Abstract
Objectives/Hypothesis
Repair of the transected facial nerve has traditionally been accomplished with microsurgical neurorrhaphy; however, fibrin adhesive coaptation (FAC) of peripheral nerves has become increasingly popular over the past decade. We compared functional recovery following suture neurorrhaphy to FAC in a rodent facial nerve model.
Study Design
Prospective, randomized animal study.
Methods
Sixteen rats underwent transection and repair of the facial nerve proximal to the pes anserinus. Eight animals underwent epineurial suture (ES) neurorrhaphy, and eight underwent repair with fibrin adhesive (FA). Surgical times were documented for all procedures. Whisking function was analyzed on a weekly basis for both groups across 15 weeks of recovery.
Results
Rats experienced whisking recovery consistent in time course and degree with prior studies of rodent facial nerve transection and repair. There were no significant differences in whisking amplitude, velocity, or acceleration between suture and FA groups. However, the neurorrhaphy time with FA was 70% shorter than for ES (P < 0.05).
Conclusion
Although we found no difference in whisking recovery between suture and FA repair of the main trunk of the rat facial nerve, the significantly shorter operative time for FA repair makes this technique an attractive option. The relative advantages of both techniques are discussed.
Keywords: Fibrin adhesive, rodent, facial nerve, neurorrhaphy
INTRODUCTION
Fibrin adhesive (FA) is a commonly used hemostatic surgical adjunct, employed in myriad specialties, including abdominal, cardiothoracic, vascular, urological and plastic surgery.1,2 FA is formulated with a combination of thrombin and fibrinogen as well as other components, which in our experiment included aprotinin, glycine, and calcium chloride. The adhesive is created by thrombin's enzymatic action on fibrinogen, which induces a conformational change of the components to form a fibrin clot. Recently, many authors have also employed FA for peripheral nerve repair as a supplement to, or replacement for, suture neurorrhaphy. Nevertheless, controversy remains regarding the substitution of FA for traditional epineurial microsurgical repair.3
Despite the relatively frequent need for facial nerve repair, there are few published reports involving FA techniques in the facial nerve. Almost all previous studies on the efficacy of FA have been performed in a sciatic nerve model. When FA repair has been applied to the facial nerve in an animal model, it has been done in the rat,3,4 which is a popular model for facial nerve regeneration due to the animal's dynamic and quantifiable vibrissal whisking. Thus far, only one study has examined whisking recovery after facial nerve repair with FA,5 and in that report, whisking movement was subjectively assessed using a 5-point scale rather than objectively quantified. The recent availability of systems for objective whisking assessment,6–8 combined with a firm understanding of the motor supply serving whisking function,9,10 warrants a comparison of FA versus suture repair in the rat facial nerve model using quantitative whisking assessment.
MATERIALS AND METHODS
Sixteen Wistar Hannover Rats (Charles River Laboratories, Wilmington, MA), 75 to 90 days old and weighing 200g to 250g were used following Massachusetts Eye and Ear Infirmary guidelines for animal care and use; food and water were available ad libitum. Animals were anesthetized with an intramuscular injection of ketamine (50 mg/kg) (Fort Dodge Animal Health, Fort Dodge, IA) and medetomidine hydrochloride (0.5 mg/kg) (Orion Corporation, Espoo, Finland) for all surgical procedures.
Head Fixation and Conditioning
One week after arrival, all animals were handled for preoperative conditioning.7 Following conditioning, titanium cranial implants were placed, which were used for head fixation during functional testing, as previously described by Hadlock et al.7 After a 2-week recovery period, animals began the dynamic conditioning process, per published protocol.7 Rats received positive reinforcement in the form of oral reward with YOO-HOO nondairy chocolate drink (Mott's LLP, Rye Brook, NY) throughout each conditioning interval.
Surgical Intervention
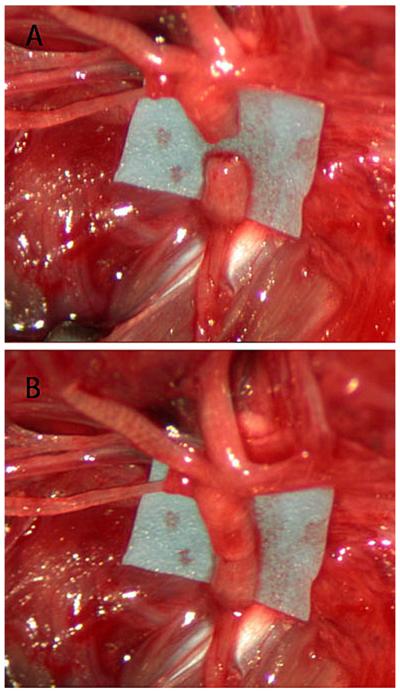
Normal vibrissal function was documented in all animals preoperatively. The rats were randomized into two groups of 8 animals. Main trunk facial nerve transection was performed in all cases; nerves were immediately repaired with one of two techniques: epineurial suture (ES) or fibrin adhesive coaptation (FAC), according to assigned group. The ES technique consisted of two epineurial 10-0 nylon sutures placed 180 degrees opposite each other. FACs were accomplished by approximating the severed nerve ends with micro forceps, pipet application of 20 μL of fibrin adhesive, and waiting for the adhesive to cure before releasing the transected nerve ends (Fig. 1). FA was made using a combination of fibrinogen and thrombin, both derived from rat plasma (Sigma-Aldrich, St. Louis, MO). Each neurorrhaphy was timed from the transection of the nerve until the last suture was completed (ES) or the nerve released after FAC. All animals were examined on postoperative day 1 to confirm absence of whisking on the operated side.
Fig. 1.
(A) The divided main trunk of the facial nerve with the pes anserinues superior to it. (B) Main facial nerve trunk after repair with fibrin adhesive.
Functional Testing
Functional whisking data were collected on a weekly basis for 15 weeks, using our previously described testing apparatus.8,11 Briefly, after animals were placed in the body restraint and head fixation apparatus, polyimide tubes (SWPT-045, SWPT-008, Small Parts Inc, Miami Lakes, FL) were used to mark the C-1 whisker. Whisking behavior was then monitored by laser micrometers (MetraLight, Santa Mateo, CA) connected to a data acquisition computer. Each data collection period lasted 5 minutes.
Data Analysis
Data were analyzed utilizing whisking software developed by Bermejo et al.6 This software calculates amplitude, velocity, and acceleration data for every vibrissal excursion in a 5-minute period. After all data were acquired, the three whisks with the greatest amplitude were identified for each animal, and then averaged. An independent, two sample, two-tailed Student's t test was used for data analysis, with a P <0.05 considered statistically significant. If fewer than three whisks were apparent, the animal's results were assigned a value of zero for data analysis purposes. Daily variation in whisking effort among rats was minimized by obtaining the ratio of the operated side whisk amplitudes to the control side values; this ratio was termed the "relative recovery."12,13
An independent, two sample, two-tailed Student's t test was used to analyze nerve repair times, with a P <0.05 considered statistically significant.
RESULTS
In all 16 animals, head fixation with subsequent facial nerve transection and repair were completed without complication. All animals demonstrated normal cage behavior, social interactions, and weight gain in the months following surgical intervention. One animal was noted to have minor eye irritation postoperatively; this was treated with ophthalmic bacitracin and resolved without apparent sequelae. One ES group animal was excluded from the study due to inability to condition to the testing apparatus. Additionally, over the study period, two animals from each group were withdrawn due to head fixation failure; their neurorrhaphy times were included in the analysis, although their functional recovery data were unavailable. One of the two excluded ES animals was able to provide whisking data until week 12, before it was withdrawn from the study due to head fixation failure; these data were included in our analysis. None of the four other excluded animals was able to provide a useful amount of data; thus, all whisking data for these animals were excluded from analysis entirely. This head fixation failure rate of 25% is consistent with rates reported in the literature.7
Both groups showed initial functional whisking recovery at postoperative day 21 (Fig. 2). The FAC group recovery did not match the functional recovery of the ES group until postoperative day 35, displaying 25% relative recovery amplitude using a one-tailed t test analysis (P <0.05) (Fig. 3). A 2 sample t test (assuming equal variances) for average relative recovery amplitude from week 3 through 15 showed no significant difference between groups (Fig. 4).
Fig. 2.
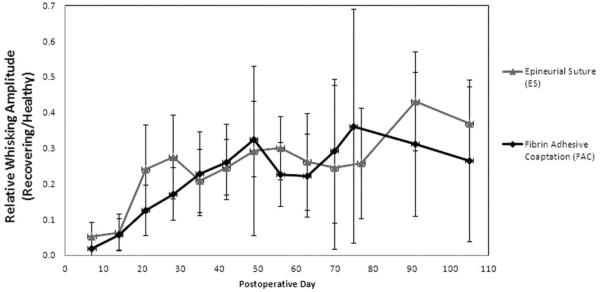
Whisking amplitude of epineurial suture (ES) and fibrin adhesive coaptation (FAC) groups plotted as recovering side amplitude divided by healthy side amplitude (i.e., relative recovery) over time. A value of 1 would represent symmetric whisking amplitude. Error bars indicate ±1 standard deviation.
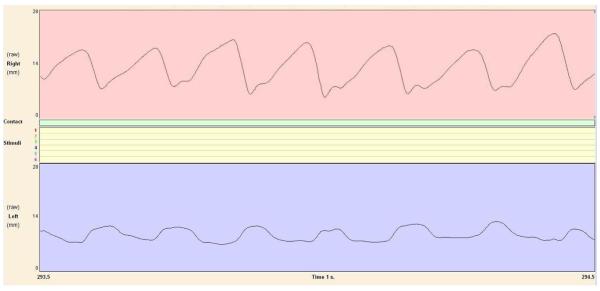
Fig. 3.
Screenshot of whisking data collection; top area depicts healthy-side of rodent face, whisking at an average of 75 degrees; bottom area depicts injured side of rodent face after fibrin adhesive (FA) facial nerve coaption, whisking an average of 18 degrees.
Fig. 4.
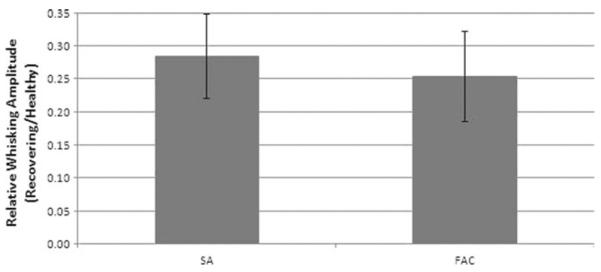
Graph demonstrating no significant difference (P <0.05) in relative recovery of whisking based on pooled data from weeks 3 to 15. Error bars indicate ± 1 standard deviation.
Measurement of operative times revealed that FAC procedures were faster than ES procedures; on average, there were statistically significant operative time differences using a two-tailed t test analysis (P <0.05) (Table I).
TABLE I.
Neurorrhaphy Times for Epineurial Suture and Fibrin Adhesive Coaptation Groups.
| Epineurial Suture (min) | Fibrin Adhesive Coaptation (min) | |
|---|---|---|
| 4.73 | 1.97 | |
| 3.58 | 1.12 | |
| 3.65 | 1.40 | |
| 3.95 | 0.80 | |
| 5.00 | 1.00 | |
| 3.27 | 0.98 | |
| 3.40 | 0.87 | |
| 3.55 | 1.03 | |
| Average | 3.89 | 1.15 |
| SD | 0.64 | 0.38 |
DISCUSSION
Our comparison of functional recovery demonstrated no statistically significant differences in whisking amplitude across weeks 3 to 15 of recovery between the FA and suture repair groups, following main trunk facial nerve transection with immediate repair. Despite the fact that numerous articles have been published on the subject of FA versus suture neurorrhaphy, the literature remains inconclusive, with evidence supporting each approach as the better method. In their 1993 review, Terris and Fee concluded that, "fibrin glue must be considered an inferior alternative to the gold standard of epineurial suture repair," after considering electrophysiological and histological evidence.14 Conversely, Suri et al. resolved 9 years later that there was no difference between FA and suture technique in the histological or walking track outcomes following sciatic nerve coaptation in rats.15 Inaloz et al. studied sciatic nerve repair with FA versus suture; their electromyography and histopathological results demonstrated that FA provided overall superior results, including less granulomatous inflammation at the site of neurorrhaphy.16 Because the epineurial sutures used in neurorrhaphy are permanent, they may cause a chronic foreign body reaction, adversely affecting healing.16,17 Martins et al. also investigated the re-approximation of the sciatic nerve with the use of FA, suture, or both; they found that neural repair with FA enhanced conditions for regeneration compared to that of suture alone.17
While there were no significant differences in whisking recovery parameters in the present report, the time required to perform FA neural coaptation was significantly shorter than that for traditional suture repair, which has important surgical implications. Reduction of operative time potentially benefits both patient and surgeon, particularly in cases involving more than one neurorrhaphy, such as facial nerve explorations or cross-face nerve grafting, in which the time savings are often multiplied. It has been shown that every minute under general anesthesia increases the risk of postoperative complications by 0.6%.18 Less time under general anesthesia has also been correlated directly with shortened length of hospital stay.18 From the surgeon's standpoint, procedures result in less fatigue if time spent at the operating microscope is reduced, especially when the neurorrhaphy comprises part of a microvascular free tissue transfer. Moreover, suture neurorrhaphy is technically challenging, and axonal contents are prone to herniate out of the epineurial sheath during passage of the needle, possibly hindering neural regeneration. Additionally, time in the operating room is expensive, approximately $1,100 per hour at our institution, making the savings from a single FA neural coaptation worth $50 in time alone. Anesthesia providers also bill in 15-minute increments, so faster neurorrhaphies may reduce the cost of care in this regard as well.
One potential advantage to suture neurorrhaphy is increased tensile strength of the repair,19 though nerve repair is ideally executed in a tensionless fashion and should not require high tensile strength. Equivalent functional recovery between ES and FAC groups in our study indicates that theoretically lower tensile strength after FA repair is not a dominant issue following tension-free neural repair under experimental conditions.
CONCLUSION
Our study suggests that the only statistically significant difference between traditional suture neurorrhaphy and FA neural coaptation was the time taken to complete the procedure. Given the reduced operative time required, and ease of application, FA maybe an acceptable alternative to suture neurorrhaphy for facial nerve repair.
Acknowledgments
This study was supported by the National Institutes of Health grant number RO1NS071067. The authors have no other funding, financial relationships, or conflicts of interest to disclose.
BIBLIOGRAPHY
- 1.Canonico S. The use of human fibrin glue in the surgical operations. Acta Biomed. 2003;74(suppl 2):21–25. [PubMed] [Google Scholar]
- 2.Dunn CJ, Goa KL. Fibrin sealant: a review of its use in surgery and endoscopy. Drugs. 1999;58:863–886. doi: 10.2165/00003495-199958050-00010. [DOI] [PubMed] [Google Scholar]
- 3.Sameem M, Wood TJ, Bain JR. A systematic review on the use of fibrin glue for peripheral nerve repair. Plast Reconstr Surg. 2011;127:2381–2390. doi: 10.1097/PRS.0b013e3182131cf5. [DOI] [PubMed] [Google Scholar]
- 4.Murray JA, Willins M, Mountain RE. A comparison of glue and a tube as an anastomotic agent to repair the divided buccal branch of the rat facial nerve. Clin Otolaryngol Allied Sci. 1994;19:190–192. doi: 10.1111/j.1365-2273.1994.tb01212.x. [DOI] [PubMed] [Google Scholar]
- 5.Farrag TY, Lehar M, Verhaegen P, Carson KA, Byrne PJ. Effect of platelet rich plasma and fibrin sealant on facial nerve regeneration in a rat model. Laryngoscope. 2007;117:157–165. doi: 10.1097/01.mlg.0000249726.98801.77. [DOI] [PubMed] [Google Scholar]
- 6.Bermejo R, Szwed M, Friedman W, Ahissar E, Zeigler HP. One whisker whisking: unit recording during conditioned whisking in rats. Somatosens Mot Res. 2004;21:183–187. doi: 10.1080/08990220400012430. [DOI] [PubMed] [Google Scholar]
- 7.Hadlock T, Kowaleski J, Mackinnon S, Heaton JT. A novel method of head fixation for the study of rodent facial function. Exp Neurol. 2007;205:279–282. doi: 10.1016/j.expneurol.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heaton JT, et al. A system for studying facial nerve function in rats through simultaneous bilateral monitoring of eyelid and whisker movements. J Neurosci Methods. 2008;171:197–206. doi: 10.1016/j.jneumeth.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henstrom D, et al. The convergence of facial nerve branches providing whisker pad motor supply in rats: implications for facial reanimation study. Muscle Nerve. 2012;45:692–697. doi: 10.1002/mus.23232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Semba K, Egger MD. The facial “motor” nerve of the rat: control of vibrissal movement and examination of motor and sensory components. J Comp Neurol. 1986;247:144–158. doi: 10.1002/cne.902470203. [DOI] [PubMed] [Google Scholar]
- 11.Hadlock T, et al. Functional assessments of the rodent facial nerve: a synkinesis model. Laryngoscope. 2008;118:1744–1749. doi: 10.1097/MLG.0b013e31817f5255. [DOI] [PubMed] [Google Scholar]
- 12.Bermejo R, Vyas A, Zeigler HP. Topography of rodent whisking—I. Two-dimensional monitoring of whisker movements. Somatosens Mot Res. 2002;19:34–346. doi: 10.1080/0899022021000037809. [DOI] [PubMed] [Google Scholar]
- 13.Bermejo R, Houben D, Zeigler HP. Optoelectronic monitoring of individual whisker movements in rats. J Neurosci Methods. 1998;83:89–96. doi: 10.1016/s0165-0270(98)00050-8. [DOI] [PubMed] [Google Scholar]
- 14.Terris DJ, Fee WE., Jr Current issues in nerve repair. Arch Otolaryngol Head Neck Surg. 1993;119:725–731. doi: 10.1001/archotol.1993.01880190021004. [DOI] [PubMed] [Google Scholar]
- 15.Suri A, Mehta VS, Sarkar C. Microneural anastomosis with fibrin glue: an experimental study. Neurol India. 2002;50:23–26. [PubMed] [Google Scholar]
- 16.Inaloz SS, et al. Comparison of microsuturing to the use of tissue adhesives in anastomosing sciatic nerve cuts in rats. Neurosurg Rev. 1997;20:250–258. doi: 10.1007/BF01105896. [DOI] [PubMed] [Google Scholar]
- 17.Martins RS, Siqueira MG, Da Silva CF, Plese JP. Overall assessment of regeneration in peripheral nerve lesion repair using fibrin glue, suture, or a combination of the 2 techniques in a rat model. Which is the ideal choice? Surg Neurol. 2005;64(suppl 1):S1, 10–16. doi: 10.1016/j.surneu.2005.04.022. discussion S11:16. [DOI] [PubMed] [Google Scholar]
- 18.Boruk M, Chernobilsky B, Rosenfeld RM, Har-El G. Age as a prognostic factor for complications of major head and neck surgery. Arch Otolaryngol Head Neck Surg. 2005;131:605–609. doi: 10.1001/archotol.131.7.605. [DOI] [PubMed] [Google Scholar]
- 19.Lin KL, et al. DuraSeal as a ligature in the anastomosis of rat sciatic nerve gap injury. J Surg Res. 2010;161:101–110. doi: 10.1016/j.jss.2008.10.020. [DOI] [PubMed] [Google Scholar]