Abstract
The reciprocal interactions between sleep and immune function are well-studied. Insufficient sleep induces innate immune responses as evidenced by increased expression of pro-inflammatory mediators in the brain and periphery. Conversely, immune challenges upregulate immunomodulator expression, which alters central nervous system-mediated processes and behaviors, including sleep. Recent studies indicate that glial cells, namely microglia and astrocytes, are active contributors to sleep and immune system interactions. Evidence suggests glial regulation of these interactions is mediated, in part, by adenosine and adenosine 5′-triphosphate actions at purinergic type 1 and type 2 receptors. Furthermore, microglia and astrocytes may modulate declines in sleep-wake behavior and immunity observed in aging.
Introduction
Chronic insufficient sleep is associated with inflammation, metabolic syndrome, cardiovascular disease, increased sensitivity and to pain stimuli, fatigue, excessive daytime sleepiness, and impaired cognitive and physical performance. Symptoms of these pathologies are associated with increased levels of endogenous pro-inflammatory cytokines such as interleukin-1β (IL-1β) and tumor necrosis factor α (TNFα), and can be experimentally elicited by peripheral or central exogenous administration of these cytokines to subjects [1,2]. Conversely, inhibition of IL-1β or TNFα attenuates many sleep-loss associated symptoms. Additionally, reduction of endogenous levels of IL-1β or TNFα, whether in mutant mice or by use of soluble receptors, antibodies, or receptor antagonists, inhibits spontaneous sleep (reviewed [2], [3], and [4]). There is a wealth of evidence indicating that IL-1β and TNFα are also involved in physiological sleep regulation and that their amplification during pathology is causative of characteristic sleep disturbances associated with many pathological states [2-4]. Indeed, the brain, including regions associated with the regulation of sleep-wake behavior, produces and is responsive to cytokines [2,3,5]. Furthermore, neuronal activity upregulates these cytokines in brain regions implicated in the regulation of sleep [6,7]. Impaired sleep also affects adaptive immune responses. Sleep deprivation attenuates antibody responses to vaccine [8,9], whereas good sleep imparts long-lasting immunoenhancing effects [10•,11]. Furthermore, sleep is a profound regulator of cellular immunity and formation of immunological memory critical for adaptive responses to immune challenges (reviewed by [11]).
We acquired substantial insight into sleep and immune system interactions during the last 30 years. However, the cellular substrates for these interactions are less well understood. Understanding the role of glia in sleep and immune system functioning is crucial because research has started to shift the traditional view of these cells as passive constituents of the central nervous system (CNS) to active contributors capable of mediating behavior (see below). Furthermore, because the aforementioned inflammatory pathologies are common in elderly individuals, identifying age-related changes in glial cell functioning may be critical in elucidating the mechanisms driving senescence of sleep and immune networks in aging. This review highlights recent findings implicating a role for glia in sleep and immune interactions and aging.
Microglia in sleep and immune function
Microglia are the resident immune cells of the CNS that are mobilized and activated in response to an immune challenge. The role of microglia in mediating responses to immune challenge has been studied exhaustively. Microglial influences on sleep-wake behavior are not extensively studied although recent data implicate microglia in sleep regulation. Microglia assume a deramified morphology, a marker of activation, in response to sleep deprivation [12]. In addition, slow wave activity is reduced following administration of minocycline, an inhibitor of microglial activation [13]. Minocycline inhibits microglial production of immunomodulators including cytokines and nitric oxide [13]. Intraperitoneal minocycline administration induces an acute increase in wakefulness and significantly reduces non-rapid eye movement sleep (NREMS) compared to saline-treated mice [14••]. Furthermore, minocycline inhibits sleep deprivation-induced augmentation of NREMS delta power, a surrogate indicator of sleep depth [14••]. Although data are limited, recent studies indicate microglia are potentially critical components of sleep regulatory mechanisms.
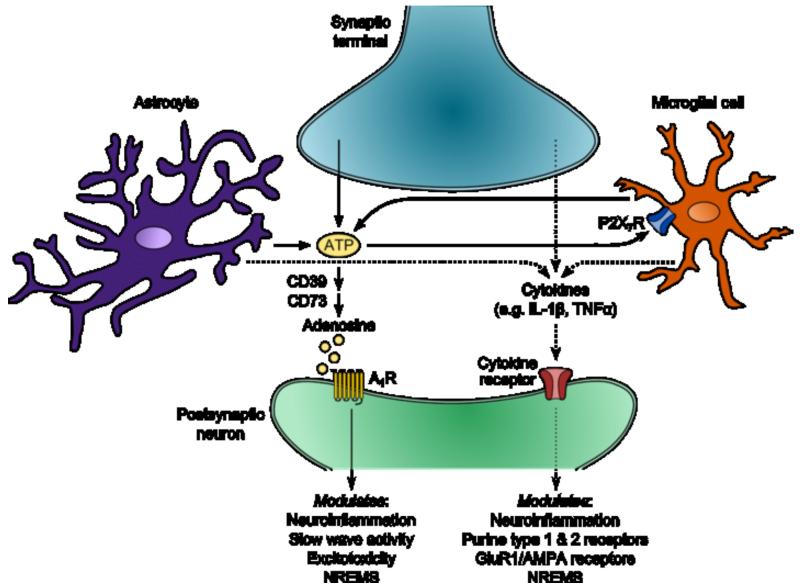
A possible effector of microglial influences on sleep-wake behavior may be extracellular adenosine-5′-triphosphate (ATP) acting at the purinergic type 2 receptor P2X7 (P2X7R). The P2X7R links increased cellular activity during waking to adenosine and cytokine sleep modulation. Activation of glial P2X7R by extracellular ATP mediates post-translational processing of sleep regulatory substances including IL-1β, TNFα, and IL-6 [3,15,16]. P2X7R expression in brain is most prominent on microglia [15,16], and neurons and glia release ATP into the extracellular space in response to cellular activity [3]. Administration of P2X7R agonists increases time spent in NREMS and enhances electroencephalographic (EEG) delta power [17]. Conversely, P2X7R inhibition reduces NREMS in rats [17]. Furthermore, mice lacking the P2X7R exhibit less robust increases in NREMS and EEG delta power in response to sleep deprivation compared to wild type animals [17]. Collectively, the data suggest a role for microglia and purinergic receptors as one component of systems and networks that mediate sleep and immune interactions (Figure 1).
Figure 1.
Glial modulation of sleep and immune interactions. Cellular activity causes ATP release into the extracellular space via neurotransmission and gliotransmission. Extracellular ATP induces rapid effects once metabolized to adenosine by ectonucleotidases such as CD39 and CD73. Adenosine binds to purine type 1 receptors like the A1R to modulate EEG slow wave activity and NREMS, as well as local neuroinflammation and excitotoxicity. Slower effects of extracellular ATP occur through direct activation of purine type 2 receptors such as the P2X7R prominently expressed on microglia. P2X7R activation induces processing and release of cytokines including, but not limited to, IL-1β and TNFα. Cytokines subsequently act on their respective receptors to activate transcription factors like nuclear factor-κB (not shown) which modulates neuroinflammation, physiological and pathological sleep, and gene transcription of receptors such as the A1R, AMPA, and the AMPA subunit GluR1. This overly simplified schematic focuses on the featured topics of this mini-review, and thus, not all cellular and molecular components are fully represented. AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors; GluR1, glutamate receptor 1.
Astrocytes in sleep and immune function
A traditional view of astrocytes was that they played a passive, supportive role for neurons. However, recent studies demonstrate that these cells are active contributors to complex behaviors and immune responses. Astrocytes are the most abundant glial cell type in the brain, respond rapidly to inflammation, express receptors for immunomodulators, and produce sleep regulatory substances in response to immune challenge [18,19]. Selectively inhibiting astrocyte gliotransmission via the dominant negative (dnSNARE) mouse reduces EEG slow wave activity during NREMS, a traditional measure of sleep pressure [20]1. Inhibition of vesicular release from astrocytes also attenuates the increase of NREMS and cognitive deficits typically observed subsequent to 6 h of sleep deprivation [20]. These data suggest astrocytic gliotransmission contributes to the modulation of sleep need.
Because altered gliotransmission of astrocytes results in reduced sleep pressure, studies have turned to astrocyte-derived adenosine, an ATP metabolite, as a potential molecular substrate of this effect. Adenosine accumulates in brain with increasing time awake [22••], and extracellular elevation of adenosine concentrations is astrocyte dependent [22••,23]. Indeed, inhibition of the adenosine 1 receptor (A1R) in wild type mice recapitulates the reductions of baseline EEG slow wave activity and responses to sleep deprivation observed in gliotransmission-impaired dnSNARE mice [20,24]. Conditional CNS A1R knockout mice also fail to demonstrate enhanced EEG delta power following intermittent sleep deprivation [25]. Conversely, mice lacking CD73, an ectonucleotidase that converts extracellular ATP to adenosine, have more spontaneous NREMS than wild type controls [26•]. Consistent with the notion that the A1R mediates sleep need, chronic sleep restriction increases A1R mRNA expression in the wake-promoting basal forebrain in rats [27] although not in the sleep-promoting hypothalamus of mice [26•].
In response to immune challenge, A1R activity is upregulated to impart neuroprotection via generating neurotrophic factors, downregulating excitotoxicity, preventing excessive astrogliosis, and inhibiting pro-inflammatory cytokines [28-31]. Blockade of this receptor increases hippocampal injury in response to hypoxia [32] and mortality to infectious disease [33]. Furthermore, lipopolysaccharide (LPS)-induced elevations of EEG slow wave activity are attenuated in gliotransmission-impaired dnSNARE mice, an effect mimicked by central inhibition of the A1R in wild type mice [34••]. Although, studies regarding the impact of inhibiting the A1R or gliotransmission on sleep-immune interactions are generally lacking, current data suggest astroglial modulation of sleep and immune function is mediated, in part, by astrocyte-derived ATP and/or adenosine and subsequent activation of purine type 1 and 2 receptors (Figure 1).
Sleep, immune function, and aging
Sleep alterations are a well-documented feature of aging. Sleep in old age is characterized by more fragmentation, less rapid eye movement sleep (REMS), reduced time in deeper stages of NREMS (i.e. stages N2 and N3), decreased EEG delta power, and more time spent in lighter stages of NREMS which results in more nighttime awakenings. Furthermore, sleep onset is progressively earlier and is accompanied by early morning wake time and more frequent daytime napping (for review see [35] and [36]). Although healthy aging need not be associated with sleep complaints, the elderly frequently indicate they have difficulty initiating or maintaining sleep [37]. Increased severity of these alterations in sleep can increase susceptibility to disease and predict age-related disease onset [38,39]. Indeed, several brain regions associated with sleep-wake behavior are impacted by neurodegenerative disease [35].
Poor sleep can also exacerbate the age-related changes in immune system functioning. “Inflammaging” is the term used to describe the homeostatic shift to a chronic, low-grade inflammatory state in aged individuals [40]. This change is manifest by increased inflammatory mediators centrally and peripherally [41], enhanced production of reactive oxygen and nitrogen species [42], as well as suppression of anti-inflammatory mediators and antioxidants [43]. A similar syndrome is elicited by chronic sleep loss in younger subjects. This shift predisposes one to exacerbated responses to immune challenge compared to that of normal, younger individuals. Indeed, the severity of this low-grade inflammatory state is predictive of all-cause mortality in the elderly [44]. Alternatively, longevity is inversely correlated with plasma concentrations of IL-6 [45], a mediator of chronic inflammation in aging [46]. Inflammation is also implicated in contributing to poor sleep observed in the elderly. However, recent data demonstrate that moderate exercise decreases pro-inflammatory cytokines IL-1β and TNFα and increases the anti-inflammatory cytokine IL-10 in aged individuals [47•,48•]. This effect is associated with improved sleep maintenance and enhanced quality of life [47•]. Exercise also increases circulating concentrations of neurotrophic factors which are associated with increased temporal lobe connectivity indicative of improved neurocognition [49]. Lastly, better sleep efficiency is associated with lower concentrations of IL-6 in elderly women [50].
Although poor sleep in aged individuals increases immunomodulators and, likewise, inflammation alters sleep, the dynamics differ from those observed in younger counterparts. For example, pre-clinical data demonstrate that intracerebroventricular administration of IL-1β reduces REMS to a similar extent in aged and middle-aged rats. However, in aged rats, there is no observed increase in NREMS characteristic of IL-1β challenge in younger animals [51]. These data indicate a potential dysregulation of interactions between sleep and immune networks in aging. Furthermore, the lack of NREMS response may contribute to increased morbidity and mortality in response to immune challenge in the elderly [52]. Studies regarding the cumulative effects of aging on sleep and immune function are generally lacking. However, understanding these interactions is increasingly important with the rapid growth of the 65 years and older population.
Glial contributions to aging
Age-related changes of the CNS are not attributed to neuronal loss per se, but are a consequence of synaptic alterations, which may be due to decreased synaptic contacts (reduced dendritic spines) or molecular alterations of intact synapses [53]. With age, there is a progressive decline in gene expression relating to vesicular function, receptor trafficking, post-synaptic density scaffolding, and neurotrophic systems [54]. Glia are strong candidates for effectors of age-related alterations in sleep and immune function as these cells are known mediators of synaptic homeostasis [55]. One of the characteristics of aging is a morphological shift of microglia and astrocytes to a primed or activated state [40,56]. Excessive and prolonged production of pro-inflammatory cytokines in aged CNS in response to systemic immune challenge with LPS is a result of primed microglia [57,58]. Also, P2X7R expression increases with age in mouse brain, an effect associated with damage to the post-synaptic density [59••]. Furthermore, astrocytes become hypersensitive after exposure to microglial-conditioned media, which may perpetuate the chronic inflammatory state in aging [60•]. Aging brain is associated with increased astrogliosis [57], and astrocytes produce 10-fold more IL-6 in aged patients relative to younger counterparts [61•]. Interestingly, peripheral concentrations of cytokines do not necessarily reflect neuroinflammatory states [57]. Collectively, these data suggest that aging may be a centrally mediated process driven by glial alterations of the CNS milieu.
Conclusions
Reciprocal influences between sleep and immune system are well-documented, but the cellular and molecular substrates modulating in these interactions are not completely understood. Current data demonstrating glial contributions to sleep regulation suggests further investigation is warranted as these cells may be critical mechanistic components of sleep and immune interactions throughout the lifespan. Future studies might aim to address the immune consequences of inhibiting glial activity and how this relates to sleep alterations. Also, the mechanisms driving dysregulation of sleep and central immune responses in aging are not well defined. Answering these, and other questions, is necessary before network components of sleep-immune interactions and their role in healthy aging may be fully elucidated.
Highlights.
Microglia modulate NREMS and slow wave activity via production of immunomodulators.
Astrocytic gliotransmission mediates NREMS and sleep pressure.
ATP (via P2X7R) and adenosine (via A1R) may be effectors of glial sleep regulation.
Aging is associated with poor sleep quality and chronic, low-grade inflammation.
Primed microglia and activated astrocytes contribute to changes of the aging CNS.
Acknowledgements
This work was supported in part by the National Institutes of Health, grants NS025378, NS031453 and HD036520 to JMK and AG041827 and MH064843 to MRO.
Footnotes
EEG slow wave activity is regulated independently from duration of NREMS [21]. There is good evidence for the involvement of extracellular adenosine in its regulation; this might occur via vasodilation induced by adenosine since cerebral blood flow alters EEG slow wave power.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
• of special interest
•• of outstanding interest
- 1.Dantzer R. Cytokine-induced sickness behavior: where do we stand? Brain, Behavior and Immunity. 2001;15:7–24. doi: 10.1006/brbi.2000.0613. [DOI] [PubMed] [Google Scholar]
- 2.Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nat.Rev.Neurosci. 2009;10:199–210. doi: 10.1038/nrn2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krueger JM, Rector DM, Roy S, Van Dongen HP, Belenky G, Panksepp J. Sleep as a fundamental property of neuronal assemblies. Nat.Rev.Neurosci. 2008;9:910–919. doi: 10.1038/nrn2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krueger JM. Translation of brain activity into sleep. Hirosaki Med J. 2011;62:S1–S16. [PMC free article] [PubMed] [Google Scholar]
- 5.Dantzer R. Cytokine, sickness behavior, and depression. Immunology and allergy clinics of North America. 2009;29:247–264. doi: 10.1016/j.iac.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Churchill L, Rector DM, Yasuda K, Fix C, Rojas MJ, Yasuda T, Krueger JM. Tumor necrosis factor alpha: activity dependent expression and promotion of cortical column sleep in rats. Neuroscience. 2008;156:71–80. doi: 10.1016/j.neuroscience.2008.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hallett H, Churchill L, Taishi P, De A, Krueger JM. Whisker stimulation increases expression of nerve growth factor- and interleukin-1beta-immunoreactivity in the rat somatosensory cortex. Brain Research. 2010;1333:48–56. doi: 10.1016/j.brainres.2010.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lange T, Perras B, Fehm H, Born J. Sleep enhances the human antibody response to hepatitis A vaccination. Psychosom Med. 2003;65:831–835. doi: 10.1097/01.psy.0000091382.61178.f1. [DOI] [PubMed] [Google Scholar]
- 9.Spiegel K, Sheridan JF, Van Cauter E. Effect of sleep deprivation on response to immunization. Journal of the American Medical Association. 2002;288:1471–1472. doi: 10.1001/jama.288.12.1471-a. [DOI] [PubMed] [Google Scholar]
- •10.Lange T, Dimitrov S, Bollinger T, Diekelmann S, Born J. Sleep after vaccination boosts immunological memory. Journal of Immunology. 2011;187:283–290. doi: 10.4049/jimmunol.1100015. Normal sleep, as opposed to sleep deprivation, during the night following vaccination against hepatitis A enhances Th1 cytokine activity, an indicator of cellular adaptive immune responses. In addition, EEG slow wave activity the night after vaccination is predictive of the subsequent T-helper cell response even at 1 year post-vaccination.
- 11.Besedovsky L, Lange T, Born J. Sleep and immune function. Pflugers Archiv: European journal of physiology. 2012;463:121–137. doi: 10.1007/s00424-011-1044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu JC, Lee YS, Chang CN, Chuang HL, Ling EA, Lan CT. Sleep deprivation inhibits expression of NADPH-d and NOS while activating microglia and astroglia in the rat hippocampus. Cells Tissues Organs. 2003;173:242–254. doi: 10.1159/000070380. [DOI] [PubMed] [Google Scholar]
- 13.Kim HS, Suh YH. Minocycline and neurodegenerative diseases. Behav Brain Res. 2009;196:168–179. doi: 10.1016/j.bbr.2008.09.040. [DOI] [PubMed] [Google Scholar]
- ••14.Wisor JP, Schmidt MA, Clegern WC. Evidence for neuroinflammatory and microglial changes in the cerebral response to sleep loss. Sleep. 2011;34:261–272. doi: 10.1093/sleep/34.3.261. Microglial inhibition by the anti-inflammatory minocycline induces a reduction in NREMS and attenuates increases in NREM delta power normally observed after sleep deprivation. These data suggest microglia contribute to the modulation of sleep need.
- 15.Choi HB, Ryu JK, Kim SU, McLarnon JG. Modulation of the purinergic P2X7 receptor attenuates lipopolysaccharide-mediated microglial activation and neuronal damage in inflamed brain. J Neurosci. 2007;27:4957–4968. doi: 10.1523/JNEUROSCI.5417-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mingam R, De Smedt V, Amedee T, Bluthe RM, Kelley KW, Dantzer R, Laye S. In vitro and in vivo evidence for a role of the P2X7 receptor in the release of IL-1 beta in the murine brain. Brain, behavior, and immunity. 2008;22:234–244. doi: 10.1016/j.bbi.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krueger JM, Taishi P, De A, Davis CJ, Winters BD, Clinton J, Szentirmai E, Zielinski MR. ATP and the purine type 2 X7 receptor affect sleep. Journal of applied physiology. 2010;109:1318–1327. doi: 10.1152/japplphysiol.00586.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends in Immunology. 2007;28:138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Dong Y, Benveniste EN. Immune function of astrocytes. GLIA. 2001;36:180–190. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- 20.Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, Abel T, Haydon PG, Frank MG. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–219. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis CJ, Clinton JM, Jewett KA, Zielinski MR, Krueger JM. Delta wave power: an independent sleep phenotype or epiphenomenon? Journal of clinical sleep medicine: JCSM: official publication of the American Academy of Sleep Medicine. 2011;7:S16–18. doi: 10.5664/JCSM.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••22.Schmitt LI, Sims RE, Dale N, Haydon PG. Wakefulness affects synaptic and network activity by increasing extracellular astrocyte-derived adenosine. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:4417–4425. doi: 10.1523/JNEUROSCI.5689-11.2012. Using a biosensor-based analysis of adenosine, this study demonstrates that extracellular adenosine increases with wakefulness. This accumulation of adenosine is dependent on astrocytic gliotransmission and mediates activity of the A1R as demonstrated by genetic inhibition of gliotransmission.
- 23.Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- 24.Florian C, Vecsey CG, Halassa MM, Haydon PG, Abel T. Astrocyte-derived adenosine and A1 receptor activity contribute to sleep loss-induced deficits in hippocampal synaptic plasticity and memory in mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:6956–6962. doi: 10.1523/JNEUROSCI.5761-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bjorness TE, Kelly CL, Gao T, Poffenberger V, Greene RW. Control and function of the homeostatic sleep response by adenosine A1 receptors. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:1267–1276. doi: 10.1523/JNEUROSCI.2942-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •26.Zielinski MR, Taishi P, Clinton JM, Krueger JM. 5′-Ectonucleotidase-knockout mice lack non-REM sleep responses to sleep deprivation. The European journal of neuroscience. 2012;35:1789–1798. doi: 10.1111/j.1460-9568.2012.08112.x. Mice lacking CD73, an ectonucleotidase that aids in the metabolism of ATP to adenosine, exhibit more spontaneous NREMS and attenuated response to sleep deprivation compared to wild type controls. These data suggest extracellular adenosine regulation of sleep is mediated, in part, by CD73.
- 27.Kim Y, Bolortuya Y, Chen L, Basheer R, McCarley RW, Strecker RE. Decoupling of sleepiness from sleep time and intensity during chronic sleep restriction: evidence for a role of the adenosine system. Sleep. 2012;35:861–869. doi: 10.5665/sleep.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lauro C, Cipriani R, Catalano M, Trettel F, Chece G, Brusadin V, Antonilli L, van Rooijen N, Eusebi F, Fredholm BB, et al. Adenosine A1 receptors and microglial cells mediate CX3CL1-induced protection of hippocampal neurons against Glu-induced death. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2010;35:1550–1559. doi: 10.1038/npp.2010.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jhaveri KA, Toth LA, Sekino Y, Ramkumar V. Nitric oxide serves as an endogenous regulator of neuronal adenosine A1 receptor expression. J Neurochem. 2006;99:42–53. doi: 10.1111/j.1471-4159.2006.04095.x. [DOI] [PubMed] [Google Scholar]
- 30.Barrie AP, Nicholls DG. Adenosine A1 receptor inhibition of glutamate exocytosis and protein kinase C-mediated decoupling. J Neurochem. 1993;60:1081–1086. doi: 10.1111/j.1471-4159.1993.tb03257.x. [DOI] [PubMed] [Google Scholar]
- 31.Ciccarelli R, Ballerini P, Sabatino G, Rathbone MP, D’Onofrio M, Caciagli F, Di Iorio P. Involvement of astrocytes in purine-mediated reparative processes in the brain. Int J Dev Neurosci. 2001;19:395–414. doi: 10.1016/s0736-5748(00)00084-8. [DOI] [PubMed] [Google Scholar]
- 32.Boissard CG, Lindner MD, Gribkoff VK. Hypoxia produces cell death in the rat hippocampus in the presence of an A1 adenosine receptor antagonist: an anatomical and behavioral study. Neuroscience. 1992;48:807–812. doi: 10.1016/0306-4522(92)90268-7. [DOI] [PubMed] [Google Scholar]
- 33.Gallos G, Ruyle TD, Emala CW, Lee HT. A1 adenosine receptor knockout mice exhibit increased mortality, renal dysfunction, and hepatic injury in murine septic peritonitis. American journal of physiology. Renal physiology. 2005;289:F369–376. doi: 10.1152/ajprenal.00470.2004. [DOI] [PubMed] [Google Scholar]
- ••34.Nadjar A, Blutstein T, Aubert A, Laye S, Haydon PG. Astrocyte-derived adenosine modulates increased sleep pressure during inflammatory response. GLIA. 2013 doi: 10.1002/glia.22465. n/a-n/a. Systemic immune challenge with LPS increases EEG slow wave activity in wild type mice but not in a mouse model of impaired astrocyte gliotransmission. This effect is mediated by astrocyte-derived adenosine activation of the A1R. However, other mechanisms are implicated in modulating LPS-induced alterations of NREMS and REMS duration.
- 35.Singletary KG, Naidoo N. Disease and Degeneration of Aging Neural Systems that Integrate Sleep Drive and Circadian Oscillations. Frontiers in neurology. 2011;2:66. doi: 10.3389/fneur.2011.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rolls A. Hypothalamic control of sleep in aging. Neuromolecular Med. 2012;14:139–153. doi: 10.1007/s12017-012-8175-0. [DOI] [PubMed] [Google Scholar]
- 37.Espiritu JR. Aging-related sleep changes. Clinics in geriatric medicine. 2008;24:1–14. v. doi: 10.1016/j.cger.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 38.Gibson EM, Williams WP, 3rd, Kriegsfeld LJ. Aging in the circadian system: considerations for health, disease prevention and longevity. Experimental gerontology. 2009;44:51–56. doi: 10.1016/j.exger.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Postuma RB, Montplaisir J. Predicting Parkinson’s disease - why, when, and how? Parkinsonism & related disorders. 2009;15(Suppl 3):S105–109. doi: 10.1016/S1353-8020(09)70793-X. [DOI] [PubMed] [Google Scholar]
- 40.Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mechanisms of Ageing and Development. 2007;128:92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 41.Ye SM, Johnson RW. Increased interleukin-6 expression by microglia from brain of aged mice. Journal of Neuroimmunology. 1999;93:139–148. doi: 10.1016/s0165-5728(98)00217-3. [DOI] [PubMed] [Google Scholar]
- 42.Vishwas DK, Mukherjee A, Haldar C, Dash D, Nayak MK. Improvement of oxidative stress and immunity by melatonin: An age dependent study in golden hamster. Experimental gerontology. 2012 doi: 10.1016/j.exger.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 43.Ye SM, Johnson RW. An age-related decline in interleukin-10 may contribute to the increased expression of interleukin-6 in brain of aged mice. Neuroimmunomodulation. 2001;9:183–192. doi: 10.1159/000049025. [DOI] [PubMed] [Google Scholar]
- 44.Bauer ME. Chronic stress and immunosenescence: a review. Neuroimmunomodulation. 2008;15:241–250. doi: 10.1159/000156467. [DOI] [PubMed] [Google Scholar]
- 45.Maggio M, Guralnik JM, Longo DL, Ferrucci L. Interleukin-6 in aging and chronic disease: a magnificent pathway. The journals of gerontology. Series A, Biological sciences and medical sciences. 2006;61:575–584. doi: 10.1093/gerona/61.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Freund A, Orjalo AV, Desprez PY, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med. 2010;16:238–246. doi: 10.1016/j.molmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •47.Santos RV, Viana VA, Boscolo RA, Marques VG, Santana MG, Lira FS, Tufik S, de Mello MT. Moderate exercise training modulates cytokine profile and sleep in elderly people. Cytokine. 2012;60:731–735. doi: 10.1016/j.cyto.2012.07.028. Moderate exercise in previously sedentary elderly adults improves sleep, reduces inflammation, and increases anti-inflammatory mediators compared to baseline measurements.
- •48.Speisman RB, Kumar A, Rani A, Foster TC, Ormerod BK. Daily exercise improves memory, stimulates hippocampal neurogenesis and modulates immune and neuroimmune cytokines in aging rats. Brain, Behavior, and Immunity. 2012 doi: 10.1016/j.bbi.2012.09.013. Daily exercise in aged rats reduces IL-1β concentrations and increases neurotrophic factors in hippocampus as well as improves cognitive function compared to non-running controls. Additionally, exercise increases neurogenesis in aged rats. This study also demonstrates that circulating cytokines do not necessarily reflect neuroinflammatory conditions.
- 49.Voss MW, Erickson KI, Prakash RS, Chaddock L, Kim JS, Alves H, Szabo A, Phillips SM, Wojcicki TR, Mailey EL, et al. Neurobiological markers of exercise-related brain plasticity in older adults. Brain, Behavior, and Immunity. 2013;28:90–99. doi: 10.1016/j.bbi.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Friedman EM, Hayney MS, Love GD, Urry HL, Rosenkranz MA, Davidson RJ, Singer BH, Ryff CD. Social relationships, sleep quality, and interleukin-6 in aging women. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:18757–18762. doi: 10.1073/pnas.0509281102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Imeri L, Ceccarelli P, Mariotti M, Manfridi A, Opp MR, Mancia M. Sleep, but not febrile responses of Fisher 344 rats to immune challenge are affected by aging. Brain, Behavior and Immunity. 2004;18:399–404. doi: 10.1016/j.bbi.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 52.Yoshikawa TT. Epidemiology and unique aspects of aging and infectious diseases. Clinical Infectious Diseases. 2000;30:931–933. doi: 10.1086/313792. [DOI] [PubMed] [Google Scholar]
- 53.Hof PR, Morrison JH. The aging brain: morphomolecular senescence of cortical circuits. Trends in Neurosciences. 2004;27:607–613. doi: 10.1016/j.tins.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 54.Berchtold NC, Coleman PD, Cribbs DH, Rogers J, Gillen DL, Cotman CW. Synaptic genes are extensively downregulated across multiple brain regions in normal human aging and Alzheimer’s disease. Neurobiology of Aging. 2012 doi: 10.1016/j.neurobiolaging.2012.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dilger RN, Johnson RW. Aging, microglial cell priming, and the discordant central inflammatory response to signals from the peripheral immune system. Journal of Leukocyte Biology. 2008;84:932–939. doi: 10.1189/jlb.0208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2005;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- 58.Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain, Behavior, and Immunity. 2009;23:309–317. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••59.Lee HG, Won SM, Gwag BJ, Lee YB. Microglial P2X(7) receptor expression is accompanied by neuronal damage in the cerebral cortex of the APPswe/PS1dE9 mouse model of Alzheimer’s disease. Exp Mol Med. 2011;43:7–14. doi: 10.3858/emm.2011.43.1.001. Microglial P2X7R expression increases with age in wild type mice, and this increase is accelerated in a mouse model of Alzheimer’s disease. Elevated P2X7R also precedes increases in reactive oxygen species and amyloid β peptide.
- •60.Henn A, Kirner S, Leist M. TLR2 hypersensitivity of astrocytes as functional consequence of previous inflammatory episodes. J Immunol. 2011;186:3237–3247. doi: 10.4049/jimmunol.1002787. Under baseline conditions, astroglial cultures are unresponsive to TLR2 ligands. However, following pre-stimulation with microglial-condition media or inflammatory substances, astrocytes become hypersensitive and demonstrate a prolonged response to TLR2 ligands.
- •61.Bhat R, Crowe EP, Bitto A, Moh M, Katsetos CD, Garcia FU, Johnson FB, Trojanowski JQ, Sell C, Torres C. Astrocyte senescence as a component of Alzheimer’s disease. PLoS One. 2012;7:e45069. doi: 10.1371/journal.pone.0045069. Senescent astrocytes produce more IL-6 and accumulate in greater numbers with age. This accumulation of senescent astrocytes is further exacerbated in Alzheimer’s disease patients.