Abstract
The stomach acts as a barrier to ingested microbes, thereby influencing the microbial ecology of the entire gastrointestinal (GI) tract. The stomach microbiota and the role of human host and environmental factors, such as health status or medications, in shaping its composition remain largely unknown. We sought to characterize the bacterial and fungal microbiota in the stomach fluid in order to gain insights into the role of the stomach in GI homeostasis. Gastric fluid was collected from 25 patients undergoing clinically indicated upper endoscopy. DNA isolates were used for PCR amplification of bacterial 16S ribosomal RNA (rRNA) genes and fungal internal transcribed spacers (ITS). RNA isolates were used for 16S rRNA cDNA generation and subsequent PCR amplification. While all stomach fluid samples are dominated by the phyla Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria and Fusobacteria (>99% of sequence reads), the transcriptionally active microbiota shows significant reduction in Actinobacteria (34%) and increase in Campylobacter (444%) (P<0.003), specifically the oral commensal and suspected intestinal pathogen Campylobacter concisus. Bacterial but not fungal diversity is reduced by antibiotic treatment (28% P<0.02), immunosuppression in transplant recipients and HIV/AIDS patients (42% P<0.001) and gastric fluid pH >4 (70% P<0.05). Immunosuppression correlates with decreased abundance of Prevotella (24%), Fusobacterium (2%) and Leptotrichia (6%) and increased abundance of Lactobacillus (3844%) (P<0.003). We have generated the first in-depth characterization of the human gastric fluid microbiota, using bacterial 16S rRNA gene and transcript, and fungal ITS amplicon sequencing and provide evidence for a significant impact of the host immune status on its composition with likely consequences for human health.
Keywords: stomach fluid, microbiome, ITS, 16S rRNA, immunosuppression, Helicobacter pylori
Introduction
The stomach plays a crucial role in the maintenance of gastrointestinal (GI) health, serving as a barrier against ingested infectious disease agents of the lower GI tract (Martinsen et al., 2005). In healthy human subjects, inactivation of ingested pathogens is mediated by gastric fluid containing a combination of hydrochloric acid and proteolytic enzymes (Tennant et al., 2008). Correspondingly, impaired gastric acid secretion is associated with an increased risk of infection (Howden and Hunt, 1987). Hypochlorhydria can result from atrophic gastritis, gastric surgery or drugs that inhibit acid secretion (Martinsen et al., 2005). The gastric environment is also subject to active control by innate and adaptive immune responses, as has been demonstrated by a large body of literature on Helicobacter pylori infection (Vorobjova et al., 2008). A protective function against pathogen infection has also been attributed to the commensal microbiota, for example, by providing direct protection against colonization (Croswell et al., 2009) or by mediating clearance (Endt et al., 2010) of invading pathogens such as Salmonella enterica. Whether the stomach microbiota performs similar tasks is unknown, as the microbial communities of the stomach have not been studied in detail. Moreover, it has not been shown if the gastric fluid supports an intrinsic microbiota different from that of adjacent locations of the GI tract, which could play a role for the stomach barrier function. Previous work has primarily focused on the microbiota of gastric mucosal biopsies in the context of H. pylori infection and applied only DNA-based methodologies that are unable to distinguish between transcriptionally active and inactive or dead bacteria (Bik et al., 2006; Dicksved et al., 2009; Maldonado-Contreras et al., 2010). The specific aim of this study was therefore to characterize the microbiota of gastric fluid, in order to test the hypothesis that complex, transcriptionally active microbial communities exist in this environment, to provide insights into the colonization resistance of the stomach and reveal which organisms are likely to enter the intestine and thus influence the human host and microbiota in the lower GI tract. Expanding the donor population beyond H. pylori-infected subjects allowed for the evaluation of the influence of multiple external or environmental factors on the microbiota.
Towards this end, we address here the function of the stomach environment for GI health by determining the microbiota composition of gastric fluid in relation to various human host parameters, including immune status, gastric fluid pH, use of proton pump inhibitors (PPIs) and antibiotic medications. Through deep-sequencing of bacterial 16S ribosomal RNA (rRNA) genes and their transcripts as well as fungal internal transcribed spacers (ITS), we identify a diverse and transcriptionally active microbiota in stomach fluid that is critically shaped by the human host immune status, with potential implications for GI and overall human health and disease.
Materials and methods
Subject enrollment and sample collection
The study was approved by the Institutional Review Board of the University of Maryland Baltimore; all subjects provided their informed consent. Twenty-five adult patients undergoing clinically indicated upper endoscopy at the University of Maryland Medical Center were enrolled between December 2010 and June 2011. All samples were obtained in a hospital-based endoscopy laboratory, which operates between the hours of 0800 and 1700 hours. All patients were required to fast for a minimum of 8 h before the procedure. Demographics, clinical features and endoscopic indications and findings were recorded (Table 1). During the endoscopy, suction was not used until the endoscope entered the stomach to avoid contamination from other body sites. Gastric fluid was aspirated and collected in a sterile container. Samples were immediately put on ice and within 2 h aliquoted (0.5 ml), combined with RNAlater (0.5 ml; Qiagen, Germantown, MD, USA) and frozen together with remaining raw samples at −80 °C until further processing. Sample pH was determined using pH strips (Sigma-Aldrich, St Louis, MO, USA).
Table 1. Overview of patient clinical data.
| # | Sex | Age (years) | Race | pH | ABx | PPI | IMM | H. pyl.a | Other pt. clin. | End. indication | End. findings |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | F | 34 | AA | 7.5 | − | − | − | − | A, alcoholic hepatitis | UGIB | EG, MWT |
| 15 | F | 33 | AA | 7.0 | BL | + | G/M/CI | − | D, renal transplant | Melena, anemia | Erosive gastritis, RE |
| 16 | M | 45 | AA | 2.0 | − | − | − | + | G | Refractory hiccups | HH |
| 26 | F | 42 | AA | 6.0 | − | − | HIV/AIDS | + | G | Diarrhea, wt loss | CE, irreg. duodenal mucosa |
| 31 | M | 52 | C | 1.5 | − | + | − | − | G | Abdominal pain | HH |
| 33 | F | 71 | C | 1.0 | − | + | − | − | D, G, hyperparathyroidism | Dysphagia | GU |
| 34 | F | 72 | AA | 8.5 | − | + | G/M | 16S | G, renal transplant | Anemia | EG |
| 38 | M | 70 | C | 3.5 | − | + | − | − | A, D, G | BE | BE, EG |
| 41 | F | 38 | C | 4.5 | − | + | − | − | A, G | Screen for BE | GU, EG, no BE |
| 42 | M | 53 | AA | 7.0 | − | + | HIV/AIDS | glmM | HCV cirrhosis, T | Screen for EV | EG, no EV |
| 44 | M | 28 | AA | 1.0 | − | − | − | + | T | Abdominal pain | None |
| 48 | M | 36 | C | 3.0 | − | + | − | − | A, G | BE, diarrhea | BE |
| 49 | F | 33 | C | 1.0 | − | + | − | − | None | Abdominal pain | None |
| 50 | F | 79 | AA | 1.0 | − | − | − | − | D, G | BE | BE |
| 52 | F | 56 | AA | 6.5 | − | + | G | − | D, G, sarcoidosis | Nausea | EG |
| 56 | M | 68 | AA | 6.0 | − | + | − | − | G, CAD, CKD | Melena | HH, RE |
| 64 | M | 42 | AA | 7.0 | FQ | + | HIV/AIDS | − | G | Abdominal pain | EG |
| 67 | F | 67 | AA | 6.5 | − | + | G/M/CI | − | D, G, renal transplant | UGIB | EG |
| 68 | F | 50 | C | 1.5 | − | − | − | − | T | Abdominal pain | None |
| 76 | M | 42 | C | 2.5 | − | − | − | − | A, T | UGIB | GU, DU |
| 77 | M | 36 | C | 4.5 | − | − | − | − | None | Hematochezia | Duodenitis |
| 84 | M | 65 | C | 5.5 | ML | + | − | − | D, G | Screen for BE | EG, gastric polyps |
| 94 | M | 81 | AA | 6.5 | − | − | − | − | Pernicious anemia | Early satiety | Atrophic gastritis |
| 96 | F | 37 | AA | 5.5 | THF+ML | + | HIV/AIDS | − | G, gastroparesis, T | Diarrhea | Retained food |
| 97 | F | 27 | C | 1.0 | − | − | − | − | T | Abdominal pain | None |
Abbreviations: A, >1 alcoholic beverage per day; AA, African American; ABx, antibiotics (within 3 months before sampling) ; BE, Barrett's esophagus; BL, β-lactam+BL inhibitor; C, Caucasian; CAD, coronary artery disease; CE, candidal esophagitis; CI, calcineurin inhibitor; CKD, chronic kidney disease; D, diabetes; DU, duodenal ulcer(s); EG, erythematous gastropathy; End., endoscopic; EV, esophageal varices; F, female; FQ, fluoroquinolone; G, gastroesophageal reflux disease; G, glucocorticoids; GU, gastric ulcer(s) ; HH, hiatal hernia; IMM, immunosuppression; M, male; M, mycophenolate; ML, macrolide; MWT, Mallory–Weiss tear; PPI, proton pump inhibitor (at least 20 mg once daily for >1 day); pt. clin., patient clinical background; RE, reflux esophagitis; T, active tobacco use; THF, Tetrahydrofolate synthesis pathway-affecting antibiotic; UGIB, upper gastrointestinal bleeding; #, patient ID.
Identified by H. pylori glmM-specific PCR (glmM), by 16S rRNA gene amplicon sequencing (16S) or both (+).
Nucleic acid isolation
DNA and RNA were isolated separately, using single aliquots per isolation. Samples were centrifuged at 5000 g for 8 min and the supernatant discarded. For DNA extraction, the cell pellets were re-suspended in 0.6 ml of 1 × phosphate-buffered saline and processed as described previously (Ravel et al., 2011). Cell lysis was initiated with two enzymatic incubations, first using 5 μl of lysozyme (10 mg ml−1; Amresco, Solon, OH, USA), 13 μl of mutanolysin (11.7 U μl−1; Sigma-Aldrich) and 3 μl of lysostaphin (4.5 U μl−1; Sigma-Aldrich) for an incubation of 30 min at 37 °C and, second, using 10 μl Proteinase K (20 mg ml−1; Research Products International, Mt Prospect, IL, USA), 50 μl 10% SDS and 2 μl RNase (10 mg ml−1) for an incubation of 45 min at 56 °C. After the enzyme treatments, cells were disrupted by bead beating in tubes with Lysing Matrix B (0.1 mm silica spheres, MP Biomedicals, Solon, OH, USA), at 6 m s−1 for 40 s at room temperature in a FastPrep-24 (MP Biomedicals). The resulting crude lysate was processed using the ZR Fecal DNA mini-prep kit (Zymo, Irvine, CA, USA) according to the manufacturer's recommendation. The samples were eluted with 100 μl of ultra pure water into separate tubes. DNA concentrations in the samples were measured using the Quant-iT PicoGreen dsDNA assay kit (Molecular Probes, Invitrogen, Carlsbad, CA, USA).
RNA isolation was performed by re-suspending pelleted cells in 1 ml TRIzol reagent (Ambion, Austin, TX, USA) and disrupting cells by bead beating as described above. The lysate was processed following the manufacturer's recommendations (Ambion) for RNA extraction with the following changes: in the phase separation step 300 μl of chloroform was added; the RNA precipitation step included 10 μg of added RNase-free glycogen (Fermentas, Waltham, MA, USA); 80% ethanol was used in RNA wash step. The RNA pellet was re-suspended in 41 μl of diethylpyrocarbonate-treated water (Ambion) without heated incubation. After pellet dissolution RNA samples were treated with 4 U DNaseI (Fermentas) and incubated for 1 h at 37 °C. DNaseI was deactivated by the addition of 3 μl 0.5 mℳ EDTA and incubation at 65 °C for 10 min.
Amplification and sequencing
Hypervariable regions V1–V2 of the bacterial 16S rRNA gene were amplified with primers 27F and 338R as described previously (Ravel et al., 2011). Fungal ITS1 regions were amplified with primers ITS1F and ITS2 (Ghannoum et al., 2010). Additional primer sets were evaluated, including EF4/Fung5 (Smit et al., 1999) and nu-SSU-0817-5/nu-SSU-1196-3 (Borneman and Hartin, 2000), which generated less consistent results than ITS1F and ITS2. Primers are shown in Supplementary Table 1.
DNA amplification of 16S rRNA genes was performed using AccuPrime Taq DNA polymerase High Fidelity (Invitrogen) and 50 ng of template DNA in a total reaction volume of 25 μl, following the AccuPrime product protocol. Reactions were run in a PTC-100 thermal controller (MJ Research, Waltham, MA, USA) using the following protocol: 3 min of denaturation at 94 °C, followed by 30 cycles of 30 s at 94 °C (denaturation), 30 s at 52 °C (annealing) and 45 s at 68 °C (elongation), with a final extension at 68 °C for 5 min. ITS amplicons were amplified as follows: 2 min of denaturation at 94 °C, followed by 35 cycles of 30 s at 94 °C (denaturing), 30 s at 50 °C (annealing) and 1 min at 68 °C (elongation), with a final extension at 68 °C for 5 min.
A two-step protocol was used to amplify 16S rRNA transcripts from RNA. All attempts to amplify ITS1 transcripts from total RNA isolates remained unsuccessful. The Phusion RT-PCR kit (Finnzymes, Waltham, MA, USA) was used to synthesize cDNA in a 20-μl volume with 250 ng RNA per reaction and the 338R primer (or ITS2) following the Phusion RT protocol. PCR amplification of the cDNA was set up as described above, using 2 μl of the cDNA template DNA and AccuPrime Buffer II as recommended by the manufacturer (Life Technologies, Carlsbad, CA, USA).
Negative controls were included for each amplification (PCR and RT-PCR) and barcoded primer pair, including amplification without template DNA and direct PCR amplification from RNA isolates. The presence of amplicons was confirmed by gel electrophoresis on a 2% agarose gel and staining with ethidium bromide. PCR and RT-PCR products were quantified using Quant-iT PicoGreen dsDNA assay. Equimolar amounts (50 ng) of the PCR amplicons were mixed in a single tube. Amplification primers and reaction buffer were removed using the AMPure Kit (Beckman Coulter, Brea, CA, USA) and purified amplicon mixtures sequenced at the Institute for Genome Sciences, University of Maryland, using 454 primer A and protocols recommended by the manufacturer (Roche, Branford, CT, USA). The 22 16S rRNA gene and 22 16S rRNA transcript amplicons (454 GS FLX adaptors) were sequenced as part of the same pool, which also contained 34 unrelated samples, on a half plate of the 454 GS FLX Titanium sequencer (Roche). The nine ITS DNA-derived amplicons (454 GS XLR adaptors) were sequenced together with 16S rRNA amplicons from 82 unrelated samples on a half plate of the 454 GS FLX Titanium sequencer. Raw sequences were deposited in the Short Read Archive Database (http://www.ncbi.nlm.nih.gov/sra, project number PRJNA168662).
Sequence processing and analysis
16S rRNA sequence reads were processed with CloVR (Angiuoli et al., 2011), using the automated CloVR-16S pipeline as described in the corresponding standard operating procedure (White et al., 2011). Briefly, sequences were binned based on sample-specific barcodes, trimmed by removal of barcode and primer sequences and filtered for quality, using a minimum sequence length of 100 nucleotides, a maximum homopolymer stretch of eight base pairs, a minimum quality score of 25 and a maximum number of ambiguous base pairs of 0. Reads were clustered into operational taxonomic units (OTUs) using a similarity threshold of 95%. On average, 20.6% of 16S rRNA reads were removed as chimeras. OTUs were classified using the RDP Naive Bayesian Classifier (Wang et al., 2007) with a score filtering threshold of 0.5. Rarefaction curves were calculated based on OTU counts using the rarefaction.single routine of the Mothur package (Schloss et al., 2009). Hierarchical clustering was performed using R. Differentially abundant OTUs were determined with Metastats (White et al., 2009).
ITS amplicon sequence data were processed using the automated CloVR-ITS pipeline (White et al., 2013) (http://www.nature.com/protocolexchange/protocols/2597). Briefly, sequence were binned, trimmed and filtered with QIIME (Caporaso et al., 2010) using similar criteria as applied for the 16S rRNA sequence analysis. Sequences were clustered at 99% similarity with UCLUST (Edgar, 2010), chimeric clusters removed with UCHIME (mean: 0.9% of ITS reads per sample) and non-chimeric clusters (OTUs) taxonomically assigned based on BLASTN searches against an ITS reference database (White et al., 2013).
Significance of phylogenetic distances (weighted and unweighted UniFrac) between sample groups was calculated in R, using Wilcoxon signed-rank tests. For these calculations, phylogenetic distances between samples assigned to the same group were compared with those between samples from different groups. P-values were calculated using two-tailed tests with a 5% significance level. Figures were generated by plotting the average distance between samples within the indicated group subtracted from the average distance between samples from the two compared groups.
Phylogenetic trees were created with FastTree2 (Price et al., 2010) using trimmed alignments generated with NAST (DeSantis et al., 2006), in case of 16S rRNA reads or Muscle (Edgar, 2004), in case of ITS reads. See Supplementary Figure legends for more details.
Results
Characterization of the gastric fluid microbiota
Microbial communities were analyzed in gastric fluid samples from 25 patients undergoing clinically indicated upper endoscopy, using bacterial 16S rRNA gene and transcript and fungal ITS amplicon sequencing (Table 1). Of the 25 patients, 15 were analyzed by high-coverage (HC) amplicon sequencing with between 4809 and 19 589 sequence reads per data set (Table 2). Additional data sets from these and the remaining patients comprised between 138 and 548 reads per sample. HC data and rarefied low-coverage (LC) data, generated from all data sets subsampled to 250 reads per sample were analyzed separately.
Table 2. Sequencing results.
| Subject | Groups | 16S DNAa | HC (LC) OTUsa | 16S RNAb | HC (LC) OTUsb | ITS DNAc | OTUsc |
|---|---|---|---|---|---|---|---|
| 2 | HC+LC | 12 755 | 358 (53) | 424 | − (48) | — | — |
| 15 | HC+LC | 11 714 | 92 (15) | — | 18 264 | 71 | |
| 16 | LC | 458 | − (46) | 336 | − (63) | — | — |
| 26 | LC | 189 | − (51) | 184 | − (39) | — | — |
| 31 | LC | 254 | − (56) | — | — | — | — |
| 33 | HC+LC | 9649 | 214 (44) | 12 847 | 241 (30) | 15 856 | 72 |
| 34 | HC+LC | 10 676 | 105 (17) | 14 071 | 86 (13) | — | — |
| 38 | HC+LC | 11 570 | 284 (42) | 513 | − (42) | 19 589 | 81 |
| 41 | LC | — | — | 343 | − (41) | — | — |
| 42 | LC | 321 | − (49) | — | — | — | — |
| 44 | HC+LC | 10 786 | 282 (39) | 14 445 | 276 (37) | 19 397 | 57 |
| 48 | LC | 548 | − (37) | 283 | − (61) | — | — |
| 49 | LC | — | — | 264 | − (42) | — | — |
| 50 | HC+LC | 11 096 | 215 (32) | 11 660 | 208 (33) | 5766 | 32 |
| 52 | HC+LC | 9973 | 172 (38) | 12 419 | 213 (35) | — | — |
| 56 | HC+LC | 8599 | 183 (42) | 301 | − (34) | — | — |
| 64 | HC+LC | 9547 | 26 (8) | 283 | − (13) | 10 737 | 42 |
| 67 | HC+LC | 4809 | 95 (32) | 239 | − (31) | — | — |
| 68 | LC | 138 | − (26) | 316 | − (40) | — | — |
| 76 | HC+LC | 5143 | 165 (48) | 298 | − (48) | — | — |
| 77 | HC+LC | 10 392 | 239 (43) | 430 | − (47) | 16 407 | 61 |
| 84 | LC | 286 | − (18) | 124 | − (17) | — | — |
| 94 | LC | 197 | − (27) | — | — | — | — |
| 96 | HC+LC | 8118 | 114 (25) | 110 | − (14) | 11 422 | 19 |
| 97 | HC+LC | 10 577 | 204 (30) | 271 | − (39) | 16 582 | 51 |
Abbreviations: HC, high-coverage; ITS, internal transcribed spacers; LC, low-coverage; OTU, operational taxonomic unit.
16S rRNA gene PCR amplicon sequence reads.
16S rRNA transcript RT-PCR amplicon sequence reads.
Internal Transcribed Spacer PCR amplicon sequence reads. Numbers refer to reads that passed binning by sample barcode, filtering for length, quality, ambiguous base pairs, homopolymers and chimeras as described in the Materials and methods section.
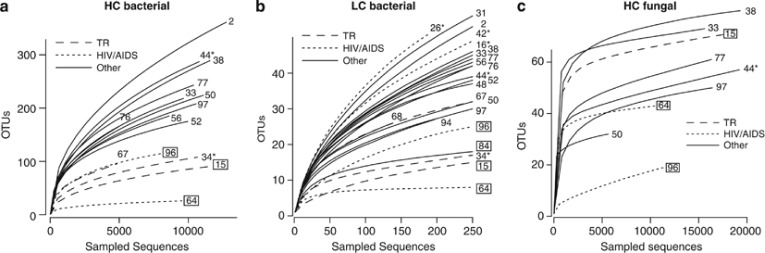
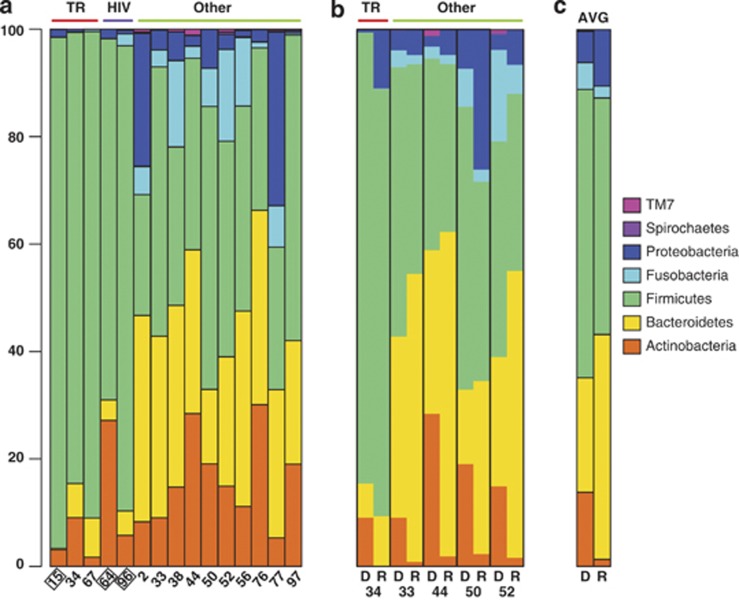
Sample pH values varied between 1.0 and 8.5 (mean: 4.3; Table 1). While, on average, pH values were higher in samples from PPI-treated (pH: 4.9) compared with all other patients (pH: 3.4), this correlation was not significant (P-value, Student's t-test: 0.13). All samples, irrespective of human host backgrounds, were found to harbor diverse microbial communities (Figure 1). For the 16S rRNA gene amplicon sequence data, HC samples contained between 26 and 358 OTUs (mean: 183) and LC samples between eight and 56 OTUs (mean: 36) per sample (see Supplementary Tables 2 and 3). On average, HC samples were dominated by members of the phyla Firmicutes (51.3%), Bacteroidetes (26.4%) and Actinobacteria (10.7%) (Figure 2a). Other prominent phyla included Proteobacteria (6.9%) and Fusobacteria (4.3%). Five samples were found to contain H. pylori, testing positive by either H. pylori-specific glmM PCR assay (Lu et al., 1999) or 16S rRNA gene amplicon sequencing (Table 1). Alignment of the 16S rRNA reads from the two HC samples identified one H. pylori genotype per patient. H. pylori was not a dominant species within the gastric fluid microbiota, accounting for <0.4% of all sequence reads in all five patient samples where it was present. Presence of H. pylori was not associated with significant differences in microbiota diversity (Figures 1a and b) or high or low sample pH (Table 1).
Figure 1.
Microbiota rarefaction curves. (a) Bacterial microbiota comparison of HC and (b) LC immunocompromised (transplant recipients and HIV/AIDS patients) and other patient samples; (c) Fungal microbiota comparison of HC immunocompromised and other patient samples. Samples from antibiotic-treated patients are boxed. H. pylori-positive samples are marked with *. AB, antibiotic-treated patient; OTU, operational taxonomic unit (equivalent of taxonomic species in the sequence space); TR, transplant recipient.
Figure 2.
Relative gastric fluid microbiota compositions at the phylum level. (a) Comparison of 15 HC 16S rRNA gene samples. (b) Comparison of 16S rRNA gene and transcript samples in five HC pairs. (c) Average compositions of 16S rRNA gene and transcript samples. Samples from antibiotic-treated patients are boxed. AVG, average; D, 16S rRNA gene amplicon data; HIV, HIV/AIDS patients; R, 16S rRNA transcript data; TR, transplant recipient.
16S rRNA transcript analysis identifies transcriptionally active microbiota
To identify stomach microbiota components that maintain metabolic activity in the stomach environment, 16S rRNA transcript amplicon sequence data were generated and compared with those from the 16S rRNA gene amplicon sequence data. For the 16S rRNA transcript analysis, total RNA isolates were used as templates for cDNA amplification with a reverse transcriptase polymerase and the 338R primer. PCR products were amplified from the cDNA with the same primers (27F and 338R) and sequence processing conditions as for the DNA-based 16S rRNA analysis. The rationale behind this approach is that 16S rRNA transcripts should be less stable under conditions detrimental for the bacterial host than 16S rRNA genes. Between 11 660 and 14 445 sequence reads were obtained from the 16S rRNA transcript analysis for five HC samples and between 110 and 513 sequence reads were obtained for 17 LC samples (Table 2).
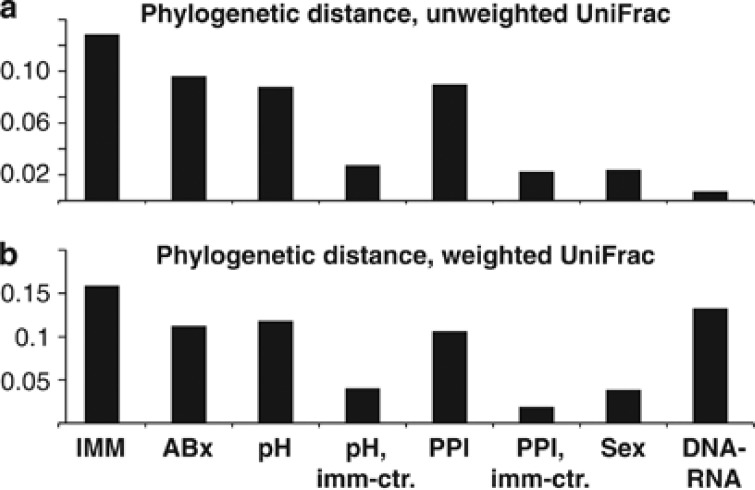
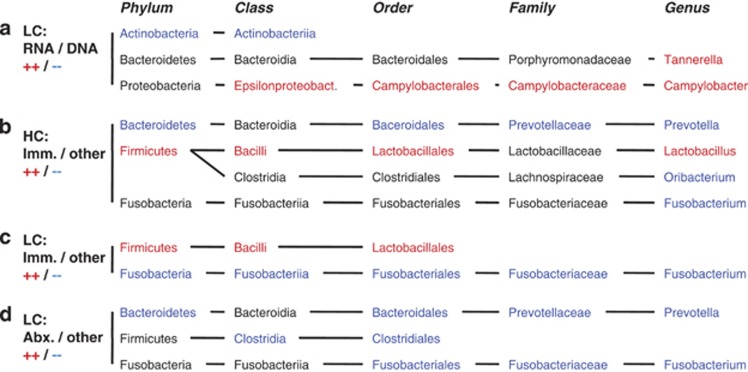
Phylogenetic distance calculations using weighted but not unweighted UniFrac analysis showed significant differences between 16S rRNA gene and transcript data (see Supplementary Tables 4 and 5 and Figure 3). In addition, consistent shifts in relative distributions of the main taxonomic phyla were apparent in the comparison of 16S rRNA transcript and gene-based amplicon sequence data (Figure 2b), including a decrease in Actinobacteria and Firmicutes and an increase in Bacteroidetes and Proteobacteria. Due to limited numbers of sample pairs, these observations could not be supported statistically based on HC sequence data. However, significant changes between DNA and RNA-based microbiota compositions confirming several of these observations were identified using the larger LC sample group (Figure 4a). Compared with the total microbiota, the transcript-based microbiota fraction shows a statistically significant (P<0.003) reduction in the class Actinobacteriia (34% phylum: Actinobacteria), and an increase in the genera Campylobacter (444% phylum: Proteobacteria) and Tannerella (680% phylum Bacteroidetes). Of the Campylobacter reads, >90% were assigned to a single species, Campylobacter concisus, based on sequence alignments and phylogenetic tree predictions (see Supplementary Figure 1). C. concisus is a known oral commensal bacterium, which has also been isolated from human feces (Van Etterijck et al., 1996). Members of the class Actinobacteriia, which had a lower relative abundance in 16S rRNA transcripts compared with genes, included known habitants of the oral cavity, such as Rothia dentocariosa (Brown et al., 1969) (on average 64% of Actinobacteriia) and Actinomyces odontolyticus (Batty, 1958) (on average 17% of Actinobacteriia). In the two HC samples that contained H. pylori (#34, #44), the relative abundance of H. pylori was, on average, 19.9 times higher in 16S rRNA transcripts compared with genes.
Figure 3.
Phylogenetic distances between HC sample groups, using unweighted (a) and weighted (b) UniFrac analysis. Plots show the average distance between samples within the indicated groups subtracted from the average distance between samples from the two compared groups. IMM, immunocompromised (transplant recipients and HIV/AIDS patients) versus other patient samples; ABx, antibiotically treated versus other patient samples, pH; low pH (1.0–4.0) versus high pH (4.0–8.5) patient samples; pH, imm-ctr., same as pH but excluding IMM; PPI, PPI-treated patient samples; PPI, imm-ctr.; same as PPI but excluding IMM; DNA–RNA, 16S rRNA gene versus transcript data.
Figure 4.
Microbiota components with significantly different relative abundance between groups. (a) LC samples: Comparison of 16S rRNA gene-based and rRNA transcript-based microbiota; (b) HC Samples: Comparison of immunocompromised (transplant recipients and HIV/AIDS patients) and other patient samples; (c) LC samples: Comparison of immunocompromised (transplant recipients and HIV/AIDS patients) and other patient samples; (d) LC samples: Comparison of antibiotic-treated and other patient samples; Significant differences were calculated on five taxonomic levels (phylum, class, order, family, genus) using Metastats (White et al., 2009) with a P-value threshold of 0.003, based on the taxonomic assignments of all reads with the RDP Bayesian classifier (Wang et al., 2007). Taxa with significantly greater relative abundance in the first compared with the second group of samples are shown in red, those with lower abundance in blue, and those without significant difference in black. Only taxonomic lineages that contain at least one component of significantly different relative abundance between the compared groups are shown.
Impact of host immune status and pH on gastric microbiota
The 15 gastric fluid samples from the HC sample group could be divided into two subsets, based on microbiota species richness (Figure 1a) and composition (Figure 5), which coincided best with differences in the human host immune status. The distinction of a sample subset from immunocompromised patients could be confirmed using phylogenetic distance (weighted and unweighted UniFrac analysis) calculations (Figures 3 and 6; Supplementary Tables 4 and 5). Two samples from this immunocompromised patient group originated from HIV/AIDS patients and three from kidney transplant recipients who were taking immunosuppressive medication at the time of sample collection (Table 1), including MMF (mycophenolate mofetil), which affects B and T lymphocyte proliferation (Villarroel et al., 2009). Among these five immunocompromised patient samples, there was a significant overlap (three out of five samples) with patients who had taken antibiotics around the time of sample collection (Figure 1a). All immunocompromised patient samples had pH values >5 (mean: 6.9). PPI usage, when controlled for immunosuppression did not show a comparable impact on microbiota richness (data not shown) or composition (Figures 3 and 5; Supplementary Tables 4 and 5). Significant differences in phylogenetic distance (weighted and unweighted UniFrac) were also measured between samples with low pH (1.0–4.0) and high pH (4.0–8.5), even when controlled for immunosuppression (Figure 3; Supplementary Tables 4 and 5; Supplementary Figure 2).
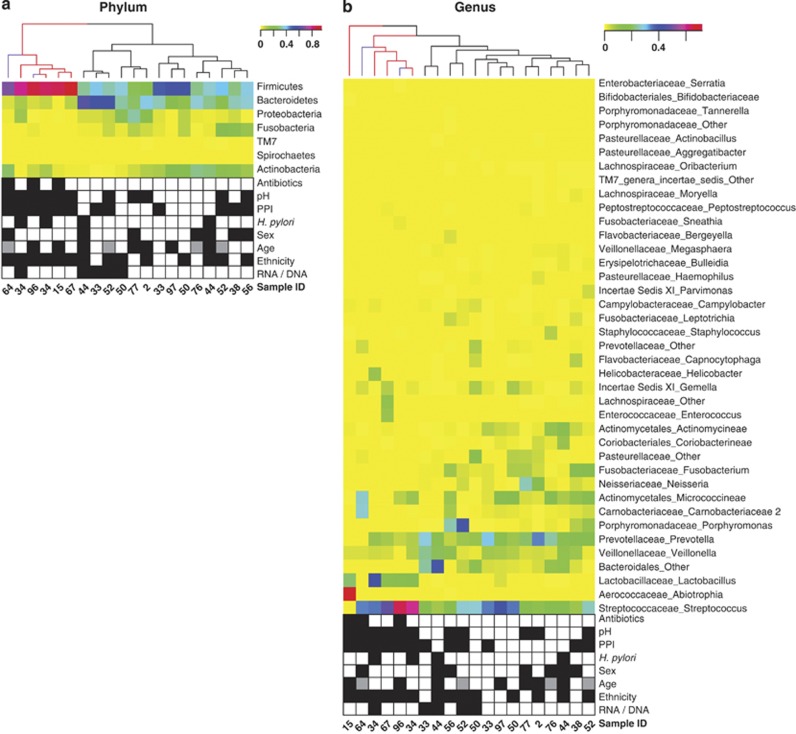
Figure 5.
Hierarchical clustering of HC 16S rRNA gene and transcript samples based on microbiota compositions. (a) Phylum level; (b) Genus level. Colors (yellow to red) show relative abundance per sample. Branches of the tree showing sample similarities based on hierarchical clustering are colored for transplant recipients (red) and HIV/AIDS patients (purple). Rows are also clustered hierarchically based on similar relative abundance values across samples (dendrogram not shown). Patient sample metadata are shown using checkerboard plots based on the following color codes: Antibiotic treatment (black) or no treatment (white) within 3 months before sample collection; high pH 4.0–8.5 (black) or low pH 1.0–4.0 (white); PPI treatment (black) or no treatment (white) within 24 h of sample collection; H. pylori presence (black) or absence (white) based on 16S rRNA gene amplicon sequencing; male (black) or female (white) sex; ethnicity: African American (black) or Caucasian (white); RNA/DNA: 16S rRNA transcript (black) or 16S rRNA gene (white) data. For age, black, gray and white boxes refer to 27–38, 42–56 and 65–81 years, respectively. See Table 1 for additional details.
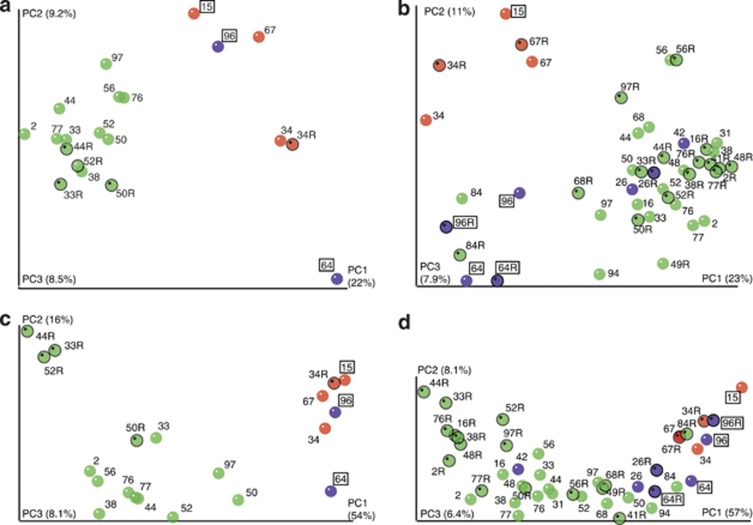
Figure 6.
Unscaled principal coordinate analysis (PCoA) plots showing unweighted (a, b) and weighted (c, d) UniFrac analysis of HC (a, c) and LC (b, d) samples. Samples are color coded as follows: Transplant recipient (red), HIV/AIDS patient (purple) or other (green) samples. 16S rRNA transcript data are marked with an ‘R' after the patient number and outlined in black. Antibiotically treated patient samples are boxed.
On average, bacterial richness in the five immunocompromised samples from transplant recipients and HIV/AIDS patients was reduced (42% P<0.001) compared with all other HC samples, measured by comparing the mean counts of OTUs per 2500 sequence reads. Statistically significant changes (P<0.003) in the microbiota composition between these two groups included, at the phylum level, increased abundances of Firmicutes (221%) and reduced abundances of Bacteroidetes (15%), and, at the genus level, increased Lactobacillus spp. (3084%) and reduced Fusobacterium spp. (1%) and Prevotella spp. (16%) (Figure 4b). The Lactobacillus populations from the immunocompromised patient samples showed heterogeneous compositions, including members from different taxonomic species, as predicted by 16S rRNA sequence alignments and phylogenetic tree predictions (see Supplementary Figure 3).
Although not significant, similar trends were identified when comparing the 23 LC 16S rRNA gene samples, that is, lower bacterial species richness (75% P<0.2) and different microbiota composition in immunocompromised patient samples (Figure 1b). In addition, similar microbiota changes were found associated with immunocompromised patient samples from the HC and LC sample groups (Figure 4c), including increased abundance of the phylum Firmicutes (193%) and reduced abundance of Fusobacteria (4%). Among the LC samples, a stronger correlation between antibiotic treatment and reduced bacterial species richness (45% P<0.001) was apparent than among the HC samples. In contrast to the HC samples, where no significant microbiota changes were found to correlate with antibiotic medication, several taxonomic groups are reduced in the LC samples from antibiotic-treated patients (Figure 4d), including the genus Prevotella (17% phylum: Bacteroidetes), the family Fusobacteriaceae (7% phylum: Fusobacteria) and the order Clostridiales (7% phylum: Firmicutes). High pH was not associated with significant reductions in bacterial diversity (81% P<0.2) or changes in the microbiota composition, except for an increase in members of the family Veillonellaceae (201% phylum: Firmicutes).
Identification of a fungal gastric fluid microbiota
To study fungal members of the gastric fluid microbiota, ITS of the rRNA gene cluster were amplified in a subset of nine randomly chosen samples, sequenced and processed using a similar methodology as for the 16S rRNA-based microbiota analysis (White et al., 2013) (see Supplementary Table 6). After sequence binning, trimming and filtering between 5766 and 19 589 reads were obtained per sample and assigned to taxonomic lineages (Table 2). Samples contained between 19 and 81 genus-level OTUs. On average, 77.5% of ITS reads could not be taxonomically assigned, even at the phylum level, most likely due to incomplete representation of human-associated fungi in available ITS reference sequence collections (White et al., 2013). Candida was one of the only two genera found in all samples, the other being Phialemonium. The known pathogens C. albicans, C. tropicalis and C. parapsilosis were identified based on phylogenetic tree predictions using publicly available Candida reference sequences (see Supplementary Figure 4). A correlation between human host immune status and fungal microbiota richness and/or composition was not found (Figure 1c; Supplementary Table 6). However, one of the two HIV/AIDS patients (#96) who was treated prophylactically against Pneumocystis pneumonia (PCP) with the tetrahydrofolate synthesis inhibitor trimethoprim/sulfamethoxazole and also took the macrolide azithromycin showed markedly reduced fungal microbiota richness (Figure 1c).
Discussion
Our results reveal that human gastric fluid harbors a diverse microbiota dominated by Actinobacteria, Bacteroidetes, Firmicutes, Fusobacteria and Proteobacteria, demonstrating a similar overall composition at the phylum level as previously found in other GI tract locations, including intraoral niches (Zaura et al., 2009; Crielaard et al., 2011), throat (Andersson et al., 2008), distal esophagus (Pei et al., 2004), stomach mucosa (Bik et al., 2006; Andersson et al., 2008) and feces (Costello et al., 2009). Fungal members of the microbiota are also identified, including the known pathogen C. albicans. Contrary to previous reports that studied the stomach environment based on biopsy samples from the mucosa (Bik et al., 2006; Andersson et al., 2008), H. pylori, if present, is not a dominant species within the gastric fluid microbiota of patients with an indication for upper endoscopy, a finding consistent with the literature showing that H. pylori colonizes the mucous layer and adheres to gastric epithelial cells (Amieva and El-Omar, 2008).
Using a 16S rRNA transcript amplicon sequencing strategy, significant differences are identified between the total (DNA) and transcriptionally active (RNA) microbiota. A similar approach was first applied by Zoetendal et al. (1998) to study fecal microbiota compositions (Zoetendal et al., 1998). Peris-Bondia et al. (2011) used flow cytometry to sort cells based on total RNA content before performing 16S rRNA amplicon pyrosequencing and reported decreases in Bacteroidetes and increases in specific families of the Clostridiales (Firmicutes) from fecal samples (Peris-Bondia et al., 2011). Typical bacterial inhabitants of the oral cavity unlikely to be adapted to the stomach, including R. dentocariosa (Brown et al., 1969) and A. odontolyticus (Batty, 1958), are reduced in 16S rRNA transcript fractions of the stomach fluid samples, supporting the utility of this method for characterization of the metabolically active gastric fluid microbiota. This characterization could have important implications for intestinal health, as only microbes that survive passage through the stomach are able to enter the lower GI tract and play a role in intestinal host/microbe homeostasis. Stomach fluid is typically believed to represent a harsh environment for bacterial growth due to low-pH acidic conditions, although our data indicate large variations in actual stomach fluid pH values, and increases in gastric pH after meals have been reported (Gardner et al., 2002). The identification of transcriptionally active C. concisus, a known oral commensal and intestinal pathogen with a suspected role in Crohn's disease (Man et al., 2010; Hess et al., 2012), represents an example of a microbial organism that could be important for intestinal disease but would not draw attention with traditional 16S rRNA gene amplicon sequencing-based techniques, due to the low relative abundance. It should be noted that C. concisus has been especially associated with intestinal pathogenicity in immunocompromised patients (Aabenhus et al., 2002) and that, among the five HC patient samples, the relative increase in C. concisus concentrations in RNA versus DNA isolates was even more pronounced in the single immunocompromised patient (#34) compared with the four other samples (1254% and 630%, respectively). In addition, all immunocompromised patient samples showed pH values above 6.0. Whether immunosuppression influences the barrier function of the stomach for intestinal pathogens needs to be further addressed in future studies.
The human host is suspected to play a major role in shaping the gastric fluid microbiota, in terms of both diversity and composition. Our results suggest that immune status is the most important determinant of gastric fluid microbiota, although antibiotic medication, which is often required in immunocompromised patients, and high gastric fluid pH, which can be caused by H. pylori infection and PPI usage among other factors, also seem to affect the microbiota diversity and composition. This is surprising since, unlike the small and large intestine, the lamina propria of the gastric mucosa typically lacks organized and diffuse lymphoid tissue. Immunocompromised HIV/AIDS patients and transplant recipients show markedly reduced diversity as well as altered composition of their gastric fluid microbiota. The effect of immunosuppressive medication on the microbiota appears restricted to transplant recipients, all of which were taking MMF and glucocorticoids at the time of sample collection. In contrast, a sample from a patient with sarcoidosis (#52), treated at the time of enrollment with a low dose (5 mg prednisone daily) of glucocorticoids alone, did not show characteristics similar to the HIV/AIDS patient and transplant recipient samples. MMF, an inhibitor of purine biosynthesis, affects T and B lymphocyte proliferation and is known to cause GI adverse side effects (Villarroel et al., 2009). Glucocorticoids exert anti-inflammatory effects through multiple often immune cell-specific mechanisms, including suppression of pro-inflammatory T cells and stimulation of regulatory T cells (Zen et al., 2011). Similarities between the histological effects of GI toxicity induced by MMF and acute GVHD (graft-versus-host-disease) affecting the GI tract as well as HIV/AIDS have been pointed out before (Papadimitriou et al., 2003). While the disease state of HIV/AIDS patients was not recorded as part of this study, CD4+ T-cell depletion of GALT (gut-associated lymphoid tissue) is an early event in the pathogenesis of human HIV infection and restoration is delayed even after highly active antiretroviral therapy (Guadalupe et al., 2003). GALT harbors the majority of T lymphocytes in the human body (Nannini and Okhuysen, 2002), further suggesting that the T-cell-mediated adaptive immune response could be responsible for the observed microbiota changes in transplant recipients and HIV/AIDS patients samples and could be important for host-controlled GI tract homeostasis.
Similarities in infectious and other complications, especially of the GI tract, between HIV/AIDS patients and transplant recipients have been previously described (Thom and Forrest, 2006). Our observations of the gastric fluid microbiota in this patient group provide the opportunity for identifying potential microbiota-associated causes of disease. For example, the observed increase in Lactobacillus spp. seen in immunocompromised patients could be responsible for an increased risk of Lactobacillus bacteremia, which is rare in immunocompetent patients but has been reported in HIV/AIDS patients (Horwitch et al., 1995) and organ transplant recipients (Patel et al., 1994). Furthermore, Jenq et al. (2012) recently described changes in the microbiota of human and mouse bone marrow transplant recipients similar to those reported here, namely reduced diversity and increases in Lactobacillales in feces (humans) and ileum and cecum (mouse) (Jenq et al., 2012). In the mouse model, the authors could also demonstrate a protective effect of Lactobacillus species against GVHD, a common complication of bone marrow transplantation, highlighting the potential therapeutic implications of the observed microbiota changes for the development of future pro- or antibiotic treatments.
Prior work suggests that acid-suppressing medications increase the risk of enteric infections (Leonard et al., 2007) and the prevalence of non-H. pylori bacteria in gastric fluid (Sanduleanu et al., 2001). Surprisingly, while high gastric fluid pH was associated with significant microbiota changes, our study did not detect a statistically significant correlation between acid-suppressing medications and high gastric fluid pH (P-value: 0.13) or a direct impact of these medications on microbiota richness or composition. Larger studies will be needed before any conclusions can be drawn.
The present work has limitations. First, while care was taken to avoid aspiration of oropharyngeal and esophageal secretions, samples could have been contaminated from these areas, as the endoscope passed from the mouth to the stomach. In addition, while endoscopes undergo high-level disinfection before every use, they are not sterile. Sample storage conditions (Lauber et al., 2010; Wu et al., 2010) and DNA extraction methods (Maukonen et al., 2012), as well as 16S rRNA gene amplification primers (Wu et al., 2010) and biases inherent in PCR amplification, have all been shown to influence the results of microbiota analyses. PCR replicates were not performed. Therefore, the true composition of the gastric fluid microbiota remains unknown and could be different from the one reported here. However, the main results presented in this study should not have been affected by potential biases, as they were based on the comparison of samples collected and processed under similar conditions. Similarly, while 16S rRNA transcript analysis suggests the presence of a metabolically active microbiota in the stomach fluid, it does not answer the question whether an intrinsic microbiota that is native to the gastric fluid environment exists, or whether extrinsic microbes were detected from other environments, which are merely adapted to persist in this environment.
In conclusion, this study provides the identification and first in-depth characterization of microbial communities from the gastric fluid environment of the stomach. With 16S rRNA transcript sequence analysis, a methodology is presented for identification of the transcriptionally active component of the total stomach fluid microbiota. The results presented here demonstrate the existence of a gastric fluid microbiota, which contains fungi and is significantly influenced by the immune status and antibiotic regimen of the human host. These findings imply an important function of these factors for human GI health and could lead to the development of new therapeutic strategies with potential to improve GI health that may be particularly relevant to immunocompromised patients.
Acknowledgments
Funding for this study was provided by the National Human Genome Research Institute (NHGRI) and the Institute of Diabetes, Digestive and Kidney Diseases (NIDDK) from the National Institutes of Health (NIH) (RC2 HG005597 and R01 DK046461).
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Aabenhus R, Permin H, On SL, Andersen LP. Prevalence of Campylobacter concisus in diarrhoea of immunocompromised patients. Scand J Infect Dis. 2002;34:248–252. doi: 10.1080/00365540110080566. [DOI] [PubMed] [Google Scholar]
- Amieva MR, El-Omar EM. Host-bacterial interactions in Helicobacter pylori infection. Gastroenterology. 2008;134:306–323. doi: 10.1053/j.gastro.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Andersson AF, Lindberg M, Jakobsson H, Backhed F, Nyren P, Engstrand L. Comparative analysis of human gut microbiota by barcoded pyrosequencing. PLoS One. 2008;3:e2836. doi: 10.1371/journal.pone.0002836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angiuoli SV, Matalka M, Gussman A, Galens K, Vangala M, Riley DR, et al. CloVR: a virtual machine for automated and portable sequence analysis from the desktop using cloud computing. BMC Bioinformatics. 2011;12:356. doi: 10.1186/1471-2105-12-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batty I. Actinomyces odontolyticus, a new species of actinomycete regularly isolated from deep carious dentine. J Pathol Bacteriol. 1958;75:455–459. doi: 10.1002/path.1700750225. [DOI] [PubMed] [Google Scholar]
- Bik EM, Eckburg PB, Gill SR, Nelson KE, Purdom EA, Francois F, et al. Molecular analysis of the bacterial microbiota in the human stomach. Proc Natl Acad Sci USA. 2006;103:732–737. doi: 10.1073/pnas.0506655103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borneman J, Hartin RJ. PCR primers that amplify fungal rRNA genes from environmental samples. Appl Environ Microbiol. 2000;66:4356–4360. doi: 10.1128/aem.66.10.4356-4360.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JM, Georg LK, Waters LC. Laboratory identification of Rothia dentocariosa and its occurrence in human clinical materials. Appl Microbiol. 1969;17:150–156. doi: 10.1128/am.17.1.150-156.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R. Bacterial community variation in human body habitats across space and time. Science. 2009;326:1694–1697. doi: 10.1126/science.1177486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crielaard W, Zaura E, Schuller AA, Huse SM, Montijn RC, Keijser BJ. Exploring the oral microbiota of children at various developmental stages of their dentition in the relation to their oral health. BMC Med Genomics. 2011;4:22. doi: 10.1186/1755-8794-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croswell A, Amir E, Teggatz P, Barman M, Salzman NH. Prolonged impact of antibiotics on intestinal microbial ecology and susceptibility to enteric Salmonella infection. Infect Immun. 2009;77:2741–2753. doi: 10.1128/IAI.00006-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis TZ, Hugenholtz P, Keller K, Brodie EL, Larsen N, Piceno YM, et al. NAST: a multiple sequence alignment server for comparative analysis of 16S rRNA genes. Nucleic Acids Res. 2006;34:W394–W399. doi: 10.1093/nar/gkl244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicksved J, Lindberg M, Rosenquist M, Enroth H, Jansson JK, Engstrand L. Molecular characterization of the stomach microbiota in patients with gastric cancer and in controls. J Med Microbiol. 2009;58:509–516. doi: 10.1099/jmm.0.007302-0. [DOI] [PubMed] [Google Scholar]
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- Endt K, Stecher B, Chaffron S, Slack E, Tchitchek N, Benecke A, et al. The microbiota mediates pathogen clearance from the gut lumen after non-typhoidal Salmonella diarrhea. PLoS Pathog. 2010;6:e1001097. doi: 10.1371/journal.ppat.1001097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner JD, Ciociola AA, Robinson M. Measurement of meal-stimulated gastric acid secretion by in vivo gastric autotitration. J Appl Physiol. 2002;92:427–434. doi: 10.1152/japplphysiol.00956.2001. [DOI] [PubMed] [Google Scholar]
- Ghannoum MA, Jurevic RJ, Mukherjee PK, Cui F, Sikaroodi M, Naqvi A, et al. Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS Pathog. 2010;6:e1000713. doi: 10.1371/journal.ppat.1000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadalupe M, Reay E, Sankaran S, Prindiville T, Flamm J, McNeil A, et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. 2003;77:11708–11717. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess DL, Pettersson AM, Rijnsburger MC, Herbrink P, van den Berg HP, Ang CW.2012Gastro-enteritis caused by Campylobacter concisus: case report and short review of literature J Med Microbiol 61(Pt 5)746–749. [DOI] [PubMed] [Google Scholar]
- Horwitch CA, Furseth HA, Larson AM, Jones TL, Olliffe JF, Spach DH. Lactobacillemia in three patients with AIDS. Clin Infect Dis. 1995;21:1460–1462. doi: 10.1093/clinids/21.6.1460. [DOI] [PubMed] [Google Scholar]
- Howden CW, Hunt RH. Relationship between gastric secretion and infection. Gut. 1987;28:96–107. doi: 10.1136/gut.28.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenq RR, Ubeda C, Taur Y, Menezes CC, Khanin R, Dudakov JA, et al. Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J Exp Med. 2012;209:903–911. doi: 10.1084/jem.20112408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauber CL, Zhou N, Gordon JI, Knight R, Fierer N. Effect of storage conditions on the assessment of bacterial community structure in soil and human-associated samples. FEMS Microbiol Lett. 2010;307:80–86. doi: 10.1111/j.1574-6968.2010.01965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard J, Marshall JK, Moayyedi P.2007Systematic review of the risk of enteric infection in patients taking acid suppression Am J Gastroenterol 1022047–2056.quiz 2057. [DOI] [PubMed] [Google Scholar]
- Lu JJ, Perng CL, Shyu RY, Chen CH, Lou Q, Chong SK, et al. Comparison of five PCR methods for detection of Helicobacter pylori DNA in gastric tissues. J Clin Microbiol. 1999;37:772–774. doi: 10.1128/jcm.37.3.772-774.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Contreras A, Goldfarb KC, Godoy-Vitorino F, Karaoz U, Contreras M, Blaser MJ, et al. Structure of the human gastric bacterial community in relation to Helicobacter pylori status. ISME J. 2011;5:574–579. doi: 10.1038/ismej.2010.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man SM, Zhang L, Day AS, Leach ST, Lemberg DA, Mitchell H. Campylobacter concisus and other Campylobacter species in children with newly diagnosed Crohn's disease. Inflamm Bowel Dis. 2010;16:1008–1016. doi: 10.1002/ibd.21157. [DOI] [PubMed] [Google Scholar]
- Martinsen TC, Bergh K, Waldum HL. Gastric juice: a barrier against infectious diseases. Basic Clin Pharmacol Toxicol. 2005;96:94–102. doi: 10.1111/j.1742-7843.2005.pto960202.x. [DOI] [PubMed] [Google Scholar]
- Maukonen J, Simoes C, Saarela M. The currently used commercial DNA-extraction methods give different results of clostridial and actinobacterial populations derived from human fecal samples. FEMS Microbiol Ecol. 2012;79:697–708. doi: 10.1111/j.1574-6941.2011.01257.x. [DOI] [PubMed] [Google Scholar]
- Nannini EC, Okhuysen PC. HIV1 and the gut in the era of highly active antiretroviral therapy. Curr Gastroenterol Rep. 2002;4:392–398. doi: 10.1007/s11894-002-0009-z. [DOI] [PubMed] [Google Scholar]
- Papadimitriou JC, Cangro CB, Lustberg A, Khaled A, Nogueira J, Wiland A, et al. Histologic features of mycophenolate mofetil-related colitis: a graft-versus-host disease-like pattern. Int J Surg Pathol. 2003;11:295–302. doi: 10.1177/106689690301100406. [DOI] [PubMed] [Google Scholar]
- Patel R, Cockerill FR, Porayko MK, Osmon DR, Ilstrup DM, Keating MR. Lactobacillemia in liver transplant patients. Clin Infect Dis. 1994;18:207–212. doi: 10.1093/clinids/18.2.207. [DOI] [PubMed] [Google Scholar]
- Pei Z, Bini EJ, Yang L, Zhou M, Francois F, Blaser MJ. Bacterial biota in the human distal esophagus. Proc Natl Acad Sci USA. 2004;101:4250–4255. doi: 10.1073/pnas.0306398101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peris-Bondia F, Latorre A, Artacho A, Moya A, D'Auria G. The active human gut microbiota differs from the total microbiota. PLoS One. 2011;6:e22448. doi: 10.1371/journal.pone.0022448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MN, Dehal PS, Arkin AP. FastTree 2—approximately maximum-likelihood trees for large alignments. PLoS One. 2010;5:e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci USA. 2011;108 (Suppl 1:4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanduleanu S, Jonkers D, De Bruine A, Hameeteman W, Stockbrugger RW. Non-Helicobacter pylori bacterial flora during acid-suppressive therapy: differential findings in gastric juice and gastric mucosa. Aliment Pharmacol Ther. 2001;15:379–388. doi: 10.1046/j.1365-2036.2001.00888.x. [DOI] [PubMed] [Google Scholar]
- Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit E, Leeflang P, Glandorf B, van Elsas JD, Wernars K. Analysis of fungal diversity in the wheat rhizosphere by sequencing of cloned PCR-amplified genes encoding 18S rRNA and temperature gradient gel electrophoresis. Appl Environ Microbiol. 1999;65:2614–2621. doi: 10.1128/aem.65.6.2614-2621.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant SM, Hartland EL, Phumoonna T, Lyras D, Rood JI, Robins-Browne RM, et al. Influence of gastric acid on susceptibility to infection with ingested bacterial pathogens. Infect Immun. 2008;76:639–645. doi: 10.1128/IAI.01138-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom K, Forrest G. Gastrointestinal infections in immunocompromised hosts. Curr Opin Gastroenterol. 2006;22:18–23. doi: 10.1097/01.mog.0000196149.29077.0d. [DOI] [PubMed] [Google Scholar]
- Van Etterijck R, Breynaert J, Revets H, Devreker T, Vandenplas Y, Vandamme P, et al. Isolation of Campylobacter concisus from feces of children with and without diarrhea. J Clin Microbiol. 1996;34:2304–2306. doi: 10.1128/jcm.34.9.2304-2306.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroel MC, Hidalgo M, Jimeno A. Mycophenolate mofetil: An update. Drugs Today (Barc) 2009;45:521–532. doi: 10.1358/dot.2009.45.7.1384878. [DOI] [PubMed] [Google Scholar]
- Vorobjova T, Watanabe T, Chiba T. Helicobacter pylori immunology and vaccines. Helicobacter. 2008;13 (Suppl 1:18–22. doi: 10.1111/j.1523-5378.2008.00636.x. [DOI] [PubMed] [Google Scholar]
- Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JR, Arze C, Matalka M, Team TC, White O, Angiuoli SV, et al. 2011CloVR-16S: Phylogenetic microbial community composition analysis based on 16S ribosomal RNA amplicon sequencing—standard operating procedure, version 1.1 Nature Preceddoi: 10.1038/npre.2011.6287.2 [DOI]
- White JR, Maddox C, White O, Angiuoli SV, Fricke WF. CloVR-ITS: automated internal transcribed spacer amplicon sequence analysis pipeline for the characterization of fungal microbiota. Microbiome. 2013;1:6. doi: 10.1186/2049-2618-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JR, Nagarajan N, Pop M. Statistical methods for detecting differentially abundant features in clinical metagenomic samples. PLoS Comput Biol. 2009;5:e1000352. doi: 10.1371/journal.pcbi.1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GD, Lewis JD, Hoffmann C, Chen YY, Knight R, Bittinger K, et al. Sampling and pyrosequencing methods for characterizing bacterial communities in the human gut using 16S sequence tags. BMC Microbiol. 2010;10:206. doi: 10.1186/1471-2180-10-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaura E, Keijser BJ, Huse SM, Crielaard W. Defining the healthy ‘core microbiome' of oral microbial communities. BMC Microbiol. 2009;9:259. doi: 10.1186/1471-2180-9-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zen M, Canova M, Campana C, Bettio S, Nalotto L, Rampudda M, et al. The kaleidoscope of glucorticoid effects on immune system. Autoimmun Rev. 2011;10:305–310. doi: 10.1016/j.autrev.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Zoetendal EG, Akkermans AD, De Vos WM. Temperature gradient gel electrophoresis analysis of 16S rRNA from human fecal samples reveals stable and host-specific communities of active bacteria. Appl Environ Microbiol. 1998;64:3854–3859. doi: 10.1128/aem.64.10.3854-3859.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.