Abstract
We evaluated the safety and efficacy of the purine nucleoside analogue, clofarabine, in patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) and mantle cell lymphoma (MCL). Six patients with DLBCL (n = 5) or MCL (n = 1) and a median age of 68 years were treated with 40 mg/m2 clofarabine IV over 2 h for 5 days, repeated every 28 days, for 1–2 cycles. The overall response rate was 50% (complete response = 1, complete response unconfirmed = 1, partial response = 1). Median progression-free survival was 3.5 months (range 1.5–10 months) and the median overall survival was 7.8 months (range 3–31 months). Grade 3–4 neutropenia and thrombocytopenia was universal, with a median of 34 (range 19–55) and 77 (range 0–275) days required for neutrophil and platelet recovery. Grade 3 non-hematologic toxicities included transaminitis, febrile neutropenia, non-neutropenic infections and orthostatic hypotension. Further accrual to the study was terminated due to prolonged Grade 3–4 myelosuppression and orthostatic hypotension in five of six patients. Clofarabine exhibits evidence of single agent activity in relapsed or refractory DLBCL. However, further study with novel administration schedules that maintain this efficacy and limit toxicity is warranted.
Keywords: Clofarabine, diffuse large B cell lymphoma, mantle cell lymphoma, nucleoside analogues, myelosuppression
Introduction
Despite the success of chemoimmunotherapy as front-line therapy for diffuse large B-cell lymphoma (DLBCL), the 5-year event-free survival is less than 50% [1–3]. Second or third-line chemotherapy regimens [4–8] produce complete response (CR) rates of 25–50% in patients with chemosensitive relapse, and 15–30% in those with chemotherapy refractory disease. Identification of new therapeutic agents for patients with relapsed diffuse large cell lymphoma is therefore needed. To date there is limited experience with nucleoside analogs such as fludarabine and cladribine in patients with relapsed or refractory DLBCL where responses have been disappointing (response rates 0–10%) [9–13].
Clofarabine (2-chloro-2′-fluoro-deoxy-9-β-D-ara-binofuranosyladenine) is a purine nucleoside analog that has been approved for the treatment of pediatric patients with relapsed or refractory acute lymphoblastic leukemia (ALL). Initial phase I–II trials of clofarabine in solid and hematologic malignancies demonstrated a maximum tolerated dose using a 5 day schedule of 2 mg/m2/day in solid tumors and 2–4 mg/m2/day in indolent lymphoproliferative disorders [14–16]. Dose limiting toxicities (DLT) in these trials consisted of Grade 3–4 thrombocytopenia and neutropenia with no evidence of clinical activity in patients with multiple myeloma, chronic lymphoid leukemia or follicular lymphoma [14–16]. In patients with ALL and acute myeloid leukemia (AML), the recommended phase II doses of clofarabine are 40 mg/m2 (adults) and 52 mg/m2 (children), daily for 5 days, with DLT at higher dose levels consisting of reversible severe hepatotoxicity and skin rash [14,17]. Subsequent phase II studies have confirmed clofarabine’s activity in ALL and AML [18–20]. Based upon the dramatic activity of clofarabine in acute leukemia that differentiates it from alternative nucleoside analogs (fludarabine and cladribine), we hypothesised that this agent might also be active in DLBCL and mantle cell lymphoma (MCL). Herein we report the results of a phase II trial of single agent clofarabine in patients with relapsed or refractory aggressive non-Hodgkin lymphoma (NHL) not only demonstrating evidence of clinical activity of clofarabine in aggressive lymphoma but also significant toxicity including prolonged myelosuppression and unexpected orthotstatic hypotension that has not been previously reported with this agent.
Patients and methods
Patients
Patients 18 years of age or older with histologically confirmed relapsed or refractory DLBCL or MCL [21] after one or more prior therapies who were not candidates for curative therapy with autologous stem cell transplant (ASCT) were eligible for study enrollment. Measurable disease ≥1.5 cm; ECOG performance status 0–2; serum creatinine and total bilirubin ≤upper limit of normal (ULN); AST, ALT and alkaline phosphatase ≤2×ULN; and an absolute neutrophil count ≥1500/mm3 and platelet count ≥100 000/mm3 were required. The protocol was approved by the Ohio State University Institutional Review Board (OSU-0442) and all patients provided written informed consent, as required by the Declaration of Helsinki.
Treatment plan
Treatment consisted of clofarabine 40 mg/m2/day administered as a 2 h intravenous infusion daily for five consecutive days, repeated every 28 days for a maximum of six cycles. All patients received 6 mg pegfilgrastim on day 6 and antibiotic prophylaxis with trimethoprim/sulfamethoxazole-DS, fluconazole and ciprofloxacin. Complete hematologic recovery (ANC ≥1500/mm3 and platelets ≥100 000/mm3) was required for re-treatment. Restaging with CT-scans of chest, abdomen, pelvis and bone marrow biopsy (only if involved at baseline) were performed after every two cycles of clofarabine.
Grade 3 or 4 non-hematologic toxicities (excluding alopecia, nausea, vomiting and transient transaminitis resolving within 48 h) required a 25% dose reduction in subsequent cycles, whereas a second event required a 50% dose reduction. Twenty-five and 50% dose reductions were required for Grade 3 or 4 infections, respectively. Patients requiring treatment delays exceeding 21 days were removed from the study.
Flow cytometric characterisation of T-lymphocyte, B-lymphocyte and natural killer (NK)-cell subsets was performed by four-colour flow cytometry technique with a gating strategy based on CD45 staining an light side scatter characteristics. Peripheral blood samples were collected pre-treatment, day 5 of cycle 1, and immediately prior to cycle 2. Whole blood staining with subsequent red cell lysis employing automated staining and processing (Prep-Plus and Q-prep instruments, Beckman-Coulter, Miami, FL) with directly conjugated antibodies for CD2, CD3, CD4, CD5, CD8 (T-cells); CD19, CD20 (B-cells); and CD16/56 (NK cells) (all from Beckman-Coulter, Miami, FL) was used. Acquisition and analysis of flow cytometry results was performed using a FC500 flow cytometer equipped with CXP software.
To investigate the potential mechanisms of clofarabine associated systemic inflammatory response (SIR) [17,19,22], cytokine levels (tumor necrosis factor [TNF]-α and interleukin [IL]-6,) were measured in plasma pre-treatment; immediately post-treatment on days 1, 3 and 5 of cycle 1; and prior to cycle 2. Peripheral blood in EDTA collection tubes was spun at 1500 g for 10 min and plasma was collected and stored at −80°C until assayed. Samples were analysed by ELISA (R&D Systems, Minneapolis, MN). Data are reported as pg/mL as determined by recombinant cytokine standard curves as directed in the manufacturer’s instructions.
Statistical analysis
Responses were assessed according 1999 international workshop NHL response criteria [23]. Toxicity was graded using National Cancer Institute Common Toxicity Criteria for Adverse Events (CTCAE) version 3.0. The primary objective of the study was to estimate overall response rate (ORR) after two cycles of therapy. A Simon 2-stage design was planned for patient accrual [24], targeting an ORR ≥30% (alternative hypothesis) versus an ORR <10% (null hypothesis). Using this design, 16 patients were required for stage 1 for the study. If <2 responses were observed in the first 16 evaluable patients, the study would be halted due to the lack of expected efficacy. If two or more responses were observed, an additional nine patients would be enrolled. If five or more responses were observed among 25 patients (i.e. ORR of > 30%), it would be concluded that clofarabine is an active regimen in this patient population, with a power of 90% and an alpha error of 0.10.
Because of the potential risks of clofarabine at this dose level, an early stopping rule was also incorporated into this trial. Specifically, study termination was planned if the observed exact binomial probability of greater than 40% of patients experiencing Grade 3–4 non-hematologic or infectious adverse events dropped below 0.10. Specifically, early closure of the trial was planned if four of the first five, five of the first six, or five of the first seven patients treated with clofarabine experienced Grade 3–4 non-hematologic toxicity.
T, NK and B-cell subsets and IL-6 and TNF-α levels collected pre-treatment and during cycles 1–2 were compared using the WIlcoxon signed-rank test or the paired t-test depending on the distribution of the data. P-values were not adjusted to control type 1 error implicit in multiple testing as this is a small preliminary study. All analyses were run using Stat 10.0, Stat Corporation, College Station, Texas.
Results
Patients and treatment
From October 2004 to February 2006, six patients with a median age of 68 (range, 50–74) who had relapsed or refractory disease after 1–2 prior therapies were enrolled (Table I). Five patients with DLBCL and one patient with MCL treated on study received a median of 1.5 clofarabine cycles (range, 1–2). Only the patient with MCL had been previously transplanted, with the other five patients ineligible for transplant due to age or refractory disease.
Table I.
Patient characteristics and clinical outcome.
| UPN | Age/sex | Stage | Histology | Prior therapy | Disease status★ | IPI★ | Pre-treatment BM cellularity (%) | No. of clofarabine cycles | Response | Days to ANC > 1000/mm3 | Days to platelets > 50 000/mm3 | Duration of response (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 50/M | IIA | DLBCL | R-CHOP, R-ICE | Refractory | 0 | 40 | 2 | SD | 34 | 194† | 4 |
| 2 | 66/M | IIA | MCL | Clinical trial, ASCT | Relapsed | 2 | 40 | 1 | SD | 34 | 275† | 8 |
| 3 | 74/M | IIA | DLBCL | R-CHOP, XRT | Relapsed | 1 | 10 | 1‡ | CRu | 28 | N/A§ | 1.5¶ |
| 4 | 69/F | IVA | DLBCL | R-CHOP, R-ICE | Relapsed | 3 | 15 | 1 | PR | 51 | 94† | 2 |
| 5 | 68/F | IIA | DLBCL | R-CHOP, XRT, R-ICE | Refractory | 1 | 65 | 2 | CR | 19 | 26 | 10★★ |
| 6 | 74/M | IIIB | DLBCL | R-CHOP | Refractory | 3 | 30 | 2 | SD | 55 | 61† | 3 |
ANC, absolute neutrophil count; UPN, unique patient number; ASCT, autologous hematopoietic stem cell transplant; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; CR, complete response; CRu, CR unconfirmed; DLBCL, diffuse large B-cell lymphoma; ICE, ifosfamide, carboplatin, etoposide; IPI, international prognostic index score; MCL, mantle cell lymphoma; R, rituximab; PR, partial response; SD, stable disease; XRT, radiation therapy.
IPI score and disease status are at time of therapy with clofarabine.
Death.
Received only one dose of cycle 2.
Patient’s platelet counts never decreased below 50 000/mm.
Patient received single agent vinorelbine, 1.5 months after receiving clofarabine while still in CRu. At the last follow-up patient had no evidence of DLBCL; however, his response duration is censored at the time of vinorelbine administration.
Response duration is censored at the time of autologous stem cell transplant.
Efficacy
The OR rate was 50% with one patient each achieving CR, complete response unconfirmed (CRu) and partial response (PR). Median progression-free survival was 3.5 months (range 1.5–10 months) and the median overall survival was 7.8 months (range 3–31 months). Among the patients achieving an objective response: one patient (with PR) died at 3 months due to disease progression, one patient (with CRu) developed stage I-A small lymphocytic lymphoma (SLL) 18 months after receiving clofarabine remains alive at 31 months with no evidence of DLBCL and requiring no therapy for SLL, and the third patient (with CR) underwent ASCT 8 months after receiving clofarabine. This third patient failed to mobilise an adequate number of CD34+ cells on the first attempt using high-dose etoposide plus filgastrim 3.5 months after clofarabine, collecting only 1.11×106 CD34+ cells/kg over 6 days. A second attempt at stem cell mobilisation with high dose G-CSF (20 μg/kg/day) 6 months after clofarabine resulted in an adequate stem cell collection (3.62×106 CD34+ cells/kg). The patient proceeded to ASCT with a cytoxan, busulfan and etoposide conditioning regimen 10 months following study enrollment and remained disease-free at last follow-up, 27 and 17 months after receiving clofarabine and ASCT, respectively.
Toxicity
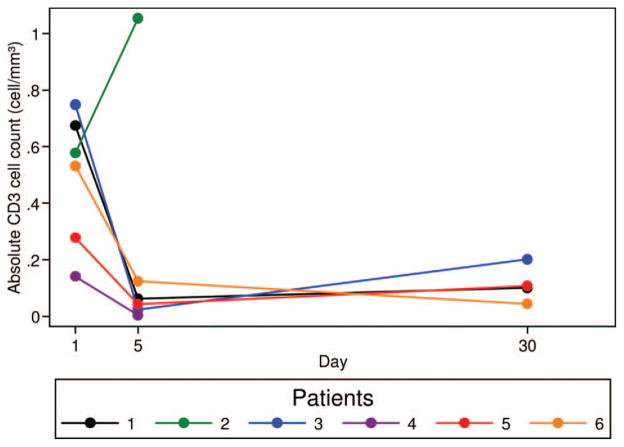
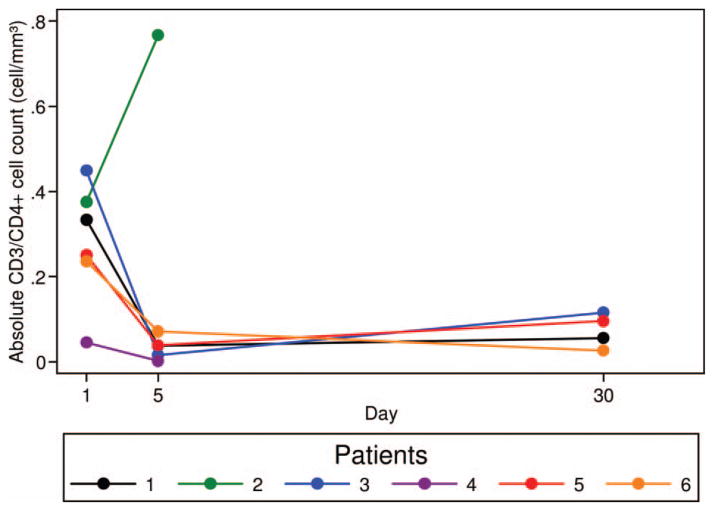
Twenty grade 3–4 hematologic adverse events occurred in six patients (Table II), with prolonged myelosuppression lasting > 28 days in four patients (Table I). Grade 3–4 neutropenia and thrombocytopenia was universal. Median time to ANC ≥1000/mm3 and platelet ≥50 000/mm3 was 34 days (range 19–55 days) and 77 days (range 0–275 days), respectively (Table I). Bone marrow biopsies performed (>30 days after clofarabine therapy) in patients (UPN 2, 4 and 6) experiencing prolonged neutropenia and/or thrombocytopenia showed a hypocellular marrow (5–10% cellularity) with marked granulocytic and megakaryocytic hypoplasia, megaloblastoid erythropoiesis, prominent monocytosis and no evidence of lymphoma. Flow cytometric analysis of T-, B- and NK-cell subsets performed on days 1 and 5 of cycle 1 and prior to cycle 2, demonstrated a 84.7% (range 69.7–96.5%) and 78.9% (range 62.0–88.7%) median decline in CD3+ and CD3/CD4+ T-cells in five and four patients, respectively (Figures 1 and 2). No significant changes in B- and NK-cell subsets (Table III) were noted; however, four patients had no circulating CD19 or CD20 positive B-lymphocytes at study enrollment due to recent rituximab therapy.
Table II.
Toxicity observed with clofarabine.
| Toxicity | No. of events
|
||
|---|---|---|---|
| Grade 1/2 | Grade 3 | Grade 4 | |
| Hematologic | |||
| ANC | 0 | 2 | 7 |
| Platelets | 1 | 4 | 5 |
| Hemoglobin | 6 | 2 | 0 |
| Infectious | |||
| Febrile neutropenia | 0 | 4 | 0 |
| Bacteremia (ANC >1000/mm3) | 0 | 1 | 0 |
| Bacteremia (ANC ≤ 1000/mm3) | 0 | 2 | 0 |
| Clostridium difficile colitis | 1 | 0 | 0 |
| Pneumonia (ANC >1000/mm3) | 1 | 0 | 0 |
| Pneumonia (ANC ≤ 1000/mm3) | 0 | 1 | 0 |
| Shingles | 1 | 0 | 0 |
| GI/Hepatic | |||
| Diarrhea | 2 | 0 | 0 |
| AST or ALT elevation | 3 | 2 | 0 |
| Nausea or vomiting | 4 | 0 | 0 |
| Renal | |||
| Creatinine elevation | 2 | 0 | 0 |
| Electrolyte abnormalities | 5 | 0 | 0 |
| Cardiovascular | |||
| Orthostatic hypotension | 1 | 2 | 0 |
| Dermatologic | |||
| Hand foot syndrome | 2 | 0 | 0 |
| Rash | 5 | 0 | 0 |
| Others | |||
| Fatigue | 3 | 0 | 0 |
| Myalgia | 2 | 0 | 0 |
| Anorexia | 2 | 0 | 0 |
ANC, absolute neutrophil count; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GI, gastrointestinal.
Figure 1.
Decline in CD3-positive T-cell subsets after 1 cycle of clofarabine. Four colour flow cytometry with directly conjugated antibodies for CD3 performed prior to clofarabine therapy, on day 5 of cycle 1, and immediately prior to the start of cycle 2 demonstrate an 84.7% (range 69.7–96.5%) median decline in absolute CD3+ T-cells. Samples were available at all three time points (pre-treatment, day 5 of cycle 1, and prior to cycle 2) in patients 1, 3–6 and from two time points (pre-treatment and day 5) in patient 2.
Figure 2.
Decline in CD3/CD4-positive T-cell subsets after one cycle of clofarabine. Four-colour flow cytometry with directly conjugated antibodies for CD3 and CD4 performed prior to clofarabine therapy, on day 5 of cycle 1, and immediately prior to the start of cycle 2 demonstrates a 78.9% (range 62.0–88.7%) median decline in absolute CD3/CD4+ T-cells. Samples were available at all three time points (pre-treatment, day 5 of cycle 1 and prior to cycle 2) in patients 1, 3–6 and from two time points (pre-treatment and day 5) in patient 2.
Table III.
Alterations in T, NK and B-cell subsets in four patients after one cycle of clofarabine.
| Cells | Median (interquartile range)
|
p-value★ | |
|---|---|---|---|
| Day 1 cells/mm3 | Prior cycle 2 cells/mm3 | ||
| Lymphocytes | 0.73 (0.52–1.13) | 0.20 (0.12–0.39) | 0.068 |
| CD3+ | 0.60 (0.40–0.71) | 0.10 (0.07–0.15) | 0.068 |
| CD3/CD4+ | 0.29 (0.24–0.39) | 0.08 (0.04–0.11) | 0.068 |
| CD5+ | 0.55 (0.39–0.63) | 0.11 (0.07–0.16) | 0.068 |
| CD3/CD8+ | 0.29 (0.19–0.32) | 0.03 (0.01–0.06) | 0.068 |
| CD16/CD56+ | 0.13 (0.06–0.29) | 0.05 (0.01–0.11) | 0.144 |
| CD19+ | 0.00 (0.00–0.20) | 0.00 (0.00–0.04) | 0.842 |
| CD20+ | 0.00 (0.00–0.20) | 0.00 (0.00–0.04) | 0.842 |
Wilxocan signed-rank.
Twelve Grade 3 non-hematologic toxicities including transaminitis, febrile neutropenia, non-neutropenic infections and orthostatic hypotension (Table II) were observed in five patients. Grade 3 transaminitis (n = 2) was transient (normalising within 10–12 days), with one patient requiring a 25% dose reduction. Infectious complications included neutropenic fever (n = 4), pneumonia (n = 2), bacteremia (Klebsiella pneumoniae, Enterococcus faecalis, Escherichia coli, n = 3) and shingles (n = 1). Orthostatic hypotension occurred in three patients (UPN 3, 4 and 6). Patient 3 developed Grade 3 orthostatic hypotension after first cycle of clofarabine, which resolved in 11 days. However, Grade 3 orthostatic hypotension recurred with the first dose of clofarabine in cycle 2, necessitating study removal. Although the patient had no clinical or laboratory evidence of adrenal insufficiency, congestive heart failure, infection or transaminitis, he symptomatically improved with fludrocortisone therapy and remained on it for persistent symptoms for 14 months. Grade 2 orthostatic hypotension in patient 4 was transient and associated with bacteremia and anemia. Patient 6 developed orthostatic hypotension 2 months after completing cycle 2 of clofarabine, during a hospitalisation for neutropenic fever; however, at the time of the orthostasis, his fevers had resolved and he had discontinued antibiotic therapy. He was treated with hydrocortisone and fludrocortisone for presumptive adrenal insufficiency in response to minimal alterations in his cortisol level (9.7–16.8 μg/dL) following ACTH stimulation, and had improvement in his symptoms.
Cytokine assays showed no statistically significant changes in TNF-α or IL-6 levels (Table IV). Although IL-6 levels increased from 0.9–7.4 pg/mL at baseline to 18.4–61.2 pg/mL post-infusion on day 5 in three patients, none of the enrolled patients developed any signs suggestive of SIR during clofarabine administration. Four patients were removed from the study because of prolonged Grade 3–4 hematologic toxicity and one patient discontinued therapy because of Grade 3 orthostatic hypotension. Further accrual to the study was terminated according to previously designated stopping rules as a result of Grade 3–4 febrile neutropenia, infection, transaminitis and orthostatic hypotension in five of six patients.
Table IV.
Comparison interleukin-6 (IL-6) and tumor necrosis factor (TNF-α) before and after treatment.
| Day | No. of patients | IL-6 mean (SD) | IL-6 p-value★ | TNF-α mean (SD) | TNF-α p-value★ |
|---|---|---|---|---|---|
| Pre-treatment day 1, cycle 1 | 4 | 15.6 (21.9) | 0.085 | 16.7 (5.8) | 0.064 |
| Post-treatment day 5, cycle 1 | 4 | 41.5 (20.8) | 7.8 (7.9) |
Paired t-test.
Discussion
Despite encouraging single agent activity (50% response rate) with clofarabine in patients with relapsed aggressive NHL, prolonged myelosuppression, orthostatic hypotension and infectious complications preclude further administration in NHL with the 40 mg/m2/day×5 day recommended phase II schedule currently used for the treatment of adult acute leukemia, even with GCSF support. In this trial, three patients with relapsed or refractory DLBCL achieved responses. However, all patients experienced Grade 3–4 neutropenia or thrombocytopenia. Sixty-seven percent of patients experienced prolonged thrombocytopenia persisting longer than 60 days after 1–2 cycles of clofarabine, preventing re-treatment with either clofarabine or other salvage regimens in the non-responders. Although neutropenia resolved more quickly, the median time to neutrophil recovery exceeded 30 days and contributed to significant infectious complications in these patients.
Although Grade 3–4 myelosuppression is expected with standard salvage therapy in patients with relapsed or refractory DLCL [4–8], persistent Grade 3–4 thrombocytopenia for a median of 77 days is uncommon without evidence of bone marrow involvement. This prolonged myelosuppression with clofarabine in the treatment of NHL also contrasts with experience in adult acute leukemia where hematologic recovery typically occurs within 30 days [18]. The median number of prior therapies in previous studies of clofarabine in patients with ALL and AML [18,19] is comparable to our study. Nevertheless, the hematopoetic reserve for patients with relapsed or refractory DLBCL on this trial may be lower due to their advanced age (median 68.5 years compared with a median age of 12 years for ALL and 54 years for AML patients treated with clofarabine [18,19]). This study also suggests that clofarabine, like fludarabine, may effect adequate stem cell mobilisation [25,26]; however, this observation needs further study since not all purine nucleoside analogs impair stem cell mobilisation [27].
In other recently published phase I/II studies of clofarabine in patients with chronic lymphocytic leukemia (CLL), multiple myeloma, and solid malignancies, profound myelosuppression has been observed at lower doses (2–4 mg/m2 IV days 1–5 without GCSF) with little or no evidence of clinical activity [14–16,28]. Specifically in eight patients with multiple myeloma, hematologic recovery following one cycle of clofarabine at 4 mg/m2 IV days 1–5 required 35–42 days and all patients receiving a second cycle were dose reduced to 2 mg/m2 due to the severity of the cytopenias [15]. Likewise, Grade 3–4 myelosuppression is universal and associated with significant infectious complications in patients with CLL receiving clofarabine 3–15 mg/m2/day daily for 5 days. Similar to our study, Nabhan et al. noted two CRs and three PRs (37% OR rate) in 14 patients with relapsed or refractory NHL; although, dose escalation was again limited by thrombocytopenia [28].
Although not statistically significant, this trial demonstrated declines in CD3+/CD4+ T-cell subsets in contrast to preliminary data reported in five patients with solid tumors receiving 103–129 mg/m2 clofarabine weekly, where no significant changes in CD4+ and CD8+ T-cell subsets but declines in CD19+ B cells were observed [29]. With the majority of patients in our trial having previously received rituximab, evaluation of alterations in the B-cell complement is difficult. On the basis of our findings and conflicting data in solid tumors, additional trials with prolonged follow-up are necessary to assess the extent of B- and T-cell depletion and risk for opportunistic infections in heavily pre-treated patients receiving clofarabine.
Severe neurotoxicity is a well-recognised adverse effect of purine nucleoside analogue (fludarabine, cladribine) therapy at higher doses, and includes encephalopathy, seizures, blindness, paralysis, coma and death [30–32]. At lower, standard therapeutic doses, these complications are rare with the purine analogs. Experience with clofarabine in AML and ALL has revealed only occasional mild and reversible neurological side effects [17,20]. Grade 3 hypotension was seen in 18% of pediatric patients in a study by Jeha et al. [19], although only one of the patients required discontinuation of therapy as in our study. The strong temporal relationship of clofarabine treatment to orthostatic hypotension observed in our trial suggests a drug relationship. In two of the three patients experiencing orthostatic hypotension in this trial, there was no clear cardiac, infectious, adrenal or hematologic etiology. Although formal tilt-table testing was not performed, the symptomatic improvement of these two patients with mineralocorticoid therapy and the lack of other etiology supports a peripheral autonomic neuropathy as the mechanism for the profound orthostasis observed.
Although toxic, our data provide support for the clinical activity of clofarabine in DLBCL. Future phase I studies examining either decreased dosing or alternative administration schedules should be pursued in patients with aggressive NHL with detailed attention to myelosuppression, alterations in lymphocyte subsets, ability to mobilise stem cells and peripheral autonomic neuropathy presenting predominately as orthostatic hypotension. Further exploration of novel pharmacologically driven dosing schedules that permit maximal dose escalation may be necessary, as low doses ranging from 2 to 4 mg/m2 IV days 1–5 appear to be ineffective in lymphoid malignancies and also associated with prolonged myelosuppression [14–16,28]. Gandhi et al. [16] in an elegant pharmacokinetic study demonstrated prolonged retention of clofarabine triphosphate (CTP, active metabolite of clofarabine) in CLL cells. Notably intracellular concentration of CTP increased in dose-dependent fashion with successive clofarabine infusions, supporting the possibility of infusing higher clofarabine doses at less frequent intervals. Cunningham et al. reported limited toxicity and clinical activity with weekly infusion schedule of clofarabine (given on days 1, 8 and 15 of a 28-day cycle) in patients with solid malignancies employing doses as high as 129 mg/m2 [32]. The encouraging single agent activity of clofarabine observed in our trial makes it an attractive potential option as pre-AHSCT salvage regimen, perhaps using a weekly dosing schedule for only 1–2 cycles. Alternative schedules with less frequent administration may permit maximal dose escalation with minimal impact on stem cell mobilisation and collection.
Acknowledgments
The authors thank Mary Beth Miller and Anne Berkal, research nurses for this trial; Sharon Cheung and Courtney Mankowski for protocol regulatory assistance; the inpatient nursing staff, and most importantly, the patients who participated in this trial and the families who supported them. This project was supported by the National Institutes of Health and the National Cancer Institute grant K23 CA109004-01A1, Leukemia and Lymphoma Society, D. Warren Brown Foundation, and Genzyme Oncology. K.A. Blum designed and performed the study; supervised patients enrolled on the described trial, analysed and interpreted the data, and authored the manuscript. M. Hamadani analysed and interpreted the data and assisted with writing the manuscript. G. Lozanski performed and analysed flow cytometry on serial peripheral blood samples to detect alterations in T, B, and NK cell subsets after clofarabine therapy. G. Phillips provided assistance with the statistical design and analysis of this trial. A.J. Johnson, D.M. Lucas, and L.L. Smith coordinated the collection and analysis of the cytokine correlative study samples and reviewed the draft manuscript. S.M. Devine, R. Baiocchi, T.S. Lin and P. Porcu enrolled and treated patients on the described trial and reviewed the draft manuscript. J.C. Byrd assisted with study design and analysis, supervised the cytokine analysis, and reviewed and edited the manuscript. Genzyme Oncology provided clofarabine and funding to support drug administration and correlative studies analysing innate immunity following clofarabine therapy.
References
- 1.Fisher RI, Gaynor ER, Dahlberg S, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin lymphoma. N Engl J Med. 1993;328:1002–1006. doi: 10.1056/NEJM199304083281404. [DOI] [PubMed] [Google Scholar]
- 2.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 3.Feugier P, Van Hoof A, Sebban C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol. 2005;23:4117–4126. doi: 10.1200/JCO.2005.09.131. [DOI] [PubMed] [Google Scholar]
- 4.Velasquez WS, Cabanillas F, Salvador P, et al. Effective salvage therapy for lymphoma with cisplatin in combination with high-dose Ara-C and dexamethasone (DHAP) Blood. 1988;71:117–122. [PubMed] [Google Scholar]
- 5.Velasquez WS, McLaughlin P, Tucker S, et al. ESHAP – an effective chemotherapy regimen in refractory and relapsing lymphoma: a 4-year follow-up study. J Clin Oncol. 1994;12:1169–1176. doi: 10.1200/JCO.1994.12.6.1169. [DOI] [PubMed] [Google Scholar]
- 6.Jermann M, Jost LM, Taverna C, et al. Rituximab-EPOCH, an effective salvage therapy for relapsed, refractory or transformed B-cell lymphomas: results of a phase II study. Ann Oncol. 2004;15:511–516. doi: 10.1093/annonc/mdh093. [DOI] [PubMed] [Google Scholar]
- 7.Kewalramani T, Zelenetz AD, Nimer SD, et al. Rituximab and ICE as second-line therapy before autologous stem cell transplantation for relapsed or primary refractory diffuse large B-cell lymphoma. Blood. 2004;103:3684–3688. doi: 10.1182/blood-2003-11-3911. [DOI] [PubMed] [Google Scholar]
- 8.El Gnaoui T, Dupuis J, Belhadj K, Jais JP, Rahmouni A, Copie-Bergman C, et al. Rituximab, gemcitabine and oxaliplatin: an effective salvage regimen for patients with relapsed or refractory B-cell lymphoma not candidates for high-dose therapy. Ann Oncol. 2007;18:1363–1368. doi: 10.1093/annonc/mdm133. [DOI] [PubMed] [Google Scholar]
- 9.Hochster HS, Kim KM, Green MD, et al. Activity of fludarabine in previously treated non-Hodgkin’s low-grade lymphoma: results of an Eastern Cooperative Oncology Group study. J Clin Oncol. 1992;10:28–32. doi: 10.1200/JCO.1992.10.1.28. [DOI] [PubMed] [Google Scholar]
- 10.Redman JR, Cabanillas F, Velasquez WS, et al. Phase II trial of fludarabine phosphate in lymphoma: an effective new agent in low-grade lymphoma. J Clin Oncol. 1992;10:790–794. doi: 10.1200/JCO.1992.10.5.790. [DOI] [PubMed] [Google Scholar]
- 11.Tsukasaki K, Tobinai K, Shimoyama M, et al. Deoxycoformycin-containing combination chemotherapy for adult T-cell leukemia-lymphoma: Japan Clinical Oncology Group Study (JCOG9109) Int J Hematol. 2003;77:164–170. doi: 10.1007/BF02983215. [DOI] [PubMed] [Google Scholar]
- 12.Robak T, Smolewski P, Cebula B, Szmigielska-Kaplon A, Chojnowski K, Blonski JZ. Rituximab combined with cladribine or with cladribine and cyclophosphamide in heavily pretreated patients with indolent lymphoproliferative disorders and mantle cell lymphoma. Cancer. 2006;107:1542–1550. doi: 10.1002/cncr.22196. [DOI] [PubMed] [Google Scholar]
- 13.Decaudin D, Bosq J, Tertian G, et al. Phase II trial of fludarabine monophosphate in patients with mantle-cell lymphomas. J Clin Oncol. 1998;16:579–583. doi: 10.1200/JCO.1998.16.2.579. [DOI] [PubMed] [Google Scholar]
- 14.Kantarjian HM, Gandhi V, Kozuch P, et al. Phase I clinical and pharmacology study of clofarabine in patients with solid and hematologic cancers. J Clin Oncol. 2003;21:1167–1173. doi: 10.1200/JCO.2003.04.031. [DOI] [PubMed] [Google Scholar]
- 15.Uy GL, Tomasson MH, Ruddell A, DiPersio JF, Vij R. The activity and toxicity of low dose clofarabine against relapsed or refractory myeloma. Haematologica. 2006;91:1581–1582. [PubMed] [Google Scholar]
- 16.Gandhi V, Plunkett W, Bonate PL, et al. Clinical and pharmacokinetic study of clofarabine in chronic lymphocytic leukemia: strategy for treatment. Clin Cancer Res. 2006;12:4011–4017. doi: 10.1158/1078-0432.CCR-05-2664. [DOI] [PubMed] [Google Scholar]
- 17.Jeha S, Gandhi V, Chan KW, et al. Clofarabine, a novel nucleoside analog, is active in pediatric patients with advanced leukemia. Blood. 2004;103:784–789. doi: 10.1182/blood-2003-06-2122. [DOI] [PubMed] [Google Scholar]
- 18.Kantarjian H, Gandhi V, Cortes J, et al. Phase 2 clinical and pharmacologic study of clofarabine in patients with refractory or relapsed acute leukemia. Blood. 2003;102:2379–2386. doi: 10.1182/blood-2003-03-0925. [DOI] [PubMed] [Google Scholar]
- 19.Jeha S, Gaynon PS, Razzouk BI, et al. Phase II study of clofarabine in pediatric patients with refractory or relapsed acute lymphoblastic leukemia. J Clin Oncol. 2006;24:1917–1923. doi: 10.1200/JCO.2005.03.8554. [DOI] [PubMed] [Google Scholar]
- 20.Faderl S, Verstovsek S, Cortes J, et al. Clofarabine and cytarabine combination as induction therapy for acute myeloid leukemia (AML) in patients 50 years of age or older. Blood. 2006;108:45–51. doi: 10.1182/blood-2005-08-3294. [DOI] [PubMed] [Google Scholar]
- 21.Harris NL, Jaffe ES, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17:3835–3849. doi: 10.1200/JCO.1999.17.12.3835. [DOI] [PubMed] [Google Scholar]
- 22.Faderl S, Gandhi V, O’Brien S, et al. Results of a phase 1–2 study of clofarabine in combination with cytarabine (ara-C) in relapsed and refractory acute leukemias. Blood. 2005;105:940–947. doi: 10.1182/blood-2004-05-1933. [DOI] [PubMed] [Google Scholar]
- 23.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 24.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 25.Tournilhac O, Cazin B, Lepretre S, et al. Impact of frontline fludarabine and cyclophosphamide combined treatment on peripheral blood stem cell mobilization in B-cell chronic lymphocytic leukemia. Blood. 2004;103:363–365. doi: 10.1182/blood-2003-05-1449. [DOI] [PubMed] [Google Scholar]
- 26.Laszlo D, Galieni P, Raspadori D, et al. Fludarabine containing-regimens may adversely affect peripheral blood stem cell collection in low-grade non Hodgkin lymphoma patients. Leuk Lymphoma. 2000;37:157–161. doi: 10.3109/10428190009057639. [DOI] [PubMed] [Google Scholar]
- 27.Holowiecki J, Grosicki S, Sadus-Wojciechowska M, et al. Addition of cladribine to induction/consolidation regimen does not impair peripheral blood stem cell mobilization and bone marrow harvest for autotransplantation in acute myeloid leukemia patients. Transplant Proc. 2005;37:4482–4487. doi: 10.1016/j.transproceed.2005.10.100. [DOI] [PubMed] [Google Scholar]
- 28.Nabhan C, Fried W, Galvez A, Venugopal P, Gozun P, Bitran JD. Phase I/II trial of clofarabine in refractory and/or relapsed non-Hodgkin’s lymphoma (NHL) Proc Am Soc Clin Oncol. 2008;26:8564. (abstr) [Google Scholar]
- 29.Cunningham C, Nemunaitis J, Senzer N, Richards D, Vukelja S, Abidchandani R, et al. Effect of clofarabine on lymphocyte populations in patients treated for solid tumors. Proc Am Soc Clin Oncol. 2007;25:21134. (abstr) [Google Scholar]
- 30.Warrell RP, Jr, Berman E. Phase I and II study of fludarabine phosphate in leukemia: therapeutic efficacy with delayed central nervous system toxicity. J Clin Oncol. 1986;4:74–79. doi: 10.1200/JCO.1986.4.1.74. [DOI] [PubMed] [Google Scholar]
- 31.Spriggs DR, Stopa E, Mayer RJ, Schoene W, Kufe DW. Fludarabine phosphate (NSC 312878) infusions for the treatment of acute leukemia: phase I and neuropathological study. Cancer Res. 1986;46:5953–5958. [PubMed] [Google Scholar]
- 32.Cunningham CC, Nemunaitis J, Senzer N, Vukelja S, Richards D, Vukovic V, et al. Clofarabine administration weekly to adult patients with advanced solid tumors in a phase I dose-finding study. Proc Am Soc Clin Oncol. 2005;23:7109. (abstr) [Google Scholar]
- 33.Parker WB, Shaddix SC, Chang CH, et al. Effects of 2-chloro-9-(2-deoxy-2-fluoro-β-D-arabinofuranosyl)adenine on K562 cellular metabolism and the inhibition of human ribonucleotide reductase and DNA polymerases by its 5′-triphosphate. Cancer Res. 1991;51:2386–2394. [PubMed] [Google Scholar]
- 34.Xie KC, Plunkett W. Deoxynucleotide pool depletion and sustained inhibition of ribonucleotide reductase and DNA synthesis after treatment of human lymphoblastoid cells with 2-chloro-9-(2-deoxy-2-fluoro-β-D-arabinofuranosyl) adenine. Cancer Res. 1996;56:3030–3037. [PubMed] [Google Scholar]
- 35.Hegde UP, Wilson WH, White T, Cheson BD. Rituximab treatment of refractory fludarabine-associated immune thrombocytopenia in chronic lymphocytic leukemia. Blood. 2002;100:2260–2262. [PubMed] [Google Scholar]
- 36.Cheson BD, Vena DA, Foss FM, Sorensen JM. Neurotoxicity of purine analogs: a review. J Clin Oncol. 1994;12:2216–2228. doi: 10.1200/JCO.1994.12.10.2216. [DOI] [PubMed] [Google Scholar]