Abstract
Noise trauma, aging, and ototoxicity preferentially damage the outer hair cells of the inner ear, leading to increased hearing thresholds and poorer frequency resolution. Whereas outer hair cells make synaptic connections with less than 10% of afferent auditory nerve fibers (type-II), inner hair cells make connections with over 90% of afferents (type-I). Despite these extensive connections, little is known about how selective inner hair cell loss impacts hearing. In chinchillas, moderate to high doses of the anticancer compound carboplatin produce selective inner hair cell and type-I afferent loss with little to no effect on outer hair cells. To determine the effects of carboplatin-induced inner hair cell loss on the most widely used clinical measure of hearing, the audiogram, pure-tone thresholds were determined behaviorally before and after 75 mg/kg carboplatin. Following carboplatin treatment, small effects on audiometric thresholds were observed even with extensive inner hair cell losses that exceed 80%. These results suggest that conventional audiometry is insensitive to inner hair cell loss and that only small populations of inner hair cells appear to be necessary for detecting tonal stimuli in a quiet background.
Keywords: Selective inner hair cell loss, carboplatin, ototoxicity, shock avoidance, chinchilla, audiometry
1.1 INTRODUCTION
The inner ear of mammals contains two distinct sensory cell types. Inner hair cells (IHC) are believed to convey nearly all of the acoustic input to the brain through extensive synaptic connections with over 90% of afferent auditory nerve fibers (type-I) whereas outer hair cells (OHC) make connections with the remaining auditory nerve afferent fibers (type-II, less than 10% of total ANF population). However, OHCs are important to hearing as these cells play a key role in cochlear non-linear amplification (Brownell et al., 1985) and frequency selectivity. Hearing impairment as a result of aging, noise exposure, or ototoxicity, typically begins with OHC loss or dysfunction, followed by a progressive loss of IHC and spiral ganglion cells (Felder et al., 1995; Harding et al., 2004; McFadden et al., 2001; Stebbins et al., 1979).
The effects of OHC loss on hearing have been well described in several species across a number of studies (Ahroon et al., 1993; Davis et al., 1989; Moore et al., 1999; Smith et al., 1987b). Primarily, OHC loss results in increased hearing thresholds at frequencies associated with OHC damage and a loss of frequency selectivity, rendering affected ears more susceptible to the masking effects of background noise (Davis et al., 1989).
In contrast to the effects of OHC loss, few models describe the effects of selective IHC loss on hearing, in part, because of the inherent difficulty in damaging IHCs in the absence of significant OHC damage. This limitation was overcome when the first model of selective IHC ototoxicity was developed in chinchillas using moderate to high doses of the anticancer drug, carboplatin (Takeno et al., 1994). This selective ototoxic effect produced IHC losses of more than 50% but spared OHCs and had no effect on distortion product otoacoustic emissions (DPOAE) and cochlear microphonics (CM); functional measures of OHC integrity (Hofstetter et al., 1997b; Salvi et al., 2000b).
Subsequent physiological studies have shown that carboplatin-induced IHC loss reduces the compound action potential (a measure of cochlear output to acoustic stimuli) that is commensurate with the extent of the IHC loss. However, responses to the same acoustic stimuli measured at the inferior colliculus (IC) showed little if any change (McFadden et al., 1998) and near-field responses in the auditory cortex (AC) were sometimes enhanced (Qiu et al., 2000; Salvi et al., 2000b). Consistent with these findings, a more recent report showed that whereas carboplatin-induced IHC loss (20–50%) reduced the amplitude of the compound action potential (CAP) and increased the CAP threshold, DPOAE and CM were unaffected and thresholds obtained from the IC were relatively unchanged (El-Badry et al., 2007). The outcomes of these studies suggest increased central gain following loss of IHC, and appear to indicate that threshold measures beyond the periphery may be inadequate for detecting IHC loss. Additional physiological studies measuring the auditory brainstem response (ABR), a clinical far-field potential for determining hearing thresholds, have shown mixed results. Two studies in chinchillas showed that ABR thresholds either did not significantly change after large carboplatin-induced IHC loss (Jock et al., 1996) or had only modest changes in threshold (El-Badry et al., 2009). More importantly, morphology, latency, repeatability, and amplitude of the ABR waveforms were relatively maintained despite large IHC losses (El-Badry et al., 2009). Similar ABR results were reported in mice with selective IHC loss (70%); thresholds did not change despite significant IHC loss (Schrott et al., 1989). The results of these experiments suggest that the ABR, a commonly used objective measure of hearing, may underestimate IHC loss even when the loss is extensive. In contrast to these results, two earlier studies reported significant ABR threshold increases in carboplatin treated chinchillas (Harrison, 1998; Wake et al., 1993). However, because these studies did not specify which waveforms were used to determine threshold, a direct comparison to the more recent reports (El-Badry et al., 2009) is not possible. In addition, it is also possible that functional differences in surviving IHC or varying extents of afferent type-I loss could explain the lack of agreement between the two sets of studies.
More recent published reports have begun to explore the effects of afferent fiber loss on ABR metrics using a noise-induced progressive type-I afferent degeneration model that does not produce hair cell loss. The results showed no changes in ABR thresholds despite significant loss of afferent fibers in both mice and guinea pigs (Kujawa et al., 2009; Lin et al., 2011). Additionally, in guinea pigs treated with the glutamate agonist AMPA, prolonged swelling of auditory nerve fibers had no long term effects on behavioral measures of hearing or ABR thresholds/amplitude, despite histological evidence of vacuoles still evident in synaptic regions (Le Prell et al., 2004). In other words, both ABR and behaviorally derived pure-tone thresholds shifted only during the most severe swelling, with complete threshold recovery despite incomplete synaptic repair. Collectively, the results of the majority of these studies appear to suggest that moderate to potentially severe losses of IHC or damage to afferent fibers have little effect on thresholds.
Whereas previous studies have evaluated the effects of both IHC and afferent auditory nerve fiber loss on physiological measures of hearing, how IHC loss impacts behaviorally derived measures of auditory performance is relatively unknown. To address this fundamental question, we trained chinchillas to respond to sound using a conditioned shock avoidance paradigm previously established to assess hearing changes associated with noise exposure or aminoglycoside ototoxicity (Giraudi-Perry et al., 1982; Giraudi et al., 1980; Graf et al., 1992; Salvi et al., 1982). Based on the aforementioned physiological threshold measures following carboplatin induced IHC loss, our working hypothesis was that behaviorally derived pure-tone thresholds would likely shift only after large IHC loss and that the rate of threshold shift would increase rapidly thereafter. Further, we sought to determine the relationship between any changes observed audiometrically and the extent of the IHC lesion. To our knowledge, this is the first report that evaluates audiometric threshold changes following carboplatin-induced IHC loss, and the first to correlate the extent of IHC loss with these changes.
1.2 METHODS
1.2.1 Subjects
Nine, healthy, adult, 1–2-year-old male chinchillas were used (400–600g). Thresholds to pure-tones (250–11,300 Hz) were assessed before and after treatment with 75 mg/kg of carboplatin (i.p.), a dose known to produce moderate to severe IHC loss (Hofstetter et al., 1997a). Subjects were housed in custom individual wire mesh cages in a temperature controlled room with a 12 hour light/dark cycle. Animals had free access to food and water. All procedures were approved by the University at Buffalo’s Institutional Animal Care and Use Committee (IACUC).
1.2.2 Equipment and Psychophysical Methods
Audiometric thresholds in quiet were obtained using a shock avoidance conditioning procedure similar to that described in earlier reports (Blakeslee et al., 1978; Giraudi-Perry et al., 1982; Giraudi et al., 1980; Salvi et al., 1978). During testing, subjects were placed in a restraining yoke that held the subject in a fixed, standing position in a calibrated sound field within a single walled sound booth (Inside dimensions: L × W × H 91.4 ×101.6 ×193 cm, Industrial Acoustics Company Inc. Bronx, NY) lined with 3-inch acoustic foam. A micro-switch mounted on the restraining yoke was used to record the animal’s behavioral response. This response consisted of a 1-cm upward movement on the restraining yoke that closed a micro-switch generating a +5 volt pulse that was delivered to a TTL input module (TDT Smartport PI2) and recorded by custom psychophysical software. For shock delivery, two silver disk electrodes covered with conductive paste (Synapse Conductive Electrode Cream, SYN 1505) were taped onto the shaved tail of the chinchilla; electrodes were placed on either side of the tail near the base. Brief current pulses (1–5 mA, 500 ms on, 500 ms off) could be delivered to the electrodes using a constant current generator (Coulbourn Instruments, Precision Regulated Animal Shocker E13–14) that was controlled by the output of a TTL module (TDT Smartport PI2) with custom software running on a personal computer. A calibrated speaker (Realistic Minimus-7 40–2030A, 40w 50–20,000 Hz) was located at the level of the subjects’ head approximately 50 cm from the left ear (270 degrees azimuth). A software controlled safety light (40-watt light bulb) was mounted on the wall of the sound booth in front of the subject (0 degrees azimuth) at a distance of 55 cm. A small piezoelectric buzzer that was paired with shock delivery was mounted on the restraining yoke. Both the light and the buzzer were used to provide response feedback during the experimental sessions. Presentation of the light provided feedback for correct responses whereas the buzzer was paired with brief shock presentation to indicate an incorrect response.
1.2.3 Tone Threshold
To determine thresholds as a function of frequency (audiogram), tone bursts (500 ms duration, 5 ms rise/fall time) were presented at 0.25, 0.5, 1, 2, 4, 8, and 11.3 kHz. Tone bursts were compiled using a signal generator [Tucker Davis Technology (TDT) AP2] running custom software (Borland C, TDT RPVDS). The output of the signal generator was fed to a D/A converter (TDT DA3), then through a headphone amplifier (TDT HB7) and a programmable attenuator (TDT PA4) before being sent to the loudspeaker. The output of the loudspeaker was calibrated using a 0.5 inch microphone (Larson Davis 2559) and sound level meter (Larson Davis 800B). Each trial within a session consisted of six tone bursts (500 ms on / 500 ms off, 5 ms rise/fall time). If the subject produced a response during the first four tone bursts, the safety light was turned on and the animal avoided tail shock, i.e., a correct response. If the subject failed to respond by the start of the 5th tone burst, the shock and buzzer were turned on and the trial was scored as incorrect (i.e., a miss). The shock stimulus and/or buzzer invariably elicited a response which then turned off the shock (escape). When the stimulus level dropped below 20 dB SPL, the shock was turned off and only the buzzer was presented when an incorrect response (miss) occurred.
Stimulus trials occurred at random inter-trial intervals (ITI) ranging from 20 s to 60 s. Thresholds in quiet were measured using a Modified Method of Limits (Carhart, 1959) with a 10-dB down and 5-dB up step size. Briefly, threshold testing began at a high stimulus level (> 60 dB SPL). If the animal made a correct response on a trial, the stimulus level decreased by 10 dB on the next trial. If the animal failed to respond to the stimulus, the level was increased 5 dB on the next trial. On 5–10% of the trials, no stimuli (blank trials) were presented to monitor false alarm rates (response in the absence of a stimulus) and correct rejection (no response in the absence of a stimulus). At each frequency tested, threshold was defined as the mean intensity of three or more 5 dB reversals. The time needed to measure threshold across all stimulus frequencies in a daily session was ~40–50 minutes. After thresholds had stabilized, five day averages were used to assess mean baseline pure-tone thresholds at each frequency. Daily threshold measures were considered valid if the false alarm rate was less than 10% during the session. Threshold data are shown in the results section.
1.2.4 Carboplatin
After baseline pure-tone thresholds were collected, each subject was injected with a single 75 mg/kg intraperitoneal (i.p.) injection of carboplatin (Sigma C2538, cis-Diammine 1,1-cyclobutanedicarboxylate platinum) dissolved in 5 ml of normal saline. Because carboplatin can also be nephrotoxic, subjects were monitored closely and received daily 10 ml subcutaneous injections of saline for two days following treatment. After a 21–28 day recovery period, pure-tone threshold assessment was repeated (5 day average).
1.2.5 Cochleograms
At the end of the post-carboplatin hearing assessments, subjects were sacrificed by carbon dioxide inhalation, decapitated, and the cochleae removed for histological analysis to determine the extent of hair cell loss (Ding et al., 1999a; Ding et al., 1999b; Hofstetter et al., 1997b; Trautwein et al., 1996). Both cochleae were removed carefully, the round window and oval window opened, and a solution of succinate dehydrogenase (SDH) (2.5 ml, 0.2 M sodium succinate, 2.5 ml, 0.2 M phosphate buffer, pH 7.6 and 5 ml 0.1% tetranitro blue tetrazolium) was perfused through the round window. The cochleae were then immersed in SDH and incubated at 37 C for 45 minutes. The cochlea were post-fixed in 10% formalin and stored in fixative for 24 hours. The basilar membrane, containing the organ of Corti, was dissected from the apex to the base as a flat surface preparation, mounted in glycerin on glass slides and cover slipped. The organ of Corti was then analyzed using light microscopy (Zeiss Standard) at 400× magnification. Successive segments (0.24 mm) were analyzed for missing IHC and OHC. Hair cells were counted as present if the SDH stained cell bodies were present and visible. Cochleograms were constructed for each ear of each animal and the percent missing hair cells was plotted as a function of percentage distance from the apex. Percent hair cell loss was determined from laboratory norms established from cochleae obtained from normal adult chinchillas (n=9). Percent distance from the apex was also converted to frequency using a cochlear frequency-place map (Greenwood, 1990). Cochleograms were constructed for both ears; however, within each animal no significant differences in the size or pattern of hair cell loss were observed between the right and left cochleae. Therefore, the data presented in the Results section represent hair cell lesions from the ear (left) facing the loudspeaker.
1.3 RESULTS
1.3.1 Moderate and Severe Carboplatin Induced IHC loss
Treatment with 75 mg/kg carboplatin produced IHC loss ranging from 30% to over 90%; this variability was consistent with previous published reports. In nearly all of the animals, IHC loss spanned across most of the cochlea. As an example of a relatively moderate loss, IHC loss of ~30% is evident in the cochlear regions corresponding to frequencies of 500 Hz, 3000 Hz, and 11,000 Hz in a representative subject (6072) (see Figure 1). The IHC lesion spans most of the cochlea, with no significant loss of OHC. Despite this carboplatin-induced IHC reduction of ~30%, there was relatively little threshold shift (Figure 2, left panel). There was no compelling relationship between threshold shifts as a function of IHC loss for this subject. At the cochlear region with maximum IHC loss of ~37%, threshold shift at corresponding frequencies was about 4 dB (Figure 2, right panel), which was within the margin of test-retest variability.
Figure 1.
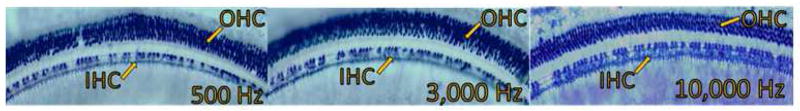
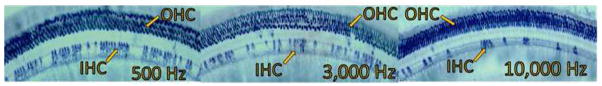
SDH stained cochlear sections from subject 6072 show carboplatin-induced inner hair cell loss in regions corresponding to the lower frequency range (500 Hz), the mid-frequency range (3,000 Hz), and the higher frequency range (10,000 Hz). The 75 mg/kg carboplatin dose produced a mean inner hair cell loss of approximately 30% across the cochlea with no evidence of outer hair cell loss.
Figure 2.
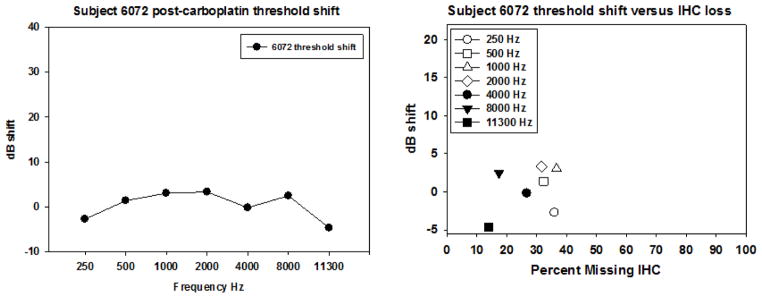
Post carboplatin threshold shift and threshold shift as a function of IHC loss are shown for subject 6072. The left panel shows essentially no evidence of any significant threshold shift across all frequencies tested. The right panel shows IHC loss ranging from 10–30%. The maximum threshold shift was less than 4 dB.
A subset of the subjects tested had more robust IHC loss. As an example of relatively severe loss, IHC loss is evident in the cochlear regions corresponding to frequencies of 500 Hz, 3000 Hz, and 11,000 Hz in a representative subject (6137) with IHC loss of 80–95% across the cochlea (see Figure 3). Threshold shift was also more evident (see Figure 4, left panel). Although there is obvious threshold shift across all frequencies, the extent of this shift is moderate (<40 dB) relative to the extent of IHC loss (which reached almost 100% in some cochlear regions, see Figure 4, right panel).
Figure 3.
SDH stained cochlear sections from subject 6137 show carboplatin-induced inner hair cell loss in regions corresponding to the lower frequency range (500 Hz), the mid-frequency range (3,000 Hz), and the higher frequency range (10,000 Hz). The 75 mg/kg carboplatin dose produced a mean inner hair cell loss of approximately 80% across the cochlea with no evidence of outer hair cell loss.
Figure 4.
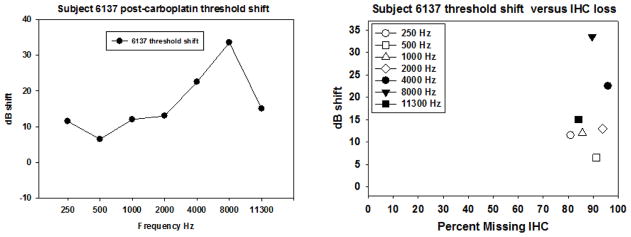
Post carboplatin threshold shift and threshold shift as a function of IHC loss are shown for subject 6137. The left panel shows threshold shift as a function of frequency. The right panel shows IHC loss ranging from 78–95%. The maximum threshold shift was 35 dB at 8000 Hz. This region had ~85% IHC loss. However, lower frequencies (500–1000 Hz) had similar levels of IHC loss with much smaller threshold shifts.
1.3.2 Average Threshold changes following carboplatin induced IHC loss
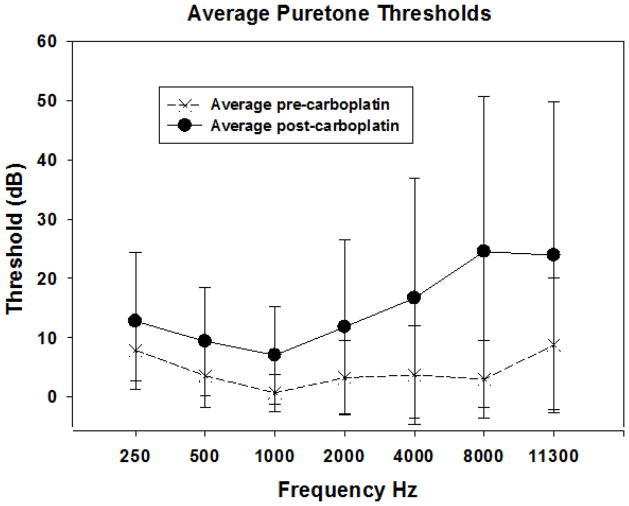
Figures 1–4 illustrated data from two animals representing the low and high end of the distribution of IHC loss. Figures 5–6 present group data. The average baseline thresholds (5 days) ranged from approximately 2 to 9 dB SPL with the highest thresholds occurring at 250 and 11,300 Hz (Figure 5). These baseline thresholds are in good agreement with previous published reports in normal chinchillas (Blakeslee et al., 1978; Miller, 1970; Salvi et al., 1978). Three to four weeks following 75 mg/kg of carboplatin treatment, thresholds (5 day averages) were consistently higher than those obtained pre-treatment (Figure 5). Post-carboplatin, thresholds increased by 5–21 dB. Threshold shifts were smaller below 4000 Hz (~5–10 dB) and larger from 4000 and 11,300 Hz (~15–20 dB). A two-way repeated measures ANOVA found a significant frequency effect (F (6, 8) = 6.839, p <.001), a significant effect of carboplatin treatment on threshold (F (1, 8) = 6.33, p=.033) and a significant interaction of frequency and carboplatin treatment (F (6, 1) = 5.748, p <.001). A Tukey post-hoc analysis showed that the increases in thresholds at 4000, 8000 and 11,300 Hz post carboplatin were statistically significant (p<.05) whereas lower frequency thresholds did not reliably differ from baseline. Although threshold shifts were statistically significant for the higher frequencies, clinical norms would categorize these 15–20 dB losses as “mild high frequency hearing loss”.
Figure 5.
Mean thresholds (+/−SD) as a function of frequency obtained using a shock avoidance procedure is shown before and after treatment with 75 mg/kg carboplatin. Carboplatin produced a mean IHC loss of ~70–80%. The post carboplatin thresholds were significantly elevated at 4,000–11,300 Hz (two-way repeated measures ANOVA, Tukey post hoc tests, P<0.05).
Figure 6.
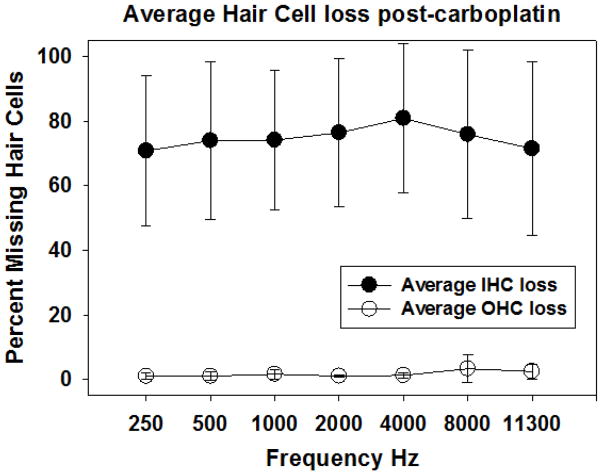
Mean IHC and OCH loss (+/−SD) are shown as a function of corresponding frequency following carboplatin treatment (75 mg/kg). Carboplatin produced significant IHC loss across the frequency regions tested behaviorally (closed circles). In contrast there was no evidence of OHC loss (open circles).
1.3.3 Threshold Changes as a Function of Hair Cell Loss
Mean IHC and OHC loss obtained from left ears are shown in Figure 6. These data were used to assess the relationship between IHC loss and threshold measures (Figure 7). The left ear was selected as the speaker was pointed at the left ear of each subject and was thus unobstructed by head shadow effects.
Figure 7.
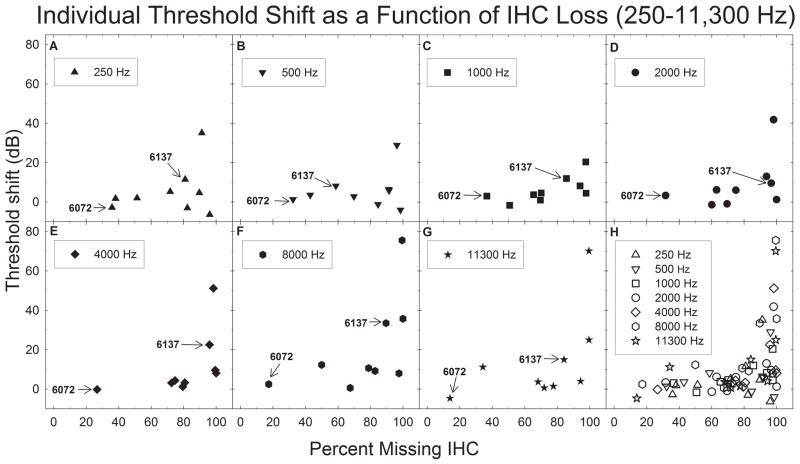
Individual threshold shifts from 250–11,300 (A–G) Hz are shown as function of IHC loss. Individual subjects from figures 1–4 are indicated in panels A–G. Post carboplatin threshold shifts were not significant 250–2000 Hz (A–D). However, carboplatin treatment significantly increased thresholds at frequencies 4000–11,300 Hz (E–G). Panel H shows individual threshold shifts for all frequencies as a function of IHC loss. Threshold shift ranged from 10–80 dB only after IHC loss exceeded 80%; results suggesting large IHC loss are needed to produce threshold shifts greater than 10 dB.
As shown in Figure 6, there is virtually no OHC loss at any test frequency, a finding that is consistent with previous reports using similar carboplatin dosing (Ding et al., 1999b; Hofstetter et al., 1997a; Hofstetter et al., 1997b; Trautwein et al., 1996; Wang et al., 1997). In contrast, mean IHC loss ranged from 65–75%, with significant variability across subjects. The size of the IHC lesion associated with the 75 mg/kg dose, and the variability across subjects, is in reasonable agreement with previous reports (Ding et al., 1999b; Hofstetter et al., 1997a; Hofstetter et al., 1997b; Trautwein et al., 1996; Wang et al., 1997). Despite considerable variability across subjects, there was no significant difference between the left and right cochleae within subjects.
To illustrate the relationship between hearing loss and IHC loss, the threshold shift at each test frequency was plotted as a function of IHC loss at regions of the cochlea corresponding to each of the test frequencies for each subject. Figure 7 (panels A–G) shows the threshold shifts at each tested frequency relative to IHC loss across all subjects.
Carboplatin failed to produce a statistically significant threshold shift at 250 Hz (p=.331) (Figure 7A), despite IHC loss that ranged from 30–95% in the cochlear region corresponding to 250 Hz. Only one subject showed a threshold shift of over 20 dB at 250 Hz. Although this subject had ~90% IHC loss in this region, other subjects with similar IHC loss did not have compelling threshold shift.
A similar relationship between threshold shift and IHC loss was found for regions corresponding to 500, 1000, and 2000 Hz (Figures 7B–7D). Carboplatin did not produce a statistically significant threshold shift (p=.252, p=.213, p=.099 respectively) despite large IHC losses.
In contrast to the small threshold effects at the low and mid frequencies, carboplatin produced statistically significant threshold shifts at 4000 (p=.018), 8000 (p<.001), and 11,300 Hz (p=.007) (Figures 7E–7G). The data at these frequencies showed two trends. First, there was more IHC loss in cochlear regions corresponding to these frequencies. Second, although the majority of subjects continued to have relatively modest threshold shifts, 2–3 subjects showed threshold shifts that were greater than 20 dB.
To better illustrate the trends observed in thresholds as a function of IHC loss and frequency, all of the individual data points are shown on Figure 7 panel H. Here, thresholds begin to increase when IHC loss exceeds 80%. However, some subjects continued to show no significant threshold shift even with IHC losses of over 85%.
1.3.4 Summary of Results
A moderately high dose of carboplatin (75 mg/kg) produced an IHC loss that ranged from moderate to severe (30–85%) across a group of nine chinchillas; results consistent with previous reports. Thresholds in quiet were relatively insensitive to moderate to severe inner hair cell loss. At low to mid frequencies (250–2000 Hz), even large IHC lesions failed to show any significant effect on threshold. In contrast, higher frequencies (4000–11,300 Hz) showed statistically significant increases in threshold following carboplatin. However, these threshold changes were only evident with very high IHC loss (>80%). Most subjects with moderate IHC loss (30–50%) showed little change in thresholds whereas severe IHC loss (>80%) appeared to produce threshold shifts >20 dB at the high frequencies in some subjects but not others. These results document for the first time the finding that significant IHC loss does not reliably impair pure-tone audiometry outcomes.
1.4 DISCUSSION
Pure-tone threshold assessment is the most widely used clinical measure of hearing. It is the basis for diagnosing the severity and configuration of hearing loss, the basis for aural rehabilitation, and the primary measure for hearing aid fittings. Poor pure-tone thresholds are indicative of hearing impairment whereas normal thresholds (<25 dB HL in humans) are typically associated with normal peripheral hearing. Clinically, hearing impairment and increased thresholds are often commensurate with the degree of OHC loss. With greater degree of hearing impairment, IHC loss is typically presumed. However, the extent of selective IHC loss necessary for hearing impairment is unknown. Physiological studies in chinchillas with carboplatin induced IHC loss have shown that the CAP is reduced as a function of IHC loss (Salvi et al., 2000a). However, physiological responses to sound at the level of the IC show little change (McFadden et al., 1998) and in the AC, the response is sometimes enhanced (Qiu et al., 2000). These results suggest that animals may be able to detect acoustic stimuli in the presence of substantial IHC loss.
In the present study we sought to determine if pure-tone audiometry was affected by moderate to severe selective IHC loss using behavioral measures in the chinchilla. Our results clearly indicate that (1) very large IHC lesions are needed in order to produce pure-tone threshold shifts, (2) audiometry appears to be relatively insensitive to moderate to large IHC loss and (3) some animals show little threshold shift even with severe IHC loss (>80%).
1.4.1 Thresholds in Quiet
Pre-carboplatin Tone Thresholds
Pre-treatment thresholds were obtained with a shock avoidance paradigm similar to one used to estimate chinchilla hearing in previous reports (Blakeslee et al., 1978; Salvi et al., 1978). The results were in good agreement to those obtained in other laboratories using different avoidance conditioning paradigms (e.g., shuttle box) (Heffner et al., 1991; Miller, 1970). The close agreement between the results obtained in the present study and earlier reports with either shock conditioning or positive reinforcement provide strong support for our methods. Further, the behavioral measures employed in the present study were relatively easy for the animals to learn, did not require food deprivation, and more importantly, allowed for stepwise procedures similar to those used clinically to determine hearing sensitivity.
1.4.2 Post-carboplatin Tone Thresholds
A number of earlier reports have shown increased tone thresholds following acoustic overstimulation or aminoglycoside treatments that preferentially damage the OHC and to a lesser extent the IHC (Blakeslee et al., 1978; Clark et al., 1974; Ryan et al., 1979; Smith et al., 1987a). In studies that have examined the effects of selective destruction of OHC (Ryan et al., 1979), threshold shifts on the order of 40–50 dB were reliably observed with extensive OHC loss. In contrast to these reports, there are no published reports on the effect of selective IHC loss on behaviorally obtained pure-tone thresholds. Our results show that selective IHC destruction did not produce significant thresholds shifts at low to mid frequencies (i.e. 250–2000 Hz). At higher frequencies (i.e. 4,000–11,300 Hz) there was evidence of threshold shift, but this shift occurred only when IHC loss was >80%. Collectively, these results suggest that pure-tone thresholds are a poor predictor of IHC loss.
The preceding results show that nearly all IHC need to be destroyed before a significant threshold shift occurs. These data could be interpreted in several ways. The most parsimonious explanation is that only a few IHC are needed to detect sounds in quiet. A second possibility is that once sound presentation levels increase, energy spreads to adjacent cochlear regions containing surviving IHC, allowing sound to be detected (off frequency hearing). Finally, a third possibility is that sounds are transmitted to the central auditory system through the OHC-type-II afferent system. In the following section, these three possibilities will be discussed in more detail.
1.4.3 Thresholds signaled from single IHC-type-I neurons
Support for the hypothesis that threshold can be mediated by a single IHC-type-I neuron comes from three physiological studies carried out in chinchillas with carboplatin induced IHC loss. In one study, recordings were made from the central nucleus of the IC in the chinchilla. Responses from single units with characteristic frequencies (CF) corresponding to regions of the cochlea with complete IHC loss could not be obtained. Alternatively, single units from regions with small residual IHC populations showed normal thresholds and sharp tuning characteristics provided that the OHC population was intact (Wake et al., 1996). Similar results were obtained from single auditory nerve fiber recordings. Neurons with CFs associated with a small number of residual IHC (<5%) had nearly normal thresholds and sharp tuning despite extensive IHC loss in surrounding regions. (Wang et al., 1997). A third evoked potential study was carried out in chinchillas with chronic round window, IC, and AC electrodes. CAP thresholds were either elevated significantly or the CAP response was in the noise floor in chinchillas with severe IHC lesions. However, at the level of the IC and the AC, near field potential thresholds were nearly normal. In addition, AC evoked response amplitudes measured at supra threshold levels tended to be larger than normal with small to moderate IHC lesions and were only slightly reduced with severe IHC lesions (Qiu et al., 2000). The evoked potential studies suggest that the amplitude decrease observed at the level of the cochlea can be compensated for in the central auditory pathway by an increase in system gain, possibly due to a reduction in inhibition. Increases in the output of the central nervous system have been observed in the AC when GABA-mediated inhibition is blocked by bicuculline (Wang et al., 2000). Additional studies, however, would be needed to determine if IHC loss produces changes in GABA mediated inhibition in the AC. Collectively, the existing physiological data appear to support the hypothesis that normal thresholds may be present in chinchillas with relatively few IHC as long as the OHC are intact.
1.4.5 Spread of Excitation and Off-Frequency Hearing
Off-frequency hearing can occur when sound levels increase resulting in broader basilar membrane excitation allowing the mechanical input to spread to adjacent regions of the cochlea where IHC may still be intact or patches of IHC remain. Whereas basilar membrane spread of excitation could explain elevated thresholds in regions with no IHC, it would be inconsistent with the low thresholds observed in this study. In addition, single unit studies, one in the auditory nerve and the other in the IC, have found sharp neural tuning in regions associated with large IHC lesions as long as the OHC remain intact (Wake et al., 1996; Wang et al., 1997). Thus, there is no evidence of degraded neural tuning with large IHC lesions in neurons located between the auditory nerve and IC. The result of these studies make it unlikely that broadened peripheral tuning could account for the low thresholds observed in the present study. However, at the level of the AC, there is evidence that some neurons receive inputs from a broad range of frequencies, i.e., spectral integration. Support for this broadened tuning comes from studies in which GABA-mediated inhibition is blocked by bicuculline. Results indicated that tuning curves shifted from having low threshold, narrowly tuned tips to low-threshold, broadly tuned tips (Wang et al., 1997). Tuning curve tips were observed to broaden considerably both above and below CF indicating that neurons in the AC receive peripheral inputs from a broader range of frequencies. In normal hearing animals, central GABA-mediated inhibition appears to sharpen the frequency response range and mask the full range of frequency inputs. If GABA-mediated inhibition were reduced or abolished by severe IHC loss, then neurons in the AC could become disinhibited and respond to a broad range of frequencies. This could explain in part the low thresholds observed in the presence of severe IHC lesion. Further studies, however, are needed to determine the role of central disinhibition and changes in central tuning in animals with large IHC lesions.
1.4.6 Thresholds signaled by single OHC-type-II neurons
Neuroanatomical studies have shown that type-II afferent fibers make synaptic contact with OHC that project to the cochlear nucleus where they branch profusely. One possibility is that thresholds in the absence of IHC may be mediated by OHC and type-II afferent fibers. However, two lines of evidence fail to support this hypothesis. First, in the few successful recordings made from type-II spiral ganglion neurons, the neurons failed to produce any spike discharges either spontaneously or in response to sound (Robertson, 1984). Second, physiological recordings in the auditory nerve or the IC could not be obtained from regions with total IHC loss (Wake et al., 1996; Wang et al., 1997) but large populations of OHC. These data, although limited, suggest that OHC are not involved in afferent signaling of acoustic information to the central auditory system.
1.4.7 IHC loss and Type-I afferent fibers
In the present study, the magnitude of the IHC lesion was confirmed by evaluating cochleae from each animal, counting remaining IHC and OHC, constructing cochleograms showing the percent hair cell loss as a function of location in the cochlea, and comparing the data to laboratory derived norms from adult chinchillas with normal hearing. However, the pattern of carboplatin ototoxicity in the chinchilla includes both IHC and type-I auditory nerve fibers (Ding et al., 1999b). It is possible that type-I afferent auditory nerve fiber survival varied in the different cochleae in remaining IHC. This could partially explain why some animals with large IHC loss had little threshold shift where others had robust shifts. Additional studies are needed to test how the number of remaining afferent fibers influences post-carboplatin thresholds.
1.5 CONCLUSIONS
1.5.1 Summary-Relationship of IHC loss to Perceptual Changes
The main finding of this study was that pure-tone thresholds were poorly correlated with IHC loss. Despite extensive IHC loss, average thresholds showed modest changes in the high frequencies relative to the extent of the loss suggesting few IHC are needed for normal pure-tone thresholds when OHC are present. These behavioral results were consistent with previously reported physiological findings showing normal or near normal thresholds in the IC and AC after severe IHC loss. (Wang et al., 1997).
1.5.2 Clinical Implications
Whereas the loss of OHC results in predictable patterns of hearing dysfunction, the functional consequences of IHC or type-I afferent fiber loss are not well understood. Based on the findings reported here, IHC or type-I afferent loss likely results in hearing deficits that are under diagnosed by pure-tone audiometry, distortion product otoacoustic emissions, or ABR thresholds (Kujawa et al., 2009; Lin et al., 2011). As such, further studies are needed to determine if other tests of hearing are sensitive to IHC loss and whether complex auditory stimuli such as signals in background noise are more susceptible to moderate to severe IHC loss. The animal model presented here provides an opportunity to study the effects of IHC loss on auditory processing in ways that would not be possible in humans.
HIGHLIGHTS.
Carboplatin produced significant inner hair cell loss with no outer hair cell loss in chinchillas
Carboplatin-induced inner hair cell loss had little impact on pure-tone thresholds measured in chinchillas
Post carboplatin thresholds suggest that chinchillas needed few inner hair cells to detect tones
Threshold shift in behaviorally trained chinchillas was only evident when inner hair cell loss exceeded 80%
Acknowledgments
The authors wish to thank the following individuals for their valuable contributions to the successful completion of this project. Dr. Wei Sun and Daniel Stolzberg designed the custom software used for the behavioral studies. Karlee Maerten and Haiyan Jiang provided important technical assistance essential to the completion of the study. Dr. Colleen Le Prell provided valuable assistance in the revisions of this manuscript. Research reported in this publication was supported by the National Institute on Deafness and Other Communication Disorders of the National Institutes of Health under Award Number R03DC011612 (Lobarinas) and R01DC006630 (Salvi). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ABBREVIATIONS
- ABR
auditory brainstem response
- AC
auditory cortex
- CAP
compound action potential
- CM
cochlear microphonics
- dB
decibel
- DPOAE
distortion product otoacoustic emissions
- IC
inferior colliculus
- IHC
inner hair cell
- OHC
outer hair cell
- SD
standard deviation
- SEM
standard error of the mean
- SPL
sound pressure level
- SDH
Succinate dehydrogenase
Footnotes
Author Contributions:
EL and RS designed the experiments, EL and DD performed data collection, analysis, and all authors contributed to writing the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahroon WA, Davis RI, Hamernik RP. The role of tuning curve variables and threshold measures in the estimation of sensory cell loss. Audiology: official organ of the International Society of Audiology. 1993;32:244–59. doi: 10.3109/00206099309072940. [DOI] [PubMed] [Google Scholar]
- Blakeslee EA, Hynson K, Hamernik RP, Henderson D. Asymptotic threshold shift in chinchillas exposed to impulse noise. The Journal of the Acoustical Society of America. 1978;63:876–82. doi: 10.1121/1.381767. [DOI] [PubMed] [Google Scholar]
- Brownell WE, Bader CR, Bertrand D, de Ribaupierre Y. Evoked mechanical responses of isolated cochlear outer hair cells. Science. 1985;227:194–6. doi: 10.1126/science.3966153. [DOI] [PubMed] [Google Scholar]
- Carhart RJF. Preferred Method For Clinical Determination Of Pure-Tone Thresholds. Journal of Speech and Hearing Disorders. 1959;24:330–345. [Google Scholar]
- Clark WW, Clark CS, Moody DB, Stebbins WC. Noise-induced hearing loss in the chinchilla, as determined by a positive-reinforcement technique. J Acoust Soc Am. 1974;56:1202–9. doi: 10.1121/1.1903409. [DOI] [PubMed] [Google Scholar]
- Davis RI, Ahroon WA, Hamernik RP. The relation among hearing loss, sensory cell loss and tuning characteristics in the chinchilla. Hearing research. 1989;41:1–14. doi: 10.1016/0378-5955(89)90173-1. [DOI] [PubMed] [Google Scholar]
- Ding D, Li M, Zheng X, Wang J, Salvi RJ. Cochleogram for assessing hair cells and efferent fibers in carboplatin-treated ear. Lin chuang er bi yan hou ke za zhi = Journal of clinical otorhinolaryngology. 1999a;13:510–2. [PubMed] [Google Scholar]
- Ding DL, Wang J, Salvi R, Henderson D, Hu BH, McFadden SL, Mueller M. Selective loss of inner hair cells and type-I ganglion neurons in carboplatin-treated chinchillas. Mechanisms of damage and protection. Annals of the New York Academy of Sciences. 1999b;884:152–70. doi: 10.1111/j.1749-6632.1999.tb08640.x. [DOI] [PubMed] [Google Scholar]
- El-Badry MM, McFadden SL. Electrophysiological correlates of progressive sensorineural pathology in carboplatin-treated chinchillas. Brain research. 2007;1134:122–30. doi: 10.1016/j.brainres.2006.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Badry MM, McFadden SL. Evaluation of inner hair cell and nerve fiber loss as sufficient pathologies underlying auditory neuropathy. Hearing research. 2009;255:84–90. doi: 10.1016/j.heares.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder E, Schrott-Fischer A. Quantitative evaluation of myelinated nerve fibres and hair cells in cochleae of humans with age-related high-tone hearing loss. Hearing research. 1995;91:19–32. doi: 10.1016/0378-5955(95)00158-1. [DOI] [PubMed] [Google Scholar]
- Giraudi-Perry DM, Salvi RJ, Henderson D. Gap detection in hearing-impaired chinchillas. The Journal of the Acoustical Society of America. 1982;72:1387–93. doi: 10.1121/1.388444. [DOI] [PubMed] [Google Scholar]
- Giraudi D, Salvi R, Henderson D, Hamernik R. Gap detection by the chinchilla. The Journal of the Acoustical Society of America. 1980;68:802–6. doi: 10.1121/1.384818. [DOI] [PubMed] [Google Scholar]
- Graf CJ, Saunders SS, Salvi RJ. Detection of intensity decrements by the chinchilla. The Journal of the Acoustical Society of America. 1992;91:1062–8. doi: 10.1121/1.402632. [DOI] [PubMed] [Google Scholar]
- Greenwood DD. A cochlear frequency-position function for several species--29 years later. The Journal of the Acoustical Society of America. 1990;87:2592–605. doi: 10.1121/1.399052. [DOI] [PubMed] [Google Scholar]
- Harding GW, Bohne BA. Noise-induced hair-cell loss and total exposure energy: analysis of a large data set. The Journal of the Acoustical Society of America. 2004;115:2207–20. doi: 10.1121/1.1689961. [DOI] [PubMed] [Google Scholar]
- Harrison RV. An animal model of auditory neuropathy. Ear and hearing. 1998;19:355–61. doi: 10.1097/00003446-199810000-00002. [DOI] [PubMed] [Google Scholar]
- Heffner RS, Heffner HE. Behavioral hearing range of the chinchilla. Hear Res. 1991;52:13–6. doi: 10.1016/0378-5955(91)90183-a. [DOI] [PubMed] [Google Scholar]
- Hofstetter P, Ding D, Salvi R. Magnitude and pattern of inner and outer hair cell loss in chinchilla as a function of carboplatin dose. Audiology: official organ of the International Society of Audiology. 1997a;36:301–11. doi: 10.3109/00206099709071981. [DOI] [PubMed] [Google Scholar]
- Hofstetter P, Ding D, Powers N, Salvi RJ. Quantitative relationship of carboplatin dose to magnitude of inner and outer hair cell loss and the reduction in distortion product otoacoustic emission amplitude in chinchillas. Hearing research. 1997b;112:199–215. doi: 10.1016/s0378-5955(97)00123-8. [DOI] [PubMed] [Google Scholar]
- Jock BM, Hamernik RP, Aldrich LG, Ahroon WA, Petriello KL, Johnson AR. Evoked-potential thresholds and cubic distortion product otoacoustic emissions in the chinchilla following carboplatin treatment and noise exposure. Hearing research. 1996;96:179–90. doi: 10.1016/0378-5955(96)00058-5. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:14077–85. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Prell CG, Yagi M, Kawamoto K, Beyer LA, Atkin G, Raphael Y, Dolan DF, Bledsoe SC, Jr, Moody DB. Chronic excitotoxicity in the guinea pig cochlea induces temporary functional deficits without disrupting otoacoustic emissions. The Journal of the Acoustical Society of America. 2004;116:1044–56. doi: 10.1121/1.1772395. [DOI] [PubMed] [Google Scholar]
- Lin HW, Furman AC, Kujawa SG, Liberman MC. Primary neural degeneration in the Guinea pig cochlea after reversible noise-induced threshold shift. Journal of the Association for Research in Otolaryngology: JARO. 2011;12:605–16. doi: 10.1007/s10162-011-0277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden SL, Ding D, Salvi R. Anatomical, metabolic and genetic aspects of age-related hearing loss in mice. Audiology: official organ of the International Society of Audiology. 2001;40:313–21. [PubMed] [Google Scholar]
- McFadden SL, Kasper C, Ostrowski J, Ding D, Salvi RJ. Effects of inner hair cell loss on inferior colliculus evoked potential thresholds, amplitudes and forward masking functions in chinchillas. Hearing research. 1998;120:121–32. doi: 10.1016/s0378-5955(98)00052-5. [DOI] [PubMed] [Google Scholar]
- Miller JD. Audibility curve of the chinchilla. The Journal of the Acoustical Society of America. 1970;48:513–23. doi: 10.1121/1.1912166. [DOI] [PubMed] [Google Scholar]
- Moore BC, Vickers DA, Plack CJ, Oxenham AJ. Inter-relationship between different psychoacoustic measures assumed to be related to the cochlear active mechanism. The Journal of the Acoustical Society of America. 1999;106:2761–78. doi: 10.1121/1.428133. [DOI] [PubMed] [Google Scholar]
- Qiu C, Salvi R, Ding D, Burkard R. Inner hair cell loss leads to enhanced response amplitudes in auditory cortex of unanesthetized chinchillas: evidence for increased system gain. Hearing research. 2000;139:153–71. doi: 10.1016/s0378-5955(99)00171-9. [DOI] [PubMed] [Google Scholar]
- Robertson D. Horseradish peroxidase injection of physiologically characterized afferent and efferent neurones in the guinea pig spiral ganglion. Hear Res. 1984;15:113–21. doi: 10.1016/0378-5955(84)90042-x. [DOI] [PubMed] [Google Scholar]
- Ryan A, Dallos P, McGee T. Psychophysical tuning curves and auditory thresholds after hair cell damage in the chinchilla. J Acoust Soc Am. 1979;66:370–8. doi: 10.1121/1.383194. [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Hamernik RP, Henderson D. Discharge patterns in the cochlear nucleus of the chinchilla following noise induced asymptotic threshold shift. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 1978;32:301–20. doi: 10.1007/BF00238704. [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Wang J, Ding D. Auditory plasticity and hyperactivity following cochlear damage. Hearing research. 2000a;147:261–74. doi: 10.1016/s0378-5955(00)00136-2. [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Giraudi DM, Henderson D, Hamernik RP. Detection of sinusoidally amplitude modulated noise by the chinchilla. The Journal of the Acoustical Society of America. 1982;71:424–9. doi: 10.1121/1.387445. [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Ding D, Wang J, Jiang HY. A review of the effects of selective inner hair cell lesions on distortion product otoacoustic emissions, cochlear function and auditory evoked potentials. Noise & health. 2000b;2:9–26. [PubMed] [Google Scholar]
- Schrott A, Stephan K, Spoendlin H. Hearing with selective inner hair cell loss. Hearing research. 1989;40:213–9. doi: 10.1016/0378-5955(89)90162-7. [DOI] [PubMed] [Google Scholar]
- Smith DW, Moody DB, Stebbins WC. Effects of changes in absolute signal level on psychophysical tuning curves in quiet and noise in patas monkeys. J Acoust Soc Am. 1987a;82:63–8. doi: 10.1121/1.395438. [DOI] [PubMed] [Google Scholar]
- Smith DW, Moody DB, Stebbins WC, Norat MA. Effects of outer hair cell loss on the frequency selectivity of the patas monkey auditory system. Hearing research. 1987b;29:125–38. doi: 10.1016/0378-5955(87)90161-4. [DOI] [PubMed] [Google Scholar]
- Stebbins WC, Hawkins JE, Jr, Johnson LG, Moody DB. Hearing thresholds with outer and inner hair cell loss. American journal of otolaryngology. 1979;1:15–27. doi: 10.1016/s0196-0709(79)80004-6. [DOI] [PubMed] [Google Scholar]
- Takeno S, Harrison RV, Mount RJ, Wake M, Harada Y. Induction of selective inner hair cell damage by carboplatin. Scanning Microsc. 1994;8:97–106. [PubMed] [Google Scholar]
- Trautwein P, Hofstetter P, Wang J, Salvi R, Nostrant A. Selective inner hair cell loss does not alter distortion product otoacoustic emissions. Hearing research. 1996;96:71–82. doi: 10.1016/0378-5955(96)00040-8. [DOI] [PubMed] [Google Scholar]
- Wake M, Takeno S, Mount RJ, Harrison RV. Recording from the inferior colliculus following cochlear inner hair cell damage. Acta oto-laryngologica. 1996;116:714–20. doi: 10.3109/00016489609137912. [DOI] [PubMed] [Google Scholar]
- Wake M, Takeno S, Ibrahim D, Harrison R, Mount R. Carboplatin ototoxicity: an animal model. The Journal of laryngology and otology. 1993;107:585–9. doi: 10.1017/s0022215100123771. [DOI] [PubMed] [Google Scholar]
- Wang J, Caspary D, Salvi RJ. GABA-A antagonist causes dramatic expansion of tuning in primary auditory cortex. Neuroreport. 2000;11:1137–40. doi: 10.1097/00001756-200004070-00045. [DOI] [PubMed] [Google Scholar]
- Wang J, Powers NL, Hofstetter P, Trautwein P, Ding D, Salvi R. Effects of selective inner hair cell loss on auditory nerve fiber threshold, tuning and spontaneous and driven discharge rate. Hearing research. 1997;107:67–82. doi: 10.1016/s0378-5955(97)00020-8. [DOI] [PubMed] [Google Scholar]