Abstract
Background and purpose
To determine the interrelationships between baseline MMSE and risk of overall dementia, post-recurrent stroke dementia and dementia without recurrent stroke among patients with a history of stroke.
Methods
Prospective cohort study among participants enrolled in the Perindopril Protection Against Recurrent Stroke Study (PROGRESS) for whom baseline Mini-Mental State Examination (MMSE) score was available. Baseline MMSE was divided into four categories: 30, 29–27, 26–24, or <24. Participants were followed for incident dementia and recurrent stroke. Logistic regression models were used to examine the association between MMSE and dementia.
Results
Of the 6080 participants included in this analysis, 2493 had MMSE=30, 1768 had MMSE=29–28, 1369 had MMSE=26–24 and 450 had MMSE<24. Average follow-up time was 3.8 years. There were 407 cases of dementia, 106 of which were preceded by a recurrent stroke. The risk of overall dementia increased with decreasing MMSE score. However, the impact of MMSE on risk of dementia without recurrent stroke was much stronger than the impact of MMSE on the risk of post-recurrent stroke dementia. For those with MMSE<24, the risk of dementia without recurrent stroke was 47.89 (95% CI: 28.57–80.26) while the risk of post-recurrent stroke dementia was only 7.17 (95% CI: 3.70–13.89). Higher MMSE scores were even less strongly associated with the risk of post-recurrent stroke dementia.
Conclusions
Stroke patients with low MMSE scores are at high risk of dementia over time, even in the absence of a recurrent stroke, and should therefore be followed closely for further cognitive decline.
Indexing Terms: cerebrovascular disease, cognitive functioning, dementia, epidemiology
Stroke and dementia are two of the largest morbidity burdens worldwide.1 Previous research has shown that cognitive status and stroke occurrence are strongly related to the risk of future dementia. Individuals who experience a stroke have double the risk of dementia, including delayed dementia, compared to those who do not experience stroke.2 Other studies have also shown that lower scores on tests of cognitive function like the Mini-Mental State Examination (MMSE) are also highly predictive of future risk of dementia.3, 4
However, little research has examined whether pre-stroke cognitive function is still a strong predictor of the risk of dementia after a stroke event because most previous studies have not assessed cognitive function prior to the stroke event. A review article on the impact of stroke on the risk of dementia concluded that pre-stroke cognitive decline did not appear to account for the association between stroke and cognitive impairment and did not find evidence for an interaction between incident stroke and pre-stroke cognition on the risk of dementia.5 However, very few studies have specifically tested this interaction or assessed the impact of baseline cognitive status on the risk of dementia preceded by or not preceded by a stroke. Learning more about the associations between baseline cognitive functioning, stroke and risk of dementia would help to determine if the risk of dementia is driven primarily by the stroke event, which would suggest a vascular pathology, or by the level of cognitive functioning prior to the stroke event, which would suggest pathologies independent of the stroke event.
The Perindopril Protection Against Recurrent Stroke Study (PROGRESS) was a randomized trial among people with a history of cerebrovascular disease in which a blood pressure regimen was tested against placebo for the secondary prevention of stroke. The study assessed participants’ cognitive function at baseline and followed them for recurrent stroke and incident dementia. Using data from this study, we aimed to determine the interrelationships between baseline MMSE and the risk of overall dementia, post-recurrent stroke dementia and dementia without the presence of a recurrent stroke. We hypothesized that recurrent stroke is a strong risk factor for the development of dementia and cognitive status prior to recurrent stroke may modify the impact of recurrent stroke on the development of dementia.
Methods
Previous studies have described the design of PROGRESS.6, 7 Briefly, PROGRESS was a randomized, double-blind, placebo-controlled trial to determine the effectiveness of a blood pressure lowering regimen to prevent recurrent stroke and dementia amongst 6105 participants with prior stroke or TIA. Participants were recruited from 172 collaborating centers in 10 countries from May 1995 to November 1997. To be eligible, participants needed to have had either a stroke or transient ischemic attack (but not subarachnoid hemorrhage) within the previous five years and have no clear indication for, nor a contraindication to, treatment with an ACE inhibitor. Additionally, dementia was an exclusion criteria. After a run-in period, participants who tolerated and adhered to perindopril therapy were randomly assigned to continued active treatment or placebo. Randomization was stratified by study center, age, sex, entry systolic blood pressure, inclusion diagnosis, and the intention to begin combination therapy or single drug therapy.
Cognitive Decline and Dementia Assessment
At baseline, the 6- and 12-month visits and annually thereafter, participants completed the MMSE.3 One point was awarded for each successfully completed item (maximum score of 30); no points were awarded for any missing item. Baseline MMSE was a priori divided into four categories: 30 (high MMSE), 29–28 (medium-high MMSE), 27–24 (medium-low MMSE), <24 (low MMSE). In the event that baseline MMSE was missing (N=32), baseline MMSE was imputed using the MMSE score from the six-month visit (N=7). Participants for whom baseline MMSE could not be imputed were excluded from the analysis (N=25).
Throughout follow-up a two-phase screening and assessment process was used to diagnose dementia.8 Participants meeting any of the following criteria were considered to be screened positive for dementia: a MMSE score ≤25 at any follow-up visit, a decline in the MMSE score of ≥3 points between any 2 follow-up visits, a MMSE score missing for ≥2 scheduled follow-up visits, or a positive response by the investigator to the question, “In your opinion, does this patient have dementia?”. All participants who screened positive were referred to a local specialist experienced in diagnosing dementia. The specialists were blinded to treatment assignment and to all clinical data. Participants who screened negative were classified as “no dementia”.
The local specialist used a checklist based on the criteria for the diagnosis of dementia as defined in the Diagnostic and Statistical Manual of Mental Disorder, Fourth Edition.9 The questionnaire included systematic questions on the presence of post-stroke focal deficits like aphasia or motor deficit and on the presence of more global problems like depressed mood that could have altered the diagnostic of dementia. Local specialists were also systematically asked whether the diagnosis of dementia was reliable. Whenever possible, the specialist examined the patient. If an interview could not be conducted, data were sought from all other available sources, including medical records, interviews with family members, and consultations with other medical professionals. After receiving the information from and diagnosis of the local specialist, a 2-person central Dementia Adjudication Committee confirmed or refuted the diagnosis and assigned each screen positive case to one of the following four categories: certain dementia, fairly certain (probable) dementia, uncertain (possible) dementia, or no dementia. No attempt was made to further classify cases into subtypes of dementia because all participants had a history of cerebrovascular disease and often had other vascular risk factors. The main outcome for this analysis was the occurrence of dementia, either “certain dementia” or “fairly certain dementia”.
Stroke Assessment
Recurrent fatal or non-fatal stroke was defined as an acute disturbance of focal neurological function with symptoms lasting more than 24 hours (or resulting in earlier death) thought to be due to either cerebral infarction or cerebral hemorrhage.7, 10 All suspected strokes and deaths were first reported by local study investigators and then reviewed by experts on the central End Point Adjudication Committee. This committee was provided with a clinical summary of the event and copies of any available investigation reports (for example, biochemistory, hematology, radiology and autopsy findings).11 Strokes were classified as cerebral hemorrhage, ischemic stroke or stroke of unknown pathological type.
If dementia occurred before the recurrent stroke event, the person was classified as dementia without recurrent stroke later denominated "Dementia without recurrent stroke". If stroke event occurred prior to dementia, the person was classified as post-recurrent stroke dementia later denominated "Post-stroke dementia".
Statistical Analysis
First, we used logistic regression models to calculate the odds ratio as a measure for the relative risk of developing dementia for the four categories of the baseline MMSE score using the highest category of MMSE as the reference category. Next, to examine the impact of MMSE on the joint outcome of recurrent stroke and dementia, we divided incident dementia into post-stroke dementia and dementia without recurrent stroke. We then used logistic regression models to assess the relative risk of both dementia types compared to no dementia for each of our MMSE categories.
All models were adjusted for variables that we believed could be potential confounders based on biological mechanisms. These variables were age (continuous), gender, height (continuous), smoking status (never smoked regularly, past, current), current alcohol consumption (currently do not drink more than once/week, currently drink <8 drinks/week currently drink ≥8 drinks/week), educational status (stopped education by age 14, 16, ≥19), diabetes status (yes/no), and systolic blood pressure (continuous). We also adjusted for randomized treatment assignment. In secondary analyses, we explored the relationship between baseline MMSE and recurrent stroke and risk of dementia stratified by the recurrent stroke subtype (ischemic versus hemorrhagic stroke). We additionally performed all analyses using only those randomized to placebo. Finally we performed separate sensitivity analyses in which we adjusted for baseline Barthel index in addition to our potential confounders, excluded patients who screened positive for dementia at baseline, and excluded patients with MMSE scores less than 18 at baseline.
No participant had missing information on age, gender, current alcohol consumption, diabetes status or baseline systolic blood pressure. Less than 100 people were missing information on height and smoking and were assigned to the median and past smoker respectively. More than 100 people had missing information on education status so we used the missing indicator method.
All statistical analyses were performed using SAS 9.2. All P values are 2-tailed and P<0.05 was considered statistically significant.
Results
Of the 6080 participants included in this analysis, 2493 (41.0%) had a MMSE score of 30, 1768 (29.1%) had an MMSE score of 29–27, 1369 (22.5%) had an MMSE score of 26–24 and 450 (7.4%) had an MMSE score of less than 24. Average follow-up time was 3.8 years. There were 407 cases of dementia, 106 of which were preceded by a recurrent stroke. A total of 709 strokes occurred either before dementia onset or the end of the follow-up.
Table 1 shows covariates by baseline MMSE categories for our study population. Those with the lowest MMSE scores were older, shorter in stature, had stopped schooling at a younger age, more likely to be female, consumed alcohol less frequently, had higher systolic blood pressure, were more likely to be diabetic and were less likely to be current smokers than participants with higher MMSE scores.
Table 1.
Baseline characteristics of participants in the PROGRESS trial by baseline MMSE score (N=6080).
| MMSE Score | ||||
|---|---|---|---|---|
| Characteristic | 30 | 29–28 | 26–24 | <24 |
| Age, mean (SE) | 61.4 (9.5) | 64.8 (9.5) | 66.0 (8.8) | 67.6 (8.9) |
| Female (%) | 27.8 | 30.7 | 32.1 | 37.6 |
| Height, mean (SE) | 167.3 (8.9) | 167.2 (8.9) | 165.4 (9.4) | 164.1 (9.0) |
| Smoking status (%) | ||||
| Never smoked regularly | 44.7 | 40.0 | 40.0 | 42.7 |
| Past smoker | 33.8 | 41.7 | 39.7 | 39.8 |
| Current smoker | 21.6 | 18.3 | 20.4 | 17.6 |
| Current alcohol consumption (%) | ||||
| Less than 1/week | 60.5 | 56.6 | 59.9 | 66.7 |
| 1 to 8 drinks/week | 18.0 | 20.8 | 20.0 | 17.1 |
| ≥8 drinks/week | 21.5 | 22.6 | 20.1 | 16.2 |
| Age at which schooling stopped | ||||
| ≤14 years old | 22.3 | 36.3 | 42.5 | 61.5 |
| 15–16 years old | 20.1 | 25.4 | 25.1 | 19.2 |
| 17–19 years old | 26.4 | 18.4 | 17.2 | 13.1 |
| >19 years old | 31.3 | 19.9 | 15.2 | 6.3 |
| Diabetes (%) | 12.0 | 11.8 | 12.5 | 17.8 |
| Systolic blood pressure, mean (SE) | 145.4 (18.8) | 147.2 (19.0) | 148.5 (18.7) | 150.4 (20.4) |
| Type of qualifying event | ||||
| TIA | 22.9 | 24.4 | 18.5 | 18.2 |
| Ischemic stroke | 64.6 | 61.4 | 64.5 | 64.7 |
| Hemorrhagic stroke | 9.8 | 8.9 | 11.8 | 10.9 |
| Unknown | 2.7 | 5.3 | 5.2 | 6.2 |
| Medication (%) | ||||
| Antihypertensive therapy | 57.6 | 57.1 | 61.5 | 61.1 |
| Antiplatelet therapy | 71.9 | 73.9 | 71.6 | 71.3 |
| Oral anticoagulants | 7.1 | 11.9 | 8.7 | 11.8 |
| Lipid-lowering therapy | 15.8 | 13.9 | 12.4 | 10.7 |
The impact of baseline MMSE on the risk of overall dementia is presented in Table 2. The risk of dementia increased with decreasing MMSE scores. Those with an MMSE score of 28–29 had a relative risk of dementia of 2.15 (95% CI: 1.43–3.24) while those with an MMSE score of less than 24 had a relative risk of dementia of 26.81 (95% CI: 18.08–39.76) compared to those with an MMSE score of 30.
Table 2.
Multivariate adjusted* relative risks of overall dementia, post-stroke dementia and dementia without recurrent stroke by baseline MMSE score.
| Baseline MMSE | No Dementia (N=5673) |
All dementia (N=407) |
Post-stroke dementia (N=106) |
Dementia without recurrent stroke (N=301) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Number of cases |
Crude incidence rate (per 1000 person- years) |
RR (95% CI) |
Number of cases |
Crude incidence rate (per 1000 person- years) |
RR (95% CI) |
Number of cases |
Crude incidence rate (per 1000 person- years) |
RR (95% CI) |
|
| 30 | 2455 | 38 | 3.86 | 1.00 | 19 | 1.93 | 1.00 | 19 | 1.93 | 1.00 |
| 28–29 | 1703 | 65 | 9.54 | 2.15 (1.43, 3.24) | 21 | 3.08 | 1.32 (0.70, 2.49) | 44 | 6.46 | 2.99 (1.73, 5.18) |
| 24–27 | 1218 | 151 | 28.87 | 6.59 (4.54, 9.55) | 44 | 8.41 | 3.67 (2.10, 6.43) | 107 | 20.45 | 9.56 (5.79. 15.80) |
| <24 | 297 | 153 | 105.08 | 26.81 (18.08, 39.76) | 22 | 15.11 | 7.17 (3.70, 13.89) | 131 | 89.97 | 47.89 (28.57, 80.26) |
Results have been adjusted for age, gender, height, smoking status, current alcohol consumption, educational status, diabetes, baseline systolic blood pressure and randomized treatment assignment.
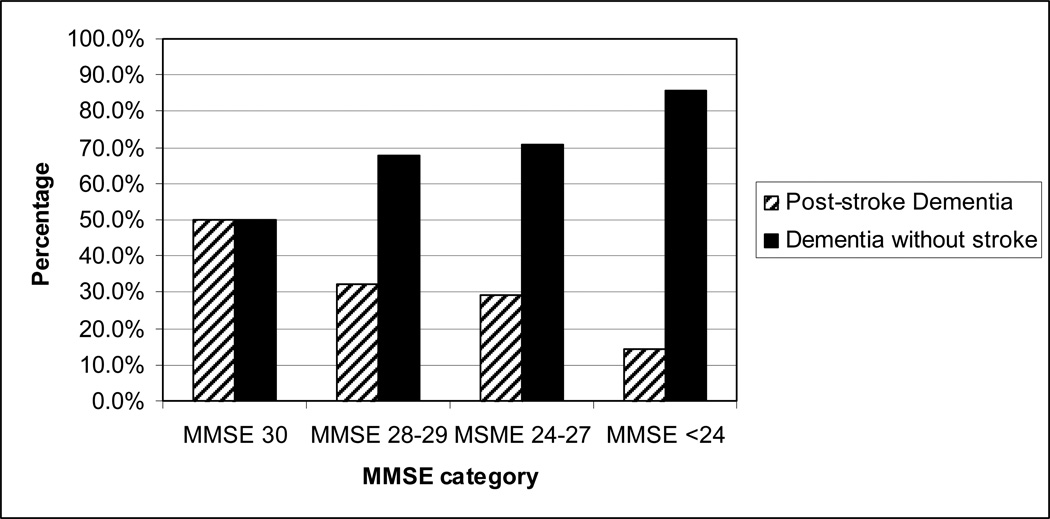
Figure 1 shows the proportion of post- stroke dementia and dementia without recurrent stroke by MMSE category. Among those with a MMSE score of 30, 50% of those who develop dementia had dementia without recurrent stroke. In contrast, among those with a low MMSE (<24) almost all of those who develop dementia had dementia without recurrent stroke.
Figure 1.
Proportion of types of dementia according to baseline MMSE
The effect estimates for the association between baseline MMSE and post-recurrent stroke dementia and between MMSE and dementia without recurrent stroke can be seen in Table 2. For both outcomes, we observed an increase in the risk of dementia with decreasing MMSE scores. However, the impact of MMSE on risk of dementia without recurrent stroke was much stronger than the impact of MMSE on the risk of post-recurrent stroke dementia. For those with MMSE less than 24, the risk of dementia without stroke was 47.89 (95% CI: 28.57–80.26) while the risk of post-recurrent stroke dementia was only 7.17 (95% CI: 3.70–13.89). Higher MMSE scores were even less strongly associated with the risk of post-recurrent stroke dementia. The risk of post-recurrent stroke dementia was only 1.32 (95% CI: 0.70–2.49) among those with an MMSE score 29–28 while the risk of dementia without stroke was 2.99 (95% CI: 1.73–5.18).
Results stratified by stroke subtype (ischemic versus hemorrhagic, results not shown) were similar to those shown above. Results among those assigned to placebo were similar to those seen for the full cohort (results not shown). Results of our other sensitivity analyses were also similar to those seen for the full cohort (please see http://stroke.ahajournals.org).
To further explore why baseline MMSE was not as strongly associated with the risk of post-recurrent stroke dementia, we examined the associations between baseline MMSE and the risk of recurrent stroke and also between recurrent stroke and the risk of dementia. Baseline MMSE was not associated with the risk of recurrent stroke (results not shown). However, recurrent stroke was significantly associated with the risk of dementia (RR = 2.93; 95% CI: 2.24, 3.82).
Discussion
In this prospective cohort of stroke patients, baseline MMSE was strongly associated with the risk of developing dementia. Analyses examining post-stroke dementia versus dementia without recurrent stroke suggested stronger associations between baseline MMSE and risk of dementia without recurrent stroke than with post-stroke dementia.
Previous studies have shown that both lower MMSE scores and stroke predict the risk of subsequent dementia.2,3, 4 However, data on the inter-relationships between pre-stroke MMSE, stroke and the risk of dementia in one study are sparse, mainly because of small numbers of stroke or dementia events not allowing the evaluation joint effects. Three studies have examined the inter-relationships between pre-stroke cognitive functioning, stroke and cognitive impairment. Data from the Framingham Heart Study showed that participants who experienced a stroke had significantly lower mean MMSE scores pre-stroke and post-stroke compared to the stroke-free participants.12 The Baltimore Longitudinal Study of Aging found that those with mild cognitive impairment who experience a stroke are at increased risk of developing dementia (odds ratio (OR)=12.4; 95% CI: 1.5–99) compared to those with mild cognitive impairment who do not experience a stroke. Additionally, those who were cognitively normal at the time of their stroke did not have an increased risk of dementia compared to those without stroke.13 Another study using data from the Health and Retirement Study found that the rate of cognitive decline was faster among those who later survived a stroke compared to those who remained stroke-free throughout follow-up. Those who died after stroke had even faster rates of decline. After the stroke event, the rate of decline among stroke survivors was similar to their rate of decline prior to the stroke event.14
While these studies were able to examine the relationships between cognitive functioning and first stroke, research on the interrelationships between cognitive function, recurrent stroke and risk of subsequent dementia is sparse. A few studies which assessed cognitive functioning in stroke patients have shown links between cognitive decline and dementia15, 16 but many could not specifically examine the effect of recurrent stroke on associations between cognitive functioning and dementia. One study directly tested the interaction between baseline cognitive status and incident stroke using participants in the Rotterdam Study who were free of dementia or a history of stroke at baseline and found no interactive effect of incident stroke and measures of pre-stroke cognitive function on the risk of dementia.17
The present study showed that among those who have already experienced a stroke or TIA, baseline MMSE is a strong predictor of the risk of dementia without recurrent stroke. Results from our study suggest that stroke patients with low MMSE are already on a trajectory of cognitive decline related to preexisting neurodegenerative lesions or other undetermined factors triggered by the initial stroke event. The stroke event may increase the risk of dementia either due to the direct impact of vascular disease on other neuropathological changes associated with Alzheimer’s disease or by synergistic effects of Alzheimer’s neuropathology and vascular neuropathology.18 This has important clinical implications because it demonstrates that for those with low MMSE, a recurrent stroke is not necessary in order to develop dementia. This result could appear counter-intuitive as, in stroke patients, one would expect a second stroke to result in a greater risk of cognitive decline and vascular dementia. A review article found that the rate of dementia was at least twice as high after recurrent stroke as it was after first-stroke and the rate of dementia after recurrent stroke may depend upon the number of recurrences.19
An important finding from the main analyses of PROGRESS is that a blood pressure-lowering regimen decreased the risk of recurrent stroke and risk of post-recurrent stroke dementia.20 In the present study, the associations between baseline MMSE and post-stroke dementia were weaker than those seen for the associations between baseline MMSE and dementia without recurrent stroke. While baseline MMSE is highly predictive of dementia in the absence of stroke, the impact of recurrent stroke on the risk of dementia outweighs the impact of baseline MMSE. Therefore, patients, especially those with high MMSE who have the lowest risk of dementia without recurrent stroke, should be delivered strong stroke prevention messages. Given the associations seen between blood-pressure lowering drugs and a reduced risk of recurrent stroke, patients with high MMSE may want to consider a blood pressure lowering regimen in order to avoid another stroke and the associated risk of dementia.
One of the main strength to this study was that unlike previous studies, we had information on MMSE score before recurrent stroke and new diagnoses of dementia after recurrent stroke. Other strengths include the large number of outcome events which allowed us to assess the interrelationships between baseline MMSE, recurrent stroke, and risk of dementia. By screening all participants at baseline for dementia, the cohort of individuals in this study were free of pre-existing dementia.
Despite the strengths to this study, some weaknesses should be noted. While this study did have a large number of outcome events, many of our confidence intervals for our effect estimates are wide. Additionally, we did not have information on MMSE prior to first stroke which prevented us from determining if first stroke may be the triggering factor for cognitive decline or if the patients had already experienced decline prior to stroke. We took several steps to obtain a consistent diagnosis of dementia across the various centers including using standardized forms and having a central Dementia Adjudication Committee to minimize heterogeneity. While it is possible that people with undiagnosed or mild cognitive impairment at baseline may have been included in our study, dementia was an exclusion criterion for entry into the trial and we used the original trial cohort for these analyses. Although four different tools were used to screen for dementia, they were mainly based on MMSE which is not sensitive to vascular cognitive impairment. It is therefore possible that some cases of dementia may have been missed.
To summarize, these results carry important messages for stroke patients and their physicians that vary according to the baseline MMSE of the stroke patient. Stroke patients with low MMSE scores are at high risk of dementia over time, even in the absence of a recurrent stroke, and should therefore be followed closely for further cognitive decline.
Supplementary Material
Acknowledgments
Study Funding: The Perindopril Protection Against Recurrent Stroke Study was funded by grants from Servier (Paris, France), the Health Research Council of New Zealand (Auckland, New Zealand, and the National Health and Medical Research Council of Australia (Canberra, Australia). The study was designed, conducted, analyzed and interpreted by the investigators independent of all sponsors. Dr. Rist was funded by a training grant from the National Institute of Aging (AG-00158).
J.C. and S.M. have received lecture fees and research grants from Servier. H.A. holds Future Fellowship from Australian Research Council. C.A. holds Senior Principal Research Fellowship from NHMRC. M.W. has received lecture fees from Servier. T.K. has received funds from Allergan and the American Academy of Neurology for educational lectures. T.C. has received fees from the ABBOTT company for participating in scientific committees.
Footnotes
Disclosures: P.M.R. has no disclosures.
References
- 1.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL. Global burden of disease and risk factors. Washington, D.C.: Oxford University Press and The World Bank; 2006. [PubMed] [Google Scholar]
- 2.Leys D, Henon H, Mackowiak-Cordoliani MA, Pasquier F. Poststroke dementia. Lancet Neurol. 2005;4:752–759. doi: 10.1016/S1474-4422(05)70221-0. [DOI] [PubMed] [Google Scholar]
- 3.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 4.Woodford HJ, George J. Cognitive assessment in the elderly: A review of clinical methods. Qjm. 2007;100:469–484. doi: 10.1093/qjmed/hcm051. [DOI] [PubMed] [Google Scholar]
- 5.Savva GM, Stephan BC. Epidemiological studies of the effect of stroke on incident dementia: A systematic review. Stroke. 2010;41:e41–e46. doi: 10.1161/STROKEAHA.109.559880. [DOI] [PubMed] [Google Scholar]
- 6.Blood pressure lowering for the secondary prevention of stroke: Rationale and design for progress. Progress management committee. Perindopril protection against recurrent stroke study. J Hypertens Suppl. 1996;14:S41–S45. discussion S45–46. [PubMed] [Google Scholar]
- 7.Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358:1033–1041. doi: 10.1016/S0140-6736(01)06178-5. [DOI] [PubMed] [Google Scholar]
- 8.Tzourio C, Anderson C. Blood pressure reduction and risk of dementia in patients with stroke: Rationale of the dementia assessment in progress (perindopril protection against recurrent stroke study). Progress management committee. J Hypertens Suppl. 2000;18:S21–S24. [PubMed] [Google Scholar]
- 9.The American Psychiatric Association. Diagnostic and statistical manual of mental disorders, fourth edition. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 10.Stroke--1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO task force on stroke and other cerebrovascular disorders. Stroke. 1989;20:1407–1431. doi: 10.1161/01.str.20.10.1407. [DOI] [PubMed] [Google Scholar]
- 11.Chapman N, Huxley R, Anderson C, Bousser MG, Chalmers J, Colman S, et al. Effects of a perindopril-based blood pressure-lowering regimen on the risk of recurrent stroke according to stroke subtype and medical history: The progress trial. Stroke. 2004;35:116–121. doi: 10.1161/01.STR.0000106480.76217.6F. [DOI] [PubMed] [Google Scholar]
- 12.Kase CS, Wolf PA, Kelly-Hayes M, Kannel WB, Beiser A, D'Agostino RB. Intellectual decline after stroke: The framingham study. Stroke. 1998;29:805–812. doi: 10.1161/01.str.29.4.805. [DOI] [PubMed] [Google Scholar]
- 13.Gamaldo A, Moghekar A, Kilada S, Resnick SM, Zonderman AB, O'Brien R. Effect of a clinical stroke on the risk of dementia in a prospective cohort. Neurology. 2006;67:1363–1369. doi: 10.1212/01.wnl.0000240285.89067.3f. [DOI] [PubMed] [Google Scholar]
- 14.Wang Q, Capistrant BD, Ehntholt A, Glymour MM. Long-term rate of change in memory functioning before and after stroke onset. Stroke. 2012;43:2561–2566. doi: 10.1161/STROKEAHA.112.661587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henon H, Durieu I, Guerouaou D, Lebert F, Pasquier F, Leys D. Poststroke dementia: Incidence and relationship to prestroke cognitive decline. Neurology. 2001;57:1216–1222. doi: 10.1212/wnl.57.7.1216. [DOI] [PubMed] [Google Scholar]
- 16.Srikanth VK, Anderson JF, Donnan GA, Saling MM, Didus E, Alpitsis R, et al. Progressive dementia after first-ever stroke: A community-based follow-up study. Neurology. 2004;63:785–792. doi: 10.1212/01.wnl.0000137042.01774.33. [DOI] [PubMed] [Google Scholar]
- 17.Reitz C, Bos MJ, Hofman A, Koudstaal PJ, Breteler MM. Prestroke cognitive performance, incident stroke, and risk of dementia: The rotterdam study. Stroke. 2008;39:36–41. doi: 10.1161/STROKEAHA.107.490334. [DOI] [PubMed] [Google Scholar]
- 18.Gottesman RF, Hillis AE. Predictors and assessment of cognitive dysfunction resulting from ischaemic stroke. Lancet Neurol. 2010;9:895–905. doi: 10.1016/S1474-4422(10)70164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: A systematic review and meta-analysis. Lancet Neurol. 2009;8:1006–1018. doi: 10.1016/S1474-4422(09)70236-4. [DOI] [PubMed] [Google Scholar]
- 20.Tzourio C, Anderson C, Chapman N, Woodward M, Neal B, MacMahon S, et al. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Intern Med. 2003;163:1069–1075. doi: 10.1001/archinte.163.9.1069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.