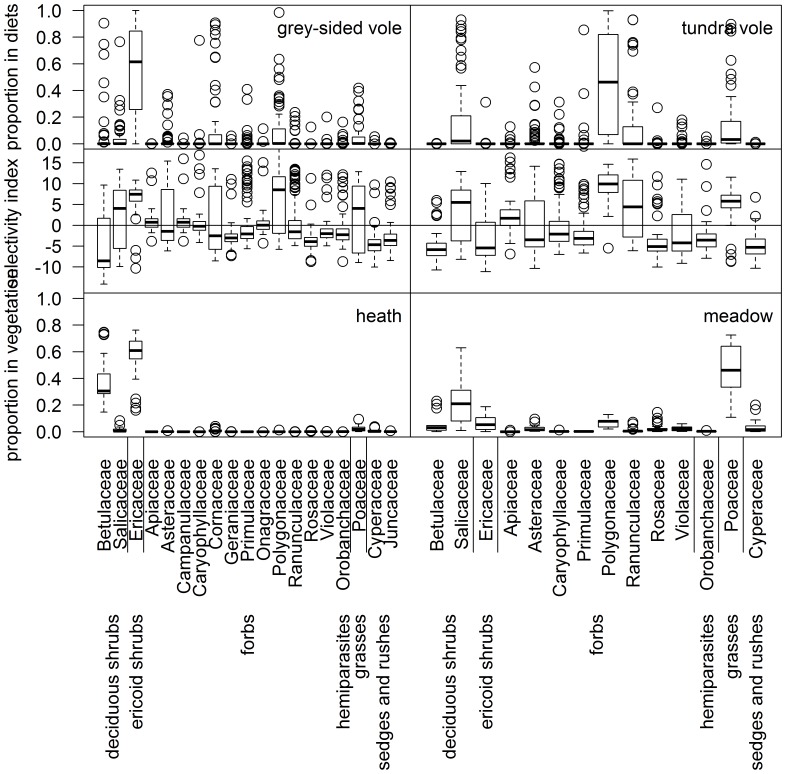
Figure 3. Vole diets, selectivity and food availability based on plant families.
To the left grey-sided voles (Myodes rufocanus, n = 81) and heath vegetation, to the right tundra voles (Microtus oeconomus, n = 66) and meadow vegetation. Upper panels show proportions in diets, middle panels selectivity index and lower panels proportions in vegetation biomass. Selectivity index has been calculated as ratio between diet and vegetation proportions using compositional analysis; see methods for details. Index values above zero indicate preference whereas values below zero avoidance. Black line represents median, boxes first and third quartiles, whiskers either maximum values or 1.5 times interquartile range (whichever is smaller) and points outliers. Plant families are grouped according to functional groups.
Both the data for diet and available food contained zeros, which have to be replaced to enable compositional analysis [58]. We followed recommendations given by [58], replacing zeros with a value three orders of magnitude smaller than any observed used proportion (0.000077) to the diet proportions. This very small replacement value ensured that minute amounts of detectable DNA were not included in the analysis. We excluded plant families which never occurred in diets from the compositional analysis at family level, as their combined biomass was on average <1% in heaths and 2% in meadows. We replaced zero availability in a given grid with average biomass of the food item in question in the same habitat and river catchment. When a food item was not recorded within a river catchment, we used average biomass of the habitat across river catchments. Campanulaceae and Apiaceae were never recorded in the heath habitat, even if they were recorded in the diets of two grey-sided voles. We replaced zero availability in these families by an order of magnitude smaller value than the smallest observed proportion of any family in the heath. We included sequences of trap bite (Avena and Vitis) in calculations of food item proportions in stomach contents, but excluded them from further analyses.
Variability in diets and selectivity: linear mixed effect models
We evaluated whether I) the proportion of a food item in diet and II), selectivity for it were related to vegetation composition using linear mixed effect models implemented by lmer-function from lme-4 library of R [61]. In order to target important food items and retain sufficient sample size for the models, we modeled separately the response of each food item which was both selected for and eaten by at least ca. 50% of the individuals of a given vole species. For grey-sided voles these were the functional group forbs and families Ericaceae, Polygonaceae, Poaceae, Salicaceae and Cornaceae, while for tundra voles they were the functional group forbs and families Polygonaceae, Poaceae, Ranunculaceae and Salicaceae. No other functional group than forbs had several families which were eaten so commonly that they could be tested separately. For all of these food items, we modeled separately proportions in diets and the selectivity index score. For diets, we used logit transformed proportions as the response variable, avoiding zeros by adding a value which was an order of magnitude smaller than the smallest value of the respective predictor variable, while selectivity index scores were already at logit-scale.
For each response variable, we created two alternative models. The first model included as predictors a) biomass of the food item itself and b) biomasses of other substantially eaten food items at family level (i.e. those listed above). However, to better fit data with the voles feeding ecology, we only used biomass of palatable deciduous Vaccinium species as predictor instead of Ericaceae. For grey-sided voles, Salicaceae was omitted from models which included Polygonaceae, as their biomasses in vegetation were highly correlated. In the alternative model we replaced the predictor(s) forb families by the forb functional group, leaving the response variable at family level. In all of these models, we evaluated the spatial variability of diets and selectivity in two ways, using river catchment (KO and VJ) as a fixed effect and grid identity as random effect. In addition, we included season (autumn and summer) as a fixed effect. When random effect variance was estimated as zero, we removed the term and present a model with fixed effects only (using lm-function of R). We then selected the better model using likelihood ratio test (model parameters estimated using ML) [62]. When neither model was significantly better, we show the model with less parameters. We present the final mixed effect models with model parameters estimated using REML. In addition to statistically significant effects (defined as 95% confidence intervals not encompassing zero), we included in our interpretation close-to-significant trends which seemed biologically interesting. These are statistically defined as having 95% confidence intervals crossing zero by <0.05 and an effect size of >0.15. After fitting fixed terms of the models, we calculated the proportion of remaining variance explained by random variable “grid identity” (i.e. grid variance/(grid variance+residual variance)) [62].
In each model, we removed those individuals which had a combination of zero availability and zero use of the response food item. We checked models for outliers and removed one heath grid where Polygonaceae biomass was approximately 8 times that of any other grid (thus removing two grey-sided vole individuals). We also verified that models showed constant variance of the residuals and approximate linearity between the fitted and observed values. For models with random effects we estimated significance of the fixed parameters with 95% confidence intervals (Markov Chain Monte Carlo estimation with 100 000 replicates using mcmcsamp -function) [61], while for models without random effects we used the confint-function of R. We used the software R for all analyses [63].
Results
Diets
At the level of plant functional groups, diet of the grey-sided voles was dominated by ericoid shrubs, followed by forbs (Figure 2). Deciduous shrubs and grasses were also eaten but less commonly (Figure 2). Within ericoid shrubs, i.e. within the family Ericaceae, deciduous species Vaccinium uliginosum (9%) and Vaccinium myrtillus (8%) were the most commonly identified but also everegreen shrubs, mainly Empetrum nigrum s. lat. (6%) were found (Table S2). Within the functional group of forbs, most abundantly eaten families were Cornaceae (10%, represented by Chamaepericlymenum suecica) and Polygonaceae (9%, represented mainly by Rumex sp.) (Figure 3, Table S2). Grey-sided voles had also consumed a range of other forb families at a lower proportion, many of which occurred in only a few individuals (Figure 3, Table S2). Species richness of grey-sided voles diet (n = 82 vole individuals) was 28 at species level, 37 at genera level and 23 at family level (Table S2).
At the level of plant functional groups, the diet of tundra voles was markedly dominated by forbs, followed by deciduous shrubs and grasses (Figure 2). The functional group of forbs was dominated by family Polygonaceae (45%, represented mainly by Rumex sp.) (Figure 3). While the family Ranunculaceae was also commonly eaten (12%), the mean proportion of other forb families was low and many of them occurred in only a few individuals (Figure 3, Table S3). Species richness of tundra vole diet (n = 67 vole individuals) was 26 at species level, 35 at genera level and 23 at family level (Table S3).
Selectivity
Both vole species had the strongest selection for forbs (Figure 2, Tables S4 and S5). After forbs, grey-sided voles selected for ericoid shrubs and thereafter grasses, whereas tundra voles selected for grasses and thereafter deciduous shrubs (Figure 2, Tables S4 and S5). The patterns of selectivity at functional group level differed somewhat from those at plant family level. For example, only one forb family, namely Polygonaceae, was more often selected than Poaceae (i.e. grasses), a pattern found for both vole species (Figure 3, Tables S6 and S7). Also within the functional group of deciduous shrubs both vole species showed a similar pattern; Salicaceae was relatively preferred whereas Betulaceae was the least preferred (Figure 3, Tables S6 and S7).
Spatio-temporal Variation of Diets and Selectivity
Both vole species showed temporal and spatial variation in their feeding habits. During summer, grey-sided voles had higher proportions of Poaceae and forbs, especially Polygonaceae in their diets than during autumn (Table 2). During autumn, they selected more for Ericaceae than during summer (Table 3). Tundra vole diets and selectivity were less modified by season than that of grey-sided voles but tundra voles also selected for Polygonaceae more during summer than autumn (Tables 4 and 5). Spatial variability in diets and selectivity was measured at two scales; river catchment and sampling grid. Of these, river catchment had little effect on the vole diets and selectivity (Tables 2, 3, 4, 5). Only grey-sided voles use of Poaceae varied at the scale of river catchment, with diet proportions and selectivity being higher at VJ than at KO (Tables 2 and 3). However, both vole species showed spatial variability in diet proportions and selectivity at the scale of sampling grids, based on percentage of residual variance explained by grid identity (Tables 2, 3, 4, 5).
Table 2. Effect of food availability, season and river catchment on grey-sided vole diets.
| Predictors | Responses | |||||||||||
| Polygonaceae (n = 52) | Cornaceae (n = 63) | Ericaceae (n = 79) | Poaceae (n = 79) | Salicaceae (n = 59) | forbs (n = 79) | |||||||
| Est. | 95% CI | Est. | 95% CI | Est. | 95% CI | Est. | 95% CI | Est. | 95% CI | Est. | 95% CI | |
| fixed effects | ||||||||||||
| intercept | −6.01 | −7.86, −4.11 | −5.89 | −8.31, −3.58 | 0.51 | −0.45, 1.47 | −9.35 | −11.02, −7.47 | −6.42 | −8.53, −4.51 | −3.84 | −5.39, −2.14 |
| Polygonaceae | 0.11 | −6.76, 7.63 | ||||||||||
| Cornaceae | 0.26 | −0.27, 0.84 | −2.17 | −4.41, 0.07 | ||||||||
| Poaceae | 0.17 | 0.01, 0.34 | −0.18 | −0.45, 0.11 | −0.04 | −0.11, 0.03 | 0.04 | −0.10, 0.22 | −0.03 | −0.23, 0.13 | 0.06 | −0.08, 0.21 |
| Salicaceae | 0.23 | −0.02, 0.53 | −0.01 | −0.10, 0.07 | 0.08 | −0.08, 0.25 | −0.09 | −0.27, 0.07 | 0.09 | −0.08, 0.23 | ||
| decidious Vaccinium | −0.007 | −0.05, 0.03 | 0.02 | −0.04, 0.09 | 0.01 | −0.006, 0.03 | 0.01 | −0.02, 0.04 | 0.005 | −0.02, 0.04 | 0.005 | 0.03, 0.03 |
| forbs | −0.30 | −0.62, 0.02 | −0.61 | −1.61, 0.28 | −0.04 | −0.36, 0.28 | 0.02 | −0.26, 0.42 | 0.13 | −0.14, 0.44 | ||
| season (summer) | 3.26 | 0.99, 5.59 | 1.06 | −1.46, 3.63 | −0.90 | −2.04, 0.23 | 1.91 | 0.10, 4.01 | 1.11 | −0.57, 3.91 | 2.30 | 0.31, 3.90 |
| river catchment (VJ) | 1.28 | −1.67, 4.24 | −1.95 | −6.59, 2.36 | 0.80 | −0.52, 2.12 | 3.38 | 0.74, 5.94 | −1.04 | −3.86, 1.80 | 0.23 | −2.18, 2.53 |
| random effects | ||||||||||||
| grid | 3.65e−05 | (n = 17, <1%) | 2.87 | (n = 15, 31%) | NA | (n = 19) | 1.65 | (n = 19, 17%) | 1.64 | (n = 19, 28%) | 1.51 | (n = 19, 17%) |
| residual | 3.55 | 4.23 | 2.16 | 3.71 | 2.65 | 3.35 | ||||||
Parameter estimates of linear mixed effect models for the effect of food availability (biomass g/m), season and river catchment on grey-sided vole stomach content proportions. Intercept is calculated for autumn, Komagelva (KO) and mean biomass of all continuous predictor variables. “Est.” refers to regression coefficients, measured at logit-scale. Random effects are presented as standard deviations, sample size (n) referring to the number of grids included in the analysis, % values to the percentage of residual variance assigned to grid. Estimates with bold indicate that 95% CI does not include 0, with italics that 95% CI includes zero at most 0.05 and effect size is >0.15. Models where data were insufficient to evaluate the random effect (NA), have been calculated as linear regressions with fixed effects only. “Forbs” as predictor variable represents availability of the functional group of forbs, except for models which have a forb family (Polygonaceae, Cornaceae) as response variable. For these, biomass of the respective family is excluded from that of forbs and used as a separate predictor. Empty cells indicate that predictor variable in question has not been included in the model. See Material and Methods for details.
Table 3. Effect of food availability, season and river catchment on grey-sided vole selectivity.
| Predictors | Responses | |||||||||||
| Polygonaceae (n = 51) | Cornacea (n = 61) | Ericaceae (n = 75) | Poaceae (n = 76) | Salicaceae (n = 58) | forbs (n = 77) | |||||||
| Est. | 95% CI | Est. | 95% CI | Est. | 95% CI | Est. | 95% CI | Est. | 95% CI | Est. | 95% CI | |
| fixed effects | ||||||||||||
| intercept | 8.34 | 5.78, 10.90 | 3.91 | 0.22, 7.71 | 7.41 | 6.66, 8.15 | −2.23 | −5.54, 1.37 | 4.14 | −1.61, 12.70 | 4.86 | 0.66, 9.32 |
| Polygonaceae | −4.93 | −14.28, 4.42 | ||||||||||
| Cornaceae | 0.43 | −0.39, 1.38 | ||||||||||
| Poaceae | 0.23 | 0.02, 0.45 | −0.29 | −0.73, 0.16 | −0.02 | −0.09, 0.04 | 0.03 | −0.27, 0.36 | −0.08 | −0.43, 0.20 | 0.03 | −0.15, 0.23 |
| Salicaceae | 0.33 | −0.07, 0.79 | −0.03 | −0.10, 0.04 | 0.16 | −0.17, 0.48 | −0.23 | −0.54, 0.04 | 0.05 | −0.16, 0.24 | ||
| deidious Vaccinium | −0.01 | −0.06, 0.04 | 0.03 | −0.07, 0.13 | 0.004 | −0.009, 0.02 | 0.01 | −0.05, 0.08 | −0.01 | −0.06, 0.06 | 0.003 | −0.03, 0.04 |
| forbs | −0.58 | −1.01, −0.16 | −1.02 | −2.57, 0.34 | −0.04 | −0.17, 0.09 | −0.03 | −0.66, 0.57 | 0.03 | −0.44, 0.74 | 0.11 | −0.28, 0.51 |
| season (summer) | 2.39 | −0.82, 5.60 | 1.25 | −3.10, 5.42 | −1.12 | −2.0, −0.22 | 3.22 | −0.22, 7.32 | 1.93 | −0.88, 6.68 | 0.63 | −1.90, 2.89 |
| river catchment (VJ) | 0.16 | −3.78, 4.09 | −3.00 | −10.33, 3.57 | 0.45 | −0.60, 1.51 | 5.94 | 0.83, 11.00 | −2.18 | −6.79, 3.05 | −0.88 | −3.95, 2.19 |
| random effects | ||||||||||||
| grid | NA | (n = 17) | 3.97 | (n = 15, 24%) | NA | (n = 19) | 3.30 | (n = 19, 18%) | 4.39 | (n = 19, 40%) | 1.50 | (n = 19, 9%) |
| residual | 4.62 | 7.12 | 1.68 | 6.98 | 5.45 | 4.58 | ||||||
Parameter estimates of linear mixed effect models for the effect of food availability (biomass g/m), season and river catchment on grey-sided vole selectivity, i.e. difference between stomach content and biomass proportions. Intercept is calculated for autumn, Komagelva (KO) and mean biomass of all continuous predictor variables. “Est.” refers to regression coefficients, measured at logit-scale. Random effects are presented as standard deviations, sample size (n) referring to the number of grids included in the analysis, % values to the percentage of residual variance assigned to grid. Estimates with bold indicate that 95% CI does not include 0, with italics that 95% CI includes zero at most 0.05 and effect size is >0.15. Models where data were insufficient to evaluate the random effect (NA), have been calculated as linear regressions with fixed effects only. “Forbs” as predictor variable represents availability of the functional group of forbs, except for models which have a forb family (Polygonaceae, Cornaceae) as response variable. For these, biomass of the respective family is excluded from that of forbs and used as a separate predictor. Empty cells indicate that predictor variable in question has not been included in the model. See Material and Methods for details.
Table 4. Effect of food availability, season and river catchment on tundra vole diets.
| Predictors | Responses | |||||||||
| Polygonaceae (n = 66) | Ranunculaceae(n = 62) | Poaceae (n = 66) | Salicaceae(n = 66) | forbs (n = 66) | ||||||
| Est. | 95% CI | Est. | 95% CI | Est. | 95% CI | Est. | 95% CI | Est. | 95% CI | |
| fixed effects | ||||||||||
| intercept | −1.54 | −3.21,0.05 | −7.50 | −10.08, −4.95 | −3.20 | −4.51, −1.86 | −4.17 | −6.48, −1.71 | −1.33 | −4.41, 1.93 |
| Polygonaceae | 0.04 | −0.13, 0.21 | ||||||||
| Poaceae | 0.01 | −0.01, 0.04 | −0.02 | −0.06, 0.03 | −0.0004 | −0.02, 0.02 | −0.02 | −0.06, 0.02 | 0.008 | −0.01, 0.03 |
| Salicaceae | −0.01 | −0.04, 0.007 | 0.01 | −0.02, 0.04 | −0.003 | −0.02, 0.01 | −0.002 | −0.03, 0.03 | 0.0004 | −0.02, 0.02 |
| Ranunculus | 0.77 | −0.18, 1.72 | ||||||||
| forbs | 0.01 | −0.05, 0.08 | −0.05 | −0.21, 0.09 | −0.02 | −0.07, 0.03 | −0.05 | −0.14, 0.05 | 0.03 | −0.03, 0.08 |
| season (summer) | 0.96 | −0.67, 2.57 | 1.17 | −1.36, 3.80 | −0.47 | −1.79, 0.90 | −0.12 | −2.46, 2.28 | 0.61 | −0.78, 1.99 |
| river catchment (VJ) | 2.09 | −1.71, 6.03 | 0.92 | −4.98, 6.82 | −0.14 | −3.06, 2.82 | −2.98 | −8.72, 2.06 | 1.50 | −1.45, 4.44 |
| random effects | ||||||||||
| grid | 5.28e−05 | (n = 21, <1%) | 5.85e−05 | (n = 21, <1%) | 3.02e-05 | (n = 21, <1%) | 1.93 | (n = 21, 16%) | 1.71e-05 | (n = 21, <1%) |
| residual | 3.18 | 4.85 | 2.64 | 4.39 | 2.72 | |||||
Parameter estimates of linear mixed effect models for the effect of food availability (biomass g/m), season and river catchment on tundra vole stomach content proportions. Intercept is calculated for autumn, Komagelva (KO) and mean biomass of all continuous predictor variables. “Est.” refers to regression coefficients, measured at logit-scale. Random effects are presented as standard deviations, sample size (n) referring to the number of grids included in the analysis, % values to the percentage of residual variance assigned to grid. Estimates with bold indicate that 95% CI does not include 0, with italics that 95% CI includes zero at most 0.05 and effect size is >0.15. Models where data were insufficient to evaluate the random effect (NA), have been calculated as linear regressions with fixed effects only. “Forbs” as predictor variable represents availability of the functional group of forbs, except for models which have a forb family (Polygonaceae, Ranunculaceae) as response variable. For these, biomass of the respective family is excluded from that of forbs and used as a separate predictor. Empty cells indicate that predictor variable in question has not been included in the model. See Material and Methods for details.
Table 5. Effect of food availability, season and river catchment on tundra vole selectivity.
| Predictors | Responses | |||||||||
| Polygonaceae (n = 65) | Ranunculaceae(n = 62) | Poaceae (n = 66) | Salicaceae(n = 66) | forbs (n = 66) | ||||||
| Est. | 95% CI | Est. | 95% CI | Est. | 95% CI | Est. | 95% CI | Est. | 95% CI | |
| fixed effects | ||||||||||
| intercept | 8.50 | 7.14, 9.95 | 3.19 | −0.36, 6.75 | 5.90 | 4.01, 7.78 | 4.97 | 1.73, 8.34 | 8.10 | 6.93, 9.26 |
| Polygonaceae | 0.04 | −0.10, 0.18 | −0.17 | −0.34, 0.02 | ||||||
| Poaceae | 0.001 | −0.02, 0.03 | −0.02 | −0.08, 0.03 | −0.004 | −0.03, 0.03 | −0.03 | −0.09, 0.02 | −0.001 | −0.02, 0.02 |
| Salicaceae | −0.01 | −0.03, 0.007 | 0.02 | −0.02, 0.06 | −0.03 | −0.06, −0.006 | −0.01 | −0.05, 0.03 | 0.004 | −0.01, 0.01 |
| Ranunculus | 1.33 | 0.02, 2.64 | 0.20 | −0.41, 0.81 | ||||||
| forbs | 0.02 | −0.04, 0.07 | −0.12 | −0.32, 0.09 | −0.06 | −0.18, 0.08 | 0.04 | −0.01, 0.08 | ||
| season (summer) | 1.99 | 0.56, 3.23 | 1.50 | −2.19, 5.20 | 0.41 | −1.64, 2.45 | −0.56 | −3.94, 2.67 | 1.08 | −0.18, 2.17 |
| river catchment (VJ) | 0.76 | −2.38, 4.25 | 1.94 | −5.99, 9.86 | −3.40 | −7.54, 0.74 | −4.90 | −12.95, 1.85 | 0.75 | −1.69, 3.48 |
| random effects | ||||||||||
| grid | 1.25 | (n = 20, 21%) | NA | (n = 21) | NA | (n = 21) | 2.25 | (n = 21, 12%) | 1.31 | (n = 21, 28%) |
| residual | 2.41 | 6.55 | 3.79 | 6.19 | 2.10 | |||||
Parameter estimates of linear mixed effect models for the effect of food availability (biomass g/m), season and river catchment on tundra vole selectivity, i.e. difference between stomach content and biomass proportions. Intercept is calculated for autumn, Komagelva (KO) and mean biomass of all continuous predictor variables. “Est.” refers to regression coefficients, measured at logit-scale. Random effects are presented as standard deviations, sample size (n) referring to the number of grids included in the analysis, % values to the percentage of residual variance assigned to grid. Estimates with bold indicate that 95% CI does not include 0, with italics that 95% CI includes zero at most 0.05 and effect size is >0.15. Models where data were insufficient to evaluate the random effect (NA), have been calculated as linear regressions with fixed effects only. “Forbs” as predictor variable represents availability of the functional group of forbs, except for models which have a forb family (Polygonaceae, Ranunculaceae) as response variable. For these, biomass of the respective family is excluded from that of forbs and used as a separate predictor. Empty cells indicate that predictor variable in question has not been included in the model. See Material and Methods for details.
Impact of Availability on Diets and Selectivity
We found few clear effects of biomass of a food item (i.e. availability) on its use (Tables 2, 3, 4, 5). The sole statistically significant effect was that tundra voles were more selective for Ranunculaceae when its biomass was higher (Table 5). In addition we found one non-significant trend whereby grey-sided voles’ selectivity for Salicaceae decreased with its biomass (Table 3). However, use of several food items decreased with the availability of alternative food items (Tables 2, 3, 4, 5). For grey-sided voles, selectivity for Polygonaceae decreased with biomass of other forbs and Polygonaceae proportion in diets had a similar trend (Tables 2 and 3). Moreover, increasing biomass of Salicaceae tended to decrease the proportion of Cornaceae in the diets of grey-sided voles (Table 2). For tundra voles, selectivity for Poaceae decreased when biomass of Salicaceae increased and had a similar trend with biomass of Polygonaceae (Table 5). We also found opposite patterns in grey-sided voles, i.e. use of a food item increasing with the availability of alternative food items (Tables 2 and 3). Of these, both diet proportions and selectivity for Polygonaceae increased when biomass of Poaceae increased, whereas diet proportions of Cornaceae increased with biomass of Salicaceae (Tables 2 and 3). Some of these indirect effects, both negative and positive ones, were caused by changes in the biomass of food items which were on average less selected for than the response food item.
The use of different forb families responded differently to their respective biomass, biomass of alternative food items and season (Tables 2, 3, 4, 5). However, combined biomass of forbs better predicted the consumption of other food items than those of separate forb families. Only the selectivity of tundra voles for Poaceae was slightly better predicted by a model which included biomass of Polygonaceae and Ranunculaceae as predictor variables than with a model using combined forb biomass ( = 3.62, d.f. = 1, p = 0.06).
Discussion
Both grey-sided voles and tundra voles consumed a diverse range of food items. Although diets and selectivity varied seasonally and spatially, the biomass of a food item had little effect on its use but sometimes influenced the use of other food items. Together, these results show that both vole species exhibit flexible feeding ecology.
New Insights into Vole Diets
Most studies on the interactions between grey-sided voles and vegetation have focused on Vaccinium myrtillus, which has been considered as the most important food item of this species [64]–[68]. However, our results show that during the snow-free period the species has a diverse diet which includes, in addition to V. myrtillus, a range of different herbaceous food items. We also found surprisingly much V. uliginosum (on average 9% of diets, eaten by 50% of individuals), even if it is relatively rare in the heath vegetation (2% of biomass in average), indicating that it is selected much more than previously observed (similar numbers for V. myrtillus are 8% in diets, eaten by 68% of individuals, 20% of biomass in average). Interestingly, V. uliginosum and E. nigrum have been suggested to be unpreferred species and eaten only when population densities are high [69]. Further, they have been suggested to produce toxins and therefore to have a negative impact on vole population growth rate even if they constitute only a small proportion of diets [69]. We found additional support for this hypothesis for V. uliginosum, which inclusion in the diets of grey-sided voles increased with population density. V. uliginosum was found in 25% of the individuals in VJ during summer while corresponding values were 42%, 45%, 62% for VJ autumn, KO summer, KO autumn, respectively (in comparison to population density index in Table 1). For E. nigrum, on the other hand, we found no such pattern (proportion of individuals that had ingested it, in same order as above, were 70%, 34%, 50% and 75%). Still, as we observed both of these plant species to be eaten by more than half of the studied grey-sided voles during a peak year, it is possible that these food items play a role in the population dynamics of this vole species at the Varanger Peninsula.
For tundra voles, we found forbs to dominate diets and be highly selected for, unlike previous microhistological stomach content studies which have emphasized the use of especially Eriophorum and Equisetum [29]–[31]. Such discrepancies between studies are probably partly due to differences in availability resulting in different diets. For example, forbs were abundant and Eriophorum absent in our study grids, whereas forbs were rare and Eriophorum abundant in habitats where tundra vole diets have been studied before [29]–[31]. Moreover, in similar habitats the closely related field voles (Microtus agrestis) have also been found to have diets dominated by dicotyledons [70], and in a cafeteria-test tundra voles showed a preference for forbs [71]. As Eriophorum was not included in that test, it remains unclear whether plant availability modifies only vole diets or also preferences. In addition, different methodology may contribute to differences in results. While results based on microhistological methods have a tendency to overestimate monocots, the DNA metabarcoding method used in this study possibly underestimates Equisetum [32], [52], [72]. In spite of such methodological discrepancies, habitat-specific food availability is likely to be an important determinant of tundra vole diets.
Both vole species selected for highly palatable functional groups, i.e. forbs and grasses, indicating that vole food preferences are related to plant nutritional quality [41], [56], [73]. However, within plant functional groups different families and species were eaten and selected to a very different degree. For example, some forb species were rarely eaten even if their availability did not greatly differ from that of other, more commonly consumed species. Moreover, the use of forb families responded differently to biomass and season. Different nutritional value may explain such differences but because only few measurements of energy, nutrients or secondary metabolites in subarctic forb species exist [64], [74], [75], we cannot judge the importance of different nutritional characteristics for voles. Within the functional group deciduous shrubs, both vole species preferred willows (Salix spp.) but avoided birches (Betula spp.), a pattern consistent with palatability of these taxa as well as previous food selection studies of voles [76], [77]. Thus, more detailed patterns of food quality than those reflected by plant functional groups, as defined in this study, seem to direct food preferences of voles. However, field measurements of detailed food-selection units have limitations especially when the food items are scarce. For example, grey-sided voles preferred forbs as a functional group even though at family level most forbs were seemingly not preferred, a pattern which could simply be due to different forbs being available to different individuals. Plant functional groups have mainly been studied from a plant ecological perspective [40], [41], [56], [73] and only few attempts have been made to evaluate them based on herbivores ecology [22], [78], [79]. However, small rodent food-selection units may be best reflected by plant functional groups defined from a herbivores perspective.
Previous analyses of diets of small rodents have used methods that are constrained to a taxonomically coarse resolution. Using DNA metabarcoding we were able to reveal that both vole species had remarkably diverse diets in terms of consuming a large number of plant taxa. In fact, diet diversity as such may be an important attribute of vole diets, as it is in general acknowledged to be an important determinant of herbivore performance [15], [80]. Accordingly, [81] found that species richness of vascular plants in the sub-arctic habitats of grey-sided voles was the most important predictor of female reproductive success. It therefore seems likely, that increased understanding of the role of food item diversity for small rodents should reveal previously unknown aspects of vegetation-small rodent interactions.
Small Rodent Functional Responses
Several earlier studies on mammalian herbivore food selection have indicated that availability, both absolute and relative of a preferred food item may increase its use [13], [82]. Seasonal effects were common even though we found that the biomass of a food item had little effect on its consumption. Nutrient content of herbaceous plants decreases towards the end of the growing season [73]–[75], [83]. Moreover, while berries produced by ericoid shrubs are more palatable than leaves of these plants they are available only in the autumn [51]. The seasonal changes in grey-sided voles feeding habits, i.e. the decrease of forbs and grasses in diets and increased selectivity for Ericaceae from summer to autumn, thus seem to be related to availability of good-quality food. However, at the resolution of our data, season was best seen as qualitative “index” of changing availability of good-quality food. In addition to availability of a food item, availability of alternative good-quality food items may modify consumption by voles [18]. Our results indicate that a food item which has such indirect effects does not have to be more preferred at the population level. For example, tundra voles selected less for grasses (Poaceae) when the biomass of willows (Salicaceae) increased, even though at population level they preferred grasses to willows. We therefore suggest that the effect of alternative food item availability for small rodent functional responses should be further evaluated. Moreover, based on the seasonal changes of voles’ diets and selectivity, relative differences of nutritional quality between different food items are probably important determinants of such effects.
The spatial effects we found, i.e. voles from the same study grids having more similar diets and preferences than those from different grids, suggest that voles from same local environment are more likely to make similar food choices than voles from different environments. In addition to food characteristics, small rodent feeding habits can be affected by competition and predation risk [6] which could therefore contribute to spatial variation in feeding habits. Vole population densities, especially those of tundra voles, differed drastically between the river catchments (Table 1). However, diets and selectivity differed little between river catchments and therefore intraspecific competition seems unlikely to have caused the spatial patterns we observed at grid level. On the other hand, population density of Norwegian lemmings was higher in VJ where vole densities were lower (Table 1) [22], [84]. Interspecific competition with lemmings may therefore have masked some of the effects of intraspecific competition on vole diets. That food biomass had little effect on diets and selectivity does not support the idea that the spatial effects would be caused by food availability either. Still, food item biomass may not necessarily reflect all vegetation characteristics which are important for determining vole feeding habits. For example, a plant species’ nutritional quality may vary, both temporally, spatially and also between plant parts [83]–[85]. Moreover, positive responses of vole selectivity on availability, evaluated via responses to biomass and season, suggest that voles do not compensate low availability with increased selectivity. This in turn indicates that voles invested little effort in searching and selecting the most preferred food. It is well established that perceived predation risk reduces time herbivores, including small rodents, spend foraging in dangerous habitats [6], [86]. Nevertheless, the interplay between food availability and perceived predation risk, ‘the landscapes of food and fear’, remains poorly understood [86], [87]. In tundra habitats vegetation cover is generally low and predation risk high, especially during small rodent population-peak years [88]. Flexible feeding habits of voles could thus at least partly be an adaptation to minimize time spent searching for food, as emphasized by [89] and [90]. The spatial variation of diets and selectivity which we observed are therefore probably caused by a combination of local vegetation characteristics and search time limitations due to predation risk. Both plant quality and search time limitations have been included in some functional response models for herbivores [2], [10] and we suggest that examining the roles of these parameters for small rodent functional response models should be attempted.
While we here show that the population level patterns in feeding habits of voles are flexible, it is possible that vole individuals are more conservative. At least some of the changes in vole diets are related to changes in gut morphology [91], [92], indicating that individual voles may have physiological limitations related to switching quickly between highly different diets. However, little is known about the flexibility of vole diets at individual level, and it is unclear how fast and drastically individual voles may change their diets.
Methodological Considerations
While few of the observed effects of food item biomass on stomach content were statistically strong, we are confident that these patterns indeed reflect the relationships between voles and their food. The methods we used to estimate food item use and availability, i.e. stomach contents and biomass of plants, have certain shortcomings. Most importantly, food passes quickly through the digestive system of voles [5] and stomach contents therefore give a snapshot of the vole diet during the last hour. In addition, food item availability to voles may be poorly represented by average g/m biomass of plant species. For example, some food items might reach a height which makes them unavailable for small-statured herbivores like voles and hence average biomass may differ from what is available for voles. Finally, we measured plant biomass during the peak of growing season but sampled vole diets during early and late growing season. Due to seasonal increase of biomass, this may have led to underestimation of selectivity in the summer in comparison to the autumn. However, the only seasonal increase of selectivity was that of grey-sided voles for Ericaceae, which can be well explained by an increase in the availability of berries. That we were able to relate patterns of diets and selectivity to patterns of food availability, such as the biomass of alternative food items or the seasonal changes in availability, in spite of biological and technical noise in the data indicates that those patterns are probably stronger in reality than suggested by our analyses. This explanation is supported by the difference between grey-sided voles and tundra voles, as the sample size was higher and the observed patterns both more abundant and statistically stronger for grey-sided voles. We therefore recommend a larger sample size and a more adapted way of measuring food availability for future studies on small rodent functional responses. Such larger sample size could be achieved by analyses of fecal samples, to avoid lethal methods. For example, DNA metabarcoding coupled with radiotelemetry could provide repeated individual-level data on diets, together with targeted locations for food availability estimates. Such estimates could be achieved by adjusting the point intercept method to better represent the vegetation actually available for small rodents, by for example counting only hits up to 10 cm from ground level and separating between leaves and woody plant parts.
Conclusions
We conclude that voles have diverse diets and flexible food preferences. Thus, viewing food preferences as a fixed ranking of a few species is likely to be insufficient for understanding small rodent feeding ecology. Diet diversity as such may be a functional trait of small rodent diets that previously has been underrated in the literature because of methodological constrains. Moreover, our results suggest that in order to understand small rodent functional responses, the roles of alternative food items and search time limitations should be further investigated.
Supporting Information
Details of the DNA metabarcoding methodology used, in order of execution, for the two datasets combined for this study. Notes: 1Soininen et al. (2009), 2available at http://www.grenoble.prabi.fr/trac/ecoPCR/, 3available at http://www.grenoble.prabi.fr/trac/OBITools/, 4in the final dataset used for analyses.
(DOCX)
Diet of Grey-sided voles (n = 82), in heath habitat at Komagelva and Vestre Jakobselva, Varanger peninsula, during summer and autumn 2007. Mean proportion with standard error and frequency of occurrence (percentage of individuals where taxa present). Abundance of taxa at species level is included in the genera, which are included in families. Column “length g-h” refers to the length of the DNA region amplified with primer pair g-h, based on Sønstebø et al. (2010).
(DOCX)
Diet of Tundra voles (n = 67), in meadow habitat at Komagelva and Vestre Jakobselva, Varanger peninsula, during summer and autumn 2007. Mean proportion with standard error and frequency of occurrence (percentage of individuals where taxa present). Abundance of taxa at species level is included in the genera, which are included in families. Column “length g-h” refers to the length of the DNA region amplified with primer pair g-h, based on Sønstebø et al. (2010).
(DOCX)
Grey-sided vole (n = 81) selectivity at plant functional group level, based on compositional analysis comparing used (plant DNA in individuals diet) against available (biomass of grid where the individual was trapped). The table is read along the rows; “+” indicates that food item on a row was used more than that in a column, “−” that it was less used. Tripled sign indicates significant differences. “dec.” refers to deciduous.
(DOCX)
Tundra vole (n = 66) selectivity at plant functional group level, based on compositional analysis comparing used (plant DNA in individuals diet) against available (biomass of grid where the individual was trapped). The table is read along the rows; “+” indicates that food item on a row was used more than that in a column, “−” that it was less used. Tripled sign indicates significant differences. “dec.” refers to deciduous.
(DOCX)
Grey-sided vole vole (n = 81) selectivity at plant family level, based on compositional analysis comparing used (plant DNA in individuals diet) against available (biomass of grid where the individual was trapped). The table is read along the rows; “+” indicates that food item on a row was used more than that in a column, “−” that it was less used. Tripled sign indicates significant differences. Columns are labeled with abbreviated family names using only the first three letters, rows are labeled with full names in the same order.
(DOCX)
Tundra vole (n = 66) selectivity at plant family level, based on compositional analysis comparing used (plant DNA in individuals diet) against available (biomass of grid where the individual was trapped). The table is read along the rows; “+” indicates that food item on a row was used more than that in a column, “−” that it was less used. Tripled sign indicates significant differences. Columns are labeled with abbreviated family names using only the first three letters, rows are labeled with full names in the same order.
(DOCX)
Acknowledgments
We thank J.-A. Henden, S. T. Killengreen, M. Nilsen and numerous field assistants for data collection, S. Kaino, D. Rioux, C. Miquel and A. Valentini for help with lab work, T. Alm for taxonomical advice, J. H. Sønstebø for help with interpreting Vaccinium-sequences, A. Tarroux, O. Huitu and two anonymous referees for comments on the manuscript and J. Stien for checking the English.
Funding Statement
This study was funded by Norwegian Research Council (http://www.forskningsradet.no/, project Ecosystem Finnmark), Oskar Huttunen Foundation http://www.oskhuttusensaatio.net/, PhD-scholarship for EMS) and Norwegian Directorate for Nature Management (http://http://www.dirnat.no/, support for EMS). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Spalinger DE, Hobbs NT (1992) Mechanisms of foraging in mammalian herbivores - New models of functional response. Am Nat 140: 325–348. [DOI] [PubMed] [Google Scholar]
- 2. Fortin D, Boyce MS, Merrill EH (2004) Multi-tasking by mammalian herbivores: Overlapping processes during foraging. Ecology 85: 2312–2322. [Google Scholar]
- 3. van Langevelde F, Drescher M, Heitkönig IMA, Prins HHT (2008) Instantaneous intake rate of herbivores as function of forage quality and mass: Effects on facilitative and competitive interactions. Ecol Modell 213: 273–284. [Google Scholar]
- 4. Ostfeld RS (1985) Limiting resources and territoriality in microtine rodents. Am Nat 126: 1–15. [Google Scholar]
- 5. Lee WB, Houston DC (1993) The role of coprophagy in digestion in voles (Microtus agrestis and Clethrionomys glareolus). Funct Ecol 7: 427–432. [Google Scholar]
- 6.Ylönen H, Brown JS (2008) Fear and the foraging, breeding and sociality of rodents. In: Wolff JO, Sherman PW, editors. Rodent Societies: An Ecological and Evolutionary Perspective. Chicago, IL, USA: University of Chicago Press. 345–358.
- 7. Solomon ME (1949) The natural control of animal populations. J Anim Ecol 18: 1–35. [Google Scholar]
- 8. Batzli GO, Jung H-J, Guntenspergen G (1981) Nutritional ecology of microtine rodents: Effects of plant extracts on the growth of arctic microtines. J Mammal 62: 286–292. [Google Scholar]
- 9. Hobbs NT, Gross JE, Shipley LA, Spalinger DE, Wunder BA (2003) Herbivore functional response in heterogeneous environments: A contest among models. Ecology 84: 666–681. [Google Scholar]
- 10.Swihart RK, DeAnglis DL, Feng Z, Bryant JP (2009) Troublesome toxins: Time to re-think plant-herbivore interactions in vertebrate ecology. BMC Ecol 9, doi:10.1186/1472-6785-9-5. [DOI] [PMC free article] [PubMed]
- 11. Lundberg P (1988) Functional response of a small mammalian herbivore - The disk equation revisited. J Anim Ecol 57: 999–1006. [Google Scholar]
- 12. Gross JE, Shipley LA, Hobbs NT, Spalinger DE, Wunder BA (1993) Functional response of herbivores in food-concentrated patches: Test of a mechanistic model. Ecology 74: 778–791. [Google Scholar]
- 13. Alm Bergvall U, Leimar O (2005) Plant secondary compounds and the frequency of food types affect food choise by mammalian herbivores. Ecology 86: 2450–2460. [Google Scholar]
- 14. Marsh KJ, Wallis IR, Andrew RL, Foley WJ (2006) The detoxification limitation hypothesis: Where did it come from and where is it going? J Chem Ecol 32: 1247–1266. [DOI] [PubMed] [Google Scholar]
- 15. Provenza FD, Villalba JJ, Haskell J, MacAdam JW, Griggs TC, et al. (2007) The value to herbivores of plant physical and chemical diversity in time and space. Crop Sci 47: 382–398. [Google Scholar]
- 16. Gilbert S, Martel J, Klemola T, Norrdahl K (2013) Increasing vole numbers cause more lethal damage to saplings in tree monocultures than in mixed stands. Basic and Applied Ecology 14: 12–19. [Google Scholar]
- 17. Vehvilainen H, Koricheva J (2006) Moose and vole browsing patterns in experimentally assembled pure and mixed forest stands. Ecography 29: 497–506. [Google Scholar]
- 18. Pusenius J, Prittinen K, Roininen H (2003) Effects of the availability of herbaceous food on vole attacks on birch seedlings. Ecoscience 10: 155–160. [Google Scholar]
- 19. Ims RA, Fuglei E (2005) Trophic interaction cycles in tundra ecosystems and the impact of climate change. BioScience 55: 311–322. [Google Scholar]
- 20. Hambäck PA, Oksanen L, Ekerholm P, Lindgren A, Oksanen T, et al. (2004) Predators indirectly protect tundra plants by reducing herbivore abundance. Oikos 106: 85–92. [Google Scholar]
- 21. Olofsson J, Hulme PE, Oksanen L, Suominen O (2004) Importance of large and small mammalian herbivores for the plant community structure in the forest tundra ecotone. Oikos 106: 324–334. [Google Scholar]
- 22. Ravolainen VT, Bråthen KA, Ims RA, Yoccoz NG, Henden J-A, et al. (2011) Rapid, landscape scale responses in riparian tundra vegetation to exclusion of small and large mammalian herbivores. Basic Appl Ecol 12: 643–653. [Google Scholar]
- 23. Olofsson J, Tømmervik H, Callaghan TV (2012) Vole and lemming activity observed from space. Nat Clim Chang 2: 880–883. [Google Scholar]
- 24. Ims RA, Henden JA, Killengreen ST (2008) Collapsing population cycles. Trends Ecol Evol 23: 79–86. [DOI] [PubMed] [Google Scholar]
- 25. Hörnfeldt B (2004) Long-term decline in numbers of cyclic voles in boreal Sweden: Analysis and presentation of hypotheses. Oikos 107: 376–392. [Google Scholar]
- 26. Ims RA, Yoccoz NG, Killengreen ST (2011) Determinants of lemming outbreaks. PNAS 108: 1970–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kalela O (1957) Regulation of reproduction rate in subarctic populations of the vole Clethrionomys rufocanus (Sund.). Ann Acad Sci Fenn 34: 1–60. [Google Scholar]
- 28. Hansson L, Larsson T-B (1978) Vole diet on experimentally managed reforestation areas in northern Sweden. Holarctic Ecology 1: 16–26. [Google Scholar]
- 29. Tast J (1974) The food and feeding habits of the root vole, Microtus oeconomus, in Finnish Lapland. Aquilo Ser Zool 15: 25–32. [Google Scholar]
- 30. Batzli GO, Jung H-JG (1980) Nutritional ecology of microtine rodents: Resource utilization near Atkasook, Alaska. Arc Alp Res 12: 483–499. [Google Scholar]
- 31. Batzli GO, Henttonen H (1990) Demography and resource use by microtine rodents near Toolik Lake, Alaska, U.S.A. Arc Alp Res. 22: 51–64. [Google Scholar]
- 32. Soininen EM, Valentini A, Coissac E, Miquel C, Gielly L, et al. (2009) Analysing diet of small herbivores: The efficiency of DNA barcoding coupled with high-throughput pyrosequencing for deciphering the composition of complex plant mixtures. Front Zool 6: 16 doi: 10.1186/1742-9994-6-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Valentini A, Pompanon F, Taberlet P (2009) DNA barcoding for ecologist. Trends Ecol Evol 24: 110–117. [DOI] [PubMed] [Google Scholar]
- 34. Taberlet P, Coissac E, Hajibabaei M, Rieseberg LH (2012) Environmental DNA Mol Ecol. 21: 1789–1793. [DOI] [PubMed] [Google Scholar]
- 35. Pegard A, Miquel C, Valentini A, Coissac E, Bouvier F, et al. (2009) Universal DNA-Based methods for assessing the diet of grazing livestock and wildlife from feces. J Agric Food Chem 57: 5700–5706. [DOI] [PubMed] [Google Scholar]
- 36. Kowalczyk R, Taberlet P, Coissac E, Valentini A, Miquel C, et al. (2011) Influence of management practices on large herbivore diet–Case of European bison in Bialowieza primeval forest (Poland). For Ecol Manage 261: 821–828. [Google Scholar]
- 37. Raye G, Miquel C, Coissac E, Redjadj C, Loison A, et al. (2011) New insights on diet variability revealed by DNA barcoding and high-throughput pyrosequencing: Chamois diet in autumn as a case study. Ecol Res 26: 265–276. [Google Scholar]
- 38. Pompanon F, Deagle BE, Symondson WOC, Brown DS, Jarman SN, et al. (2012) Who is eating what: Diet assessment using next generation sequencing. Mol Ecol 21: 1931–1950. [DOI] [PubMed] [Google Scholar]
- 39. Yoccoz NG (2012) The future of environmental DNA in ecology. Mol Ecol 21: 2031–2038. [DOI] [PubMed] [Google Scholar]
- 40. Aerts R, Chapin FS (2000) The mineral nutrition of wild plants revisited: A re-evaluation of processes and patterns. Adv Ecol Res 30: 1–67. [Google Scholar]
- 41. Cornelissen JHC, Quested HM, Gwyn-Jones D, van Logestijn RSP, de Beus MAH, et al. (2004) Leaf digestibility and litter decomposability are related in a wide range of subarctic plant species and types. Ecology 18: 779–786. [Google Scholar]
- 42. Henden J-A, Ims R, Yoccoz N, Sørensen R, Killengreen S (2011) Population dynamics of tundra voles in relation to configuration of willow thickets in southern arctic tundra. Polar Biol 34: 533–540. [Google Scholar]
- 43. Yoccoz N, Ims RA (2004) Spatial population dynamics of small mammals: Some methodological and practical issues. Anim Biodivers Conserv 27: 427–435. [Google Scholar]
- 44. Oksanen T, Oksanen L, Dahlgren J, Olofsson J (2008) Arctic lemmings, Lemmus spp. and Dicrostonyx spp.: Integrating ecological and evolutionary perspectives. Evol Ecol Res 10: 415–434. [Google Scholar]
- 45. Henden JA, Ims R, Yoccoz NG, Killengreen ST (2011) Declining willow ptarmigan populations: The role of habitat structure and community dynamics. Basic Appl Ecol 12: 413–422. [Google Scholar]
- 46. Myllymäki A, Paasikalio A, Pankakoski E, Kanevo V (1971) Removal experiments on small quadrats as a means of rapid assessment of the abundance of small mammals. Ann Zool Fennici 8: 177–185. [Google Scholar]
- 47.Jensen PM, Stenseth NC, Framstad E (1993) Trappability of Norwegian lemming (Lemmus lemmus). In: Stenseth NC, Ims RA, editors. The biology of lemmings. London: Academic Press. 547–554.
- 48. Jonasson S (1988) Evaluation of the point intercept method for the estimation of plant biomass. Oikos 52: 101–106. [Google Scholar]
- 49. Bråthen KA, Hagberg O (2004) More efficient estimation of plant biomass. J Veg Sci 15: 653–660. [Google Scholar]
- 50. Ravolainen VT, Yoccoz NG, Brathen KA, Ims RA, Iversen M, et al. (2010) Additive partitioning of diversity reveals no scale-dependent impacts of large ungulates on the structure of tundra plant communities. Ecosystems 13: 157–170. [Google Scholar]
- 51. Iversen M, Brathen KA, Yoccoz NG, Ims RA (2009) Predictors of plant phenology in a diverse high-latitude alpine landscape: growth forms and topography. Journal of Vegetation Science 20: 903–915. [Google Scholar]
- 52. Taberlet P, Coissac E, Pompanon F, Gielly L, Miquel C, et al. (2007) Power and limitations of the chloroplast trnL (UAA) intron for plant DNA barcoding. Nucleic Acids Res 35: e14 doi: 10.1093/nar/gkl1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sønstebø JH, Gielly L, Brysting A, Elven R, Edwards M, et al. (2010) Using next-generation sequencing for molecular reconstruction of past Arctic vegetation and climate. Mol Ecol Res 10: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 54. Deagle BE, Eveson JP, Jarman SN (2006) Quantification of damage in DNA recovered from highly degraded samples - A case study on DNA in faeces. Front Zool 3: 11 doi: 10.1186/1742-9994-1183-1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mossberg B, Stenberg L (2005) Suuri Pohjolan Kasvio. Helsinki: Tammi.
- 56. Quested HM, Cornelissen JHC, Press MC, Callaghan TV, Aerts R, et al. (2003) Decomposition of sub-arctic plants with differing nitrogen economies: A functional role for hemiparasites. Ecology 84: 3209–3221. [Google Scholar]
- 57. Bråthen K, Ims R, Yoccoz N, Fauchald P, Tveraa T, et al. (2007) Induced shift in ecosystem productivity? Extensive scale effects of abundant large herbivores. Ecosystems 10: 773–789. [Google Scholar]
- 58. Aebischer NJ, Robertson PA, Kenward RE (1993) Compositional analysis of habitat use from animal radio-tracking data. Ecology 74: 1313–1325. [Google Scholar]
- 59.van den Boogaart KG, Tolosana R, Bren M (2011) compositions: Compositional Data Analysis. R package version 1.10–2. R-project website: http://CRAN.R-project.org/package=compositions. Accessed 03.06.2013.
- 60. Calenge C (2006) The package “adehabitat” for the R software: A tool for the analysis of space and habitat use by animals. Ecol Modell 197: 516–519. [Google Scholar]
- 61.Bates D, Maechler M, Dai B (2008) Lme4: Linear mixed-effects models using s4 classes [computer software].
- 62.Zuur AF, Ieno EN, Walker N, Saveliev AA, Smith GM (2009) Mixed Effects Models and Extensions in Ecology with R: Springer. 562 p.
- 63.R Development Core Team (2011) R: a language and environment for statistical computing. R foundation for Statistical Computing. version 2.12.2 (2011–02–25). Vienna, Austria. ISBN 3–900051–07–0. R-project website: http://www.r-project.org/. Accessed 03.06.2013.
- 64. Andersson M, Jonasson S (1986) Rodent cycles in relation to food resources on an alpine heath. Oikos 46: 93–106. [Google Scholar]
- 65. Laine KM, Henttonen H (1987) Phenolics/nitrogen ratios in the blueberry Vaccinium myrtillus in relation to temperature and microtine density in Finnish Lapland. Oikos 50: 389–395. [Google Scholar]
- 66. Hambäck PA, Ekerholm P (1997) Mechanisms of apparent competition in seasonal environments: An example with vole herbivory. Oikos 80: 276–288. [Google Scholar]
- 67. Hansen TF, Stenseth NC, Henttonen H, Tast J (1999) Interspecific and intraspecific competition as causes of direct and delayed density dependence in a fluctuating vole population. PNAS 96: 986–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dahlgren J, Oksanen L, Sjodin M, Olofsson J (2007) Interactions between gray-sided voles (Clethrionomys rufucanus) and bilberry (Vaccinium myrtillus), their main winter food plant. Oecologia 152: 525–532. [DOI] [PubMed] [Google Scholar]
- 69. Plesner Jensen S, Doncaster CP (1999) Lethal toxins in non-preferred foods: How plant chemical defences can drive microtine cycles. J Theor Biol 199: 63–85. [DOI] [PubMed] [Google Scholar]
- 70. Saetnan ER, Gjershaug JO, Batzli GO (2009) Habitat use and diet composition of Norwegian lemmings and field voles in Central Norway. J Mammal 90: 183–188. [Google Scholar]
- 71. Gebczy?ska Z (1970) Bioenergetics of a root vole population. Acta Theriol (Warsz) 15: 33–66. [Google Scholar]
- 72. Alipayo D, Valdez R, Holechek JL, Cardenas M (1992) Evaluation of microhistological analysis for determining ruminant diet botanical composition. Journal of Range Management 45: 148–152. [Google Scholar]
- 73. Chapin FS, Shaver GR (1989) Differences in growth and nutrient use among arctic plant-growth forms. Funct Ecol 3: 73–80. [Google Scholar]
- 74. Mårell A, Hofgaard A, Danell K (2006) Nutrient dynamics of reindeer forage species along snowmelt gradients at different ecological scales. Basic Appl Ecol 7: 13–30. [Google Scholar]
- 75. Mysterud A, Hessen DO, Mobaek R, Martinsen V, Mulder J, et al. (2011) Plant quality, seasonality and sheep grazing in an alpine ecosystem. Basic Appl Ecol 12: 195–206. [Google Scholar]
- 76. Palo RT (1984) Distribution of birch (Betula spp), willow (Salix spp), and poplar (Populus spp) secondary metabolites and their potential role as chemical defense against herbivores. J Chem Ecol 10: 499–520. [DOI] [PubMed] [Google Scholar]
- 77.Harju A (1996) Food selection and performance of Microtus voles in relation to dietary protein and woody bark [PhD thesis]: University of Joensuu.
- 78. Hjalten J, Danell K, Ericson L (1996) Food Selection by Two Vole Species in Relation to Plant Growth Strategies and Plant Chemistry. Oikos 76: 181–190. [Google Scholar]
- 79. Speed JDM, Cooper EJ, Jonsdottir IS, van der Wal R, Woodin SJ (2010) Plant community properties predict vegetation resilience to herbivore disturbance in the Arctic. J Ecol 98: 1002–1013. [Google Scholar]
- 80. Nersesian CL, Banks PB, Simpson SJ, McArthur C (2012) Mixing nutrients mitigates the intake constraints of a plant toxin in a generalist herbivore. Behav Ecol 23: 879–888. [Google Scholar]
- 81. Ims RA (1987) Differential reproductive success in a peak population of the greysided vole Clethrionomys Rufocanus . Oikos 50: 103–113. [Google Scholar]
- 82. Williams LR, Cameron GN (1990) Intraspecific response to variation in food resources by Attwater’s pocket gopher. Ecology 71: 797–810. [Google Scholar]
- 83. Chapin FS, Johnson DA, McKendrick JD (1980) Seasonal movement of nutrients in plants of differing growth forms in an Alaskan tundra ecosystem, implications for herbivory. J Ecol 68: 189–209. [Google Scholar]
- 84. Soininen EM, Bråthen KA, Jusdado JGH, Reidinger S, Hartley SE (2013) More than herbivory: Levels of silica-based defences in grasses vary with plant species, genotype and location. Oikos 122: 30–41. [Google Scholar]
- 85. Chapin FS, Mckendrick JD, Johnson DA (1986) Seasonal changes in carbon fractions in Alaskan tundra plants of differing growth form - implications for herbivory. J Ecol 74: 707–731. [Google Scholar]
- 86. Searle KR, Stokes CJ, Gordon IJ (2008) When foraging and fear meet: Using foraging hierarchies to inform assessments of landscapes of fear. Behav Ecol 19: 475–482. [Google Scholar]
- 87. Bolnick D, Preisser E (2005) Resource competition modifies the strength of trait-mediated predator-prey interactions: A meta-analysis. Ecology 86: 2771–2779. [Google Scholar]
- 88. Gilg O, Sittler B, Sabard B, Hurstel A, Sane R, et al. (2006) Functional and numerical responses of four lemming predators in high arctic Greenland. Oikos 113: 193–216. [Google Scholar]
- 89. Predavec M, Krebs CJ (2000) Microhabitat utilisation, home ranges, and movement patterns of the collared lemming (Dicrostonyx groenlandicus) in the central Canadian Arctic. Can J Zool 78: 1885–1890. [Google Scholar]
- 90.Berg TB (2003) The collared lemming (Dicrostonyx groenlandicus) in Greenland: Population dynamics and habitat selection in relation to food quality [PhD thesis]: University of Copenhagen.
- 91. Owl MY, Batzli GO (1998) The integrated processing response of voles to fibre content of natural diets. Funct Ecol 12: 4–13. [Google Scholar]
- 92. Lee WB, Houston DC (1993) The effect of diet quality on gut anatomy in british voles (Microtinae). J Comp Physiol B 163: 337–339. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Details of the DNA metabarcoding methodology used, in order of execution, for the two datasets combined for this study. Notes: 1Soininen et al. (2009), 2available at http://www.grenoble.prabi.fr/trac/ecoPCR/, 3available at http://www.grenoble.prabi.fr/trac/OBITools/, 4in the final dataset used for analyses.
(DOCX)
Diet of Grey-sided voles (n = 82), in heath habitat at Komagelva and Vestre Jakobselva, Varanger peninsula, during summer and autumn 2007. Mean proportion with standard error and frequency of occurrence (percentage of individuals where taxa present). Abundance of taxa at species level is included in the genera, which are included in families. Column “length g-h” refers to the length of the DNA region amplified with primer pair g-h, based on Sønstebø et al. (2010).
(DOCX)
Diet of Tundra voles (n = 67), in meadow habitat at Komagelva and Vestre Jakobselva, Varanger peninsula, during summer and autumn 2007. Mean proportion with standard error and frequency of occurrence (percentage of individuals where taxa present). Abundance of taxa at species level is included in the genera, which are included in families. Column “length g-h” refers to the length of the DNA region amplified with primer pair g-h, based on Sønstebø et al. (2010).
(DOCX)
Grey-sided vole (n = 81) selectivity at plant functional group level, based on compositional analysis comparing used (plant DNA in individuals diet) against available (biomass of grid where the individual was trapped). The table is read along the rows; “+” indicates that food item on a row was used more than that in a column, “−” that it was less used. Tripled sign indicates significant differences. “dec.” refers to deciduous.
(DOCX)
Tundra vole (n = 66) selectivity at plant functional group level, based on compositional analysis comparing used (plant DNA in individuals diet) against available (biomass of grid where the individual was trapped). The table is read along the rows; “+” indicates that food item on a row was used more than that in a column, “−” that it was less used. Tripled sign indicates significant differences. “dec.” refers to deciduous.
(DOCX)
Grey-sided vole vole (n = 81) selectivity at plant family level, based on compositional analysis comparing used (plant DNA in individuals diet) against available (biomass of grid where the individual was trapped). The table is read along the rows; “+” indicates that food item on a row was used more than that in a column, “−” that it was less used. Tripled sign indicates significant differences. Columns are labeled with abbreviated family names using only the first three letters, rows are labeled with full names in the same order.
(DOCX)
Tundra vole (n = 66) selectivity at plant family level, based on compositional analysis comparing used (plant DNA in individuals diet) against available (biomass of grid where the individual was trapped). The table is read along the rows; “+” indicates that food item on a row was used more than that in a column, “−” that it was less used. Tripled sign indicates significant differences. Columns are labeled with abbreviated family names using only the first three letters, rows are labeled with full names in the same order.
(DOCX)