Abstract
Background
The relationship between cigarette smoking and neonatal and maternal clinical outcomes among opioid-agonist-treated pregnant patients is sparse.
Objectives
(1) Is smoking measured at study entry related to neonatal and maternal outcomes in pregnant women receiving opioid-agonist medication? (2) Is it more informative to use a multi-item measure of smoking dependence or a single-item measure of daily smoking? (3) Is the relationship between smoking at study entry and outcomes different between methadone and buprenorphine?
Methods
Secondary analyses examined the ability of the Tobacco Dependence Screener (TDS) and self-reported past 30-day daily average number of cigarettes smoked, both measured at study entry, to predict 12 neonatal and 9 maternal outcomes in 131 opioid-agonist-maintained pregnant participants.
Results
Past 30-day daily average number of cigarettes smoked was significantly positively associated with total amount of morphine (mg) needed to treat neonatal abstinence syndrome (NAS), Adjusted Odds Ratio (AOR)=1.06(95% CI: 1.02,1.09), number of days medicated for NAS, AOR=1.04(95% CI: 1.01,1.06), neonatal length of hospital stay in days, AOR=1.03(95% CI: 1.01,1.05), and negatively associated with 1- AOR=.995(95% CI: .991,.999) and 5-minute Apgar scores, AOR=.996(95% CI: .994,.998). Simple effect tests of the two significant TDS X medication condition effects found TDS was unrelated to non-normal presentation and amount of voucher money earned in the methadone [AORs=.90 (95%CI: .74,1.08, p>.24) and 1.0 (95%CI: .97, 1.03, p>.9)] but significant in the buprenorphine condition, [AORs=1.57 (95%CI: 1.01,2.45, p<.05) and 1.08 (95%CI: 1.04,1.12, p<.01)].
Conclusions
Regardless of prenatal methadone or buprenorphine exposure, heavier smoking was associated with more compromised birth outcomes.
Keywords: opioid dependence, pregnancy, smoking, neonate, agonist treatment, neonatal abstinence syndrome
1. INTRODUCTION
Approximately 21% of U.S. women of childbearing age are current cigarette smokers (Center for Disease Control, 2011). Approximately 20% of these smokers quit upon learning of their pregnancy, but the vast majority, especially heavier smokers, continue smoking throughout their pregnancy (Solomon and Quinn, 2004). Smoking is the leading preventable cause of poor pregnancy outcomes, increased risk for infertility, catastrophic pregnancy complications, intrauterine growth restriction, birth defects, infant death, and later-in-life metabolic diseases (Cohen et al., 2010; Dietz et al., 2010; Guerrero-Preston et al., 2010; Hackshaw et al., 2011; Rogers, 2009).
Among pregnant opioid agonist-maintained women, the rate of smoking (e.g., 88%; Jones et al., 2009; (Haug et al., 2001) is four times higher than in the general population of pregnant women. Consistent with the larger literature on smoking and poor pregnancy outcomes, one study on this topic reported that heavier smoking among opioid-maintained pregnant women was associated with lower neonatal birth weight and smaller birth length (Winklbaur et al., 2009).
One factor unique to in utero opioid-exposed neonates is neonatal abstinence syndrome (NAS). NAS is characterized by hyperirritability of the central nervous system and dysfunction in the autonomic nervous system, gastrointestinal tract, and respiratory system (Kaltenbach et al., 1998). Heavier cigarette smoking has been found to be related to peak NAS score and amount of time to reach peak NAS score in neonates of 29 methadone-maintained women (Choo et al., 2004), and higher NAS severity scores in the neonates of 139 opioid-maintained (methadone, buprenorphine, or slow-release morphine) pregnant women (Winklbaur et al., 2009). Heavy cigarette smoking has also been associated with longer duration of NAS treatment in 26 neonates of methadone-maintained pregnant women. However, this association was not found in 12 neonates of buprenorphine-maintained pregnant women (Bakstad et al., 2009). Lastly, heavy cigarette smoking was associated with increased number of NAS symptoms, but not duration of hospitalization, in 64 neonates of methadone-maintained women (Jansson et al., 2010).
The purpose of the present secondary analysis study was to determine the relationship between cigarette smoking and neonatal and maternal outcomes in a sample of 131 opioid-dependent pregnant women who were participants in the MOTHER (Maternal Opioid Treatment: Human Experimental Research) study, a randomized clinical trial comparing buprenorphine and methadone (Jones et al., 2010). The MOTHER study sample is ideal for examining the relationship between maternal cigarette smoking at study entry and neonatal and maternal outcomes due to limited concomitant illicit drug use. Current alcohol and/or benzodiazepine regular use, abuse or dependence were exclusion criteria in the study. Furthermore, patients earned monetary vouchers for providing urine samples negative for opioids and other illicit drug use to minimize use of these substances. This intervention produced a low level of illicit drug use throughout the study: 73% and 81% of urine samples were opioid- and cocaine-negative, respectively, throughout the period of thrice-weekly prenatal testing, and only 13% of the samples were positive for opioids other than their respective study medication at delivery.
This study was intended to answer three specific questions: (1) Is smoking measured at study entry related to neonatal and maternal outcomes in opioid-dependent pregnant women receiving opioid agonist medication? (2) Is it more informative to use a multi-item measure of smoking dependence or a single-item self-report measure of recent daily smoking to determine the relationship between smoking at study entry and neonatal and maternal outcomes? and (3) Does it make a difference in the relationship between smoking at study entry and neonatal and maternal outcomes if the medication is methadone or buprenorphine?
2. METHODS
2.1 The MOTHER Study
The MOTHER (Maternal Opioid Treatment: Human Experimental Research) study (Jones et al., 2012, 2010) was a double-blind, double-dummy, flexible-dosing, two-group randomized clinical trial comparing methadone and buprenorphine pharmacotherapy to opioid-dependent women within the context of comprehensive care. Findings supported the comparative efficacy of buprenorphine, revealing that, on average, neonates exposed to buprenorphine in utero needed 89% less morphine to treat NAS, spent 43% less time in the hospital, and 58% less time in the hospital being medicated for NAS than neonates exposed to methadone in utero.
Further details concerning the MOTHER study can be found in Jones et al. (2012, 2010). The information that follows overviews those aspects of the MOTHER study that are central to the present paper.
2.2 MOTHER Sites
Seven university hospital sites contributed outcome data. Because the number of participants at some of the US sites was quite small, the 7 sites were merged into three categories: US Urban (Baltimore, MD; Philadelphia, PA: Detroit MI: Providence, RI) vs. US Rural (Burlington, VT; Nashville, TN) vs. European (Vienna). Moreover, merging the 7 sites into 3 categories reduced the potential for site heterogeneity to negatively impact the statistical analyses by adversely affecting the power of the tests of significance.
2.3 MOTHER Participants
Opioid-dependent pregnant women with a singleton fetus between 6 and 30 weeks gestation were recruited for possible participation. Women who met eligibility criteria were randomized to receive either methadone or buprenorphine in a double-blind, double-dummy format in which study medication was administered daily, first with sublingual tablets (placebo or buprenorphine), followed by oral liquid (placebo or methadone). Dosing was flexible in the range of 20–140 mg for methadone, and 2–32 mg for buprenorphine. Observed urine drug screening was conducted three times per week, and participants earned monetary vouchers for test results that were negative for opioids other than their respective study medication (for which testing was not conducted) and other illicit drugs to reduce concomitant drug use. One-hundred-seventy-five maternal participants were enrolled in the study, of whom 131 delivered newborns, 73 in the methadone condition and 58 in the buprenorphine condition. The secondary analyses in this paper use the data from the maternal and neonatal outcomes from these 131 participants.
2.4 Smoking Measures
Two self-report measures of smoking were administered at study entry: 1) The Tobacco Dependence Screener, a multi-item measure of smoking dependence and 2) a single item that was included as part of the Addiction Severity Index. These two measures were utilized in the present study in order to ascertain the relative predictive validity of a multi-item self-report measure of smoking dependence in comparison to a single-item self-report measure of past 30-day daily average number of cigarettes smoked.
2.4.1 Tobacco Dependence Screener (TDS)
The self-administered TDS (Kawakami et al., 1999) is composed of 10 questions: “Have you often had periods of days when you smoked a lot more than you intended to?”; “Have you ever tried to quit or cut down on tobacco and found you could not?”; “Did you crave tobacco after you quit or cut down on it?”; “Did you have any of the following problems when you quit or cut down on tobacco: irritation, nervousness, restlessness, trouble concentrating, headache, drowsiness, upset stomach, heart slow down, increased appetite or body weight, hand shakes, depression?”; “Did you ever start using tobacco again to keep from having such problems?”; “Have you ever continued to smoke when you had a serious illness that you knew made it unwise to use tobacco?”; “Did you continue to use tobacco after you knew that it caused you health problems?”; “Did you continue to use tobacco after you knew that it caused you mental problems?”; “Have you ever felt like you were dependent on tobacco?”; “Have you ever given up work or social activities so you could use tobacco?”. Answers are categorized as yes (1) or no or not applicable (0). TDS scores could range between 0 and 10, inclusive. The TDS has good reliability and validity and better psychometric properties than the Fagerstrom Tolerance Questionnaire (Fagerstrom, 1978), the most commonly used screening questionnaire for tobacco/nicotine dependence. TDS scores are strongly correlated with the severity of nicotine/tobacco dependence derived from standardized diagnostic interviews (e.g., ICD-10, DSM-III-R, DSM-IV) and are also significantly and positively correlated with the number of years smoking. TDS scores also were correlated with the number of cigarettes smoked per day for men, but not women (Kawakami et al., 1999). The lack of significant association between TDS scores and number of cigarettes per day for women may have been due to the fact that women smoked fewer cigarettes per day, on average, than did men, [13.0 (SD=7.3) vs. 23.1 (SD=10.5)], thereby reducing the variance for women in comparison to men.
2.4.2 Past 30-day Daily Average Number of Cigarettes Smoked
As part of the MOTHER study, an item assessing past 30-day daily average number of cigarettes smoked was added to the drug domain of the Addiction Severity Index (McLellan et al., 1992). Two additional items that inquired about smoking history, age at first cigarette use and lifetime number of months of regular cigarette use, were also added to this instrument.
2.5 Measurement of Neonatal Abstinence Syndrome (NAS)
The Finnegan Scale (Finnegan and Kaltenbach, 1992), used to assess NAS, was modified for the MOTHER study. Briefly, the MOTHER NAS Scale contains 28 items, with 19 items used for scoring and medication decisions. Scores range from 0 to a maximum withdrawal score of 42. A peak NAS score, the highest NAS score the neonate attained during the first 10 days of monitoring, was calculated for each neonate. Trained staff administered the MOTHER NAS scale for a minimum of 10 days. Details concerning rater training and agreement for the MOTHER NAS scale can be found in Jones et al. (2010).
2.6 MOTHER Outcome Measures
The 5 primary neonatal outcome measures in the MOTHER study were: treated for NAS (yes vs. no), peak NAS score, total amount of oral morphine sulfate (mg) needed to treat NAS, length of hospital stay (days), and head circumference (cm). In addition, there were 7 secondary neonatal outcomes: days medicated for NAS, birthweight (gm), infant length (cm), pre-term (<37 weeks) birth (yes vs. no), estimated gestational age at delivery (weeks), and Apgar scores (a summation of five items measured 0, 1, or 2, and so ranging from 0 to 10, inclusive) at 1 and 5 minutes; and 9 secondary maternal outcomes: cesarean section (yes vs. no), maternal weight gain from study entry until delivery (kg), normal presentation (yes vs. no), anesthesia during delivery (yes vs. no), positive drug screen at delivery (yes vs. no), medical complications at delivery (yes vs. no), study discontinuance (yes vs. no), amount of voucher money earned ($) during the course of the study, and number of prenatal obstetrical visits. All 21 of these outcome variables were analyzed in the present study.
More complete details about all variables can be found in Jones et al. (2010).
2.7 Statistical Analyses
Consistent with Jones et al. (2010), the assumption was that peak score on the MOTHER NAS scale during the assessment period, and infant head circumference, birth weight, and length, and maternal weight gain during the course of the study were normally distributed, and so ordinary least squares regression analyses were therefore conducted for these variables. The assumption was that total amount of morphine needed to treat NAS, number of days medicated for NAS, infant length of stay in the hospital, estimated gestational age at delivery, Apgar scores at 1 and 5 minutes, and amount of voucher money earned during the course of the study followed a Poisson distribution, so an overdispersed Poisson regression was utilized for these outcomes. The remaining outcomes were assumed to follow a binomial distribution, and so logistic regression analysis was utilized for these variables.
Two separate sets of analyses were conducted. Medication condition and a three-level factor representing site were included as fixed effects to control for design features of the MOTHER study in all analyses, while estimated gestational age at study entry was included as a fixed covariate to control for differential length of exposure to study medication. In the first set of analyses, the TDS score and the interaction between the TDS and medication condition were included as additional predictor variables, while in the second set, past 30-day daily average number of cigarettes smoked and its interaction with medication condition were included in addition to the medication condition and site main effects. Thus, the two sets of analyses evaluate the relative abilities of self-reported dependence in comparison to self-reported smoking behavior at study entry to predict neonatal and maternal outcomes. Due to missing data, analyses for the TDS measured at study entry used data from 124 participants while analyses for past 30-day daily average number of cigarettes smoked at study entry used data from 128 participants. The Type I error rate was set to .05, two-tailed, for all analyses.
3. RESULTS
3.1 Participant Characteristics
The 131 MOTHER participants were, on average, in their mid-20 s, white, had less than a high school education, unmarried, and unemployed. Table 1 in Jones et al. (2010) contains more complete details regarding this sample.
In terms of cigarette smoking, 127/131 (97%) of the sample smoked cigarettes at study entry, the average age at first cigarette use was 14.4 years old (SD=2.8), and lifetime months of regular cigarette use was 128.2 (SD=76.5). Mean TDS scores were 7.2 (SD=3.7) and 7.3 (SD=3.7) while mean self-reported past 30-day daily average number of cigarettes smoked was 13.1 (SD=7.5) and 14.0 (SD=8.0) in the buprenorphine and methadone conditions, respectively.
As a way of providing a more complete summary of the participants on whom the secondary analyses were conducted, among the 44 women who discontinued study participation prior to delivery, 8 were missing Tobacco Dependence Screener scores, and 6 were missing self-reported past 30-day daily average number of cigarettes smoked. The mean TDS score and mean self-reported past 30-day daily average number of cigarettes smoked within this group were 7.3 (SD=3.5) and 14.3 (SD=8.6), respectively, neither of which were significantly different from the corresponding means for the participants who delivered while enrolled in the study (both p-values>.6).
3.2 Results for Medication Condition Main Effect
Findings for the main effect for medication condition are reported in Jones et al. (2010) and therefore are not repeated here. (Inclusion of either the TDS or the past 30-day daily average number of cigarettes smoked in the analyses did not impact any conclusion drawn in Jones et al. (2010) regarding a medication condition finding.)
3.3 Results for Estimated Gestational Age at Study Entry
Estimated gestational age at study entry (in weeks) was negatively related to head circumference (cm), b=−.09 (SE=.03), η2=.06, p<.01, birthweight (gm), b=−23.81 (SE=8.04), η2=.08, p<.01, estimated gestational age at delivery (weeks), b=−.002, adjusted odds ratio (AOR)=.998 (95%CI: .996, .999), p<.05, maternal weight gain from study entry until delivery (kg), b=−.31 (SE=.10), η2=.08, p<.01, amount of voucher money earned ($) during the course of the study, b=−.07, AOR=.93 (95%CI: .91, .94), p<.01, and number of prenatal obstetrical visits, b=−.04, AOR=.96 (95%CI: .95, .97), p<.01, and positively related to maternal drug screen at delivery (positive), b=.11, AOR=1.11 (95%CI: 1.01, 1.23), p<.05.
3.4 Results for the Tobacco Dependence Screener
3.4.1 Tobacco Dependence Screener X Medication Condition Interaction
Table 1 reveals that there were two significant TDS X medication condition interaction effects. Tests of the simple effects of TDS within medication condition for both outcomes revealed that the AOR was non-significant in the methadone condition but significant in the buprenorphine condition, for non-normal presentation, AOR=.90 (95%CI: .74, 1.08, p>.24) and AOR=1.57 (95%CI: 1.01, 2.45, p<.05), respectively, and for amount of voucher money earned ($) during the course of the study, AOR=1.0 (95%CI: .97, 1.03, p>.9) and AOR=1.08 (95%CI: 1.04, 1.12, p<.01), respectively.
Table 1.
Parameter estimates and their standard errors, adjusted odds ratios and their 95% confidence intervals, semi-partial η2, and p-values associated with the Tobacco Dependence Screener and Tobacco Dependence Screener X medication condition interaction effects (N=124)
| Effect: | Tobacco Dependence Screener | Tobacco Dependence Screener X medication condition interaction | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| b | SE | AOR | 95% CI | η2 | p | b | SE | AOR | 95% CI | η2 | p | |
| Neonatal Outcomes | ||||||||||||
| Treated for NAS [yes] | .91 | .81, 1.02 | .11 | 1.11 | .89, 1.40 | .33 | ||||||
| NAS peak score | .11 | .12 | .01 | .33 | −.05 | .24 | .00 | .93 | ||||
| Total amount of morphine (mg) | 1.03 | .93, 1.14 | .59 | .92 | .61, 1.40 | .72 | ||||||
| Infant length of hospital stay (days) | 1.0 | .97, 1.07 | .31 | .99 | .90, 1.08 | .78 | ||||||
| Head circumference (cm) | .03 | .05 | .00 | .60 | −.05 | .10 | .00 | .63 | ||||
| Days medicated for NAS (days) | 1.04 | .07, 1.10 | .26 | .92 | .79, 1.07 | .28 | ||||||
| Birthweight (gm) | 12.22 | 12.91 | .01 | .35 | −9.9 | 26.12 | .00 | .70 | ||||
| Length (cm) | .02 | .01 | .00 | .85 | −.06 | .20 | .00 | .77 | ||||
| Pre-term birth [yes] | .99 | .85, 1.16 | .98 | .87 | .62, 1.23 | .43 | ||||||
| Gestational age at delivery (weeks) | 1.00 | 1.00, 1.00 | .91 | .99 | .99, 1.00 | .76 | ||||||
| Apgar score at 1 minute | 1.00 | .99, 1.01 | .85 | 1.00 | .98, 1.02 | .86 | ||||||
| Apgar score at 5 minutes | 1.00 | 1.00, 1.00 | .43 | 1.00 | .99, 1.01 | .96 | ||||||
| Maternal Outcomes | ||||||||||||
| Cesarean section [yes] | .93 | .84, 1.04 | .20 | .98 | .78, 1.22 | .84 | ||||||
| Maternal weight gain (kg) | −.10 | .17 | .00 | .56 | .01 | .34 | .00 | .97 | ||||
| Non-normal presentation [yes] | 1.01 | .85, 1.20 | .94 | .57 | .35, .92 | .05 | ||||||
| Analgesia during delivery [yes] | 1.00 | .86, 1.16 | .99 | 1.28 | .95, 1.73 | .11 | ||||||
| Maternal drug screen at delivery [positive] | 1.03 | .86, 1.24 | .75 | 1.29 | .93, 1.80 | .13 | ||||||
| Medical complications at delivery [yes] | .96 | .87, 1.07 | .47 | .99 | .79, 1.22 | .92 | ||||||
| Treatment discontinuation [yes] | 1.00 | .90, 1.11 | .97 | 1.11 | .99, 1.25 | .07 | ||||||
| Income from negative drug tests ($) | 1.03 | 1.004, 1.05 | .02 | .93 | .89, .97 | <.01 | ||||||
| Number of pre-natal visits | 1.01 | 1.00, 1.03 | .14 | 1.01 | .97, 1.04 | .73 | ||||||
Notes: b = unstandardized partial regression coefficient; SE = standard error for the regression coefficent; AOR = adjusted odds ratio; 95% CI = 95% Confidence Interval for the adjusted odds ratio; η2 = semi-partial η2 (a measure of the unique variance in the outcome measure explained by the effect of interest); p = p-value associated with the test of the regression coefficient. AORs and 95% CIs are reported for logistic and Poisson regression analyses, while η2 is reported for ordinary least squares analyses. In the case of logistic regression analysis, the reference group is indicated in [brackets].
3.4.2 Tobacco Dependence Screener Main Effect
Table 1 indicates that there was one significant TDS effect, for income from negative drug tests. Because the corresponding TDS X medication condition interaction is significant for this same outcome, as presented directly above, interpretation of the TDS main effect in the presence of this significant interaction would be misleading, so the parameter estimates are reported in Table 1 but no interpretation is offered beyond the interpretation of the simple main effects found in the above paragraph.
3.5 Results for Past 30-day Daily Average Number of Cigarettes Smoked
3.5.1 Past 30-day Daily Average Number of Cigarettes Smoked X Medication Condition Interaction
Table 2 reveals that there were no significant effects for the past 30-day daily average number of cigarettes smoked X medication condition interaction for any neonatal or maternal outcome measure (all p-values>.05).
Table 2.
Parameter estimates and their standard errors, adjusted odds ratios and their 95% confidence intervals, semi-partial η2, and p-values associated with the self-reported past 30-day daily average number of cigarettes smoked and self-reported past 30-day daily average number of cigarettes smoked X medication condition interaction effects (N=128)
| Effect: | Self-reported past 30-day daily average number of cigarettes smoked | Self-reported past 30-day daily average number of cigarettes smoked X medication condition interaction | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| b | SE | AOR | 95% CI | η2 | p | b | SE | AOR | 95% CI | η2 | p | |
| Neonatal Outcomes | ||||||||||||
| Treated for NAS [yes] | .96 | .91, 1.02 | .17 | 1.06 | .95, 1.19 | .26 | ||||||
| NAS peak score | .04 | .05 | .00 | .46 | −.08 | .11 | .00 | .48 | ||||
| Total amount of morphine (mg) | 1.06 | 1.02, 1.09 | <.01 | 1.00 | .85, 1.18 | .98 | ||||||
| Infant length of hospital stay (days) | 1.03 | 1.01, 1.05 | <.01 | .99 | .95, 1.03 | .58 | ||||||
| Head circumference (cm) | −.03 | .02 | .01 | .25 | .04 | .04 | .01 | .37 | ||||
| Days medicated for NAS (days) | 1.04 | 1.01, 1.06 | <.01 | .98 | .92, 1.04 | .46 | ||||||
| Birthweight (gm) | −10.03 | 6.13 | .02 | .10 | 11.88 | 12.65 | .01 | .35 | ||||
| Length (cm) | −.07 | .04 | .02 | .15 | .06 | .09 | .01 | .49 | ||||
| Pre-term birth [yes] | .97 | .91, 1.04 | .39 | 1.04 | .87, 1.23 | .72 | ||||||
| Gestational age at delivery (weeks) | 1.00 | 1.00, 1.00 | .44 | 1.00 | 1.00, 1.00 | .66 | ||||||
| Apgar score at 1 minute | .995 | .991, .999 | .03 | 1.00 | .99, 1.00 | .99 | ||||||
| Apgar score at 5 minutes | .996 | .994, .998 | <.01 | 1.00 | .99, 1.00 | .66 | ||||||
| Maternal Outcomes | ||||||||||||
| Cesarean section [yes] | .96 | .91, 1.01 | .06 | .97 | .87, 1.08 | .61 | ||||||
| Maternal weight gain (kg) | −.13 | .08 | .02 | .10 | −.02 | .16 | .00 | .92 | ||||
| Non-normal presentation [yes] | .95 | .87, 1.04 | .11 | .85 | .69, 1.07 | .18 | ||||||
| Analgesia during delivery [yes] | 1.00 | .93, 1.06 | .07 | 1.04 | .91, 1.20 | .54 | ||||||
| Maternal drug screen at delivery [positive] | 1.05 | .98, 1.12 | .07 | .96 | .83, 1.11 | .61 | ||||||
| Medical complications at delivery [yes] | .98 | .93, 1.03 | .05 | .97 | .88, 1.09 | .69 | ||||||
| Treatment discontinuation [yes] | .98 | .93, 1.03 | .06 | 1.11 | .99, 1.25 | .07 | ||||||
| Income from negative drug tests ($) | 1.00 | .99, 1.01 | .01 | 1.00 | .98, 1.02 | .99 | ||||||
| Number of pre-natal visits | 1.00 | 1.00, 1.01 | .01 | 1.00 | .98, 1.01 | .91 | ||||||
Notes: b = unstandardized partial regression coefficient; SE = standard error for the regression coefficent; AOR = adjusted odds ratio; 95% CI = 95% Confidence Interval for the adjusted odds ratio; η2 = semi-partial η2 (a measure of the unique variance in the outcome measure explained by the effect of interest); p = p-value associated with the test of the regression coefficient. AORs and 95% CIs are reported for logistic and Poisson regression analyses, while η2 is reported for ordinary least squares analyses. In the case of logistic regression analysis, the reference group is indicated in [brackets].
3.5.2 Past 30-day Daily Average Number of Cigarettes Smoked Main Effect
3.5.2.1 Neonatal Outcomes
Past 30-day daily average number of cigarettes smoked at study entry was positively associated with: the total amount of morphine needed to treat NAS, AOR=1.06 (95% CI: 1.02, 1.09), indicating each additional cigarette smoked at study entry was related to a 6% increase in the total amount of morphine needed to treat NAS; the number of days medicated for NAS, AOR=1.04 (95% CI: 1.01, 1.06), indicating each additional cigarette smoked at study entry was related to a 4% increase in the number of days medicated for NAS; the neonatal length of hospital stay, AOR=1.03 (95% CI: 1.01, 1.05), indicating each additional cigarette smoked at study entry was related to a 3% increase in the number of days neonates spent hospitalized. Past 30-day daily average number of cigarettes smoked at study entry was negatively associated with Apgar scores at 1, AOR=.995 (95% CI: .991, .999), and 5 minutes, AOR=.996 (95%CI: .994, .998), respectively, indicating each additional cigarette smoked at study entry was related to an approximate 1% decrease in Apgar scores at 1 and at 5 minutes. Past 30-day daily average number of cigarettes smoked at study entry was unrelated to the remaining neonatal outcomes (all p-values>.05).
Figure 1 depicts the estimated relationship between the past 30-day daily average number of cigarettes smoked at study entry and each of the significant neonatal outcomes at 4 levels of smoking: no smoking in the past 30 days; below average smoking (1 SD below the mean: 5.88 cigarettes smoked daily on average in the past 30 days); average smoking (mean amount of smoking: 13.66 cigarettes smoked daily on average in the past 30 days); and above-average smoking (1 SD above the mean: 21.44 cigarettes smoked daily on average in the past 30 days).
Figure 1. Bar Charts of Neonatal Outcome Measures at Selected Values of Past 30-Day Average Number of Cigarettes Smoked.
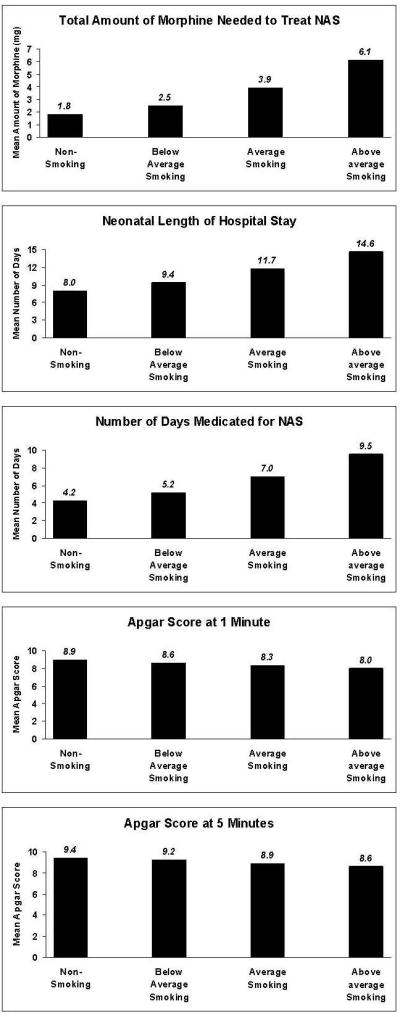
Poisson regression analyses were used to test the relationship between these 5 outcome variables and the past-30 day daily average number of cigarettes smoked, adjusting for medication condition, site, and estimated gestational age at study entry. Estimates are exponentiated parameter estimates. Estimates were calculated at 4 values of the predictor variable of past-30 day daily average number of cigarettes smoked: No cigarette smoking; below-average cigarette smoking, defined as 5.88 cigarettes/day (−1 SD); average cigarette smoking, defined as 13.66 cigarettes/day (Mean); and, above-average cigarette smoking, defined as 21.44 cigarettes/day (+1 SD).
3.5.2.2 Maternal Outcomes
Table 2 shows that there were no significant effects for past 30-day daily average number of cigarettes smoked for the maternal outcomes (all p-values>.09).
4. DISCUSSION
Findings from the present study do clearly support three conclusions. First, the failure to find an interaction between medication condition and past 30-day daily average number of cigarettes smoked indicates that the choice of agonist medication for treating opioid dependence in pregnant women does not need to take into consideration information regarding level of cigarette smoking at treatment entry.
Second, there is little support for the utility of tobacco dependence measure in predicting maternal outcomes, with the inexplicable exceptions that higher tobacco dependence scores predicted a greater likelihood of a normal presentation and a higher amount of voucher money earned during the course of the study in the buprenorphine condition but not in the methadone condition, and no support for its utility in predicting neonatal outcomes. The general failure of the tobacco dependence measure may be due in part to the fact that the past 30-day daily average number of cigarettes smoked in the MOTHER sample was quite similar to that reported in the Kawakami sample, resulting in a very restricted range of dependence in the sample, which in turn, negatively impacted power to detect a relationship.
Third, in contrast to the findings regarding tobacco dependence, a simple single self-report item of the past 30-day daily average number of cigarettes smoked at study entry proved to be a relatively robust predictor of neonatal outcomes. It might be argued that a tobacco dependence measure such as the TDS would show utility in prediction relative to a single self-report item of daily average number of cigarettes smoked in the past 30 days at study entry. However, in addition to the possibility of low power noted above, it may be that a measure of dependence is more subjective in its focus on smoking, confounding frequency of smoking and psychological distress. Thus, this measure may reflect more on the desire to smoke rather than the amount of nicotine ingested, as would be reflected by a measure of number of cigarettes smoked in the past 30 days, irrespective of how likely the latter measure might be subject to possible recall bias and impression management.
None of the findings regarding estimated gestational age at study entry are surprising. Later study entry would almost by necessity yield a lower amount of voucher money earned during the course of the study, and fewer prenatal obstetrical visits. Moreover, later study entry also is likely to be associated with a shorter period of quality prenatal care, resulting in a lower gestational age at delivery, smaller head circumference, lower birthweight, and lower maternal weight gain from study entry until delivery. Finally, the finding that estimated gestational age at study entry is positively related to maternal drug screen at delivery likely reflects the fact that those women who entered the study early in their pregnancy had more opportunity within treatment to reduce their substance use than women who entered the study later in their pregnancy.
4.1 Implications
It is critical to understand that the relationships observed between cigarette smoking and neonatal and maternal outcomes in the current study were found in a sample of pregnant women in treatment for opioid dependence. It is likely that the impact of smoking on the neonate is underestimated in the present sample relative to the population of opioid-dependent women who are not receiving pharmacotherapy and who are using heroin and/or other opioids and other substances. Furthermore, inclusion criteria for the parent study yielded a sample that had low levels of alcohol, cocaine, and benzodiazepine misuse during the course of their pregnancies. While these exclusion criteria yielded a sample in which it was optimal to explore the impact of cigarette smoking in the absence of these substances, it is quite likely that the combination of any of these drugs with smoking would prove even more deleterious to the neonate and mother. Finally, given the relationship between the putative quantity of cigarette smoking and NAS outcomes, it may be even more informative to examine the relationship between actual nicotine exposure (perhaps maternal cotinine levels) and neonatal outcomes.
4.2 Limitations
The present study involved a secondary analysis of data collected as part of a study whose primary aim did not include an examination of cigarette smoking. A study whose primary focus was the examination and/or treatment of cigarette smoking in the sample population might have produced quite different results. Moreover, better and more objective measures of smoking might have been employed, with the possibility of yielding different findings. More precise estimates of the impact of smoking on neonatal and maternal outcomes might have been possible were the relationship between smoking and neonatal and maternal outcomes assessed throughout pregnancy. However, reporting on smoking in this same sample (Chisolm et al., submitted), found that number of cigarettes smoked per day during the course of the study was similar in the methadone and buprenorphine conditions, and neither condition demonstrated any reduction in smoking over the course of pregnancy. These findings strongly suggest that use of a measure of smoking over the course of the pregnancy rather than at study entry would not have achieved substantially different findings than those reported in the present paper.
4.3 Conclusions
The present research is consistent with previous studies showing a relationship between cigarette smoking and compromised neonatal outcomes (Choo et al., 2004; Winklbaur et al., 2009; Bakstad et al., 2009; Jansson et al., 2010). Findings suggest that, in opioid-agonist-maintained pregnant women, assessment of actual smoking behavior rather than craving or feelings of withdrawal may be more informative for the development of smoking cessation interventions. Moreover, it may be that smoking cessation interventions in opioid-maintained pregnant women should focus on decreasing the quantity of cigarettes smoked, rather than cessation alone, because reductions in smoking can lead to improved neonatal outcomes. More research is needed to determine the level and rate of smoking reduction needed to maximize outcomes for the mother and neonate.
Acknowledgments
Role of Funding Source
The MOTHER clinical trial was registered with ClinicalTrials.gov (Identifier: NCT00271219; Title: RCT Comparing Methadone and Buprenorphine in Pregnant Women).
All MOTHER grants are from the National Institute on Drug Abuse (NIDA) unless noted otherwise: Brown University, R01 DA015778; Johns Hopkins University, R01 DA015764; Medical University of Vienna, R01 DA018417; Thomas Jefferson University, R01 DA015738; University of Toronto, R01 DA015741; University of Vermont, R01 DA018410 and M01 RR109; Vanderbilt University, R01 DA017513 and M01 RR00095, and Wayne State University, R01 DA15832.
NIDA played no role in the: 1) study design; 2) collection, analysis, and interpretation of data; 3) writing of the report; and 4) decision to submit or where to submit the paper for publication.
Footnotes
Contributors
HEJ planned this secondary analysis study, and wrote the initial draft of the manuscript. SHH, MT, MSC, and KK provided substantive revisions to the initial draft. JMM conducted the literature searches. KEO’G conducted the statistical analyses. All authors discussed the results and implications and commented on the final versions of the manuscript.
All authors have approved the final manuscript.
The authors alone are responsible for the content and writing of this article.
No honorarium, grant, or other form of payment was given to any author or any other individual to produce the manuscript.
Conflict of Interest
H.E.J. discloses that she has received reimbursement for time and travel from Reckitt Benckiser. K.E.O’G. discloses that he has received reimbursement for time from Reckitt Benckiser. The remaining authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bakstad B, Sarfi M, Welle-Strand GK, Ravndal E. Opioid maintenance treatment during pregnancy: occurrence and severity of neonatal abstinence syndrome. A national prospective study. Eur Addict Res. 2009;15:128–134. doi: 10.1159/000210042. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control. Vital signs: current cigarette smoking among adults aged ≥18 Years --- United States, 2005--2010. MMWR. 2011;60:1207–1212. [PubMed] [Google Scholar]
- Chisolm MS, Brigham EP, Lookatch SJ, Tuten M, Strain EC, Jones HE. Cigarette smoking knowledge, attitudes, and practices of patients and staff at a perinatal substance abuse treatment center. J Subst Abuse Treat. 2010;39:298–305. doi: 10.1016/j.jsat.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisolm MS, Fitzsimons H, Leoutsakos J-MS, Acquavita SP, Heil SH, Murphy MW, Tuten M, Kaltenbach K, Martin PM, Winklbaur B, Jansson LM, Jones HE. A comparison of cigarette smoking profiles in opioid-dependent pregnant patients receiving methadone or buprenorphine. doi: 10.1093/ntr/nts274. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo RE, Huestis MA, Schroeder JR, Shin AS, Jones HE. Neonatal abstinence syndrome in methadone-exposed infants is altered by level of prenatal tobacco exposure. Drug Alcohol Depend. 2004;75:253–260. doi: 10.1016/j.drugalcdep.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Cohen G, Jeffery H, Lagercrantz H, Katz-Salamon M. Long-term reprogramming of cardiovascular function in infants of active smokers. Hypertension. 2010;55:722–728. doi: 10.1161/HYPERTENSIONAHA.109.142695. [DOI] [PubMed] [Google Scholar]
- Dietz PM, England LJ, Shapiro-Mendoza CK, Tong VT, Farr SL, Callaghan WM. Infant morbidity and mortality attributable to prenatal smoking in the U.S. Am J Prev Med. 2010;39:45–52. doi: 10.1016/j.amepre.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Fagerstrom KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav. 1978;3:235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- Finnegan LP, Kaltenbach K. Neonatal abstinence syndrome. In: Hoekelman RA, Friedman SB, Nelson NM, Seidel HM, editors. Primary Pediatric Care. Mosby; St. Louis: 1992. pp. 1367–1378. [Google Scholar]
- Guerrero-Preston R, Goldman LR, Brebi-Mieville P, Ili-Gangas C, Lebron C, Witter FR, Apelberg BJ, Hernandez-Roystacher M, Jaffe A, Halden RU, Sidransky D. Global DNA hypomethylation is associated with in utero exposure to cotinine and perfluorinated alkyl compounds. Epigenetics. 2010;5:539–546. doi: 10.4161/epi.5.6.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackshaw A, Rodeck C, Boniface S. Maternal smoking in pregnancy and birth defects: a systematic review based on 173 687 malformed cases and 11.7 million controls. Hum Reprod Update. 2011;17:589–604. doi: 10.1093/humupd/dmr022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug NA, Stitzer ML, Svikis DS. Smoking during pregnancy and intention to quit: a profile of methadone-maintained women. Nicotine Tob Res. 2001;3:333–339. doi: 10.1080/14622200110050493. [DOI] [PubMed] [Google Scholar]
- Haug NA, Svikis DS, Diclemente C. Motivational enhancement therapy for nicotine dependence in methadone-maintained pregnant women. Psychol Addict Behav. 2004;18:289–292. doi: 10.1037/0893-164X.18.3.289. [DOI] [PubMed] [Google Scholar]
- Jansson LM, Dipietro JA, Elko A, Velez M. Infant autonomic functioning and neonatal abstinence syndrome. Drug Alcohol Depend. 2010;109:198–204. doi: 10.1016/j.drugalcdep.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HE, Fischer G, Heil SH, Kaltenbach K, Martin PR, Coyle MG, Selby P, Stine SM, O’Grady KE, Arria AM. Maternal Opioid Treatment: Human Experimental Research (MOTHER): approach, issues, and lessons learned. Addiction. doi: 10.1111/j.1360-0443.2012.04036.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HE, Heil SH, O’Grady KE, Martin PR, Kaltenbach K, Coyle MG, Stine SM, Selby P, Arria AM, Fischer G. Smoking in pregnant women screened for an opioid agonist medication study compared to related pregnant and non-pregnant patient samples. Am J Drug Alcohol Abuse. 2009;35:375–380. doi: 10.1080/00952990903125235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HE, Kaltenbach K, Heil SH, Stine SM, Coyle MG, Arria AM, O’Grady KE, Selby P, Martin PR, Fischer G. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N Engl J Med. 2010;363:2320–2331. doi: 10.1056/NEJMoa1005359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltenbach K, Berghella V, Finnegan L. Opioid dependence during pregnancy. Effects and management. Obstet Gynecol Clin North Am. 1998;25:139–151. doi: 10.1016/s0889-8545(05)70362-4. [DOI] [PubMed] [Google Scholar]
- Kawakami N, Takatsuka N, Inaba S, Shimizu H. Development of a screening questionnaire for tobacco/nicotine dependence according to ICD-10, DSM-III-R, and DSM-IV. Addict Behav. 1999;24:155–166. doi: 10.1016/s0306-4603(98)00127-0. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Rogers JM. Tobacco and pregnancy. Reprod Toxicol. 2009;28:152–160. doi: 10.1016/j.reprotox.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Solomon L, Quinn V. Spontaneous quitting: self-initiated smoking cessation in early pregnancy. Nicotine Tob Res. 2004;6(Suppl 2):S203–216. doi: 10.1080/14622200410001669132. [DOI] [PubMed] [Google Scholar]
- Tuten M, Fitzsimons H, Chisolm MS, Nuzzo PA, Jones HE. Contingent incentives reduce cigarette smoking among pregnant, methadone-maintained women: results of an initial feasibility and efficacy randomized clinical trial. Addiction. 2012;107:1868–1877. doi: 10.1111/j.1360-0443.2012.03923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winklbaur B, Baewert A, Jagsch R, Rohrmeister K, Metz V, Aeschbach Jachmann C, Thau K, Fischer G. Association between prenatal tobacco exposure and outcome of neonates born to opioid-maintained mothers. Implications for treatment. Eur Addict Res. 2009;15:150–156. doi: 10.1159/000216466. [DOI] [PubMed] [Google Scholar]


