Abstract
Background
The brain undergoes major remodeling during adolescence, resulting in improved cognitive control and decision-making, and reduced impulsivity, components of behavior mediated in part by the maturing frontal lobe. Gamma-amino butyric acid (GABA), the main inhibitory neurotransmitter system, also matures during adolescence, with frontal lobe GABA receptors reaching adult levels late in adolescence. Thus, the objective of this study was to characterize in vivo developmental differences in brain GABA levels.
Methods
Proton magnetic resonance spectroscopy (MRS) was employed at 4 Tesla to acquire metabolite data from the anterior cingulate cortex (ACC) and the parieto-occipital (POC) cortex in adolescents (n=30) and emerging adults (n=20).
Results
ACC GABA/Cr levels were significantly lower in adolescents relative to emerging adults, whereas no age differences were observed in the POC. Lower ACC GABA/Cr levels were significantly associated with greater impulsivity and worse response inhibition, with relationships being most pronounced for ACC GABA/Cr and No-Go response inhibition in adolescent males.
Conclusions
These data provide the first human developmental in vivo evidence confirming frontal lobe GABA maturation, which was linked to impulsiveness and cognitive control. These findings suggest that reduced GABA may be an important neurobiological mechanism in the immature adolescent brain, contributing to the reduced, yet rapidly developing, ability to inhibit risky behaviors and to make decisions, which could compromise adolescent health and safety.
Keywords: adolescent, emerging adult, MRS, GABA, ACC, menstrual cycle
Introduction
The brain undergoes major remodeling during adolescence (1), including alterations in cerebral structure and function (2,3) that lead to improved cognitive control and decision-making (4,5), as well as reduced impulsivity and risk taking (6). These behavioral components are mediated in part by the frontal lobe (7–9), which is the last brain region to mature in humans (10). Gamma-amino butyric acid (GABA), the major inhibitory neural system in mammalian brain, also undergoes marked maturation during adolescence (11). Thus, developmental GABA alterations may be involved in age-related improvements on response inhibition tasks, or the ability to “hold back” less optimal or inappropriate responding, mediated in part by the maturing frontal cortex.
In rats, GABA concentrations at birth are 50% of adult levels (12). Glutamic acid decarboxylase (GAD), which catalyzes the decarboxylation of glutamate to GABA and carbon dioxide, and the density of postsynaptic GABA receptors also increase linearly to reach adults levels, but lag behind the increase in GABA concentrations (12–14). Given that GABA also plays a prominent role in the metabolism of glucose and fatty acids and is taken up by non-neuronal cells early in development, GABA concentrations may be a more liberal index of GABA development than enzymatic activity or receptor density (12). In non-human primates, chandelier inhibitory interneurons undergo marked developmental increases in terminal density in the prefrontal cortex (PFC), while GABA plasma membrane transporters (GAT) remain stable (15). Postnatal changes in GABA binding are more pronounced in humans, with GABAergic receptor density increasing fivefold during the perinatal period, followed by an additional 100% increase several weeks thereafter (16). GABAA receptors reach adult levels by age 18 in frontal cortex and age 19.5 in the prefrontal cortex (PFC), unlike subcortical structures that reach adult levels earlier (e.g., basal ganglia, age 14) (17). In addition, a recent human post mortem study examining expression of interneuron markers in the dorsolateral PFC (DLPFC) provides support that GABAergic neurons continue to differentiate and mature through the second decade of life (18).
Recent advances in proton magnetic resonance spectroscopy (1H-MRS) have significantly improved the ability to detect and quantify in vivo brain GABA (19–25). The development of specialized editing techniques was necessary given that the concentration of GABA is near the lower limit of detection and is obscured by metabolite peaks of higher concentrations, especially creatine (Cr). Low GABA, measured using 1H-MRS, has been observed in a number of pathological conditions in adults including epilepsy, anxiety, depression, obsessive-compulsive disorder, and alcohol and cocaine dependence (26–37). More recently, adolescents with major depressive disorder were reported to have lower GABA in the anterior cingulate cortex (ACC) compared to healthy comparison subjects (38). Importantly, some pharmacologic treatments for these psychiatric and neurological conditions, as well as natural interventions such as yoga, have been reported to increase GABA (39–41).
To date, there are no published developmental data comparing in vivo brain GABA levels in healthy human adolescents with adults. Based on previous structural and functional developmental findings (3,4,10,18), the à priori hypotheses of this study were that 12–14 year old adolescents (ADO) would exhibit lower GABA than 18–24 year old emerging adults (EA) in the ACC, but no differences would be observed in a comparison region in the parieto-occipital cortex (POC). There are some published data available relating GABA in the DLPFC to impulsivity and unconscious motor control, however, these reports are limited to adults (42,43). Although investigations of the ACC have failed to show a relationship between GABA and impulsivity in adults, the ACC was chosen for examination in the present study because this region has been implicated in the development of higher-order cognitive processes, including cognitive control, response selection, and decision-making (44–52). Therefore, we hypothesized that lower ACC GABA would predict greater impulsivity on the Barratt Impulsivity Scale and worse response inhibition on Go No-Go (GNG) and Stroop Color-Word tasks in both our subject groups. Given that menstrual cycle phase has been shown to influence GABA (29), an exploratory aim of the study was to investigate the influence of menstrual cycle phase on the research findings.
Methods and Materials
Participants
Participants included 30 healthy ADO (12–14 years, 15 females) and 20 healthy EA (18–24 years, 10 females), with middle-upper class socioeconomic status (53) (Table 1). The clinical research protocol was approved by the Institutional Review Board of McLean Hospital. After complete study description, all subjects and ADO parent(s)/guardian(s) provided written informed assent/consent. Participants completed urine screening prior to scanning to rule out current psychoactive substance use and pregnancy. Participants were free of psychiatric diagnoses based on Kiddie-Schedule for Affective Disorders and Schizophrenia interviews (ADO) (54) or the Structured Clinical Interview for DSM-IV Non-Patient Edition (EA) (55). Participants had no prior head trauma or loss of consciousness, and were free of radiologic brain abnormalities, MR scanning contraindications, or current psychoactive substance use, including nicotine. Less than 3 lifetime episodes of alcohol use and no history of drug use were reported in ADO, and EA average alcohol use was 1.3±1.1 alcoholic drinks on 2.2±1.8 occasions/month. Menstrual cycle status was determined by self-report: 33% of ADO and 60% of EA females were in the follicular phase (cycle days 2–9); 33% of ADO and 30% of EA females were in the luteal phase (cycle days 13–32); 20% of ADO females had not yet begun cycling; and in 14% of ADO and 10% of EA, menstrual cycle information was unavailable.
Table 1.
Demographic and Clinical Data
| Subject Demographics | ADO (n=30) | EA (n=20) | p |
|---|---|---|---|
| Age | 13.6 ± 0.9 | 21.6 ± 1.7 | .0001 |
| Education | 7.3 ± 0.9 | 14.7 ± 1.3 | .0001 |
| Female | 50% | 50% | ns |
| Handedness | 27R, 3L | 20R, 0L | - |
| Ethnicity | 87% Caucasian | 75% Caucasian | - |
|
| |||
| Barratt Impulsivity | ADO (n=30) | EA (n=20) | p |
| Attention | 15.3 ± 4.2 | 14.6 ± 3.4 | ns |
| Motor | 24.2 ± 4.1 | 18.7 ± 2.9 | .0001 |
| Non-Planning | 26.3 ± 4.8 | 21.6 ± 4.3 | .001 |
| Total Score | 65.8 ± 10.3 | 54.8 ± 8.5 | .0001 |
Data represent mean values ± SD.
Abbreviations: ADO, adolescent; EA, emerging adult; ns, not statistically significant, p >.05.
Clinical and Cognitive Measures
Subjects completed the Barratt Impulsiveness Scale (BIS-11) (56,57), a self-report measure of impulsivity yielding a total score for trait impulsivity, and subscale impulsivity scores: attention (rapid shifts in attention/impatience with complexity), motor (impetuous action), and non-planning (lack of future orientation). The adult and adolescent BIS-11 each consist of 30 questions, with 14 identical questions and 16 questions age-appropriately modified (e.g., adolescent: “I change my mind about what I will do when I grow up”; adult: “I change jobs”). Versions were scored using the identical procedure and internal consistency was similar between groups, Cronbach’s alpha = .696 and .708, ADO and EA, respectively.
The Wechsler Abbreviated Scale of Intelligence (58) vocabulary subtest was administered to obtain an estimate of general intelligence. The California Verbal Learning test (CVLT-C, adolescent (59); CVLT-II, adult (60)) was used to assess working memory/auditory attention span, verbal learning and memory, and verbal recognition (Table 2). A modified Stroop test (61) was used to assess inhibition of inappropriate responses and resisting interference using three subtests: Color Naming (CN), Word Reading (WR) and Interference (INTF). Derived interference was calculated as [INTF time – CN time] to account for information processing speed differences. Difficulty with response inhibition is reflected by longer duration to completion or a high error rate on the Interference condition. A computerized GNG task (62) consisted of the serial presentation of Go signals (large circles, small circles, large squares) and No-Go signals (small squares). To enhance prepotent response tendency, No-Go trials occurred less frequently than Go trials. Difficulty with inhibition is reflected by low percent accuracy or failing to inhibit responding to No-Go stimuli (63).
Table 2.
Cognitive Performance Data
| WASI | ADO (n=30) | EA (n=20) | p |
|---|---|---|---|
| Vocabulary T-Score | 62.2 ± 9.6 | 61.7 ± 7.3 | ns |
|
| |||
| CVLT-C/CVLT-II | ADO (n=30) | EA (n=19)a | p |
| Trial 1 % correct | 51.1 ± 11.0 | 52.0 ± 16.3 | ns |
| Trials 1 – 5 % correct | 73.3 ± 9.3 | 75.4 ± 11.5 | ns |
| Recognition % correct | 95.6 ± 7.7 | 98.0 ± 3.0 | ns |
|
| |||
| Stroop Color-Word Task | ADO (n=30) | EA (n=20) | p |
| Color Naming Time (sec) | 67.4 ± 11.0 | 56.8 ± 10.2 | .001 |
| Errors | 2.3 ± 1.7 | 1.1 ± 1.1 | .007 |
| Word Reading Time (sec) | 51.8 ± 8.1 | 45.1 ± 7.6 | .005 |
| Errors | 1.3 ± 1.2 | 1.3 ± 1.2 | ns |
| Interference Time (sec) | 120.2 ± 28.9 | 103.4 ± 28.3 | .047 |
| Errors | 4.4 ± 4.0 | 3.0 ± 2.5 | ns |
| Derived Interference Time (sec) | 52.8 ± 21.4 | 46.6 ± 24.4 | ns |
|
| |||
| Go No-Go Task | ADO (n=30) | EA (n=18)b | p-value |
| Go Total Percent Accuracy | 90.6 ± 4.8 | 95.4 ± 4.4 | .001 |
| Go Reaction Time (msec) | 412.6 ± 77.0 | 397.5 ± 81.8 | ns |
| No-Go Total Percent Accuracy | 71.1 ± 15.8 | 87.9 ± 6.2 | .0001 |
Data represent mean scores ± standard deviation.
CVLT data were unavailable from one EA subject.
Data from two EA subjects for No-Go trials were determined to be statistical outliers and were not included in the univariate ANOVAs.
Abbreviations: ADO, adolescent; EA, emerging adult; ns, not statistically significant, p >.05, WASI, Wechsler Abbreviated Scale of Intelligence, CVLT, California Verbal Learning Task
Magnetic Resonance Imaging/Magnetic Resonance Spectroscopy
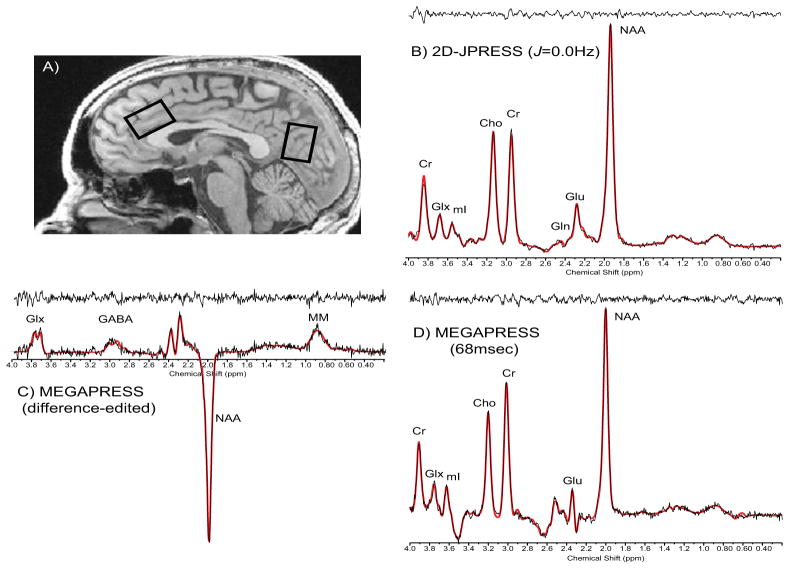
Magnetic resonance imaging (MRI) and 1H-MRS were performed using a 4.0Tesla (T) Varian Unity/INOVA whole-body MRI/MRS scanner (Varian Inc., Palo Alto, CA) and a volumetric head coil (XLR Imaging, London, Canada). Subject head placement was confirmed using three-plane scout images. After global shimming, high-contrast 3D fast low-angle shot T1-weighted images were collected for voxel placement. A 20mm x 20mm x 30mm voxel was placed in the ACC along the midline, with the inferior edge of the voxel parallel to the descending surface of the corpus callosum (Fig 1a) and a 20mm x 20mm x 30mm voxel in POC, with the inferior edge of the voxel aligned with the anterior commissure – posterior commissure (Fig 1a).
Figure 1. MRS Voxel Placement and Proton Spectra.
A) Sagittal image illustrating the placement of 20mm x 30mm x 20 mm single voxels in the ACC and POC. Sample raw (no exponential filter) spectral ACC data: B) 2D-JPRESS J=0.0Hz, C) difference-edited GABA and D) 68msec spectrum from MEGAPRESS. Abbreviations: ACC, anterior cingulate cortex; POC, parieto-occipital cortex; Cr, creatine; GLX, glutamate + glutamine; mI, myo-Inositol; Cho, choline; Gln, glutamine; Glu, glutamate; NAA, N-acetyl-aspartate; GABA, gamma-aminobutyric acid; MM, macromolecule.
Manual voxel shimming yielded water linewidths ranging from 9–12Hz. MEGAPRESS (22,23) (Fig 1c,d) was used to obtain difference-edited GABA-optimized spectra, followed by Point-Resolved Echo Spectroscopy Sequence (64) modified for the current J-resolved 1H-MRS protocol (2D-JPRESS, Fig 1b) to collect 24 TE-stepped spectra in ACC and POC (65,66). MEGAPRESS and 2D-JPRESS acquisition times were 13 minutes/sequence/voxel, total scan time=90 minutes.
The 3.00ppm GABA doublet resonance and co-edited resonance structures of glutamate (Glu), glutamine (Gln), N-acetyl-aspartate (NAA) and the 0.93ppm macromolecule (MM) resonance from MEGAPRESS difference-edited spectra were fitted using LCModel (67). For 2D-JPRESS data, the 24 TE-stepped free-induction decay series (FIDs) was zero-filled out to 64 points, Gaussian-filtered, and Fourier-Transformed. GAMMA-simulated J-resolved basis sets were used with LCModel (66),(67). Test-retest reliability from 6 healthy young adults, scanned twice at a 1-week interval, demonstrated intra-subject coefficient-of-variance for GABA=14.8± 14.7% (MEGAPRESS), and choline (Cho)=3.3±3.8%, Glu=5.3±3.1%, Gln=14.0±4.8%, myo-Inositol (mI)=8.4±3.7%, and NAA=4.4±2.0% (2D-JPRESS). Average Cramer Rao lower bounds (CRLB) were 25.0±13.0% and 2.0±0.4% for MEGAPRESS GABA and 68msec Cr, respectively, which did not differ between groups or regions. For 2D-JPRESS, CRLB were Cr=1.9±0.6%, Cho=3.2±0.8%, Glu=5.2±1.7%, Gln=11.5±3.0%, mI=4.7±1.7%, and NAA=2.1±0.7%.
CrT2 values were derived for each voxel using TE-stepped datasets and a least-squares algorithm, to test for an age-related bias associated with using Cr as an internal reference for normalizing metabolites. Cr was subsequently T2-corrected by multiplying raw Cr integral by 1/exp (−TE/T2) (TE=30ms, T2=derived CrT2 decay-constant, msec). T1-weighted axial image sets were segmented into grey matter (GM), white matter (WM) and cerebrospinal fluid (CSF) binary-tissue maps (FSL, Oxford, UK), with partial tissue percentages extracted for each voxel (66,68), to quantitatively estimate potential tissue-percentage differences on GABA:Cr ratios, which only correct for total tissue content (20,69).
Statistical Analyses
Two group (ADO, EA) univariate analyses of variance (ANOVAs) were conducted to examine age differences on the à priori measures of interest: ACC and POC GABA/Cr, BIS impulsivity measures, No-Go percent accuracy, and Stroop INTF time and derived-INTF as measures of response inhibition. In addition, a 2 group (ADO, EA) x 2 region (ACC, POC) repeated measures ANOVA was conducted to verify the hypothesized group x region interaction for ACC and POC GABA/Cr levels. Effect size f (ES) was calculated for significant main effects or interactions observed in ANOVAs using G*power (Version 3.0.6). Spearman’s rho correlation coefficients (one-tailed based on à priori hypotheses) were used to examine relationships between GABA, BIS impulsivity and response inhibition. Bootstrap confidence intervals (BSCI) at 90% were calculated for significant correlations. A p-value of ≤0.05 was considered statistically significant, except when the Bonferroni method was used to correct for multiple comparisons analyses for impulsivity (BIS Attention, Motor, Non-Planning, Total; p<.013), response inhibition (No-Go percent accuracy, Stroop INTF, derived-INTF; p<.017), 2D-JPRESSS metabolites (Cho, Glu, Gln, Gln/Glu, mI and NAA; p<.008), and correlations with ACC GABA/Cr, BIS, GNG and Stroop response inhibition (p<.007). Statistical analyses were conducted using SPSS 18.0 (SPSS, Chicago, IL).
Results
Impulsivity
Significantly higher BIS impulsivity scores (Table 1) were observed for ADO versus EA on Motor [F(1,48)=26.97, p=.0001, ES=.77] and Non-Planning [F(1,48)=13.16, p=.001; ES=.51] subscales, and total BIS [F(1,48)=15.70, p=.0001; ES=.57].
Response Inhibition
EA took significantly less time than ADO to complete each Stroop subtest, CN [F(1,48)=11.80, p=.001; ES=.49], WR [F(1,48)=8.66, p=.005; ES=.42], and INTF [F(1,48)=4.17, p=.047; ES=.29], although group differences for derived INTF were not significant (Table 2). Significantly more errors were made by ADO, but only on CN [F(1,48)=7.93, p=.007; ES=.42]. On the GNG task, EA had a higher percent accuracy on Go Trials than ADO [F(1,48)=12.83, p=.001; ES=.52], although reaction times to respond on Go trials did not differ significantly between groups. On No-Go trials, EA demonstrated significantly better accuracy than ADO [F(1,46)=18.60, p=.0001; ES=.74] (Table 2).
MRI/MRS Measures
No significant group differences were observed for Cr/total proton signal in ACC [ADO=0.127±0.006; EA=0.125±0.007; p=.22] or POC [ADO=0.144±0.010; EA=0.144±0.009; p=.96] or for Cr T2 in ACC [ADO=147.7±20.0msec; EA=148.0±12.3msec; p=.95] or POC [ADO=140.8±25.4msec; EA=129.9±10.5msec; p=.08]. Thus, MEGAPRESS Cr was used to determine GABA ratios and 2D-JPRESS Cr was used to determine all other metabolite ratios (Table 3). Relationships between MEGAPRESS and 2D-JPRESS Cr integrals were highly correlated [ACC: r(50)=.850, p<.0001, BSCI r=0.768 to .904; POC r(47)=.925, p<.0001, BSCI r=0.880 to .954]. Neither full width half max (FWHM: ACC 0.04±0.01, POC 0.04±0.01) nor signal-to-noise ratios (SNR, height of NAA/noise standard deviation in the residual: ACC 27.3±6.1, POC 37.2±6.3) differed between groups or regions.
Table 3.
Metabolite:Cr Ratios
| ACC | POC | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| MEGAPRESS | ADO (n=30) | EA (n=19)a | p | ADO (n=23)a | EA (n=18)a | p |
| GABA/Cr | 0.130 ± 0.055 | 0.168 ± 0.063 | .029 | 0.256 ± 0.086 | 0.213 ± 0.093 | ns |
|
| ||||||
| 2D-JPRESS | ADO (n=30) | EA (n=20) | p | ADO (n=28)a | EA (n=19)a | p |
| Cho/Cr | 0.244 ± 0.022 | 0.253 ± 0.045 | ns | 0.147 ± 0.015 | 0.153 ± 0.022 | ns |
| Glu/Cr | 0.946 ± 0.113 | 0.881 ± 0.175 | .11 | 0.868 ± 0.129 | 0.797 ± 0.115 | ns |
| Gln/Cr | 0.261 ± 0.055 | 0.287 ± 0.055 | .10 | 0.278 ± 0.096 | 0.281 ± 0.063 | ns |
| Gln/Glu | 0.280 ± 0.073 | 0.345 ± 0.118 | .02 | 0.317 ± 0.073 | 0.359 ± 0.093 | .09 |
| mI/Cr | 0.772 ± 0.093 | 0.818 ± 0.061 | ns | 0.699 ± 0.058 | 0.689 ± 0.116 | ns |
| NAA/Cr | 1.130 ± 0.110 | 1.154 ± 0.128 | ns | 1.328 ± 0.163 | 1.385 ± 0.068 | ns |
Data represent mean values ± standard deviation.
Metabolite data were not available for some subjects.
Abbreviations: ADO, adolescent; EA, emerging adult; ACC, anterior cingulate cortex; POC, parieto-occipital cortex; Cr, creatine; Cho, choline; Glu, glutamate; Gln, glutamine; mI, myo-Inositol; NAA, N-acetyl-aspartate; ns, not statistically significant, p >.05
Significantly lower ACC GABA/Cr was observed in ADO compared to EA [F(1,47)=5.10, p=.029; ES=.32] (Table 3), with no significant group differences observed for GM, WM, or CSF, or tissue percentage in the ACC (Table 4). Significantly lower GABA was also observed when GABA was examined relative to 2D-JPRESS Cr [F(1,47)=4.66, p=.036; ES=.31] or total proton signal [F(1,47)=4.14, p=.047; ES=.27]. No significant differences were observed in the POC for GABA/Cr (p=.14) (Table 3). Repeated measures ANOVA confirmed significant age differences in ACC GABA/Cr but not in POC [F(1,38)=4.12, p=.049; ES=.33]. Significant group tissue differences were observed in POC, with ADO exhibiting less GM [F(1,45)=5.23, p=.027; ES=.33], more WM [F(1,45)=6.45, p=.015; ES=.36], and less CSF [F(1,45)=4.44, p=.041; ES=.30], although tissue percentage did not differ between groups (Table 4). Even when GM content was included as a covariate in the POC GABA/Cr ANOVA, no significant POC GABA/Cr group differences were observed (p=.24).
Table 4.
Tissue Segmentation
| ACC | POC | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Tissue Segmentation | ADO (n=30) | EA (n=20) | p | ADO (n=28)a | EA (n=19)a | p |
| GM | 57.4 ± 5.8 | 59.4 ± 6.6 | ns | 37.0 ± 4.6 | 40.6 ± 6.3 | .03 |
| WM | 34.6 ± 6.0 | 31.9 ± 8.4 | ns | 57.9 ± 5.8 | 52.5 ± 8.8 | .02 |
| CSF | 8.0 ± 2.0 | 8.6 ± 2.6 | ns | 5.2 ± 2.3 | 6.9 ± 3.5 | .04 |
| Tissue Percentage | 92.0 ± 1.9 | 91.5 ± 2.6 | ns | 94.8 ± 2.3 | 93.1 ± 3.6 | ns |
Data represent mean values ± standard deviation.
Tissue segmentation data were not available for some subjects.
Abbreviations: ADO, adolescent; EA, emerging adult; ACC, anterior cingulate cortex; POC, parieto-occipital cortex; GM, grey matter; WM, white matter; CSF, cerebral spinal fluid; ns, not statistically significant, p >.05. Tissue percentage was calculated as (((GM+WM)/(GM + WM + CSF)) * 100).
Significantly higher ratios of ACC MM/Cr were evident in ADO compared to EA [ADO=0.261±0.046; EA=0.224±0.054; F(1,47)=6.67, p=.013; ES=.36], but no significant age differences were observed for the POC [ADO=0.245±0.038; EA=0.242±0.046]. ACC MM/Cr did not correlate significantly with impulsivity or response inhibition on the GNG or Stroop tasks.
Detailed examination of other proton metabolites and their relationships with impulsivity and response inhibition measures were beyond the main scope of this a priori investigation examining GABA. Briefly, Glu/Cr was higher and Gln/Cr was lower in the ACC of ADO relative to EA, although age differences did not reach statistical significance (Table 3). The Gln/Glu ratio was significantly lower in ADO than EA, but only in the ACC [F(1,48)=5.85, p=.019; ES=.33]. However, this analysis did not survive a Bonferroni correction of six multiple comparisons (p<.008) that was required for 2D-JPRESS metabolite comparisons.
Correlations between ACC Metabolites, Impulsivity and Response Inhibition
Significant correlations were observed between ACC GABA/Cr, BIS impulsivity and percent accuracy on No-Go inhibition trials for the total sample. Specifically, higher ACC GABA/Cr was associated with lower BIS Motor [r(49)=−.399, p=.002, BSCI r = −0.582 to −0.178], Non-Planning [r(49)=−.262, p=.035, BSCI r = −0.471 to −0.026], and Total impulsivity [r(49)=−.301, p=.018, BSCI r=−0.503 to −0.068], and higher percent accuracy on No-Go trials of the GNG task [r(49)=.282, p=.026, BSCI r=0.047 to 0.487]. Of these measures, only the ACC GABA/Cr relationship with BIS motor impulsivity survived multiple comparison correction. In contrast, the relationship between ACC GABA/Cr and Stroop INTF was not significant. Correlations with POC GABA/Cr also did not reach significance. Exploratory correlations of other proton metabolites revealed only that lower ACC Glu/Cr was significantly associated with higher attention impulsivity [r(50)=−.246, p=.042, BSCI r=−0.455 to −0.011], although this finding would not survive correction for multiple comparisons.
Menstrual Cycle Effects
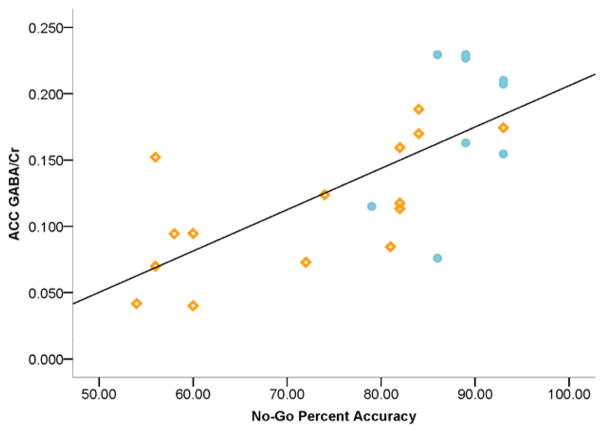
Exploratory analyses revealed significantly higher ACC and POC GABA/Cr of females in the follicular phase compared to those in the luteal phase: ACC follicular =0.179±0.056 (n=11); luteal=0.110±0.048 (n=8) [F(1,17)=7.96, p=.012; ES=.66]; POC follicular=0.270±0.096 (n=11); luteal=0.159±0.074 (n=8) [F(1,17)=7.38, p=.015; ES=.65]. Given the variability associated with menstrual cycle phase on GABA, ANOVAs were repeated for males only: group differences for ACC GABA/Cr and correlations with impulsivity and response inhibition remained statistically significant and yielded larger effect sizes: ACC GABA/Cr: ADO=0.113±0.048; EA=0.179±0.055 [F(1,22)=9.51, p=.005; ES=.62]; ACC GABA/Cr and BIS Motor [r(24)=−.568, p=.002, BSCI r = −0.763 to −0.278], Non-Planning [r(24)=−.399, p=.027, BSCI r=−0.654 to −0.0631] and Total impulsivity [r(24)=−.409, p=.024, BSCI r=−0.660 to −0.075]; and No-Go percent accuracy: [r(24)=.758, p<.0001, BSCI r=0.560 to 0.874]. Significant correlations between ACC GABA/Cr and Stroop INTF also emerged in males: ACC GABA/Cr and INTF time [r(24)=−.451, p=.013, BSCI r=−0.688 to −0.126] and derived-INTF [r(24)=−.386, p=.031, BSCI r=−0.645 to −0.048]. However, only the relationships between ACC GABA/Cr, BIS Motor and No-Go percent accuracy survived multiple comparison corrections. Notably, the ACC GABA/Cr and No-Go percent accuracy correlation remained significant when adolescents were examined alone, r(15)=.787, p=.001 (Figure 2), which also survived multiple comparison correction. ADO correlations were not significant for BIS or Stroop, and no correlations were significant in EA examined alone. No significant group differences were observed for POC GABA/Cr, ADO=0.250±0.091, EA=0.237±0.075; and no significant correlations were evident between POC GABA/Cr, BIS, GNG or Stroop measures.
Figure 2. Correlation: ACC GABA/Cr and Response Inhibition.
Scatterplot representing individual male subject data (n=15 ADO, n=10 EA) and linear regressions of the relationship between ACC GABA/Cr and No-Go percent accuracy, where higher percent accuracy scores reflect better response inhibition, [r(24)=.758, p<.0001] (not shown: whole sample [r(49)=.282, p=.026]). Orange open diamonds represent ADO individual subject data and blue filled circles represent EA individual subject data.
Discussion
This study provides the first in vivo human evidence of lower GABA in ADO relative to EA, which is consistent with previous developmental findings (17,18). Lower adolescent GABA/Cr was observed in ACC but not POC, and was evident in the absence of tissue contribution differences. There were significant differences in POC tissue contributions but not tissue percentage, the relevance of which is unclear with regard to the use of the POC as a comparison region. Consistent with the à priori study hypotheses, lower ACC GABA/Cr was significantly associated with greater impulsiveness and less cognitive control, with lower ACC GABA/Cr most strongly predicting worse accuracy on No-Go trials in adolescent males. Thus, ACC GABAergic contributions may not necessarily generalize across impulse control indices, especially when response inhibition tasks require different sensory or response demands (70). ACC GABA/Cr was not significantly related to other aspects of cognition, such as attention or memory, or general intelligence. These findings suggest that the observed brain and behavior relationships have unique regional significance, as similar relationships with impulsivity and cognitive control were not observed for other metabolites or for POC GABA.
Consistent with previous work by Epperson and colleagues (71), significantly lower GABA/Cr levels were observed in ADO and EA females tested during the luteal phase than in the follicular phase of the menstrual cycle, in both the ACC and POC. Indeed, group analyses limited to males demonstrated greater statistical precision (smaller p-values, larger effect sizes) for GABA differences and correlations. While pubertal stage was not assessed in the present study, ADO were 12–14 years old, a span overlapping with pubertal endocrine events (72). Thus, subtle pubertal stage differences may have existed within the ADO sample, however, EA were clearly outside of the pubertal window. It is therefore important that age-related differences in ACC GABA be replicated in a study that empirically investigates the role of menstrual cycle phase, sex-specific hormones and pubertal stage on impulsivity and response inhibition.
Significant relationships between individual variations in frontal lobe GABA and impulsivity and automatic motor control have previously been reported adults (42,43,73–75). Lower DLPFC GABA was associated with higher urgency (42) and lower supplementary motor cortex GABA significantly predicted worse automatic motor control, but not response inhibition (43). Notably, adult studies have failed to find significant relationships between GNG performance and impulsivity (76,77). While BIS Motor scores have been reported to load strongly on the ‘Feeling of Urgency’ factor (42,78), ACC GABA was not correlated with impulsivity or response inhibition previously in adults. However, relationships between ACC GABA/Cr and BIS motor and No-Go percent accuracy were significantly correlated after multiple comparison correction across the ages currently examined. Furthermore, only the ACC GABA/Cr and No-Go percent accuracy relationship remained significant after multiple comparison correction when adolescents, but not adults, were examined alone. This highlights the regional specificity of GABA in the developing ACC to better predict response inhibition than impulsivity in adolescents. It is plausible that developmental DLPFC GABA/Cr differences may have been even greater than those observed in the ACC, and as in previous work, DLPFC GABA/Cr may have been more strongly correlated with impulsivity measures (43) in the present adolescent sample.
Brain GABA measured in the current study likely reflects intracellular rather than intrasynaptic levels, since the majority of the GABA pool exists within GABAergic neurons (79), although determining such specificity is beyond the capabilities of current in vivo MRS methods. Given that the ACC is involved in detecting conflict, i.e., identifying that a prepotent response needs to be inhibited, the relative immaturity of the ACC GABA circuitry is likely ineffective for inhibiting incorrect responses. A potential mechanistic framework by which reduced GABA is associated with less motor inhibition and cognitive control during adolescence involves reduced modulation of glutamatergic activity (over excitation), presumably due to the protracted maturation of frontal lobe GABA receptors, along with reduced GABAergic tone in the rapidly developing ACC of adolescents. Clearly, additional investigations are necessary to further elucidate the neurobiological mechanism underlying the role of GABA in response inhibition and impulsivity during adolescence.
Notable strengths of the study include acquisition of spectral data using specialized MEGAPRESS and 2D-JPRESS sequences within the same scanning session at 4.0T, which permit improved spatial and spectral resolution, high SNR afforded by high field MRS, optimal detection of GABA, Glu and Gln and measurement of metabolite T2. The current study included a large number of subjects for an MRS study, 30 ADO and 20 EA, who were well characterized to be healthy, and with no or minimal alcohol use, and no other substance use, including nicotine. Finally, impulsivity and cognitive measures, selected to examine the a priori study hypotheses, permitted the investigation of brain and behavior relationships during the critical period of adolescent brain development.
Normalizing GABA to Cr has its disadvantages (80,81), however Cr T2 and Cr/total ratios did not differ significantly between groups, which is consistent with previous developmental studies (81,82). It would have been ideal to calculate absolute metabolite concentrations (20,33), however, given the developmental aspect of this study, multiple T1 and T2 unknowns would have invalidated this approach. Further, co-editing of MM, which resonate near the detected GABA peak, complicates accurate quantification of the edited GABA signal (23,83–86). Some evidence exists for a smaller MM peak at 3.00ppm in adolescents compared to adults (87), however significantly higher ACC MM/Cr was observed in ADO than EA in the current study, which has limited relevance for interpreting GABA differences but may reflect MM in general (83,84,86). Future studies should correct for MM to reliably interpret age differences in GABA.
It is possible that accidental findings may have occurred, given the multiple measures examined. This is unlikely for the à priori, regionally-specific developmental GABA hypothesis, which was statistically supported with medium-large effect sizes. While the analyses of other proton metabolites and correlations between ACC GABA/Cr, BIS and Stroop measures did not survive multiple comparison corrections, the significant ACC GABA/Cr and No-Go percent accuracy correlation in adolescents survived Bonferroni correction (p<.008). Given that the major brain and behavior findings from this study are correlational in nature, future studies that include GABA manipulations would strengthen the current causal interpretations. Reduced occipital GABA was observed in young adult social drinkers after an acute alcohol infusion, consistent with GABAA receptor facilitation by alcohol (88). Drug challenges that alter GABA, however, would be unethical in adolescents given the potential neurotoxicity of substances on the developing brain (89). Yet activities that naturally boost GABAergic activity, such as yoga (40,41), may bolster adolescent GABA maturation, which in turn could help adolescents manage the natural course of impulsivity while optimizing decision-making.
These results suggest that the immature frontal cortex is only beginning to integrate inhibitory GABAergic neurotransmission with inhibitory control in the healthy, yet vulnerable, adolescent brain. These GABA findings have important public health relevance, as greater impulsivity and reduced motor control and inhibition could lead to increased risk-taking and poor decision-making during a critical period of brain development, thereby compromising adolescent health and safety. Alcohol and drug experimentation during adolescence (90) could perpetuate an already heightened level of risk-taking and impulsivity typically observed at this age, when GABA levels are developmentally low and there is risk of altering the healthy maturation of this very important neural system. These findings are also relevant given that reduced GABA has been implicated in anxiety disorders (34), depression (26), obsessive-compulsive disorder (36), and alcohol dependence (32,39) in adults, and in adolescents with major depressive disorder (38). These developmental GABA data may therefore contribute to the development of novel GABAergic interventions that have increased efficacy in treating adolescent substance abuse and psychiatric illnesses.
Acknowledgments
This study was supported by K01 AA014651 and R01 AA018153 grants (MMS). The authors wish to acknowledge Dr. Deborah Yurgelun-Todd for her role as the K-award mentor, Dr. William D.S. Killgore for development of the GNG paradigm, and Ms. Alexandra McCaffrey for early contributions to study coordination.
Footnotes
Financial Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 2.Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, et al. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cereb Cortex. 1996;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- 3.Sowell ER, Thompson PM, Toga AW. Mapping changes in the human cortex throughout the span of life. Neuroscientist. 2004;10:372–392. doi: 10.1177/1073858404263960. [DOI] [PubMed] [Google Scholar]
- 4.Casey BJ, Galvan A, Hare TA. Changes in cerebral functional organization during cognitive development. Curr Opin Neurobiol. 2005;15:239–244. doi: 10.1016/j.conb.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Luna B, Sweeney JA. The emergence of collaborative brain function: FMRI studies of the development of response inhibition. Ann N Y Acad Sci. 2004;1021:296–309. doi: 10.1196/annals.1308.035. [DOI] [PubMed] [Google Scholar]
- 6.Arnett J. Reckless behavior in adolescence - A developmental perspective. Dev Rev. 1992;12:339–373. [Google Scholar]
- 7.Goldman-Rakic PS. Topography of cognition: parallel distributed networks in primate association cortex. Annu Rev Neurosci. 1988;11:137–156. doi: 10.1146/annurev.ne.11.030188.001033. [DOI] [PubMed] [Google Scholar]
- 8.Marsh R, Zhu H, Schultz RT, Quackenbush G, Royal J, Skudlarski P, et al. A developmental fMRI study of self-regulatory control. Hum Brain Mapp. 2006;27:848–863. doi: 10.1002/hbm.20225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silveri MM, Rogowska J, McCaffrey A, Yurgelun-Todd DA. Adolescents at risk for alcohol abuse demonstrate altered frontal lobe activation during stroop performance. Alcohol Clin Exp Res. 2011;35:218–228. doi: 10.1111/j.1530-0277.2010.01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCormick DA. GABA as an inhibitory neurotransmitter in human cerebral cortex. J Neurophysiol. 1989;62:1018–1027. doi: 10.1152/jn.1989.62.5.1018. [DOI] [PubMed] [Google Scholar]
- 12.Coyle JT, Enna SJ. Neurochemical aspects of the ontogenesis of GABAergic neurons in the rat brain. Brain Res. 1976;111:119–133. doi: 10.1016/0006-8993(76)91053-2. [DOI] [PubMed] [Google Scholar]
- 13.Johnston MV, Coyle JT. Development of central neurotransmitter systems. Ciba Found Symp. 1981;86:251–270. doi: 10.1002/9780470720684.ch12. [DOI] [PubMed] [Google Scholar]
- 14.Candy JM, Martin IL. The postnatal development of the benzodiazepine receptor in the cerebral cortex and cerebellum of rat. J Neurochem. 1979;32:655–658. doi: 10.1111/j.1471-4159.1979.tb00402.x. [DOI] [PubMed] [Google Scholar]
- 15.Erickson SL, Lewis DA. Postnatal development of parvalbumin- and GABA transporter-immunoreactive axon terminals in monkey prefrontal cortex. J Comp Neurol. 2002;448:186–202. doi: 10.1002/cne.10249. [DOI] [PubMed] [Google Scholar]
- 16.Brooksbank BW, Atkinson DJ, Balazs R. Biochemical development of the human brain. II. some parameters of the GABA-ergic system. Dev Neurosci. 1981;4:188–200. doi: 10.1159/000112756. [DOI] [PubMed] [Google Scholar]
- 17.Chugani DC, Muzik O, Juhasz C, Janisse JJ, Ager J, Chugani HT. Postnatal maturation of human GABAA receptors measured with positron emission tomography. Ann Neurol. 2001;49:618–626. [PubMed] [Google Scholar]
- 18.Fung SJ, Webster MJ, Sivagnanasundaram S, Duncan C, Elashoff M, Weickert CS. Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. Am J Psychiatry. 2010;167:1479–1488. doi: 10.1176/appi.ajp.2010.09060784. [DOI] [PubMed] [Google Scholar]
- 19.Hetherington HP, Newcomer BR, Pan JW. Measurements of human cerebral GABA at 4.1 T using numerically optimized editing pulses. Magn Reson Med. 1998;39:6–10. doi: 10.1002/mrm.1910390103. [DOI] [PubMed] [Google Scholar]
- 20.Jensen JE, de Frederick BB, Renshaw PF. Grey and white matter GABA level differences in the human brain using two-dimensional, J-resolved spectroscopic imaging. NMR Biomed. 2005;18:570–576. doi: 10.1002/nbm.994. [DOI] [PubMed] [Google Scholar]
- 21.Keltner JR, Wald LL, Frederick BB, Renshaw PF. In vivo detection of GABA in human brain using a localized double-quantum filter technique. Magn Reson Med. 1997;37:366–371. doi: 10.1002/mrm.1910370312. [DOI] [PubMed] [Google Scholar]
- 22.Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11:266–272. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 23.Rothman DL, Petroff OAC, Behar KL, Mattson RH. Localized 1H NMR measurements of (gamma)-aminobutyric acid in human brain in vivo. Proc Natl Acad Sci U S A. 1993;90:5662–5666. doi: 10.1073/pnas.90.12.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen J, Shungu DC, Rothman DL. In vivo chemical shift imaging of (gamma)-aminobutyric acid in the human brain. Mag Res Med. 1999;41:35–42. doi: 10.1002/(sici)1522-2594(199901)41:1<35::aid-mrm7>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 25.Puts NA, Edden RA. In vivo magnetic resonance spectroscopy of GABA: a methodological review. Prog Nucl Magn Reson Spectrosc. 2012;60:29–41. doi: 10.1016/j.pnmrs.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhagwagar Z, Wylezinska M, Jezzard P, Evans J, Boorman E, PMM, et al. Low GABA concentrations in occipital cortex and anterior cingulate cortex in medication-free, recovered depressed patients. Int J Neuropsychopharmacol. 2008;11:255–260. doi: 10.1017/S1461145707007924. [DOI] [PubMed] [Google Scholar]
- 27.Chang L, Cloak CC, Ernst T. Magnetic resonance spectroscopy studies of GABA in neuropsychiatric disorders. J Clin Psychiatry. 2003;64(Suppl 3):7–14. [PubMed] [Google Scholar]
- 28.Cosgrove KP, Esterlis I, Mason GF, Bois F, O’Malley SS, Krystal JH. Neuroimaging insights into the role of cortical GABA systems and the influence of nicotine on the recovery from alcohol dependence. Neuropharmacology. 2011;60:1318–1325. doi: 10.1016/j.neuropharm.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Epperson CN, Gueorguieva R, Czarkowski KA, Stiklus S, Sellers E, Krystal JH, et al. Preliminary evidence of reduced occipital GABA concentrations in puerperal women: a 1H-MRS study. Psychopharmacology (Berl) 2006;186:425–433. doi: 10.1007/s00213-006-0313-7. [DOI] [PubMed] [Google Scholar]
- 30.Goddard AW, Mason GF, Almai A, Rothman DL, Behar KL, Petroff OA, et al. Reductions in occipital cortex GABA levels in panic disorder detected with 1h-magnetic resonance spectroscopy. Arch Gen Psychiatry. 2001;58:556–561. doi: 10.1001/archpsyc.58.6.556. [DOI] [PubMed] [Google Scholar]
- 31.Ke Y, Streeter CC, Nassar LE, Sarid-Segal O, Hennen J, Yurgelun-Todd DA, et al. Frontal lobe GABA levels in cocaine dependence: a two-dimensional, J-resolved magnetic resonance spectroscopy study. Psychiatry Res. 2004;130:283–293. doi: 10.1016/j.pscychresns.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Mason GF, Appel M, de Graaf RA, Petrakis IL, Rothman DL. Reduction in cortical GABA levels over the first month of sobriety. Alcohol Clin Exp Res. 2003;27(suppl):56A. [Google Scholar]
- 33.Mon A, Durazzo TC, Meyerhoff DJ. Glutamate, GABA, and other cortical metabolite concentrations during early abstinence from alcohol and their associations with neurocognitive changes. Drug Alcohol Depend. 2012;125:27–36. doi: 10.1016/j.drugalcdep.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pollack MH, Jensen JE, Simon NM, Kaufman RE, Renshaw PF. High-field MRS study of GABA, glutamate and glutamine in social anxiety disorder: response to treatment with levetiracetam. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:739–743. doi: 10.1016/j.pnpbp.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 35.Sanacora G, Mason GF, Rothman DL, Krystal JH. Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am J Psychiatry. 2002;159:663–665. doi: 10.1176/appi.ajp.159.4.663. [DOI] [PubMed] [Google Scholar]
- 36.Simpson HB, Shungu DC, Bender J, Jr, Mao X, Xu X, Slifstein M, et al. Investigation of Cortical Glutamate-Glutamine and gamma-Aminobutyric Acid in Obsessive-Compulsive Disorder by Proton Magnetic Resonance Spectroscopy. Neuropsychopharmacology. 2012;37:2684–2692. doi: 10.1038/npp.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Streeter CC, Hennen J, Ke Y, Jensen JE, Sarid-Segal O, Nassar LE, et al. Prefrontal GABA levels in cocaine-dependent subjects increase with pramipexole and venlafaxine treatment. Psychopharmacology (Berl) 2005;182:516–526. doi: 10.1007/s00213-005-0121-5. [DOI] [PubMed] [Google Scholar]
- 38.Gabbay V, Mao X, Klein RG, Ely BA, Babb JS, Panzer AM, et al. Anterior cingulate cortex {gamma}-Aminobutyric Acid in depressed adolescents: Relationship to anhedonia. Arch Gen Psychiatry. 2011;69:139–149. doi: 10.1001/archgenpsychiatry.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanacora G, Mason GF, Rothman DL, Hyder F, Ciarcia JJ, Ostroff RB, et al. Increased cortical GABA concentrations in depressed patients receiving ECT. Am J Psychiatry. 2003;160:577–579. doi: 10.1176/appi.ajp.160.3.577. [DOI] [PubMed] [Google Scholar]
- 40.Streeter CC, Jensen JE, Perlmutter RM, Cabral HJ, Tian H, Terhune DB, et al. Yoga Asana sessions increase brain GABA levels: a pilot study. J Altern Complement Med. 2007;13:419–426. doi: 10.1089/acm.2007.6338. [DOI] [PubMed] [Google Scholar]
- 41.Streeter CC, Whitfield TH, Owen L, Rein T, Karri SK, Yakhkind A, et al. Effects of yoga versus walking on mood, anxiety, and brain GABA levels: a randomized controlled MRS study. J Altern Complement Med. 2010;16:1145–1152. doi: 10.1089/acm.2010.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boy F, Evans CJ, Edden RA, Lawrence AD, Singh KD, Husain M, et al. Dorsolateral prefrontal gamma-aminobutyric acid in men predicts individual differences in rash impulsivity. Biol Psychiatry. 2011;70:866–872. doi: 10.1016/j.biopsych.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boy F, Evans CJ, Edden RA, Singh KD, Husain M, Sumner P. Individual differences in subconscious motor control predicted by GABA concentration in SMA. Curr Biol. 2010;20:1779–1785. doi: 10.1016/j.cub.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Leijenhorst L, Crone EA, Bunge SA. Neural correlates of developmental differences in risk estimation and feedback processing. Neuropsychologia. 2006;44:2158–2170. doi: 10.1016/j.neuropsychologia.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 45.Rubia K, Smith AB, Taylor E, Brammer M. Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Hum Brain Mapp. 2007;28:1163–1177. doi: 10.1002/hbm.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Leijenhorst L, Gunther Moor B, Op de Macks ZA, Rombouts SA, Westenberg PM, Crone EA. Adolescent risky decision-making: neurocognitive development of reward and control regions. Neuroimage. 2010;51:345–355. doi: 10.1016/j.neuroimage.2010.02.038. [DOI] [PubMed] [Google Scholar]
- 47.Kelly AM, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, et al. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cereb Cortex. 2009;19:640–657. doi: 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- 48.Adleman NE, Menon V, Blasey CM, White CD, Warsofsky IS, Glover GH, et al. A Developmental fMRI Study of the Stroop Color-Word Task. Neuroimage. 2002;16:61–75. doi: 10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- 49.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 50.Sowell ER, Delis D, Stiles J, Jernigan TL. Improved memory functioning and frontal lobe maturation between childhood and adolescence: a structural MRI study. J Int Neuropsychol Soc. 2001;7:312–322. doi: 10.1017/s135561770173305x. [DOI] [PubMed] [Google Scholar]
- 51.Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci. 1999;2:859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- 52.Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, et al. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Hum Brain Mapp. 2006;27:973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hollingshead AB. Two factor index of social position. New Haven: Author; 1957. [Google Scholar]
- 54.Puig-Antich J, Orvaschel H, Tabrizi M, Chambers W. The Schedule for Affective Disorders and Schizophrenia for School-Aged Children - Epidemiologic Version (Kiddie-SADS-E) New York: New York State Psychiatric Institute and Yale University School of Medicine; 1980. [Google Scholar]
- 55.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP) New York: New York Psychiatric Institute; 2002. [Google Scholar]
- 56.Fossati A, Barratt ES, Acquarini E, Di Ceglie A. Psychometric properties of an adolescent version of the Barratt Impulsiveness Scale-11 for a sample of Italian high school students. Percept Mot Skills. 2002;95:621–635. doi: 10.2466/pms.2002.95.2.621. [DOI] [PubMed] [Google Scholar]
- 57.Patton JM, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 58.Wechsler D. Wechsler Abbreviated Scale of Intelligence Manual. San Antonio: The Psychological Corporation, Hartcourt Brace and Company; 1999. [Google Scholar]
- 59.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test-Children’s Version. San Antonio: Psychological Corporation; 1994. [Google Scholar]
- 60.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test-Adult Version. 2. San Antonio: Psychological Corporation; 2000. [Google Scholar]
- 61.Comalli PE, Wapner S, Werner H. Interference effects of Stroop color-word test in childhood, adulthood, and aging. J Genet Psychol. 1962;100:47–53. doi: 10.1080/00221325.1962.10533572. [DOI] [PubMed] [Google Scholar]
- 62.Leland DS, Arce E, Miller DA, Paulus MP. Anterior cingulate cortex and benefit of predictive cueing on response inhibition in stimulant dependent individuals. Biol Psychiatry. 2008;63:184–190. doi: 10.1016/j.biopsych.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 63.Finn PR, Justus A, Mazas C, Steinmetz JE. Working memory, executive processes and the effects of alcohol on Go/No-Go learning: testing a model of behavioral regulation and impulsivity. Psychopharmacology (Berl) 1999;146:465–472. doi: 10.1007/pl00005492. [DOI] [PubMed] [Google Scholar]
- 64.Bottomley PA. Spatial localization in NMR spectroscopy in vivo. Ann N Y Acad Sci. 1987;508:333–348. doi: 10.1111/j.1749-6632.1987.tb32915.x. [DOI] [PubMed] [Google Scholar]
- 65.Licata SC, Jensen JE, Penetar DM, Prescot AP, Lukas SE, Renshaw PF. A therapeutic dose of zolpidem reduces thalamic GABA in healthy volunteers: a proton MRS study at 4 T. Psychopharmacology (Berl) 2009;203:539–546. doi: 10.1007/s00213-008-1431-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jensen JE, Licata SC, Ongur D, Friedman SD, Prescot AP, Henry ME, et al. Quantification of J-resolved proton spectra in two-dimensions with LCModel using GAMMA-simulated basis sets at 4 Tesla. NMR Biomed. 2009;22:762–769. doi: 10.1002/nbm.1390. [DOI] [PubMed] [Google Scholar]
- 67.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 68.Licata SC, Jensen JE, Penetar DM, Prescot AP, Lukas SE, Renshaw PF. A therapeutic dose of zolpidem reduces thalamic GABA in healthy volunteers: a proton MRS study at 4 T. Psychopharmacology (Berl) 2009 doi: 10.1007/s00213-008-1431-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Choi IY, Lee SP, Merkle H, Shen J. In vivo detection of gray and white matter differences in GABA concentration in the human brain. Neuroimage. 2006;33:85–93. doi: 10.1016/j.neuroimage.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 70.Stevens MC, Kiehl KA, Pearlson GD, Calhoun VD. Functional neural networks underlying response inhibition in adolescents and adults. Behav Brain Res. 2007;181:12–22. doi: 10.1016/j.bbr.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Epperson CN, O’Malley S, Czarkowski KA, Gueorguieva R, Jatlow P, Sanacora G, et al. Sex, GABA, and nicotine: the impact of smoking on cortical GABA levels across the menstrual cycle as measured with proton magnetic resonance spectroscopy. Biol Psychiatry. 2005;57:44–48. doi: 10.1016/j.biopsych.2004.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blakemore SJ, Burnett S, Dahl RE. The role of puberty in the developing adolescent brain. Hum Brain Mapp. 2010;31:926–933. doi: 10.1002/hbm.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stagg CJ, Bachtiar V, Johansen-Berg H. The role of GABA in human motor learning. Curr Biol. 2011;21:480–484. doi: 10.1016/j.cub.2011.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stagg CJ, Bestmann S, Constantinescu AO, Moreno LM, Allman C, Mekle R, et al. Relationship between physiological measures of excitability and levels of glutamate and GABA in the human motor cortex. J Physiol. 2011;589:5845–5855. doi: 10.1113/jphysiol.2011.216978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sumner P, Edden RA, Bompas A, Evans CJ, Singh KD. More GABA, less distraction: a neurochemical predictor of motor decision speed. Nat Neurosci. 2010;13:825–827. doi: 10.1038/nn.2559. [DOI] [PubMed] [Google Scholar]
- 76.Goya-Maldonado R, Walther S, Simon J, Stippich C, Weisbrod M, Kaiser S. Motor impulsivity and the ventrolateral prefrontal cortex. Psychiatry Res. 2010;183:89–91. doi: 10.1016/j.pscychresns.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 77.Asahi S, Okamoto Y, Okada G, Yamawaki S, Yokota N. Negative correlation between right prefrontal activity during response inhibition and impulsiveness: a fMRI study. Eur Arch Psychiatry Clin Neurosci. 2004;254:245–251. doi: 10.1007/s00406-004-0488-z. [DOI] [PubMed] [Google Scholar]
- 78.Whiteside SP, Lynam DR. Understanding the role of impulsivity and externalizing psychopathology in alcohol abuse: application of the UPPS impulsive behavior scale. Exp Clin Psychopharmacol. 2003;11:210–217. doi: 10.1037/1064-1297.11.3.210. [DOI] [PubMed] [Google Scholar]
- 79.Petroff OA. GABA and glutamate in the human brain. Neuroscientist. 2002;8:562–573. doi: 10.1177/1073858402238515. [DOI] [PubMed] [Google Scholar]
- 80.Knight-Scott J, Haley AP, Rossmiller SR, Farace E, Mai VM, Christopher JM, et al. Molality as a unit of measure for expressing 1H MRS brain metabolite concentrations in vivo. Magn Reson Imaging. 2003;21:787–797. doi: 10.1016/s0730-725x(03)00179-6. [DOI] [PubMed] [Google Scholar]
- 81.Kreis R, Ernst T, Ross BD. Development of the human brain: in vivo quantification of metabolite and water content with proton magnetic resonance spectroscopy. Magn Reson Med. 1993;30:424–437. doi: 10.1002/mrm.1910300405. [DOI] [PubMed] [Google Scholar]
- 82.Raininko R, Mattsson P. Metabolite concentrations in supraventricular white matter from teenage to early old age: A short echo time 1H magnetic resonance spectroscopy (MRS) study. Acta Radiol. 2010;51:309–315. doi: 10.3109/02841850903476564. [DOI] [PubMed] [Google Scholar]
- 83.Behar KL, Rothman DL, Spencer DD, Petroff OA. Analysis of macromolecule resonances in 1H NMR spectra of human brain. Magn Reson Med. 1994;32:294–302. doi: 10.1002/mrm.1910320304. [DOI] [PubMed] [Google Scholar]
- 84.Behar KL, Ogino T. Characterization of macromolecule resonances in the 1H NMR spectrum of rat brain. Magn Reson Med. 1993;30:38–44. doi: 10.1002/mrm.1910300107. [DOI] [PubMed] [Google Scholar]
- 85.Choi C, Bhardwaj PP, Kalra S, Casault CA, Yasmin US, Allen PS, et al. Measurement of GABA and contaminants in gray and white matter in human brain in vivo. Magn Reson Med. 2007;58:27–33. doi: 10.1002/mrm.21275. [DOI] [PubMed] [Google Scholar]
- 86.Cudalbu C, Mlynarik V, Gruetter R. Handling macromolecule signals in the quantification of the neurochemical profile. J Alzheimers Dis. 2012;31:S101–115. doi: 10.3233/JAD-2012-120100. [DOI] [PubMed] [Google Scholar]
- 87.Hofmann L, Slotboom J, Boesch C, Kreis R. Characterization of the macromolecule baseline in localized (1)H-MR spectra of human brain. Magn Reson Med. 2001;46:855–863. doi: 10.1002/mrm.1269. [DOI] [PubMed] [Google Scholar]
- 88.Gomez R, Behar KL, Watzl J, Weinzimer SA, Gulanski B, Sanacora G, et al. Intravenous ethanol infusion decreases human cortical gamma-aminobutyric acid and N-acetylaspartate as measured with proton magnetic resonance spectroscopy at 4 tesla. Biol Psychiatry. 2012;71:239–246. doi: 10.1016/j.biopsych.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Silveri MM. Adolescent brain development and underage drinking in the United States: identifying risks of alcohol use in college populations. Harv Rev Psychiatry. 2012;20:189–200. doi: 10.3109/10673229.2012.714642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bates ME, Labouvie EW. Adolescent risk factors and the prediction of persistent alcohol and drug use into adulthood. Alcohol Clin Exp Res. 1997;21:944–950. [PubMed] [Google Scholar]