Abstract
Background
Experimental animal work shows that prenatal stress has a persisting effect on the Hypothalamic-Pituitary-Adrenal (HPA) axis of offspring. The implications of these findings for human health and development are not yet clear.
Methods
The data are based on the ALSPAC cohort, a prospective longitudinal study of a community sample that has followed mothers and children from pregnancy. When the children were aged 15 years, diurnal cortisol samples were collected at wake-up, 30 minutes post-awakening and at afternoon and evening times on up to three consecutive days on n=889 adolescents. Diurnal cortisol was predicted from prenatal anxiety and depression, obstetric, life-style, socio-demographic, and postnatal covariates.
Results
Multilevel model analysis indicated that maternal prenatal anxiety was associated with a modest alteration of diurnal cortisol, indexed by a reduced cortisol awakening response and flatter diurnal slope. The effects were independent of psychosocial and obstetric covariates and measures of maternal postnatal anxiety; effects were similar for prenatal maternal depression. There was no association between adolescent cortisol and paternal prenatal anxiety.
Conclusions
There are small but persisting associations between maternal prenatal mood and diurnal cortisol in the child that persist into adolescence and may constitute a programming effect.
Keywords: prenatal anxiety, HPA axis, ALSPAC, cortisol, developmental programming
Research findings from experimental animal studies suggest that in utero exposures may have a lasting effect on the health and development of the offspring. One prominent model emerging from this work, which is now being translated in humans, is developmental programming, or the hypothesis that exposures early in development instigate an adaptive response of the organism that is carried forward with persisting effects on behavior and biology(1, 2). A leading paradigm in this research is prenatal maternal stress or anxiety. Numerous animal experiments show that, consistent with the programming hypothesis, experimentally induced prenatal stress has sizable and lasting effects on offspring fear, reproductive behavior, immunity, neurogenesis, and stress physiology(3, 4). Several research groups are now investigating the implications for human health. The current study, based on a large, community prospective longitudinal design, extends this work by examining the link between prenatal maternal mood (anxiety and depression) and adolescent diurnal cortisol pattern, a proposed mechanism in the developmental programming model with relevance to mental disorder and somatic health outcomes.
Prenatal maternal anxiety is linked with a wide range of outcomes in the child, including temperament emotionality(5, 6), reduced cognitive ability(7), adverse neuropsychological outcomes(8), altered sleep(9), and behavioral problems(10, 11), including schizophrenia(12). The findings appear robust, but questions remain about persistence past childhood and the mechanisms of effect. The current study addressed both of these limitations by examining the long-term connection between prenatal mood and a leading candidate mechanism for its effect on the child, via the HPA axis.
A leading biological model driving the work is that elevated levels of cortisol from the (anxious) mother may cross the placenta, perhaps by altering the barrier enzyme 11β-HSD2(13), to affect obstetric outcomes as well as fetal and child development via the influence on the developing HPA axis(14). Direct evidence for this model has been reported in animal studies(15), but human evidence is limited. Our earlier study, based on a subsample of 10 year-olds (n=74), showed that prenatal maternal anxiety predicted raised diurnal cortisol(16). Other studies link prenatal maternal anxiety or an index of fetal exposure to glucocorticoids (e.g., from amniotic fluid) with altered cortisol response (17-20).
The current study indexes adolescent HPA axis function from diurnal cortisol output, which provides an index of the total output of the system; this index has attracted considerable research attention (21-24). We assessed cortisol on four occasions within a day on three typical days in the adolescent’s life. Within this measurement model, variation in cortisol can be assessed according to initial wake-up, cortisol awakening response, and diurnal decline. What is not yet clear is which of these indicators is reliably altered by early exposure; each has been linked to stress exposure(22, 25), and these may not index independent effects. In addition to assessing which aspect of the diurnal rhythm is affected by prenatal maternal mood, the current study also extends prior work by examining the nature of the effect. To date, studies linking (early) stress exposure to alterations in HPA axis function report disparate effects, namely, both low basal cortisol and hypo-reactivity(26, 27) and hyper-reactivity(25, 28, 29). These discrepant findings may be explained by several factors, including age of assessment, age of onset and chronicity of exposure, severity of stress, and current behavior. Prenatal anxiety exposure, as a potential predictor of a programming effect, may be a special case of stress exposure; however, that does not lead to a clear prediction about the nature of the effect. Experimental animal work suggests that prenatal stress leads to hyper-activation of the HPA axis(30), but limited human studies of prenatal maternal anxiety or other stresses do not yet offer a consistent pattern(18, 31). The current study, which includes a large sample size, prospective longitudinal design from pregnancy to age 15 years, and detailed diurnal cortisol assessment allows us to test the hypothesis there is a persisting and particular effect of maternal prenatal anxiety or depression on diurnal cortisol rhythm in adolescence, and to clarify the nature of that effect.
Methods
Data for this study were obtained as part of the Avon Longitudinal Study of Parents and Children (ALSPAC), an ongoing population-based study designed to investigate the effects of a wide range of influences on the health and development of children(32); see http://www.bris.ac.uk/alspac. Pregnant women residing in the Avon area of south-west England who had an estimated date of delivery between April 1, 1991, and December 31, 1992, were invited to participate in the study. It was estimated that 85-90% of the eligible population participated. The study cohort consisted of 14,541 pregnancies and 13,988 children who were still alive at 12 months of age. Ethical approval for all measures was obtained from the ALSPAC Ethics and Law Committee and from Local Research Ethics Committees.
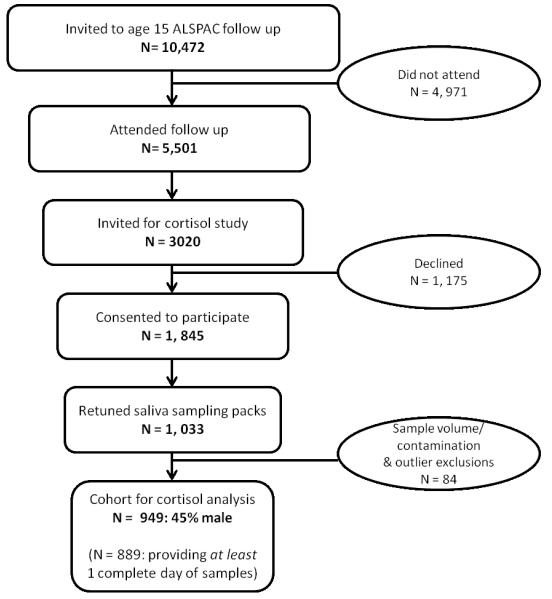
For the current study, we proposed to assess a random subsample of 1,000 of the adolescents who participated in the clinic visit scheduled when the adolescents were aged 15 years. Figure 1 shows the rate of participation from the entire cohort to the subset of children invited to participate in the cortisol sub-study. We then applied the following inclusion criteria: a) product of a singleton pregnancy, 2) gestation at birth >32 weeks, 3) birth weight >1500g, 4) no current exposure to steroid medication. In addition, from the cortisol data collected at age 15 years we applied the following exclusion criteria for particular samples (i.e., not individuals): 1) undetectable/contaminated; 2) values > 4 SD above the sample mean for that time point; 3) for the cortisol awakening response sample, a sample that was provided > 1 hour after waking. Following these exclusions a total of n=899 participants (403 males, 496 females; average age 15 years 4 months) remained for analyses.
Figure 1.
Consort Diagram for Cortisol Collection.
Procedure
Mothers completed measures of anxiety on two occasions in pregnancy (18 and 32 weeks gestation) and on multiple occasions in the postnatal period; we include in these analyses anxiety measures collected in pregnancy and when the children were 8 weeks, 8 months, and 81 months of age, the closest available assessment to the cortisol collection in the adolescents. Three postnatal assessments of anxiety were included as a control for a postnatal effect and to provide a strong test of whether or not there was a particular effect of prenatal anxiety on HPA axis function in adolescence. In addition to reporting on anxiety, mothers also completed measures of depressive symptoms and psychosocial and socio-demographic data (see below). Maternal data were collected via postal questionnaires. At age 15 years, adolescents were seen for an in-person assessment at the university. At the visit, a random subsample of adolescents was approached about the cortisol component of the study (Figure 1). Those agreeing to take part in the cortisol sub-study were given full instructions and provided with a saliva sampling pack that was returned to the laboratory in pre-paid envelopes and stored at −20C until assayed.
Measures
Maternal anxiety in pregnancy and the 8-week and 8-month postpartum period was measured using the Crown-Crisp experiential index (CCEI), a validated self-rating inventory(33); maternal anxiety at 85 months of age was based on the Spielberger State-Trait Anxiety Inventory(STAI)(34). The CCEI has been shown to correlate with the State (0.70) and Trait (0.76) subscales of the STAI. For supplementary analyses, we include paternal prenatal anxiety as a check on the programming hypothesis. Fathers’ completed that CCEI measure at a single time point in pregnancy corresponding to the 18-week maternal prenatal questionnaire assessment. Maternal depression at each occasion was assessed using the Edinburgh Postnatal Depression Scale (EPDS), a 10-item self-report questionnaire shown to be valid in and outside the postnatal period(35).
Cortisol has a strong diurnal pattern and so assessments must include multiple occasions within the day to capture this variation. Our assessment included four samples: wake-up (immediately after wake-up and before getting out of bed), 30 minutes following wake-up (to assess the cortisol awakening response), afternoon and before bedtime. These times were chosen because they index the diurnal pattern and could be collected with minimal inconvenience. Adolescents were instructed to provide samples on three typical school days. At the age 15-year clinic visit, adolescents were shown how to collect saliva by a research assistant and were given detailed instructions (e.g., avoiding brushing teeth prior to providing samples, an adequate number of salivettes, a time log for recording the day and time for each assessment, and pre-paid envelope in which to store the samples prior to sending to the laboratory. Adolescents were instructed to keep the envelope with used salivettes in the refrigerator until they were mailed to the laboratory. Phone calls were made to remind adolescents to return envelopes with saliva. All samples were assayed for saliva cortisol using a commercially available immuno-assay (Salimetrics, UK). Inter- and intra-assay variation were 7.9% and 8.9%, respectively.
Data quality of the cortisol was checked in several ways. All individual samples were visually inspected for contamination; time logs were also checked for completeness and correspondence to intended sampling times. Salivary assays were run in duplicate except for a small minority of cases with minimal volume. The raw data indicated the predicted strong diurnal pattern (Table 1). The numbers of adolescents who provided cortisol data on day1 was n=852; for day 2, n=624; for day 3, n=395. There was a comparatively high rate of return for at least one full day (Figure 1); analyses indicated few outliers (<1% for each time point).
Table 1.
Descriptive information for Adolescents in the Cortisol Sub-study (with at least 1 full day of cortisol data, n=889)
| Sub-study participants | ALSPAC cohort | F(df), | p | |
|---|---|---|---|---|
|
|
||||
| Maternal age | 28.52 (4.38) | 27.10 (4.99) | 65.05(1, 13038), | <0.001 |
| SES | 2.76 (1.04) | 2.95 (1.60) | 9.01(1, 9397), | <0.02 |
| Prenatal smoking | 0.83 (3.24) | 1.96 (5.00) | 42.92(1, 12224), | <0.001 |
| Prenatal alcohol use | 2.09 (4.34) | 1.69 (3.74) | 3.94(1, 6383), | <0.05 |
| Birth weight(g) | 3464 (490) | 3429 (507) | 5.81(1, 13038), | <0.05 |
| Gestational age(wks) | 39.60 (1.62) | 39.57 (1.58) | 0.53(1, 13038), | ns |
| Maternal anxiety | ||||
| 32 wks gestation | 4.55 (3.47) | 5.15 (3.60) | 22.61(1, 10955), | <0.001 |
| 8 wks postnatal | 3.17 (3.04) | 3.42 (3.34) | 5.44(1, 10805), | <0.05 |
| Percent female | 56% | 48% | 13.72(1, 9875), | <0.001 |
| Cortisol | values (nmoL/l) | Time | ||
|
|
||||
| Waking | 7.19 (4.12) | 7:03am | ||
| Waking + 30’ | 11.54 (6.04) | 7:41am | ||
| Afternoon | 2.32 (1.98) | 4:43pm | ||
| Evening | 1.12 (1.55) | 9:45pm | ||
Note: N’s vary across measure because of missing data. Average cortisol values and times are for day 1.
Age 15-year covariates were adolescent body mass index (BMI), contraceptive and psychotropic medication use, and current smoking and alcohol use (variation in these variables was quite skewed so we included any current use as a covariate in analyses). Puberty was not included as a covariate because, e.g., the vast majority (>97%) of girls were post-menarche. Also considered were obstetric, prenatal life-style and psychosocial risks, including smoking in pregnancy (number of reported cigarettes per day at 18-weeks gestation), alcohol use in pregnancy (number of units drunk in the week prior to the 18 week gestation assessment), birth weight, and gestational age. We also include a composite measure of socio-economic status from available parental education and occupation data.
Data analysis
We first report descriptive data and preliminary analyses assessing the representativeness of the subsample to the larger cohort. Following general practice, cortisol values were log transformed for analyses testing study hypotheses. The data have a 3-level structure: occasions (Level 1), nested within day (level 2), nested within person (level 3). Total variance in cortisol is partitioned into within-day variance (i.e., diurnal variation), between-day variance, and between-person variance. Our focus is on between-person variance, i.e., why adolescents differ from one another. Three distinct between-person parameters are estimated for cortisol using this measurement approach: 1) the initial or wake-up value, 2) cortisol awakening response (CAR), and 3) diurnal decline in across the day, i.e., the slope (see below). We did not center the cortisol data so that we could interpret the effect of predictors on the initial value. We focus in the main analyses on prenatal anxiety measured at 32 weeks gestation because of its role in predicting behavioral outcomes in previous analyses with this sample; we note briefly in the supplementary analyses section the results with prenatal anxiety at 18 weeks gestation and prenatal depression.
Covariates (see below) are included as a control for alternative explanations and because they account for bias in estimates that may derive from missing data. In addition, we report findings from a model that used multiple imputation as a strategy for managing missing data. This procedure involves the creation of multiple data sets where the missing values in each data set are replaced with plausible values derived from observed data. The model is then fitted to each complete data set in the usual way; multiple sets of model results (n=10 datasets) are combined to produce estimates and standard errors that reflect the uncertainty in the imputation process(36). The imputation program within SPSS (version 19) was used and data were imputed at the occasion, day and person levels. Newgard and Haukoos(37) note that when interaction terms are central to an investigation, as in the current analysis, they must be considered in the imputation phase. It is ideal to impute separately at high and low levels of a particular moderator variable, which arises if the imputation model has a single categorical moderator variable. That is not so in the current case, however. An alternative approach is to compute interaction terms among continuous variables but this approach is less efficient and can lead to biased estimates because multiple imputation assumes a multivariate normal distribution and suggests that any missing variable is predictable based on a linear regression of predictors without interactions. That is, estimates from multiple imputation may lead to biased estimates if the model includes multiple interactions, as in the current case. Accordingly, the main study results are based on the full available data but we present estimates from the imputation for comparison. Finally, we include several sets of supplementary analyses to test the robustness of the prenatal effect.
Results
Attrition analyses compared the sample on whom we had at least one full day of cortisol compared to those who were originally part of the ALSPAC cohort but did not participate in the research at age 15 years (Table 1). Analyses indicated small but, given the large sample size, significant differences. Variables were included as covariates if they had been previously shown to predict the cortisol, if there was a sizable empirical literature, and if it was associated with bias in the subsample. The final list of covariates included adolescent sex, birth weight, gestational age, socioeconomic status, body mass index, medication use, current smoking and drinking.
The base model shows cortisol as a function of wake-up time, time since wake-up (i.e., diurnal slope), a quadratic term for time since waking to account for a non-linear decline, and a dummy variable for the cortisol awakening response to distinguish the second occasion of measurement from the other measurements. The equation for the base model is as follows:
Cortisol sample i on day j for child k, cortisolijk, is equal to the intercept, β0ijk or the grand mean cortisol that is permitted to vary randomly at the sample, e0ijk, day u0jk, and person v0k levels, plus the fixed effect of time since wake, β1k, time since wake squared, β2, wake-up time, β3, and CAR, β4. In addition to the random effect associated with the intercept, the effects of time since wake (i.e., slope), and CAR, were also permitted to vary randomly at the person level, indicated by v1k, v4k, respectively. The residual variation or random effects are summarized in variance terms: σ2 ε0 , σ2 μ0 , and σ2 v0 estimate the within-day, within-individual and between-individual variation in the intercept, respectively, and σ2 v1 and σ2 v4, reflect the individual variation for the effects of time since waking and the individual variation for the cortisol awakening response, respectively. At the person level, variation in the intercept, slope, and CAR were permitted to co-vary. Covariates and hypothesized predictors were added to the base model as fixed effects. Fixed effects that are approximately twice the size of their standard errors are statistically significant at p<0.05; significance of model comparisons is conducted by comparing the number of parameter differences between the models evaluated with the change in loglikelihood.
The base model included wake-up time, linear and quadratic terms for time since waking or diurnal slope (because the diurnal decline is non-linear), and CAR; the estimates were wake-up time (−0.03, SE=.01), time since waking (−0.61, SE=.01) time since waking2 (0.02, SE=.01), and CAR (0.43, SE=.01). These results indicate, respectively, that cortisol values were lower with later waking times, that there is a substantial decline in cortisol values with time since waking which flattened over time (quadratic effect), and that the cortisol values collected at 30 minutes post awakening (i.e., the CAR) were significantly greater than other values. In this base model, most of the variance was at the occasion level (76%), with the remainder at the person level (24%); between-day level variance was negligible.
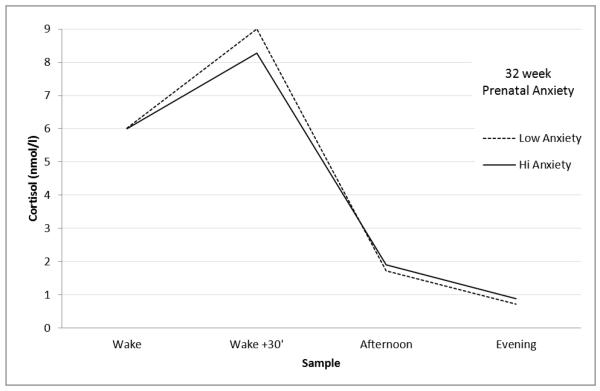
The prediction model is provided in Table 2. We include estimates [SE] only for adolescent sex (females had significantly higher values) and the main parameters of interest, namely prenatal and postnatal anxiety measures; the full model results, which include parameters for all covariates and random effects coefficients are presented in Supplemental Table S1. Prenatal anxiety was associated with initial cortisol, after adjusting for covariates. Two significant separate effects were detected. Higher prenatal anxiety was associated with a lower CAR (estimate −0.042 [.021]); extrapolating back to the original scale, this translates to a reduction of approximately 1nmol/l cortisol. Additionally, prenatal anxiety was associated with a flatter diurnal decline (estimate −0.028 [.013]). These effects are illustrated in Figure 2, which shows the diurnal pattern of adolescents who had mothers who did (+1 SD) and did not (−1 SD) experience anxiety in pregnancy, adjusting for covariates. Additional analyses (not reported) indicated that the prenatal anxiety effect was not moderated by adolescent sex; neither was there evidence that the prenatal maternal anxiety effect was significantly moderated by postnatal maternal anxiety. When the model was re-analyzed using multiple imputation, somewhat weaker effects were obtained. Specifically, for the prenatal anxiety X CAR effect, the estimate (−0.024) translated to, extrapolating back to the original scale, 0.98nmol/l cortisol; the estimate for diurnal decline in the imputed model was −0.012.
Table 2.
Prenatal anxiety predicts diurnal cortisol rhythm in adolescence.
| Fixed effects | Estimate | SE | p |
|---|---|---|---|
| Wake time | −.043 | .009 | .001 |
| Cortisol awakening response (CAR) | .380 | .025 | .001 |
| Time Since Wake | −.587 | .015 | .001 |
| Time Since wake2 | .014 | .007 | .05 |
| Female | .107 | .025 | .001 |
| Female X CAR | .123 | .035 | .001 |
| Female X Time since wake | −.031 | .020 | ns |
| Prenatal anxiety (32wks) | .020 | .015 | ns |
| Prenatal anxiety X CAR | −.042 | .021 | .05 |
| Prenatal anxiety X Time Since wake | −.028 | .013 | .05 |
| Prenatal anxiety X Time Since wake2 | .006 | .008 | ns |
| Postnatal maternal anxiety (8 wks) | −.020 | .015 | ns |
| Postnatal maternal anxiety X CAR | .021 | .021 | ns |
| Postnatal maternal anxiety X Time Since wake | .012 | .013 | ns |
| Postnatal maternal anxiety X Time Since wake2 | .012 | .008 | ns |
| Maternal anxiety (81 mos) | −.005 | .015 | ns |
| Maternal anxiety X CAR | −.032 | .021 | ns |
| Maternal anxiety X Time Since wake | −.001 | .013 | ns |
| Maternal anxiety X Time Since wake2 | .006 | .008 | ns |
Note. Also included in the model were gestational age, birth weight, social class, adolescent BMI, smoking and alcohol use. See Supplemental Table S1 for full model results.
Figure 2.
Diurnal cortisol values for adolescents whose mothers were anxious (+1 SD) and not anxious (−1 SD) at 32 weeks gestation, controlling for covariates.
Supplementary analyses
Several sets of supplementary analyses were carried out to examine the nature of the prenatal prediction and the robustness of the maternal prenatal, i.e., in utero, effect. First, the correlation between maternal prenatal anxiety at 18 and 32 weeks gestation was r= 0.66; the moderately large overlap diminishes the likelihood of finding distinct effects of prenatal anxiety at either time. In fact, analyses including prenatal anxiety at 18 weeks instead of 32 weeks (and identical predictors in the model in Table 2) indicated very similar effects. For example, the coefficient for prenatal anxiety at 18 weeks on the cortisol awakening response was −0.039 (SE 0.021) compared with −0.042 for prenatal anxiety at 32 weeks. Supplementary analyses (not tabled) showed that the effects of maternal prenatal anxiety at 18 and 32 weeks gestation overlapped; neither measure of maternal prenatal anxiety was significantly associated with adolescent diurnal cortisol rhythm when both measures were in the model. Second, analyses based on a parallel model to that given in Table 2 indicated that paternal prenatal anxiety was not significantly associated with any indicator of adolescent diurnal cortisol rhythm (not tabled). Moreover, after adjusting for paternal prenatal anxiety, maternal prenatal anxiety continued to significantly predict a reduced cortisol awakening response and a flatter diurnal decline (p’s <.05), suggesting no confound of paternal prenatal anxiety.
A final set of supplementary analyses considered depression rather than anxiety, an important additional consideration given that the overlap between constructs in pregnancy was > r =.70. Analyses for maternal depression, based on a parallel model to that given in Table 2 for anxiety, indicated that maternal prenatal depression at 32 weeks gestation was significantly associated with a decrease in adolescent CAR (estimate −0.052 [SE 0.021]), comparable in effect size to that found for prenatal anxiety. Follow-up analyses (not tabled) indicated that the prenatal depression and anxiety effects on CAR were not independent of each other.
Discussion
Findings from this study provide the strongest evidence to date that prenatal mood has a small but persisting effect on the HPA axis in human development. The findings help to substantiate and further translate a leading model for understanding how early stress exposures, including in utero, may have a lasting effect on offspring behavior and biology. Adolescents whose mothers reported elevated levels of prenatal anxiety exhibited a diminished CAR and flatter diurnal rhythm. The effect size was small, but significant after controlling multiple confounds, including measures of obstetric, life-style, and psychosocial risk, postnatal anxiety, and current indicators of adolescent health and behavior. Evidence that the association may reflect an in utero programming mechanism (e.g., rather than simple genetic transmission effect) was substantiated by the absence of any confound (or any effect at all) from paternal prenatal anxiety. We did not demonstrate that the prediction is specific to maternal prenatal anxiety, as comparable predictions were found for prenatal depression, implying a broader risk phenotype; similarly, the prediction was not particular to late pregnancy but was comparable in mid-pregnancy, likely because of the stability of individual differences in prenatal anxiety.
The key observation is that maternal prenatal mood influences the cortisol diurnal variation of her child 15 years subsequent to exposure, and independent of paternal and postnatal factors. The mechanisms by which this occurs are not yet known and are likely complex. Salivary cortisol is an endpoint measurement of a complex system with many levels of control. The prefrontal cortex, hippocampus and amygdala influence HPA activity(38) and are also sensitive to the effects of early life stress(39). Similarly, GR-mediated negative feedback, which modulates HPA axis activity, is regulated by the early environment through epigenetic modifications, transcriptional and posttranslational events(40).
A novel finding in the current study is the link between prenatal anxiety and depression and a blunted CAR. The very few previous studies assessing prenatal mood and diurnal cortisol have not explicitly distinguished the CAR from the diurnal profile, and there is potentially important variation in the age of the children assessed(16). There may be a developmental shift in which adrenocortical counter-regulation is induced following an extended period of HPA hyperactivity, a notion which has some support from studies of early trauma(41) and PTSD in adulthood(42) (in the current sample, there were too few cases with diurnal cortisol at age 10 and 15 years to test this directly). There is also accumulating evidence that early stress exposure may be associated with elevated or hyper-activation of the HPA axis that, over time, leads to adrenocortical counter-regulation and hypo-activation(43), but see(44). More broadly, there is accumulating evidence that chronic stress is associated with blunted response to acute laboratory stress(45) and may reflect a pathophysiological process that may be as clinically important as hyper-activation of the HPA axis. That possibility derives from the notion of allostatic load and especially the proposal that optimal functioning is characterized by the ability to mount a physiological response to environmental demand. A reduced capacity to respond to environmental demands, which may be indexed in this study by reduced CAR and flattened diurnal rhythm, may therefore constitute a potential risk for metabolic disease or psychiatric disorder(46); this possibility requires further follow-up study. On the other hand, other investigators have noted that elevated CAR response has been linked with risk for subsequent depression and related conditions (22) . One model with which these findings are congruent is the chronic stress paradigm, which emphasizes that prolonged exposure to stress is associated with a down-regulation of the HPA axis. Not all studies of early stress exposure report lower or dampened HPA axis function, such as CAR(47), but the persisting effects of stress exposure leading to dampened cortisol reactivity has been widely reported(45, 48) and is considered a significant potential marker for psychopathology and allostatic load.
There was a sizable sex difference in the diurnal profile (elevated in females in overall levels and CAR), which is consistent with other reports. However, there was no sex moderation of the link between prenatal anxiety exposure and cortisol diurnal profile. The implication is that the mechanisms accounting for altered HPA axis function in a post-pubertal sample, which would likely include the relative roles of estrogen and progesterone in girls and testosterone in boys, are not themselves altered by prenatal anxiety. Importantly, the absence of a sex moderation effect is consistent with human studies of the impact of prenatal stress on a range of behavioral and biological outcomes – but is not consistent with experimental animal work(49).
Several limitations of the work should be noted. The first is that the assessment of HPA axis was limited to diurnal cortisol. Other aspects of HPA axis function, such as upstream release of CRH and ACTH, sensitivity to glucocorticoids, reactivity to laboratory stresses, and the negative feedback loop that characterizes the system were not directly assessed. The research literature is not yet clear about whether or not findings from diurnal assessments of cortisol are comparable to what has been observed in response to an acute stress, e.g.,(45). A second limitation is that we did not have access to maternal physiology, and particularly maternal HPA axis function, in pregnancy. Prenatal maternal anxiety or depression may alter a range of biological systems in the child, and an impact on cortisol, as an index of the HPA axis, might be direct or indirect. Third, we had limited data on quality of childrearing and other potentially relevant factors such as traumatic stress in childhood that may modify the prenatal effects observed here; experimental animal data using cross-fostering designs and human data(50) indicate that early caregiving may be an important moderating factor. Fourth, mild discrepancies were observed between the raw and imputed results, which may result from sample attrition or may be confounded by the number of interactions in the model; also error of measurement in cortisol would have diluted the predicted effects(51). Finally, several possible causal mechanisms, including genetic and epigenetic factors, were not included in the study. These limitations are offset by several strengths of the study, including a 15-year follow-up period, large community sampling frame, inclusion of paternal prenatal anxiety (at one time point), detailed assessment of diurnal cortisol, and a conservative analytic approach that included multiple different kinds of covariates.
Given the small effect size reported in this study, it may be premature to derive direct and immediate clinical applications. Nonetheless, the findings fit into a broader and rapidly developing clinical literature linking prenatal mood to several different kinds of outcomes in the child, as cited above. Collectively, these findings provide robust, reliable evidence that there may be something particular about prenatal mood on the developing fetus and child, and that there is now sufficient evidence to consider how and if interventions in pregnancy prevent adverse outcomes in the child.
Supplementary Material
Acknowledgements
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses. The UK Medical Research Council (Grant ref: 74882) the Wellcome Trust (Grant ref: 076467) and the University of Bristol provide core support for ALSPAC. This publication is the work of the authors and T.G. O’Connor, K.J. O’Donnell, and V. Glover will serve as guarantors for the contents of this paper. This research was specifically funded by NIH grant R01 MH073842.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Conflict of interest: The authors declare no financial or other conflicts of interest.
The funding source played no role in the study design, writing, or analysis of the paper and results. Authors
TG O’Connor contributed to the study design, obtained funding, authored the first and revised drafts, and contributed to the data analyses.
K O’Donnell contributed to the data management and conducted all laboratory work and contributed to the writing and analyses.
V Glover contributed to the study design and writing.
J Jenkins contributed to the data analyses and writing.
D Browne contributed to the data analyses and writing.
Y Ben Shlomo contributed to the study design and writing.
J Golding contributed to the study design and writing.
All authors have contributed and approved the final manuscript.
References
- 1.Barker DJ. Fetal origins of cardiovascular disease. Ann Med. 1999;31(Suppl 1):3–6. [PubMed] [Google Scholar]
- 2.Gluckman PD, Hanson MA. Developmental origins of disease paradigm: a mechanistic and evolutionary perspective. Pediatr Res. 2004;56(3):311–7. doi: 10.1203/01.PDR.0000135998.08025.FB. [DOI] [PubMed] [Google Scholar]
- 3.Coe CL, Kramer M, Czeh B, Gould E, Reeves AJ, Kirschbaum C, et al. Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile rhesus monkeys. Biol Psychiatry. 2003;54(10):1025–34. doi: 10.1016/s0006-3223(03)00698-x. [DOI] [PubMed] [Google Scholar]
- 4.Maccari S, Darnaudery M, Morley-Fletcher S, Zuena AR, Cinque C, Van Reeth O. Prenatal stress and long-term consequences: implications of glucocorticoid hormones. Neurosci Biobehav Rev. 2003;27(1-2):119–27. doi: 10.1016/s0149-7634(03)00014-9. [DOI] [PubMed] [Google Scholar]
- 5.Glynn LM, Davis EP, Schetter CD, Chicz-Demet A, Hobel CJ, Sandman CA. Postnatal maternal cortisol levels predict temperament in healthy breastfed infants. Early Hum Dev. 2007;83(10):675–81. doi: 10.1016/j.earlhumdev.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Bergman K, Sarkar P, O’Connor TG, Modi N, Glover V. Maternal stress during pregnancy predicts cognitive ability and fearfulness in infancy. J Am Acad Child Adolesc Psychiatry. 2007;46(11):1454–63. doi: 10.1097/chi.0b013e31814a62f6. [DOI] [PubMed] [Google Scholar]
- 7.Davis EP, Sandman CA. The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Dev. 2010;81(1):131–48. doi: 10.1111/j.1467-8624.2009.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glover V, O’Connor TG, Heron J, Golding J. Antenatal maternal anxiety is linked with atypical handedness in the child. Early Hum Dev. 2004;79(2):107–18. doi: 10.1016/j.earlhumdev.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 9.O’Connor TG, Caprariello P, Blackmore ER, Gregory AM, Glover V, Fleming P. Prenatal mood disturbance predicts sleep problems in infancy and toddlerhood. Early Hum Dev. 2007;83(7):451–8. doi: 10.1016/j.earlhumdev.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Connor TG, Heron J, Golding J, Beveridge M, Glover V. Maternal antenatal anxiety and children’s behavioural/emotional problems at 4 years. Report from the Avon Longitudinal Study of Parents and Children. Br J Psychiatry. 2002;180:502–8. doi: 10.1192/bjp.180.6.502. [DOI] [PubMed] [Google Scholar]
- 11.Van den Bergh BR, Marcoen A. High antenatal maternal anxiety is related to ADHD symptoms, externalizing problems, and anxiety in 8- and 9-year-olds. Child Dev. 2004;75(4):1085–97. doi: 10.1111/j.1467-8624.2004.00727.x. [DOI] [PubMed] [Google Scholar]
- 12.Khashan AS, Abel KM, McNamee R, Pedersen MG, Webb RT, Baker PN, et al. Higher risk of offspring schizophrenia following antenatal maternal exposure to severe adverse life events. Archives of general psychiatry. 2008;65(2):146–52. doi: 10.1001/archgenpsychiatry.2007.20. [DOI] [PubMed] [Google Scholar]
- 13.O’Donnell KJ, Bugge Jensen A, Freeman L, Khalife N, O’Connor TG, Glover V. Maternal prenatal anxiety and downregulation of placental 11beta-HSD2. Psychoneuroendocrinology. 2012;37(6):818–26. doi: 10.1016/j.psyneuen.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Swanson JM, Entringer S, Buss C, Wadhwa PD. Developmental origins of health and disease: environmental exposures. Semin Reprod Med. 2009;27(5):391–402. doi: 10.1055/s-0029-1237427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneider ML, Roughton EC, Koehler AJ, Lubach GR. Growth and development following prenatal stress exposure in primates: an examination of ontogenetic vulnerability. Child Dev. 1999;70(2):263–74. doi: 10.1111/1467-8624.00020. [DOI] [PubMed] [Google Scholar]
- 16.O’Connor TG, Ben-Shlomo Y, Heron J, Golding J, Adams D, Glover V. Prenatal anxiety predicts individual differences in cortisol in pre-adolescent children. Biol Psychiatry. 2005;58(3):211–7. doi: 10.1016/j.biopsych.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 17.Saridjan NS, Huizink AC, Koetsier JA, Jaddoe VW, Mackenbach JP, Hofman A, et al. Do social disadvantage and early family adversity affect the diurnal cortisol rhythm in infants? The Generation R Study. Horm Behav. 2010;57(2):247–54. doi: 10.1016/j.yhbeh.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Davis EP, Glynn LM, Waffarn F, Sandman CA. Prenatal maternal stress programs infant stress regulation. J Child Psychol Psychiatry. 2010 doi: 10.1111/j.1469-7610.2010.02314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gutteling BM, de Weerth C, Buitelaar JK. Maternal prenatal stress and 4-6 year old children’s salivary cortisol concentrations pre- and post-vaccination. Stress. 2004;7(4):257–60. doi: 10.1080/10253890500044521. [DOI] [PubMed] [Google Scholar]
- 20.O’Connor TG, Bergman K, Sarkar P, Glover V. Prenatal cortisol exposure predicts infant cortisol response to acute stress. Developmental Psychobiology. 2012 doi: 10.1002/dev.21007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Essex MJ, Shirtcliff EA, Burk LR, Ruttle PL, Klein MH, Slattery MJ, et al. Influence of early life stress on later hypothalamic-pituitary-adrenal axis functioning and its covariation with mental health symptoms: a study of the allostatic process from childhood into adolescence. Dev Psychopathol. 2011;23(4):1039–58. doi: 10.1017/S0954579411000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adam EK, Doane LD, Zinbarg RE, Mineka S, Craske MG, Griffith JW. Prospective prediction of major depressive disorder from cortisol awakening responses in adolescence. Psychoneuroendocrinology. 2010;35(6):921–31. doi: 10.1016/j.psyneuen.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cicchetti D, Rogosch FA, Gunnar MR, Toth SL. The differential impacts of early physical and sexual abuse and internalizing problems on daytime cortisol rhythm in school-aged children. Child development. 2010;81(1):252–69. doi: 10.1111/j.1467-8624.2009.01393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gunnar MR, Quevedo KM. Early care experiences and HPA axis regulation in children: a mechanism for later trauma vulnerability. Progress in brain research. 2008;167:137–49. doi: 10.1016/S0079-6123(07)67010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halligan SL, Herbert J, Goodyer IM, Murray L. Exposure to postnatal depression predicts elevated cortisol in adolescent offspring. Biological psychiatry. 2004;55(4):376–81. doi: 10.1016/j.biopsych.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 26.Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25(1):1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- 27.Goldman-Mellor S, Hamer M, Steptoe A. Early-life stress and recurrent psychological distress over the lifecourse predict divergent cortisol reactivity patterns in adulthood. Psychoneuroendocrinology. 2012 doi: 10.1016/j.psyneuen.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 28.Rao U, Hammen C, Ortiz LR, Chen LA, Poland RE. Effects of early and recent adverse experiences on adrenal response to psychosocial stress in depressed adolescents. Biological psychiatry. 2008;64(6):521–6. doi: 10.1016/j.biopsych.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, et al. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284(5):592–7. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- 30.Henry C, Kabbaj M, Simon H, Le Moal M, Maccari S. Prenatal stress increases the hypothalamo-pituitary-adrenal axis response in young and adult rats. J Neuroendocrinol. 1994;6(3):341–5. doi: 10.1111/j.1365-2826.1994.tb00591.x. [DOI] [PubMed] [Google Scholar]
- 31.Entringer S, Kumsta R, Hellhammer DH, Wadhwa PD, Wust S. Prenatal exposure to maternal psychosocial stress and HPA axis regulation in young adults. Horm Behav. 2009;55(2):292–8. doi: 10.1016/j.yhbeh.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Golding J. The Avon Longitudinal Study of Parents and Children (ALSPAC)--study design and collaborative opportunities. European journal of endocrinology / European Federation of Endocrine Societies. 2004;151(Suppl 3):U119–23. doi: 10.1530/eje.0.151u119. [DOI] [PubMed] [Google Scholar]
- 33.Birtchnell J, Evans C, Kennard J. The total score of the Crown-Crisp Experiential Index: a useful and valid measure of psychoneurotic pathology. The British journal of medical psychology. 1988;61(Pt 3):255–66. doi: 10.1111/j.2044-8341.1988.tb02787.x. [DOI] [PubMed] [Google Scholar]
- 34.Spielberger CD, Gorusch I, Lushene RE. Manual for the State-Trait Anxiety Inventory. Consulting Psychologist Press; Palo Alto, CA: 1983. [Google Scholar]
- 35.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–6. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 36.Little RJ, Rubin DB. Statistical analysis with missing data. 2nd edition Wiley; New York: 2002. [Google Scholar]
- 37.Newgard CD, Haukoos JS. Advanced statistics: missing data in clinical research--part 2: multiple imputation. Academic emergency medicine : official journal of the Society for Academic Emergency Medicine. 2007;14(7):669–78. doi: 10.1197/j.aem.2006.11.038. [DOI] [PubMed] [Google Scholar]
- 38.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10(6):434–45. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 39.Lupien SJ, Parent S, Evans AC, Tremblay RE, Zelazo PD, Corbo V, et al. Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(34):14324–9. doi: 10.1073/pnas.1105371108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonte B, Szyf M, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature neuroscience. 2009;12(3):342–8. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am J Psychiatry. 2001;158(4):575–81. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- 42.Luo H, Hu X, Liu X, Ma X, Guo W, Qiu C, et al. Hair Cortisol Level as a Biomarker for Altered Hypothalamic-Pituitary-Adrenal Activity in Female Adolescents with Posttraumatic Stress Disorder After the 2008 Wenchuan Earthquake. Biological psychiatry. 2012 doi: 10.1016/j.biopsych.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 43.Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007;133(1):25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- 44.Hankin BL, Badanes LS, Abela JR, Watamura SE. Hypothalamic-pituitary-adrenal axis dysregulation in dysphoric children and adolescents: cortisol reactivity to psychosocial stress from preschool through middle adolescence. Biological psychiatry. 2010;68(5):484–90. doi: 10.1016/j.biopsych.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lovallo WR, Farag NH, Sorocco KH, Cohoon AJ, Vincent AS. Lifetime adversity leads to blunted stress axis reactivity: studies from the Oklahoma Family Health Patterns Project. Biological psychiatry. 2012;71(4):344–9. doi: 10.1016/j.biopsych.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Papadopoulos AS, Cleare AJ. Hypothalamic-pituitary-adrenal axis dysfunction in chronic fatigue syndrome. Nature reviews Endocrinology. 2012;8(1):22–32. doi: 10.1038/nrendo.2011.153. [DOI] [PubMed] [Google Scholar]
- 47.Gustafsson PE, Janlert U, Theorell T, Hammarstrom A. Life-course socioeconomic trajectories and diurnal cortisol regulation in adulthood. Psychoneuroendocrinology. 2010;35(4):613–23. doi: 10.1016/j.psyneuen.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 48.Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: potential indices of risk in human development. Development and Psychopathology. 2001;13(3):515–38. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- 49.Zuena AR, Mairesse J, Casolini P, Cinque C, Alema GS, Morley-Fletcher S, et al. Prenatal restraint stress generates two distinct behavioral and neurochemical profiles in male and female rats. PLoS One. 2008;3(5):e2170. doi: 10.1371/journal.pone.0002170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bergman K, Sarkar P, Glover V, O’Connor TG. Maternal prenatal cortisol and infant cognitive development: moderation by infant-mother attachment. Biol Psychiatry. 2010;67(11):1026–32. doi: 10.1016/j.biopsych.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Halpern CT, Whitsel EA, Wagner B, Harris KM. Challenges of measuring diurnal cortisol concentrations in a large population-based field study. Psychoneuroendocrinology. 2012;37(4):499–508. doi: 10.1016/j.psyneuen.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.