Abstract
Background
Alcohol abuse is the second leading cause of dilated cardiomyopathy, a disorder specifically referred to as Alcoholic Cardiomyopathy (ACM). Rodent and human studies have revealed cardiac fibrosis to be a consequence of ACM and prior studies by this lab have associated this occurrence with elevated transforming growth factor-beta (TGF-β) and activated fibroblasts (myofibroblasts). To date there have been no other studies to investigate the direct effect of alcohol on the cardiac fibroblast.
Methods
Primary rat cardiac fibroblasts were cultured in the presence of ethanol and assayed for fibroblast activation by collagen gel contraction, alpha smooth muscle- actin (α-SMA) expression, migration, proliferation, apoptosis, collagen I & III and TGF-β expression. The TGF-β receptor type 1 inhibitor compound SB 431542 and a soluble recombinant TGF-βII receptor (RbII) were used to assess the role of of TGF-β in the response of cardiac fibroblasts to ethanol.
Results
Treatment of cardiac fibroblasts with ethanol at concentrations of 100 mg/dl or higher resulted in fibroblast activation and fibrogenic activity after 24 hours including an increase in contraction, α-SMA expression, migration, and expression of collagen I and TGF-β. No changes in fibroblast proliferation or apoptosis were observed. Inhibition of TGF-β by SB 431542 and RbII attenuated the ethanol-induced fibroblast activation.
Conclusions
Ethanol treatment directly promotes cardiac fibroblast activation by stimulating TGF-β release from fibroblasts. Inhibiting the action of TGF-β decreases the fibrogenic effect induced by ethanol treatment. The results of this study support TGF-β to be an important component in cardiac fibrosis induced by exposure to ethanol.
Keywords: Ethanol, Cardiac Fibrosis, TGF-β, Myofibroblasts, Collagen
Introduction
Alcoholic Cardiomyopathy (ACM) is a complex disorder that leads to maladaptive myocardial remodeling and can eventually lead to heart failure. ACM includes a number of pathological processes including significant cardiac fibrosis (Dancy and Maxwell, 1986; Law et al., 2012). ACM has been characterized as primarily left ventricular systolic dysfunction but human studies have shown that diastolic dysfunction, a parameter affected by fibrosis, predicts mortality in those suffering from heart failure (Karaahmet et al., 2010; Laonigro et al., 2009). Studies done by this lab and others have reported myocardial fibrosis in rodent models of alcohol abuse; however, many questions remain regarding the mechanisms responsible for this process (Law et al., 2012). Though the known functions of cardiac fibroblasts have expanded since their early characterization, they are still the cells primarily responsible for modulating the extracellular matrix (ECM) in the mammalian heart (Lajiness and Conway, 2012).
Studies investigating the liver response to alcohol abuse have indicated that myofibroblasts are the cell type predominantly responsible for producing fibrotic scarring in hepatic fibrosis (Kisseleva et al., 2012). In the liver, these myofibroblasts are derived from hepatic stellate cells, a transdifferentiation that has been shown to be largely dependent on transforming growth factor-beta (TGF-β) (Unanue, 2007). In other cardiovascular disease states, such as myocardial infarction, fibroblasts and their activated state of myofibroblasts play a pivotal role in pathological ECM deposition but to our knowledge there have been no studies undertaken to delineate the mechanisms of cardiovascular fibrosis due to alcohol abuse (van Nieuwenhoven and Turner, 2012). Previous work by this lab has associated the presence of non-vascular alpha-smooth muscle actin (α-SMA) positive cells (myofibroblasts) and elevated TGF-β with cardiac interstitial fibrosis in mice chronically consuming ethanol (Law et al., 2012).
In the present studies, cells isolated from adult rat hearts were used to evaluate the effects of alcohol exposure on fibroblast function and gene expression. These studies illustrated that ethanol promotes the activation to a myofibroblast phenotype including enhanced contractile activity and increased collagen gene expression. Using pharmacological inhibitors, these studies also illustrated that the response of fibroblasts to ethanol is at least partly dependent upon autocrine/paracrine activity of TGF-β produced by fibroblasts in response to ethanol.
Materials and Methods
Isolation of Cardiac Fibroblasts
Eight week old adult male Sprague Dawley rats (200–250 g body weight) were purchased from Harlan and housed in an AAALAC-approved animal facility. All animals were provided food and water ad libitum. All experiments were performed in accordance to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication No. 85-23, revised 1996) and were approved by the University of South Carolina Institutional Animal Use and Care Committee (IACUC). Animals were euthanized, hearts removed and rinsed in sterile, physiological saline. Extracardiac tissue was discarded, heart tissues were minced in sterile saline and fibroblasts were isolated by digestion of the minced tissue with Liberase 3 (Roche Applied Science; Indianapolis, IN) as described previously (Burgess et al., 2002; Diaz-Araya et al., 2003). Cells collected from digested tissue were plated into T75 flasks in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% neonatal calf serum, 5% fetal bovine serum and antibiotics (hereafter called normal fibroblast medium). Fibroblasts were purified by their rapid attachment to tissue culture plastic. Cells were passaged at approximately 80% confluency following detachment with a 0.25% trypsin/0.1% ethylenediaminetetraacetic acid (trypsin/EDTA) solution. Fibroblasts were used from passages 3 through 7.
Cardiac Fibroblast Treatment
Cells were allowed to grow for 24 h prior to experimental treatment on dishes coated with type 1 collagen(10μg/mL ), medium was removed and replaced with low serum DMEM (1.5% fetal bovine serum) containing antibiotics. Fibroblasts were treated with varying doses of ethanol (0 to 400 mg/dl) (Henderson et al., 1989; Ranzer et al., 2011) in low serum medium for 24 h. Cells were maintained in a humid environment at 37 degrees Centigrade in covered dishes. 10 μM of the TGF-β receptor antagonist, SB 431542 (Sigma; St. Louis, MO), was used to block TGF-β receptor signaling. The concentration of SB 431542 was utilized based on previously published efficacy (Inman et al., 2002). Additionally, 0.3μg/mL recombinant receptor TGF-βII (RbII) (R&D Systems) was utilized 1 h prior to ethanol treatment as another inhibitor of TGF- β. The concentration of RbII utilized was based on previously published efficacy (Tsang et al., 1995). 1ng/mL TGF-β1 (R&D Systems; Minneapolis, MN) was used as a positive control and an equal amount of DMSO (Sigma) used as a vehicle control for SB 431542. After 24 hours treatment and incubation, an ethanol assay kit (Abcam, Cambridge, MA, USA) was used to measure alcohol concentration. After 24 hours, approximately 80% of ethanol remained in cell media.
Collagen Gel Contraction
The three-dimensional collagen gel system has been extensively used to examine interactions between cardiac fibroblasts and the collagenous ECM (Bell et al., 1979; Guidry and Grinnell, 1985; 1987). Fibroblasts were cultured as described above in normal fibroblast medium. Fibroblasts were detached from culture plates with a trypsin/EDTA solution. Cells were rinsed, pelleted by centrifugation and resuspended in low serum medium (1.5% fetal bovine serum). An equal volume of cells was combined with a 1.25 mg/ml collagen solution to yield a final concentration of 150,000 cells per milliliter. The collagen: cell mixture was added to wells of 24- well plates that had been precoated with bovine serum albumin to prevent collagen gels from attaching to the culture plastic. Collagen gels were allowed to polymerize 1 h at 37 °C. Following polymerization, low serum medium (with or without ethanol) was added to each well of the plate and collagen gels dislodged from the well to allow the gels to float in the medium. Collagen gels were photographed and the relative contraction of the collagen gel was determined by measuring the top surface of the gels at 24 h relative to their initial size. Collagen gels were performed in triplicate in each of at least six independent experiments.
Fibroblast Proliferation, Apoptosis, and Myofibroblast Detection
For analysis of proliferation, apoptosis, and myofibroblast formation, cells were cultured on glass coverslips that were precoated with collagen type I. Following culture and treatment (described above), coverslips were rinsed with phosphate-buffered saline and fixed in absolute ethanol containing 10 mM glycine (pH 2.0) for 30 min at −20 °C.
The proliferation of isolated cardiac fibroblasts was assayed by BrdU incorporation, where BrdU was added alongside ethanol treatment (Roche Applied Science). BrdU incorporation was detected by immunocytochemical staining with anti-BrdU serum and fluorescien-conjugated secondary antibodies (Roche Applied Sciences). Cells were simultaneously stained with DAPI to detect all nuclei. Cells were examined and the percentage of BrdU-positive fibroblasts determined using a Nikon E600 microscope equipped for epifluorescence. Proliferation assays were performed with at least three independent experiments.
Immunocytochemical staining of fibroblasts with an α-SMA antibody was employed to identify myofibroblasts (Abcam, Cambridge, MA, USA) A fluorescein isothiocyanate conjugated secondary antibody (Santa Cruz Biotechnology) was used to visualize immunostaining. Cells were simultaneously stained with DAPI (Sigma) to detect all nuclei. Cells were examined and the percentage of α-SMA-positive fibroblasts determined using a Nikon E600 microscope equipped for epifluorescence. α-SMA assays were performed with at least four independent experiments.
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) (Roche Diagnostics; Mannheim, Germany) was applied to fibroblasts, and the amount of apoptotic nuclei per cells was quantified according to manufacturer’s protocol. Slides were also co-stained with DAPI (Sigma) to identify all nuclei in the section. A ratio of TUNEL positive cell number to DAPI positive cells was used to quantify changes in apoptosis. Apoptosis assays were performed with at least three independent experiments.
Fibroblast Migration Assay
The relative migratory ability of fibroblasts was determined using a scratch assay as previously described (Lijnen et al., 2007). Fibroblasts were plated into wells of 6-well plates precoated with collagen type I (10 μg/ml). After 24 h of culture, normal fibroblast medium was replaced with DMEM containing 1.5% fetal bovine serum. A scratch was gently created through the confluent cells with a pipet tip. Fibroblasts were cultured and treated as described above. The area devoid of cells was photographed at the time the scratch was created and 24 h later. The relative migration of fibroblasts into the denuded area was measured using Infinity Analyze software (Ottawa, ON CA). Migration experiments were performed in triplicate wells with each of at least three independent experiments.
Western Blots
The effect of alcohol treatment on the expression of TGF-β precursor and collagens type I and III in isolated cardiac fibroblasts was determined by Western blot analysis. Fibroblasts were cultured and treated as described above in normal fibroblast medium in 150 mm dishes coated with collagen type I. Following treatment of cells, conditioned medium was removed for analysis of collagens. Total protein content of the media was determined using the BCA assay (Pierce; Rockford, IL). Equal amounts of protein were separated on 4–20% gradient polyacrylamide gels (Pierce; Rockford, IL). Proteins were transferred onto nitrocellulose membranes, which were stained with Fast Green to verify efficient transfer of proteins. Membranes containing conditioned medium proteins or cell lysate proteins were incubated in primary antisera (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) against GAPDH, TGF-β1, collagen type I or III (1:1000, overnight, 4°C). Secondary HRP-conjugated antibodies were utilized for detection (1:10,000, 2 h, room temperature). Signals from these antisera were normalized to the Fast Green stained membrane.
Western blots were developed with SuperSignal reagent (Pierce; Rockford, IL) and exposed to X-ray film. Relative protein expression was determined by image analysis of X-ray films using Adobe Photoshop analysis of band integrated densities. Protein expression was normalized to GAPDH in cell lysates and fast green band of 75 KDa in cell medium.
Reverse Transcriptase Polymerase Chain Reaction
The expression of mRNAs encoding ECM components was assayed by semiquantitative reverse transcriptase-polymerase chain reaction (RT-PCR). Adult cardiac fibroblasts were cultured and treated as described above in normal fibroblast medium. Cells were rinsed with phosphate-buffered saline and extracted in TRIzol Reagent (Invitrogen; Carlsbad, CA). RNA was precipitated, resuspended in nuclease-free water, column cleaned using an RNeasy Mini Kit (Qiagen; Valencia, CA) and quantified spectrophotometrically. cDNA was produced from 2 μg of RNA using the iScript cDNA kit (BioRad; Hercules, CA). Semiquantitative PCR was carried out using primers specific for markers of fibrosis including collagen type I, collagen type III, αSMA, and TGF-β. The relative expression of target mRNA was normalized to the acidic ribosomal phosphoprotein PO (Akamine et al., 2007). Preliminary experiments were conducted to determine the appropriate amplification cycle number for each of the mRNAs of interest (Rameckers et al., 1997).
TGF-β ELISA
A TGF-β ELISA was used to quantify the amount of TGF-β in cell supernatant samples (R&D Systems). Fibroblasts were cultured and treated as described above. Conditioned medium was removed for analysis and assayed according to manufacturer instructions.
Statistical analysis
Results are shown ±SEM. Statistical significance was determined by ANOVA with a Tukey post hoc test (p < 0.05). Analyses were performed using GraphPad Prism Software.
Results
Cardiac fibroblasts exposed to ethanol exhibit increased transdifferentiation to myofibroblasts in vitro, which is blocked by inhibition of TGF-β signaling
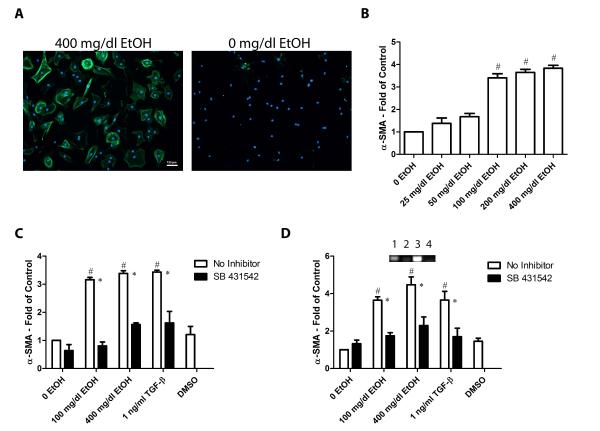
Transition from fibroblasts to the more activated form of myofibroblasts is an important step in the progression of many diseases including alcohol-induced fibrosis. Myofibroblasts have been shown to be marked by expression of α-SMA (Desmoulière et al., 1993). Fibroblasts were treated with varying doses of ethanol (0 to 400 mg/dl) and α-SMA expression detected by immunocytochemical staining. Treatment of isolated adult cardiac fibroblasts with 100, 200 and 400 mg/dl ethanol resulted in a significant increase in α-SMA positive cells compared with controls not exposed to ethanol (Figure 1A and 1B). Treatment with lower doses of ethanol (25 and 50 mg/dl) did not have significant effects on α-SMA expression. Based upon these data, subsequent experiments were performed with 100 and 400 mg/dl ethanol. A significant increase in myofibroblasts was also observed in cells treated with TGF-β1. Addition of TGF-β inhibitor SB 431542 successfully blocked this elevated trandifferentiation in ethanol-treated and TGF-β1-treated cells. There was no significant difference between cells treated with DMSO and the untreated control (Figure 1C). PCR analysis of fibroblast RNA collected from cells that were treated with 100 and 400 mg/dl ethanol revealed significant increases in α-SMA (Figure 1D). Significant increases in these transcripts were also observed in cells treated with TGF-β. Treatment with the TGF-β type I receptor inhibitor compound SB 431542 resulted in a prevention of these observed increases. No significant difference between DMSO vehicle treatment and negative controls was observed (Figure 1D).
Figure 1.
Treatment with ethanol induces myofibroblast transdifferentiation and TGF-β inhibitors block this effect. Panel A exhibits example images of cardiac fibroblasts immunocytochemically stained for α-SMA. From left to right the images show examples of cells incubated with 400 mg/dl EtOH and 0 mg/dl EtOH. Green staining denotes α-SMA and blue, DAPI. Panel B shows a graphical dose response of α-SMA expression in 25, 50, 100, 200, and 400 mg/dl ethanol treatments. Panel C shows the quantification of the ratio of myofibroblasts to total nuclei. Panel D shows the quantification and inset examples of fibroblast RNA analysis for α-SMA. On inset 1 is no treatment, 2 is SB 431542, 3 is 400 mg/dl ethanol and 4 is 400 mg/dl ethanol + SB 431542. Each value represents the mean ± SEM as a ratio to non-treated 0 EtOH control, n=4. # p <0.05 comparing to 0 EtOH control and *p<0.05 comparing treatments of SB 431542 to conditions with no inhibitor.
Fibroblasts treated with ethanol exhibit increased contraction when seeded in a collagen matrix and TGF-β inhibition reduces this effect
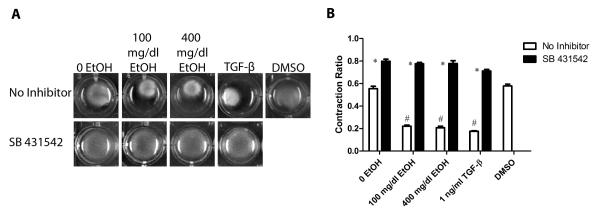
The 3-dimensional collagen gel model has been utilized by this lab (Fix et al., 2011) and others for examining the ability of fibroblasts to remodel the collagen matrix. The effects of ethanol (100 and 400 mg/dl) and TGF-β1 on the ability of fibroblasts obtained from adult male rat hearts to remodel and contract 3-dimensional collagen gels were assayed. SB 431542 was utilized as an inhibitor of TGF-β. TGF-β1, DMSO, and collagen gels with no treatment were used as controls. After 24 h, these treatments showed that addition of 100 and 400 mg/dl ethanol yielded increased contraction of fibroblast-seeded 3-dimensional gels in comparison to untreated control samples (Figure 2). The degree of collagen gel contraction in ethanol-treated cells was similar to what was observed in gels treated with TGF-β1 which has been shown previously to promote contraction of 3-dimensional collagen gels ( Kobayashi et al., 2005; Lijnen et al., 2003; Tingström et al., 1992) (Figure 2B). Addition of the TGF-β type I receptor activin receptor-like kinase inhibitor, SB 431542, significantly decreased contraction in both ethanol-treated gels and untreated controls. This suggests that ethanol-induced collagen gel contraction by heart fibroblasts is mediated by activation of TGF-β signaling pathways.
Figure 2.
Exposure to ethanol increases fibroblast contraction, which is reduced by TGF-β inhibition. Panel A shows representative images of cardiac fibroblast-seeded collagen gels with their respective treatments after 24 hours of incubation. Panel B shows the quantification of the change in collagen gel contraction as a ratio of size at 24 to 0 hours. Each value represents the mean ± SEM, n=3 to 6. # p<0.05 comparing to 0 EtOH control and *p<0.05 comparing treatments of SB 431542 to corresponding conditions without SB 431542.
Ethanol- treated cardiac fibroblasts show enhanced migration and treatment with SB 431542 attenuates this effect
In addition to enhanced contractility, myofibroblasts characteristically demonstrate increased migratory activity (Porter and Turner, 2009). Cardiac fibroblasts were subjected to a wound healing assay in order to assay the effect of ethanol on migration. Cells treated with 100 and 400 mg/dl ethanol exhibited increased migration after being subjected to a scratch through the confluent fibroblast layer (Figure 3). This increase was significant compared to the untreated controls. A more robust increase in migration was observed in cells treated with TGF-β1. Treatment with SB 431542 completely prevented fibroblast migration in ethanol-treated and TGF-β1-treated cultures. No significant difference was observed between fibroblasts treated with DMSO and untreated cells (Figure 3).
Figure 3.
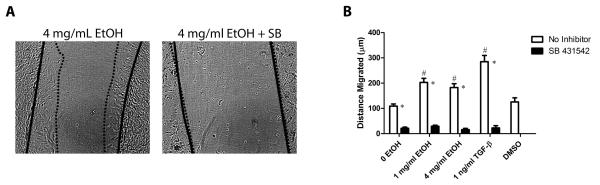
Ethanol treatment increases cardiac fibroblast migration in a wound healing assay and the presence of SB 431542 eliminates this effect. Panel A shows representative examples of the wound healing assay. The solid black line traces the edge of fibroblasts along the denuded area at 0 hours and the dotted line after 24. Images were taken at 4X magnification. Panel B shows the quantification of the distance migrated by cells after 24 hours of treatment. Each value represents the mean ± SEM, n=6. # p <0.05 comparing to 0 EtOH control, and *p<0.05 comparing treatments of SB 431542 to conditions with no inhibitor.
Treatment with ethanol and TGF-β have no effect on cardiac fibroblast proliferation or apoptosis
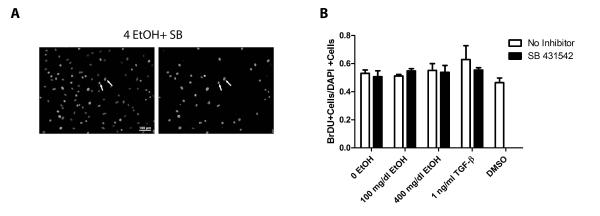
A hallmark of fibrosis is increased density of fibroblasts and myofibroblasts. Experiments were performed to evaluate the effects of ethanol on fibroblast proliferation. Rat heart fibroblasts were treated with 100 and 400 mg/dl ethanol and subsequently fixed and assayed for proliferation via BrdU incorporation and for apoptosis by TUNEL. Treatment with 100 and 400 mg/dl ethanol resulted in no significant change in fibroblast proliferation compared to untreated controls (Figure 4). Similarly, no change was observed in cells treated with TGF-β1. Addition of SB 431542 had no effect on fibroblast proliferation in vitro. Vehicle treatment with DMSO exhibited no significant changes in BrdU incorporation compared to the untreated control. TUNEL analysis revealed that no significant apoptosis occurred in fibroblasts across all treatments (not shown).
Figure 4.
Exposure to ethanol does not affect fibroblast proliferation in vitro nor does treatment with SB 431542. Panel A shows a representative image of fibroblasts treated with SB 431542. Left image shows total nuclei stained with DAPI, and the right image shows only BrdU-positive nuclei. Arrows indicate two cells that exhibit BrdU incorporation. Panel B shows the quantification of the BrdU positive cells as a ratio to DAPI positive cells. Each value represents the mean ± SEM, n=4. SB = SB 431542
Ethanol treatment increases expression of collagen I by cardiac fibroblasts and SB 431542 blocked this increase
The presence of increased deposition of fibrillar collagens is associated with fibroblast activation to myofibroblasts (Porter and Turner, 2009). Western blot analysis of fibroblast conditioned medium from dishes treated with 100 and 400 mg/dl ethanol revealed a significant increase in accumulation of collagen type I protein, but not collagen type III compared to the untreated control (Figure 5A). Cells treated with TGF-β1 alone presented a significant increase in collagen type I deposition. Addition of SB 431542 in cells treated with ethanol inhibited this elevation in collagen accumulation. PCR analysis of fibroblast RNA collected from cells that were treated with 100 and 400 mg/dl ethanol revealed significant increases in col1a1 but not col3a1 (Figure 5A). Significant increases in these transcripts were also observed in cells treated with TGF-β1. Treatment with the TGF-β receptor inhibitor compound SB 431542 resulted in a prevention of these observed increases. No significant difference between DMSO vehicle treatment and negative controls was observed (Figure 5B).
Figure 5.

Protein and RNA analysis indicates that ethanol treatment increases the amount of collagen I, but not collagen III in fibroblasts and this effect is rescued by inhibition of TGF-β by SB 431542. Panel A shows the quantification and example insets of western blot analysis on protein extracted from cell medium after treatments. Panel B shows graphical quantifications of PCR on RNA and example insets collected from cells after being treated with ethanol, controls, and inhibitors. On insets 1 is no treatment, 2 is SB 431542, 3 is 400 mg/dl ethanol and 4 is 400 mg/dl ethanol + SB 431542. Each value represents the mean ± SEM, n=3 to 6. # p <0.05 comparing to 0 EtOH control, and *p<0.05 comparing treatments of SB 431542 to conditions with no inhibitor.
Treatment with ethanol increases expression of TGF-β by fibroblasts
TGF-β has been associated with fibroblast to myofibroblast activation (Desmoulière et al., 1993). Cells treated with 100 and 400 mg/dl show an increase in the TGF-β precursor compared to untreated controls in fibroblast lysates. Treatment with the activin receptor-like kinase inhibitor compound SB 431542 inhibited the elevation of TGF-β’s latent form. No significance was observed between DMSO and untreated controls (Figure 6A). Similar to what was observed in protein, an increase in TGF-β RNA was observed in fibroblasts treated with 100 and 400 mg/dl alcohol. The increase in TGF-β transcripts was also observed in cells treated with 1 ng/ml TGF-β1 as a positive control. No significant difference was observed between fibroblasts treated with DMSO and untreated controls (Figure 6B). ELISA analysis performed on cultured cell medium treated with 100 and 400 mg/dl ethanol resulted in a 5 fold increase of TGF-β compared to untreated controls (Figure 6C).
Figure 6.

Analysis of protein and RNA shows that treatment with ethanol increases the expression of TGF-β and that inhibition of TGF-β reduces this effect. Panel A shows the quantification and inset of examples of western blot analysis for TGF-β latent precursor. Panel B shows the graphical quantification and inset of examples RNA extracted from cells treated with ethanol and probed for TGF-β by PCR. Panel C displays the results of a TGF-β ELISA from cell treated media. On insets 1 is no treatment, 2 is SB 431542, 3 is 400 mg/dl ethanol and 4 is 4 mg/dl ethanol + SB 431542. Each value represents the mean ± SEM, n=3. # p<0.05 comparing to 0 EtOH control and *p<0.05 comparing treatments of SB 431542 to conditions with no treatment. SB = SB 431542
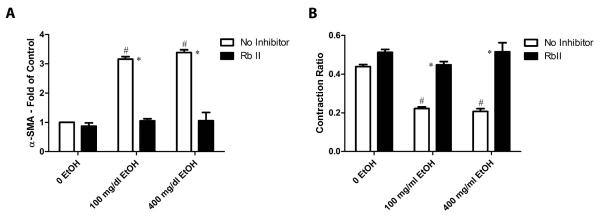
Treatment with recombinant receptor TGF-βII (RbII) inhibited the ethanol effects on collagen gel contraction and myofibroblast formation
Data presented above suggests that exposure of cardiac fibroblasts to ethanol results in increased expression and secretion of TGF-β, which in turn promotes fibroblast activation in an autocrine/paracrine manner. Experiments were performed with a soluble recombinant TGF-β receptor (RbII) to test this. It has been demonstrated previously that RbII has a high affinity for binding and inhibiting the actions of TGF-β isotypes 1, 3, and 5 in cell culture (Tsang et al., 1995). Fibroblast seeded collagen gels pre-incubated with RbII showed significantly reduced contraction compared with gels treated with alcohol alone (Figure 7A). The inhibition of contraction by RbII was less robust than what was observed with cells treated with SB 431542 (compare to Figure 2). RbII pre-incubation also significantly decreased the presence of α-SMA positive myofibroblasts in cells treated with ethanol at a similar level to what was observed with the activin receptor-like kinase inhibitor SB 431542 (Figure 7B).
Figure 7.
TGF-β inhibition by recombinant receptor TGF-β II decreases collagen gel contraction and the presence of myofibroblasts induced by ethanol treatment. Panel A shows the quantification of contraction ratio in untreated and treated fibroblast seeded collagen gels. Panel B exhibits the graphical quantification of the number of myofibroblasts in treated cultures compared to untreated controls. Each value represents the mean ± SEM, n=3-6. # p<0.05 comparing to 0 EtOH control and *p<0.05 comparing treatments of SB 431542 to conditions with no treatment.
Discussion
Though light to moderate use of alcohol has been shown to be cardioprotective, chronic consumption results in detrimental damage to most organs including the heart (Collins et al., 2009). ACM has received far less attention than other cardiac disorders even though according to the Center for Disease Control, heavy alcohol abuse is the second leading cause of dilated cardiomyopathy (Piano, 2002). As cardiac fibrosis has been reported to be a consequence of ACM in humans and rodents, this study was undertaken to investigate the effect ethanol treatment has directly on cardiac fibroblasts in vitro. Under certain stimuli, cardiac fibroblasts can undergo transdifferentiation into a more active and fibrogenic form: the myofibroblast (Santiago et al., 2010). Primary rat cardiac fibroblasts were treated with 100 and 400 mg/dl ethanol for 24 h and assayed for alterations in fibroblast activity and gene expression. The alcohol concentrations used in the present study were chosen based upon dose response experiments and previously published doses used in cell and tissue culture. These doses are within the high range for blood alcohol content in ethanol-consuming humans (Henderson et al., 1989; Schaffert et al., 2010). These studies illustrated that ethanol exposure induces changes in fibroblasts consistent with transformation to a myofibroblast phenotype including increased contractility, migration and α-SMA expression. Utilizing a TGF-β receptor antagonist, these studies also illustrated a fundamental role of activation of TGF-β signaling in the response of cardiac fibroblasts to ethanol. Furthermore, use of soluble TGF-β receptor to bind and inactivate secreted TGF-β illustrated that this cytokine acts in an autocrine/paracrine manner to mediate the ethanol response in cardiac fibroblasts.
TGF-β, a known profibrogenic cytokine, has been shown to be secreted by several cell types, especially those of immune cell lineage (Moustakas et al., 2002). Cardiac fibroblasts have been described as sentinel cells that secrete various biochemical factors in response to myocardial stress (Porter and Turner, 2009). TGF-β is among the cytokines and growth factors secreted by cardiac fibroblasts (Ruwhof et al., 2000). The data presented in the present study indicates that treatment of cardiac fibroblasts with relatively high concentrations of ethanol in vitro results in increased expression and secretion of TGF-β by nearly 5-fold compared to untreated control cells. The mechanism whereby ethanol exposure stimulates TGF-β production was not evaluated in the present experiments. Ethanol is rapidly metabolized into acetaldehyde in many cell types. It has been somewhat controversial whether or not fibroblasts are able to metabolize ethanol but others have shown that fibroblasts are immunohistochemically positive for alcohol dehydrogenase (Brenner and Chojkier, 1987; Buehler et al., 1982). Treatment with both ethanol and its first metabolite acetaldehyde has also been shown to induce TGF-β secretion in hepatic cell types such as stellate and HepG2 cells (Chen, 2002; Gutierrez-Ruiz et al., 2001). Studies in the liver have supported that Kupffer cells may release TGF-β in response to hepatocyte stress and subsequently activate hepatic stellate cells (Purohit and Brenner, 2006), but the mechanisms behind ethanol and acetaldehyde induction of TGF-β in stellate cells and cardiac fibroblasts directly remain un-elucidated.
Based upon the data presented herein, we propose that ethanol exposure stimulates the expression and secretion of TGF-β, which in turn acts in a paracrine manner to promote myofibroblast formation. Myofibroblasts, also known as “contractile fibroblasts,” display increased contraction as a consequence of expressing α-SMA (Baudino et al., 2006). In this study, cardiac fibroblasts seeded in collagen matrices displayed a significant increase in contraction when treated with ethanol compared to untreated controls. Treatment with the TGF-β receptor 1/activin receptor like kinase inhibitor SB 431542 significantly reduced collagen gel contraction in ethanol- treated cells as well as in TGF-β- treated and untreated fibroblast gels. This indicates that TGF-β signaling is necessary for ethanol-stimulated collagen contraction by cardiac fibroblasts. A soluble recombinant TGF-βII receptor has been shown to efficiently bind and inhibit the activity of TGF-β isotypes 1, 3 and 5 and also bind type 2 at ~50% efficiency. 0.3μg/mL of recombinant receptor TGF-βII (RbII) was used to treat fibroblasts and sequester TGF-β based on previously published efficiency (Tsang et al., 1995). Treatment with the soluble TGF-β receptor to competitively bind secreted TGF-β also affectively attenuated ethanol-induced collagen gel contraction. This suggests that TGF-β produced by the fibroblasts in response to ethanol-exposure is acting in a paracrine manner to promote collagen gel contraction. The fact that SB 431542 inhibited not only ethanol-stimulated collagen gel contraction, but also contraction of unstimulated cells suggests that TGF-β signaling is also necessary for basal contraction.
The most widely accepted indicator of myofibroblast presence within the heart is expression of α-SMA in non-vascular cells. Fibroblasts treated with 100 and 400 mg/dl ethanol displayed significantly more α-SMA positive cells than untreated controls. In addition to increased α-SMA protein expression, elevated α-SMA transcripts were also observed in ethanol-treated cells. Treatment with the TGF-β inhibitor SB 431542 attenuated the increase in both α-SMA protein and RNA in cells treated with ethanol. Unlike the effect SB 431542 had on contraction in non-ethanol treated cells, there was no significant difference between cells treated with the inhibitor and untreated cells in α-SMA expression. Formation of myofibroblasts by ethanol was also prevented by incubation of the cells with the recombinant TGF-β receptor. Again this supports the concept that the effects of ethanol on cardiac fibroblasts is at least in part via the secretion and paracrine activity of TGF-β.
There are a number of discrepancies in the literature regarding the response of cells to ethanol, particularly regarding cell proliferation and apoptosis. Studies with fibroblasts isolated from skin and mouse embryos have reported decreases in proliferation and apoptosis when exposed to ethanol (Ranzer et al., 2011; Wang et al., 2008). The amount of ethanol used for treatment may be important as well as the origin of the fibroblasts as another study utilizing embryonic fibroblasts and a smaller concentration of ethanol reported no apoptosis or fibrogenic effects caused by ethanol alone (Brenner and Chojkier, 1987). In the present study, no changes in proliferation or apoptosis were observed in cardiac fibroblasts treated with 100 and 400 mg/dl ethanol. This is consistent with the positive control, as TGF-β is a known activator of fibroblasts and no differences in proliferation (or apoptosis) were observed (Baudino et al., 2006). The presence of no apoptosis supports the hypothesis of fibroblast activation when treated with alcohol as we are suggesting fibroblasts transdifferentiate into an activated state in response to ethanol stress rather than succumb to cell death.
A number of biochemical and mechanical factors including ethanol promote myocardial fibrosis. Fibrillar collagens type I and III have been attributed as being responsible for stiffness in myocardial scarring (Chapman et al., 1990). In a study focusing on the anti-fibrogenic effect fibroblasts of the skin experience when exposed to ethanol, they reported a reduction in collagen I and an increase in collagen III after skin fibroblasts were acutely exposed to ethanol (Ranzer et al., 2011). Conversely, acetaldehyde has been shown to upregulate col1a1 and col1a2 genes in hepatic stellate cells (Greenwel et al., 2000; Svegliati-Baroni et al., 2005). In the present study where cardiac fibroblasts exposed to 100 and 400 mg/dl ethanol showed an increase in collagen type I transcripts and protein and no change in collagen III. Studies in hypertensive hearts have attributed myocardial stiffness to be affected not only by collagen content but the ratio of high tensile strength collagen (collagen type I) to collagen type III (Norton et al., 1997). Ethanol’s stimulus on fibroblasts to produce more collagen I could disturb this ratio in the alcoholic heart. TGF-β inhibition by SB 431542 significantly reduced the upregulation of collagen I at the RNA and protein level in fibroblasts treated with ethanol and TGF-β as a positive control. These results support the necessity of TGF-β in ethanol- induced collagen type I deposition by fibroblasts (a major constituent of fibrosis) in the heart.
Evidence from this study strongly supports that ethanol treatment directly promotes cardiac fibroblast activation and that this event is dependent on TGF-β produced from the fibroblasts themselves. It is likely that other cells in the heart are stimulated to secrete TGF-β in response to ethanol and the role of these cells has been reported as being important by others in the development of fibrosis in ACM (Liu et al., 2011). However, the ability of fibroblasts to produce TGF-β in response to ethanol stimulus is vital when considering potential therapeutic targets to reverse or prevent this phenotype.
Acknowledgments
We would like to thank Charity Fix for her helpful advice and assistance. This publication was supported by NIH grants: HL083441 and F31AA020162. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NHBLI, NIAAA, or NIH.
Contributor Information
Brittany A. Law, University of South Carolina School of Medicine Department of Cell Biology and Anatomy 6311 Garners Ferry Road Columbia, SC 29208
Wayne E. Carver, University of South Carolina School of Medicine Department of Cell Biology and Anatomy 6311 Garners Ferry Road Columbia, SC 29208.
References
- Akamine R, Yamamoto T, Watanabe M, Yamazaki N, Kataoka M, Ishikawa M, Ooie T, Baba Y, Shinohara Y. Usefulness of the 5 region of the cDNA encoding acidic ribosomal phosphoprotein P0 conserved among rats, mice, and humans as a standard probe for gene expression analysis in different tissues and animal species. J Biochem Biophys Methods. 2007;70:481–486. doi: 10.1016/j.jbbm.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Baudino TA, Carver W, Giles W, Borg TK. Cardiac fibroblasts: friend or foe? Am J Physiol Heart Circ Physiol. 2006;291:H1015–1026. doi: 10.1152/ajpheart.00023.2006. [DOI] [PubMed] [Google Scholar]
- Bell E, Ivarsson B, Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci U S A. 1979;76:1274–1278. doi: 10.1073/pnas.76.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner DA, Chojkier M. Acetaldehyde increases collagen gene transcription in cultured human fibroblasts. J Biol Chem. 1987;262:17690–17695. [PubMed] [Google Scholar]
- Buehler R, Hess M, Von Wartburg JP. Immunohistochemical localization of human liver alcohol dehydrogenase in liver tissue, cultured fibroblasts, and HeLa cells. Am J Pathol. 1982;108:89–99. [PMC free article] [PubMed] [Google Scholar]
- Burgess ML, Terracio L, Hirozane T, Borg TK. Differential integrin expression by cardiac fibroblasts from hypertensive and exercise-trained rat hearts. Cardiovasc Pathol. 2002;11:78–87. doi: 10.1016/s1054-8807(01)00104-1. [DOI] [PubMed] [Google Scholar]
- Chapman D, Weber KT, Eghbali M. Regulation of fibrillar collagen types I and III and basement membrane type IV collagen gene expression in pressure overloaded rat myocardium. Circ Res. 1990;67:787–794. doi: 10.1161/01.res.67.4.787. [DOI] [PubMed] [Google Scholar]
- Chen A. Acetaldehyde stimulates the activation of latent transforming growth factor-beta1 and induces expression of the type II receptor of the cytokine in rat cultured hepatic stellate cells. Biochem J. 2002;368:683–693. doi: 10.1042/BJ20020949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins MA, Neafsey EJ, Mukamal KJ, Gray MO, Parks DA, Das DK, Korthuis RJ. Alcohol in moderation, cardioprotection, and neuroprotection: epidemiological considerations and mechanistic studies. Alcohol Clin Exp Res. 2009;33:206–219. doi: 10.1111/j.1530-0277.2008.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancy M, Maxwell JD. Alcohol and dilated cardiomyopathy. Alcohol Alcohol. 1986;21:185–198. doi: 10.7748/ns2008.05.22.38.42.c6565. [DOI] [PubMed] [Google Scholar]
- Desmoulière A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Araya G, Borg TK, Lavandero S, Loftis MJ, Carver W. IGF-1 modulation of rat cardiac fibroblast behavior and gene expression is age-dependent. Cell Commun Adhes. 2003;10:155–165. [PubMed] [Google Scholar]
- Fix C, Bingham K, Carver W. Effects of interleukin-18 on cardiac fibroblast function and gene expression. Cytokine. 2011;53:19–28. doi: 10.1016/j.cyto.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwel P, Domínguez-Rosales JA, Mavi G, Rivas-Estilla AM, Rojkind M. Hydrogen peroxide: a link between acetaldehyde-elicited alpha1(I) collagen gene up-regulation and oxidative stress in mouse hepatic stellate cells. Hepatology. 2000;31:109–116. doi: 10.1002/hep.510310118. [DOI] [PubMed] [Google Scholar]
- Guidry C, Grinnell F. Studies on the mechanism of hydrated collagen gel reorganization by human skin fibroblasts. J Cell Sci. 1985;79:67–81. doi: 10.1242/jcs.79.1.67. [DOI] [PubMed] [Google Scholar]
- Guidry C, Grinnell F. Contraction of hydrated collagen gels by fibroblasts: evidence for two mechanisms by which collagen fibrils are stabilized. Coll Relat Res. 1987;6:515–529. doi: 10.1016/s0174-173x(87)80050-x. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Ruiz MC, Gomez Quiroz LE, Hernandez E, Bucio L, Souza V, Llorente L, Kershenobich D. Cytokine response and oxidative stress produced by ethanol, acetaldehyde and endotoxin treatment in HepG2 cells. Isr Med Assoc J. 2001;3:131–136. [PubMed] [Google Scholar]
- Henderson GI, Baskin GS, Horbach J, Porter P, Schenker S. Arrest of epidermal growth factor-dependent growth in fetal hepatocytes after ethanol exposure. J Clin Invest. 1989;84:1287–1294. doi: 10.1172/JCI114296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman GJ, Nicolás FJ, Hill CS. Nucleocytoplasmic shuttling of Smads 2, 3, and 4 permits sensing of TGF-beta receptor activity. Mol Cell. 2002;10:283–294. doi: 10.1016/s1097-2765(02)00585-3. [DOI] [PubMed] [Google Scholar]
- Karaahmet T, Tigen K, Dundar C, Pala S, Guler A, Kilicgedik A, Cevik C, Mahmutyazicioglu K, Isiklar I, Basaran Y. The effect of cardiac fibrosis on left ventricular remodeling, diastolic function, and N-terminal pro-B-type natriuretic peptide levels in patients with nonischemic dilated cardiomyopathy. Echocardiography. 2010;27:954–960. doi: 10.1111/j.1540-8175.2010.01170.x. [DOI] [PubMed] [Google Scholar]
- Kisseleva T, Cong M, Paik Y, Scholten D, Jiang C, Benner C, Iwaisako K, Moore-Morris T, Scott B, Tsukamoto H, Evans SM, Dillmann W, Glass CK, Brenner DA. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc Natl Acad Sci U S A. 2012;109:9448–9453. doi: 10.1073/pnas.1201840109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Liu X, Kim HJ, Kohyama T, Wen FQ, Abe S, Fang Q, Zhu YK, Spurzem JR, Bitterman P, Rennard SI. TGF-beta1 and serum both stimulate contraction but differentially affect apoptosis in 3D collagen gels. Respir Res. 2005;6:141. doi: 10.1186/1465-9921-6-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajiness JD, Conway SJ. The Dynamic Role of Cardiac Fibroblasts in Development and Disease. J Cardiovasc Transl Res. 2012 Aug 10; doi: 10.1007/s12265-012-9394-3. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laonigro I, Correale M, Di Biase M, Altomare E. Alcohol abuse and heart failure. Eur J Heart Fail. 2009;11:453–462. doi: 10.1093/eurjhf/hfp037. [DOI] [PubMed] [Google Scholar]
- Law BA, Levick SP, Carver WE. Alterations in cardiac structure and function in a murine model of chronic alcohol consumption. Microsc Microanal. 2012;18:453–461. doi: 10.1017/S1431927612000372. [DOI] [PubMed] [Google Scholar]
- Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- Lijnen P, Petrov V, Fagard R. Transforming growth factor-beta 1-mediated collagen gel contraction by cardiac fibroblasts. J Renin Angiotensin Aldosterone Syst. 2003;4:113–118. doi: 10.3317/jraas.2003.011. [DOI] [PubMed] [Google Scholar]
- Liu W, Li J, Tian W, Xu T, Zhang Z. Chronic alcohol consumption induces cardiac remodeling in mice from Th1 or Th2 background. Exp Mol Pathol. 2011;91:761–767. doi: 10.1016/j.yexmp.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Moustakas A, Pardali K, Gaal A, Heldin CH. Mechanisms of TGF-beta signaling in regulation of cell growth and differentiation. Immunol Lett. 2002;82:85–91. doi: 10.1016/s0165-2478(02)00023-8. [DOI] [PubMed] [Google Scholar]
- Norton GR, Tsotetsi J, Trifunovic B, Hartford C, Candy GP, Woodiwiss AJ. Myocardial stiffness is attributed to alterations in cross-linked collagen rather than total collagen or phenotypes in spontaneously hypertensive rats. Circulation. 1997;96:1991–1998. doi: 10.1161/01.cir.96.6.1991. [DOI] [PubMed] [Google Scholar]
- Piano MR. Alcoholic cardiomyopathy: incidence, clinical characteristics, and pathophysiology. Chest. 2002;121:1638–1650. doi: 10.1378/chest.121.5.1638. [DOI] [PubMed] [Google Scholar]
- Porter KE, Turner NA. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther. 2009;123:255–278. doi: 10.1016/j.pharmthera.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Purohit V, Brenner DA. Mechanisms of alcohol-induced hepatic fibrosis: a summary of the Ron Thurman Symposium. Hepatology. 2006;43:872–878. doi: 10.1002/hep.21107. [DOI] [PubMed] [Google Scholar]
- Rameckers J, Hummel S, Herrmann B. How many cycles does a PCR need? Determinations of cycle numbers depending on the number of targets and the reaction efficiency factor. Naturwissenschaften. 1997;84:259–262. doi: 10.1007/s001140050393. [DOI] [PubMed] [Google Scholar]
- Ranzer MJ, Chen L, DiPietro LA. Fibroblast function and wound breaking strength is impaired by acute ethanol intoxication. Alcohol Clin Exp Res. 2011;35:83–90. doi: 10.1111/j.1530-0277.2010.01324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruwhof C, van Wamel AE, Egas JM, van der Laarse A. Cyclic stretch induces the release of growth promoting factors from cultured neonatal cardiomyocytes and cardiac fibroblasts. Mol Cell Biochem. 2000;208:89–98. doi: 10.1023/a:1007046105745. [DOI] [PubMed] [Google Scholar]
- Santiago JJ, Dangerfield AL, Rattan SG, Bathe KL, Cunnington RH, Raizman JE, Bedosky KM, Freed DH, Kardami E, Dixon IM. Cardiac fibroblast to myofibroblast differentiation in vivo and in vitro: expression of focal adhesion components in neonatal and adult rat ventricular myofibroblasts. Dev Dyn. 2010;239:1573–1584. doi: 10.1002/dvdy.22280. [DOI] [PubMed] [Google Scholar]
- Schaffert CS, Duryee MJ, Bennett RG, DeVeney AL, Tuma DJ, Olinga P, Easterling KC, Thiele GM, Klassen LW. Exposure of precision-cut rat liver slices to ethanol accelerates fibrogenesis. Am J Physiol Gastrointest Liver Physiol. 2010;299:G661–668. doi: 10.1152/ajpgi.00287.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svegliati-Baroni G, Inagaki Y, Rincon-Sanchez AR, Else C, Saccomanno S, Benedetti A, Ramirez F, Rojkind M. Early response of alpha2(I) collagen to acetaldehyde in human hepatic stellate cells is TGF-beta independent. Hepatology. 2005;42:343–352. doi: 10.1002/hep.20798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingström A, Heldin CH, Rubin K. Regulation of fibroblast-mediated collagen gel contraction by platelet-derived growth factor, interleukin-1 alpha and transforming growth factor-beta 1. J Cell Sci. 1992;102:315–322. doi: 10.1242/jcs.102.2.315. [DOI] [PubMed] [Google Scholar]
- Tsang ML, Zhou L, Zheng BL, Wenker J, Fransen G, Humphrey J, Smith JM, O’Connor-McCourt M, Lucas R, Weatherbee JA. Characterization of recombinant soluble human transforming growth factor-beta receptor type II (rhTGF-beta sRII) Cytokine. 1995;7:389–397. doi: 10.1006/cyto.1995.0054. [DOI] [PubMed] [Google Scholar]
- Unanue ER. Ito cells, stellate cells, and myofibroblasts: new actors in antigen presentation. Immunity. 2007;26:9–10. doi: 10.1016/j.immuni.2007.01.001. [DOI] [PubMed] [Google Scholar]
- van Nieuwenhoven FA, Turner NA. The role of cardiac fibroblasts in the transition from inflammation to fibrosis following myocardial infarction. Vascul Pharmacol. 2012 Aug 3; doi: 10.1016/j.vph.2012.07.003. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Wang LH, Yang JY, Cui W, Shin YK, Wu CF. Involvement of promyelocytic leukemia protein in the ethanol-induced apoptosis in mouse embryo fibroblasts. Yakugaku Zasshi. 2008;128:1067–1071. doi: 10.1248/yakushi.128.1067. [DOI] [PubMed] [Google Scholar]