Abstract
Adult stem cells are multipotent and persist in small numbers in adult tissues throughout the lifespan of an organism. Unlike differentiated cells, adult stem cells are intrinsically resistant to senescence. It is unclear how adult stem cells in solid organs respond to oncogenic stimulation and whether these cells have a role in tumor initiation. We report here that expression of BRAFV600E in human neural crest progenitor cells (hNCPCs) did not induce growth arrest as seen in human melanocytes, but instead, increased their cell proliferation capacity. These cells (hNCPCsV600E) acquired anchorage-independent growth ability and were weakly tumorigenic in vivo. Unlike in human melanocytes, BRAFV600E expression in hNCPCs did not induce p16INK4a expression. BRAFV600E induced elevated expression of CDK2, CDK4, MITF and EST1/2 protein in hNCPCs, and also induced melanocytic differentiation of these cells. Furthermore, overexpression of MITF in hNCPCsV600E dramatically increased their tumorigenicity and resulted in fully transformed tumor cells. These findings indicate that hNCPCs are susceptible to BRAFV600E-induced transformation, and MITF potentiates the oncogenic effect of BRAFV600E in these progenitor cells. These results suggest that the hNCPCs are potential targets for BRAFV600E-induced melanocytic tumor formation.
Keywords: melanoma, neural crest, transformation, melanocytes, BRAFV600E
INTRODUCTION
Neural crest progenitor cells (NCPCs) persist in the adult in a number of differentiated tissues, such as dermis, hair follicles, peripheral nerve, gut and the heart.1–5 Cell fate mapping studies using transgenic mice confirm that neural crest cells are present in the bulge and dermal papilla of hair follicles.6 Hair follicles are a niche for NCPCs in the skin.1,5,7,8 We and others have shown that adult NCPCs can be isolated from rodent and human hair follicles5,7–10 and they can give rise to multiple cell types, including melanocytes, Schwann cells and smooth muscle cells.5,8,10 Hair follicle-derived mouse neural crest stem cells have similar gene expression profiles as embryonic neural crest stem cells.11
A number of cancers, such as neuroblastoma, medullary thyroid cancer, Ewing sarcoma and melanoma, are known to be of neural crest origin. Adult stem cells including NCPCs have characteristics that are similar to malignant cells.12,13 For example, adult stem cells have remarkable longevity and self-renewal capacities and NCPCs in particular can migrate to distant body parts where they frequently form new tissues and biological structures.14 Long-lived stem cells have inherent characteristics that inhibit their own senescence and allow these cells to survive under stress.15,16 It is possible that these changes in adult stem cells circumvent protective mechanisms (such as oncogene-induced senescence) that would normally prevent cancer development. Perhaps these properties of adult stem cells mean that fewer steps are required to transform these cells into cancer cells, compared with more differentiated cells. Indeed, it has been reported that the mutation of a single gene in hematopoietic stem cells can lead to increased stem cell self-renewal and transformation in vivo.17 Compared with hematopoietic stem cells, adult stem cells from solid organs are technically more difficult to isolate, and it is not clear how these cells respond to oncogene-induced transformation.
Melanocytic nevi often grow for a period of time, then stop growth and persist locally.18 A majority of nevi harbor BRAF mutation. The most common BRAF mutation (90%) results in a valine-to-glutamic acid change at amino acid 600 (V600E).19 It is well documented that expression of BRAFV600E in foreskin primary human melanocytes induced cell cycle arrest and senescence;20 and it is unknown that how BRAFV600E induces initial growth of human nevi. On the other hand, expression of BRAFV600E under MITF or tyrosinase promoter induces nevus-like growth of melanocytes in transgenic zebrafish or mouse.21,22 We hypothesize that the timing of mutant BRAF acquisition during neural crest lineage development determines its function in melanocytic lesion formation. In this work, we test this hypothesis by investigating the role of BRAFV600E in the transformation of human skin NCPCs.
RESULTS
BRAFV600E increases cell proliferation capacity of hNCPCs
We previously showed that human NCPCs (hNCPCs) can be isolated from hair follicles by culturing disassociated follicular epithelium in human embryonic stem cell (hESC) culture conditions.8,23 Human NCPCs were infected with BRAFV600E/p Babe viral vectors and expanded in blasticidin containing Tu 2% medium (2% serum without other growth factors). We performed RT–PCR analysis using primers specific for BRAFV600E,20 and confirmed the expression of BRAFV600E in hNCPCs (hNCPCsV600E, data not shown). To assay mitogen-activated protein kinase (MAPK) pathway activation, we performed western blotting analysis and the results showed that phosphorylated MEK and ERK levels were increased in hNCPCsV600E, compared with controls (Figure 1a).
Figure 1.
BRAFV600E increases hNCPC proliferative capacity. (a) BRAFV600E activates ERK pathway in hNCPCs. Western blotting analysis was used to measure MEK and ERK activation. There was no significant change in total MEK or p42/44 expression, but phosphorylated p42/44 and MEK were increased in hNCPCsV600E. The blot represents a typical result from three independent experiments. (b) BRAFV600E fails to induce oncogene-induced senescence (OIS) in hNCPCs. Cellular senescence was measured by β-galactosidase activity. Only rare cells in passage-8 control and hNCPCsV600E cultures showed β-galactosidase activity. Thirty percent of passage-16 control hNCPCs showed features of senescence, and 3% of passage-31 hNCPCsV600E had features of senescence; n=3. *indicates P<0.01 compared with control. (c) BRAFV600E increases hNCPC proliferation capacity. Cell proliferation was measured by MTT assays. Passage-8 control and hNCPCsV600E showed similar proliferative rates. Passage-16 control hNCPCs grew slower in the Tu 2% medium, while passage-31 hNCPCsV600E proliferated at a similar rate as passage-8 hNCPCsV600E; n=3. *Indicates P<0.05 comparing passage-16 control cells with passage-31 hNCPCsV600E. **Indicates P<0.01 comparing passage-16 control cells with passage-31 hNCPCsV600E. (d) Cell cycle analysis. Cell cycle analysis was performed by FACS using passage-16 control cells and passage-31 hNCPCsV600E; hNCPCs isolated from three different individuals were tested and a representative flow chart is shown.
As sustained expression of BRAFV600E-induced senescence in primary human melanocytes and fibroblasts,20 we analyzed senescent responses to BRAFV600E in hNCPCs. Less than 1% of the cells were positive for senescence-associated acidic β-galactosidase activity (SA-β-Gal) in passage-8 control hNCPCs or hNCPCsV600E, indicating that BRAFV600E does not induce senescence in hNCPCs. At passage-16, 32% of control hNCPCs showed SA-β-Gal activity in Tu 2% medium. In contrast, <5% of hNCPCsV600E at passage-31 were positive for SA-β-Gal activity (Figure 1b). These results support that BRAFV600E does not induce senescence in hNCPCs. We then compared the proliferation rate of hNCPCsV600E with control hNCPCs. There was no significant difference in early passage cells (Figure 1c). However, hNCPCs at passage-16 grew significantly slower than hNCPCs at passage-8 in the Tu 2% medium, while hNCPCsV600E at passage-31 still grew at a similar rate as hNCPCsV600E at passage-8 (Figure 1c). This result was further supported by cell cycle analysis of passage-31 hNCPCsV600E and passage-16 control hNCPCs: 23.77% of hNCPCsV600E were in S phase and 12.23% were in G2–M. In contrast, 11.38% of hNCPCs were in S1 phase and 4.98% were in G2–M phase (Figure 1d). Although hNCPCs grew in the hESC medium for over 8 months and survived 70 passages (data not shown), the hNCPCs stopped proliferating in the Tu 2% medium after just 20 passages, in contrast hNCPCsV600E continued to grow after 50 passages in the Tu 2% medium (data not shown).
To demonstrate that the effect of BRAFV600E on hNCPCs depends on functional BRAFV600E, we inhibited the function of BRAFV600E in hNCPCsV600E using either a BRAFV600E-specific inhibitor (PLX-4720) or a small interfering RNA to BRAFV600E as previously described.24 Western blotting analysis showed that PLX-4720 inhibited ERK phosphorylation (Figure 2a). We have previous shown that this small interfering RNA can efficiently knock down BRAFV600E expression.24 Inhibition of BRAFV600E by either PLX-4720 or BRAFV600E small interfering RNA resulted in significantly decreased proliferation of hNCPCsV600E (Figures 2b and c), further supporting that BRAFV600E increases proliferative capacity of hNCPCs.
Figure 2.

BRAFV600E dependent proliferation of hNCPCsV600E. (a) Effect of BRAFV600E inhibitor (PLX-4720). Western blotting analysis was performed for ERK phosphorylation in the presence of PLX-4720 (1 and 10 μM). Shown are typical results from two independent experiments. (b) hNCPCsV600E proliferation in the presence of PLX-4720. *Indicates P<0.05 compared with controls (n=3). (c) hNCPCsV600E proliferation after BRAFV600E knockdown. BRAFV600E-specific small interfering RNA (siRNA) was used to knockdown BRAFV600E expression. *Indicates P<0.05 comparing with control (n=3).
Human NCPCsV600E acquire transformed phenotype in vitro
Anchorage-independent growth is an important feature for cell transformation in vitro. We found that hNCPCsV600E formed colonies in soft agar, although the number of colonies was significantly less than colonies formed by WM793 human melanoma cells (Figures 3a and c). In contrast, control hNCPCs did not form any colonies in the soft agar (Figure 3a). In soft agar, the hNCPCsV600E exhibited enlarged nuclei with increased nucleus/cytoplasm ratios (Figure 3b, H&E stain). In addition, many cells in these colonies were positive for Ki67, indicating that they are proliferating (Figure 3b, Ki67 stain). These cells expressed MITF and tyrosinase but not cytokeratins (Figure 3b).
Figure 3.
hNCPCsV600E acquire anchorage-independent growth capacity in vitro. (a) hNCPCsV600E acquired anchorage-independent growth in soft agar. Control hNCPCs were not able to form colonies in soft agar. hNCPCsV600E and WM793 melanoma cells formed colonies in soft agar. Insets, colony size at × 100. Bar: 2 mm; n=5. (b) Morphology and lineage marker expression by hNCPCsV600E. hNCPCsV600E showed enlarged nuclei in soft agar (H&E). A majority of cells were positive for Ki67 (Ki67 staining). Many cells were positive for MITF (MITF staining) and tyrosinase (tyrosinase staining). These cells were negative for cytokeratins (PanCK staining). Bar: 50 μm. (c) Density of colonies in soft agar assay. The number of colonies were counted and averaged. **Indicates P<0.01 comparing with control.
Human NCPCsV600E are weakly tumorigenic in vivo
To determine if hNCPCsV600E are tumorigenic in vivo, 2×106 control cells or hNCPCsV600E were mixed with Matrigel and injected s.c. into the flanks of NON-severe combined immunodeficiency mice. Tumor volume was then monitored for 8 weeks, after which, the animals were killed and tissues were preserved for analysis. Mice injected with wild-type hNCPCs did not form any tumors, while mice injected with hNCPCsV600E formed palpable masses after 2 weeks (Figure 4a). In these mice, tumor growth reached a plateau after 6 weeks (Figure 4b). Histology revealed that the tumor cells in the xenografts were confined to the areas containing Matrigel and they did not invade into surrounding tissues (Figure 4c). The tumor cells exhibited transformed cell morphology with enlarged nuclei (Figure 4d), and a few tumor cells were positive for Ki67 staining (Figure 4e). The tumor cells were positive for S-100 (Supplementary Figure 1). There was no evidence of metastatic tumors in any of these mice.
Figure 4.
hNCPCsV600E are weakly tumorigenic in vivo. Control and hNCPCsV600E were injected s.c. into non-obese diabetic (NOD)/severe combined immunodeficiency (SCID) mice; n=8. Mice injected with control hNCPCs did not form tumors. (a) hNCPCsV600E formed small xenografts in mice. (b) Tumor growth curve. The tumors became palpable after 2 weeks and continued to grow for 6 weeks after which they reached a growth plateau. (c) Histology of xenografts, the tumor was circumscribed. Bar indicates 1 mm. (d) Cytology of tumor cells in xenografts. The tumor cells showed enlarged nuclei. Bar indicates 50 μm. (e) Ki67 staining, a few tumor cells were positive for Ki67, arrows point to the nuclei of positive cells. (f) Array CGH analysis. Array CGH were performed on two pairs of hNCPCs before and after BRAFV600E infection. Data from four samples were compiled and no significant alterations were observed.
Copy number aberrations in hNCPCsV600E
To rule out genetic alterations in the donor tissues and study whether BRAFV600E induces additional genetic alterations in hNCPCs, we performed array comparative genomic hybridization (CGH) on hNCPCs. This analysis was performed on two hNCPC samples from different donors before and after BRAFV600E viral infection. The two pairs of hNCPC samples showed few DNA copy number changes; the changes detected were concentrated within telomere regions (Figure 4f). Despite these sporadic telomeric changes, there were no consistent gene copy number changes within these samples, suggesting that the few genetic alterations observed in these cells are likely the result of cell culture-induced alterations and not BRAFV600E expression.25
Mechanisms underlying BRAFV600E-induced transformation and differentiation of hNCPCs
We performed western blotting analysis to measure the expression of Cyclin-dependent kinases 2 and 4 (CDK2 and CDK4) in hNCPCsV600E. The expression of both CDK2 and CDK4 proteins was elevated compared with the expression in control cells (Figure 5a). BRAFV600E expression in human melanocytes has been shown to induce cell cycle arrest, which is accompanied by the induction of p16INK4a.20 Therefore, we next examined p16INK4a expression in both human foreskin melanocytes (FOM) and hNCPCsV600E. Passage 4 FOM cells expressed little p16INK4a, but at passage 12 or following BRAFV600E infection, p16INK4a expression increased significantly (Figure 5b). In contrast, hNCPCs expressed little p16INK4a, similar to WM451 melanoma cells. Expression of BRAFV600E in hNCPCs did not induce p16INK4a expression, even after 40 passages (Figure 5b). Similarly, the hNCPCsV600E in the xenografts also expressed little p16INK4a (Supplementary Figure 2). The expression of phosphorylated RB protein increased in hNCPCs after BRAFV600E expression (Figure 5c), whereas no significant changes were detected in the expression levels of p21 and p27 (data not shown). Pten expression level did not change in hNCPCsV600E (Figure 5c). Moreover, no change was observed in telomerase activity in these cells (data not shown).
Figure 5.
BRAFV600E induces cell cycle progression and melanocytic differentiation of hNCPCs. (a) CDK expression. Western blotting analysis of CDK2 and CDK4 expression before and after BRAFV600E infection. Typical results from three independent experiments. (b) p16INK4a expression in melanocytes and hNCPCs. Western blotting analysis for p16INK4a was performed on passage 4 or 12 FOM and FOM after BRAFV600E infection as well as passage 19 and passage 40 hNCPCsV600E. WM451 melanoma cells were used as controls. Shown are typical results from three independent experiments. (c) Protein expression. Western blotting analyses were performed before and after BRAFV600E infection for phosphorylated RB, Ets1/2, MITF and PTEN. Shown are typical results from three independent experiments. (d) MITF gene expression. Quantitative RT–PCR was performed to measure MITF mRNA level before and after BRAFV600E infection. *Indicates P<0.05 comparing with control. **indicates P<0.01 comparing to control. (e) MITF promoter-luciferase assay. Luciferase assays were performed to measure BRAFV600E-induced activation of the MITF promoter (n=3). (f) Tyrosinase gene expression. Quantitative RT–PCR was performed to measure tyrosinase message level before and after BRAFV600E infection. *Indicates P<0.05 comparing with control.
Ets1/2 is a transcription factor involved in pigmented cell differentiation and is a downstream target of MAPK/ERK activation.26 We found that Ets1/2 expression level increased in hNCPCsV600E. Our western blotting and RT–PCR analysis showed that MITF protein and mRNA levels were elevated in hNCPCsV600E cells (Figures 5c and d). Meanwhile, luciferase assays revealed that BRAFV600E increased MITF promoter-luciferase activity (Figure 5e). hNCPCsV600E expressed higher level of tyrosinase mRNA compared with the control cells (Figure 5f), supporting that BRAFV600E induces melanocytic differentiation of hNCPCs.
MITF cooperates with BRAFV600E to increase proliferative capacity of hNCPCs in vitro
It has been shown that oncogenic BRAFV600E upregulates MITF transcription through transcription factor BRN2 (N-Oct3).27 We found that BRN2 was expressed in hNCPCs and its expression level was significantly increased after BRAFV600E lentivirus infection (Figure 6a). As MITF is a known oncogene in melanoma and regulates the expression of key cell cycle regulatory proteins such as CDK2 and CDK4, we studied whether MITF cooperates with BRAFV600E in hNCPC transformation. To this end, we infected hNCPCs and hNCPCsV600E with viruses carrying control or MITF vectors (these cells were designated as hNCPCsMITF and hNCPCsV600E+MITF). MITF overexpression was confirmed by quantitative RT–PCR (data not shown) and western blotting analysis (Figure 6b). Cell proliferation assays showed that MITF alone had little effect on hNCPC proliferation (data not shown). When we performed soft agar assays, we found that hNCPCsV600E+MITF were able to form significantly more colonies in the soft agar than hNCPCsV600E. Control cells or hNCPCsMITF did not form colonies in soft agar (Figures 6c and d).
Figure 6.
MITF cooperates with BRAFV600E to induce transformation in vitro. (a) BRN2 expression. Quantitative RT–PCR was performed to measure BRN2 mRNA level before and after BRAFV600E infection. *Indicates P<0.05 comparing with control. (b) MITF expression. Western blotting analysis of MITF in hNCPCs infected with control, MITF, BRAFV600E or MITF plus BRAFV600E vectors. (c) Soft agar assay. Soft agar assays using hNCPCs infected with control vectors, MITF, BRAFV600E or MITF plus BRAFV600E. Shown are typical results from three independent experiments. (d) Quantification of colony formation in soft agar. The number of well-formed colonies was counted. *Indicates P<0.05 comparing with control and #indicates P<0.05 comparing with hNCPCsV600E.
MITF overexpression fully transforms hNCPCsV600Ein vivo
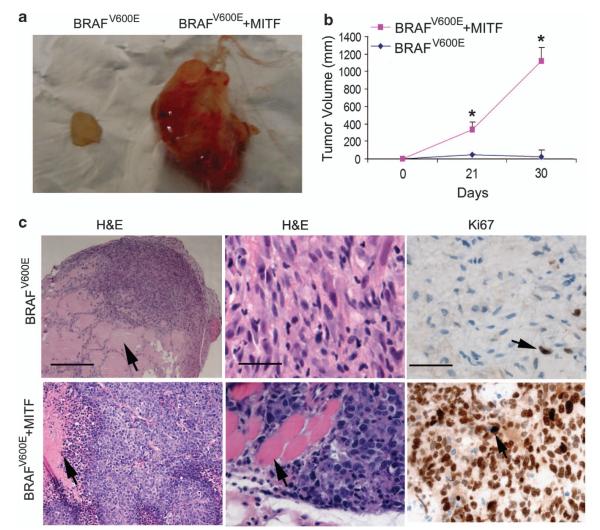
To study the tumorigenicity of hNCPCsV600E+MITFin vivo, we injected hNCPCsV600E and hNCPCsV600E+MITF (2×106) s.c. into the flanks of non-obese diabetic.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice. Tumor volume was then monitored weekly once tumors became apparent. Mice injected with hNCPCsV600E formed small s.c. tumor nodules, whereas mice injected with hNCPCsV600E+MITF formed significantly larger tumors (Figure 7a). Because of the rapid growth of the hNCPCsV600E+MITF xenografts, these mice had to be killed 5 weeks post-injection (Figure 7b). Upon euthanasia, necropsy was performed and all the major organs were collected for histology. Histologically, hNCPCsV600E were confined to areas containing Matrigel, whereas hNCPCsV600E+MITF formed expansile dermal tumors with focal tumor necrosis, had high grade atypical cytological features similar to human malignant melanoma cells and invaded into surrounding skeletal muscle (Figure 7c). Most of the hNCPCsV600E+MITF in the xenografts were positive for Ki67 staining. Nevertheless, none of these mice developed metastasis, suggesting that these cells do not possess metastatic capacity.
Figure 7.
MITF cooperates with BRAFV600E to transform hNCPCs in vivo. hNCPCsV600E and hNCPCsV600E+MITF (2 million) were injected s.c. into NSG mice; n=5. These mice were followed for 5 weeks. (a) Mice injected with hNCPCsV600E formed small xenografts, whereas mice injected with hNCPCsV600E+MITF formed larger tumors. (b) Tumor growth curve. Tumors in mice injected with hNCPCsV600E+MITF grew rapidly in vivo and these mice had to be killed after 5 weeks. (c) Histology and Ki67 staining of tumor cells in the mouse xenografts. In mice injected with hNCPCsV600E, tumors were confined to areas with Matrigel (arrow points to the Matrigel), whereas mice injected with hNCPCsV600E+MITF had tumor morphologies similar to human melanoma tumors, with some of the tumor cells infiltrating skeletal muscle (arrow points to the skeletal muscle). The bars in the left panels indicate 150 μm, bars in the middle and right panels indicate 30 μm. Ki67 stains shows most of the hNCPCsV600E+MITF were proliferative, whereas fewer hNCPCsV600E were proliferative.
DISCUSSION
In this study, our findings support that skin NCPCs are susceptible to BRAFV600E-induced transformation. Specifically, we found that BRAFV600E expression did not induce growth arrest in skin hNCPCs, in contrast to what occurs in either melanocytes or fibroblasts.20 Rather, BRAFV600E expression in hNCPCs induced anchorage-independent growth and weak tumorigenecity in vivo. Our studies suggest that lack of p16INK4a induction and inactivation of RB in hNCPCs have a key role in bypassing BRAFV600E-induced senescence. BRAFV600E also induces the expression of MITF, which contributes to melanocytic differentiation of hNCPCs. MITF potentiates the transforming effect of BRAFV600E in hNCPCs. These results suggest that BRAFV600E mutation occurring in NCPCs results in early transformation, whereas occurring in mature melanocytes results in cellular senescence.
Adult stem cells are normally located in a niche microenvironment that usually protects them from oncogenic insults.28 NCPCs in the skin are concentrated in the hair bulge5,6,10 or dermal papilla,1 well-defined stem cell niches.29 It has been shown that hematopoietic stem cells can be transformed by inactivation of a single gene, such as Fbxw7.30 Genetic mutations in normal hematopoietic stem cells may give rise to tumor stem cells and lead to leukemia.31,32 It is controversial whether adult stem cells in solid organs are susceptible to transformation. It has been shown that human adult stem cells can spontaneously transform after long-term culture in vitro.33,34 Other studies have revealed that bone marrow-derived mesenchymal stem cells do not undergo transformation after long-term culture35 and neural stem cells are resistant to Myc and Ras oncogene-induced transformation.36 Our results indicate that skin NCPCs are susceptible to BRAFV600E-induced transformation, however, additional genetic alteration, such as MITF overexpression, are necessary to induce fully malignant phenotypes.
Oncogene-induced senescence, a paradoxical growth arrest that occurs after aberrant activation of oncogenes, is recognized as a major barrier to tumor development.37–41 Melanocytic nevi (moles), frequently harbor BRAFV600E, yet only a minority progress to malignant melanomas.19 Overexpression of BRAFV600E in cultured human melanocytes induces oncogene-induced senescence, and senescence is a common feature seen in nevi.20 We and others have previously shown that a minor population of human melanocytes with elevated levels of nestin or BrdU-retaining capacity survives and proliferates for months in vitro after BRAFV600E lentivirus infection,42,43 suggesting that melanocytes with features of progenitor cells are less susceptible to BRAFV600E-induced senescence. Our current study further demonstrates that BRAFV600E expression in hNCPCs does not induce senescence and but results in early transformation of these cells. Nevertheless, the tumorigenecity is rather weak in vivo. These data demonstrate that activation of BRAFV600E in hNCSCs results in different outcome as that in human melanocytes.
p16INK4a is a critical cell cycle regulator and its expression is elevated in replicative senescence. p16INK4a induction is a critical component of BRAFV600E-induced senescence in human melanocytes.20 p16INK4a is known to inhibits CDK4- and CDK6-mediated phosphorylation of pRB, resulting in cell growth arrest.44 Our results confirmed that expression of p16INK4a increases with passages in cultured human FOM and BRAFV600E also induces expression of p16INK4a in these cells. However, we found that hNCPCs express little p16INK4a and p16INK4a expression is not significantly increased after BRAFV600E expression in vitro or in vivo. The lack of p16INK4a induction in hNCPCs after BRAFV600E expression is associated with increased CDK2 and CDK4 expression, as well as inactivation of RB. These data support that intrinsic stem cell characters of hNCPCs have a critical role in bypassing BRAFV600E-induced senescence.
MITF is a transcription factor that has a pivotal role in the melanocyte differentiation, proliferation and transformation; and it is a lineage survival oncogene that is often amplified in malignant melanoma.45 MITF is known to upregulate CDK2 and CDK4, as well as other survival factors, such as BCL2.45,46 It has been shown that nevi with BRAF mutations have elevated MITF expression.47 Wellbrock et al.27 also reported that BRAFV600E, but not wild-type BRAF, upregulate MITF transcription in the presence of BRN2. In this study, we found that hNCPCs express BRN2 and BRAFV600E increases MITF expression in hNCPCs. To study the interaction between MITF and mutant BRAF, we overexpressed MITF in hNCPCsV600E. hNCPCsV600E+MITF displayed features of fully transformed tumor cells, such as severe cytological atypia, tumorigenicity and capacity of invading surrounding tissues. MITF has been shown to be an anti-proliferative transcription factor able to induce a G1 cell-cycle arrest that is dependent on Mitf-mediated activation of the p21Cip1 in melanocytes48 and BRAFV600E is a senescence inducer in adult melanocytes.20 However, MITF and BRAFV600E co-expression confers factor-independent growth in hTERT/CDK4(R24C)/p53DD melanocytes.45 Our data demonstrated that MITF and BRAFV600E co-expression fully transforms hNCPCs. These data suggest that function of MITF and BRAFV600E depends on the recipient cell proliferation or differentiation state. Despite the dramatic increase of tumorigenicity, hNCPCsV600E+MITF were not capable of metastasis, indicating additional genetic or epigenetic alterations are required for tumor metastasis.
Although the role of adult stem cells in solid cancer initiation is yet to be fully defined, our findings underscore crucial difference between adult stem cells and differentiated cells in response to oncogenic stimulus. Our results indicate that hNCPCs are susceptible to the transforming effects of BRAFV600E, and suggest that skin NCPCs are a potent target of mutant BRAF-induced melanocytic tumor formation.
MATERIALS AND METHODS
Plasmids and reagents
We used the pBABE-puro retroviral backbone (courtesy of M Herlyn; The Wistar Institute, Philadelphia, PA, USA) to express human BRAFV600E as previously described.43 Production of retrovirus was achieved as previously described.43 The myc-tagged BRAFV600E cDNA in the pEFP expression vector was kindly provided by Richard Marais from the Institute of Cancer Research, London, UK; and the MITF promoter-luciferase vector was kindly provided by S Carreira from Marie Curie Research Institute, UK. The QCXIP-MITF and QCXIP vectors were kindly provided by Feng Liu from the University of California, Irvine, Orange, CA, USA. All chemicals were purchased from Sigma-Aldrich (St Louis, MO, USA).
Human NCSC isolation and viral infection
Hair follicles were plucked from dispase-treated fetal (Advanced Bioscience Resources, Alameda, CA, USA) or adult scalp tissue (Cooperative Human Tissue Network). The protocol was approved by the University of Pennsylvania Institutional Review Board. The hair follicle epithelium was disassociated into single-cell suspensions using trypsin as previously described.8 Disassociated follicular epithelium was cultured in conditioned hESC medium,49 and hNCPCs grew in this medium as both spheroid aggregates and attached cells and reached confluence after 3–4 weeks.
The spheroids were enzymatically-dissociated into single cells before plating onto tissue culture-grade plastics, coated with 10 ng/ml fibronectin for transfection. BRAFV600E/pBabe, empty pBabe, GFP control, QCXIP-MITF or QCXIP control vectors were used to infect hNCPCs. After infection, BRAFV600E/pBabe infected hNCPCs (hNCPCsV600E) were selected in Tu 2% medium (MCDB153/L15 medium (v/v: 4/1) supplemented with 2% fetal bovine serum, insulin (5 units/ml), CaCl2 (2 mmol/l), 100 U/ml penicillin and 100 mg/ml streptomycin) with 2 μg/ml Blasticidin. hNCPCsV600E primary cell cultures were established from five fetal scalp tissues, and one adult scalp tissue for comparison. Human NCPCs derived from adult scalp tissue showed similar responses to mutant BRAF as fetal hNCPCs.
Immunoblotting, Immunocytochemistry and immunohistochemistry
Western blotting was done as described previously.50 Membranes were probed with antibodies against phosphorylated-MAPK, pMEK, MAPK, MEK, CDK2, CDK4, pRB, Ets1/2, PTEN, p16ink4a (Cell Signaling Technology Inc, Danvers, MA, USA), MITF or β-actin (Sigma Chemicals, St Louis, MO, USA). Blots were probed with horseradish peroxidase-conjugated secondary antibodies (Amersham, Piscataway, NJ, USA) and subjected to enhanced chemiluminescence (Amersham). For immunocytochemistry, hNCPCsV600E or melanoma cells were plated on glass chamber slides until they reached 80% confluency. The cells were fixed with 4% paraformaldehyde in phosphate buffer for 20 min and washed. Cells were then permeablized with 0.2% Triton X100 for 10 min. The cells were then blocked with 10% normal goat serum and then incubated with antibodies against Ki67 (1:50, Dako, Carpinteria, CA, USA), MITF (1:200; NeoMarkers, Fremont, CA, USA), tyrosinase (1:75; Novocastra Laboratories, Newcastle upon Tyne, UK) or PanCK (1:25; Novocastra Laboratories) for 1 h at room temperature or 4 °C overnight. The cells were then washed and incubated with secondary antibodies. For immunohistochemistry, tissues were fixed in 10% formalin overnight and embedded in paraffin. Tissue sections were incubated with antibody against Ki67 (1:50, Dako). Appropriate positive and negative controls were used for each antibody staining.
RNA isolation and quantitative PCR
Total RNA was prepared using the RNeasy kit (Qiagen, Valencia, CA, USA), and cDNAs were prepared using SuperScript First-Strand Synthesis system (Invitrogen, Carlsbad, CA, USA) for RT–PCR according to the manufacture’s instruction. RT–PCR analysis was performed using the primers specific for BRAFV600E, MITF and tyrosinase as described previously.20 β-actin was used as a control.
Luciferase assay
293T cells were seeded in 24-well plates, and transient transfections were performed on the following day using 1.5 μl TurboFectin 8.0 Transfection Reagent (OriGene, Rockville, MD, USA) and MITF promoter-luciferase vectors with or without BRAFV600E vectors in a 50-μl final volume. pRLTK (Renilla) was co-transfected with the plasmids to control transfection efficiency. 24 h after transfection, cells were washed with ice cold 1× phosphate-buffered saline (PBS) and cell extracts were prepared and luciferase activity was measured according to the manufacturer’s instructions (Promega, Madison, WI, USA).
WST-1 Cell Proliferation assay
Cell proliferation assay was performed according to the manufacture’s instructions. Briefly, cultured cells were washed with PBS and suspended to a final concentration of 1× 105/ml in assay medium. Aliquots (50 μl) of cell suspensions were dispensed into 96-well plates. The plates were incubated at 37 °C for 24 h in a humidified CO2 incubator. After the incubation period, WST-1 reagent was added to the cell culture medium (10μl in 100 μl media) and incubated at 37°C for 20 min. Plates were shaken vigorously on an orbital shaker for 1 min, and absorbance was measured at 450 nm using a 96-well spectrophotomer plate reader. Experiments were carried out in triplicate.
Cellular senescence assay
Cellular senescence is characterized by the appearance of senescence-associated β-galactosidase activity in vitro. The cellular senescence assay was performed using a Cellular Senescence Assay Kit (Chemicon, Temecula, CA, USA). Briefly, cells were cultured in 35 mm dishes overnight and then washed once with PBS (pH7.2), incubated with 1× fixing solution for 10–15 min and washed twice with PBS. Cells were then stained in 1× SA-β-gal detection solution at 37 °C overnight. The detection solution was removed and cells were washed twice with PBS, and blue-stained cells were counted under light microscopy.
Soft agar colony formation assay
Five to ten thousand control or BRAFV600E-infected hNCPCs were suspended in 3 ml 1.8% (w/v) BactoAgar solution containing MCDB with 20% fetal bovine serum. The mixtures were overlaid onto a 3.3% (w/v) BactoAgar solution in six-well dishes. On the following day, 2ml of MCDB supplemented with 10% fetal bovine serum was added. Colonies were counted under a microscope after 15 days. Colony forming efficiency was calculated as: the number of colonies × 100 divided by the number of cells plated.
Matrigel-GFR invasion assay
Twenty-four-well plates with precoated growth factor reduced Matrigel (Matrigel-GFR) and chambers (BD Biosciences, San Jose, CA, USA) were prepared according to the manufacturer’s instructions. The lower well of the chamber was filled with MCDB medium and 10% serum. Cell suspensions (1×105 cells) were added to the upper chamber with no growth factors. The plates were incubated for 24 h at 37 °C, non invasive cells were removed from the filter top and the filter was fixed in 1% formaldehyde in PBS. A dilution of the cell samples placed in a 96-well plate served as the loading control. Invading cells and loading controls were stained with 1% crystal violet. Filters were washed in PBS, solubilized in 2% SDS overnight and optical density was determined at 570nm using a spectrophotometer. Mean±s.d. values were calculated from three repeated observations in each of four separate experiments.
Array CGH
CGH was performed using the ‘1Mb-array’ platform developed at the University of Pennsylvania.51 For hybridization, 1 μg of test DNA and 1 μg of sex-match pooled normal human DNA (obtained from a set of 10 healthy female or male volunteers) were labeled with either Cy3-dCTP or Cy5-dCTP (Amersham Biosciences, Little Chalfont, UK) incorporated by random priming (Bioprime Labeling Kit, Invitrogen). The reaction was then cleaned using the Qiagen MiniElute PCR kit (Qiagen, Valencia, CA, USA). Equal amounts of test and reference DNA (1 μg each) were mixed and precipitated with Cot1-DNA, 3 M NaOAc (pH 7.0) and ethanol. Arrays were hybridized with labeled probes in 50% deionized formamide, 2× SSC, 2% SDS, 10% dextran sulfate and 100 μg/μl of yeast tRNA for 72 h at 37 °C in a moist chamber on a slowly rocking table. Slides were washed and arrays were scanned on a GenePix 4000B dual scanner (Axon Instruments, Downingtown, PA, USA).
Data analysis
Fluorescent data from hybridization images were processed and analyzed with Gene Pix Pro 5.0 (Axon Instruments, Union City, CA, USA) to obtain the log2ratios (tumor/reference) of each slide. aCGH data were processed through print-tip loess normalization, using the DNMAD application52 that also allowed us to merge and filter clones between replicates in a slide and in the dye-swap experiment. We filtered out inconsistent replicates (those ones with a log2 ratio distant to the median log2 ratio of the replicates >0.3) and those clones that did not have available data in >70% of the cases. For visualization and detection of copy number alteration, ‘Analysis of Copy number Errors’ algorithm in the CGH-Explorer v. 3.1b software (Softgenetics, State College, PA, USA) was employed. Analysis of copy number errors estimates copy number calls based on genomic regions rather than on individual BACs. The significance is expressed by the estimation of the positive false discovery rate: in our case, the positive false discovery rate chosen was 0.1.53
Tumorigenicity analysis
We injected 2×106 control hNCPCs or hNCPCsV600E s.c. into the flanks of non-obese diabetic-severe combined immunodeficiency mice (5 mice each group) in 100 μl growth factor reduced Matrigel (BD Biosciences). After tumors were palpable (~2 weeks), caliper measurements were obtained every 3 days. Tumor volume was monitored closely for 8 weeks, at which time the animals were killed. In a second set of experiments, we injected 2×106 hNCPCsV600E or hNCPCsV600E+MITF s.c. into the flanks of NSG mice (5 mice each group) in 100 μl growth factor reduced Matrigel. Owing to rapid tumor growth of the hNCPCsV600E+MITF, these mice were killed after 5 weeks. All the organs were examined for metastasis grossly and histologically. The tumors were excised and processed for histology and additional analysis.
Statistical analysis
Student’s t-test or one-way analysis of variance was used to analyze gene expression, cell viability proliferation data and tumor growth. Statistical significance was determined if two-sided tests yielded P<0.05.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Drs M Herlyn from the Wistar Institute, Philadelphia, PA for melanoma cell lines, R Marais from the Institute of Cancer Research, London, UK for BRAFV600E plasmids, S Carreira from Marie Curie Research Institute, UK for MITF promoter-luciferase plasmids, F Liu from university of California for QCXIP-MiTF and QCXIP vectors, K Huang for editing the manuscript. CHTN for providing tissues and the immunohistochemical lab at the Department of Pathology and Laboratory medicine, University of Pennsylvania for immunohistochemical stains. This work is supported by US National Institutes of Health grants AR-054593, CA-116103 to XX.
Footnotes
CONFLICT OF INTEREST The authors declare no conflict of interest.
REFERENCES
- 1.Fernandes KJ, McKenzie IA, Mill P, Smith KM, Akhavan M, Barnabe-Heider F, et al. A dermal niche for multipotent adult skin-derived precursor cells. Nat Cell Biol. 2004;6:1082–1093. doi: 10.1038/ncb1181. [DOI] [PubMed] [Google Scholar]
- 2.Kruger GM, Mosher JT, Bixby S, Joseph N, Iwashita T, Morrison SJ. Neural crest stem cells persist in the adult gut but undergo changes in self-renewal, neuronal subtype potential, and factor responsiveness. Neuron. 2002;35:657–669. doi: 10.1016/s0896-6273(02)00827-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morrison SJ, White PM, Zock C, Anderson DJ. Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell. 1999;96:737–749. doi: 10.1016/s0092-8674(00)80583-8. [DOI] [PubMed] [Google Scholar]
- 4.Sieber-Blum M. Cardiac neural crest stem cells. Anat Rec A Discov Mol Cell Evol Biol. 2004;276:34–42. doi: 10.1002/ar.a.10132. [DOI] [PubMed] [Google Scholar]
- 5.Sieber-Blum M, Grim M, Hu YF, Szeder V. Pluripotent neural crest stem cells in the adult hair follicle. Dev Dyn. 2004;231:258–269. doi: 10.1002/dvdy.20129. [DOI] [PubMed] [Google Scholar]
- 6.Wong CE, Paratore C, Dours-Zimmermann MT, Rochat A, Pietri T, Suter U, et al. Neural crest-derived cells with stem cell features can be traced back to multiple lineages in the adult skin. J Cell Biol. 2006;175:1005–1015. doi: 10.1083/jcb.200606062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amoh Y, Li L, Katsuoka K, Penman S, Hoffman RM. Multipotent nestin-positive, keratin-negative hair-follicle bulge stem cells can form neurons. Proc Natl Acad Sci USA. 2005;102:5530–5534. doi: 10.1073/pnas.0501263102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu H, Fang D, Kumar SM, Li L, Nguyen TK, Acs G, et al. Isolation of a novel population of multipotent adult stem cells from human hair follicles. Am J Pathol. 2006;168:1879–1888. doi: 10.2353/ajpath.2006.051170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sieber-Blum M, Grim M. The adult hair follicle: cradle for pluripotent neural crest stem cells. Birth Defects Res C Embryo Today. 2004;72:162–172. doi: 10.1002/bdrc.20008. [DOI] [PubMed] [Google Scholar]
- 10.Li L, Mignone J, Yang M, Matic M, Penman S, Enikolopov G, et al. Nestin expression in hair follicle sheath progenitor cells. Proc Natl Acad Sci USA. 2003;100:9958–9961. doi: 10.1073/pnas.1733025100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu YF, Zhang ZJ, Sieber-Blum M. An epidermal neural crest stem cell (EPI-NCSC) molecular signature. Stem Cells. 2006;24:2692–2702. doi: 10.1634/stemcells.2006-0233. [DOI] [PubMed] [Google Scholar]
- 12.Clarke MF, Fuller M. Stem cells and cancer: two faces of eve. Cell. 2006;124:1111–1115. doi: 10.1016/j.cell.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 13.Polyak K, Hahn WC. Roots and stems: stem cells in cancer. Nat Med. 2006;12:296–300. doi: 10.1038/nm1379. [DOI] [PubMed] [Google Scholar]
- 14.Duband JL. Neural crest delamination and migration: integrating regulations of cell interactions, locomotion, survival and fate. Adv Exp Med Biol. 2006;589:45–77. doi: 10.1007/978-0-387-46954-6_4. [DOI] [PubMed] [Google Scholar]
- 15.Hilton MJ, Tu X, Wu X, Bai S, Zhao H, Kobayashi T, et al. Notch signaling maintains bone marrow mesenchymal progenitors by suppressing osteoblast differentiation. Nat Med. 2008;14:306–314. doi: 10.1038/nm1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossi DJ, Jamieson CH, Weissman IL. Stems cells and the pathways to aging and cancer. Cell. 2008;132:681–696. doi: 10.1016/j.cell.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 17.Moran-Crusio K, Reavie L, Shih A, Abdel-Wahab O, Ndiaye-Lobry D, Lobry C, et al. Tet2 loss leads to increased hematopoietic stem cell self-renewal and myeloid transformation. Cancer Cell. 2011;20:11–24. doi: 10.1016/j.ccr.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elder DE. Tumorigenic melanocytic proliferations. Demos Medical Publishing; 2009. [Google Scholar]
- 19.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 20.Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, van der Horst CM, et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436:720–724. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- 21.Patton EE, Widlund HR, Kutok JL, Kopani KR, Amatruda JF, Murphey RD, et al. BRAF mutations are sufficient to promote nevi formation and cooperate with p53 in the genesis of melanoma. Curr Biol. 2005;15:249–254. doi: 10.1016/j.cub.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 22.Milagre C, Dhomen N, Geyer FC, Hayward R, Lambros M, Reis-Filho JS, et al. A mouse model of melanoma driven by oncogenic KRAS. Cancer Res. 2010;70:5549–5557. doi: 10.1158/0008-5472.CAN-09-4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu H, Kumar SM, Kossenkov AV, Showe L, Xu X. Stem cells with neural crest characteristics derived from the bulge region of cultured human hair follicles. J Invest Dermatol. 2010;130:1227–1236. doi: 10.1038/jid.2009.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar SM, Yu H, Edwards R, Chen L, Kazianis S, Brafford P, et al. Mutant V600E BRAF increases hypoxia inducible factor-1alpha expression in melanoma. Cancer Res. 2007;67:3177–3184. doi: 10.1158/0008-5472.CAN-06-3312. [DOI] [PubMed] [Google Scholar]
- 25.Baird DM. Telomere dynamics in human cells. Biochimie. 2008;90:116–121. doi: 10.1016/j.biochi.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Squarzoni P, Parveen F, Zanetti L, Ristoratore F, Spagnuolo A. FGF/MAPK/Ets signaling renders pigment cell precursors competent to respond to Wnt signal by directly controlling Ci-Tcf transcription. Development. 2011;138:1421–1432. doi: 10.1242/dev.057323. [DOI] [PubMed] [Google Scholar]
- 27.Wellbrock C, Rana S, Paterson H, Pickersgill H, Brummelkamp T, Marais R. Oncogenic BRAF regulates melanoma proliferation through the lineage specific factor MITF. PLoS One. 2008;3:e2734. doi: 10.1371/journal.pone.0002734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- 30.Matsuoka S, Oike Y, Onoyama I, Iwama A, Arai F, Takubo K, et al. Fbxw7 acts as a critical fail-safe against premature loss of hematopoietic stem cells and development of T-ALL. Genes Dev. 2008;22:986–991. doi: 10.1101/gad.1621808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Passegue E, Jamieson CH, Ailles LE, Weissman IL. Normal and leukemic hematopoiesis: are leukemias a stem cell disorder or a reacquisition of stem cell characteristics? Proc Natl Acad Sci USA. 2003;100(Suppl 1):11842–11849. doi: 10.1073/pnas.2034201100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jamieson CH, Ailles LE, Dylla SJ, Muijtjens M, Jones C, Zehnder JL, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med. 2004;351:657–667. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- 33.Rubio D, Garcia-Castro J, Martin MC, de la Fuente R, Cigudosa JC, Lloyd AC, et al. Spontaneous human adult stem cell transformation. Cancer Res. 2005;65:3035–3039. doi: 10.1158/0008-5472.CAN-04-4194. [DOI] [PubMed] [Google Scholar]
- 34.Shiras A, Chettiar ST, Shepal V, Rajendran G, Prasad GR, Shastry P. Spontaneous transformation of human adult nontumorigenic stem cells to cancer stem cells is driven by genomic instability in a human model of glioblastoma. Stem Cells. 2007;25:1478–1489. doi: 10.1634/stemcells.2006-0585. [DOI] [PubMed] [Google Scholar]
- 35.Bernardo ME, Zaffaroni N, Novara F, Cometa AM, Avanzini MA, Moretta A, et al. Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res. 2007;67:9142–9149. doi: 10.1158/0008-5472.CAN-06-4690. [DOI] [PubMed] [Google Scholar]
- 36.Foroni C, Galli R, Cipelletti B, Caumo A, Alberti S, Fiocco R, et al. Resilience to transformation and inherent genetic and functional stability of adult neural stem cells ex vivo. Cancer Res. 2007;67:3725–3733. doi: 10.1158/0008-5472.CAN-06-4577. [DOI] [PubMed] [Google Scholar]
- 37.Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N, et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444:633–637. doi: 10.1038/nature05268. [DOI] [PubMed] [Google Scholar]
- 38.Braig M, Lee S, Loddenkemper C, Rudolph C, Peters AH, Schlegelberger B, et al. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature. 2005;436:660–665. doi: 10.1038/nature03841. [DOI] [PubMed] [Google Scholar]
- 39.Di Micco R, Fumagalli M, Cicalese A, Piccinin S, Gasparini P, Luise C, et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature. 2006;444:638–642. doi: 10.1038/nature05327. [DOI] [PubMed] [Google Scholar]
- 40.Mooi WJ, Peeper DS. Oncogene-induced cell senescence—halting on the road to cancer. N Engl J Med. 2006;355:1037–1046. doi: 10.1056/NEJMra062285. [DOI] [PubMed] [Google Scholar]
- 41.Yaswen P, Campisi J. Oncogene-induced senescence pathways weave an intricate tapestry. Cell. 2007;128:233–234. doi: 10.1016/j.cell.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 42.Wajapeyee N, Serra RW, Zhu X, Mahalingam M, Green MR. Role for IGFBP7 in senescence induction by BRAF. Cell. 2010;141:746–747. doi: 10.1016/j.cell.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu H, McDaid R, Lee J, Possik P, Li L, Kumar SM, et al. The role of BRAF mutation and p53 inactivation during transformation of a subpopulation of primary human melanocytes. Am J Pathol. 2009;174:2367–2377. doi: 10.2353/ajpath.2009.081057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Witkiewicz AK, Knudsen KE, Dicker AP, Knudsen ES. The meaning of p16(ink4a) expression in tumors: functional significance, clinical associations and future developments. Cell Cycle. 2011;10:2497–2503. doi: 10.4161/cc.10.15.16776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garraway LA, Widlund HR, Rubin MA, Getz G, Berger AJ, Ramaswamy S, et al. Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma. Nature. 2005;436:117–122. doi: 10.1038/nature03664. [DOI] [PubMed] [Google Scholar]
- 46.Du J, Widlund HR, Horstmann MA, Ramaswamy S, Ross K, Huber WE, et al. Critical role of CDK2 for melanoma growth linked to its melanocyte-specific transcriptional regulation by MITF. Cancer Cell. 2004;6:565–576. doi: 10.1016/j.ccr.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 47.Bloethner S, Snellman E, Bermejo JL, Hiripi E, Gast A, Thirumaran RK, et al. Differential gene expression in melanocytic nevi with the V600E BRAF mutation. Genes Chromosomes Cancer. 2007;46:1019–1027. doi: 10.1002/gcc.20488. [DOI] [PubMed] [Google Scholar]
- 48.Carreira S, Goodall J, Aksan I, La Rocca SA, Galibert MD, Denat L, et al. Mitf cooperates with Rb1 and activates p21Cip1 expression to regulate cell cycle progression. Nature. 2005;433:764–769. doi: 10.1038/nature03269. [DOI] [PubMed] [Google Scholar]
- 49.Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 50.Kumar SM, Acs G, Fang D, Herlyn M, Elder DE, Xu X. Functional erythropoietin autocrine loop in melanoma. Am J Pathol. 2005;166:823–830. doi: 10.1016/S0002-9440(10)62303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Greshock J, Naylor TL, Margolin A, Diskin S, Cleaver SH, Futreal PA, et al. 1-Mb resolution array-based comparative genomic hybridization using a BAC clone set optimized for cancer gene analysis. Genome Res. 2004;14:179–187. doi: 10.1101/gr.1847304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vaquerizas JM, Dopazo J, Diaz-Uriarte R. DNMAD: web-based diagnosis and normalization for microarray data. Bioinformatics. 2004;20:3656–3658. doi: 10.1093/bioinformatics/bth401. [DOI] [PubMed] [Google Scholar]
- 53.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci USA. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.