Abstract
Background
Health status measure is not only important for clinical research studies but also for clinical practices of chronic obstructive pulmonary disease (COPD) patients. The objective of this study is to evaluate the validity of the Korean Version of COPD Assessment Test (CAT) in primary care clinics as well as in referral hospitals.
Methods
Smokers or ex-smokers, aged 40 years or older, with a smoking history of >10 pack-years; and a COPD diagnosis in the past 6 months or more, were recruited from 4 primary care clinics and 2 referral hospitals. Demographic, medical, and spirometry data was collected from patients who completed the CAT and St. George Respiratory Questionnaire (SGRQ), and had their dyspnea been assessed. The primary endpoint was the correlation between of the Korean version of CAT with SGRQ in patients with COPD.
Results
A total 100 patients were enrolled. The mean age and smoking amounts were 69.2±8.4 years and 40.6±22.3 pack-years, respectively. Sixty-seven percent of the patients reported at least one exacerbation in the past year. The mean CAT score was 16.9±8.0. The internal consistency assessed by Cronbach's alpha was 0.85. The CAT score was positively correlated with the SGRQ score (r=0.76, p<0.0001) and each component of SGRQ: symptoms, activity and impacts; r=0.68, r=0.61, and r=0.72, respectively (all p<0.0001). These positive correlations were preserved in the different groups (r=0.86, p<0.0001 in primary care clinic group; r=0.69, p<0.0001 in hospital group). The CAT score was also positively correlated to the Medical Research Council dyspnoea scale (r=0.46, p<0.0001).
Conclusion
The Korean version of CAT had good internal consistency and showed good correlations with SGRQ. It can be used for assessing the impacts of COPD on the patient's health including primary care setting.
Keywords: Pulmonary Disease, Chronic Obstructive; Health Status Indicators: Questionnaires
Introduction
Chronic obstructive pulmonary disease (COPD), a common preventable and treatable disease, is characterised by persistent airflow limitation that is usually progressive1. COPD accounted for 5.1% of all deaths in 20042, and is expected to be third cause of death in 20203. In Korea, the prevalence of COPD is 12.4% from the data of fourth Korean National Health and Nutritional Survey4. COPD was reported to be seventh cause of death in Korea in 20115. Unfortunately, there is no pharmacologic treatment that slows the rate of decline of airflow limitation or reduces the mortality of COPD.
Quality of life is one of the treatment goals for COPD. Recently revised Global Initiative for Chronic Obstructive Lung Disease (GOLD) guideline emphasized the assessment of health status of patients with COPD1. Our previous study showed that only 11% of COPD patients feel good in their health status6.
Therefore, health status measurement is now important not only in clinical research studies but also in clinical practice for COPD patients. Existing health status measures such as the St. George's Respiratory Questionnaire (SGRQ) and the Chronic Respiratory Disease Questionnaire (CRQ)-while reliable, valid, and useful for clinical trials-are lengthy, with scoring algorithms less than ideal for routine use in clinical practice7.
The COPD Assessment Test (CAT) is an 8-item, self-administered, disease-specific questionnaire, which is recently developed8 and is recommended for the assessment of COPD disease severity in the GOLD guidelines1. Initially, it was developed and validated in the USA and Europe9,10, and has been translated into many other languages11. The aim of this study was to validate the Korean version of CAT in clinical practice not only in the referral hospitals but also in the primary care clinics.
Materials and Methods
The primary endpoint of this study was to evaluate the correlation between of the Korean version of CAT with SGRQ in patients with COPD. Secondary endpoint was to reveal the correlation between the CAT with forced expiratory volume in one second (FEV1) values and Medical Research Council (MRC) dyspnea scale. This study was approved by the Institutional Review Board of Hallym University Sacred Heart Hospital.
1. Study subjects
Patients were recruited from six investigational sites in South Korea. The sites comprised of 4 primary care clinics and 2 referral hospitals. Written informed consent was obtained from each patient prior to the participation in the study. We included the subjects who were outpatients of age 40 years or older with a smoking history of 10 pack-years or more. Subjects also had a diagnosis of COPD at least 6 months. Those with an active respiratory disorder other than COPD such as lung cancer or tuberculosis; or a current diagnosis of asthma; or were unable to complete the questionnaires were excluded.
2. Study design
This study was a cross-sectional study. We collected the subjects' information including demographic characteristics, smoking history, medication history of COPD and comorbidities. We also asked the subjects about the history of exacerbation. The comorbidities included diabetes, peripheral artery diseases and rheumatoid disease, heart failure (any causes) and ischemic heart disease. Medication history other than COPD such as anti-hypertensive and other cardiovascular drugs was also collected.
Patients were asked to complete the SGRQ and CAT questionnaires during the study visit. Validated translations of the standard SGRQ questionnaire with three domains for symptoms, activity and impacts, was used12. The CAT questionnaire had 8 items, covering cough, phlegm, chest tightness, breathlessness, activity limitation, confidence, sleep, and energy. It was translated into Korean by a professional translational centre, in accordance with the International Society for Pharmacoeconomics and Outcome Research (ISPOR) translation and cultural adaptation process for patient-reported outcomes13. During the same visit, the patient's breathlessness was assessed using the MRC dyspnea scale14. Spirometry was performed if there were no prior documented results within 6 months of the study visit.
3. Statistical methods
Demographic data and other baseline characteristics including CAT, MRC, and SGRQ scores and post-bronchodilator FEV1 % predicted values were presented using descriptive statistics. Disease severity was assessed using the GOLD severity classification1 based on the subject's post-bronchodilator FEV1 % predicted value. Associations between the CAT and SGRQ, MRC, and FEV1 % predicted were tested using Pearson's correlations upon normality assumptions. All the statistical analyses were performed by using the SPSS version 18 (SPSS Inc., Chicago, IL, USA). A p<0.05 was regarded as statistically significant.
Results
1. Patient characteristics
A total 100 subjects were enrolled in this study. Forty subjects were enrolled from the primary care clinics and the rest sixty subjects were from 2 referral hospitals. The clinical characteristics of the subjects are summarized in Table 1. The mean age was 69.2±8.4 years and 97% of them were male. The subjects had a mean smoking history of 40.6±22.3 pack-years and 31% of them were current smoker. The mean duration of COPD diagnosis was 55.7 months. A total 16% of the subjects had the comorbidities. Diabetes was the most frequent one (13% of the subjects). Long acting anticholinergics were the most commonly prescribed treatment (73.0% of the patients), followed by fixed combination of glucocorticoid and longt-acting β2-agonists (60.0%) and theophylline (60.0%). A flu vaccination was given in 71% of the subjects in the past year. The mean postbronchodilator FEV1 was 63.6±21.1% of the predicted value. A total 75.0% of the subjects were classified as GOLD grade I or II. More subjects from the primary care clinics were classified into GOLD grade 1 than form the referral hospitals (35.0% vs. 8.3%, p=0.0019). Sixty-seven percent of the patients reported at least one exacerbation in the past year. The mean number of acute exacerbation was 1.56. Subjects from the primary care clinic experienced more exacerbation than subject from the referral hospital in the past year, which was statistically significant (2.80 vs. 0.73, p<0.0009). About half of subjects from the referral hospital experienced no exacerbation in the past year (Table 1).
Table 1.
Demographic and clinical characteristics
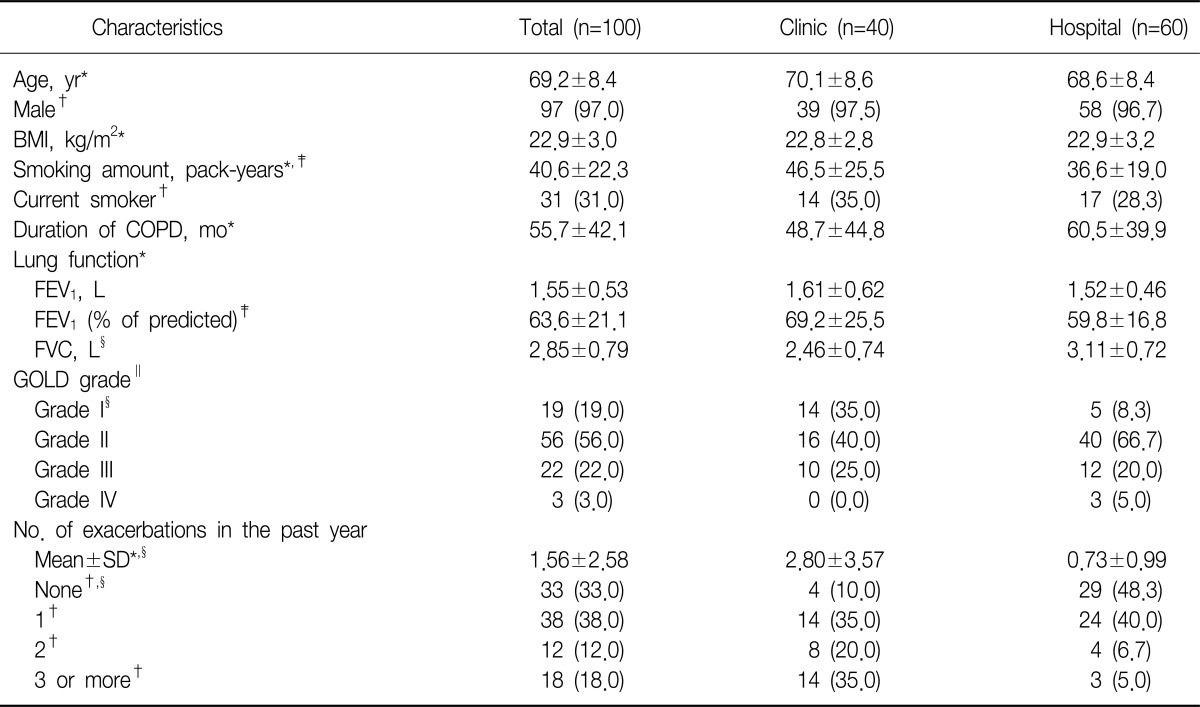
Values are presented as mean±SD or number (%).
*t-test. †Chi-square test. ‡p<0.05. §p<0.01. ∥Fisher's exact test.
BMI: body mass index; COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; GOLD: Global Initiative for Chronic Obstructive Lung Disease.
2. Result of questionnaires
The mean CAT score for the study subjects was 16.9±8.0. The internal consistency assessed by Cronbach's alpha was 0.85. The total CAT score was not different between the subjects form the primary care clinics and referral hospitals. Subjects from the primary care clinics reported more cough. The mean total score of SGRQ was 39.9±18.9. The mean symptom score, activity score and impact score were 49.2, 52.9, and 29.6, respectively. The SGRQ score was not significantly different between the groups. Fifty-five percent of the subjects responded to MRC grade 2 as their severity of breathlessness. The distributions of MRC score as follows: 12% in MRC 1, 55% in MRC 2, 23% in MRC 3, 9% in MRC 4, and 1% in MRC 5. More subjects experienced stopping for a breath after walking 100 m or after a few minutes on the level from the primary care clinics (Table 2).
Table 2.
Summary of COPD Assessment Tests, St. George Respiratory Questionnaires and Medical Research Council dyspnea scales
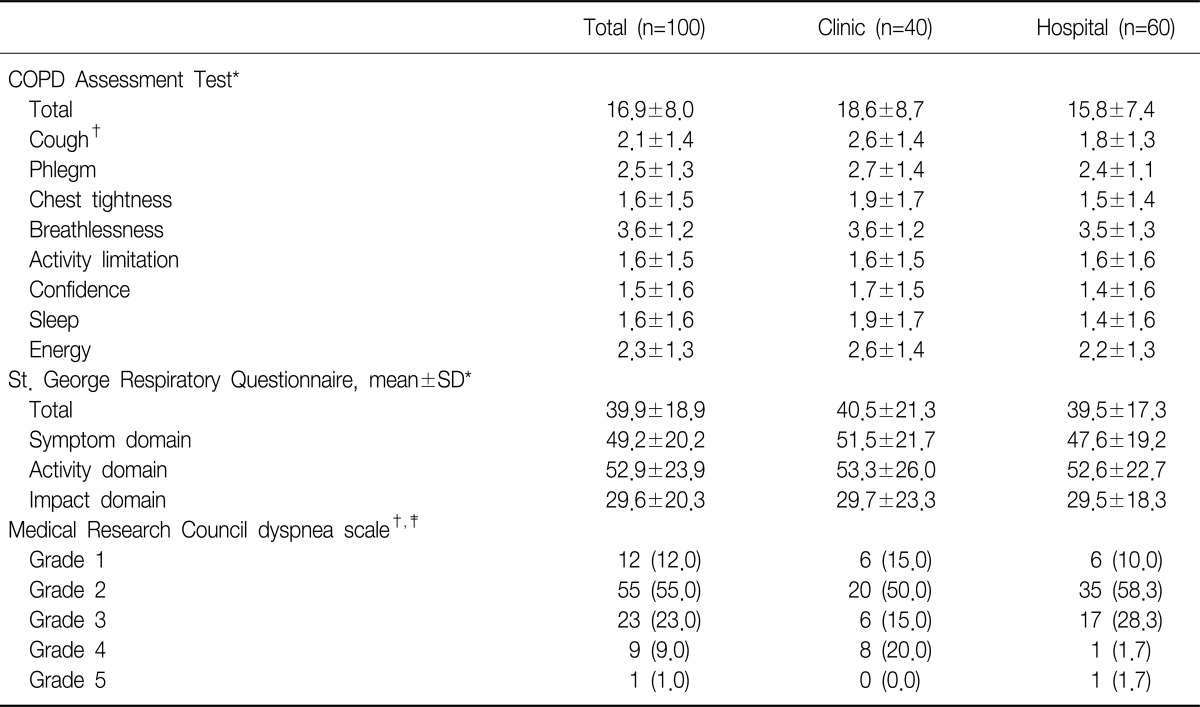
Values are presented as mean±SD or number (%).
*t-test. †p<0.05. ‡Fisher's exact test.
COPD: chronic obstructive pulmonary disease.
3. Correlation between CAT score and SGRQ, MRC dyspnea scale and FEV1
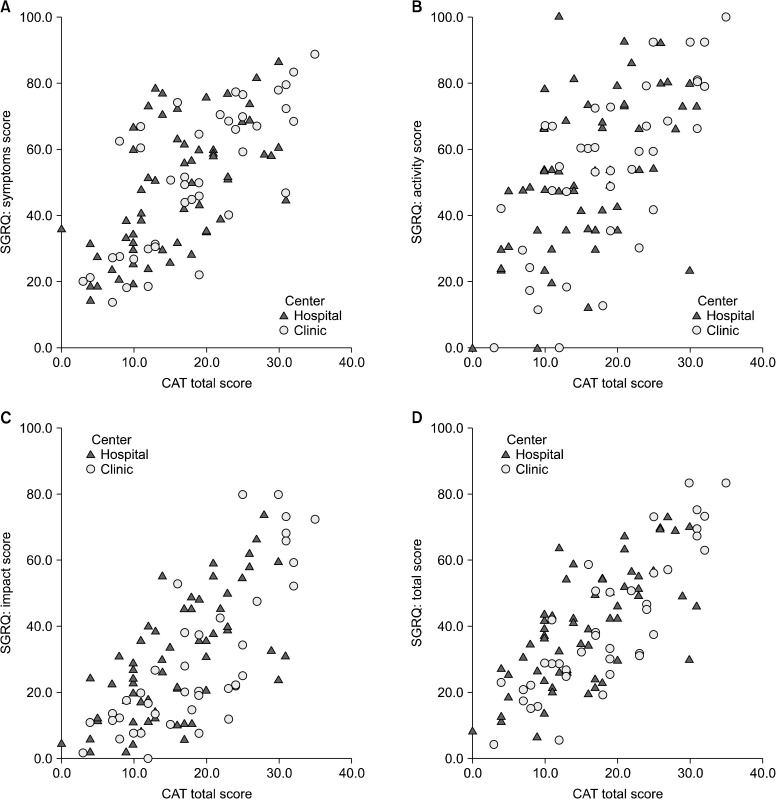
The CAT score was positively correlated with the SGRQ total score (r=0.7641, p<0.0001) and its three component domains: symptoms, activity, and impacts; r=0.6767, r=0.6076, and r=0.7224, respectively, all p<0.0001. The CAT score also positively correlated with MRC dyspnea scale (r=0.46, p<0.0001) (Table 3, Figure 1). This tendency was preserved when comparing between subgroups.
Table 3.
Correlation of COPD Assessment Test with other parameters
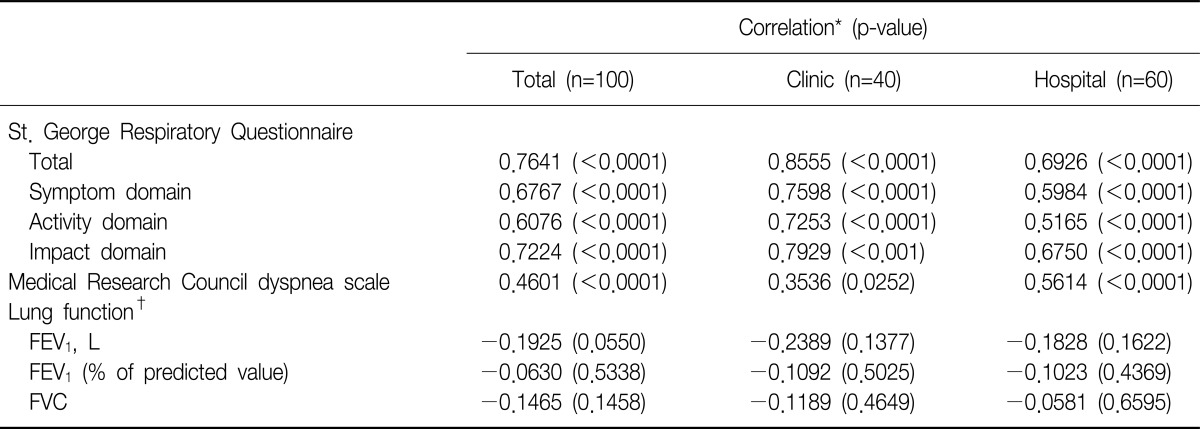
*Pearson's correlation. †Post bronchodilator spirometry.
COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in one second; FVC: forced vital capacity.
Figure 1.
Correlation with COPD Assessment Test (CAT) and with St. George Respiratory Questionnaire (SGRQ). (A) CAT scores and SGRQ symptoms scores. (B) CAT scores and SGRQ activity scores. (C) CAT scores and SGRQ impacts scores. (D) CAT scores and SGRQ total scores. COPD: chronic obstructive pulmonary disease.
However, there was no significant correlation between CAT score and post-bronchodilator FEV1 % predicted (r=-0.063, p>0.05). SGRQ correlated with post-bronchodilator FEV1 % predicted but the correlation co-efficient was small (r=-0.2809, p=0.0046).
Discussion
Improving health status is one of the treatment goals for COPD. While spirometry is critical for diagnosis of COPD and determination of disease severity, lung function testing alone does not provide a measurement of the overall impact of COPD on health status7. However, the instruments for health status of COPD patients such as the SGRQ9 and CRQ, although comprehensive, are lengthy and time-consuming10,11. It is not easy to use these instruments in routine practice. The CAT, which has been developed recently, is an 8-item, self-administered questionnaire to assess the condition of patients and overall impact of COPD, and to improve patient-physician communication8. In this study, the Korean version of CAT showed good internal consistency of Cronabch alpha 0.85. This figure is consistent with that of the original version of CAT validated in Western countries8 and that of another validation study in Korea15. Since the previous Korean validation study was conducted at a single referral hospital, it could not reveal the usefulness of CAT in primary care setting where majority of COPD patients are treated. Our study was conducted in 2 referral hospitals and 4 primary clinics.
We compared not only the mean CAT and SGRQ scores but also the demographic and clinical characteristics of the subjects according to the treatment site. The CAT and SGRQ sores were not different between the groups, which meant COPD equally affects the patients' quality of life irrespective of treatment site. As expected, there were more mild subjects in the primary clinic subgroup. However, more subjects experienced exacerbations in this group. This means that mild COPD patients experienced more exacerbation in primary care clinics. This confirms again that lung function testing alone does not provide a measurement of the overall impact of COPD on health status7. Also, it is not easy to use spirometry in the primary care clinics16. It is necessary to use a simple instrument for assessing COPD patients in primary care clinics. Therefore, use of Korean version of CAT can be extended to the primary clinic.
Scores for each item of CAT range from 0 to 5. Total scores between 10 and 20 indicate that the impact of COPD to the patients is medium, which means COPD is one of the most important problems that the patients have. Scores between 21 and 30 indicate that the impact level of COPD is high. Scores above 30 indicate the very high impact level17. So, a CAT score ≥10 indicates a high level of symptoms1. The mean CAT score in this study was 16.9, which meant COPD significantly impaired the health status of the patients. This impact level was similar to those of the original CAT validation study and the European study which enrolled more severe and exacerbated patients8,10.
The Korean version of CAT significantly correlated with total SGRQ score (r=0.74). This is concordant with previous findings8,10,15. Also, it had significant correlation with the three domains of the SGRQ, which address COPD symptoms, disturbance of physical activity and psycho-social impact of the disease. These correlations were not different between the groups. This supports findings obtained in American patients which showed that the CAT correlated with each of the four domains of the CRQ18. Therefore the CAT addresses a wide range of effects of COPD on the patient's health1.
The CAT and MRC had positive correlation with moderate degree. As it is known that there is a significant disparity between patient perceptions of their disease severity and the degree of severity indicated by the MRC dyspnea scale19, the CAT has a broader coverage of the impact of COPD on patient's daily life and well-being than MRC dyspnea scale. In this study, more than half of the subjects responded to MRC grade 2 as their severity of dyspnea. Only 10% of the subjects had severe dyspnea such as MRC grade 4 or 5. However, almost of these severe dyspneic subjects were from the primary care clinics. The mean scores of chest tightness, breathless and activity limitation domain of the CAT were significantly different between the groups. This confirms again the usefulness of CAT for the assessment of COPD patients. The CAT score showed a trend of negative correlation with FEV1, but was not statistically significant. This finding was concordant with previous Korean validation study15.
Taken altogether, the findings of our study confirm that spirometry alone has limitation for assessing the health status of COPD patients, and there is considerable heterogeneity in health-related quality of life impairment among patients COPD. An understanding of the impact of COPD on an individual patient needs to combine the symptomatic assessment with the patient's spirometric classification and risk of exacerbation1.
We did not directly compare the CAT score according to GOLD grade, exacerbation history and presence of co-morbidity in this study. Such analyses were beyond scope of our validation study. Future study will be needed in this context.
In conclusion, the Korean version of CAT had good internal consistency and showed good correlation with SGRQ. It can be used for assessing the impact of COPD on the patient's health including primary care setting. Use of this instrument should lead to better recognition of the impact of this disease, better assessment and therefore more effective treatment.
Acknowledgements
This study was supported by GlaxoSmithKline for the funding of the study and data analysis.
References
- 1.Global Initiative for Chronic Obstructive Pulmonary Disease (GOLD) Global strategy for the diagnosis, management and prevent of chronic obstructive pulmonary disease [Internet] GOLD; 2011. [cited 2012 Feb 22]. Available from: http://www.goldcopd.org. [Google Scholar]
- 2.World Health Organization. The global burden of disease. 2004 update. Geneva: World Health Organization; 2008. [Google Scholar]
- 3.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet. 1997;349:1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 4.Hwang YI, Yoo KH, Sheen SS, Park JH, Kim SH, Yoon HI, et al. Prevalence of chronic obstructive pulmonary disease in Korea: the result of Forth Korean National Health and Nutrition Examination Survey. Tuberc Respir Dis. 2011;71:328–334. doi: 10.1111/j.1440-1843.2011.01951.x. [DOI] [PubMed] [Google Scholar]
- 5.Statistics Korea. Causes of death statistics in 2011 [Internet] Daejeon: Statistics Korea; [cited 2013 Jan 5]. Available from: http://kostat.go.kr/portal/english/news. [Google Scholar]
- 6.Hwang YI, Kwon OJ, Kim YW, Kim YS, Park YB, Lee MG, et al. Awareness and impact of COPD in Korea: an epidemiologic insight survey. Tuberc Respir Dis. 2011;71:400–407. [Google Scholar]
- 7.Jones P, Harding G, Wiklund I, Berry P, Leidy N. Improving the process and outcome of care in COPD: development of a standardised assessment tool. Prim Care Respir J. 2009;18:208–215. doi: 10.4104/pcrj.2009.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34:648–654. doi: 10.1183/09031936.00102509. [DOI] [PubMed] [Google Scholar]
- 9.Jones PW, Brusselle G, Dal Negro RW, Ferrer M, Kardos P, Levy ML, et al. Properties of the COPD assessment test in a cross-sectional European study. Eur Respir J. 2011;38:29–35. doi: 10.1183/09031936.00177210. [DOI] [PubMed] [Google Scholar]
- 10.Jones PW, Brusselle G, Dal Negro RW, Ferrer M, Kardos P, Levy ML, et al. Health-related quality of life in patients by COPD severity within primary care in Europe. Respir Med. 2011;105:57–66. doi: 10.1016/j.rmed.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 11.COPD Assessment Test [Internet] Brentford: GlaxoSmithKline; c2009. [cited 2012 Feb 22]. Available from: http://www.catestonline.org. [Google Scholar]
- 12.Kim YS, Byun MK, Jung WY, Jeong JH, Choi SB, Kang SM, et al. Validation of the Korean version of the St. George's Respiratory Questionnaire for patients with chronic respiratory disease. Tuberc Respir Dis. 2006;61:121–128. [Google Scholar]
- 13.Wild D, Grove A, Martin M, Eremenco S, McElroy S, Verjee-Lorenz A, et al. Principles of good practice for the translation and cultural adaptation process for patient-reported outcomes (PRO) measures: report of the ISPOR Task Force for Translation and Cultural Adaptation. Value Health. 2005;8:94–104. doi: 10.1111/j.1524-4733.2005.04054.x. [DOI] [PubMed] [Google Scholar]
- 14.Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54:581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee S, Lee JS, Song JW, Choi CM, Shim TS, Kim TB, et al. Validation of the Korean version of Chronic Obstructive Pulmonary Disease Assessment Test (CAT) and Dyspnea-12 Questionnaire. Tuberc Respir Dis. 2010;69:171–176. [Google Scholar]
- 16.Park MJ, Choi CW, Kim SJ, Kim YK, Lee SY, Kang KH, et al. Survey of COPD management among the primary care physicians in Korea. Tuberc Respir Dis. 2008;64:109–124. [Google Scholar]
- 17.The COPD Assessment Test: healthcare professional user guide: expert guidance on frequently asked questions [Internet] Brentford: GlaxoSmithKline; c2009. [cited 2012 Feb 22]. Available from: http://www.catestonline.co.uk/ [Google Scholar]
- 18.Jones PW, Harding G, Wiklund I, Berry P, Tabberer M, Yu R, et al. Tests of the responsiveness of the COPD Assessment Test following acute exacerbation and pulmonary rehabilitation. Chest. 2012;142:134–140. doi: 10.1378/chest.11-0309. [DOI] [PubMed] [Google Scholar]
- 19.AACR. Confronting COPD in America: executive summary [Internet] Philadelphia: American Association for Cancer Research; [cited 2012 Feb 22]. Available from: http://www.aacr.org/ [Google Scholar]