Abstract
Patients with leukemia relapsing after allogeneic hematopoietic stem cell transplantation (SCT) have a dismal prognosis. A second SCT offers a further opportunity for cure, but has a high rate of treatment failure. To determine the utility of this option, we analyzed 59 consecutive patients relapsing after a myeloablative HLA-matched sibling T cell depleted SCT. Twenty five patients (13 relapsing within 6 months and 12 relapsing between 6 – 170 months after the first SCT) received a T-replete second SCT. Thirty-eight patients relapsing early had a shorter survival than the 21 patients relapsing later (median 96 vs 298 days, p = 0.0002). In patients relapsing early, the second SCT did not improve overall survival compared to patients receiving non-SCT treatments (median survival 109 vs 80 days, p = 0.41). In patients relapsing late, despite an early trend in favor of second SCT, overall survival was comparable for patients receiving a second SCT compared with patients not retransplanted (median survival 363.5 vs 162 days, p = 0.49). Disappointingly our results do not demonstrate an important survival benefit for a second T-replete allogeneic SCT to treat relapse following a T cell depleted SCT.
Keywords: Second stem cell transplantation, Leukemia, Relapse, T-depleted, Survival
Introduction
Leukemic relapse is the single biggest cause of treatment failure after hematopoietic stem cell transplant (SCT) for hematological malignancies, and its management remains largely unsuccessful.1 Patients relapsing with acute leukemia within 6 months of SCT have a one year survival of less than 20%.2, 3 In comparison, later relapse of acute leukemia after SCT carries a better prognosis, but is still plagued by a high mortality from progressive disease.4 Consequently, no standard approach exists for management of relapsed disease after SCT. Typically most patients will receive chemotherapy and/or a donor lymphocyte infusion, while others may only be offered palliative treatment.5–8 Selected patients with a good performance status, in whom some disease control is achieved, may receive a second SCT. However, a second SCT is not without risk. Offering a second opportunity for cure logically requires either intensification of the conditioning regimen or enhancement of the graft-versus-leukemia (GVL) effect, which can be achieved by selecting an alternative donor or by promoting a more rapid and complete immune reconstitution.1, 9
At our institution we offered a second SCT to selected patients deemed fit enough and with sufficient disease control to survive the procedure without early death from disease progression or treatment related mortality. In the setting of myeloablative T cell depleted SCT we sought to augment the GVL effect with a T cell-replete second transplant and reduced immune suppression. Here we describe our single institute experience of T cell-replete, reduced intensity, second allogeneic SCT from the original donor in 25 patients drawn from a cohort of 59 patients who relapsed after a matched sibling T cell depleted SCT.
Patients, materials, and methods
Patients
Between 1997 and 2011, 220 consecutive patients with a hematological malignancy underwent a myeloablative T cell depleted (TCD) SCT from an HLA-identical sibling on National Heart, Lung and Blood Institute (NHLBI) institutional review board–approved protocols. Patients and donors provided written informed consent before enrolling in the transplantation protocols.
First Transplant
All patients received a TCD granulocyte colony-stimulating factor (G-CSF) – mobilized peripheral blood stem cell transplant. G-CSF was administered to all donors at a targeted dose of 10–12 μg/kg of body weight subcutaneously for 5 consecutive days prior to collection. The conditioning regimen consisted of 1200 or 1360 cGy total body irradiation (TBI), cyclophosphamide (Cy, 120 mg/kg over 2 days), with or without fludarabine (Flu, 125mg/m2 over 5 days). Depletion of T cells from the stem cell transplant products was accomplished by selection of CD34+ cells using either the CellPro system (CellPro Inc., Bothel, WA), Isolex system (Nexell Therapeutics Inc, Irvine, CA), or the Miltenyi CliniMacs system (MiltenyiBiotec Inc., Auburn, CA). On the day of SCT all patients received this enriched CD34+ stem cell product with a target dose of 5 × 106 CD34+ cells/kg, accompanied by a predetermined, protocol-specific dose of 0.2 – 1 × 105 T cells/kg that was obtained by supplementing the final product with T cells from the original unmanipulated peripheral blood stem cell component. All patients received low-dose cyclosporine A (CSA) (target plasma level, 100–200 mcg/L), starting on day −4 per protocol. In the absence of significant acute GVHD (grade > 1), a donor lymphocytes infusion (DLI, 1× 107 CD3+ cells/kg) was administered between days 45 to 100 after transplantation. Standard prophylaxis against infection included fluconazole and trimethoprim/sulfamethoxazole, which were administered for at least 6 months after transplantation, and weekly surveillance was performed for cytomegalovirus (CMV) antigenemia as described previously.10, 11
Second Transplant
Second transplant regimens are detailed in Table 2. GVHD prophylaxis consisted of cyclosporine alone (target plasma level, 100–200 mcg/L). Standard prophylaxis against infection and virus surveillance are the same as for the first transplant.
Table 2.
Profile of second transplanted patients
| Sex | Age | Diagnosis at 1st SCT | Cytogenetics at diagnosis | Day of relapse | DLI | Chemo pre 2nd SCT | Disease status prior to 2nd SCT | Conditioning for 2nd SCT | Outcome | Survival Days Post relapse |
|---|---|---|---|---|---|---|---|---|---|---|
| Early relapse | ||||||||||
| M | 25 | AML CR1 | Complex | 133 | + | FLAG-I | NR | Bu | VOD | 41 |
| F | 31 | AML CR1 | +8, t(5;9) | 91 | + | FLAG-I | NR | Flu/Bu | Relapse | 211 |
| F | 27 | ALL Relapse | UA | 77 | − | V+P | NR | BC/VP/Cy | Relapse | 216 |
| M | 20 | ALL Relapse | complex | 84 | − | - | NR | Mel/VP | Relapse | 96 |
| F | 36 | AML/MDS NR | UA | 65 | − | - | NR | Flu/Mel | Relapse | 337 |
| F | 48 | ALL CR3 | UA | 92 | − | HyperCVAD | NR | Bu | Relapse | 71 |
| M | 36 | AML CR2 | UA | 57 | − | - | NR | FLAG | Relapse | 182 |
| F | 49 | AML/MDS NR | Complex | 80 | + | - | NR | FLAG-I | Relapse | 208 |
| F | 16 | AML CR1 | +8, t(11;19) | 85 | − | - | NR | FLAG | Relapse | 159 |
| F | 36 | AML CR2 | t(9:22), inv3 | 56 | − | - | NR | Flu/Bu | Relapse | 65 |
| M | 60 | AML/MDS NR | UA | 78 | − | Flu | NR | Bu | Relapse | 94 |
| F | 35 | ALL Relapse | normal | 99 | − | HyperCVAD | CR | Mel | GVHD | 109 |
| M | 24 | ALL Relaplse | UA | NR | − | VAD | NR | Flu/VP/Mel | Relapse | 67 |
| Late relapse | ||||||||||
| M | 66 | AML CR1 | complex | 220 | − | - | NR | Flu/Bu | Alive | >921 |
| F | 21 | AML/MDS CRi | +8, +14p | 270 | − | - | NR | Flu/TBI | MOF | 73 |
| F | 38 | AML CR1 | normal | 1120 | + | - | NR | FLAG-I | Relapse | 367 |
| M | 13 | AML/MDS NR | −7 | 1149 | − | - | NR | Flu/Bu | Alive | 1378 |
| F | 44 | CML BC | Complex | 1346 | + | - | NR | FLAG-IM | Relapse | 219 |
| F | 40 | AML/MDS NR | UA | 1788 | − | - | NR | Flu/Mel | Relapse | 360 |
| M | 31 | AML Relapse | T(8;21) | 303 | + | FLAG-I | NR | Flu/Bu | Relapse | 298 |
| M | 38 | AML/MDS NR | −7, +8 | 363 | − | IA | CRi | Flu/Bu | Relapse | 591 |
| M | 25 | ALL CR2 | Normal | 374 | − | FLAG-I | CR | Flu/Bu | Pulm Failure | 681 |
| M | 17 | AML CR1 | normal | 465 | − | - | NR | FLAG-I | RSV | 28 |
| M | 37 | CLL RAI stage 4 | −17p, −13q | 731 | + | Ritux + other | NR | Flu/Bu | Relapse | 190 |
| F | 35 | AML/MDS NR | complex | 5091 | + | FLAG-I | CR | Flu/Bu | Relapse | 703 |
Abbreviations: UA = unavailable; FLAG-IM = fludarabine, cytarabine, G-CSF, idarubicin, gemtuzumab; V+P = vincristine + prednisone; HyperCVAD = cyclophosphamide, vincristine, adriamycin, dexamethasone; Flu = fludarabine; VAD = vincristine, doxorubicin, dexamethasone; IA = idarubicin; Ritux = rituximab; Bu = busulfan; Mel = melphalan; VP = etopside; TBI = total body irradiation; VOD = hepatic veno-occlusive disease; MOF = multiple organ failure; Pulm = pulmonary; RSV = respiratory syncytial virus infection
Definition of Clinical Terms
Overall survival (OS) was calculated from the interval between the date of transplantation and death, or last follow-up visit. Relapsed disease for acute leukemia, myelodysplastic syndrome (MDS), chronic lymphocytic leukemia (CLL), multiple myeloma (MM), and non-Hodgkin’s lymphoma (NHL) was defined by morphologic or cytogenetic evidence, either in peripheral blood or in bone marrow.
Statistical analysis
Survival analysis for time-to-event data was used to analyze the effects of baseline risk factors relapse and overall survival. For relapse, patient who did not relapse before death or by the end of the study were treated as censored. For overall survival, patients who were alive by the end of the study were treated as censored. Survival was measured from time of relapse after the first SCT to the last contact date or death. Effects of the baseline risk factors relapse and overall survival were evaluated using the univariate Cox proportional hazard models. Kruskal-Wallis test was used for the treatment of non-transplanted patients. The overall effects of the risk factors were evaluated using the Log-rank p-values, and effects of the individual risk factors were evaluated using p-values based on the two-sided t-tests with 0.05 significance level. Statistical analyses were performed with SPSS 15.0 (IBM SPSS, New York) and Prism 5 (GraphPad Software, San Diego, CA) software.
Results
Patient Characteristics
General patient characteristics after first SCT are shown in table 1a and 1b. Fifty-nine patients relapsed with cytogenetic or morphological evidence of disease recurrence after their first SCT. Relapses occurred in 31 AML/MDS, 17 ALL, 4 B cell malignancies (2 CLL, 1 NHL, 1 MM), 6 CML blast phase and 1 CML accelerated phase (Molecular relapses in CML patients were not included in this study, and no chronic phase CML patient had a hematological relapse). The median age at time of first SCT for the 30 males and 29 females that relapsed was 36 (range 9 – 66) years. Thirty-eight patients relapsed within 6 months (early relapse) at a median of 85 days and 21 patients relapsed after 6 months (late relapse) at a median of 465 days after their first transplant. Patient characteristics of SCT recipients vs other treatments did not show significant differences in gender, age, disease status and risk classification at first SCT, Karnofsky performance status, days of relapse post first SCT in both early and relapse groups. All of our patients were from other institutions or overseas. As a result, cytogenetics were not always available limiting a full description of the kinetics of the disease and chemorefractoriness.
Table 1a.
Characteristics of relapsed patients after first SCT: Early Relapse < 180 days
| No 2nd SCT | Received 2nd SCT | P | |
|---|---|---|---|
| Number | 25 | 13 | |
| Gender (M/F) | 12/13 | 8/5 | NS |
| Age median/range (year) | 33 (14–55) | 35 (16–60) | NS |
| Disease status at 1st SCT | NS | ||
| AML CR1 | - | 3 | |
| CR2 | 3 | 2 | |
| >CR2/MDS | 8 | 3 | |
| CML BC | 5 | - | |
| ALL CR2/3 | 4 | 1 | |
| Relapse | 3 | 4 | |
| Other (MM NHL) | 2 | - | |
| Risk classification | NS | ||
| High risk | 18 (72%) | 7 (54%) | |
| Standard and Intermediate | 7 (28%) | 6 (46%) | |
| Karnofsky % median (range) | 90 (20–90) | 90 (50–90) | NS |
| Day of relapse post 1st SCT median (range) | 91 (0–170) | 80 (0–133) | NS |
| Treatment (excluding SCT) | |||
| DLI | 16 | 3 | 0.02 |
| Chemo – remission intent | 10 | 7 | NS |
| Palliative or None | 6 | 5 | NS |
Abbreviations: SCT = stem cell transplantation; CML BC = CML in blast phase; DLI = donor lymphocyte infusion; NS = not statistically significant
Table 1b.
Characteristics of relapse patients after second SCT: Late Relapse ≥ 180 days
| No 2nd SCT | Received 2nd SCT | P | |
|---|---|---|---|
| Number | 9 | 12 | |
| Gender (M/F) | 3/6 | 7/5 | NS |
| Age median/range (year) | 37 (9–44) | 36 (13–66) | NS |
| Disease status at 1st SCT | NS | ||
| AML CR1 | 1 | 3 | |
| CR2 | 1 | - | |
| >CR2/MDS | 1 | 6 | |
| CML AP | 1 | - | |
| BC | - | 1 | |
| ALL CR1 | 1 | - | |
| CR2/3 | 3 | 1 | |
| CLL | 1 | 1 | |
| Risk classification | NS | ||
| High risk | 2 (22%) | 7 (58%) | |
| Standard and Intermediate | 7 (78%) | 5 (42%) | |
| Karnofsky % median (range) | 90 (50–90) | 90 (50–90) | NS |
| Day of relapse post 1st SCT median (range) | 254 (181–3678) | 598 (220–5091) | NS |
| Treatment (excluding SCT) | |||
| DLI | 3 | 5 | NS |
| Chemo – remission intent | 5 | 5 | NS |
| Palliative or None | 3 | 5 | NS |
Abbreviations: SCT = stem cell transplantation; CML AP = CML in accelerated phase; CML BC = CML in blast phase; DLI = donor lymphocyte infusion; NS = not statistically significant
Second Transplant Characteristics
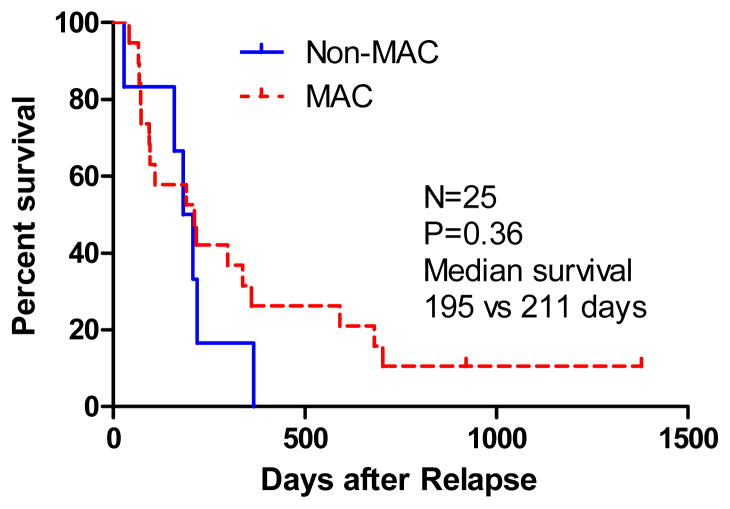
Characteristics of second transplant procedures are shown in table 2. A second T-replete SCT from the original donor using a reduced-intensity conditioning regimen was performed in 25 patients: 13/38 early relapses and 12/21 late relapses. The median number of CD34+ cells infused was 5.76 × 106 cells/kg. Nineteen of 25 patients received myeloablative conditioning (MAC) with either Busulphan or Melphalan, and 6 received a non-MAC specific to their disease. There was no statistically significant difference in survival outcome in patients who received MAC or non-MAC SCT (median 211 vs 195 days, p=0.36) (see figure 3).
Figure 3.
Overall Survival for Myeloablative (MAC) versus Non-Myeloablative (Non-MAC) Conditioning Regimen of second SCT
Prior to second transplant, 1 patient relapsing early and 3 patients relapsing late achieved complete remission (2 CR and 1 CRi) after chemotherapy alone or in combination with DLI. However, no survival benefit of obtaining a remission prior to the second HSCT was noted (p=0.55, data not shown).
Non transplant treatment
The treatment for patients not receiving a second SCT is summarized in table 3. Of these 34 patients (25 early and 9 late relapse), 9 received chemotherapy with intent to obtain remission in combination with DLI, 6 received chemotherapy alone, 10 received DLI alone, and 9 received palliative chemotherapy or hospice care only. In early relapsed patients DLI were given more frequently than those given a second SCT (p=0.02). Otherwise, there were no differences regarding treatment options between patients that received or did not received a second transplant in either early or late relapse groups. Amongst non-transplant recipients no treatment emerged as superior for overall survival (p=0.19).
Table 3.
Treatment of non-second transplanted patients
| Number | Survival post relapse (days median range) | P | |
|---|---|---|---|
| Early relapse | 0.19 | ||
| Therapy with intent to obtain remission with DLI | 7 | 93 (35–941) | |
| Therapy with intent to obtain remission no DLI | 3 | 107 (75–149) | |
| DLI alone | 9 | 130 (11–333) | |
| Palliative (to support blood counts) or none | 6 | 38 (3–110) | |
| Late relapse | |||
| Therapy with intent to obtain remission with DLI | 2 | 473 (165, 781) | |
| Therapy with intent to obtain remission no DLI | 3 | 64 (34–1799) | |
| DLI alone | 1 | 346 | |
| Palliative (to support blood counts) or none | 3 | 64 (34–192) |
Abbreviations: DLI = donor lymphocyte infusion
Relapse and Overall Survival
The distribution of hematological disease type and overall survival for patients relapsing after the first SCT is shown in figure 1. The overall survival for early versus late relapse after SCT is shown in figure 2. Patients experiencing an early relapse included AML/MDS (50%), ALL (32%), CML blast phase (13%), and B cell malignancies (NHL and MM 5%). Patients experiencing a late relapse included AML/MDS (57%), ALL (24%), CML blast or accelerated phase (9%), and B cell malignancies (CLL 10%). Patients relapsing late had a longer overall survival compared with patients relapsing early (p=0.0002). No patient relapsing early after the first SCT survived beyond 1 year following relapse, while the probability of one year survival for patients with a late relapse was 33% (HR=2.97, 95% CI 1.683–5.241 p=0.0002). At 3 years after relapse, the actuarial survival was 0 for early relapsed patients (with or without a second SCT). In contrast, the Kaplan-Meier estimate of actuarial survival for late relapsed patients was 17% after second SCT and 0% without. However, a second SCT in early relapse did not significantly prolong survival when compared with patients receiving other treatments (median 109 vs 80 days, HR = 1.308, 95% CI 0.69–2.17, p = 0.41). Similarly a second SCT for late relapse only modestly prolonged survival (median survival 363.5 vs 162 days, HR = 1.44, 95% CI 0.52 – 4.0, p = 0.49). Among 21 patients relapsing late not receiving SCT, 1 patient with CLL, was a long term survivor at a median follow-up of 6.3 years while 2 of 12 transplanted patients (with AML and MDS RAEB2) were long-term survivors beyond 5 years.
Figure 1.
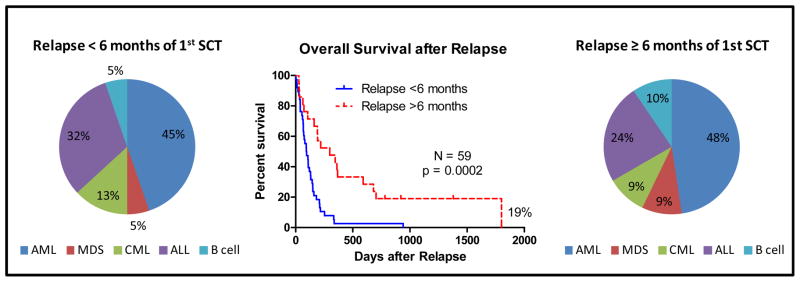
Overall Survival after Relapse and Type of Hematologic Malignancy
Figure 2.
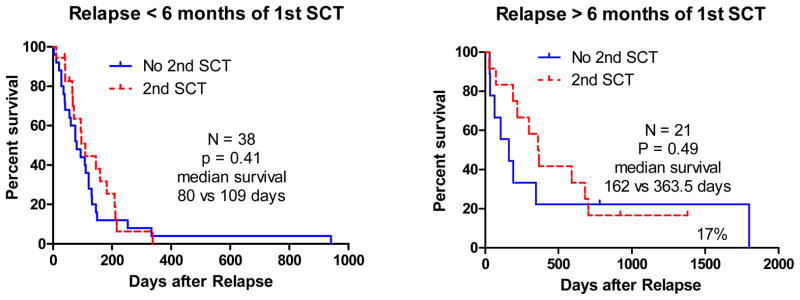
Overall Survival for Early versus Late Relapse after SCT
In 13 early relapsed patients receiving second HSCT the cause of treatment failure was relapse in all but two patients (1 death from grade IV gastrointestinal GVHD and 1 from severe hepatic veno-occlusive disease). Four of the 13 patients developed acute GVHD, and no survival benefit was noted from acute GVHD in these patients (p=0.5041, see supplementary figure). In contrast, in the 12 patients relapsing late after SCT there was a 58% probability of dying from relapse and a 25% probability of non-relapse mortality (organ failure and infection, Table 2). In this group, 4 out of the 12 patients developed acute GVHD after second HSCT. However, again no survival benefit was noted in patients with acute GVHD (p=0.5841, see supplementary figure). There was no observed chronic GVHD in all patients received second SCT.
Discussion
Relapse of a hematological malignancy after an SCT given with intent to cure the disease represents a devastating treatment failure with no ideal treatment options. While there is some consensus that chemotherapy associated with a donor lymphocyte infusion can prolong survival, only a small minority of patients become long term survivors.12, 13 A second SCT may bring a second chance of cure if the second transplant strategy provides a more intensive antileukemic conditioning regimen or a stronger GVL effect.9 Nevertheless, second SCT carries a high risk of treatment failure, both from relapse and non-relapse mortality.14 Because patients relapsing after SCT show diversity in the aggressiveness and chemosensitivity of their disease, and vary widely in their performance status, second transplants tend to be offered on an individual basis to selected patients, usually those with better disease control and good performance status. An additional factor determining treatment for relapse is dictated by patient preference for either further intensive treatment or palliative measures, and the availability of donor. As a consequence there are no prospective randomized studies comparing transplant and non-transplant treatments for hematological malignancy relapsing after SCT.
We review here our approach to treatment of relapse after allogeneic SCT in selected patients with at least partial control of their disease who could tolerate a second SCT. Because of the better outcomes described for late relapse disease,2, 4 a greater proportion of second transplants were performed in patients relapsing after six months of their first SCT (57% vs 34%). The second SCT was performed with a T cell-replete transplant using only cyclosporine as GVHD prophylaxis in order to enhance the GVL effect over the original T cell depleted transplant. In this way it was hoped that a second transplant from the same donor could prolong survival and achieve a second chance of cure of malignancy. While presenting data for relapsed patients not receiving SCT, it must be pointed out that the groups are not matched - there was an inevitable bias in selecting patients for second SCT based on their general fitness, degree of disease control after relapse treatment, patient’s choice to undergo further intensive treatment, and the availability of donor. Nevertheless, despite more favorable features of the second transplant group, it was disappointing that such patients did not experience an important prolongation of survival with the procedure. This disappointing outcome was shared among all patients regardless of the ability to successfully obtain a complete remission prior to a second SCT. Patients relapsing early after their first SCT had at best a 3 months survival advantage with a second SCT, offset by inevitably prolonged hospitalization. Comparable to other experience, patients relapsing late after their first SCT generally had a more favorable outcome.2, 15 The one year probability of survival reached 33% for late relapsed patients undergoing second SCT. Overall there was 17% survival for late relapsed patients given a second SCT maintained over the 6 years of follow-up in our study.
This is the first study to our knowledge of patients receiving a T cell-replete transplant for disease relapsing after an initial T cell depleted SCT. However, our study is limited by the sample size, selection bias for second transplantation, and availability of donors for SCT or DLI.
In conclusion these results indicate that the additional GVL effect from the unmanipulated second SCT was not sufficient to control relapsed disease in most patients who originally received a T cell-depleted SCT. A meaningful survival benefit from second SCT was only observed in late relapsed patients, and may have been due to selection bias. Based on this 14 year observation we no longer offer second SCT for patients relapsing within 6 months of transplant, and plan only to further study second SCT for late relapsed patients in the context of randomized trials comparing SCT with other treatments. It should be noted, however, that these findings specifically relate to the myeloablative regimens used in this patient group. Better outcomes after relapse might be observed in patients who received reduced intensity conditioning for the first SCT and more intensive conditioning for the second SCT. Consistent with this observation, recent studies have demonstrated that simply repeating the first SCT maneuver in treating patients with relapsed ALL provides minimal benefit, and that intensifying the conditioning or selecting an alternative donor may confer a greater antileukemic effect.16
Strategies that include a combination of therapeutic modalities to promote GVL while minimizing risks of toxicity and GVHD may improve outcomes for patients with relapsed disease after SCT. Recent studies have demonstrated the efficacy of azacitidine in the treatment of relapsed AML. Azacitidine up-regulates the expression of epigenetically silenced tumor antigens to promote cytotoxic CD8+ T cell responses and augment the GVL effect. Complete remission rates of 20% – 40% have been noted when used as a single agent to treat patients with AML that have relapsed after SCT. Azacitidine also appears well tolerated, and is associated with an expansion of regulatory T cells and low rates of GVHD when used early after SCT.17 Recently, the combination of azacitidine and DLI as first salvage therapy for relapsed AML or MDS after SCT has been explored and appears safe with low rates of acute GVHD (grades III–IV of 10%). An overall response rate of 30% was observed, with 17% durable complete remissions (median duration, 777 days), demonstrating the feasibility of combining azacitidine with cellular therapies to augment anti-leukemic responses while minimizing toxicity.18 In this context, the combination of epigenetic modifying agents, such as azacitidine, lenalidomide and histone deacetylase inhibitors, with novel cellular therapy to include prophylactic DLIs, natural killer cell infusions, and leukemia-specific T cells or leukemia vaccines provides an exciting new avenue of research that may lead to improved treatment of leukemia relapse after allogeneic SCT.19, 20
Supplementary Material
Acknowledgments
The study was sponsored by National Heart, Lung, and Blood Institute and registered as ClinicalTrials.gov-registered trials NCT00001623, NCT00001873, NCT00353860, NCT00066300, NCT00079391, NCT00398346, and NCT00467961.
Footnotes
Conflict of interest disclosure: The authors declare no conflicts of interests.
Supplementary information is available at BMT’s website.
References
- 1.Barrett AJ, Battiwalla M. Relapse after allogeneic stem cell transplantation. Expert review of hematology. 2010;3(4):429–41. doi: 10.1586/ehm.10.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaw BE, Mufti GJ, Mackinnon S, Cavenagh JD, Pearce RM, Towlson KE, et al. Outcome of second allogeneic transplants using reduced-intensity conditioning following relapse of haematological malignancy after an initial allogeneic transplant. Bone marrow transplantation. 2008;42(12):783–9. doi: 10.1038/bmt.2008.255. [DOI] [PubMed] [Google Scholar]
- 3.Pollyea DA, Artz AS, Stock W, Daugherty C, Godley L, Odenike OM, et al. Outcomes of patients with AML and MDS who relapse or progress after reduced intensity allogeneic hematopoietic cell transplantation. Bone marrow transplantation. 2007;40(11):1027–32. doi: 10.1038/sj.bmt.1705852. [DOI] [PubMed] [Google Scholar]
- 4.Mielcarek M, Storer BE, Flowers ME, Storb R, Sandmaier BM, Martin PJ. Outcomes among patients with recurrent high-risk hematologic malignancies after allogeneic hematopoietic cell transplantation. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2007;13(10):1160–8. doi: 10.1016/j.bbmt.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Arellano ML, Langston A, Winton E, Flowers CR, Waller EK. Treatment of relapsed acute leukemia after allogeneic transplantation: a single center experience. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2007;13(1):116–23. doi: 10.1016/j.bbmt.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Barrett AJ, Joshi R, Tew C. How should acute lymphoblastic leukaemia relapsing after bone-marrow transplantation be treated? Lancet. 1985;1(8439):1188–91. doi: 10.1016/s0140-6736(85)92865-x. [DOI] [PubMed] [Google Scholar]
- 7.Hijiya N, Gaynon P, Barry E, Silverman L, Thomson B, Chu R, et al. A multi-center phase I study of clofarabine, etoposide and cyclophosphamide in combination in pediatric patients with refractory or relapsed acute leukemia. Leukemia: official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2009;23(12):2259–64. doi: 10.1038/leu.2009.185. [DOI] [PubMed] [Google Scholar]
- 8.Loren AW, Porter DL. Donor leukocyte infusions for the treatment of relapsed acute leukemia after allogeneic stem cell transplantation. Bone marrow transplantation. 2008;41(5):483–93. doi: 10.1038/sj.bmt.1705898. [DOI] [PubMed] [Google Scholar]
- 9.Oran B, de Lima M. Prevention and treatment of acute myeloid leukemia relapse after allogeneic stem cell transplantation. Current opinion in hematology. 2011;18(6):388–94. doi: 10.1097/MOH.0b013e32834b6158. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura R, Battiwalla M, Solomon S, Follmann D, Chakrabarti S, Cortez K, et al. Persisting posttransplantation cytomegalovirus antigenemia correlates with poor lymphocyte proliferation to cytomegalovirus antigen and predicts for increased late relapse and treatment failure. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2004;10(1):49–57. doi: 10.1016/j.bbmt.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Solomon SR, Nakamura R, Read EJ, Leitman SF, Carter C, Childs R, et al. Cyclosporine is required to prevent severe acute GVHD following T-cell-depleted peripheral blood stem cell transplantation. Bone marrow transplantation. 2003;31(9):783–8. doi: 10.1038/sj.bmt.1703928. [DOI] [PubMed] [Google Scholar]
- 12.Spyridonidis A, Labopin M, Schmid C, Volin L, Yakoub-Agha I, Stadler M, et al. Outcomes and prognostic factors of adults with acute lymphoblastic leukemia who relapse after allogeneic hematopoietic cell transplantation. An analysis on behalf of the Acute Leukemia Working Party of EBMT. Leukemia: official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2012 doi: 10.1038/leu.2011.351. [DOI] [PubMed] [Google Scholar]
- 13.Roddie C, Peggs KS. Donor lymphocyte infusion following allogeneic hematopoietic stem cell transplantation. Expert opinion on biological therapy. 2011;11(4):473–87. doi: 10.1517/14712598.2011.554811. [DOI] [PubMed] [Google Scholar]
- 14.Hartwig M, Ocheni S, Asenova S, Wiedemann B, Zabelina T, Ayuk F, et al. Second allogeneic stem cell transplantation in myeloid malignancies. Acta haematologica. 2009;122(4):185–92. doi: 10.1159/000253025. [DOI] [PubMed] [Google Scholar]
- 15.Oran B, Giralt S, Couriel D, Hosing C, Shpall EJ, de Meis E, et al. Treatment of AML and MDS relapsing after reduced-intensity conditioning and allogeneic hematopoietic stem cell transplantation. Leukemia: official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2007;21 (12):2540–4. doi: 10.1038/sj.leu.2404828. [DOI] [PubMed] [Google Scholar]
- 16.Poon LM, Bassett R, Jr, Rondon G, Hamdi A, Qazilbash M, Hosing C, et al. Outcomes of second allogeneic hematopoietic stem cell transplantation for patients with acute lymphoblastic leukemia. Bone marrow transplantation. 2012 doi: 10.1038/bmt.2012.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodyear OC, Dennis M, Jilani NY, Loke J, Siddique S, Ryan G, et al. Azacitidine augments expansion of regulatory T cells after allogeneic stem cell transplantation in patients with acute myeloid leukemia (AML) Blood. 2012;119(14):3361–9. doi: 10.1182/blood-2011-09-377044. [DOI] [PubMed] [Google Scholar]
- 18.Schroeder T, Czibere A, Platzbecker U, Bug G, Uharek L, Luft T, et al. Azacitidine and donor lymphocyte infusions as first salvage therapy for relapse of AML or MDS after allogeneic stem cell transplantation. Leukemia: official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2013 doi: 10.1038/leu.2013.7. [DOI] [PubMed] [Google Scholar]
- 19.Gupta V, Tallman MS, Weisdorf DJ. Allogeneic hematopoietic cell transplantation for adults with acute myeloid leukemia: myths, controversies, and unknowns. Blood. 2011;117(8):2307–18. doi: 10.1182/blood-2010-10-265603. [DOI] [PubMed] [Google Scholar]
- 20.Warren EH, Fujii N, Akatsuka Y, Chaney CN, Mito JK, Loeb KR, et al. Therapy of relapsed leukemia after allogeneic hematopoietic cell transplantation with T cells specific for minor histocompatibility antigens. Blood. 2010;115(19):3869–78. doi: 10.1182/blood-2009-10-248997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.