Abstract
Objective
To examine the role of hypertension, hyperglycemia and dyslipidemia in potentially accounting for obesity-related brain vulnerability in the form of altered cerebral neurochemistry.
Design and Methods
Sixty-four adults, ages 40 to 60 years, underwent a health screen and proton magnetic resonance spectroscopy (1H MRS) of occipitoparietal grey matter to measure N-acetyl aspartate (NAA), choline (Cho), myo-inositol (mI) and glutamate (Glu) relative to creatine (Cr). The causal steps approach and non-parametric bootstrapping were utilized to assess if fasting glucose, mean arterial pressure or peripheral lipid/lipoprotein levels mediate the relationship between body mass index (BMI) and cerebral neurochemistry.
Results
Higher BMI was significantly related to higher mI/Cr, independent of age and sex. BMI was also significantly related to two of the proposed mediators, triglyceride and HDL-cholesterol, which were also independently related to increased mI/Cr. Finally, the relationship between BMI and mI/Cr, was significantly attenuated after inclusion of triglyceride and HDL-cholesterol into the model, one at a time, indicating statistical mediation.
Conclusions
Higher triglyceride and lower HDL levels statistically account for the association between BMI and myo-inositol, pointing towards a potentially critical role for dyslipidemia in the development of cerebral neurochemical alterations in obesity.
Keywords: obesity, magnetic resonance spectroscopy, myo-inositol, triglycerides, cholesterol
Introduction
Obesity is the leading cause of preventable death and arguably one of the most – if not the most – serious public health concerns of the 21st century (1). If current trends continue, by the year 2015, 1.5 billion people worldwide, an estimated 21% of the world’s population, will be overweight (2). In addition to being at an increased risk for heart disease, diabetes and cancer, individuals who are obese in midlife have a 74% higher chance of developing dementia (3) with 36% increase of risk for each point increase in body mass index (BMI) (4, 5). Even in adults with intact cognitive function, obesity is associated with reduced grey matter volumes (6), diminished resting cerebral glucose metabolism (7) and altered cerebral neurochemical profiles, decreased N-acetyl aspartate and increased myo-inositol levels in particular (8–10). Among these biological markers of brain vulnerability, the organic osmolyte and glial marker (11), myo-inositol, is of special interest as a recent study demonstrated that increases in BMI are related to poorer memory performance in midlife through elevated concentrations of that particular neurometabolite (10). This finding joined a large body of evidence indicating that myo-inositol elevations occur early in the neurodegenerative process and appear to foreshadow cognitive decline in several conditions (12–14). In addition, myo-inositol elevations are linked to increases in the peripheral inflammatory marker C-reactive protein (15), highlighting a potential link between cerebral myo-inositol alterations and obesity, a condition known for inducing oxidative stress and inflammation (16). To further explore the mechanisms of obesity-related brain vulnerability, here we examined the role of obesity-related complications such as elevated blood pressure (hypertension), elevated fasting blood glucose (hyperglycemia) and the abnormal accumulation of lipids in the blood (dyslipidemia) in contributing to cerebral myo-inositol elevations in midlife.
Hypertension, hyperglycemia and dyslipidemia are among the most common features of obesity, significantly linked to obesity-related morbidity and mortality (17). Suspected causal links between increases in BMI and pathological alterations in peripheral glucose, blood pressure and lipid levels have been confirmed in longitudinal studies and further supported by evidence that weight loss leads to normalization in all of these physiological parameters (18). In addition to their links to cardiovascular disease, hypertension, hyperglycemia and dyslipidemia have been independently associated with cognitive decline (19, 20). Finally, hypertension and hyperglycemia have each been related to increased cerebral myo-inositol (21, 22). A logical next step in obesity research is to examine to what extent these common complications account for the documented myo-inositol increases in obesity (10).
In this study, we hypothesized that midlife increases in BMI induce early brain vulnerability in the form of increased cerebral myo-inositol through changes in peripheral physiological parameters such as blood pressure, blood glucose and serum lipid/lipoprotein levels. To test this hypothesis, we employed a statistical mediation model, a procedure that allows one to explore the pathways through which obesity may exert its deleterious effects on the brain within a cross-sectional study design.
Methods and Procedures
Participants
Community-dwelling adults between the ages of 40 and 60 years old were recruited through advertisements in local newspapers and classified advertisements websites. The study sample included sixty-four participants (33 women; 31 men; mean age 50.5 ± 6.6; mean BMI 29.39 ± 5.70 kg/m2). Minority participants were especially solicited to ensure an accurate representation of the state’s demographic characteristics. Participants in the study sample identified themselves as follows: 41% Caucasian, 41% Hispanic, 11% African-American, and 7% Other/Did Not Specify individuals.
To be included in the study, participants had to be free of neurological disease (i.e., large vessel stroke, seizure disorder, Parkinson’s disease, clinically significant traumatic brain injury, multiple sclerosis, or brain infection/meningitis); major psychiatric illness (e.g., schizophrenia, bipolar disorder); and substance abuse (diagnosed abuse and/or previous hospitalization for substance abuse) as indicated by participant self-report. Participants also had to demonstrate global cognitive function within the normal range during screening (Wechsler Abbreviated Scale of Intelligence full scale intelligence quotient, FSIQ>84, n=3 were excluded for low FSIQ scores and n=2 were excluded for missing FSIQ scores). Finally, they had to report no MRI contraindications. MRS data also had to pass quality control (Cramér-Rao Lower Bounds for NAA/Cr, mI/Cr, Cho/Cr or Glu/Cr>12 were excluded, n=1). Demographic and physiological characteristics of the sample are reported in Table 1.
Table 1.
Selected demographic and physiologic characteristics of the study sample
| n=64 Mean±SD |
|
|---|---|
| Female/Male | 33/31 |
| Age (years) | 51±7 |
| Education Level (years) | 15±2 |
| Systolic Blood Pressure (mmHg) | 125±17 |
| Diastolic Blood Pressure (mmHg) | 75±10 |
| Body Mass Index (kg/m2) | 29.4±5.7 |
| HDL-Cholesterol (mg/dL) | 47.7±16.1 |
| Triglyceride (mg/dL) | 163.8±91.8 |
| LDL-Cholesterol (mg/dL) | 122.3±33.7 |
| Fasting Glucose (mg/dL) | 109.7±44.8 |
HDL=high-density lipoprotein; LDL=low-density lipoprotein
Procedures
The study was conducted according to the ethical principles of medical research with human participants outlined in the Helsinki Declaration, with approval by the local Institutional Review Board. All enrollees provided written informed consent prior to participation. A health screening, a neuropsychological evaluation, and brain MRI imaging were conducted on separate days.
General health assessment and BMI calculations
Details regarding the health assessment procedures have been previously published (23). Briefly, height and weight were measured on a physician’s beam balance scale for the subsequent calculations of BMI. BMI was calculated by dividing weight in kilograms by height in meters squared. Fasting blood concentrations of glucose, triglyceride, HDL-cholesterol, and LDL-cholesterol were determined using standard enzymatic techniques; arterial blood pressure was measured using a standard oscillometric blood pressure monitor after at least 15 minutes of rest; and prior medical diagnoses and prescription medications were coded as present or absent according to participant self-report. A trained research assistant coded medications as antihypertensive, lipid lowering, hypoglycemic, antiplatelet, anti-inflammatory, antidepressant, antihistamine, hormone replacement, or bisphosphonates.
1H-Magnetic Resonance Spectroscopy
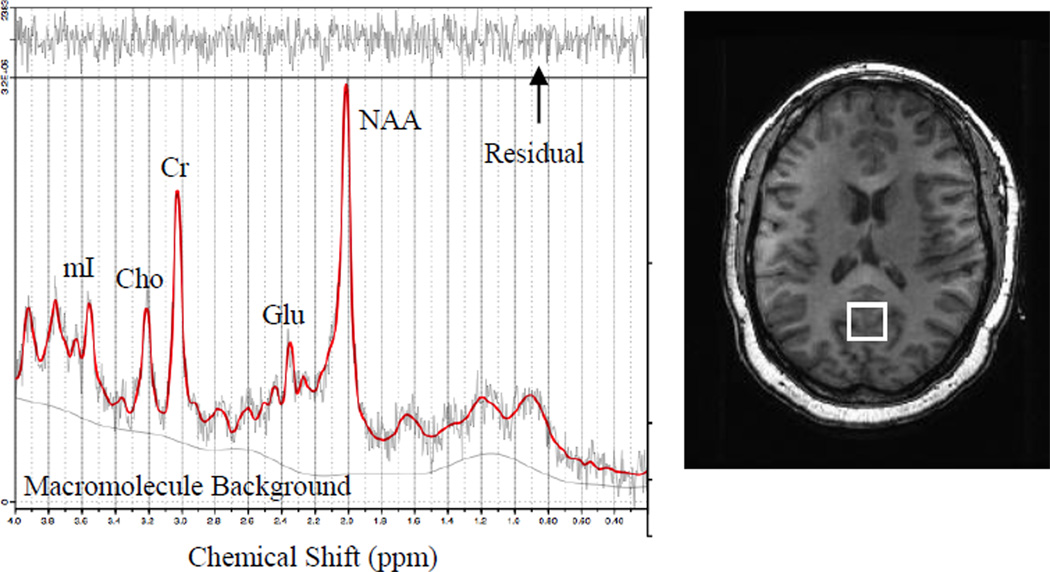
A 3T GE Signa Excite MRI scanner equipped with a standard head coil was used in all brain imaging sessions. We employed a standard PROBE-P 1H MRS sequence with the following parameters: echo time/ repetition time TE/TR = 35/3000 ms, 128 excitations, 5000 Hz spectral width. A volume of interest (~6 cm3) was localized within occipitoparietal grey matter (Fig. 1) using 3D high-resolution Spoiled Gradient Echo (SPGR) sagittal images of the entire brain (256 × 256 matrix, FOV = 24 × 24 cm2, 1 mm slice thickness, 0 gap). The volume of interest was specifically positioned to include the posterior cingulate gyrus. As obese persons are at risk for all-cause dementia (3), we selected a region of interest documented to show early metabolic and functional alterations in both young adults at risk for Alzheimer’s disease (24) and young adults at risk for vascular cognitive impairment due to family history of early onset hypertension (25).
Figure 1.
Volume of interest borders on high-resolution anatomy (A) and representative spectrum (B)
NAA = N-acetyl-aspartate; Glu = glutamate; Cr = creatine and phosphocreatine; Cho = choline and phosophocholine; mI = myo-inositol
Metabolite concentrations were derived through identification and quantification of metabolite resonances with LCModel (26). All neurometabolite concentrations are reported as ratios relative to creatine (Cr) as is customary in clinical investigations (13). Metabolites of interest included: N-acetyl-aspartate (NAA), a marker of neuronal viability; choline containing compounds (free choline, phosphocholine, and glycerophosphocholine, Cho), markers of membrane breakdown and turn over; myo-inositol (mI), an organic osmolyte, marker of glial proliferation and substrate for the synthesis of the secondary messenger, inositol triphosphate; and glutamate (Glu), an excitatory neurotransmitter and marker of synaptic integrity (supplementary reference 1).
Data analyses
All statistical analyses were performed using SPSS 19.0 (IBM Corporation, Chicago, IL). Liner regression analyses were used to test for BMI-related changes in cerebral metabolism, controlling for age and sex. Four separate analyses were conducted, one for each neurometabolite ratio (NAA/Cr, Cho/Cr, mI/Cr and Glu/Cr). Linear regression analyses were also used to examine the relationships between BMI and peripheral physiological parameters such as mean arterial pressure, fasting blood glucose, triglyceride, HDL- and LDL-cholesterol levels, again controlling for age and sex. Finally, linear regression analyses controlling for age and sex were used to examine the relationships between the aforementioned physiological parameters and the cerebral neurometabolite ratio significantly related to BMI (mI/Cr). Regression residuals were examined using Shapiro-Wilk test of normality.
Follow up mediation analysis was conducted only for mI/Cr where a significant direct effect of BMI was detected. Only triglyceride and HDL-cholesterol levels were further explored as mediators as they were the physiological parameters that fulfilled conditions for mediation by being significantly related to both the independent variable (BMI) and the outcome variable (mI/Cr). Age, sex, mean arterial pressure, fasting glucose and history of smoking were used as covariates in the mediation analysis as they have exhibited significant relationships to either BMI or mI/Cr in prior studies (17, 21, 22).
The direct and indirect effects of BMI on cerebral mI/Cr concentration through peripheral lipid/lipoprotein levels were tested using the traditional causal steps approach proposed by Baron and Kenny (supplementary reference 2) and non-parametric bootstrapping procedures. For full mediation to occur in this study, the causal steps approach would posit that BMI should significantly predict cerebral mI/Cr and serum lipid/lipoprotein levels. Furthermore, serum lipid/lipoprotein levels should significantly predict cerebral mI/Cr. Finally, the effect of BMI on cerebral mI/Cr should be significantly attenuated after controlling for serum lipid/lipoprotein levels.
In addition, we formally tested the significance of the indirect effect of BMI on cerebral mI/Cr through the proposed mediators (serum lipid/lipoprotein levels). These tests were accomplished through the INDIRECT macro for SPSS provided by Preacher and Hayes (27). They were conducted using both normal theory and a non-parametric bootstrapping procedure, which makes no assumptions about the shape of the distributions of the variables or the sampling distribution of the statistic, thus lowering the likelihood of Type 2 error. We utilized a resample procedure with 5000 bootstrap samples and report the 95% bias corrected confidence interval (CI). As recommended by Preacher and Hayes, indirect effects were interpreted as significant if zero was not included in the 95% CI (supplementary reference 3). Indirect effects were assessed separately for triglyceride level (TG) and HDL-cholesterol.
Results
Descriptive statistics
Details regarding the demographic and physiological characteristics of the sample can be found in Table 1. According to World Health Organization criteria, 25% of all participants could be classified as normal weight (BMI: 18.5 – 24.99 kg/m2), 37.5% as overweight (25 – 29.99 kg/m2), and 37.5% as obese (BMI: 30 kg/m2). Twenty-three participants reported a diagnosis of hypertension (36%); fourteen participants reported a diagnosis of dyslipidemia (22%) and ten participants, a diagnosis of diabetes (16%). Thirteen participants were currently being treated with antihypertensive medication, four participants were taking lipid-lowering drugs, and eight participants were being treated with hypoglycemic agents. Thyroid medication, bisphosphonates, psychoactive medication and vitamins were reported at low frequency (≤3).
Peripheral physiological parameters and cerebral metabolism
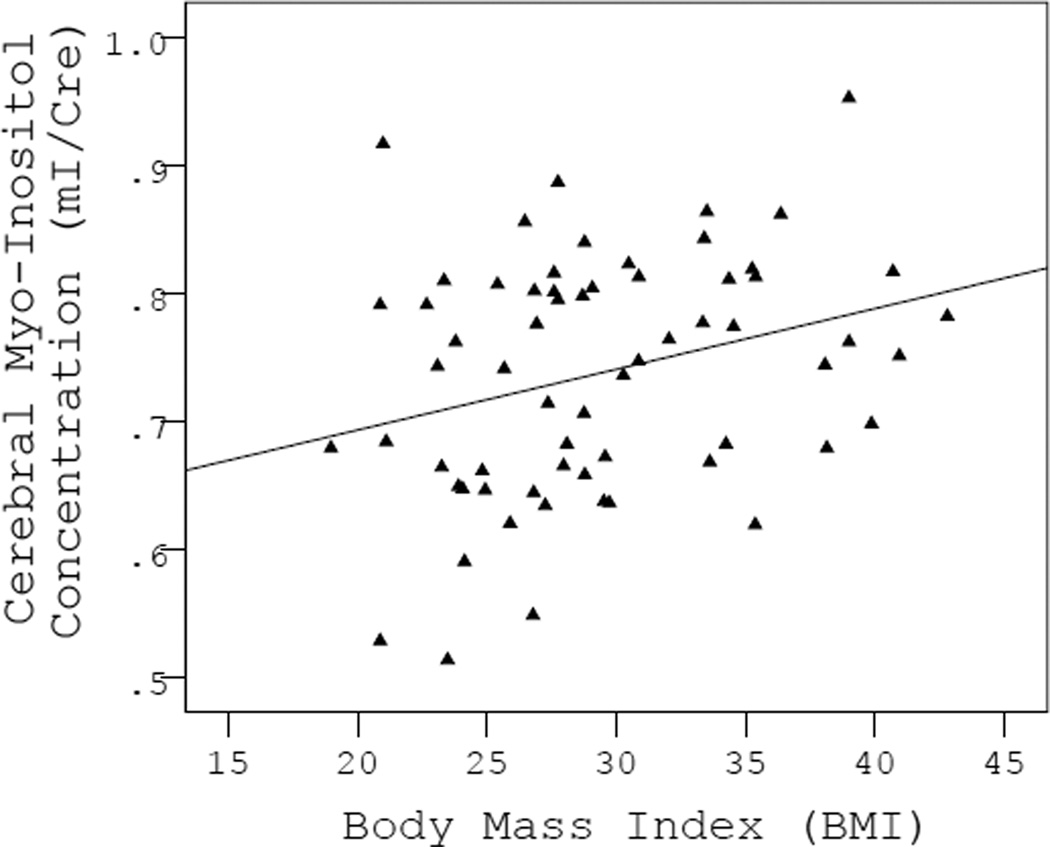
The bivariate correlations between all cerebral metabolite ratios, age, sex and BMI are presented in Table 2. Independent of age and sex, higher BMI was significantly related to higher cerebral mI/Cr (F(3,60)=2.89, p=0.043, BMI β=0.27, p=0.030, Fig. 2). Over and above the effects of age and sex, BMI was not significantly related to changes in any of the other metabolite ratios: NAA/Cr (F(3,60)=0.64, p=0.594), Cho/Cr (F(3,60)=0.43, p=0.730), or Glu/Cr (F(3,60)=1.39, p=0.256).
Table 2.
Bivariate correlations between cerebral metabolite concentrations and demographic variables
| n=64 Mean±SD |
Age r (p) |
Sex r (p) |
BMI r (p) |
|
|---|---|---|---|---|
| NAA/Cr | 1.41±0.10 | 0.15 (.239) | 0.12 (.348) | 0.04 (.748) |
| Cho/Cr | 0.19±0.04 | −0.08 (.553) | 0.20 (.106) | 0.07 (.601) |
| mI/Cr | 0.74±0.09 | −0.04 (.731) | 0.25 (.050) | 0.29 (.020) |
| Glu/Cr | 1.45±0.16 | 0.07 (.559) | −0.06 (.656) | 0.23 (.063) |
NAA=N-acetyl-aspartate; Cr=creatine+phosphocreatine; Cho=choline+phosphocholine; mI=myo-inositol; Glu=glutamate
Figure 2.
Scatterplot showing a significant direct effect of increased BMI on cerebral myo-inositol levels
Body mass index and peripheral physiological parameters
Independent of age and sex, higher BMI was significantly related to higher triglyceride (F(3,60)=4.39, p=0.007, TG β=0.41, p=0.001), significantly lower HDL-cholesterol (F(3,60)=5.03, p=0.004, HDL β=−0.50, p=0.000) and a trend towards higher mean arterial pressure (F(3,50)=2.42, p=0.075). Higher BMI was not related to significantly higher fasting glucose values (F(3,60)=0.39, p=0.760) or LDL-cholesterol levels (F(3,59)=0.88, p=0.456) over and above the effects of age and sex.
Cerebral mI/Cr and peripheral physiological parameters
Independent of age and sex, higher cerebral mI/Cr was related to significantly higher triglyceride (F(3,60)=3.24, p=0.028, TG β=0.29, p=0.018), significantly lower HDL-cholesterol (F(3,60)=3.917, p=0.013, HDL β=−0.38, p=0.007) and significantly higher fasting glucose levels (F(3,60)=2.89, p=0.043, glucose β=0.27, p=0.030). Higher mI/Cr was not related to mean arterial pressure (F(3,60)=1.60, p=0.200) or LDL-cholesterol levels (F(3,59)=1.49, p=0.228) over and above the effects of age and sex. The partial correlations between mI/Cr, blood pressure, fasting glucose and plasma lipid/lipoprotein levels adjusted for age and sex are presented in Table 3.
Table 3.
Partial correlations between myo-inositol concentration and selected physiologic variables adjusted for age and sex
| mI/Cr r (p) |
|
|---|---|
| Systolic Blood Pressure (mmHg) | −0.05 (.701) |
| Diastolic Blood Pressure (mmHg) | −0.18 (.172) |
| Body Mass Index (kg/m2) | 0.28 (.030) |
| HDL-Cholesterol (mg/dL) | −0.38 (.007) |
| LDL-Cholesterol (mg/dL) | −0.09 (.477) |
| Triglyceride (mg/dL) | 0.29 (.018) |
| Fasting Glucose (mg/dL) | 0.27 (.030) |
Mediation analysis
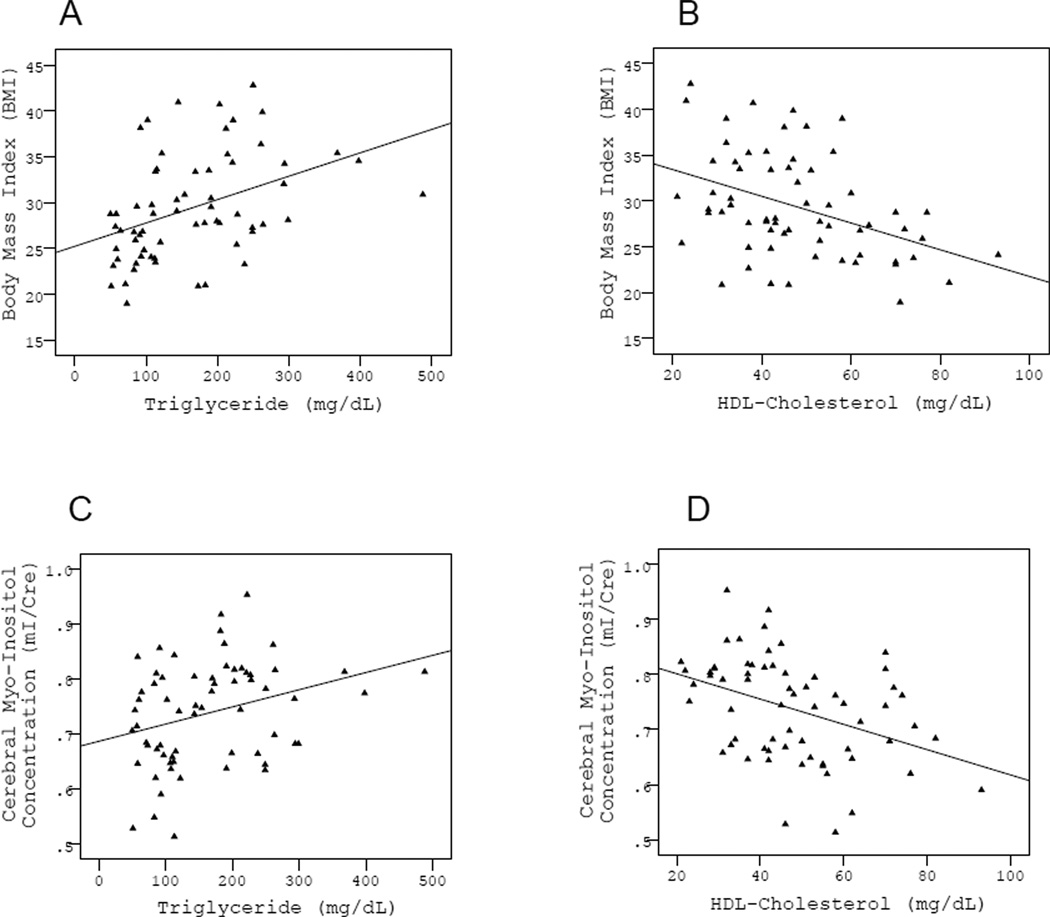
Follow-up mediation analysis was conducted only for the significant direct effect between BMI and mI/Cr. Only triglyceride and HDL-cholesterol were explored as mediators as they were the only two peripheral physiological variables to fulfill conditions for mediation by exhibiting a significant relationship with both the proposed independent variable (BMI) and proposed outcome (mI/Cr). Age, sex, mean arterial pressure, fasting glucose and history of smoking were included as covariates as they have exhibited significant relationships to either BMI or mI/Cr in prior studies (17, 21, 22). Following the causal steps approach, we examined each mediator in turn. BMI had a significant effect on mI/Cr, independent of age, sex, mean arterial pressure, fasting glucose and history of smoking (β=0.32, p=0.014, Fig. 2). BMI was also significantly related to triglyceride (β=0.36, p=0.008, Fig. 3A) and HDL-cholesterol (β=−0.49, p=0.001, Fig 3B). Triglyceride and HDL-cholesterol were also significantly related to mI/Cr (β=0.39, p=0.003 and β=−0.45, p=0.002, respectively, Fig. 3C, 3D). Finally, the relationship between BMI and mI/Cr was significantly attenuated after the proposed mediators were entered into the model, one at a time (β=0.21, p=0.116 for triglyceride and β=0.18, p=0.184 for HDL-cholesterol) indicating statistical mediation. The variance in mI/Cr accounted for by triglyceride and HDL-cholesterol appeared to be largely shared between the two variables as controlling, for each in turn attenuated the effect of the other. This finding is not surprising as triglyceride and HDL-cholesterol are known to have a very strong inverse relationship to each other, which was also the case in this study (r=−0.49, p=.000).
Figure 3.
Scatterplots showing a significant relationships between BMI and the proposed mediators, triglyceride and HDL-cholesterol levels (panels A & B), as well as significant relationships between the proposed mediators, triglyceride and HDL-cholesterol levels, and the outcome variable, cerebral myo-inositol concentration (panels C & D).
Indirect effects
The indirect effects of BMI on mI/Cr through triglyceride and HDL-cholesterol were also significant when assessed by the non-parametric bootstrapping procedure (triglyceride 95% CI: 0.0005–0.0041 and HDL-cholesterol 95% CI: 0.0006–0.0051). Additional adjustments for lipid lowering, antihypertensive and hypoglycemic medication did not change the outcome (triglyceride 95% CI: 0.0004–0.0046 and HDL-cholesterol 95% CI: 0.0007–0.0056).
Discussion
Our main finding was that higher BMI in midlife was significantly related to elevated cerebral myo-inositol levels, independent of age and sex. More importantly, this relationship was accounted for by dyslipidemia, potentially implicating changes in serum lipid and lipoprotein metabolism as mechanisms behind obesity-related alterations in cerebral neurochemistry. Since elevated myo-inositol appears to be predictive of future cognitive impairment in patients with Mild Cognitive Impairment, Down syndrome, HIV and Multiple Sclerosis (12–14), these results point toward a potentially crucial role for peripheral lipid and lipoprotein metabolism in the development of early cognitive vulnerability in overweight and obese individuals. Thus, our study is among the first to propose a viable biological mechanism that can, at least in part, account for the observed associations between midlife obesity and late-life cognitive decline in epidemiological studies (3, 5). Somewhat unexpectedly, we did not find an association between BMI and fasting blood glucose or mean arterial pressure, possibly because our sample included only participants with well-controlled hypertension and diabetes. Fasting glucose and mean arterial pressure were retained as covariates in all subsequent analyses as both have been independently associated with increased cerebral myo-inositol in prior studies (21, 22). However, replacing fasting glucose values with measurements of hemoglobin A1c is worth considering in future studies as hemoglobin A1c measurements reflect the integrated glucose concentration over time and are much less susceptible to diurnal fluctuations than blood glucose concentration (28).
In this study, elevated triglyceride and reduced HDL levels were the markers of dyslipidemia that accounted for the association between BMI and alterations in cerebral myo-inositol levels. High triglyceride and low HDL levels are the most typical dyslipidemic profile associated with obesity (29). Hypertriglyceridemia has been associated with cognitive impairment in animals (30) as well as humans (31–33). Obese hypertriglyceridemic mice exhibit impaired performance in several cognitive paradigms including active avoidance, T-maze, the Morris water maze and food reward lever press (30). In human subjects, elevated triglyceride levels have related to poorer short-term memory and attention-executive task performance in non-insulin dependent diabetics (32), lower memory performance in individuals with high inflammation (33) and steeper longitudinal declines in verbal knowledge in older adults participating in long-term follow-up studies (31). One of the proposed mechanisms for this association is impaired maintenance of NMDA-dependent hippocampal long-term synaptic potentiation (30). Interestingly, a prior study also found that higher BMI may have an indirect effect on poorer memory performance through elevations in myo-inositol (10). Thus, obesity-related hypertriglyceridemia may affect cerebral neurochemistry, increasing cognitive vulnerability. Elevations in myo-inositol at midlife may be an early marker of this vulnerability in obese individuals similar to its role as a biomarker in pre-clinical Alzheimer’s disease.
The second significant mediator of the relationship between increased BMI and elevated cerebral myo-inositol levels in our study was HDL cholesterol level. In addition to its important role in reverse cholesterol transport, plasma HDL (34), has been shown to modulate pathways involved in oxidation (35) and inflammation (36, 37), providing yet another potential mechanisms to link dyslipidemia to brain vulnerability and cognitive impairment. HDLs inhibit the production of pro-inflammatory cytokines such as interleukin-1β and tumor necrosis factor (36). HDLs also have the ability to neutralize upregulation of inflammatory adhesion molecules induced by C-reactive protein, another sensitive marker of inflammatory disease (37). Since C-reactive protein is known to be associated with both poorer cognitive function and greater brain vulnerability in the form of increased cerebral myo-inositol levels (15), it is not surprising that higher plasma HDL levels have correlated with better cognitive function in individuals with exceptional longevity (38).
Thus, we present new evidence that overweight and obesity in midlife can be linked to early brain vulnerability in the form of neurochemical alterations through disturbances in peripheral lipid and lipoprotein metabolism. This evidence extends the previous literature reporting links between midlife obesity and late-life cognitive impairment by providing a potential mechanism and adding to our understanding of the complex interactions between peripheral metabolism and brain function. It also re-affirms that the deleterious effects of obesity extend well beyond the periphery. However, it is important to acknowledge the complexity of the proposed mechanisms. The HDL particle carries over 40 different proteins (39), and we are unable at this point to identify which components may be more directly linked to the observed effects. It has been recommended that laboratory assessments beyond a simple measurement of HDL mass in order to account for the functional heterogeneity of HDL (40).
Finally, the limitations of this study need to be noted. First and foremost, the reported effect represents the results of a statistical mediation procedure. While it is plausible to suggest that dyslipidemia may be driving the observed cerebral neurochemical alterations, it is also possible that both may be related to early development or genetic influences. Confidence in the reported mechanisms can be gained through longitudinal studies where the temporal precedence between dyslipidemia and cerebral neurochemistry can be clearly established. In addition, much can be gained by experimental studies where triglyceride and HDL levels are systematically manipulated to test if these changes will have a direct impact on cerebral myo-inositol concentrations. On the other hand, a larger, randomly selected community sample can help establish the external validity of the results as our study was based on a relatively small sample of self-selected volunteers with relatively high level of education (~15 years). Finally, the imaging methods could be improved by the inclusion of additional regions of interest and tissue segmentation. Absolute quantitation of cerebral metabolites could serve to alleviate concerns that potential pathology-related changes in creatine concentrations might be exaggerating the changes in myo-inositol concentrations reported here as ratios to creatine.
One last important point to make is that while we detected a strong association between BMI and cerebral myo-inositol in occipitoparietal grey matter, another team reported no BMI-related neurochemical alterations in posterior brain regions (8, 9). The discrepancy in these findings could be due to methodological differences as the different studies utilized magnets of different field strength as well as different acquisition parameters. Alternatively, the discrepancy might be accounted for by differences in the sample characteristics. While the other two studies included only a small percentage of individuals with obese BMI, 10% and 0%, respectively, in our study, 38% of all participants were classified as obese. Therefore, it is possible that significant increases in cerebral myo-inositol in posterior brain areas can be detected only at higher BMIs or at higher prevalence of obesity-related complications. Studies that experimentally examine the separate and combined influences of obesity and obesity-related complications on brain composition could shed light on the relative contributions of these conditions to altered cerebral neurochemistry.
Summary and Conclusions
In summary, we found that higher midlife BMI was linked to cerebral neurochemical alterations through disturbances in peripheral lipid and lipoprotein concentrations. Higher BMI was related to higher cerebral myo-inositol levels and the relationship was statistically accounted for by higher triglyceride and lower HDL levels pointing towards a potentially critical role for dyslipidemia in linking midlife obesity to late-life cognitive impairment.
Supplementary Material
Acknowledgements
This study was funded in part by the American Heart Association (09BGIA2060722, APH), the American Federation for Aging Research (8A0024, APH), the National Institute of Neurological Disorders and Stroke (R01NS75565, APH) and the National Institute on Aging (F31AG040890, MMG.).
Footnotes
Disclosures
Dr. Haley is funded by the American Heart Association, the American Federation for Aging Research, and the National Institute of Neurological Disorders and Stroke.
Ms. Gonzales is funded by the National Institute on Aging.
Mr. Tarumi reports no disclosures.
Dr. Tanaka has received research support from the American Heart Association.
References
- 1.Barness LA, Opitz JM, Gilbert-Barness E. Obesity: Genetic, molecular, and environmental aspects. Am J Med Genet A. 2007;143A:3016–3034. doi: 10.1002/ajmg.a.32035. [DOI] [PubMed] [Google Scholar]
- 2.WHO. Switzerland: Geneva; 2005. Preventing chronic diseases: a vital investment: WHO global report. [Google Scholar]
- 3.Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP, Yaffe K. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. BMJ. 2005;330:1360. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gustafson D, Lissner L, Bengtsson C, Bjorkelund C, Skoog I. A 24-year follow-up of body mass index and cerebral atrophy. Neurology. 2004;63:1876–1881. doi: 10.1212/01.wnl.0000141850.47773.5f. [DOI] [PubMed] [Google Scholar]
- 5.Kivipelto M, Ngandu T, Fratiglioni L, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62:1556–1560. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- 6.Ward MA, Carlsson CM, Trivedi MA, Sager MA, Johnson SC. The effect of body mass index on global brain volume in middle-aged adults: a cross sectional study. Bmc Neurology. 2005;5 doi: 10.1186/1471-2377-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volkow ND, Wang GJ, Telang F, et al. Inverse Association Between BMI and Prefrontal Metabolic Activity in Healthy Adults. Obesity. 2009;17:60–65. doi: 10.1038/oby.2008.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gazdzinski S, Kornak J, Weiner MW, Meyerhoff DJ, Nat R. Body mass index and magnetic resonance markers of brain integrity in adults. Ann Neurol. 2008;63:652–657. doi: 10.1002/ana.21377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gazdzinski S, Millin R, Kaiser LG, et al. BMI and Neuronal Integrity in Healthy, Cognitively Normal Elderly: A Proton Magnetic Resonance Spectroscopy Study. Obesity. 2010;18:743–748. doi: 10.1038/oby.2009.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzales MM, Tarumi T, Eagan D, Tanaka H, Vaghasia M, Haley AP. Indirect effects of elevated Body Mass Index on memory performance through altered cerebral metabolite concentrations. Psychosom Med. 2012;74:691–698. doi: 10.1097/PSY.0b013e31825ff1de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brand A, Richter-Landsberg C, Leibfritz D. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev Neurosci. 1993;15:289–298. doi: 10.1159/000111347. [DOI] [PubMed] [Google Scholar]
- 12.Cloak CC, Chang L, Ernst T. Increased frontal white matter diffusion is associated with glial metabolites and psychomotor slowing in HIV. J Neuroimmunol. 2004;157:147–152. doi: 10.1016/j.jneuroim.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 13.Kantarci K, Jack CR, Xu YC, et al. Regional metabolic patterns in mild cognitive impairment and Alzheimer's disease: A 1H MRS study. Neurology. 2000;55:210–217. doi: 10.1212/wnl.55.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Summers M, Swanton J, Fernando K, et al. Cognitive impairment in multiple sclerosis can be predicted by imaging early in the disease. J Neurol Neurosur Ps. 2008;79:955–958. doi: 10.1136/jnnp.2007.138685. [DOI] [PubMed] [Google Scholar]
- 15.Eagan DE, Gonzales MM, Tarumi T, Tanaka H, Stautberg S, Haley AP. Elevated serum C-reactive protein relates to increased cerebral myo-inositol levels in middle-aged adults. Cardiovasc Psychiatry Neurol. 2012 doi: 10.1155/2012/120540. Epub 2012 Feb 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bailey-Downs LC, Tucsek Z, Toth P, et al. Aging Exacerbates Obesity-Induced Oxidative Stress and Inflammation in Perivascular Adipose Tissue in Mice: A Paracrine Mechanism Contributing to Vascular Redox Dysregulation and Inflammation. J Gerontol A Biol Sci Med Sci. 2012 doi: 10.1093/gerona/gls238. Epub ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 18.NIH. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: The evidence report. National Institutes of Health; 1998. [PubMed] [Google Scholar]
- 19.Knopman D, Boland LL, Mosley T, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology. 2001;56:42–48. doi: 10.1212/wnl.56.1.42. [DOI] [PubMed] [Google Scholar]
- 20.Meyer JS, Rauch GM, Crawford K, et al. Risk factors accelerating cerebral degenerative changes, cognitive decline and dementia. Int J Geriatr Psych. 1999;14:1050–1061. doi: 10.1002/(sici)1099-1166(199912)14:12<1050::aid-gps56>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 21.García Santos JM, Fuentes LJ, Vidal JB, et al. Posterior paralimbic and frontal metabolite impairments in asymptomatic hypertension with different treatment outcomes. Hypertens Res. 2010;33:67–75. doi: 10.1038/hr.2009.176. [DOI] [PubMed] [Google Scholar]
- 22.Geissler A, Fründ R, Schölmerich J, Feuerbach S, Zietz B. Alterations of cerebral metabolism in patients with diabetes mellitus studied by proton magnetic resonance spectroscopy. Exp Clin Endocrinol Diabetes. 2003;111:421–427. doi: 10.1055/s-2003-44289. [DOI] [PubMed] [Google Scholar]
- 23.Haley AP, Gonzales MM, Tarumi T, Miles SC, Goudarzi K, Tanaka H. Elevated cerebral glutamate and myo-inositol levels in cognitively normal middle-aged adults with metabolic syndrome. Metab Brain Dis. 2010;25:397–405. doi: 10.1007/s11011-010-9221-y. [DOI] [PubMed] [Google Scholar]
- 24.Reiman EM, Chen KW, Alexander GE, et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer's dementia. P Natl Acad Sci USA. 2004;101:284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haley AP, Gunstad J, Cohen RA, Jerskey BA, Mulligan R, Sweet LH. Neural correlates of visuospatial working memory in healthy young adults at risk for hypertension. Brain Imaging Behav. 2008;2:192–199. [Google Scholar]
- 26.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 27.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 28.Greenberg RA, Sacks DB. Screening for diabetes: is it warranted? Clin Chim Acta. 2002;315:61–69. doi: 10.1016/s0009-8981(01)00722-7. [DOI] [PubMed] [Google Scholar]
- 29.Franssen R, Monajemi H, Stroes ESG, Kastelein JJP. Obesity and Dyslipidemia. Med Clin North Am. 2011;95:893–902. doi: 10.1016/j.mcna.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Farr SA, Yamada KA, Butterfield DA, et al. Obesity and Hypertriglyceridemia produce cognitive impairment. Endocrinology. 2008;149:2628–2636. doi: 10.1210/en.2007-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Frias CA, Bunce D, Wahlin A, et al. Cholesterol and triglycerides moderate the effect of apolipoprotein E on memory functioning in older adults. J Gerontol B-Psychol. 2007;62:P112–P118. doi: 10.1093/geronb/62.2.p112. [DOI] [PubMed] [Google Scholar]
- 32.Perlmuter LC, Nathan DM, Goldfinger SH, Russo PA, Yates J, Larkin M. Triglyceride levels affect cognitive function in noninsulin-dependent diabetics. J Diabet Complications. 1988;2:210–213. doi: 10.1016/s0891-6632(88)80011-4. [DOI] [PubMed] [Google Scholar]
- 33.van den Kommer TN, Dik MG, Comijs HC, Jonker C, Deeg DJH. The role of lipoproteins and inflammation in cognitive decline: Do they interact? Neurobiol Aging. 2012;33 doi: 10.1016/j.neurobiolaging.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 34.Toth PP. The "good cholesterol" - High-density lipoprotein. Circulation. 2005;111:E89–E91. doi: 10.1161/01.CIR.0000154555.07002.CA. [DOI] [PubMed] [Google Scholar]
- 35.Navab M, Ananthramaiah GM, Reddy ST, et al. The oxidation hypothesis of atherogenesis: the role of oxidized phospholipids and HDL. J Lipid Res. 2004;45:993–1007. doi: 10.1194/jlr.R400001-JLR200. [DOI] [PubMed] [Google Scholar]
- 36.Scanu A, Molnarfi N, Brandt KJ, Gruaz L, Dayer JM, Burger D. Stimulated T cells generate microparticles, which mimic cellular contact activation of human monocytes: differential regulation of pro- and anti-inflammatory cytokine production by high-density lipoproteins. J Leukoc Biol. 2008;83:921–927. doi: 10.1189/jlb.0807551. [DOI] [PubMed] [Google Scholar]
- 37.Wadham C, Albanese N, Roberts J, et al. High-density lipoproteins neutralize C-reactive protein proinflammatory activity. Circulation. 2004;109:2116–2122. doi: 10.1161/01.CIR.0000127419.45975.26. [DOI] [PubMed] [Google Scholar]
- 38.Atzmon G, Gabriely I, Greiner W, Davidson D, Schechter C, Barzilai N. Plasma HDL levels highly correlate with cognitive function in exceptional longevity. J Gerontol a-Biol. 2002;57:M712–M715. doi: 10.1093/gerona/57.11.m712. [DOI] [PubMed] [Google Scholar]
- 39.Vaisar T, Pennathur S, Green PS, et al. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J Clin Invest. 2007;117:746–756. doi: 10.1172/JCI26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Movva R, Rader DJ. Laboratory assessment of HDL heterogeneity and function. Clin Chem. 2008;54:788–800. doi: 10.1373/clinchem.2007.101923. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.