Abstract
Kaposi's sarcoma-associated herpesvirus (KSHV) has been implicated in Kaposi's sarcoma, as well as in primary effusion lymphoma and multicentric Castleman's disease. The K1 protein of KSHV has been shown to induce cellular transformation and focus formation and to deregulate B-lymphocyte signaling pathways by functionally mimicking the activated B-cell receptor complex. Here we show that expression of K1 in B lymphocytes targets the phosphatidylinositol-3 kinase pathway, leading to the activation of the Akt kinase and the inhibition of the phosphatase PTEN. We also demonstrate that activation of Akt by the K1 protein leads to the phosphorylation and inhibition of members of the forkhead (FKHR) transcription factor family, which are key regulators of cell cycle progression and apoptosis. We demonstrate that K1 can inhibit apoptosis induced by the FKHR proteins and by stimulation of the Fas receptor. Our observations suggest that the K1 viral protein promotes cell survival pathways and may contribute to KSHV pathogenesis by preventing virally infected cells from undergoing apoptosis prematurely.
Kaposi's sarcoma-associated herpesvirus (KSHV; also known as human herpesvirus 8) is a gammaherpesvirus that was first identified in Kaposi's sarcoma (KS) biopsies (14). KSHV has since been found in all epidemiological forms of KS. Viral DNA has been consistently isolated from cases of AIDS-associated KS as well as human immunodeficiency virus-negative classic and transplant-associated KS (21). KSHV has also been associated with lymphoproliferative diseases, such as pleural effusion lymphomas and multicentric Castleman's disease (52), both of which originate in B cells. However, the molecular mechanism by which KSHV induces malignancy in its host remains to be resolved.
At the far left end of its genome (5′ end), KSHV encodes a 46-kDa transmembrane glycoprotein called K1. This position is equivalent to that of the herpesvirus saimiri (HVS) transforming protein (STP) and the R1 gene of rhesus monkey rhadinovirus (16). K1 expression has previously been shown to deregulate cell growth, inducing focus formation and morphological changes in Rat-1 fibroblasts (35). Additionally, K1 can functionally substitute for STP of HVS for transforming common marmoset T lymphocytes to interleukin-2-independent growth (35). Furthermore, transgenic K1 mice develop tumors with features of spindle-cell sarcomatoid and malignant plasmablastic lymphomas (43).
K1 is structurally similar to the B-cell receptor (BCR). The cytoplasmic tail contains an immunoreceptor tyrosine-based activation motif (ITAM) which has been shown to be capable of activating signaling pathways (31, 34) similar to those activated by the BCR complex in B lymphocytes. However, unlike the BCR, it is thought that K1 is constitutively active through oligomerization via conserved, extracellular cysteine residues (31). Activation of K1 leads to phosphorylation of the ITAM and recruitment of the major B-cell kinase, Syk. This initiates a signaling cascade in K1-expressing B cells (31, 34). Additionally, the cytoplasmic tail of the K1 protein has been shown to induce the phosphorylation of several different signaling molecules, including Syk, Vav, Cbl, and the p85 subunit of phosphatidylinositol-3′-OH kinase (PI3K), leading to lymphocyte activation, as measured by calcium mobilization, phosphorylation of tyrosine residues, and up-regulation of the NFAT and AP-1 transcription factors (34). K1 has also been shown to inhibit intracellular transport of BCR complexes and to have effects on viral lytic reactivation and replication (30, 32, 33). Furthermore, lymphocytes isolated from transgenic mice expressing K1 showed constitutive activation of NF-κB and Oct-2 as well as enhanced Lyn kinase activity (43).
The PI3K/Akt pathway is one of the key signaling pathways that are activated upon engagement of the BCR complex. PI3Ks are heterodimeric enzymes consisting of a regulatory subunit, p85, and a catalytic subunit, p110 (11). Consequent to BCR activation, p85 is recruited to the BCR-Syk complex, followed by the recruitment of the p110 subunit (5, 51). This results in the activation of PI3K and phosphorylation of the lipid membrane-associated moiety phosphatidylinositol 4,5-bisphosphate to yield phosphatidylinositol 3,4,5-triphosphate (PIP3). The Akt kinase specifically binds PIP3 through its pleckstrin homology domain, and this event recruits Akt to the plasma membrane (3, 22, 56). The PTEN phosphatase has previously been shown to be a negative regulator of this pathway (53, 59). PTEN catalyzes dephosphorylation at the D3 position of PIP3, serving to counter the effects of PI3K and resulting in the inhibition of Akt. Conversely, PTEN is itself inactivated by phosphorylation, leading to the activation of Akt kinase (57, 58). Active Akt kinase promotes cellular survival mechanisms by directly phosphorylating and inactivating proapoptotic factors such as Bad and caspase-9 (10, 17, 19). Additionally, Akt phosphorylates a family of transcription factors known as the forkhead transcription factors (FKHR) (8, 28, 55). Members of this family include FKHR, FKHRL1, and AFX. The net result of phosphorylation of the downstream targets of Akt is cell survival via inactivation of the FKHR family, glycogen synthase kinase 3β (GSK-3β), caspase-9, and Bad (10, 15, 17, 19).
At the present time, the exact mechanism by which the KSHV K1 viral protein transforms cells and activates B-cell signaling pathways remains to be elucidated. Given the facts that K1 elicits B-cell signaling events and that the cytoplasmic tail of K1 can induce the phosphorylation of a number of different kinases, including the p85 subunit of PI3K (34), we attempted to dissect the downstream effects of K1 signaling. Here we show that K1 up-regulates the PI3K pathway in B lymphocytes, resulting in the phosphorylation of Akt and PTEN. Further, this event appears to be significantly dependent on an intact K1 ITAM motif. The activation of Akt leads to an increase in phosphorylation of FKHR transcription factors. Phosphorylation of FKHR family members promotes their nuclear exclusion and inhibits their transcriptional activation properties (8, 28, 55). FKHR family members modulate transcription of several classes of genes involved in cell cycle regulation, including p27Kip (37, 39), p130 (29), and cyclin D1 (47), as well as genes that promote cell death, including Bim (20) and Fas ligand (8). We present data demonstrating that expression of the K1 viral protein in B lymphocytes enhances cell survival signals and protects cells from FKHR- and Fas-mediated apoptosis.
MATERIALS AND METHODS
Plasmid constructs.
EF-K1 was constructed as previously described (31). The CD8 signal peptide and Flag M2 epitope were fused in frame to the amino-terminal end of the K1 open reading frame. Three K1 mutants, EF-K1YY/FF, EF-K1Y282F, and EF-K1ITAM−, were created by using a QuikChange site-directed mutagenesis kit from Stratagene. The tyrosines at positions 270, 271(EF-K1YY/FF and EF-K1ITAM−), and 282 (EF-K1Y282F and EF-K1ITAM−) from EF-K1 were changed to phenylalanines and verified by sequencing. pGL2-3xIRS, pcDNA3-FKHR, pcDNA3-GFP-FKHR, and pcDNA3-GFP-FKHRAAA were previously described elsewhere (39). pCDNA3-Bcl-2 was a gift from John Reed.
Cell lines, cell culture, and transfections.
BJAB cells, a human B-cell line that is KSHV and Epstein-Barr virus negative, were maintained in RPMI 1640 medium supplemented with 10% calf serum, penicillin, and streptomycin. Forty micrograms of EF-K1 plasmid or empty vector was electroporated in serum-free medium into BJAB cells at 300 V and 950 μF. At 24 h postelectroporation, cells were rinsed in phosphate-buffered saline and transferred to serum-free medium for an additional 48 h. For luciferase assays, 293 cells were transfected with 1 μg of pcDNA3-GFP-FKHR or pcDNA3-GFP-FKHRAAA plus 2 μg of EF-K1 or empty vector by use of GenePorter 2 reagent (Gene Therapy Systems) as directed by the manufacturer. Cells were harvested at 48 h postelectroporation, and luciferase assays were performed as previously described (7). BJAB cells were electroporated with 5 or 10 μg of EF-K1 or empty vector EF and 5 μg of pCDNA3-FKHR or pCDNA3. A similar procedure was performed for transfection of BCBL-1 cells with the GenePorter 2 reagent.
Cell fractionation.
For cell fractionation, BJAB cells were electroporated and incubated as described above. At 72 h postelectroporation, cells were harvested and fractionated into cytoplasmic and nuclear lysates by use of OptiPrep (Nycomed Pharma) as described by the manufacturer, with the following modifications: harvested cells were rinsed in phosphate-buffered saline, pelleted, resuspended in 350 μl of cold buffer A (10 mM HEPES [pH 7.8], 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, complete protease inhibitor cocktail, and phosphatase inhibitor cocktail [Sigma]), and incubated on ice for 15 min prior to the addition of 40 μl of 10% NP-40. Samples were vortexed and then centrifuged, and cytoplasmic supernatants were reserved. Pelleted nuclei were rinsed in homogenization buffer (0.25 M sucrose, 25 mM KCl, 5 mM MgCl2, 20 mM Tris [pH 7.8], and phosphatase and protease inhibitors) and were resuspended in a final volume of 400 μl in homogenization buffer. Nuclei were isolated by underlaying them with 600 μl of 30% OptiPrep and 800 μl of 35% OptiPrep and centrifugation at 10,000 × g for 20 min. Isolated nuclei were rinsed in homogenization buffer, pelleted, and lysed in NE buffer (20 mM Tris [pH 8.0], 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 25% glycerol, 0.5 mM phenylmethylsulfonyl fluoride, and protease and phosphatase inhibitors). Lysates were incubated on ice for 10 min and then centrifuged to pellet debris. Supernatants were reserved as nuclear lysates.
Immunoblotting and immunofluorescence.
At 72 h posttransfection, cells were harvested and lysed in RIPA buffer containing a phosphatase inhibitor cocktail and protease inhibitors. For K1 expression, cells were freeze-thawed three times. Protein concentrations were determined by use of the Bradford assay. Eighty micrograms of protein was loaded per lane, resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to nitrocellulose. Each blot was stained with Ponceau S to ensure that equivalent amounts of protein samples were loaded. Primary antibodies were used at 1:1,000 and secondary antibodies were used at 1:2,000. K1 expression was verified by using horseradish peroxidase (HRP)-conjugated anti-Flag M2 (Sigma). Anti-Akt, anti-phospho-Akt (T308), anti-phospho-Akt (S473), anti-PTEN, anti-phospho-PTEN, anti-FKHR, anti-phospho-FKHR (S256), anti-phospho-PDK1, anti-phospho-GSK3β, anti-phospho-Bad, and HRP-conjugated anti-mouse and anti-rabbit antibodies were all purchased from Cell Signaling Technologies. The anti-PI3K (p85) antibody and the anti-phosphotyrosine HRP-conjugated antibody (4G10) were obtained from Upstate Biotechnology. Anti-caspase-9 antibody was a gift from M. Deshmukh. Immunoblotting detection was performed by using the ECL Plus kit (Amersham).
For immunofluorescence assays, 293 cells were transfected as described above, rinsed with Tris-buffered saline (TBS), fixed at room temperature in methanol-acetone (1:1), rinsed with TBS, and incubated for 1 h with anti-Flag M2-Cy3 (Sigma) antibody at room temperature. Cells were rinsed with TBS and viewed under a fluorescence Leica Axiovert microscope.
Apoptosis assay.
For FKHR-mediated apoptosis, 293 cells were transfected with the indicated amounts of EF-K1 or EF vector, 3 μg of pCDNA3-FKHR, and 1 μg of β-galactosidase (β-Gal) expression vector by using Superfect (Qiagen). Cells were harvested and analyzed at 72 h posttransfection. For Fas-mediated apoptosis, 293, BJAB, and BCBL-1 cells were transfected with the indicated amounts of EF-K1 and EF vector. Superfect (Qiagen) was used for transfection of 293 cells, electroporation was used for transfection of BJAB cells, and GenePorter 2 reagent was used for transfection of BCBL-1 cells. At 48 h posttransfection, 1.0 mg of anti-Fas antibody (Upstate Biotechnology)/ml was added in 1% fetal bovine serum, and cells were harvested 24 h later. Apoptosis was analyzed by using an ApoAlert caspase-3 colorimetric assay kit from Clontech. The transfection efficiency was normalized to β-Gal expression (Galacto-Star; Tropix).
TUNEL assay and flow cytometry.
BJAB cells were electroporated with 20 μg of EF or EF-K1 expression plasmid. At 48 h postelectroporation, anti-Fas antibody was added at 1 μg/ml in RPMI with 1% fetal bovine serum and was incubated for 24 h. Cells were then stained for terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) by using an in situ cell death detection kit (Roche) as directed by the manufacturer. Briefly, cells were fixed in 2% paraformaldehyde, permeabilized with 0.1% Triton X-100 in 0.1% sodium citrate, and incubated with the TUNEL reaction mixture for 1 h. Cells were rinsed and analyzed by use of a FACScan instrument (Becton-Dickinson), and data were acquired by use of Cytomation, Inc.
RESULTS
K1 activates the Akt signaling pathway.
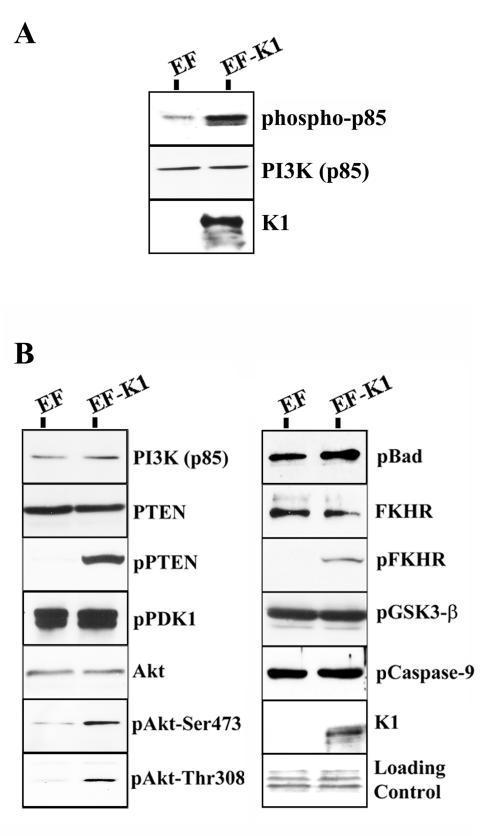
The KSHV K1 protein has previously been shown to induce phosphorylation of the p85 subunit of PI3K (34). BJAB cells were transfected with EF or EF-K1 expression plasmids. Cells were harvested and cell lysates were subjected to immunoprecipitation, with an anti-p85 antibody used to pull down PI3K. Immunoprecipitates were subjected to SDS-PAGE, and Western blotting was performed with an anti-pTyr-HRP antibody. As shown in Fig. 1A, we observed an increase in the phosphorylation of the p85 subunit of PI3K in K1-expressing cells, as previously described (34).
FIG. 1.
K1 activates the Akt pathway in B cells. (A) BJAB cells were transfected with the EF or EF-K1 expression plasmid as indicated. Cells were harvested, lysed, and subjected to immunoprecipitation with an anti-p85 antibody to pull down PI3K. A Western blot analysis was performed on the immunoprecipitate reactions, using an anti-phospho-Tyr-HRP antibody to detect the phosphorylated p85 subunit of PI3K. (B) BJAB cells were transfected with empty vector (EF) or a K1 expression vector (EF-K1). Equal amounts of proteins were separated by SDS-PAGE, transferred to nitrocellulose, and probed with the indicated antibodies. Ponceau S staining was used to evaluate equivalent loading of the samples. K1 expression was determined by probing with an anti-Flag antibody.
To understand the functional consequence of this induction, we analyzed the effects of K1 expression on downstream effectors in the PI3K pathway. We transfected BJAB cells with either an EF-K1 expression plasmid or empty EF vector. Equal amounts of total protein per sample were subjected to SDS-PAGE, and Western blot analyses were performed. Each blot was also stained with Ponceau S to ensure that equivalent amounts of protein samples were loaded and transferred. As shown in Fig. 1B, EF- and EF-K1-transfected cells had equal amounts of the p85 subunit of PI3K and total amounts of PTEN. However, only the K1-expressing cells showed a significant increase in the amount of phospho-PTEN on serine 380, as measured by a phospho-specific anti-PTEN antibody. Phosphorylation of PTEN at serine 380 is known to inhibit it from actively dephosphorylating PIP3. It has been shown that this inhibition allows for an increase in PIP3, which recruits Akt to the membrane, where it can be activated by the phosphoinositide-dependent kinase 1 (PDK1) (2, 22). Interestingly, although we observed no increase in the phosphorylation of PDK1 itself, we did see an increase in the phosphorylation of Akt at residues serine 473 (S473) and threonine 308 (T208) in K1-expressing cells (Fig. 1B). It has been widely reported that phosphorylation of Akt correlates with its activation (22, 27). Hence, the expression of K1 in B cells resulted in the activation of Akt, while the total levels of this kinase remained unchanged (Fig. 1B). Upon dual phosphorylation, Akt has been previously demonstrated to become fully activated, detach from the plasma membrane, and phosphorylate target substrates, such as Bad, FKHRs, GSK-3β, and caspase-9 (8, 10, 15, 17, 19, 28, 39).
Expression of K1 results in phosphorylation of the FKHR transcription factor family.
Since K1 activated Akt kinase in B cells, we investigated the effects on the downstream targets of Akt. Of the four Akt targets we analyzed, only the phosphorylation of FKHR was increased in K1-expressing cells (Fig. 2). There was a very marginal increase in phosphorylation of the proapoptotic protein Bad (Fig. 2).
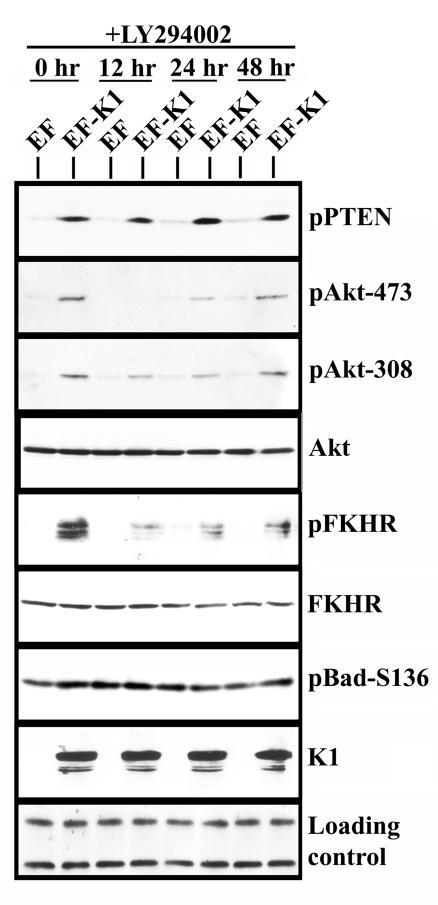
FIG. 2.
Activation of Akt by K1 is inhibited by LY294002. EF- or EF-K1-transfected BJAB cells were incubated for 12, 24, or 48 h in the presence of 10 μM LY294002. Lysates were subjected to Western blot analysis and probed with the indicated antibodies.
To determine if the changes in protein phosphorylation state in K1-expressing B cells are directly attributable to the activation of Akt by PI3K, we inhibited PI3K activation by using the specific chemical inhibitor LY294002. BJAB cells were transfected as described above, and LY294002 was added to the culture for a period of 12 h as previously reported (41). Upon exposure to LY294002, phosphorylation of Akt at serine 473 was completely eliminated, while phosphorylation of Akt at Thr 308 was inhibited slightly. It has been shown that the phosphorylation of Akt at S473 is much more sensitive to PI3K inhibition by LY294002 than that at the T308 residue (36), which may account for the different phosphorylation states of the two residues in this experiment. Phosphorylation of Akt reappeared after an additional 12 h (Fig. 2), which was most likely due to the drug losing its effect. There was no inhibition of PTEN phosphorylation. We also saw a slight inhibition of FKHR phosphorylation after 12 h of exposure to LY294002 in K1-expressing cells, and similar to the case with Akt, this was reversed at 24 h. The total levels of FKHR remained unaffected by the presence of LY294002. Curiously, LY294002 did not affect the phosphorylation of Bad. However, it has been shown that there are other kinases in addition to Akt that are capable of phosphorylating Bad (62).
Activation of the PI3K/Akt pathway and phosphorylation of FKHR by a panel of K1 mutants.
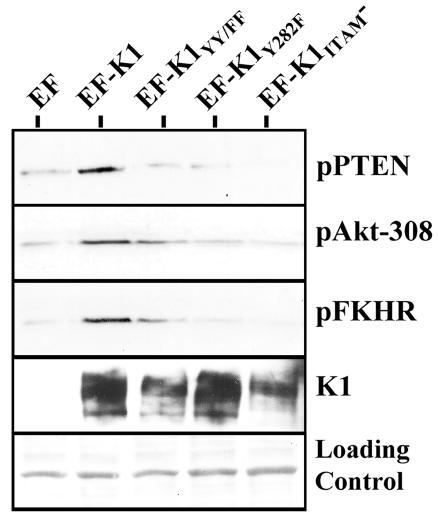
Much importance has been attributed to the presence of the ITAM in the cytoplasmic tail of K1. Despite significant variations in K1 sequences from different KSHV isolates from around the world, more than 60 K1 isolates sequenced to date show that the ITAM in the K1 cytoplasmic domain is always conserved (65). It has been demonstrated that the K1 ITAM is capable of activating signaling pathways (31, 34) similar to those activated by the BCR complex in B lymphocytes. In order to investigate whether the K1 ITAM was responsible for activating the Akt signaling cascade described above, we analyzed three mutants of K1 in which the tyrosine residues in the ITAM were individually or dually mutated to phenylalanine. These mutants were cloned into the same EF vector as wild-type K1 and were named EF-K1Y282F, EF-K1YY/FF, and EF-K1ITAM−, respectively. We next determined whether these tyrosine-altering mutations in the K1 cytoplasmic tail were capable of activating the Akt pathway in BJAB cells. Cells were electroporated with the aforementioned expression plasmids, and Western blot analysis was performed to analyze the phosphorylation status of PTEN, Akt, and FKHR. As shown in Fig. 3, all three K1 mutants, K1Y282F, K1YY/FF, and K1ITAM−, were unable to phosphorylate PTEN and thus unable to inactivate PTEN compared to wild-type K1. In addition, an analysis of the phosphorylation and activation state of Akt suggested that wild-type K1, and to some extent, the K1YY/FF mutant could still activate Akt, as measured by phosphorylation of T308, but that the K1Y282F and K1ITAM− mutants could not activate this kinase. Lee et al. previously showed that the K1YY/FF mutant can weakly induce cellular tyrosine phosphorylation, although at a much reduced level compared to that of wild-type K1 (34). The same pattern was seen when we looked at the FKHR transcription factor (Fig. 3). Wild-type K1, and to some degree, the K1YY/FF mutant could still induce inactivation of FKHR, as measured by phosphorylation of FKHR, but the K1Y282F and K1ITAM− mutants could not inactivate FKHR (Fig. 3).
FIG. 3.
Activation of the PI3K/Akt pathway by K1 mutants. BJAB cells were electroporated with the indicated expression plasmids. Cells were harvested as previously described. Equal amounts of proteins were separated by SDS-PAGE, transferred to nitrocellulose, and probed with the indicated antibodies. The individual panels, from top to bottom, represent Western blots probed with an anti-pPTEN, anti-pAKT (T308), and anti-pFKHR antibody, respectively. Ponceau S staining was used to evaluate equivalent loading of the samples. K1 expression was determined by probing with an anti-Flag antibody.
Expression of K1 results in the cytoplasmic sequestration of FKHR.
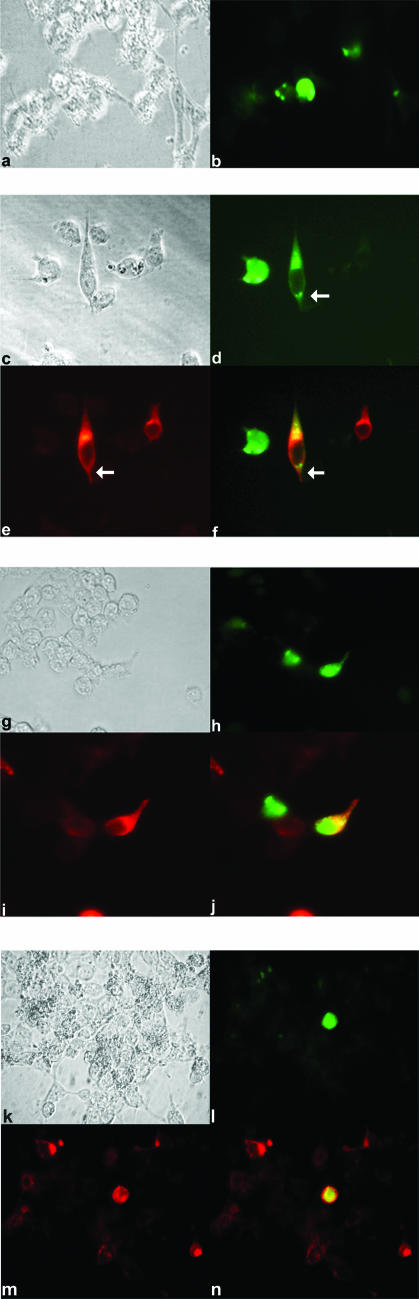
Direct phosphorylation of FKHR by Akt has been previously shown to result in cytoplasmic retention and inactivation of FKHR family members (6, 39, 55). We attempted to determine the cytoplasmic versus nuclear localization of FKHR in response to K1 expression. As we were limited by the rounded morphology of B cells, we analyzed the localization of FKHR in K1-expressing 293 cells, which have a flatter morphology and a more demarcated nuclear and cytoplasmic compartment than B cells. 293 cells were cotransfected with either empty vector EF or an EF-K1 plasmid, as well as with an FKHR-green fluorescent protein (GFP) fusion expression plasmid (39, 55). In Fig. 4, panels a and b depict the localization of FKHR-GFP in the presence of empty EF vector. The FKHR-GFP protein resides in both the cytoplasm and the nucleus, with the majority of the protein being in the nucleus. This correlates with what has been previously shown for this protein (39). Since FKHR in the nucleus activates the transcription of proapoptotic genes, these cells also appear to be undergoing apoptosis, as is evident from their morphology. However, in cells in which FKHR-GFP was coexpressed with K1 (Fig. 4c to f), the FKHR-GFP protein was predominantly excluded from the nucleus (see arrows in Fig. 4d to f). This was true for the majority of cells expressing both FKHR-GFP and the K1 protein. This conclusion was strengthened by the observation that the adjacent cell, which did not express K1, had FKHR-GFP localized to both the nucleus and the cytoplasm and appeared to be undergoing apoptosis. To determine if the localization of FKHR is dependent on the phosphorylation of FKHR by Akt, we cotransfected a mutant FKHR (FKHRAAA) lacking the three Akt phospho-acceptor sites, threonine 24A, serine 256A, and serine 319A (39, 55), along with the EF-K1 expression vector (Fig. 4g to j). The FKHRAAA-GFP protein was predominantly localized in the nucleus, regardless of whether or not the cell also expressed K1 (Fig. 4g to j), corroborating our observation that K1's effects on FKHR are dependent on Akt phosphorylation of the three aforementioned phospho-acceptor sites. Finally, we also analyzed the ability of the K1ITAM− mutant and found that it was unable to sequester the FKHR-GFP fusion protein in the cytoplasm of the cell (Fig. 4k to n). Thus, we suggest that FKHR phosphorylation and localization in K1-expressing cells are dependent on the ITAM.
FIG. 4.
K1 promotes cytoplasmic localization of FKHR. (a) 293 cells were transfected with empty vector (EF) and FKHR-GFP. Transfected cells were fixed and examined by bright-field microscopy. (b) The same cells as in panel a examined for expression and distribution of FKHR-GFP (green) by immunofluorescence microscopy. Cells expressing FKHR-GFP appear to be undergoing apoptosis based on their rounded morphology. (c) Cells were transfected with EF-K1 and FKHR-GFP expression plasmids. (d) The same cells as in panel c fixed and examined for expression and distribution of FKHR-GFP (green). (e) The same cells as depicted in panels c and d stained for expression of K1 (red) and examined by immunofluorescence microscopy. (f) Merged image of the same cells shown in panels c, d, and e. Yellow represents the colocalization of K1 (red) and FKHR-GFP (green). The white arrow indicates a cell that is coexpressing K1 and FKHR-GFP. The adjacent cell to its left expresses FKHR-GFP, but not K1, and appears to be undergoing apoptosis. (g) Cells were transfected with EF-K1 and the mutant FKHRAAA-GFP expression plasmid. (h) The same cells shown in panel g analyzed for expression and distribution of FKHRAAA-GFP (green). Based on their morphology, these cells appear to be undergoing apoptosis. (i) Cells were examined for expression of K1 (red) by immunofluorescence microscopy. (j) Merged image of the same cells shown in panels g, h, and i. Yellow represents the colocalization of FKHRAAA-GFP (green) and K1 (red). (k) Cells were transfected with FKHR-GFP and the EF-K1ITAM− expression plasmid. Transfected cells were fixed and examined by bright-field microscopy. (l) The same cells shown in panel k analyzed for expression and distribution of FKHR-GFP (green). (m) Cells were examined for expression of the K1ITAM− mutant (red). (n) Merged image of the same cells shown in panels k, l, and m. Yellow represents the colocalization of FKHR-GFP (green) and the K1ITAM− mutant (red).
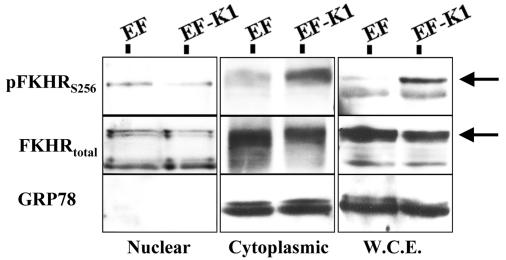
To confirm the localization of endogenous FKHR in B cells in response to K1 expression, we transfected BJAB cells with either the EF vector or an EF-K1 expression plasmid. At 72 h posttransfection, cells were harvested and lysed. Cell lysates were subjected to a nuclear and cytoplasmic fractionation scheme, as described in Materials and Methods, and Western blot analyses were performed (Fig. 5). The fractions were analyzed for both total FKHR and phosphorylated FKHR. The amount of phospho-FKHR present in the nucleus of K1-expressing cells was less than that in the empty vector transfected cells (Fig. 5, left panels). This is further supported by the observation that K1 expression results in an increase in phospho-FKHR protein in the corresponding cytoplasmic fraction of K1-expressing cells compared to empty vector controls (Fig. 5, middle panels). Taken together, our data suggest that the majority of FKHR in K1-expressing cells is phosphorylated on residue S256, resulting in the cytoplasmic retention of FKHR and thereby causing inhibition of its transcriptional activity (8, 25, 28). GRP78, a cytoplasmic protein, was used as a marker to assess the purity of the nuclear and cytoplasmic fractions.
FIG. 5.
Phospho-FKHR is retained in the cytoplasm of K1-expressing B cells. BJAB cells were transfected with EF or EF-K1, and the whole-cell extract (W.C.E.) was fractionated into a nuclear and a cytoplasmic fraction. Equal amounts of cytoplasmic and nuclear fractions from each sample were subjected to Western blot analysis with the indicated antibodies. Left panels, nuclear fraction; middle panels, cytoplasmic fraction; right panels, whole-cell extract. GRP78, a cytoplasmic protein, was used as a marker to assess the purity of the nuclear and cytoplasmic fractions. The arrows point to the FKHR-specific band.
K1 represses FKHR-responsive promoters and can inhibit both FKHR- and Fas-mediated apoptosis.
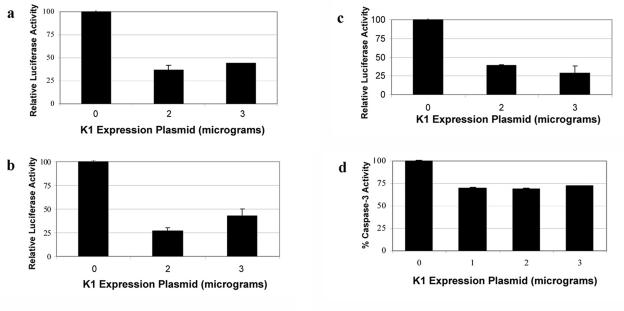
The localization and phosphorylation of FKHR suggest that the transcriptional function of FKHR may be inhibited in K1-expressing cells. The observation that FKHR is retained in the cytoplasm by the expression of K1 in B lymphocytes prompted us to investigate whether K1 could prevent the activation of FKHR-responsive promoters. FKHR has been shown to activate transcription from a minimal promoter element contained within insulin-like growth factor binding protein 1 known as the insulin response sequence (IRS) and from a forkhead-responsive element (FHRE) within the FasL promoter (8, 25, 55). Cells were cotransfected with various amounts of an EF-K1 plasmid or EF empty vector and a luciferase reporter plasmid, pFHRE-Luc, which contains three FHREs upstream of luciferase. A β-Gal expression vector was used to control for transfection efficiency. K1 repressed the FHRE-luciferase promoter threefold (Fig. 6a). The three FHREs are naturally present in the FasL promoter itself (8, 39) and are activated upon Fas receptor ligation. We next performed the identical experiment again, but stimulated the transfected cells with an anti-Fas receptor antibody to simulate the Fas receptor-dependent death pathway (Fig. 6b). K1 inhibited the FHRE-luciferase promoter fourfold in the presence of Fas-receptor engagement (Fig. 6b). Cells were also cotransfected with various amounts of EF-K1 plasmid or EF empty vector and a different FHRE reporter plasmid, 3XIRS-luciferase, which is comprised of three insulin-response elements upstream of the luciferase reporter gene (8, 25, 28). Again, K1 repressed the 3XIRS-luciferase promoter threefold (Fig. 6c).
FIG. 6.
K1 represses forkhead-regulated promoters and protects cells from FKHR-mediated apoptosis. (a) 293 cells were transfected with 0, 2, or 3 μg of EF-K1 or EF empty vector and 3 μg of a 3XFHRE-luciferase plasmid. A β-Gal construct was also cotransfected to normalize for transfection efficiency. At 48 h posttransfection, cells were lysed and assayed for luciferase expression. Luciferase activity in the absence of K1 was set to 100%, and relative luciferase activities in the presence of different amounts of the K1 expression plasmid were calculated as percentages of this luciferase activity. Error bars represent variations from the means. (b) Cells were transfected similar to the case for panel a, except that anti-Fas antibody was used to stimulate apoptosis with the FHRE-luciferase promoter. Luciferase activity in the absence of K1 and the presence of anti-Fas antibody was set to 100%, and relative luciferase activities in the presence of different amounts of the K1 expression plasmid were calculated as percentages of this luciferase activity. Error bars represent variations from the means. (c) 293 cells were transfected with 0, 2, or 3 μg of EF-K1 or EF empty vector and 3 μg of a 3XIRS-luciferase plasmid. Luciferase activity in the absence of K1 was set to 100%, and relative luciferase activities in the presence of different amounts of the K1 expression plasmid were calculated as percentages of this luciferase activity. Error bars represent variations from the means. (d) Cells were transfected with 0, 1, 2, or 3 μg of EF-K1 or EF empty vector and pCDNA3-FKHR. At 48 h posttransfection, cells were lysed and assayed for caspase-3 activity. Caspase-3 activity in the absence of K1 was set to 100% activity, and relative caspase-3 activities in the presence of different amounts of the K1 expression plasmid were calculated as percentages of this caspase-3 activity. Error bars represent variations from the means.
The forkhead family of transcription factors has been implicated in cell survival through the regulation of several proapoptotic genes, e.g., FasL and Bim. Our observation that the expression of K1 modulated FKHR expression and localization as well as its transcriptional activity on the FHRE-luciferase promoter led us to investigate whether K1 could increase cell survival under conditions of apoptotic stimuli, such as overexpression of FKHR or engagement of the Fas receptor by FasL or anti-Fas receptor antibody.
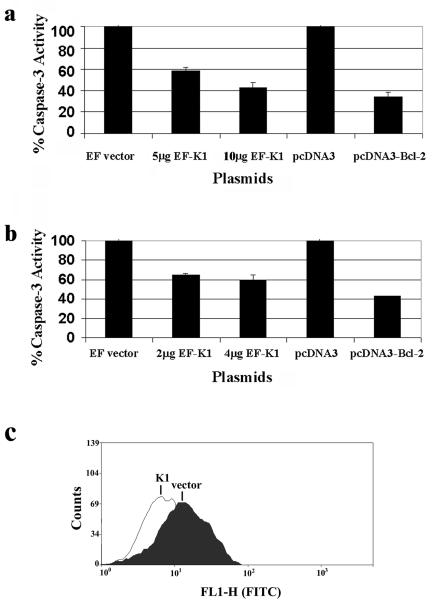
Caspase-3 is a downstream effector of both receptor-dependent and receptor-independent apoptotic stimuli, and its activation has served as a marker for cells undergoing apoptosis, as has been widely reported in the literature (42). We analyzed caspase-3 activity in EF- versus EF-K1-expressing cells that were cotransfected with a pCDNA3-FKHR expression plasmid to induce FKHR-mediated apoptosis. We observed that expression of K1 in these cells resulted in an ∼30% decrease in caspase-3 activity (Fig. 6d), indicating that K1 is able to thwart FKHR-mediated apoptosis. To determine whether K1 could inhibit Fas-mediated apoptosis, we transfected cells with EF or EF-K1 vector and pcDNA3 or pcDNA3-Bcl-2 vector, and at 48 h posttransfection, we stimulated the cells with anti-Fas receptor antibody. We observed that K1 expression resulted in a 50% decrease in caspase-3 activity induced by engagement of the Fas receptor compared to cells transfected with EF vector alone (Fig. 7a). BJAB cells transfected with Bcl-2 showed an ∼60% decrease in caspase-3 activity, which corresponds well with published literature that demonstrated that Bcl-2 antagonizes Fas receptor-induced apoptosis in B lymphocytes (1). Finally, in order to determine whether K1 could protect KSHV-positive B cells from Fas-mediated apoptosis, we repeated the experiment in BCBL-1 cells. We observed that expression of K1 in BCBL-1 cells resulted in an ∼40% decrease in caspase-3 activity induced by engagement of the Fas receptor, while Bcl-2 resulted in an ∼60% decrease in caspase-3 activity (Fig. 7b), again suggesting that K1 is able to inhibit Fas death receptor-dependent apoptosis. The K1ITAM− mutant was also tested in the caspase-3 cell death assays and only showed a 10% decrease in protection against Fas-mediated apoptosis (data not shown). We also performed a TUNEL assay with BJAB cells transfected with either EF vector or EF-K1 expression plasmid (Fig. 7c). Cells were incubated with anti-Fas antibody for 24 h to induce Fas receptor-dependent apoptosis, and a TUNEL assay was used to measure DNA fragmentation. BJAB cells were transfected with the EF or EF-K1 expression plasmid. The results showed that 14.86% of cells transfected with EF vector alone were fluorescein isothiocyanate (FITC) or TUNEL positive, while only 7.5% of cells transfected with the EF-K1 plasmid were FITC or TUNEL positive by flow cytometry, confirming our findings that K1 is able to protect against Fas-mediated apoptosis.
FIG. 7.
K1 protects cells from Fas-mediated apoptosis. (a) KSHV-negative BJAB cells were transfected with 0, 5, or 10 μg of EF-K1 or EF empty vector or pcDNA3 or pcDNA3-Bcl-2 expression plasmid by electroporation. At 48 h posttransfection, cells were stimulated with 1 μg of anti-Fas antibody/ml for 24 h. Cells were lysed and assayed for caspase-3 activity. Caspase-3 activity in the absence of K1 was set to 100%, and relative caspase-3 activities in the presence of different amounts of the K1 expression plasmid were calculated as percentages of this caspase-3 activity. Error bars represent variations from the means. (b) KSHV-positive BCBL-1 cells were transfected with 0, 2, or 4 μg of EF-K1 or EF vector or pcDNA3 or pcDNA3-Bcl-2 expression plasmid by using GenePorter 2 reagent. At 48 h posttransfection, cells were stimulated with 1 μg of anti-Fas antibody/ml for 24 h. Cells were lysed and assayed for caspase-3 activity. Caspase-3 activity in the absence of K1 was set to 100%, and relative caspase-3 activities in the presence of different amounts of the K1 expression plasmid were calculated as percentages of this caspase-3 activity. Error bars represent variations from the means. (c) BJAB cells were transfected with the EF or EF-K1 expression plasmid. Cells were treated with anti-Fas antibody to simulate Fas receptor-dependent apoptosis. Twenty-four hours later, a TUNEL assay was performed. Cells were stained for fragmented DNA by enzymatically labeling the nicked ends with FITC-conjugated dUTP and were assayed by flow cytometry. A total of 14.86% of cells transfected with EF vector alone were FITC or TUNEL positive, while only 7.5% of cells transfected with EF-K1 plasmid were FITC or TUNEL positive.
In order to confirm our findings, we also performed an in situ TUNEL assay to measure DNA fragmentation. We found that 15% of cells expressing wild-type K1 stained positive for DNA fragmentation, while 39% of cells expressing the K1ITAM− mutant protein stained positive for TUNEL. Thus, we conclude that wild-type K1 is able to protect cells from Fas-mediated apoptosis.
DISCUSSION
The KSHV K1 protein has been shown to have transforming potential by a wide variety of different assays, including in vitro focus formation assays, the induction of tumors in K1 transgenic animals and in nude mice injected with K1-expressing cells, and studies of the ability of K1 to substitute for the STP oncogene in HVS to immortalize T cells to interleukin-2-independent growth and to induce lymphomas in common marmosets (35, 43). Although K1 mRNA is induced upon lytic reactivation, its expression in latent cells cannot be definitively ruled out. K1 mRNA has been shown to be transcribed in KS lesions and in KSHV-infected B lymphoma cell lines, although in the latter case this may be a result of lytic K1 expression in the 3 to 5% of cells that undergo spontaneous reactivation (7, 46). Regardless of whether K1 is expressed during latency, the expression of K1 during the KSHV lytic cycle may contribute to the paracrine stimulation that is thought to sustain proliferation in most KSHV-associated malignancies (13, 24, 46). It is possible that K1, similar to the KSHV vGPCR protein (13), activates expression of a number of cytokines and growth factors needed for expansion of KSHV-associated neoplasms. Hence, an understanding of K1's signaling properties is important to the understanding of KSHV pathogenesis.
The KSHV K1 protein bears a marked structural resemblance to members of the immunoreceptor superfamily. Functional analysis of the K1 protein in B cells has demonstrated that K1 can elicit B-lymphocyte signaling events. For K1-mediated activation, there is a requirement for the major B-cell kinase, Syk. In addition, K1 has been shown to induce the phosphorylation of several signaling molecules, including the p85 subunit of PI3K. Given the fact that the PI3K pathway is linked not only to cell activation events but also to cell survival, our analysis here indicates that K1 is able to modulate the PI3K pathway by eliciting cell activation and survival responses.
In B lymphocytes, Akt kinase is a downstream target of activated PI3K. The activation of Akt itself plays an important role in B-cell survival, proliferation, and differentiation (41). Here we demonstrate that K1 expression leads to the activation of Akt. When expressed in B lymphocytes, K1 activates Akt by inducing phosphorylation at two separate residues, Thr308 and Ser473. Interestingly, K1 simultaneously appears to target the phosphatase PTEN, which is a negative regulator of the Akt pathway. The expression of K1 results in increased PTEN phosphorylation, which is indicative of its inactivation. Thus, K1 appears to activate Akt by a two-pronged approach that involves phosphorylation of the kinase itself as well as phosphorylation of its regulatory phosphatase, PTEN. In addition, Akt activation by K1 is inhibited by the PI3K-specific inhibitor, LY294002, confirming that K1 modulates Akt via the PI3K pathway.
As a key regulator of cell survival events, Akt targets a number of different cytoplasmic proteins, including GSK3β, caspase-9, Bad, and the FKHR family of transcription factors. As is widely reported, phosphorylation of these proteins by Akt generally results in their inactivation and inability to activate proapoptotic pathways. While K1 expression in B lymphocytes did not change the phosphorylation of GSK3β or caspase-9, it resulted in the marked phosphorylation of FKHR transcription factors and altered their cellular localization. Curiously, the marginal inhibition of FKHR phosphorylation levels by LY294002 suggests that although K1 targets Akt, its effect on FKHR may be mediated by other kinases besides Akt. Indeed, it is known that serum- and glucocorticoid-inducible kinase can also phosphorylate FKHR family members (9). A panel of K1 mutants in which the tyrosine residues in the ITAM were individually or dually mutated to phenylalanine (K1Y282F, K1YY/FF and K1ITAM−) were unable or very much inhibited in the ability to activate Akt or inactivate PTEN and FKHR, suggesting that activation of this pathway is dependent to a significant extent upon the ITAM present in the K1 cytoplasmic tail.
A wide variety of reports have shown that Akt kinase controls the subcellular localization of the members of the FKHR family via phosphorylation. Phosphorylation of FKHRs results in the preferential sequestration of these proteins in the cytoplasm, thus preventing them from activating their target genes, such as FasL and Bim. The exact mechanism by which this occurs has yet to be investigated. Phosphorylation may cause FKHR to interact with 14-3-3 proteins, resulting in nuclear export and cytoplasmic sequestration. It has also been observed that a decrease in DNA binding results when FKHR is phosphorylated at Ser256, which is located in the FKHR DNA-binding domain (63). Similar to the work of other laboratories, we observed that upon transient transfection of an FKHR-GFP expression plasmid into cells, FKHR was localized mainly in the nucleus. However, upon K1 coexpression in these cells, the FKHR protein was redirected to the cytoplasm. This localization was dependent on phosphorylation, since an FKHRAAA-GFP mutant in which all three Akt phospho-acceptor sites were mutated to alanine accumulated in the nucleus regardless of K1 coexpression. This observation was further supported by the fact that B lymphocytes expressing K1 showed a decrease in the levels of phospho-FKHR in the nuclear fraction accompanied by a concomitant increase in phospho-FKHR in the cytoplasmic fraction. Interestingly, although we saw a specific effect of K1 expression on the FKHR family, we did not see an effect on other downstream targets of Akt, such as GSK-3β and caspase-9. This suggests that these targets may be regulated by counteractive signals which prevent their phosphorylation, and it is possible that they may be targets of other KSHV viral proteins, such as the latency-associated nuclear antigen (23).
The biological significance of FKHR retention in the cytoplasm of K1-expressing cells was established by analyzing the functional targets of FKHR. One important target of the forkhead family of transcription factors is the Fas ligand. Specifically, FKHRL1 mediates the transcription of FasL in response to apoptotic stimuli. This activation is mediated through binding of FKHRL1 to the three FHREs in the FasL promoter. Secreted FasL binds to the Fas receptor on the surface of the cell and establishes a positive feedback loop, resulting in cell death. We observed that K1 protects cells from both FKHR-mediated apoptosis and Fas-mediated apoptosis, as analyzed by several different apoptosis assays.
Signaling through the BCR can disrupt the Fas pathway and inhibit apoptosis in both primary and established B-cell lines (12, 45). Hinshaw et al. (26) have shown that this protection is mediated upstream of caspase activation. Although Fas-mediated apoptosis has been well studied, FKHR-induced apoptosis is not completely understood. While we did observe that K1 protected cells from FKHR-induced apoptosis, it was to a lesser extent than that seen with Fas-mediated apoptosis. FKHR can transactivate several proapoptotic genes but has also been shown to repress gene transcription (44). In addition, the FKHR family can regulate genes directly by binding to their promoters or indirectly through interactions with other cellular factors (49, 64). Importantly, an FKHR mutant that is unable to bind DNA to induce cell death could still induce cell cycle arrest. Hence, although K1 prevents FKHR from translocating to the nucleus, it may not preclude FKHR's ability to interact with other cellular proteins, and although K1 may be able to repress the FKHR-regulated arm of the Fas-FasL apoptotic pathway, it may not be effective at preventing other types of apoptosis that are regulated by various members of the FKHR family. Conversely, although K1 can prevent FKHR from activating FasL transcription, it is possible that it can also inhibit other arms of the Fas-FasL pathway as well, which may explain why K1 protects cells from Fas-mediated apoptosis more effectively.
Our data indicate that the K1 protein can activate the Akt pathway in B lymphocytes and that this activation event is mediated by PI3K. This is consistent with recent reports indicating that Akt is a target for other transforming viral gene products, namely simian virus 40 large and small T antigens and Epstein-Barr virus LMP1 and LMP2A proteins (18, 48, 54, 60, 61). Similar to K1, the KSHV vGPCR protein has also been shown to transform cells and to target Akt kinase (4, 38, 40, 50). In addition, we have shown that K1 inhibits FKHR. Given the role of the FKHR family in activating expression of the proapoptotic FasL and Bim-1 genes and repressing cyclin D1 expression, the FKHR family members seem likely candidates for inactivation by viruses to prevent infected cells from undergoing apoptosis.
In summary, we suggest that the role of the KSHV K1 protein in the viral life cycle is to protect KSHV-infected cells from undergoing premature apoptosis by initiating cell survival signals. Thus, K1's ability to enhance B-cell survival may play an important role in the KSHV viral life cycle.
Acknowledgments
We are grateful to W. Sellers for providing the 3XIRS-luciferase vector and FKHR, FKHRAAA-, and FKHR-GFP expression plasmids and M. Greenberg for providing the FHRE-luciferase plasmid. We thank J. Jung for the original K1 expression plasmid; C. Patterson, E. Cutrone, and S. Krall for the 293 cell line; and N. Raab-Traub for protocols. We also thank J. Griffith and D. Dittmer for manuscript reading and members of the Damania lab for informative discussions.
This work was supported in part by grants from the V Foundation, the American Heart Association, and by NIH grant CA096500 to B.D. C.C.T. is supported in part by NIH training grant 5P30AI050410.
REFERENCES
- 1.Alam, M. K., S. Davison, N. Siddiqui, J. D. Norton, and J. J. Murphy. 1997. Ectopic expression of Bcl-2, but not Bcl-xL, rescues Ramos B cells from Fas-mediated apoptosis. Eur. J. Immunol. 27:3485-3491. [DOI] [PubMed] [Google Scholar]
- 2.Alessi, D. R., S. R. James, C. P. Downes, A. B. Holmes, P. R. Gaffney, C. B. Reese, and P. Cohen. 1997. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase B alpha. Curr. Biol. 7:261-269. [DOI] [PubMed] [Google Scholar]
- 3.Andjelkovic, M., D. R. Alessi, R. Meier, A. Fernandez, N. J. Lamb, M. Frech, P. Cron, P. Cohen, J. M. Lucocq, and B. A. Hemmings. 1997. Role of translocation in the activation and function of protein kinase B. J. Biol. Chem. 272:31515-31524. [DOI] [PubMed] [Google Scholar]
- 4.Bais, C., A. Van Geelen, P. Eroles, A. Mutlu, C. Chiozzini, S. Dias, R. L. Silverstein, S. Rafii, and E. A. Mesri. 2003. Kaposi's sarcoma associated herpesvirus G protein-coupled receptor immortalizes human endothelial cells by activation of the VEGF receptor-2/ KDR. Cancer Cell 3:131-143. [DOI] [PubMed] [Google Scholar]
- 5.Beitz, L. O., D. A. Fruman, T. Kurosaki, L. C. Cantley, and A. M. Scharenberg. 1999. SYK is upstream of phosphoinositide 3-kinase in B cell receptor signaling. J. Biol. Chem. 274:32662-32666. [DOI] [PubMed] [Google Scholar]
- 6.Biggs, W. H., III, J. Meisenhelder, T. Hunter, W. K. Cavenee, and K. C. Arden. 1999. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc. Natl. Acad. Sci. USA 96:7421-7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowser, B. S., S. M. DeWire, and B. Damania. 2002. Transcriptional regulation of the K1 gene product of Kaposi's sarcoma-associated herpesvirus. J. Virol. 76:12574-12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunet, A., A. Bonni, M. J. Zigmond, M. Z. Lin, P. Juo, L. S. Hu, M. J. Anderson, K. C. Arden, J. Blenis, and M. E. Greenberg. 1999. Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell 96:857-868. [DOI] [PubMed] [Google Scholar]
- 9.Brunet, A., J. Park, H. Tran, L. S. Hu, B. A. Hemmings, and M. E. Greenberg. 2001. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a). Mol. Cell. Biol. 21:952-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cardone, M. H., N. Roy, H. R. Stennicke, G. S. Salvesen, T. F. Franke, E. Stanbridge, S. Frisch, and J. C. Reed. 1998. Regulation of cell death protease caspase-9 by phosphorylation. Science 282:1318-1321. [DOI] [PubMed] [Google Scholar]
- 11.Carpenter, C. L., K. R. Auger, M. Chanudhuri, M. Yoakim, B. Schaffhausen, S. Shoelson, and L. C. Cantley. 1993. Phosphoinositide 3-kinase is activated by phosphopeptides that bind to the SH2 domains of the 85-kDa subunit. J. Biol. Chem. 268:9478-9483. [PubMed] [Google Scholar]
- 12.Catlett, I. M., and G. A. Bishop. 1999. Cutting edge: a novel mechanism for rescue of B cells from CD95/Fas-mediated apoptosis. J. Immunol. 163:2378-2381. [PubMed] [Google Scholar]
- 13.Cesarman, E., E. A. Mesri, and M. C. Gershengorn. 2000. Viral G protein-coupled receptor and Kaposi's sarcoma: a model of paracrine neoplasia? J. Exp. Med. 191:417-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 15.Cross, D. A., D. R. Alessi, P. Cohen, M. Andjelkovich, and B. A. Hemmings. 1995. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378:785-789. [DOI] [PubMed] [Google Scholar]
- 16.Damania, B., M. Li, J. K. Choi, L. Alexander, J. U. Jung, and R. C. Desrosiers. 1999. Identification of the R1 oncogene and its protein product from the rhadinovirus of rhesus monkeys. J. Virol. 73:5123-5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Datta, S. R., H. Dudek, X. Tao, S. Masters, H. Fu, Y. Gotoh, and M. E. Greenberg. 1997. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91:231-241. [DOI] [PubMed] [Google Scholar]
- 18.Dawson, C. W., G. Tramountanis, A. G. Eliopoulos, and L. S. Young. 2003. Epstein-Barr virus latent membrane protein 1 (LMP1) activates the phosphatidylinositol 3-kinase/Akt pathway to promote cell survival and induce actin filament remodeling. J. Biol. Chem. 278:3694-3704. [DOI] [PubMed] [Google Scholar]
- 19.del Peso, L., M. Gonzalez-Garcia, C. Page, R. Herrera, and G. Nunez. 1997. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science 278:687-689. [DOI] [PubMed] [Google Scholar]
- 20.Dijkers, P. F., R. H. Medema, J. W. Lammers, L. Koenderman, and P. J. Coffer. 2000. Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr. Biol. 10:1201-1204. [DOI] [PubMed]
- 21.Dupin, N., M. Grandadam, V. Calvez, I. Gorin, J. T. Aubin, S. Havard, F. Lamy, M. Leibowitch, J. M. Huraux, J. P. Escande, and H. Agut. 1995. Herpesvirus-like DNA sequences in patients with Mediterranean Kaposi's sarcoma. Lancet 345:761-762. [DOI] [PubMed] [Google Scholar]
- 22.Franke, T. F., D. R. Kaplan, L. C. Cantley, and A. Toker. 1997. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science 275:665-668. [DOI] [PubMed] [Google Scholar]
- 23.Fujimuro, M., F. Y. Wu, C. ApRhys, H. Kajumbula, D. B. Young, G. S. Hayward, and S. D. Hayward. 2003. A novel viral mechanism for dysregulation of beta-catenin in Kaposi's sarcoma-associated herpesvirus latency. Nat. Med. 9:300-306. [DOI] [PubMed] [Google Scholar]
- 24.Ganem, D. 1995. AIDS. Viruses, cytokines and Kaposi's sarcoma. Curr. Biol. 5:469-471. [DOI] [PubMed] [Google Scholar]
- 25.Guo, S., G. Rena, S. Cichy, X. He, P. Cohen, and T. Unterman. 1999. Phosphorylation of serine 256 by protein kinase B disrupts transactivation by FKHR and mediates effects of insulin on insulin-like growth factor-binding protein-1 promoter activity through a conserved insulin response sequence. J. Biol. Chem. 274:17184-17192. [DOI] [PubMed] [Google Scholar]
- 26.Hinshaw, J. A., C. M. Mueller, D. W. Scott, and M. S. Williams. 2003. B cell receptor signaling mediates immediate protection from Fas-induced apoptosis upstream of caspase activation through an atypical protein kinase C isozyme and de novo protein synthesis. Eur. J. Immunol. 33:2490-2500. [DOI] [PubMed] [Google Scholar]
- 27.Kohn, A. D., F. Takeuchi, and R. A. Roth. 1996. Akt, a pleckstrin homology domain containing kinase, is activated primarily by phosphorylation. J. Biol. Chem. 271:21920-21926. [DOI] [PubMed] [Google Scholar]
- 28.Kops, G. J., N. D. de Ruiter, A. M. De Vries-Smits, D. R. Powell, J. L. Bos, and B. M. Burgering. 1999. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature 398:630-634. [DOI] [PubMed] [Google Scholar]
- 29.Kops, G. J., R. H. Medema, J. Glassford, M. A. Essers, P. F. Dijkers, P. J. Coffer, E. W. Lam, and B. M. Burgering. 2002. Control of cell cycle exit and entry by protein kinase B-regulated forkhead transcription factors. Mol. Cell. Biol. 22:2025-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lagunoff, M., D. M. Lukac, and D. Ganem. 2001. Immunoreceptor tyrosine-based activation motif-dependent signaling by Kaposi's sarcoma-associated herpesvirus K1 protein: effects on lytic viral replication. J. Virol. 75:5891-5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lagunoff, M., R. Majeti, A. Weiss, and D. Ganem. 1999. Deregulated signal transduction by the K1 gene product of Kaposi's sarcoma-associated herpesvirus. Proc. Natl. Acad. Sci. USA 96:5704-5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee, B. S., X. Alvarez, S. Ishido, A. A. Lackner, and J. U. Jung. 2000. Inhibition of intracellular transport of B cell antigen receptor complexes by Kaposi's sarcoma-associated herpesvirus K1. J. Exp. Med. 192:11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee, B. S., M. Paulose-Murphy, Y. H. Chung, M. Connlole, S. Zeichner, and J. U. Jung. 2002. Suppression of tetradecanoyl phorbol acetate-induced lytic reactivation of Kaposi's sarcoma-associated herpesvirus by K1 signal transduction. J. Virol. 76:12185-12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee, H., J. Guo, M. Li, J. K. Choi, M. DeMaria, M. Rosenzweig, and J. U. Jung. 1998. Identification of an immunoreceptor tyrosine-based activation motif of K1 transforming protein of Kaposi's sarcoma-associated herpesvirus. Mol. Cell. Biol. 18:5219-5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee, H., R. Veazey, K. Williams, M. Li, J. Guo, F. Neipel, B. Fleckenstein, A. Lackner, R. C. Desrosiers, and J. U. Jung. 1998. Deregulation of cell growth by the K1 gene of Kaposi's sarcoma-associated herpesvirus. Nat. Med. 4:435-440. [DOI] [PubMed] [Google Scholar]
- 36.Mao, M., X. Fang, Y. Lu, R. Lapushin, R. C. Bast, Jr., and G. B. Mills. 2000. Inhibition of growth-factor-induced phosphorylation and activation of protein kinase B/Akt by atypical protein kinase C in breast cancer cells. Biochem. J. 352:475-482. [PMC free article] [PubMed] [Google Scholar]
- 37.Medema, R. H., G. J. Kops, J. L. Bos, and B. M. Burgering. 2000. AFX-like forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature 404:782-787. [DOI] [PubMed] [Google Scholar]
- 38.Montaner, S., A. Sodhi, S. Pece, E. A. Mesri, and J. S. Gutkind. 2001. The Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor promotes endothelial cell survival through the activation of Akt/protein kinase B. Cancer Res. 61:2641-2648. [PubMed] [Google Scholar]
- 39.Nakamura, N., S. Ramaswamy, F. Vazquez, S. Signoretti, M. Loda, and W. R. Sellers. 2000. Forkhead transcription factors are critical effectors of cell death and cell cycle arrest downstream of PTEN. Mol. Cell. Biol. 20:8969-8982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pati, S., M. Cavrois, H. G. Guo, J. S. Foulke, Jr., J. Kim, R. A. Feldman, and M. Reitz. 2001. Activation of NF-kappaB by the human herpesvirus 8 chemokine receptor ORF74: evidence for a paracrine model of Kaposi's sarcoma pathogenesis. J. Virol. 75:8660-8673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pogue, S. L., T. Kurosaki, J. Bolen, and R. Herbst. 2000. B cell antigen receptor-induced activation of Akt promotes B cell survival and is dependent on Syk kinase. J. Immunol. 165:1300-1306. [DOI] [PubMed] [Google Scholar]
- 42.Porter, A. G., and R. U. Janicke. 1999. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 6:99-104. [DOI] [PubMed] [Google Scholar]
- 43.Prakash, O., Z. Y. Tang, X. Peng, R. Coleman, J. Gill, G. Farr, and F. Samaniego. 2002. Tumorigenesis and aberrant signaling in transgenic mice expressing the human herpesvirus-8 K1 gene. J. Natl. Cancer Inst. 94:926-935. [DOI] [PubMed] [Google Scholar]
- 44.Ramaswamy, S., N. Nakamura, I. Sansal, L. Bergeron, and W. R. Sellers. 2002. A novel mechanism of gene regulation and tumor suppression by the transcription factor FKHR. Cancer Cell 2:81-91. [DOI] [PubMed] [Google Scholar]
- 45.Rothstein, T. L., J. K. Wang, D. J. Panka, L. C. Foote, Z. Wang, B. Stanger, H. Cui, S. T. Ju, and A. Marshak-Rothstein. 1995. Protection against Fas-dependent Th1-mediated apoptosis by antigen receptor engagement in B cells. Nature 374:163-165. [DOI] [PubMed] [Google Scholar]
- 46.Samaniego, F., S. Pati, J. Karp, O. Prakash, and D. Bose. 2001. Human herpesvirus 8 k1-associated nuclear factor-kappa b-dependent promoter activity: role in Kaposi's sarcoma inflammation? J. Natl. Cancer Inst. Monogr. 28:15-23. [DOI] [PubMed] [Google Scholar]
- 47.Schmidt, M., S. F. de Mattos, A. van der Horst, R. Klompmaker, G. J. Kops, E. W. Lam, B. M. Burgering, and R. H. Medema. 2002. Cell cycle inhibition by FoxO forkhead transcription factors involves downregulation of cyclin D. Mol. Cell. Biol. 22:7842-7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scholle, F., K. M. Bendt, and N. Raab-Traub. 2000. Epstein-Barr virus LMP2A transforms epithelial cells, inhibits cell differentiation, and activates Akt. J. Virol. 74:10681-10689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schuur, E. R., A. V. Loktev, M. Sharma, Z. Sun, R. A. Roth, and R. J. Weigel. 2001. Ligand-dependent interaction of estrogen receptor-alpha with members of the forkhead transcription factor family. J. Biol. Chem. 276:33554-33560. [DOI] [PubMed] [Google Scholar]
- 50.Smit, M. J., D. Verzijl, P. Casarosa, M. Navis, H. Timmerman, and R. Leurs. 2002. Kaposi's sarcoma-associated herpesvirus-encoded G protein-coupled receptor ORF74 constitutively activates p44/p42 MAPK and Akt via G(i) and phospholipase C-dependent signaling pathways. J. Virol. 76:1744-1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Songyang, Z., S. E. Shoelson, J. McGlade, P. Olivier, T. Pawson, X. R. Bustelo, M. Barbacid, H. Sabe, H. Hanafusa, and T. Yi. 1994. Specific motifs recognized by the SH2 domains of Csk, 3BP2, fps/fes, GRB-2, HCP, SHC, Syk, and Vav. Mol. Cell. Biol 14:2777-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soulier, J., L. Grollet, E. Oksenhendler, P. Cacoub, D. Cazals-Hatem, P. Babinet, M. F. d'Agay, J. P. Clauvel, M. Raphael, L. Degos, et al. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 86:1276-1280. [PubMed] [Google Scholar]
- 53.Stambolic, V., A. Suzuki, J. L. de la Pompa, G. M. Brothers, C. Mirtsos, T. Sasaki, J. Ruland, J. M. Penninger, D. P. Siderovski, and T. W. Mak. 1998. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell 95:29-39. [DOI] [PubMed] [Google Scholar]
- 54.Swart, R., I. K. Ruf, J. Sample, and R. Longnecker. 2000. Latent membrane protein 2A-mediated effects on the phosphatidylinositol 3-kinase/Akt pathway. J. Virol. 74:10838-10845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tang, E. D., G. Nunez, F. G. Barr, and K. L. Guan. 1999. Negative regulation of the forkhead transcription factor FKHR by Akt. J. Biol. Chem. 274:16741-16746. [DOI] [PubMed] [Google Scholar]
- 56.Toker, A., and L. C. Cantley. 1997. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature 387:673-676. [DOI] [PubMed] [Google Scholar]
- 57.Torres, J., and R. Pulido. 2001. The tumor suppressor PTEN is phosphorylated by the protein kinase CK2 at its C terminus. Implications for PTEN stability to proteasome-mediated degradation. J. Biol. Chem. 276:993-998. [DOI] [PubMed] [Google Scholar]
- 58.Vazquez, F., S. R. Grossman, Y. Takahashi, M. V. Rokas, N. Nakamura, and W. R. Sellers. 2001. Phosphorylation of the PTEN tail acts as an inhibitory switch by preventing its recruitment into a protein complex. J. Biol. Chem. 276:48627-48630. [DOI] [PubMed] [Google Scholar]
- 59.Wu, X., K. Senechal, M. S. Neshat, Y. E. Whang, and C. L. Sawyers. 1998. The PTEN/MMAC1 tumor suppressor phosphatase functions as a negative regulator of the phosphoinositide 3-kinase/Akt pathway. Proc. Natl. Acad. Sci. USA 95:15587-15591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu, Y., and J. C. Alwine. 2002. Human cytomegalovirus major immediate-early proteins and simian virus 40 large T antigen can inhibit apoptosis through activation of the phosphatidylinositide 3′-OH kinase pathway and the cellular kinase Akt. J. Virol. 76:3731-3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yuan, H., T. Veldman, K. Rundell, and R. Schlegel. 2002. Simian virus 40 small tumor antigen activates AKT and telomerase and induces anchorage-independent growth of human epithelial cells. J. Virol. 76:10685-10691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zha, J., H. Harada, E. Yang, J. Jockel, and S. J. Korsmeyer. 1996. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L). Cell 87:619-628. [DOI] [PubMed] [Google Scholar]
- 63.Zhang, X., L. Gan, H. Pan, S. Guo, X. He, S. T. Olson, A. Mesecar, S. Adam, and T. G. Unterman. 2002. Phosphorylation of serine 256 suppresses transactivation by FKHR (FOXO1) by multiple mechanisms: direct and indirect effects on nuclear/cytoplasmic shuttling and DNA binding. J. Biol. Chem. 277:45276-45284. [DOI] [PubMed] [Google Scholar]
- 64.Zhao, H. H., R. E. Herrera, E. Coronado-Heinsohn, M. C. Yang, J. H. Ludes-Meyers, K. J. Seybold-Tilson, Z. Nawaz, D. Yee, F. G. Barr, S. G. Diab, P. H. Brown, S. A. Fuqua, and C. K. Osborne. 2001. Forkhead homologue in rhabdomyosarcoma functions as a bifunctional nuclear receptor-interacting protein with both coactivator and corepressor functions. J. Biol. Chem. 276:27907-27912. [DOI] [PubMed] [Google Scholar]
- 65.Zong, J. C., D. M. Ciufo, D. J. Alcendor, X. Wan, J. Nicholas, P. J. Browning, P. L. Rady, S. K. Tyring, J. M. Orenstein, C. S. Rabkin, I. J. Su, K. F. Powell, M. Croxson, K. E. Foreman, B. J. Nickoloff, S. Alkan, and G. S. Hayward. 1999. High-level variability in the ORF-K1 membrane protein gene at the left end of the Kaposi's sarcoma-associated herpesvirus genome defines four major virus subtypes and multiple variants or clades in different human populations. J. Virol. 73:4156-4170. [DOI] [PMC free article] [PubMed] [Google Scholar]