Abstract
Heightened sensitivity to threat and reduced sensitivity to reward are potential mechanisms of dysfunction in anxiety and depressive disorders, respectively. However, few studies have simultaneously examined whether these mechanisms are unique or common to these disorders. In this study, sensitivity to predictable and unpredictable threat (measured by startle response during threat anticipation) and sensitivity to reward (measured by frontal electroencephalographic [EEG] asymmetry during reward anticipation) were assessed in 4 groups (N = 191): those with (1) panic disorder (PD) without a lifetime history of depression, (2) major depression (MDD) without a lifetime history of an anxiety disorder, (3) comorbid PD and MDD, and (4) controls. General distress/negative temperament (NT) was also assessed via self-report. Results indicated that PD (with or without comorbid MDD) was uniquely associated with heightened startle to predictable and unpredictable threat, and MDD (with or without comorbid PD) was uniquely associated with reduced frontal EEG asymmetry. Both psychophysiological measures of threat and reward sensitivity were stable on retest approximately 9 days later in a subsample of participants. Whereas the comorbid group did not respond differently on the tasks relative to the PD-only and MDD-only groups, they did report greater NT than these 2 groups (which did not differ from each other). Results suggest that heightened sensitivity to threat and reduced sensitivity to reward may be specific components of PD and MDD, respectively. In addition, relative to noncomorbid depression and PD, comorbid MDD and PD may be characterized by heightened NT, but not abnormal levels of these “specific” components.
Keywords: depression, anxiety, comorbidity, startle, electroencephalography
For most psychiatric constructs, comorbidity is the norm rather than the exception (Kessler, Chiu, Demler, & Walters, 2005). However, few comorbidities have received as much attention in the literature as that between depressive and anxiety disorders (Kendler, Prescott, Myers, & Neale, 2003; Krueger, 1999; Vollebergh et al., 2001).
There have been several theoretical models that have attempted to explain the relation between depressive and anxiety disorders and identify common and specific features of the disorders (e.g., Clark & Watson, 1991; Davidson, 1998; Heller, Nitschke, Etienne, & Miller, 1997; see Shankman & Klein, 2003).1 One of the most prominent models is the tripartite model (Clark & Watson, 1991; Clark, Watson, & Mineka, 1994). The original 1991 model posited that high general distress/negative affectivity was a common component to both depression and anxiety, and low positive affectivity (PA; e.g., joy, interest, and excitement) was specific to depression, and heightened physiological arousal (e.g., racing heart, shortness of breath) was specific to anxiety.
The tripartite model has undergone several revisions since its inception (e.g., Mineka, Watson, & Clark, 1998; Watson, 2009). Although the model still conceptualizes low PA as specific to depression and high general distress as common to both depression and anxiety, based on extant research, the revised model posited that heightened physiological arousal may not be the specific component associated with all anxiety disorders (Brown, Chorpita, & Barlow, 1998; Brown & McNiff, 2009; Greaves-Lord et al., 2007). Rather, given the heterogeneity of anxiety disorders, the revised model theorized that there might be other constructs (or set of symptoms; Watson, 2009) that delineate particular anxiety disorders from depression (also see Heller & Nitschke, 1998).
Decades of research have examined many aspects of the tripartite model (Cook, Orvaschel, Simco, Hersen, & Joiner, 2004; Gershuny & Sher, 1998; Lonigan, Phillips, & Hooe, 2003; Roberts & Kendler, 1999). However, few studies have examined whether particular laboratory-induced affects are common versus unique to depression or anxiety. These studies are critical as they can provide experimentally controlled evidence of emotional mechanisms that are deficient or intact in depressive and anxiety disorders (Coan & Allen, 2007).
What Is Specific to Anxiety?
The tripartite model posits that there may be various constructs that delineate anxiety disorders from depression. One possibility is that anxiety disorders may be characterized by a heightened sensitivity to particular threatening stimuli (e.g., trauma-related cues in posttraumatic stress disorder [PTSD], social-evaluative cues in social phobia; Barlow, 2000; Craske et al., 2009). However, anxiety disorders have been shown to vary in their responses to threat (e.g., Cuthbert et al., 2003). Thus, particular characteristics of threat may be important moderators of responsivity.
One characteristic that has been examined in the anxiety literature is whether the threat is predictable or unpredictable. Compared with predictable threat, unpredictable threat is associated with greater vigilance and defensive preparedness (Barlow, 2000; Grillon, Lissek, et al., 2008; Mineka & Kihlstrom, 1978). Animal studies also suggest that responses to predictable versus unpredictable threat are mediated by overlapping but separable neuroanatomical systems, specifically the central nucleus of the amygdala for predictable threat and the bed nucleus of the stria terminalis for unpredictable threat (Davis, 1998, 2006; Gray & McNaughton, 2000). The distinction between responses to predictable versus unpredictable threat has been further supported by pharmacological challenge (Grillon et al., 2006; Grillon, Pine, et al., 2009; Moberg & Curtin, 2009) and human functional MRI (fMRI) studies (Alvarez, Chen, Bodurka, Kaplan, & Grillon, 2011). Based on these findings, researchers have often used the labels fear and anxiety to describe the emotions elicited by predictable and unpredictable threat, respectively (Barlow, 2000; Davis, 2006; Gray & McNaughton, 2000).
The distinction between fear and anxiety is especially relevant for panic disorder (PD)—a disorder characterized by periods of intense fear (i.e., panic attacks) and anxiety (i.e., anxious apprehension between panic attacks; Barlow, 2000). Laboratory-based studies have shown that individuals with PD exhibit greater aversive responding to predictable (e.g., carbon dioxide inhalation; Gorman et al., 2001; Griez, Lousberg, Van der Hout, & Van der Molen, 1987; Perna Battaglia, Garberi, & Arancio, 1994) and unpredictable threat (e.g., electric shocks; Melzig, Weike, Zimmermann, & Hamm, 2007). However, one study by Grillon and colleagues (2008) examined response to both types of threat and found that compared with controls, PD was associated with a heightened response to only unpredictable and not predictable threat. It is interesting, however, that Grillon et al. used loud noises as the aversive stimuli, and this may have limited their ability to detect group differences in responsivity to predictable threat. Several laboratory studies have demonstrated that the type of aversive stimulus is critical in studies of anxiety/fear reactivity (Cuthbert et al., 2003) and bodily or interoceptive cues are particularly potent triggers of fear in PD (e.g., Ehlers & Breuer, 1992; Pauli et al., 1997). Indeed, learning theories of PD suggest that interoceptive cues associated with panic attacks elicit anticipatory anxiety, which may in turn potentiate future panic attacks and the downward spiral into PD (Bouton, Mineka, & Barlow, 2001). Thus, individuals with PD may exhibit abnormal responses to predictable aversive cues if those cues are bodily/interoceptive.
To examine responses to predictable and unpredictable threat in those with PD, the aforementioned study by Grillon et al. (2008) used a novel startle paradigm called the NPU-threat paradigm (Grillon, Baas, Lissek, Smith, & Milstein, 2004; Schmitz & Grillon, 2012). The NPU-threat paradigm consists of three conditions: (1) no threat (subjects are safe from an aversive stimulus), (2) predictable threat (aversive stimuli are signaled by short duration cue), and (3) unpredictable threat (aversive stimuli are not signaled). Besides PD, the task has also been used to study other anxiety disorders, such as PTSD and generalized anxiety disorder (GAD; Grillon, Pine, et al., 2009). It is interesting that responses elicited by this paradigm have not been uniform across all anxiety disorders. Whereas those with PTSD and PD exhibited heightened responses to unpredictable threat relative to controls, those with GAD did not differ from controls on response to either type of threat (Grillon et al., 2008; Grillon, Pine, et al., 2009). These findings support the revised tripartite model’s theory that anxiety disorders are heterogeneous and that there are different components associated with each of them (Mineka et al., 1998; Watson, 2009). In addition, given that startle responding is sensitive to valence rather than arousal (Lang, Greenwald, Bradley, & Hamm, 1993), these findings suggest that heightened physiological arousal may not be the feature that is specific to anxiety (or at least to particular anxiety disorders). To the best of our knowledge, however, no study has examined whether depression is also associated with an abnormal response to predictable or unpredictable threat (i.e., whether threat sensitivity is specific to certain anxiety disorders).
What Is Specific to Depression?
The tripartite model hypothesizes that low PA distinguishes depression from anxiety. Given that PA is broad, it may be important to examine particular features of PA to better understand what components are specific to depression (Naragon-Gainey, Watson, & Markon, 2009; Watson & Clark, 1997). One aspect of PA is reward sensitivity, which can be further parsed into two distinct temporal components: anticipation and consummation of reward (Berridge & Robison, 2003; Gard, Gard, Kring, & John, 2006). Deficits in reward anticipation have long been considered a fundamental feature of depression (D. F. Klein, 1974), and numerous behavioral studies have supported its specificity to depression relative to anxiety (Forbes, Shaw, & Dahl, 2007; Pizzagalli, Jahn, & O’Shea, 2005). Notably, a recent study suggests that decreased reward sensitivity in depression may be driven primarily by a deficit in reward anticipation and not reward consummation (Sherdell, Waugh, & Gotlib, 2012).
One psychophysiological indicator that has been shown to relate to reward sensitivity (and approach motivation more broadly) is an asymmetry in electroencephalogram (EEG) activity between right and left frontal brain regions (i.e., reduced left relative to right; Davidson, 1994, 1998). Most studies examining frontal EEG asymmetry have used alpha power as an inverse measure of brain activity. Although the use of alpha power as a measure of cortical inhibition has been controversial (Klimesch, Sauseng, & Hanslmayr, 2007; Tenke & Kayser, 2005), several studies have shown that alpha power is inversely correlated with other measures of brain activity, such as fMRI (Goldman, Stern, Engel, & Cohen, 2002) and positron emission tomography (Oakes et al., 2004).
EEG asymmetry studies typically record data while the participant is at rest (Henriques & Davidson, 1991; Stewart, Bismark, Towers, Coan, & Allen, 2010; Tomarken, Dichter, Garber, & Simien, 2004). However, EEG asymmetry has been shown to be sensitive to affective state and can thus be modulated experimentally (J. J. B. Allen, Coan, & Nazarian, 2004; Hagemann, Hewig, Seifert, Naumann, & Bartussek, 2005). To test whether EEG asymmetry is sensitive to reward anticipation, Shankman, Klein, Tenke, and Bruder (2007) recorded EEG while individuals anticipated winning money in a slot machine game. Consistent with the hypothesis that depression is associated with reduced reward anticipation, individuals with major depressive disorder (MDD) exhibited a reduced relative left frontal EEG asymmetry compared with controls. However, this effect was not found for all individuals with MDD, but only for those with early onset depression (i.e., childhood or adolescent onset), whereas those with adult onset depression were comparable to healthy controls. This suggests that a deficit in reward anticipation may be only for those with early onset depression (Hirschfeld, 1990; D. N. Klein, Durbin, & Shankman, 2009).
It is important to note that several anxiety disorders have also been associated with an abnormal frontal EEG asymmetry characterized by greater relative right activation (Kemp et al., 2010; Nitschke, Heller, Palmieri, & Miller, 1999; Wiedemann et al., 1999). However, according to Heller and Nitschke’s (1998) neuropsychological model of frontal asymmetry, this association may be present only in anxiety disorders characterized by heightened anxious arousal (e.g., PD) and not anxious apprehension (e.g., GAD; Heller et al., 1997). On the other hand, most studies that have measured frontal EEG asymmetry in anxiety disorders have done so while participants are at rest. It is therefore unclear whether the frontal asymmetry will be present in PD while anticipating reward.
Present Study
The present study examined whether heightened sensitivity to (predictable or unpredictable) threat and reduced sensitivity to reward are specific to PD and MDD, respectively, using the aforementioned NPU-threat task (Grillon et al., 2004) and slot task (Shankman et al., 2007). The two tasks complement each other as they both assess anticipatory processes (i.e., anticipating threat and reward). Four groups of individuals were assessed: those with current (1) PD without a lifetime history of depressive disorder, (2) MDD without a lifetime history of an anxiety disorder, (3) co-occurring PD and MDD, and (4) controls. The present study focused on PD given the heterogeneity of anxiety disorders (Craske et al., 2009), and, as discussed above, because there are likely to be different constructs that delineate the various anxiety disorders from depression (Watson, 2009). Similarly, given the heterogeneity in MDD (D. N. Klein, 2008) and the aforementioned finding that low reward anticipation may be evident only for those with early onset depression (Shankman et al., 2007), those in the MDD groups were required to have their onset of depression prior to age 18. There were three potential patterns of results for the present study.
Specificity: If reduced sensitivity to reward is specific to depression, then participants with MDD will differ from non-MDD individuals in frontal EEG asymmetry while anticipating reward. Similarly, if heightened sensitivity to threat is specific to PD, then participants with PD will have greater startle responding while anticipating threat compared with non-PD participants. These results would be largely consistent with the revised tripartite model (Mineka et al., 1998; Watson, 2009), but only if increased sensitivity to threat is a component specific to PD (something that has not been examined in relation to MDD).
Commonality: If reduced sensitivity to reward is common to depression and PD, then MDD and PD participants will differ from controls (but not each other) in their frontal EEG asymmetry while anticipating reward. Similarly, if heightened sensitivity to threat is common across depression and PD, then PD and MDD participants will have greater startle responding while anticipating threat relative to controls (but will not differ from each other).
Interaction: Lastly, there could also be an interaction between MDD and PD on responding. For example, those with comorbid MDD and PD could exhibit different responses on the threat or reward task compared with those with MDD only or PD only. These results could elucidate whether those with comorbid MDD and PD are different from those with MDD or PD alone.
Method
Participants
The sample consisted of 28 individuals with current PD (i.e., PD only), 40 individuals with current MDD (i.e., MDD only), 58 individuals with current PD and current MDD (i.e., comorbids), and 65 controls (N = 191). All diagnoses were made via the Structured Clinical Interview for DSM–IV (SCID; First, Spitzer, Gibbon, & Williams, 1996). SCIDs were conducted by the first author and advanced clinical psychology doctoral students. Diagnosticians were trained to criterion by viewing the SCID-101 training videos (Biometrics Research Department, New York, NY), observing two or three joint SCID interviews with the first author, and completing three SCID interviews (observed by the first author or an advanced interviewer) in which diagnoses were in agreement with the observer. Twenty SCIDs were audio recorded and scored by a second rater blind to original diagnoses to determine reliability of diagnoses. Interrater reliability indicated perfect agreement for PD and MDD diagnoses (kappas = 1.00).
Both depressed groups were required to have an age of onset of first affective disorder (dysthymia or MDD) before 18 years as Shankman et al. (2007) found that it was only those with an early onset depression who exhibited an abnormal frontal EEG asymmetry during the slot task. Participants in the MDD-only group were required to have no current or past history of an anxiety disorder (PD, social phobia, etc.). Participants in the PD-only and comorbid groups were allowed to meet criteria for additional current and past anxiety disorders. PD-only participants also met criteria for social phobia (n = 2), specific phobia (n = 6), PTSD (n = 3), GAD (n = 7), and obsessive– compulsive disorder (n = 1). Comorbid participants also met criteria for social phobia (n = 20), specific phobia (n = 11), PTSD (n = 18), GAD (n = 1), and obsessive– compulsive disorder (n = 9). Comorbid (63.8%) and PD-only (46.4%) participants did not differ in the rate of other lifetime anxiety disorders, χ2(1, N = 86) = 2.34, ns. Control participants were not allowed to have a lifetime diagnosis of Axis I psychopathology, with the exception of a past diagnosis of alcohol or cannabis abuse (but not dependence and not current; n = 4). Control participants were also required to have scores of less than 8 on both the 24-item Hamilton Rating Scale for Depression (HRSD; Hamilton, 1960) and Beck Anxiety Inventory (BAI; Beck, Epstein, Brown, & Steer, 1988).
Participants were excluded from the study if they had a lifetime diagnosis of a psychotic disorder, bipolar disorder, or dementia; were unable to read or write English; had a history of head trauma with loss of consciousness; or were left-handed (as confirmed by the Edinburgh Handedness Inventory; range of laterality quotient: +20 to +100; Oldfield, 1971). Participants were recruited from the community (via fliers, Internet postings, etc.) and area mental health clinics. All procedures were approved by University of Illinois–Chicago Institutional Review Board.
Current Symptom Severity and Personality Measures
Current depression and anxiety severity were assessed via the HRSD and BAI, respectively. Both measures assess the severity of symptoms over the previous week and are widely used measures in research and clinical settings. Cronbach’s alphas for the HRSD and BAI were .92 and .95, respectively. Eight participants (three controls, two PD only, two MDD only, and one comorbid) did not complete the BAI.
Participants also completed the General Temperament Survey (GTS; Clark & Watson, 1990). The GTS is a 90-item true-or-false questionnaire developed to assess general aspects of temperament; it contains factor analytically derived scales of Negative Temperament (GTS-NT) and Positive Temperament (GTS-PT). The GTS-NT and PT scales were later adopted into Clark’s (1993) Schedule for Nonadaptive and Adaptive Personality. High scores on the GTS-NT indicate a tendency to experience emotions such as anger, guilt, and sadness. High scores on the GTS-PT indicate a tendency to experience emotions such as cheerfulness, enthusiasm, and excitement. Cronbach’s alphas for the GTS-PT and GTS-NT were .89 and .92, respectively. Eight participants (three controls, two PD only, two MDD only, and one comorbid) did not complete the GTS.
Procedure
Participants completed the following experimental tasks designed to measure sensitivity to threat (NPU-threat task) and reward (slot task). The two tasks were presented in a counterbalanced order, and order did not differ between groups. For both tasks, participants were seated in an electrically shielded, sound-attenuated booth approximately 3.5 ft from a 19-in. computer monitor.
NPU-threat task and physiological recordings
To prevent early exaggerated startle responding, participants completed a 2.5-min baseline habituation task prior to the NPU-threat task in which nine acoustic startle probes were administered (data not presented). Next, a shock work-up procedure was completed in which participants received increasing levels of shock intensity until they reached a level that they described as feeling “highly annoying but not painful.” Ideographic shock levels were used to ensure equality in perceived shock aversiveness (Rollman & Harris, 1987) and to be consistent with prior studies (Grillon et al., 2004). The maximum shock level a participant could achieve was 5 mA. Within our sample, the mean shock level was 2.08 mA (SD = 1.20), and these scores did not differ between diagnostic groups (ps > .18).
The NPU-threat task was modeled after that used by Grillon and colleagues (Grillon et al., 2004, 2006, 2008; Schmitz & Grillon, 2012) and included three within-subjects conditions: no shock (N), predictable shock (P), and unpredictable shock (U). Text at the bottom of the computer monitor informed participants of the current threat condition by displaying the following information: “no shock” (N), “shock possible during square” (P), or “shock possible at any time” (U). Each condition lasted 90 s, during which an 8-s geometric cue (blue circle for N, red square for P, and green star for U) was presented four times. Different shapes were used for each condition to ensure that participants were aware of which condition they were in (see Schmitz & Grillon, 2012). Interstimulus intervals (ISIs) ranged from 7 to 15 s (M = 11.6 s), during which only the text describing the condition was on the screen. In the N condition, no shocks were delivered. In the P condition, participants could receive a shock only when the cue (red square) was on the screen. In the U condition, shocks were administered at any time (i.e., during the cue or ISI). Startle probes were presented both during the cue (2–7 s following cue onset) and ISI (4 –12 s following ISI onset). No more than one startle probe was delivered during each presentation of the cue or each ISI.
The experiment consisted of two recording blocks, with a 5-min rest period between blocks. Each block consisted of two presentations of each 90-s condition, during which the cue appeared four times, in the following orders (counterbalanced): PNUNPU or UPNUNP. In between the blocks, participants reported on their emotional state during the task (see below). All participants received 12 electric shocks (six during P and six during U) and 72 startle probes (24 during N, 24 during P, and 24 during U). The time interval between a shock and a subsequent startle probe was always greater than 10 s to ensure that startle responses were not affected by an immediately preceding shock.
All stimuli for the task (shocks, startle probes, visual stimuli) were administered using PSYLAB (Contact Precision Instruments, London, U.K.) and psychophysiological data were acquired using Neuroscan 4.4 (Compumedics, Charlotte, NC). Acoustic startle probes were 40-ms duration, 103-dB bursts of white noise with near-instantaneous rise time presented binaurally through headphones. Electric shocks lasted 400 ms and were administered to the wrist of the participants’ left (nondominant) hand.
Startle response was recorded from two 4-mm Ag/AgCl electrodes placed over the orbicularis oculi muscle below the right eye and the ground electrode was at the frontal pole (AFZ). As per published guidelines (Blumenthal et al., 2005), one electrode was 1 cm below the pupil and the other was 1 cm lateral of that electrode. Data were collected using a bandpass filter of DC-200 Hz at a sampling rate of 1000 Hz. Although the upper end of this frequency band is below the Blumenthal et al. (2005) recommendation of 500 Hz, the missing bandwidth (200 –500 Hz) was not likely to affect the experimental manipulation or the reliability of the results (A. Van Boxtel and T. Blumenthal, personal communications, December 14, 2009).
Slot task and physiological recordings
A computerized slot machine paradigm previously used by Shankman et al. (2007) was used to assess reward sensitivity. The task consisted of three reels of numbers and fruit, which “spun” simultaneously for 11 s and then “landed” on a result. To start the reels spinning, participants pressed a button with both thumbs that pulled a lever on the computer screen. The task included 60 “spins” that were divided into two possible outcomes of 30 trials each: a reward condition (R) in which participants won money if the reels landed on three fruits, and a no-incentive condition (NI) in which participants were ineligible to win money no matter the outcome. Thus, the R condition was designed to elicit reward anticipation, and the NI condition served as a control for several aspects of the R condition (e.g., visual input, anticipating an outcome). The amount of money that could be won during each R trial ranged from $0.50 to $3. Notably, in both conditions participants did not lose money if the reels did not land on three pieces of fruit.2
Trials were presented in a pseudorandom order, and there were never more than two consecutive trials of similar type or outcome. Participants began the game with $2 and were told the specific condition (R or NI) prior to each trial, but not the potential dollar amount in each R condition. Unbeknownst to the participant, half of the trials in each condition “landed” on three fruits. Trials were divided into three blocks. Participants completed retrospective ratings of their emotional state for each condition after the first and second blocks (see below). At the end of the task, all participants were given their winnings ($12) in cash.
EEG data were recorded from Ag/AgCl electrodes in a 64-channel stretch-lycra electrode cap (Compumedics Neuroscan 4.4, Charlotte, NC). The ground electrode was at the frontal pole (AFZ) and the online reference was near the vertex (between CZ and CPZ). Electrodes placed at the right supra- and infraorbital sites were used to monitor vertical eye movements (VEOG) and electrodes placed at the right and left outer canthi were used to monitor horizontal eye movements (HEOG). Electrode impedances were under 5,000 ohms, and homologous sites (e.g., F3/F4) were within 1,500 ohms of each other. Data were recorded through a Neuroscan Synamp2 data acquisition system at a gain of 10K (5K for eye channels) with a bandpass of DC-200 Hz. Data were acquired and digitized continuously at a rate of 1000 Hz. EEG data were rereferenced offline by computing a digitally derived “linked mastoids” reference using data from the left and right mastoid.
Emotion ratings
After each block of the NPU-threat task, participants rated their level of nervousness/anxiety during the cues and ISIs for each condition on a scale ranging from 1 (not at all) to 7 (extremely). Participants also rated how intense, annoying, and anxiety provoking the shocks were on a scale ranging from 1 (not at all) to 7 (extremely), and the degree to which they would avoid the shocks on a scale ranging from 1 (would definitely not avoid) to 7 (would definitely avoid). Similarly, after the first two blocks of the slot task, participants rated how much they “looked forward to three pieces of fruit” during both the R and NI conditions on a scale ranging from 1 (not at all) to 7 (extremely). Participants also rated how much they would like to play the slot game again after the end of the second block of trials on a scale ranging from 1 (not at all) to 7 (definitely would). Twenty-five participants (13.1%) did not answer this last question as it was added after the start of the study. Separate analysis of the Time 1 and Time 2 emotion ratings yielded nearly identical results, so the present study used the average rating of the two administrations.
Physiological data processing
Startle blinks were scored according to published guidelines (Blumenthal et al., 2005). Data were first rectified and then smoothed using a FIR filter with a band pass of 28 – 40 Hz. Blink response was defined as the peak amplitude of electromyogram (EMG) activity within the 20- to 150-ms period following startle probe onset relative to baseline (average baseline EMG level for the 50 ms preceding the startle probe onset). Each peak was identified by software but was examined by hand to ensure acceptability (e.g., not a double blink). Blinks were scored as nonresponses if EMG activity during the 20- to 150-ms poststimulus timeframe did not produce a blink peak that was visually differentiated from baseline activity. Blinks were scored as missing if the baseline period was contaminated with noise, movement artifact, or if a spontaneous or voluntary blink began before minimal onset latency and thus interfered with the startle probe-elicited blink response. Analyses were conducted using both blink magnitude (i.e., condition averages that include values of zero for nonresponse trials) and amplitude (i.e., condition averages that do not include nonresponse trials). However, blink amplitude and magnitude yielded nearly identical results, so only startle magnitude results are presented as these are a more conservative estimate of average blink response (Blumenthal et al., 2005). Seven participants (three MDD only, three comorbids, and one control) were excluded from analyses because they produced fewer than two scorable blinks in any single condition (i.e., NISI, NCue, PISI, PCue, UISI, UCue) and three participants (MDD only) were excluded because of equipment failure, refusal to complete the task, or deafness in one ear.3 Diagnostic groups did not differ in the number of missing blinks (ps > .29). This left a final sample of 181 (28 PD only, 34 MDD only, 55 comorbids, and 64 controls). Participants who were included versus excluded from analyses did not differ on any demographic or clinical characteristics.
EEG data from the 11-s period while the slot machine reels were spinning were segmented into consecutive 1.024-s epochs every 0.512 s (50% overlap). After referencing to a linked mastoid reference offline and then applying a baseline correction, we manually excluded epochs contaminated by blinks, eye movements, and movement-related artifacts from analyses by direct visual inspection of the data. The EEG was tapered over the entire 1.024-s epoch by a Hanning window to suppress spectral side lobes. Artifact-free data were recovered in adjacent (overlapping) epochs and power spectra were computed offline from EEG data by using a fast Fourier transform. Subsequently, the average absolute alpha power was computed for each electrode site and then natural log transformed to normalize the data. Consistent with previous studies (e.g., Bruder, Fong, Tenke, & Leite, 1997), we defined the alpha band as 7.81–12.70 Hz and used it as an inverse measure of regional brain activity. Frontal asymmetry scores were computed for the R and NI conditions by subtracting power at left frontal electrodes from power at homologous right electrodes (e.g., F8 –F7), so that the higher values reflect greater activity in left relative to right frontal regions. The present study examined three frontal (F3/4, F5/6, and F7/8) and three parietal (P3/4, P5/6, and P7/8) EEG asymmetries. Parietal EEG asymmetries were included to test topographical specificity of any effect. These analyses are especially important as depression has also been shown to be associated with an abnormal EEG asymmetry over parietal regions (e.g., Bruder et al., 1997; Heller & Nitschke, 1998).
The sample of participants for the EEG analyses was taken from the 181 participants with good startle data (all participants with good EEG data had good startle data, but not vice versa). Twenty-five participants (13%; four PD only, five MDD only, seven comorbids, and nine controls) were excluded from EEG analyses because of excessive artifacts in electrodes of interest (i.e., F3, F4, F5, F6, F7, F8, P3, P4, P5, P6, P7, P8) in the NI or R condition, leaving a sample of 156 for the EEG analyses (24 PD only, 29 MDD only, 48 comorbids, and 55 controls). To determine whether groups or conditions differed in the number of EEG epochs that were accepted, we conducted a three-way analysis of variance (ANOVA) with condition (NI vs. R) as the within-subjects factor and depression status (present vs. absent) and panic status (present vs. absent) as between-subjects factors. Results indicated a main effect of condition, F(1, 153) = 27.88, p < .001, , such that the R condition (M = 376.41, SD = 110.56) had more accepted epochs than the NI condition (M = 357.83, SD = 110.10), but there were no group differences in accepted EEG epochs (ps > .10).
Test–retest reliability of threat and reward sensitivity
To examine the stability of threat and reward sensitivity, 32 participants returned to the lab 5–17 days later (M = 9.46 days, SD = 3.71) and completed the experimental tasks a second time. Two participants (one PD only and one comorbid) were excluded from analyses because of poor-quality EEG data at one of the two lab visits, leaving a final sample of 30 (four PD only, seven MDD only, nine comorbids, and 10 controls). For the NPU-threat task, participants completed identical blocks of trials, but in the alternate order as the first lab session (i.e., if PNUNPU at Time 1, then UPNUNP at Time 2). For the slot task, participants completed an identical number of trials, but in an alternate order and with a different final payout ($15).
Data Analysis
An important initial step in studying group differences on reactivity is to examine group differences during the control condition (i.e., N condition for NPU-threat task; NI condition for slot task). These analyses rule out that group effects on “reactivity” are not due to baseline differences (Nelson, Shankman, Olino, & Klein, 2011). The NPU-threat model included cue (cue vs. ISI— both during the N condition) as a within-subjects factor and depression status (present vs. absent) and panic status (present vs. absent) as between-subjects factors.4 The slot task model included location (F3/4 vs. F5/6 vs. F7/8 —all during the NI condition) as a within-subjects factor and depression status and panic status as between-subjects factors. Results indicated no main effects or interactions involving depression status or panic status for both psychophysiological and verbal measures (ps > .12), suggesting that there was no effect of group on the tasks’ respective control conditions. Consequently, for the NPU-threat task, potentiation scores were computed for both the P (i.e., PCue – NCue, PISI – NISI) and U (i.e., UCue – NCue, PISI – NISI) threat conditions. These potentiation scores represented response potentiation to threat from the N condition. Similarly, for the slot task, potentiation scores (R asymmetry – NI asymmetry) were computed for each region (F3/4, F5/6, and F7/8). These potentiation scores represented response potentiation to reward from the NI condition.
Group differences on startle potentiation were conducted using a four-way analysis of covariance (ANCOVA) with condition (P vs. U) and cue (Cue vs. ISI) entered as within-subjects factors and depression status (present vs. absent) and panic status (present vs. absent) entered as between-subjects factors. Identical analyses were conducted for verbal anxiety. Group differences in EEG asymmetry potentiation were conducted using a three-way ANCOVA with location (F3/4, F5/6, F7/8) entered as the within-subjects factor and depression status and panic status entered as between-subjects factors. For verbal excitement, a two-way ANCOVA was conducted with depression status and panic status entered as between-subjects factors. For all analyses, age (mean-centered based on the whole sample) and gender were included as covariates (see below).
Group differences in GTS-NT/GTS-PT were examined using a Depression Status × Panic Status ANCOVA (with gender and age as covariates).
Startle and EEG data were processed and scored using identical approaches described in the aforementioned sections. To examine test–retest reliability, we calculated Pearson’s correlation coefficients between Time 1 and Time 2 psychophysiological (i.e., startle and EEG) and verbal (i.e., anxiety and excitement) measures.
Results
Demographics and Clinical Characteristics
Participant demographics and clinical characteristics are presented in Table 1. Groups did not differ on gender, education, or ethnicity (ps > .14). Comorbid participants were older than MDD-only participants at a trend level, F(1, 96) = 3.74, p < .06, , and age was found to be related to startle and EEG responding. Therefore, age (mean-centered) was entered as a covariate (along with gender) in all subsequent analyses involving depression status and panic status.
Table 1.
Participant Demographics and Clinical Characteristics
| Variable | Control (n = 65) | MDD only (n = 40) | PD only (n = 28) | Comorbid (n = 58) |
|---|---|---|---|---|
| Mean (SD) age (years) | 32.4 (13.2)a | 31.4 (12.5)a | 34.0 (13.1)a | 36.1 (11.2)a |
| Sex (% female) | 60.0a | 62.5a | 60.7a | 70.7a |
| Race (% Caucasian) | 41.5a | 47.5a | 46.4a | 48.3a |
| Education (%) | ||||
| Grade 7 to 12 (without graduating high school) | 0.0 | 0.0 | 3.6 | 5.2 |
| Graduated high school or high school equivalent | 7.7 | 0.0 | 3.6 | 3.4 |
| Part college or graduated 2-year college | 29.2 | 60.0 | 32.1 | 46.6 |
| Graduated 4-year college | 35.4 | 27.5 | 42.9 | 27.6 |
| Part or completed graduate/professional school | 27.7 | 12.5 | 17.9 | 17.2 |
| Mean (SD) clinical variables | ||||
| Global Assessment of Functioning | 88.7 (7.5)a | 53.5 (7.5)c | 58.1 (8.9)b | 52.3 (6.4)c |
| Hamilton Rating Scale of Depression | 1.5 (1.8)a | 24.2 (8.1)c | 8.6 (7.2)b | 26.5 (8.6)c |
| Beck Anxiety Inventory | 1.7 (2.0)a | 13.9 (10.8)b | 15.5 (11.8)b,c | 20.4 (13.3)c |
| Age of onset of first depressive disorder (years) | – | 13.6 (3.3)a | – | 13.4 (4.0)a |
| Age of onset of first anxiety disorder (years) | – | – | 21.2 (9.0)a | 16.0 (8.9)b |
| Lifetime alcohol abuse/dependence disorder | 6.2a | 42.5b,c | 28.6b | 50.0c |
| Lifetime drug abuse/dependence disorder | 1.5a | 27.5b,c | 17.9b | 39.7c |
| Current psychiatric medications (%) | ||||
| Any medication | 1.6a | 30.0b,c | 21.4b | 44.8c |
| SSRI/SNRI | 0.0 | 17.5 | 25.0 | 19.0 |
| Tricyclic/tetracyclic antidepressant | 0.0 | 2.5 | 0.0 | 5.2 |
| Atypical antidepressant | 0.0 | 0.0 | 0.0 | 6.9 |
| Atypical antipsychotic | 0.0 | 0.0 | 0.0 | 5.2 |
| Benzodiazepine | 0.0 | 7.5 | 7.1 | 22.4 |
| Other | 1.5 | 7.5 | 0.0 | 12.1 |
Note. Means or percentages with different subscripts across rows were significantly different in pairwise comparisons (p < .05, chi-square test for categorical variables and Tukey’s honestly significant difference test for continuous variables). MDD = major depressive disorder; PD = panic disorder; SSRI = selective serotonin reuptake inhibitor; SNRI = serotonin-norepinephrine reuptake inhibitor. “Other” medications included stimulants (n = 1 control, n = 2 comorbids), serotonin modulators (n = 3 comorbids), tryptophan (n = 1 comorbid), s-adenosylmethionine (n = 1 comorbid), opiate analgesics (n = 1 MDD), and hypnotics (n = 2 MDD, n = 1 comorbid).
Within the three PD and/or MDD groups, comorbid participants had higher rates of lifetime alcohol abuse/dependence disorder, lifetime substance abuse/dependence disorder, and current psychiatric medication use relative to PD-only participants. Comorbid participants had an earlier age of onset of first anxiety disorder relative to PD-only participants, but did not differ from MDD-only participants on age of onset of first depressive disorder.
As previously mentioned, MDD participants were required to have an age of onset before 18 years. This fact, along with a large age range, introduced the potential for a great deal of variability in the course of depression between onset and the time of study. Therefore, additional analyses were conducted within the MDD participants (i.e., MDD only and comorbids) examining whether (a) the number of lifetime depressive episodes and (b) the difference between current age and age of onset of MDD (i.e., current age – age of onset of MDD) were associated with psychophysiological and/or verbal responding. All analyses were nonsignificant (ps > .21).
As expected, control participants had higher Global Assessment of Functioning (GAF; as assessed by diagnosticians during the SCID) and lower HRSD and BAI scores relative to the other three groups. PD-only participants had higher GAF and lower HRSD scores relative to MDD-only and comorbid participants (who did not differ). Comorbid participants had higher BAI scores relative to MDD-only participants, but PD-only participants did not differ from MDD-only and comorbid participants.5
To address potential effects of group differences in symptom severity, we examined whether HRSD or BAI scores were associated with psychophysiological and/or verbal responding within MDD (i.e., MDD only and comorbids) and PD participants (i.e., PD only and comorbids), respectively. None of these results were significant (ps > .12).
NPU-Threat Task
Aversiveness of the electric shocks
Participants rated the shocks as moderate to extremely intense (M = 4.93, SD = 1.16), annoying (M = 5.20, SD = 1.29), and anxiety provoking (M = 4.95, SD = 1.50). Participants also rated that they would avoid receiving the shocks again to a high degree (M = 5.61, SD = 1.41). Thus, the shocks were sufficiently aversive for participants. Depression status or panic status was not associated with any of the shock ratings (ps > .13).
Diagnostic group comparisons
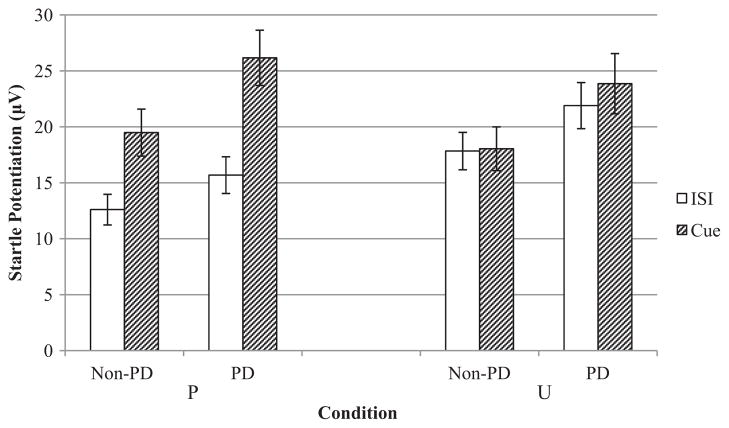
Results indicated a trend-level main effect for condition, F(1, 175) = 3.48, p < .07, , that was qualified by a Condition × Cue interaction, F(1, 175) = 4.37, p < .05, , such that startle potentiation was greater during the threat (i.e., PCue, UISI, UCue) relative to threat-free periods (i.e., PISI). Results also indicated a main effect for panic status, F(1, 175) = 4.72, p < .05, , such that PD participants (PD only and comorbids) had greater startle potentiation relative to non-PD participants (controls and MDD only) during the P and U threat conditions. There was no main effect or interactions involving depression status (ps > .19). Figure 1 displays means (and standard errors) for startle potentiation (i.e., difference from the N condition) across different levels of condition, cue, and panic status.
Figure 1.
Mean startle potentiation (i.e., difference from N condition) at different levels of condition, cue, and panic status. Error bars represent standard error. PD = panic disorder; N = no shock; P = predictable shock; U = unpredictable shock; ISI = interstimulus interval; μV = microvolts.
As previously mentioned, groups did not differ in startle responding to the N condition (ps > .12). Therefore, to examine whether group differences in startle potentiation were due to heightened responding during the threat conditions or the relative difference between N and threat conditions, we conducted a Condition (P vs. U) × Cue (ISI vs. cue) × Panic Status (present vs. absent) mixed measures ANCOVA with age and gender entered as covariates for raw startle responding (i.e., PISI, PCue, UISI, UCue). Results again indicated a main effect for panic status, F(1, 175) = 4.52, p < .05, , suggesting that group differences in startle potentiation scores were due to heightened startle responding to P and U threat, and not the N condition.
Even though the nonsignificant Condition × Cue × Panic Status interaction suggests that PD individuals did not exhibit a differential sensitivity to unpredictable versus predictable threat, we explored whether there were group differences in potentiation to the threat cues (PCue and UCue). Specifically, a Condition (P vs. U) × Panic Status (present vs. absent) ANCOVA was conducted with condition as the within-subjects factor and panic status as the between-subjects factor and startle potentiation to the cue (i.e., PCue – NCue, UCue – NCue) as the dependent variable. Age and gender were again included as covariates. Results indicated a main effect for panic status, F(1, 177) = 5.35, p < .05, , such that participants with PD had greater startle potentiation to PCue and UCue relative to potentiation in non-PD participants. However, there was no Condition × Panic Status interaction (p = .78), suggesting that PD participants’ heightened sensitivity to threat was not significantly different across P and U threats.
For verbal anxiety, results indicated main effects for condition, F(1, 175) = 9.22, p < .01, , and cue, F(1, 175) = 6.68, p < .05, , that were qualified by a Condition × Cue interaction, F(1, 175) = 10.88, p < .001, , such that verbal anxiety was greater during threat (i.e., PCue, UISI, UCue) relative to threat-free periods (i.e., PISI). Results also indicated a Panic Status × Cue interaction, F(1, 175) = 4.49, p < .05, , such that non-PD participants reported greater anxiety potentiation during the cue relative to ISI period, F(1, 95) = 5.87, p < .05, , but PD participants reported comparable anxiety potentiation during the cue and ISI periods, F(1, 180) = 1.74, ns, . There was no main effect or interactions involving depression status (ps > .28).
Slot Task
Results for frontal EEG asymmetry (i.e., F3/4, F5/6, F7/8) indicated a main effect for depression status, F(1, 150) = 7.05, p < .01, , that was qualified by a Depression Status × Location interaction that approached significance, F(2, 300) = 3.27, p = .06, . To follow-up the interaction, we conducted separate one-way ANCOVAs for each region (i.e., F3/4, F5/6, F7/8) with depression status as the between-subjects factor. Across all three electrode pairs, MDD participants (MDD only and comorbids) had nearly symmetrical frontal EEG activation (M = −0.002, SD = 0.067) compared with non-MDD participants (controls and PD only), who had greater left relative to right frontal EEG activation (M = 0.024, SD = 0.054). Group differences were most pronounced in the lateral electrode pair (i.e., F7/8). There was no panic status main effect, F(1, 150) = 0.65, ns, Panic Status × Depression Status interaction, F(1, 150) = 0.01, ns, or any interaction involving panic status and location (ps > .84).
As previously mentioned, groups did not differ in frontal EEG asymmetry during the NI condition (ps > .12). Therefore, to examine whether group differences in frontal EEG potentiation were due to responding during the R condition or to the relative difference between conditions, we conducted a Location (F3/4, F5/6, F7/8) × Depression Status (present vs. absent) ANCOVA with age and gender entered as covariates using EEG asymmetry during the R condition as the dependent variable. Results indicated no depression status main effect (p = .83) or Location × Depression Status interaction (p = .38), suggesting that MDD and non-MDD group differences in frontal EEG asymmetry potentiation were due to the relative difference between the R and NI conditions.
To examine regional specificity, we added region (frontal vs. parietal) to the omnibus slot task ANCOVA. Results indicated a Region × Location × Depression Status three-way interaction, F(2, 298) = 3.50, p = .05, , suggesting different effects for the frontal compared with parietal regions. Follow-up analyses in the parietal region indicated no main effects or interactions (ps > .12). Thus, the effects for depression appeared to be largely due to differences in the frontal region.
Analyses for verbal excitement indicated no main effects or interactions involving depression status or panic status (ps > .11). However, analyses for verbal reports of how much participants would like to play the game again indicated a trend level main effect for depression status, F(1, 134) = 3.48, p < .07, , such that MDD participants (M = 4.54, SD = 1.87) had less desire to play the game again relative to non-MDD participants (M = 3.88, SD = 1.97).
Reliability of Threat and Reward Sensitivity
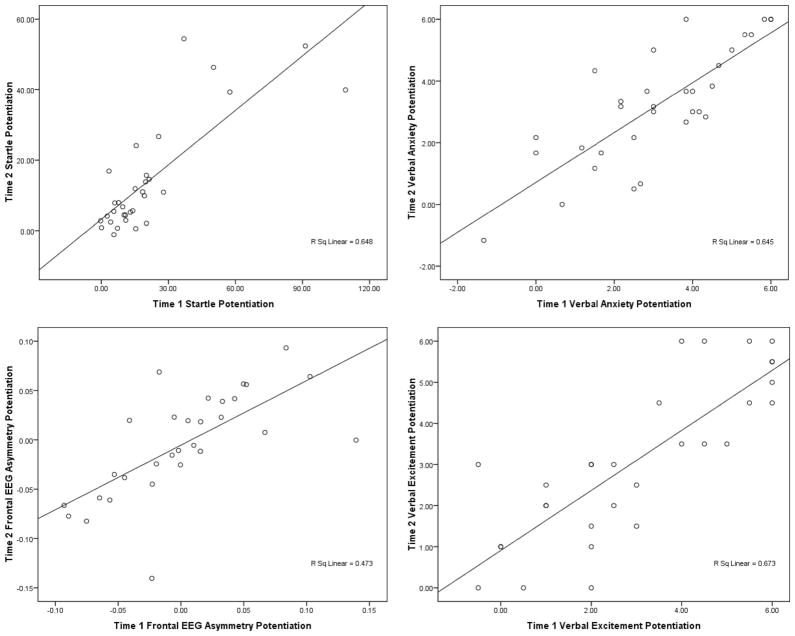
To examine the reliability of threat and reward sensitivity, we calculated Pearson’s correlation coefficients for startle and verbal anxiety potentiation (i.e., difference from N condition) and frontal EEG asymmetry and verbal excitement potentiation (i.e., R – NI) between Time 1 and Time 2 (see Figure 2). For threat sensitivity, results indicated that startle potentiation at Time 1 was strongly correlated with startle potentiation at Time 2 during UCue, r(30) = .71, p < .001; UISI, r(30) = .80, p < .001; PCue, r(30) = .69, p < .001; and PISI, r(30) = .36, p = .05. Similarly, verbal anxiety potentiation was also strongly correlated during UISI, r(30) = .71, p < .001; UCue, r(30) = .78, p < .001; PCue, r(30) = .76, p < .001; and PISI, r(30) = .56, p = .001. For reward sensitivity, EEG asymmetry potentiation (R – NI) at Time 1 was strongly correlated with EEG asymmetry potentiation at Time 2 for the F3/4, r(30) = .74, p < .001; F5/6, r(30) = .74, p < .001; and F7/8 electrode pairs, r(30) = .70, p < .001. Similarly, verbal excitement potentiation was strongly correlated across the two time points, r(30) = .82, p < .001.
Figure 2.
Test–retest reliabilities of Time 1 (x-axis) and Time 2 (y-axis) psychophysiological and verbal responding measures for NPU-threat (top row) and slot tasks (bottom row). Given the similarity of test–retest across electrode pairs (F3/4, F5/6, and F7/8) for frontal electroencephalography (EEG) asymmetry and startle potentiation (see text), frontal EEG asymmetry potentiation was averaged across F3/4, F5/6, and F7/8 asymmetries and startle potentiation was averaged across PCue, UISI, and UCue conditions. N = no shock; P = predictable shock; U = unpredictable shock; ISI = interstimulus interval.
Self-Report Personality Measures of GTS-NT and GTS-PT
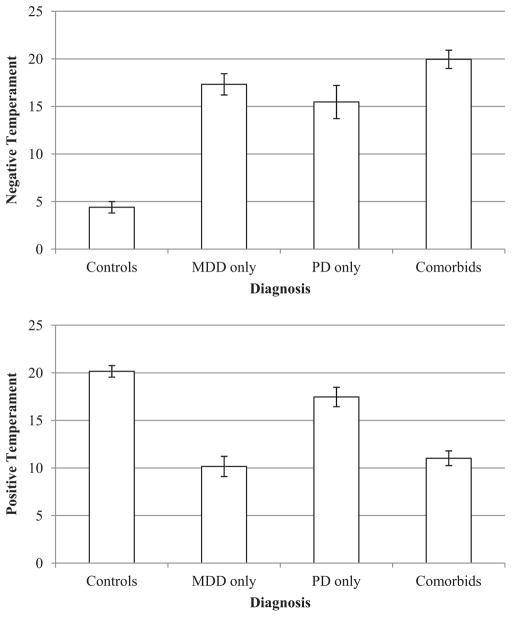
Figure 3 displays means (and standard errors) for self-report measures of GTS-NT (top) and GTS-PT (bottom) at different levels of depression status and panic status. For GTS-NT (see top of Figure 3), results indicated main effects for depression status, F(1, 177) = 69.47, p < .001, , and panic status, F(1, 177) = 44.32, p < .001, , and a Depression Status × Panic Status interaction, F(1, 177) = 14.95, p < .001, . Follow-up analyses indicated that comorbid participants reported greater GTS-NT relative to controls, F(1, 115) = 192.72, p < .001, ; MDD-only, F(1, 91) = 3.82, p = .05, ; and PD-only participants, F(1, 79) = 7.22, p < .01, . MDD-only and PD-only participants reported greater GTS-NT relative to controls, F(1, 96) = 119.80, p < .001, , and F(1, 84) = 57.79, p < .001, , respectively. It is interesting that MDD-only and PD-only participants did not differ from each other on GTS-NT, F(1, 60) = 0.69, ns.
Figure 3.
Mean Negative Temperament (top) and Positive Temperament (bottom) scores from the General Temperament Survey at different levels of diagnosis. Error bars represent standard error. MDD = major depressive disorder; PD = panic disorder.
For GTS-PT (see bottom of Figure 3), results indicated a main effect for depression status, F(1, 177) = 86.37, p < .001, , and a Depression Status × Panic Status interaction, F(1, 177) = 4.31, p < .05, . Follow-up analyses indicated that MDD-only and comorbid participants reported less GTS-PT relative to controls, F(1, 96) = 75.28, p < .001, , and F(1, 115) = 81.45, p < .001, , respectively, and PD-only participants, F(1, 60) = 22.20, p < .001, , and F(1, 79) = 21.33, p < .001, , respectively. MDD-only and comorbid participants did not differ from each other, F(1, 91) = 0.22, ns. In addition, PD-only participants had less GTS-PT relative to controls, F(1, 84) = 5.26, p < .05, .
Discussion
The present study examined whether heightened sensitivity to threat and reduced sensitivity to reward are common or unique to PD and MDD, respectively. Participants with current PD and/or early onset MDD and controls completed two experimental tasks designed to assess sensitivity to predictable and unpredictable threat (NPU-threat task; Grillon et al., 2004) and sensitivity to reward (slot task; Shankman et al., 2007). In sum, the psychophysiology results suggested specificity: A heightened sensitivity to predictable and unpredictable threat was related to PD (but not MDD) and a reduced sensitivity to reward was related to MDD (but not PD). In addition, there were no significant PD = MDD interactions for reactivity to the tasks. Although one cannot rule out the presence of interactions based on these nonsignificant results (i.e., Type II error), they do provide suggestive evidence that those with comorbid PD and MDD did not exhibit different responses than those with PD and MDD only.
Heightened Sensitivity to Threat
Clark and Watson’s original tripartite model (Clark & Watson, 1991; Clark et al., 1994) hypothesized that heightened physiological arousal was specifically associated with anxiety (relative to depression). However, revisions to the model (Mineka et al., 1998; Watson, 2009) have highlighted the heterogeneity of anxiety disorders and suggested that heightened physiological arousal may not be the specific component for all anxiety disorders. Rather, the revised model (Mineka et al., 1998) posited that each anxiety disorder might have its own specific component that differentiates it from depression, and heightened physiological arousal may specifically relate to PD (although see Brown & McNiff, 2009). Watson (2009) extended this further by arguing that particular symptoms may vary on their specificity to depression versus anxiety disorders.
Consistent with the revised tripartite model, the present study found that PD, but not depression, was associated with heightened physiological responding (i.e., startle) during anticipation of threat. Moreover, this effect was largely observed for both predictable and unpredictable threat. It has been suggested that predictability is one feature of aversive stimuli that differentiates the emotional states of fear (elicited by predictable threat) and anxiety (elicited by unpredictable threat; Davis, 2006; Grillon, et al., 2008). It is therefore interesting that PD was associated with heightened reactivity to both types of aversive stimuli as individuals with PD experience periods of intense fear during panic attacks and anticipatory anxiety (i.e., anxious apprehension) between panic attacks (Barlow, 2000).
The results are not entirely consistent with the only other study that used the NPU-threat task with PD individuals. Grillon et al. (2008) found that PD was associated with heightened startle responding to unpredictable but not predictable threat. The present study therefore replicated the finding for unpredictable threat, but it differed from Grillon et al. in that it found that PD was also associated with heightened startle to predictable threat. There were several important methodological differences between Grillon et al. and the present study that may account for this discrepancy. First, the aversive stimuli in the present study were electric shocks with ideographically determined intensity levels, whereas Grillon et al. used standardized unpleasant noises (e.g., smoke alarm, car alarm). Although unpleasant noises have been shown to be effective aversive stimuli in several studies that employed the NPU-threat task (Grillon, Pine, et al., 2009; Lissek et al., 2005), tactile stimuli (such as shocks) may be especially aversive for those with PD given the disorder’s association with fear of bodily sensations (De Cort, Griez, Büchler, & Schruers, 2002; Meuret, Rosenfield, Hofmann, Suvak, & Roth, 2009). Moreover, studies in PD that have examined startle responses to other predictably administered aversive stimuli (e.g., fear imagery scripts) have also yielded conflicting findings (Cuthbert et al., 2003; Lang & McTeague, 2009; McTeague, Lang, Laplante, & Bradley, 2011). Therefore, for individuals with PD, the type of threat may be an important determinant of responding when the threat is predictable, but not when it is unpredictable. Another difference between the present study and Grillon et al. is that Grillon et al. required PD participants to be medication-free and excluded those with comorbid depression, whereas the present study did not. As the present study found that the comorbid group was more severe and more likely to be taking medication than the PD-only group, it is possible that Grillon et al. may have excluded more severe PD participants who exhibit a heightened sensitivity to both predictable and unpredictable threat.
Results from the present study also indicated that sensitivity to threat was not associated with depression—those with MDD (MDD only plus comorbids) did not differ from those without MDD (controls plus PD only) on startle potentiation to either predictable or unpredictable threat. In addition, the PD-only and comorbid participants did not differ from each other on startle potentiation to predictable and unpredictable threat, as there was no PD × MDD interaction. The literature on startle reactivity in anxiety is considerably larger than that for depression (Grillon & Baas, 2003). However, the startle studies that have been conducted with depression generally show comparable and possibly reduced startle modulation compared with controls (N. B. Allen, Trinder, & Brennen, 1999; Dichter & Tomarken, 2008; Dichter, Tomarken, Shelton, & Sutton, 2004; Kaviani et al., 2004; Taylor-Clift, Morris, Rottenberg, & Kovacs, 2011). Results from the present study therefore extend this literature and suggest that depression may not be associated with abnormal startle potentiation to predictable or unpredictable threat (either on its own or in co-occurrence with PD). This pattern of results may be somewhat surprising given that the amygdala plays a role in startle responding (Koch, 1999) and amygdala dysfunction has been observed in individuals with depression (see Davidson, Pizzagalli, & Nitschke, 2009, for review). On the other hand, Beesdo et al. (2009) reported that depression and anxiety exhibit overlapping, yet distinguishable patterns of amygdala functioning. Thus, the amygdala (or different nuclei in the amygdala) may play a different role in depression and anxiety conditions.
Reduced Sensitivity to Reward
Both the original and revised tripartite model posited that low PA distinguishes depression from anxiety (Clark & Watson, 1991; Mineka et al., 1998). However, given the broad nature of PA, the current study focused on the specific component of reduced reward anticipation as this has long been considered to be a fundamental feature of depression (D. F. Klein, 1974; Pizzagalli et al., 2005; Sherdell et al., 2012).
Replicating previous results (Shankman et al., 2007), the present study found that early onset MDD was associated with an abnormal frontal EEG asymmetry while anticipating reward. More specifically, non-MDD participants (controls and PD only) exhibited greater relative left frontal EEG activity while anticipating reward, whereas MDD participants (MDD only and comorbids) exhibited a nearly “symmetrical” frontal EEG pattern. These results add to the literature on frontal EEG asymmetry as a potential biomarker for depression (Coan & Allen, 2004; Davidson, 1998; Thibodeau, Jorgensen, & Kim, 2006). It must be noted, however, that studies have not always shown an association between frontal EEG asymmetry and depression (e.g., Reid, Duke, & Allen, 1998) and the literature is not without controversies (J. J. B. Allen, 2009). One of the controversies is that EEG asymmetry is usually recorded at rest and thus “state” effects are often left uncontrolled. As a large portion of the variance in EEG asymmetry may be due to state effects (Hagemann et al., 2005), EEG asymmetry may be a more valid indicator of affective processes (and marker for depression) if measured during an experimentally controlled context (i.e., adopting a capability model approach to measure individual differences; Cervone, 2004; Coan et al., 2006; Mischel, 1973).
PD was not associated with an abnormal frontal EEG pattern during the reward task. This is noteworthy given that an abnormal frontal EEG asymmetry has been associated with anxiety (Blackhart, Minnix, & Kline, 2006; Heller & Nitscke, 1998; Kemp et al., 2010; Nitschke et al., 1999) and PD more specifically (Wiedemann et al., 1999). However, most of the studies on anxiety and EEG asymmetry either did not take current (or past) depression into account and/or only examined EEG asymmetry at rest and not during a lab induction of reward sensitivity. Thus, it is possible that these asymmetry studies may have been assessing constructs besides reward sensitivity, such as the broad negative affectivity component that is common to depression and anxiety (Clark & Watson, 1991).
Replicability and Test–Retest Reliability of Tasks
The results of this study add to the validity of the NPU-threat and slot tasks as measures of reward and threat sensitivity, respectively (Schmitz & Grillon, 2012; Shankman et al., 2007). Although replication is clearly important, biological indicators of psychological processes have often been criticized for not being stable over time (e.g., Bennett & Miller, 2010). Therefore, in a subsample of participants, we tested whether individual differences in reactivity were stable over an approximately 9-day period. Interestingly, we found that both psychophysiological and verbal indices of reactivity were strongly correlated with reactivity assessed at a later date. Although these results cannot address whether reactivity represents a trait/preexisting factor (or even whether it remains stable when participants are in remission), it does suggest that the tasks measure reliable indicators of threat and reward sensitivity.
What Characterizes Those Who Are Comorbid?
The majority of studies and theoretical models on the comorbidity between depression and anxiety examine common versus specific components of the two classes of disorders (Clark & Watson, 1991; Davidson, 1998; Heller & Nitschke, 1998). An often unaddressed question in these studies is which of these components characterizes individuals who have both disorders (and, relatedly, whether these components account for why they co-occur so often; Shankman & Klein, 2003). That is, do depression and anxiety co-occur frequently only because of elevated levels of general distress/negative affectivity or also because of elevated levels of the specific components of the respective disorder?
The present study found that comorbid participants had greater general distress/negative affectivity (the component common to depression and anxiety; Clark & Watson, 1990) relative to MDD-only and PD-only participants, who did not differ. In addition, as stated above, there were no significant effects of comorbidity on the results for the NPU-threat and slot tasks. Taken together, results from the present study suggest that MDD and PD may only co-occur because of heightened levels of general distress/negative affectivity shared by the two disorders and not by abnormalities on components specific to the individual disorders: low reward sensitivity and heightened threat sensitivity. There are of course many caveats to this conclusion. First, and most notably, the cross-sectional nature of the analyses introduces the plausible explanation that high general distress/negative affectivity may be the result (rather than underlying cause) of having comorbid disorders. Second, the specific components were assessed using laboratory tasks, whereas general distress/negative affectivity was assessed with a self-report personality questionnaire (GTS). Third, one cannot reject the hypothesis that comorbidity plays a role in sensitivity to reward and threat based on the nonsignificant PD = MDD interactions found in this study (i.e., this would be a Type II error). Fourth, there are likely different mechanisms to comorbidity depending on the anxiety disorder (and the particular symptom; Watson, 2009). Nevertheless, the present results do provide suggestive evidence for this important issue. As those with comorbid depression and anxiety are generally more severe and harder to treat than those with only one disorder (Belzer & Schneier, 2004; Johnson & Lydiard, 1998), more research on why the disorders co-occur is critical.
Discrepancy Between Psychophysiology and Verbal Emotions Ratings
Both experimental tasks were largely effective at changing participants’ self-report emotions ratings. For the NPU-threat task, participants reported greater anxiety during the cue of the P and U conditions compared with the N condition and greater anxiety during the ISI of the U condition compared with the P and N conditions. For the slot task, participants reported more excitement while anticipating the outcome of trials during the R condition compared with the NI condition. However, unlike the results for startle and EEG, the diagnostic groups did not differ on verbal emotions ratings. One potential explanation for these findings is that the experimental tasks may have induced a ceiling effect on the emotion ratings because of the tasks being “strong situations.” Situational strength has long been identified as an important factor that moderates the relationship between individual differences and behavior, particularly self-reported behavior (Caspi & Moffitt, 1993; Cooper & Withey, 2009; Mischel, 1977). Strong situations provide unambiguous stimuli that generally yield uniform reactions across individuals. In contrast, weak situations are more ambiguous events that attenuate the influence of the situation and increase the contribution of individual differences (such as diagnosis) on subsequent responses. Within the present study, the experimental tasks may have been so effective at eliciting verbal anxiety and excitement responses that they produced a restricted range of verbal responses. For example, during the R condition of the slot task, even though the scale ranged from 1 to 7, 61.9% of participants rated their excitement between 5 and 7. Similarly, during the U condition of the NPU-threat task, 58.0% of participants rated their anxiety between 5 and 7 during the ISI, and 56.4% made ratings in this range during the cue.
Another potential explanation for the lack of group differences in verbal ratings is that the self-report measure of anxiety/nervousness may have reflected the participants’ level of arousal and not valence. In contrast, startle responding has been shown to be sensitive to valence and not arousal (Lang et al., 1993). Therefore, it is not surprising that diagnostic groups differed on startle but not verbal anxiety/nervousness ratings. This finding also suggests that heightened sensitivity to particular features of aversive stimuli (e.g., predictability) may be what differentiates certain anxiety disorders from depression rather than physiological arousal (as was proposed in the original tripartite model; Clark & Watson, 1991; Clark et al., 1994).
Developmental Perspective
There are several possible developmental explanations for the observed associations between depression and a reduced sensitivity to reward and anxiety and a heightened sensitivity to threat. One possibility is that the abnormal sensitivities are vulnerability factors for the development of particular disorders. Consistent with this perspective, several family studies have reported that children of parents with anxiety disorders exhibit heightened aversive responding to unpredictable threat (Grillon, Dierker, & Merikangas, 1998; Pine et al., 2005) and children of depressed mothers exhibit abnormal frontal EEG asymmetries (Dawson, Frey, Panagiotides, Osterling, & Hessl, 1997; Field, Fox, Pickens, & Nawrocki, 1995; Jones, Field, Fox, Lundy, & Davalos, 1997; Tomarken et al., 2004). In addition, several longitudinal studies have shown that these indicators predict the onset of depressive disorders (Craske et al., 2012; Nusslock et al., 2011).
A second possibility is that reduced sensitivity to reward and heightened sensitivity to threat are “scars” of depression and anxiety, respectively. This explanation, however, is inconsistent with several of the aforementioned family studies, which observed these risk markers in those with no personal history of depression or anxiety (e.g., Tomarken et al., 2004), as well as several of the longitudinal studies that found that these markers predicted first onset (e.g., Nusslock et al., 2011).
A final possibility is that a third variable caused both the abnormal sensitivity and depressive/anxious episode. For example, early life trauma may disrupt the development of reward sensitivity, which in turn may increase risk for depression (Pickett, Bardeen, & Orcutt, 2011). Results from the present study were cross-sectional in nature, and therefore cannot differentiate these three perspectives. Longitudinal research is therefore needed to determine when and how reduced sensitivity to reward and heightened sensitivity to threat emerge in the context of depression and anxiety, respectively.
Strengths and Limitations
The present study had several strengths. First, the sample was a well-characterized, relatively large (N = 191) sample of individuals diagnosed using the SCID. Relatedly, the “pure” PD-only and MDD-only groups did not meet current or past criteria for depressive or anxiety disorders, respectively. Second, two established psychophysiological measures of threat and reward sensitivity were used, and both elicited the expected pattern of results. Third, in a subsample, test–retest reliabilities over approximately 9 days indicated that reactivity was relatively stability over time (and comparably stable across the two tasks).
The present study also had several limitations. First, depressed participants were limited to those with child or adolescent onset depression, and results from the present study may not generalize to all types of depression (e.g., adult onset depression). Second, most comorbid participants had an earlier onset of depression than anxiety disorder. There is some literature suggesting that anxiety is more likely to precede the onset of depression in comorbid individuals than the reverse (Merikangas et al., 1996; although see Wittchen, Kessler, Pfister, & Lieb, 2000). Thus, results from the present study may not generalize to all comorbid individuals, but may rather be specific to those for which depression precedes anxiety. Third, participants with PD (i.e., PD only and comorbids) were allowed to meet criteria for other current and lifetime anxiety disorders, adding heterogeneity to the sample. However, only requiring one anxiety disorder may have resulted in a less representative sample given the large comorbidity among anxiety disorders (Kessler et al., 2005). Nevertheless, it is possible that the results for PD were not due to PD specifically, but anxiety disorders more generally (although see footnote 5). Fourth, many participants were on various psychiatric medications; however, results remained largely the same after controlling for medication use (see footnote 5). Finally, group differences in threat and reward sensitivity were limited to the psychophysiological indices and not the self-report emotions ratings. The use of retrospective emotions ratings may have limited the ability to detect group differences, and it is possible that online verbal measures (e.g., affective rating dial; Mauss, Wilhelm, & Gross, 2004) would have been more sensitive to group differences.
Conclusion
In summary, the present study found that PD (but not MDD) was associated with heightened startle responding to predictable and unpredictable threat, and MDD (but not PD) was associated with an abnormal frontal EEG asymmetry while anticipating reward. It is noteworthy that the affective constructs examined in the present study (reward anticipation, response to predictable and unpredictable threat) are several of the dimensions put forth by the recent National Institute of Mental Health Research Domain Criteria (RDoC) initiative as basic, transdiagnostic dimensions of psychopathology (Insel et al., 2010; Sanislow et al., 2010). Although we very much agree with the spirit of the RDoC initiative, our results suggest that the RDoC domains may demonstrate discriminant validity in their association with particular psychopathological constructs. This is not to say that a particular RDoC dimension will be related to only one psychopathology (e.g., studies show that reduced reward sensitivity is related to other conditions besides depression, such as psychosis; Herbener, Harrow, & Hill, 2005). Rather, our recommendation is that studies under this initiative should examine multiple RDoC dimensions simultaneously in order to determine whether different dimensions relate to the same or different constructs and neurobiology.
Acknowledgments
This study was supported by National Institute of Mental Health Grant R21 MH080689 awarded to Stewart A. Shankman. We would also like to thank Jon Kassel and Jeffrey Bishop for their assistance with this project.
Footnotes
Throughout this study, the terms specific and unique were not intended to mean “specific/unique compared with all other conditions (e.g., schizophrenia, anorexia, etc.),” but only to mean specific/unique to depression versus anxiety (and vice versa).
There were also 12 loss trials during which participants lost money if the reels landed on three pieces of fruit (data not presented). Loss trials were included because pilot testing suggested that the slot task was less engaging if there were only NI and R trials, and interspersing loss trials during the game made the R trials feel “more exciting.” Examination of the loss trials using a Location (F3/4, F5/6, F7/8) × Depression Status (present vs. absent) × Panic Status (present vs. absent) mixed measures ANCOVA with age and sex entered as covariates revealed no main effects or interactions (ps > .19).
The present study chose to include all participants who had at least two scorable blinks per condition to increase power for detecting a Depression Status × Panic Status interaction. The pattern of results was identical when the minimum blink requirement was increased to three blinks per condition and four blinks per condition.
The effect of diagnosis was examined as two 2-level factors (Depression Status and Panic Status) instead of one 4-level factor in order to examine main effects of MDD and PD as well as the interaction of MDD and PD on the variables of interest.
There were several group differences in clinical characteristics (medication status, lifetime alcohol/drug use disorder, and other comorbid anxiety disorders [e.g., PTSD, GAD]). Several exploratory analyses were therefore conducted to determine whether these characteristics affected the pattern of results. When medication status (present vs. absent) was entered as a covariate, the results were nearly identical, although several effects were reduced to trends (e.g., effect of panic status in the NPU-threat, p < .06). In addition, there were also no main effects or interactions involving lifetime alcohol use disorder (present vs. absent) or lifetime drug use disorder (present vs. absent) for either task (ps < .17). Thus, results suggest that medication, alcohol, or drug use did not affect the pattern of results.
We next examined the potential effects of additional comorbid anxiety disorders besides PD (i.e., specific phobia, social anxiety disorder, obsessive– compulsive disorder, PTSD, and GAD). First, we examined whether PD participants (i.e., PD only and comorbids) with versus without another comorbid anxiety disorder differed on psychophysiological and verbal responding. Separate analyses were conducted for lifetime comorbid anxiety disorder and current comorbid anxiety disorder. For both sets of analyses, results indicated that PD participants with versus without another comorbid anxiety disorder did not differ on psychophysiological or verbal measures of threat or reward sensitivity (ps > .16). Second, given the large number of participants with comorbid PTSD, similar analyses were conducted for just PTSD. Results indicated that participants with comorbid PD + PTSD did not differ from those with PD without PTSD on any psychophysiological or verbal measures (ps < .11). Finally, given Heller and colleagues’ (1997) prediction that anxiety disorders characterized by anxious arousal (e.g., PD) are associated with different profiles of frontal EEG asymmetry than anxiety disorders characterized by anxious apprehension (e.g., GAD), we reran analyses excluding the seven participants with GAD and results were nearly identical to those that included these participants.
References
- Allen JJB. Frontal EEG asymmetry as an endophenotype for depression: A skeptic’s journey. Paper presented at the meeting of the Society for Psychophysiological Research; Berlin, Germany. 2009. Oct, [Google Scholar]
- Allen JJB, Coan JA, Nazarian M. Issues and assumptions on the road from raw signals to metrics of frontal EEG asymmetry in emotion. Biological Psychology. 2004;67:183–218. doi: 10.1016/j.biopsycho.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Allen NB, Trinder J, Brennen C. Affective startle modulation in clinical depression: Preliminary findings. Biological Psychiatry. 1999;46:542–550. doi: 10.1016/S0006-3223(99)00025-6. [DOI] [PubMed] [Google Scholar]
- Alvarez RP, Chen G, Bodurka J, Kaplan R, Grillon C. Phasic and sustained fear in humans elicits distinct patterns of brain activity. NeuroImage. 2011;55:389– 400. doi: 10.1016/j.neuroimage.2010.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow DH. Unraveling the mysteries of anxiety and its disorders from the perspective of emotion theory. American Psychologist. 2000;55:1247–1263. doi: 10.1037/0003-066X.55.11.1247. [DOI] [PubMed] [Google Scholar]
- Beck A, Epstein N, Brown G, Steer R. An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology. 1988;56:893– 897. doi: 10.1037/0022-006X.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beesdo K, Lau JYF, Guyer AE, McClure-Tone EB, Monk CS, Nelson EE, Pine DS. Common and distinct amygdala-function perturbations in depressed vs. anxious adolescents. Archives of General Psychiatry. 2009;66:275–285. doi: 10.1001/archgenpsychiatry.2008.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzer K, Schneier FR. Comorbidity of anxiety and depressive disorders: Issues in conceptualization, assessment, and treatment. Journal of Psychiatric Practice. 2004;10:296–306. doi: 10.1097/00131746-200409000-00003. [DOI] [PubMed] [Google Scholar]
- Bennett CM, Miller MB. How reliable are the results from functional magnetic resonance imaging? Annals of the New York Academy of Sciences. 2010;1191:133–155. doi: 10.1111/j.1749-6632.2010.05446.x. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends in Neurosciences. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Blackhart GC, Minnix JA, Kline JP. Can EEG asymmetry patterns predict future development of anxiety and depression? A preliminary study. Biological Psychology. 2006;72:46–50. doi: 10.1016/j.biopsycho.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, van Boxtel A. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42:1–15. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Mineka S, Barlow DH. A modern learning theory perspective on the etiology of panic disorder. Psychological Review. 2001;108:4–32. doi: 10.1037/0033-295X.108.1.4. [DOI] [PubMed] [Google Scholar]
- Brown TA, Chorpita BF, Barlow DH. Structural relationships among dimensions of the DSM–IV anxiety and mood disorders and dimensions of negative affect, positive affect, and autonomic arousal. Journal of Abnormal Psychology. 1998;107:179–192. doi: 10.1037/0021-843X.107.2.179. [DOI] [PubMed] [Google Scholar]
- Brown TA, McNiff J. Specificity of autonomic arousal to DSM–IV panic disorder and posttraumatic stress disorder. Behaviour Research and Therapy. 2009;47:487– 493. doi: 10.1016/j.brat.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder GE, Fong R, Tenke CE, Leite P. Regional brain asymmetries in major depression with or without an anxiety disorder: A quantitative electroencephalographic study. Biological Psychiatry. 1997;41:939–948. doi: 10.1016/S0006-3223(96)00260-0. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE. When do individual differences matter? A paradoxical theory of personality coherence. Psychological Inquiry. 1993;4:247–271. doi: 10.1207/s15327965pli0404_1. [DOI] [Google Scholar]
- Cervone D. The architecture of personality. Psychological Review. 2004;111:183–204. doi: 10.1037/0033-295X.111.1.183. [DOI] [PubMed] [Google Scholar]
- Clark LA. Manual for the Schedule for Nonadaptive and Adaptive Personality (SNAP) Minneapolis, MN: University of Minnesota Press; 1993. [Google Scholar]
- Clark LA, Watson D. Unpublished manuscript. Southern Methodist University; Dallas, TX: 1990. The General Temperament Survey. [Google Scholar]
- Clark LA, Watson D. Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. Journal of Abnormal Psychology. 1991;100:316–336. doi: 10.1037/0021-843X.100.3.316. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D, Mineka S. Temperament, personality, and the mood and anxiety disorders. Journal of Abnormal Psychology. 1994;103:103–116. doi: 10.1037/0021-843X.103.1.103. [DOI] [PubMed] [Google Scholar]
- Coan JA, Allen JJB. Frontal EEG asymmetry as a moderator and mediator of emotion. Biological Psychology. 2004;67:7– 49. doi: 10.1016/j.biopsycho.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Coan JA, Allen JJB. Handbook of emotion elicitation and assessment. New York, NY: Oxford University Press; 2007. [Google Scholar]
- Coan JA, Allen JJB, McKnight PE. A capability model of individual differences in frontal EEG asymmetry. Biological Psychology. 2006;72:198–207. doi: 10.1016/j.biopsycho.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JM, Orvaschel H, Simco E, Hersen M, Joiner T. A test of the tripartite model of depression and anxiety in older adult psychiatric outpatients. Psychology and Aging. 2004;19:444– 451. doi: 10.1037/0882-7974.19.3.444. [DOI] [PubMed] [Google Scholar]
- Cooper WH, Withey MJ. The strong situation hypothesis. Personality and Social Psychology Review. 2009;13:62–72. doi: 10.1177/1088868308329378. [DOI] [PubMed] [Google Scholar]
- Craske MG, Rauch SL, Ursano R, Prenoveau J, Pine DS, Zinbarg RE. What is anxiety disorder? Depression and Anxiety. 2009;26:1066–1085. doi: 10.1002/da.20633. [DOI] [PubMed] [Google Scholar]
- Craske MG, Wolitzky-Taylor KB, Mineka S, Zinbarg R, Waters AM, Ornitz E. Elevated responding to safe conditions as a specific risk factor for anxiety versus depressive disorders: Evidence from a longitudinal investigation. Journal of Abnormal Psychology. 2012;121:315–324. doi: 10.1037/a0025738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN, Lang PJ, Strauss C, Drobes D, Patrick CJ, Bradley MM. The psychophysiology of anxiety disorder: Fear memory imagery. Psychophysiology. 2003;40:407– 422. doi: 10.1111/1469-8986.00043. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Asymmetric brain function, affective style, and psychopathology: The role of early experience and plasticity. Development and Psychopathology. 1994;6:741–758. doi: 10.1017/S0954579400004764. [DOI] [Google Scholar]
- Davidson RJ. Affective style and affective disorders: Perspectives from affective neuroscience. Cognition & Emotion. 1998;12:307–330. doi: 10.1080/026999398379628. [DOI] [Google Scholar]
- Davidson RJ, Pizzagalli DA, Nitschke JB. Representation and regulation of emotion in depression: Perspectives from affective neuroscience. New York, NY: Guilford Press; 2009. [Google Scholar]
- Davis M. Are different parts of the extended amygdala involved in fear versus anxiety? Biological Psychiatry. 1998;44:1239–1247. doi: 10.1016/S0006-3223(98)00288-1. [DOI] [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. American Psychologist. 2006;61:741–756. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- Dawson G, Frey K, Panagiotides H, Osterling J, Hessl D. Infants of depressed mothers exhibit atypical frontal brain activity: A replication and extension of previous findings. Journal of Child Psychology and Psychiatry. 1997;38:179–186. doi: 10.1111/j.1469-7610.1997.tb01852.x. [DOI] [PubMed] [Google Scholar]
- De Cort K, Griez E, Büchler M, Schruers K. The role of “interoceptive” fear conditioning in the development of panic disorder. Behavior Therapy. 2012;43:203–215. doi: 10.1016/j.beth.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Tomarken AJ. The chronometry of affective startle modulation in unipolar depression. Journal of Abnormal Psychology. 2008;117:1–15. doi: 10.1037/0021-843X.117.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Tomarken AJ, Shelton RC, Sutton SK. Early- and late-onset startle modulation in unipolar depression. Psychophysiology. 2004;41:433– 440. doi: 10.1111/j.1469-8986.00162.x. [DOI] [PubMed] [Google Scholar]
- Ehlers A, Breuer P. Increased cardiac awareness in panic disorder. Journal of Abnormal Psychology. 1992;101:371–382. doi: 10.1037/0021-843X.101.3.371. [DOI] [PubMed] [Google Scholar]
- Field T, Fox NA, Pickens J, Nawrocki T. Relative right frontal EEG activation in 3- to 6-month-old infants of “depressed” mothers. Developmental Psychology. 1995;31:358–363. doi: 10.1037/0012-1649.31.3.358. [DOI] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM–IV Axis I Disorders, Clinician Version (SCID-CV) Washington, DC: American Psychiatric Press; 1996. [Google Scholar]
- Forbes EE, Shaw DS, Dahl RE. Alterations in reward-related decision making in boys with recent and future depression. Biological Psychiatry. 2007;61:633– 639. doi: 10.1016/j.biopsych.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Gard DE, Gard MG, Kring AM, John OP. Anticipatory and consummatory components of the experience of pleasure: A scale development study. Journal of Research in Personality. 2006;40:1086–1102. doi: 10.1016/j.jrp.2005.11.001. [DOI] [Google Scholar]
- Gershuny BS, Sher KJ. The relation between personality and anxiety: Findings from a 3-year prospective study. Journal of Abnormal Psychology. 1998;107:252–262. doi: 10.1037/0021-843X.107.2.252. [DOI] [PubMed] [Google Scholar]
- Goldman RI, Stern JM, Engel J, Cohen MS. Simultaneous EEG and fMRI of the alpha rhythm. NeuroReport. 2002;13:2487–2492. doi: 10.1097/00001756-200212200-00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman JM, Kent J, Martinez J, Browne S, Coplan J, Papp LA. Physiological changes during carbon dioxide inhalation in patients with panic disorder, major depression, and premenstrual dysphoric disorder: Evidence for a central fear mechanism. Archives of General Psychiatry. 2001;58:125–131. doi: 10.1001/archpsyc.58.2.125. [DOI] [PubMed] [Google Scholar]
- Gray JA, McNaughton N. The neuropsychology of anxiety: An enquiry into the functions of the septohippocampal system. 2. Oxford, England: Oxford University Press; 2000. [Google Scholar]
- Greaves-Lord K, Ferdinand RF, Sondeijker FEPL, Dietrich A, Oldehinkel AJ, Rosmalen JGM, Verhulst FC. Testing the tripartite model in young adolescents: Is hyperarousal specific for anxiety and not depression? Journal of Affective Disorders. 2007;102:55–63. doi: 10.1016/j.jad.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Griez EJ, Lousberg H, Van den Hout MA, Van der Molen GM. CO2 vulnerability in panic disorder. Psychiatry Research. 1987;20:87–95. doi: 10.1016/0165-1781(87)90001-1. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas J. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clinical Neurophysiology. 2003;114:1557–1579. doi: 10.1016/S1388-2457(03)00202-5. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JP, Lissek S, Smith K, Milstein J. Anxious responses to predictable and unpredictable aversive events. Behavioral Neuroscience. 2004;118:916–924. doi: 10.1037/0735-7044.118.5.916. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JMP, Pine DS, Lissek S, Lawley M, Ellis V, Levine J. The benzodiazepine alprazolam dissociates contextual fear from cued fear in humans as assessed by fear-potentiated startle. Biological Psychiatry. 2006;60:760–766. doi: 10.1016/j.biopsych.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Grillon C, Dierker L, Merikangas KR. Fear-potentiated startle in adolescent offspring of parents with anxiety disorders. Biological Psychiatry. 1998;44:990–997. doi: 10.1016/S0006-3223(98)00188-7. [DOI] [PubMed] [Google Scholar]
- Grillon C, Lissek S, Rabin S, McDowell D, Dvir S, Pine DS. Increased anxiety during anticipation of unpredictable but not predictable aversive stimuli as a psychophysiologic marker of panic disorder. The American Journal of Psychiatry. 2008;165:898–904. doi: 10.1176/appi.ajp.2007.07101581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Pine DS, Lissek S, Rabin S, Bonne O, Vythilingam M. Increased anxiety during anticipation of unpredictable aversive stimuli in posttraumatic stress disorder but not in generalized anxiety disorder. Biological Psychiatry. 2009;66:47–53. doi: 10.1016/j.biopsych.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemann D, Hewig J, Seifert J, Naumann E, Bartussek D. The latent state-trait structure of resting EEG asymmetry: Replication and extension. Psychophysiology. 2005;42:740–752. doi: 10.1111/j.1469-8986.2005.00367.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery & Psychiatry. 1960;23:56– 62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller W, Nitscke JB. The puzzle of regional brain activity in depression and anxiety: The importance of subtypes and comorbidity. Cognition & Emotion. 1998;12:421– 447. doi: 10.1080/026999398379664. [DOI] [Google Scholar]
- Heller W, Nitschke JB, Etienne MA, Miller GA. Patterns of regional brain activity differentiate types of anxiety. Journal of Abnormal Psychology. 1997;106:376–385. doi: 10.1037/0021-843X.106.3.376. [DOI] [PubMed] [Google Scholar]
- Henriques JB, Davidson RJ. Left frontal hypoactivation in depression. Journal of Abnormal Psychology. 1991;100:535–545. doi: 10.1037/0021-843X.100.4.535. [DOI] [PubMed] [Google Scholar]
- Herbener ES, Harrow M, Hill SK. Change in the relationship between anhedonia and functional deficits over a 20-year period in individuals with schizophrenia. Schizophrenia Research. 2005;75:97–105. doi: 10.1016/j.schres.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Hirschfeld RMA. Personality and dysthymia. In: Burton SW, Akiskal HS, editors. Dysthymic disorder. London, England: Royal College of Psychiatrists; 1990. pp. 69–77. (formerly Gaskell) [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Wang P. Research Domain Criteria (RDoC): Developing a valid diagnostic framework for research on mental disorders. The American Journal of Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Johnson MR, Lydiard RB. Comorbidity of major depression and panic disorder. Journal of Clinical Psychology. 1998;54:201–210. doi: 10.1002/(SICI)1097-4679(199802)54:2<201::AID-JCLP9<3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Jones NA, Field T, Fox NA, Lundy B, Davalos M. EEG activation in one-month-old infants of depressed mothers. Development and Psychopathology. 1997;9:491–505. doi: 10.1017/S0954579497001260. [DOI] [PubMed] [Google Scholar]
- Kaviani H, Gray JA, Checkley SA, Raven PW, Wilson GD, Kumari V. Affective modulation of the startle response in depression: Influence of the severity of depression, anhedonia, and anxiety. Journal of Affective Disorders. 2004;83:21–31. doi: 10.1016/j.jad.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Griffiths K, Felmingham KL, Shankman SA, Drinkenburg W, Arns M, Bryant RA. Disorder specificity despite comorbidity: Resting EEG alpha asymmetry in major depressive disorder and post-traumatic stress disorder. Biological Psychology. 2010;85:350–354. doi: 10.1016/j.biopsycho.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of General Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kessler R, Chiu W, Demler O, Walters E. Prevalence, severity, and comorbidity of 12-month DSM–IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:617– 627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DF. Endogenomorphic depression: A conceptual and terminological revision. Archives of General Psychiatry. 1974;31:447– 454. doi: 10.1001/archpsyc.1974.01760160005001. [DOI] [PubMed] [Google Scholar]
- Klein DN. Classification of depressive disorders in the DSM–V: Proposal for a two-dimension system. Journal of Abnormal Psychology. 2008;117:552–560. doi: 10.1037/0021-843X.117.3.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DN, Durbin CE, Shankman SA. Personality and mood disorders. New York, NY: Guilford Press; 2009. [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: The inhibition-timing hypothesis. Brain Research Reviews. 2007;53:63– 88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Koch M. The neurobiology of startle. Progress in Neurobiology. 1999;59:107–128. doi: 10.1016/S0301-0082(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Krueger RF. The structure of common mental disorders. Archives of General Psychiatry. 1999;56:921–926. doi: 10.1001/archpsyc.56.10.921. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: Affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30:261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Lang PJ, McTeague LM. The anxiety disorder spectrum: Fear imagery, physiological reactivity, and differential diagnosis. Anxiety, Stress, & Coping. 2009;22:5–25. doi: 10.1080/10615800802478247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Baas JMP, Pine DS, Orme K, Dvir S, Rosenberger E, Grillon C. Sensation seeking and the aversive motivational system. Emotion. 2005;5:396– 407. doi: 10.1037/1528-3542.5.4.396. [DOI] [PubMed] [Google Scholar]
- Lonigan CJ, Phillips BM, Hooe ES. Relations of positive and negative affectivity to anxiety and depression in children: Evidence from a latent variable longitudinal study. Journal of Consulting and Clinical Psychology. 2003;71:465– 481. doi: 10.1037/0022-006X.71.3.465. [DOI] [PubMed] [Google Scholar]
- Mauss IB, Wilhelm FH, Gross JJ. Is there less to social anxiety than meets the eye? Emotion experience, expression, and bodily responding. Cognition & Emotion. 2004;18:631– 642. doi: 10.1080/02699930341000112. [DOI] [Google Scholar]
- McTeague LM, Lang PJ, Laplante M, Bradley MM. Aversive imagery in panic disorder: Agoraphobia severity, comorbidity, and defensive physiology. Biological Psychiatry. 2011;70:415– 424. doi: 10.1016/j.biopsych.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzig CA, Weike AI, Zimmermann J, Hamm AO. Startle reflex modulation and autonomic responding during anxious apprehension in panic disorder patients. Psychophysiology. 2007;44:846–854. doi: 10.1111/j.1469-8986.2007.00560.x. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Angst J, Eaton W, Canino G, Rubio-Stipec M, Wacker H, Kupfer D. Comorbidity and boundaries of affective disorders with anxiety disorders and substance misuse: Results of an international task force. British Journal of Psychiatry. 1996;168:58– 67. [PubMed] [Google Scholar]
- Meuret AE, Rosenfield D, Hofmann SG, Suvak MK, Roth WT. Changes in respiration mediate changes in fear of bodily sensations in panic disorder. Journal of Psychiatric Research. 2009;43:634–641. doi: 10.1016/j.jpsychires.2008.08.003. doi:10.1016.j.jpsychires.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineka S, Kihlstrom JF. Unpredictable and uncontrollable events: A new perspective on experimental neurosis. Journal of Abnormal Psychology. 1978;87:256–271. doi: 10.1037/0021-843X.87.2.256. [DOI] [PubMed] [Google Scholar]
- Mineka S, Watson D, Clark LA. Comorbidity of anxiety and unipolar mood disorders. Annual Review of Psychology. 1998;49:377– 412. doi: 10.1146/annurev.psych.49.1.377. [DOI] [PubMed] [Google Scholar]
- Mischel W. Toward a cognitive social learning reconceptualization of personality. Psychological Review. 1973;80:252–283. doi: 10.1037/h0035002. [DOI] [PubMed] [Google Scholar]
- Mischel W. The interaction of person and situation. In: Magnusson D, Endler NS, editors. Personality at the crossroads: Current issues in interactional psychology. Hillsdale, NJ: Erlbaum; 1977. pp. 333–352. [Google Scholar]
- Moberg CA, Curtin JJ. Alcohol selectively reduces anxiety but not fear: Startle response during unpredictable versus predictable threat. Journal of Abnormal Psychology. 2009;118:335–347. doi: 10.1037/a0015636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naragon-Gainey K, Watson D, Markon KE. Differential relations of depression and social anxiety symptoms to the facets of extraversion/positive emotionality. Journal of Abnormal Psychology. 2009;118:299–310. doi: 10.1037/a0015637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BD, Shankman SA, Olino TM, Klein DN. Defining reactivity: How several methodological decisions can affect conclusions about emotional reactivity in psychopathology. Cognition & Emotion. 2011;25:1439–1459. doi: 10.1080/02699931.2010.551185. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Heller W, Palmieri PA, Miller GA. Contrasting patterns of brain activity in anxious apprehension and anxious arousal. Psychophysiology. 1999;36:628– 637. doi: 10.1111/1469-8986.3650628. [DOI] [PubMed] [Google Scholar]
- Nusslock R, Shackman AJ, Harmon-Jones E, Alloy LB, Coan JA, Abramson LY. Cognitive vulnerability and frontal brain asymmetry: Common predictors of first prospective depressive episode. Journal of Abnormal Psychology. 2011;120:497–503. doi: 10.1037/a0022940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes TR, Pizzagalli DA, Hendrick AM, Horras KA, Larson CL, Abercrombie HC, Davidson RJ. Functional coupling of simultaneous electrical and metabolic activity in the human brain. Human Brain Mapping. 2004;21:257–270. doi: 10.1002/hbm.20004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pauli P, Dengler W, Wiedemann G, Montoya P, Flor H, Birbaumer N, Buchkremer G. Behavioral and neurophysiological evidence for altered processing of anxiety-related words in panic disorder. Journal of Abnormal Psychology. 1997;106:213–220. doi: 10.1037/0021-843X.106.2.213. [DOI] [PubMed] [Google Scholar]
- Perna G, Battaglia M, Garberi A, Arancio C. Carbon dioxide/oxygen challenge test in panic disorder. Psychiatry Research. 1994;52:159–171. doi: 10.1016/0165-1781(94)90085-X. [DOI] [PubMed] [Google Scholar]
- Pickett SM, Bardeen JR, Orcutt HK. Experiential avoidance as a moderator of the relationship between behavioral inhibition system sensitivity and posttraumatic stress symptoms. Journal of Anxiety Disorders. 2011;25:1038–1045. doi: 10.1016/j.janxdis.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Pine DS, Klein RG, Roberson-Nay R, Mannuzza S, Moulton JL, Woldehawariat G, Guardino M. Response to 5% carbon dioxide in children and adolescents: Relationship to panic disorder in parents and anxiety disorders in subjects. Archives of General Psychiatry. 2005;62:73– 80. doi: 10.1001/archpsyc.62.1.73. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Jahn AL, O’Shea JP. Toward an objective characterization of an anhedonic phenotype: A signal-detection approach. Biological Psychiatry. 2005;57:319–327. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid SA, Duke LM, Allen JJB. Resting frontal electroencephalographic asymmetry in depression: Inconsistencies suggest the need to identify mediating factors. Psychophysiology. 1998;35:389–404. doi: 10.1017/S0048577298970986. [DOI] [PubMed] [Google Scholar]
- Roberts SB, Kendler KS. Neuroticism and self-esteem as indices of the vulnerability to major depression in women. Psychological Medicine. 1999;29:1101–1109. doi: 10.1017/S0033291799008739. [DOI] [PubMed] [Google Scholar]
- Rollman GB, Harris G. The detectability, discriminability, and perceived magnitude of painful electrical shock. Perception & Psychophysics. 1987;42:257–268. doi: 10.3758/BF03203077. [DOI] [PubMed] [Google Scholar]
- Sanislow CA, Pine DS, Quinn KJ, Kozak MJ, Garvey MA, Heinssen RK, Cuthbert BN. Developing constructs for psychopathology research: Research domain criteria. Journal of Abnormal Psychology. 2010;119:631– 639. doi: 10.1037/a0020909. [DOI] [PubMed] [Google Scholar]
- Schmitz A, Grillon C. Assessing fear and anxiety in humans using the threat of predictable and unpredictable aversive events (the NPU-threat test) Nature Protocols. 2012;7:527–532. doi: 10.1038/nprot.2012.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman SA, Klein DN. The relation between depression and anxiety: An evaluation of the tripartite, approach-withdrawal and valence-arousal models. Clinical Psychology Review. 2003;23:605– 637. doi: 10.1016/S0272-7358(03)00038-2. [DOI] [PubMed] [Google Scholar]
- Shankman SA, Klein DN, Tenke CE, Bruder GE. Reward sensitivity in depression: A biobehavioral study. Journal of Abnormal Psychology. 2007;116:95–104. doi: 10.1037/0021-843X.116.1.95. [DOI] [PubMed] [Google Scholar]
- Sherdell L, Waugh CE, Gotlib IH. Anticipatory pleasure predicts motivation for reward in major depression. Journal of Abnormal Psychology. 2012;121:51– 60. doi: 10.1037/a0024945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, Bismark AW, Towers DN, Coan JA, Allen JJB. Resting frontal EEG asymmetry as an endophenotype for depression risk: Sex-specific patterns of frontal brain asymmetry. Journal of Abnormal Psychology. 2010;119:502–512. doi: 10.1037/a0019196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Clift A, Morris BH, Rottenberg J, Kovacs M. Emotion-modulated startle in anxiety disorders is blunted by co-morbid depressive episodes. Psychological Medicine. 2011;41:129–139. doi: 10.1017/S003329171000036X. [DOI] [PubMed] [Google Scholar]
- Tenke C, Kayser J. Reference-free quantification of EEG spectra: Combining current source density (CSD) and frequency principal components analysis (fPCA) Clinical Neurophysiology. 2005;116:2826–2846. doi: 10.1016/j.clinph.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Thibodeau R, Jorgensen RS, Kim S. Depression, anxiety, and resting frontal EEG asymmetry: A meta-analytic review. Journal of Abnormal Psychology. 2006;115:715–729. doi: 10.1037/0021-843X.115.4.715. [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Dichter GS, Garber J, Simien C. Resting frontal brain activity: Linkages to maternal depression and socioeconomic status among adolescents. Biological Psychology. 2004;67:77–102. doi: 10.1016/j.biopsycho.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Vollebergh WAM, Iedema J, Bijl RV, de Graaf R, Smit F, Ormel J. The structure and stability of common mental disorders: The NEMESIS study. Archives of General Psychiatry. 2001;58:597–603. doi: 10.1001/archpsyc.58.6.597. [DOI] [PubMed] [Google Scholar]
- Watson D. Differentiating the mood and anxiety disorders: A quadripartite model. Annual Review of Clinical Psychology. 2009;5:221–247. doi: 10.1146/annurev.clinpsy.032408.153510. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA. Extraversion and its positive emotional core. San Diego, CA: Academic Press; 1997. [DOI] [Google Scholar]
- Wiedemann G, Pauli P, Dengler W, Lutzenberger W, Birbaumer N, Buchkremer G. Frontal brain asymmetry as a biological substrate of emotions in patients with panic disorders. Archives of General Psychiatry. 1999;56:78– 84. doi: 10.1001/archpsyc.56.1.78. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Kessler RC, Pfister H, Lieb M. Why do people with anxiety disorders become depressed? A prospective-longitudinal community study. Acta Psychiatrica Scandinavica. 2000;102:14–23. doi: 10.1111/j.0065-1591.2000.acp29-03.x. [DOI] [PubMed] [Google Scholar]