Abstract
Molecular and behavioral studies corroborate a pivotal role for the innate immune system in mediating the acute and chronic effects of alcohol and support a neuroimmune hypothesis of alcohol addiction. Changes in expression of neuroimmune genes and microglial transcripts occur in post-mortem brain from alcoholics and animals exposed to alcohol, and null mutant animals lacking certain innate immune genes show decreased alcohol-mediated responses. Many of the differentially expressed genes are part of the toll like receptor signaling pathway and culminate in increased expression of pro-inflammatory immune genes. Compounds known to inhibit inflammation, microglial activation, and neuroimmune gene expression have shown promising results in reducing alcohol-mediated behaviors in animal models, indicating that neuroimmune signaling pathways offer unexplored targets in the treatment of alcohol abuse.
Introduction
The interplay between brain, behavior, and immunity in the etiology and progression of drug abuse is a rapidly expanding area of interest for addiction research. Evidence is accumulating that the neuroimmune system, encompassing innate immune responses within the peripheral and central nervous systems, contributes to drug abuse and dependence. Recent studies point to a role for immune responses in all three stages of the addiction model, from binge/intoxication, withdrawal/negative affect, to preoccupation/anticipation or craving [1*–3].
In the case of alcohol abuse, there is strong evidence for a neuroimmune role of addiction, with the innate immune system being linked to brain changes associated with acute and chronic alcohol exposure. An array of behavioral and genetic studies within the past several years supports a role for innate immunity in alcohol abuse and also highlights neuroimmune pathways as potential targets in the treatment of alcohol addiction.
Innate Immunity
The innate immune system is also known as the non-specific immune system and is the first line of defense against pathogens. It defends the host in a rather generic, albeit immediate manner, by acting as a physical or chemical barrier to infection but does not provide long-lasting immunity, which is the role of the adaptive immune system.
Innate immune cells outside of the brain consist of macrophages (including liver Kupffer cells), dendritic cells, mast cells, neutrophils, and other leukocytes. Microglia are brain-specific macrophages and are the main immune-derived cells in the brain while astrocytes, a subtype of glial cells, are also involved in mediating innate immunity in the CNS. Although microglia activation can be pro- or anti-inflammatory, it is the pro-inflammatory mechanisms induced by alcohol that will be discussed here. Innate immune signaling pathways are shared among major tissues; thus brain microglia respond to and initiate innate immune signaling via similar pathways to immune cells in the liver, intestines, and lungs.
Activation of innate immune cells stimulates endogenous toll like receptors (TLRs), a family of highly conserved pattern recognition receptors found in invertebrates and vertebrates. TLRs have been implicated in everything from neural plasticity to disease, demonstrating their dichotomous role from neurogenesis to pathogenesis [4]. The most widely studied TLR to date is TLR4 (the receptor for bacterial endotoxin), although 13 TLRs have now been identified [5]. Microglial cells express high levels of TLR4 and respond rapidly to the gram-negative bacterial endotoxin lipopolysaccharide (LPS) to produce inflammatory mediators [6]. Microglial activation of TLR4 is required for astrocyte pro-inflammatory responses [7*]. Neurons have also been shown to express TLR4 [4,8–10] and propagate LPS-induced signaling [11], indicating an unexpected role for neurons in innate immunity and eluding to significant cross-communication among microglia, astrocytes, and neurons that likely characterizes innate immune signaling in the CNS. Brain endothelial cells also express TLR4 and are able to receive neuroimmune stimulation from the brain side and secrete cytokines into the blood or receive stimulation from the blood and secrete cytokines into the brain, suggesting that the blood brain barrier (BBB) may be a fourth component involved in the cross-talk between neurons, microglia, and astrocytes [12]. Further study is needed to determine the exact cellular location of TLR4 in the brain and to decipher the contribution of neurons versus glia in innate immune responses. Nonetheless, the diverse roles of TLRs no doubt depend on the specific TLR, its agonists, mediators, and cellular location.
In addition to recognizing conserved molecular components of microbes (such as the endotoxin LPS), TLRs across the innate immune system respond to other cellular stressors called danger signals [13]. Danger signals include endogenous TLR agonists, such as high-mobility group box 1 (HMGB1) protein (Fig. 1). HMGB1 is a nuclear protein with cytokine-like actions that activates microglia-TLR signaling, further fueling expression of innate immune genes via activation of NF-κB, nuclear factor κ light-chain-enhancer of activated B cells (Fig. 1). Pro-inflammatory signals spread through signaling loops that amplify within and across peripheral and central immune cells. The extent to which CNS inflammation and immune gene expression rely on central or peripheral TLR4 signaling cascades remains unknown, but there is evidence for peripheral TLR4 signaling, at least partly, mediating CNS immune responses [14,15].
Figure 1. Summary diagram of TLR4 signaling cascade.
TLRs signal as dimers and heterodimers that recruit adaptor proteins such as CD14 and MD2. Depending on the adaptors recruited by the activated TLR, different pathways can be triggered, all of which culminate in activation of the pro-inflammatory transcription factor NF-κB. One pathway involves MyD88 and TIRAP and results in activation of NF-κB via IκB kinase. Another pathway uses NADPH oxidase that can activate NF-κB through ROS. TRIF and TRAM signaling proteins also initiate signal cascades, culminating in the activation of NF-κB and other pro-inflammatory transcription factors. RAGE is another transmembrane receptor operating in innate immune cells that is known to respond to HMGB1, and this pathway also induces pro-inflammatory gene transcription via NF-κB activation. The release of cytokines such as TNF-α, HMGB1, IL-1β, chemokines, proteases, and ROS activate adjacent cells. These cytokines affect the brain and are thought to contribute to the etiology, progression, and persistence of alcohol addiction. “Off-the-shelf” FDA-approved drugs (shown here in boxes along with their site of action) are anti-inflammatory and interfere with the TLR4 signaling cascade. These examples are discussed in the text and represented here because they have been shown to decrease alcohol consumption and modify other alcohol behaviors. Bold red font indicates a gene that has been manipulated and shown to affect ethanol-related behavior.
NF-κB: Nuclear factor κ light-chain-enhancer of activated B cells
MyD88: Myeloid differentiation primary response gene 88
TIRAP: Toll-interleukin 1 receptor (TIR) domain containing adaptor protein
ROS: Reactive oxygen species
TRIF: TIR-domain-containing adaptor-inducing IFNβ
TRAM: Trif-related adaptor molecule
RAGE: Receptor for advanced glycation endproducts
TNF-α: Tumor necrosis factor-α
IL-1β: Interleukin-1 beta
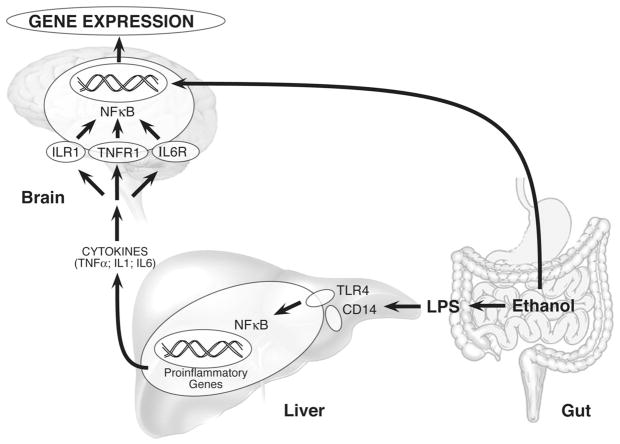
Since LPS is a large molecule, it is unlikely that it crosses the BBB and has a direct effect on the brain [16]. Instead, LPS is thought to activate peripheral TLR4 signaling cascades (including TLR4 on cerebral endothelial cells) that initiate the release of pro-inflammatory cytokines and other immune mediators (Fig. 1) that then cross the BBB by diffusion or active transport mechanisms [12]. Alcohol compromises the tight junctions in the gut epithelium and increases its permeability to LPS [17] which initiates immune responses in liver, blood, and other tissues. In fact, intestinal permeability, LPS, and peripheral pro-inflammatory cytokines were largely increased in non-cirrhotic alcohol-dependent subjects compared to healthy controls and the increased pro-inflammatory cytokines in the alcohol-dependent subjects were correlated with alcohol craving [18*]. Once these cytokines have crossed the BBB, they affect the brain and have been linked to sickness behavior and depression in rodent models [14], suggesting a role for the gut-brain axis in alcohol dependence (Fig. 2).
Figure 2. Gut-brain axis and alcohol dependence.
Lipopolysaccharide (LPS), a gram-negative bacterial endotoxin, is normally localized to the gut. Ethanol jeopardizes the tight junctions of the intestinal mucosa allowing LPS to enter systemic circulation. Once in the bloodstream, LPS binds to TLR4 receptors on liver macrophages, Kupffer, and stellate cells and activates signaling cascades that result in an increase of pro-inflammatory genes (cytokines, chemokines, proteases, ROS) via activation of the transcription factor NF-κB. This process is known to be a key factor in the development of alcoholic liver disease. These cytokines then enter the bloodstream, cross the BBB, and activate microglia, the brain’s resident macrophages. Microglial activation increases the expression of pro-inflammatory genes in the brain which is hypothesized to increase alcohol consumption behavior.
Genomic and Behavioral Evidence for a Role of Alcohol in Innate Immunity
The influence of innate immunity on the etiology and progression of alcohol abuse is a rapidly expanding area of interest in alcohol research. Changes in expression of neuroimmune genes and microglial transcripts were first identified in post-mortem brains from alcoholics [19–22]. In addition, alcoholics showed altered levels of microRNAs that are known to regulate immune function [23] and which may also contribute to changes in neuroimmune gene expression.
In agreement with human studies, brain gene expression studies in animal models indicate a role for immune and microglial transcripts in mediating alcohol action. Chronic alcohol treatment in mice induced pro-inflammatory gene expression that persisted for at least one week of abstinence [24], while LPS-induced neuroimmune activation persisted for months [25]. LPS treatment in mice also produced a prolonged increase in alcohol drinking and preference [26]. Moreover, animals lacking specific innate immune genes showed reduced preference for drinking alcohol [2,3,27] as well as altered acute responses to the motor and sedative effects of alcohol [28*]. In a multivariate analysis of 37 different mutant mice chronically treated with alcohol using a two-bottle choice drinking paradigm, a highly correlated phenotypic cluster was identified, suggesting a role for genes involved in GABA (Gad2, Gabra1), glycine (Glra1), and neuroimmune signaling (Ccr2, Il6) [29].
Many of the differentially expressed genes found in the human and mouse studies are part of the TLR signaling pathway. In fact, ethanol-mediated increases in glial activation, inflammatory mediators, and apoptosis are prevented in microglia lacking TLR4 and in microglia from TLR4-deficient mice [30*,31]. Ethanol activation of NF-κB in brain microglia is dependent on TLR4 [30*,31] and roles for TLR4 accessory proteins, MD2 and CD14, have been demonstrated following alcohol exposure in TLR4-deficient cells [30*]. Blockade of TLR4 receptors prevented ethanol-induced inflammatory signaling in astrocytes, including prevention of NF-κB activation and cell death [32]. Also, brain protein expression of TLR2, TLR3, and TLR4 increased in post-mortem alcoholic brain and in mouse brain following ethanol treatment [19]. The association between TLRs and alcohol has even been reported in Drosophila, where an upregulation of genes in toll and immune deficiency pathways was produced by alcohol exposure [33]. Thus, there is substantial support for alcohol action on innate immune genes and specific evidence for an overlap of TLR signaling by alcohol among different species.
Behavioral studies provide in vivo validation for the pivotal role of TLR4 in mediating alcohol action. For example, C3H/HeJ mice have defective TLR4 function and showed decreased alcohol consumption [3]; although there were no changes reported in alcohol intake and preference in TLR4 knockout mice, the learning and memory deficits observed in wild type mice following ethanol treatment were not observed in TLR4 knockout mice [34]. In addition to preventing cognitive impairments, mice lacking TLR4 were protected against alcohol-induced glial activation and anxiety [34]. Genetic deficiency of either TLR4 or MyD88 reduced acute alcohol-induced sedation and motor impairment in mice, suggesting involvement of the TLR4-MyD88 dependent pathway in the acute behavioral actions of alcohol [28*]. Moreover, deficiency of TLR4 prevents LPS-induced sickness behavior and provides resistance to chronic alcohol consumption [35]. When TLR4 is targeted in rat amygdala using small inhibitory RNAs, self-administration of alcohol is reduced [36*], showing that reduction of TLR4 in a single brain region is sufficient to reduce alcohol administration. Furthermore, knockout mice lacking the TLR4 adaptor protein, CD14, drink less alcohol [26] and are resistant to the LPS-induced increase in alcohol consumption, consistent with TLR4 being the site of action of LPS and mediating the behavioral actions of alcohol [26].
Alcohol-induced TLR signaling culminates in increased expression of pro-inflammatory immune genes via NF-κB activation (Fig. 1). Although NF-κB is expressed in most cells, it is transcriptionally active in brain primarily in glia [37]. Ethanol treatment activates brain microglia and NF-κB-induced transcription of pro-inflammatory immune genes, increasing expression of cytokines, proteases, and oxidases. For example, chronic ethanol increases expression of TNFα, monocyte chemoattractant protein (MCP-1), interleukin-1β (IL-1β), interleukin-6 (IL-6), and NOX (NADPH oxidase) in mouse and rat brain slice cultures [24,38,39]. Increased expression of IL-1β[40] and MCP-1 (CCl2) [20] was also found in post-mortem brains from alcoholics. Chronic ethanol treatment in rat brain cultures increases HMGB1 and TLRs in vitro in agreement with increased in vivo protein expression of HMGB1 and TLRs in mouse brain following ethanol treatment and in post-mortem brains from alcoholics [19]. Increased frontal cortical HMBG1, TLR3, and TLR4 were also observed in rats exposed to intermittent alcohol using a binge-drinking model [41]. A role for cytokines and NF-κB in innate immune gene expression was reported in rat brain slice cultures given that blockade of the cytokine TNFα or blockade of NF-κB reduced ethanol induction of pro-inflammatory target genes [39]. There is also evidence in humans for involvement of the NF-κB system in prefrontal cortex of alcoholics [42]. Furthermore, ethanol consumption and preference in mice was decreased slightly following administration of caffeic acid phenethyl ester (CAPE), an inhibitor of NF-κB activation, providing behavioral evidence for NF-κB activation mediating alcohol behavior [3].
Further evidence for innate immunity in alcohol abuse and dependence comes from human genetic association and linkage studies. For example, human studies found a link between NFKB1 [43] and TNF [44,45] polymorphisms and alcohol abuse. Genetic linkage of the IL-1 and IL-1 receptor antagonist genes to alcoholism has also been reported [46,47]. In addition, CYP2E1, a gene involved in alcohol metabolism, is associated with risk of alcoholism [48]. CYP2E1 leads to increased expression of reactive oxygen species and propagation of inflammatory NF-κB responses in liver Kupffer cells [49]. Collectively, an array of molecular, behavioral, and genomic studies substantiate a prominent role for the innate immune system in the neurobiology of alcohol action.
Neuroimmune Targets for Potential Treatment of Alcohol Abuse
Given the limited treatment options currently available for alcohol abuse, effective therapeutic targets for medication development are of critical importance. Since neuroinflammation is implicated in the etiology of alcoholism and other brain diseases, neuroimmune pathways offer unexplored targets for treating alcohol abuse.
Several FDA-approved drugs with anti-inflammatory and immune inhibitory actions (Fig. 1) have been shown to modulate alcohol responses in animal models. For example, the antibiotics minocycline and doxycycline are anti-inflammatory and modestly decrease alcohol consumption in mice, increase sensitivity to the motor-impairing effects of alcohol, and decrease alcohol-induced sedation [50–52]. Anakinra, the IL-1 receptor antagonist used in the treatment of rheumatoid arthritis, also reduces alcohol-induced sedation in mice [52]. The anticonvulsant topiramate is anti-inflammatory and decreases alcohol consumption in alcohol-preferring rats [53–55] and improves treatment outcome in alcoholics [56,57]. Moreover, indomethacin, a cyclooxygenase enzyme inhibitor and non-steroidal anti-inflammatory, reduces induction of innate immune genes and decreases behavioral deficits in rats exposed to ethanol [58]. Pioglitazone treats insulin resistance and diabetes and is an agonist of peroxisome proliferator-activated type gamma receptors (PPAR) located on neurons and glia in brain. PPAR activation reduces innate immune signaling, and pioglitazone suppresses alcohol drinking and relapse to alcohol seeking in rats [59]. Additional subtypes of PPAR agonists may prove effective in the treatment of addictions given that clofibrate, a PPAR alpha agonist, blocks the rewarding effects of nicotine in monkeys and rats [60], and another PPAR alpha agonist prevents fatty liver in ethanol-fed animals [61]. Naltrexone is an opioid inhibitor approved for the treatment of alcoholism and is also known to inhibit ethanol-induced microglial activation and neurodegeneration in mice [62]. The commercially available (−) isomers of naltrexone and naloxone interact with both opioid and TLR4 receptors, whereas the synthetic (+) isomers interact specifically with TLR4 [63]. (+) Naloxone reduces alcohol-induced sedation and motor impairment in mice, indicating a specific role of TLR4 signaling in mediating the behavioral action of acute alcohol [28*]. It should be noted that while there is evidence that the anti-inflammatory properties of these drugs are responsible for modifying the ethanol-related behaviors above, other mechanisms cannot be ruled out without further investigation. These examples indicate there are FDA-approved drugs currently available that inhibit innate immune signaling and, therefore, may offer promise in treating the neuroimmune component of alcohol abuse.
Summary and Future Directions
The role of neuroimmune signaling in alcohol and other addictions has evolved as a key area of future research in the treatment of addiction disorders. A neuroimmune hypothesis of addiction may be a common mechanism for alcohol and other drugs of abuse [1*]. It is important to note that this mechanism must work in conjunction with the neurocircuitry of the extended amygdala and mesolimbic dopamine reward pathways and is unlikely to promote drug abuse and dependence solely by activation of microglia. For example, pretreatment of mice with LPS reduces the neuronal firing rate of dopamine neurons in the ventral tegmental area [26], providing an example of neuroimmune signaling directly affecting neuronal reward circuitry.
The next several years hold promise for important advances in the treatment of alcoholism, from preventing alcoholic liver disease to targeting non-neuronal central neuroimmune responses associated with chronic alcohol abuse. Further investigation is needed to determine whether indirect effects from peripheral cytokines crossing the BBB or direct effects of TLR signaling in the brain are responsible for neuroinflammation. Another limitation that needs to be addressed is an incomplete knowledge of the cellular location of cytokine receptors in the brain, which impedes our ability to tease apart glial and neuronal contributions in neuroimmune modulation of the development, progression, and persistence of alcohol addiction.
Given the role of neuroimmune activation in drug reward, dependence [1*–3], depression-negative affect [64], and neurodegeneration [65] and the involvement of all of these pathologies in alcohol abuse, the possibility of an overactive immune system promoting alcohol consumption is a hypothesis that we can now consider. Interestingly, abstinence from alcohol and other drugs of abuse is regulated by a common gene network that includes the transcription factor NF-κB [66], which may also regulate development of dependence to alcohol [42]. Thus, common gene networks containing neuroimmune genes may be a hallmark of adaptive molecular mechanisms of drug dependence and abstinence. Although the translocation and amplification of neuroimmune signaling complicates our understanding of alcohol’s direct effect on innate immunity, the role of alcoholism as a chronic inflammatory disease opens a new chapter in alcohol research. Treatment strategies that target innate immune responses in the peripheral and central nervous systems may uncover revolutionary therapies for this neurodegenerative disease and address the pivotal role of neuroimmune signaling in the neurobiology of alcoholism.
Highlights.
Alcohol exposure causes differential expression of neuroimmune genes.
Genetic deficiency of innate immune genes reduces alcohol action.
Toll like receptor signaling mediates alcohol action and produces pro-inflammatory gene expression.
Anti-inflammatory compounds show potential for treating the neuroimmune component of alcohol abuse.
Innate immune signaling mediates alcohol action, suggesting a neuroimmune hypothesis of alcohol addiction.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
* are of special interest.
- *1.Coller JK, Hutchinson MR. Implications of central immune signaling caused by drugs of abuse: Mechanisms, mediators and new therapeutic approaches for prediction and treatment of drug dependence. Pharmacol Ther. 2012;134(2):219–245. doi: 10.1016/j.pharmthera.2012.01.008. This paper is the first to review the evidence for central immune signaling pathways in the development and perpetuation of dependence across many types of addictions (opioids, alcohol, cocaine, methamphetamine, and 3,4-methylenedioxymethamphetamine (MDMA), and their metabolites). Similar cellular and molecular themes are identified for these drugs of abuse, suggesting a common neuroimmune hypothesis of drug addiction and dependence. [DOI] [PubMed] [Google Scholar]
- 2.Crews FT, Zou J, Qin L. Induction of innate immune genes in brain create the neurobiology of addiction. Brain Behav Immun. 2011;25 (Suppl 1):S4–S12. doi: 10.1016/j.bbi.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris R, Blednov Y. Neuroimmune genes and alcohol drinking behavior. In: Cui C, Grandison L, Noronha A, editors. Neural-Immune Interactions in Brain Function and Alcohol Related Disorders. Springer; US: 2013. pp. 425–440. [Google Scholar]
- 4.Okun E, Griffioen KJ, Mattson MP. Toll-like receptor signaling in neural plasticity and disease. Trends Neurosci. 2011;34(5):269–281. doi: 10.1016/j.tins.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawai T, Akira S. Toll-like receptor and rig-i-like receptor signaling. Ann N Y Acad Sci. 2008;1143:1–20. doi: 10.1196/annals.1443.020. [DOI] [PubMed] [Google Scholar]
- 6.Lehnardt S. Innate immunity and neuroinflammation in the cns: The role of microglia in toll-like receptor-mediated neuronal injury. Glia. 2010;58(3):253–263. doi: 10.1002/glia.20928. [DOI] [PubMed] [Google Scholar]
- *7.Holm TH, Draeby D, Owens T. Microglia are required for astroglial toll-like receptor 4 response and for optimal tlr2 and tlr3 response. Glia. 2012;60(4):630–638. doi: 10.1002/glia.22296. Using ow cytometry and differential adhesion as well as a myeloid lineage-specific suicide gene to purify astrocytes from mixed glial cultures, the authors found that functional microglia are required for astrocytic response toTLR4 agonists. This finding supports the role of glial cross-communication in neuroimmune responses. [DOI] [PubMed] [Google Scholar]
- 8.Hua F, Ma J, Ha T, Xia Y, Kelley J, Williams DL, Kao RL, Browder IW, Schweitzer JB, Kalbfleisch JH, Li C. Activation of toll-like receptor 4 signaling contributes to hippocampal neuronal death following global cerebral ischemia/reperfusion. J Neuroimmunol. 2007;190(1–2):101–111. doi: 10.1016/j.jneuroim.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang SC, Arumugam TV, Xu X, Cheng A, Mughal MR, Jo DG, Lathia JD, Siler DA, Chigurupati S, Ouyang X, Magnus T, et al. Pivotal role for neuronal toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci U S A. 2007;104(34):13798–13803. doi: 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoo KY, Yoo DY, Hwang IK, Park JH, Lee CH, Choi JH, Kwon SH, Her S, Lee YL, Won MH. Time-course alterations of toll-like receptor 4 and nf-kappab p65, and their co-expression in the gerbil hippocampal ca1 region after transient cerebral ischemia. Neurochem Res. 2011;36(12):2417–2426. doi: 10.1007/s11064-011-0569-0. [DOI] [PubMed] [Google Scholar]
- 11.Leow-Dyke S, Allen C, Denes A, Nilsson O, Maysami S, Bowie AG, Rothwell NJ, Pinteaux E. Neuronal toll-like receptor 4 signaling induces brain endothelial activation and neutrophil transmigration in vitro. J Neuroinflammation. 2012;9:230. doi: 10.1186/1742-2094-9-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banks WA. The blood-brain barrier in psychoneuroimmunology. Immunol Allergy Clin North Am. 2009;29(2):223–228. doi: 10.1016/j.iac.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Matzinger P. The danger model: A renewed sense of self. Science. 2002;296(5566):301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 14.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones KA, Thomsen C. The role of the innate immune system in psychiatric disorders. Mol Cell Neurosci. 2012 doi: 10.1016/j.mcn.2012.10.002. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Singh AK, Jiang Y. How does peripheral lipopolysaccharide induce gene expression in the brain of rats? Toxicology. 2004;201(1–3):197–207. doi: 10.1016/j.tox.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 17.Mandrekar P, Szabo G. Signalling pathways in alcohol-induced liver inflammation. J Hepatol. 2009;50(6):1258–1266. doi: 10.1016/j.jhep.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *18.Leclercq S, Cani PD, Neyrinck AM, Starkel P, Jamar F, Mikolajczak M, Delzenne NM, de Timary P. Role of intestinal permeability and inflammation in the biological and behavioral control of alcohol-dependent subjects. Brain Behav Immun. 2012;26(6):911–918. doi: 10.1016/j.bbi.2012.04.001. The authors measured intestinal permeability and blood LPS and cytokines in alcohol dependent non-cirrhotic subjects at the onset and end of a 3-week detoxification and rehabilitation program and found increased intestinal permeability, blood LPS and cytokines when compared to controls. Intestinal permeability fully recovered while cytokine levels only partially recovered during the detoxification. Pro-inflammatory cytokines were positively correlated with alcohol craving while the anti-inflammatory cytokine, IL-10, was negatively correlated with alcohol craving. This is the first study to correlate serum cytokines to alcohol craving in humans. [DOI] [PubMed] [Google Scholar]
- 19.Crews FT, Qin L, Sheedy D, Vetreno RP, Zou J. High mobility group box 1/toll-like receptor danger signaling increases brain neuroimmune activation in alcohol dependence. Biol Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.09.030. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He J, Crews FT. Increased mcp-1 and microglia in various regions of the human alcoholic brain. Exp Neurol. 2008;210(2):349–358. doi: 10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, Lewohl JM, Harris RA, Iyer VR, Dodd PR, Randall PK, Mayfield RD. Patterns of gene expression in the frontal cortex discriminate alcoholic from nonalcoholic individuals. Neuropsychopharmacology. 2006;31(7):1574–1582. doi: 10.1038/sj.npp.1300947. [DOI] [PubMed] [Google Scholar]
- 22.Ponomarev I, Wang S, Zhang L, Harris RA, Mayfield RD. Gene coexpression networks in human brain identify epigenetic modifications in alcohol dependence. J Neurosci. 2012;32(5):1884–1897. doi: 10.1523/JNEUROSCI.3136-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewohl JM, Nunez YO, Dodd PR, Tiwari GR, Harris RA, Mayfield RD. Up-regulation of micrornas in brain of human alcoholics. Alcohol Clin Exp Res. 2011;35(11):1928–1937. doi: 10.1111/j.1530-0277.2011.01544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin L, He J, Hanes RN, Pluzarev O, Hong JS, Crews FT. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J Neuroinflammation. 2008;5:10. doi: 10.1186/1742-2094-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic lps causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55 (5):453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blednov YA, Benavidez JM, Geil C, Perra S, Morikawa H, Harris RA. Activation of inflammatory signaling by lipopolysaccharide produces a prolonged increase of voluntary alcohol intake in mice. Brain Behav Immun. 2011;25 (Suppl 1):S92–S105. doi: 10.1016/j.bbi.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blednov YA, Ponomarev I, Geil C, Bergeson S, Koob GF, Harris RA. Neuroimmune regulation of alcohol consumption: Behavioral validation of genes obtained from genomic studies. Addict Biol. 2012;17(1):108–120. doi: 10.1111/j.1369-1600.2010.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *28.Wu Y, Lousberg EL, Moldenhauer LM, Hayball JD, Coller JK, Rice KC, Watkins LR, Somogyi AA, Hutchinson MR. Inhibiting the tlr4-myd88 signalling cascade by genetic or pharmacological strategies reduces acute alcohol-induced sedation and motor impairment in mice. Br J Pharmacol. 2012;165(5):1319–1329. doi: 10.1111/j.1476-5381.2011.01572.x. The authors used the TLR4 antagonist (+)-naloxone and mice with null mutations in TLR4 and MyD88 genes to demonstrate involvement of the TLR4 pathway in the acute behavioral actions of alcohol. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wu Y, Lousberg EL, Moldenhauer LM, Hayball JD, Robertson SA, Coller JK, Watkins LR, Somogyi AA, Hutchinson MR. Attenuation of microglial and il-1 signaling protects mice from acute alcohol-induced sedation and/or motor impairment. Brain Behav Immun. 2011;25 (Suppl 1):S155–164. doi: 10.1016/j.bbi.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Blednov YA, Mayfield RD, Belknap J, Harris RA. Behavioral actions of alcohol: Phenotypic relations from multivariate analysis of mutant mouse data. Genes Brain Behav. 2012;11(4):424–435. doi: 10.1111/j.1601-183X.2012.00780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *30.Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I, Guerri C. Pivotal role of tlr4 receptors in alcohol-induced neuroinflammation and brain damage. J Neurosci. 2010;30(24):8285–8295. doi: 10.1523/JNEUROSCI.0976-10.2010. The authors use rat astroglial cell cultures with siRNA-mediated knock down of TLR4 and astroglial cell cultures from TLR4−/− mice to examine the role of TLR4 in mediating the deleterious effects of chronic ethanol treatment. This was the first paper to show that TLR4 is specifically required for ethanol-induced neuroinflammation and brain damage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernandez-Lizarbe S, Pascual M, Guerri C. Critical role of tlr4 response in the activation of microglia induced by ethanol. J Immunol. 2009;183(7):4733–4744. doi: 10.4049/jimmunol.0803590. [DOI] [PubMed] [Google Scholar]
- 32.Blanco AM, Valles SL, Pascual M, Guerri C. Involvement of tlr4/type i il-1 receptor signaling in the induction of inflammatory mediators and cell death induced by ethanol in cultured astrocytes. J Immunol. 2005;175(10):6893–6899. doi: 10.4049/jimmunol.175.10.6893. [DOI] [PubMed] [Google Scholar]
- 33.Kong EC, Allouche L, Chapot PA, Vranizan K, Moore MS, Heberlein U, Wolf FW. Ethanol-regulated genes that contribute to ethanol sensitivity and rapid tolerance in drosophila. Alcohol Clin Exp Res. 2010;34(2):302–316. doi: 10.1111/j.1530-0277.2009.01093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pascual M, Balino P, Alfonso-Loeches S, Aragon CM, Guerri C. Impact of tlr4 on behavioral and cognitive dysfunctions associated with alcohol-induced neuroinflammatory damage. Brain Behav Immun. 2011;25 (Suppl 1):S80–91. doi: 10.1016/j.bbi.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 35.Kelley KW, Dantzer R. Alcoholism and inflammation: Neuroimmunology of behavioral and mood disorders. Brain Behav Immun. 2011;25 (Suppl 1):S13–20. doi: 10.1016/j.bbi.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *36.Liu J, Yang AR, Kelly T, Puche A, Esoga C, June HL, Jr, Elnabawi A, Merchenthaler I, Sieghart W, June HL, Sr, Aurelian L. Binge alcohol drinking is associated with gabaa alpha2-regulated toll-like receptor 4 (tlr4) expression in the central amygdala. Proc Natl Acad Sci U S A. 2011;108(11):4465–4470. doi: 10.1073/pnas.1019020108. The authors inject siRNA for TLR4 into the central amygdala of alcohol-preferring (P) rats and evaluate its effects on self-administration of ethanol and sucrose. Previous studies have used global knockouts of TLR4 making it difficult to determine whether the outcome is due to compromises in peripheral or central immune function. This paper is the first to show that reduction of TLR4 in a single brain region is sufficient to reduce operant self-administration of ethanol but not sucrose in P rats. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mao XR, Moerman-Herzog AM, Chen Y, Barger SW. Unique aspects of transcriptional regulation in neurons--nuances in nfkappab and sp1-related factors. J Neuroinflammation. 2009;6:16. doi: 10.1186/1742-2094-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qin L, Crews FT. Nadph oxidase and reactive oxygen species contribute to alcohol-induced microglial activation and neurodegeneration. J Neuroinflammation. 2012;9:5. doi: 10.1186/1742-2094-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zou J, Crews F. Induction of innate immune gene expression cascades in brain slice cultures by ethanol: Key role of nf-kappab and proinflammatory cytokines. Alcohol Clin Exp Res. 2010;34(5):777–789. doi: 10.1111/j.1530-0277.2010.01150.x. [DOI] [PubMed] [Google Scholar]
- 40.Zou J, Crews FT. Inflammasome-il-1beta signaling mediates ethanol inhibition of hippocampal neurogenesis. Front Neurosci. 2012;6:77. doi: 10.3389/fnins.2012.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vetreno RP, Crews FT. Adolescent binge drinking increases expression of the danger signal receptor agonist hmgb1 and toll-like receptors in the adult prefrontal cortex. Neuroscience. 2012;226:475–488. doi: 10.1016/j.neuroscience.2012.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okvist A, Johansson S, Kuzmin A, Bazov I, Merino-Martinez R, Ponomarev I, Mayfield RD, Harris RA, Sheedy D, Garrick T, Harper C, et al. Neuroadaptations in human chronic alcoholics: Dysregulation of the nf-kappab system. PLoS One. 2007;2 (9):e930. doi: 10.1371/journal.pone.0000930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Edenberg HJ, Xuei X, Wetherill LF, Bierut L, Bucholz K, Dick DM, Hesselbrock V, Kuperman S, Porjesz B, Schuckit MA, Tischfield JA, et al. Association of nfkb1, which encodes a subunit of the transcription factor nf-kappab, with alcohol dependence. Hum Mol Genet. 2008;17(7):963–970. doi: 10.1093/hmg/ddm368. [DOI] [PubMed] [Google Scholar]
- 44.Gonzalez-Quintela A, Dominguez-Santalla MJ, Loidi L, Quinteiro C, Perez LF. Relation of tumor necrosis factor (tnf) gene polymorphisms with serum concentrations and in vitro production of tnf-alpha and interleukin-8 in heavy drinkers. Alcohol. 2004;34(2–3):273–277. doi: 10.1016/j.alcohol.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 45.Kebir O, Gorsane MA, Blecha L, Krebs MO, Reynaud M, Benyamina A. Association of inflammation genes with alcohol dependence/abuse: A systematic review and a meta-analysis. Eur Addict Res. 2011;17(3):146–153. doi: 10.1159/000324849. [DOI] [PubMed] [Google Scholar]
- 46.Pastor IJ, Laso FJ, Avila JJ, Rodriguez RE, Gonzalez-Sarmiento R. Polymorphism in the interleukin-1 receptor antagonist gene is associated with alcoholism in spanish men. Alcohol Clin Exp Res. 2000;24(10):1479–1482. [PubMed] [Google Scholar]
- 47.Saiz PA, Garcia-Portilla MP, Florez G, Corcoran P, Arango C, Morales B, Leza JC, Alvarez S, Diaz EM, Alvarez V, Coto E, et al. Polymorphisms of the il-1 gene complex are associated with alcohol dependence in spanish caucasians: Data from an association study. Alcohol Clin Exp Res. 2009;33(12):2147–2153. doi: 10.1111/j.1530-0277.2009.01058.x. [DOI] [PubMed] [Google Scholar]
- 48.Webb A, Lind PA, Kalmijn J, Feiler HS, Smith TL, Schuckit MA, Wilhelmsen K. The investigation into cyp2e1 in relation to the level of response to alcohol through a combination of linkage and association analysis. Alcohol Clin Exp Res. 2011;35 (1):10–18. doi: 10.1111/j.1530-0277.2010.01317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cao Q, Mak KM, Lieber CS. Cytochrome p4502e1 primes macrophages to increase tnf-alpha production in response to lipopolysaccharide. Am J Physiol Gastrointest Liver Physiol. 2005;289(1):G95–107. doi: 10.1152/ajpgi.00383.2004. [DOI] [PubMed] [Google Scholar]
- 50.Agrawal RG, Hewetson A, George CM, Syapin PJ, Bergeson SE. Minocycline reduces ethanol drinking. Brain Behav Immun. 2011;25 (Suppl 1):S165–169. doi: 10.1016/j.bbi.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McIver SR, Muccigrosso MM, Haydon PG. The effect of doxycycline on alcohol consumption and sensitivity: Consideration for inducible transgenic mouse models. Exp Biol Med (Maywood) 2012;237(10):1129–1133. doi: 10.1258/ebm.2012.012029. [DOI] [PubMed] [Google Scholar]
- 52.Wu Y, Lousberg EL, Moldenhauer LM, Hayball JD, Robertson SA, Coller JK, Watkins LR, Somogyi AA, Hutchinson MR. Attenuation of microglial and il-1 signaling protects mice from acute alcohol-induced sedation and/or motor impairment. Brain Behav Immun. 2011;25 (Suppl 1):S155–164. doi: 10.1016/j.bbi.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 53.Breslin FJ, Johnson BA, Lynch WJ. Effect of topiramate treatment on ethanol consumption in rats. Psychopharmacology (Berl) 2010;207(4):529–534. doi: 10.1007/s00213-009-1683-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lynch WJ, Bond C, Breslin FJ, Johnson BA. Severity of drinking as a predictor of efficacy of the combination of ondansetron and topiramate in rat models of ethanol consumption and relapse. Psychopharmacology (Berl) 2011;217(1):3–12. doi: 10.1007/s00213-011-2253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zalewska-Kaszubska J, Bajer B, Gorska D, Andrzejczak D, Dyr W, Bienkowski P. Effect of repeated treatment with topiramate on voluntary alcohol intake and beta-endorphin plasma level in warsaw alcohol high-preferring rats. Psychopharmacology (Berl) 2013;225(2):275–281. doi: 10.1007/s00213-012-2812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnson BA, Rosenthal N, Capece JA, Wiegand F, Mao L, Beyers K, McKay A, Ait-Daoud N, Anton RF, Ciraulo DA, Kranzler HR, et al. Topiramate for treating alcohol dependence: A randomized controlled trial. JAMA. 2007;298(14):1641–1651. doi: 10.1001/jama.298.14.1641. [DOI] [PubMed] [Google Scholar]
- 57.Miranda R, Jr, MacKillop J, Monti PM, Rohsenow DJ, Tidey J, Gwaltney C, Swift R, Ray L, McGeary J. Effects of topiramate on urge to drink and the subjective effects of alcohol: A preliminary laboratory study. Alcohol Clin Exp Res. 2008;32(3):489–497. doi: 10.1111/j.1530-0277.2007.00592.x. [DOI] [PubMed] [Google Scholar]
- 58.Pascual M, Blanco AM, Cauli O, Minarro J, Guerri C. Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioural alterations in adolescent rats. Eur J Neurosci. 2007;25(2):541–550. doi: 10.1111/j.1460-9568.2006.05298.x. [DOI] [PubMed] [Google Scholar]
- 59.Stopponi S, Somaini L, Cippitelli A, Cannella N, Braconi S, Kallupi M, Ruggeri B, Heilig M, Demopulos G, Gaitanaris G, Massi M, et al. Activation of nuclear ppargamma receptors by the antidiabetic agent pioglitazone suppresses alcohol drinking and relapse to alcohol seeking. Biol Psychiatry. 2011;69(7):642–649. doi: 10.1016/j.biopsych.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 60.Panlilio LV, Justinova Z, Mascia P, Pistis M, Luchicchi A, Lecca S, Barnes C, Redhi GH, Adair J, Heishman SJ, Yasar S, et al. Novel use of a lipid-lowering fibrate medication to prevent nicotine reward and relapse: Preclinical findings. Neuropsychopharmacology. 2012;37(8):1838–1847. doi: 10.1038/npp.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Crabb DW, Galli A, Fischer M, You M. Molecular mechanisms of alcoholic fatty liver: Role of peroxisome proliferator-activated receptor alpha. Alcohol. 2004;34 (1):35–38. doi: 10.1016/j.alcohol.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 62.Qin L, Crews FT. Chronic ethanol increases systemic tlr3 agonist-induced neuroinflammation and neurodegeneration. J Neuroinflammation. 2012;9:130. doi: 10.1186/1742-2094-9-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hutchinson MR, Shavit Y, Grace PM, Rice KC, Maier SF, Watkins LR. Exploring the neuroimmunopharmacology of opioids: An integrative review of mechanisms of central immune signaling and their implications for opioid analgesia. Pharmacol Rev. 2011;63(3):772–810. doi: 10.1124/pr.110.004135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eyre H, Baune BT. Neuroplastic changes in depression: A role for the immune system. Psychoneuroendocrinology. 2012;37(9):1397–1416. doi: 10.1016/j.psyneuen.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 65.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140(6):918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Le Merrer J, Befort K, Gardon O, Filliol D, Darcq E, Dembele D, Becker JA, Kieffer BL. Protracted abstinence from distinct drugs of abuse shows regulation of a common gene network. Addict Biol. 2012;17(1):1–12. doi: 10.1111/j.1369-1600.2011.00365.x. [DOI] [PubMed] [Google Scholar]