Abstract
This review describes effects of miso with reference to prevention of radiation injury, cancer and hypertension with a twin focus on epidemiological and experimental evidence. Miso with a longer fermentation time increased crypt survival against radiation injury in mice. When evaluating different types of miso provided by different areas in Japan, miso fermented for a longer period increased the number of surviving crypts, and 180 days of fermentation was the most significant. Dietary administration of 180-day fermented miso inhibits the development of azoxymethane (AOM)-induced aberrant crypt foci (ACF) and rat colon cancers in F344 rats. Miso was also effective in suppression of lung tumors, breast tumors in rats and liver tumors in mice. The incidence of gastric tumors of groups of rats given NaCl was higher than those of the groups given miso fermented for longer periods. Moreover, the systolic blood pressure of the Dahl male rat on 2.3% NaCl was significantly increased but that of the SD rat was not. However, the blood pressures of the rats on a diet of miso or commercial control diet (MF) did not increase. Even though miso contains 2.3% NaCl, their blood pressures were as stable as those of rats fed commercial diet containing 0.3% salt. So we considered that sodium in miso might behave differently compared with NaCl alone. These biological effects might be caused by longer fermentation periods.
Keywords: miso, biological effects, experimental, ethological
Introduction
Miso (fermented soy bean paste), a traditional ingredient of the Japanese diet, is fermented from a mixture of soybeans with rice, wheat or oats and contains vitamins, microorganisms, salts, minerals, plant proteins, carbohydrates, and fat. Saponin inhibiting lipid peroxides, trypsin inhibitor, isoflavones, lecithin, colin, prostaglandin E and others are additional substances1. Miso is used on a daily basis as a flavor in soup and solid food in Japan and other parts of Asia and is an essential ingredient for Japanese cuisine. Even though there is no equivalent product in Western cooking, those who are familiar with miso have prized its almost unlimited versatility for cooking. For example, it can be used as bouillon, a rich meat stock in soups and stews2. It is considered to exert health-promoting benefits, relieving fatigue, regulating intestinal functions, aiding digestion, protecting against gastric ulcer, decreasing cholesterol and blood pressure, and preventing diseases associated with the lifestyles, like cancers.
As previously reported by Ito et al.3 in a mini review, effects of miso with reference to prevention of radiation injury, cancer and hypertension are described with a twin focus on recent epidemiological and experimental evidence in this review.
Radiation Protection by Miso
When the 2nd atomic bomb was dropped in Nagasaki on August 9th, 1945, physician Tatuichiro Akizuki, along with 20 employees, was taking care of 70 tuberculosis patients at “Uragami Daiichi Hospital” (St. Francis Hospital) about 1.4 km away from the hypocenter. However, these people including Dr. Akizuki did not have any acute radiation disease. Dr. Akizuki considered that this was the result of consuming cups of wakame miso soup (miso soup with garnish of wakame seaweed) every day4. Later, this was translated into English and became known in the West. In the Chernobyl of nuclear power plant accident on April 26, 1986, in the Ukraine, many Europeans consumed miso soup as a preventive measure for radiation diseases. Therefore, Dr. Akizuki can be considered to be the first person in Japan to point out radioprotective effects of miso for maintaining health.
Based on this case, we commenced our experimental research studies on radioprotective effects of miso against radiation5,6,7,8.
A week before irradiation, five-week-old male B6C3F1 mice were fed a diet of MF commercially available from Oriental Yeast (Tokyo, Japan) and MF with 10% dried red rice miso obtained from the Miso Central Institute (Tokyo, Japan). Six-week-old male mice were irradiated with 7–12 Gy (dose rate 4 Gy/min) without anesthesia. They were autopsied for histological observation of intestinal crypt survival at 3.5 days after irradiation. No protection effect against radiation was seen in 2% NaCl or MF diet groups, but the numbers of surviving crypts (Fig. 1) was significantly increased in the miso diet group (Table 1). However, this phenomenon was not observed when miso was given on 0, 1 or 2 days after irradiation. So it was concluded that a certain concentration of the effective substance(s) must exist in the blood before exposure5.
Fig. 1.
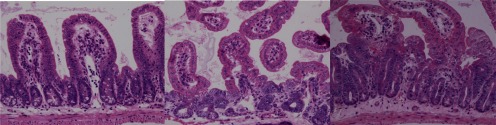
Small intestine 3 days after irradiation ×200, HE staining. Left, nonirradiated; middle, 10 Gy Cs-irradiation; right, miso+10 Gy irradiation. Note that there is an increase in regenerated crypts in miso group.
Table 1. Number of Surviving Crypts after X-irradiation by Miso7.
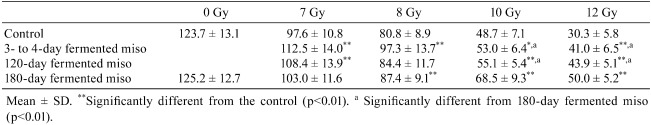
An investigation was conducted to determine whether protective effects were caused by soybean itself or a substance produced during fermentation of miso. One week before irradiation, the mice were fed a diet supplemented with 10% miso at different stages of fermentation: 3 to 4 days, 120 days and 180 days (Table 1). After 7 Gy irradiation, the numbers of surviving crypts with 120-day fermented miso and 3- to 4-day fermented miso were significantly greater as compared with the MF group. With 8 Gy, the numbers of surviving crypts in the 3- to 4-day and 180-day fermented miso groups were also significantly increased compared with the MF group. Crypt survival with 10 Gy was significantly different in the 180-day fermented miso group as compared with the 3-to 4-day fermented miso, 120-day fermented miso and MF groups7, 8.
Using miso produced by the Miso Central Institute (Tokyo) and the Hiroshima Prefectural Food Technology Research Center (Hiroshima), crypt survival was further studied at a dose of 12 Gy of X-irradiation. There were no significant differences in the numbers of surviving crypts with 180-day fermented miso from either source. However, when evaluating different types of miso provided by different areas of each institute, miso fermented for longer resulted in increased the numbers of surviving crypts, with the period of 180 days fermentation being considered significant. The mechanism of the radioprotective effect of miso is considered to be closely related to substances produced during fermentation stages9.
Clearly, the dose of radiation is of paramount importance. With 15 Gy, the survival rate (dose rate 2 Gy/min), started to go down on the 5th day in animals, and on the 7th day, there were no surviving mice, showing that there was no significant protective effect of miso. However, with 8 Gy, the number of surviving mice in the MF group started to decrease on the 10th day, and in the 120-day and 180-day fermentation groups, it began to decrease on the 11th and 13th days, respectively. A delay in mortality was obvious in all three miso groups, with a significantly increased survival rate in the 3- to 4-day (p=0.048), 120-day (p=0.026) and 180-day fermented miso groups (p=0.011) as compared with the MF group by the Cox model6. Houchen et al.10 reported that expression of FGF-2 is induced with radiation injury and that recombinant human FGF-2 markedly enhanced the crypt survival rate. Takahama et al.11 also and that reported that a replication-deficient adenovirus containing the HST-1 gene acted as a potent protector against lethal irradiation associated with injury of the intestinal tract as well as myelosuppression in the bone marrow and spleen. Ferrel et al.12 described that recombinant human keratinocyte growth factor can protect mice from chemotherapy- and radiation-induced gastrointestinal damage but not when the whole body was exposed to radiation, at least in terms of death from intestinal and marrow toxicity. We also found VEGF to be protective (Katoh and Watanabe, unpublished data). The cytokine-like substance in miso may conceivably play an important role in the protection and/or the recovery and repopulation of critical tissue elements when they are given prior to and during radiation exposure. However, to our knowledge, there have been no reports of such effect using an extract of cytokines from miso10,11,12.
In our continuing search for active ingredients, water-soluble and ether-soluble fractions as well as residues from 10-day and 260-day fermented miso were investigated. Only the water-soluble fraction of 10-day fermented miso increased crypt survival, but all fractions of 10-day and 260-day fermented products exerted a beneficial influence. As for mortality, only the water-soluble faction of 260-day fermented miso increased the survival rate as compared with the control (MF). This suggests that the effective substances in this fraction might be produced or present in 10-day fermented miso and produced in greater quantity during 260 days of fermentation9.
It is known that aglycon-type isoflavones and melanoidin are produced during fermentation of miso. Watanabe et al. found that the former did not have any radiation protective effects (unpublished data), but chemically synthesized melanoidin and a melanoidin-like substance extracted from 7-month fermented miso showed a protective effect13.
Further study is needed to elucidate the substances responsible for increased crypt survival, crypt lengths and prolongation of average time to death after a high dose of irradiation in mice, the mechanisms and any associated changes in bacterial flora in the gastrointestinal tract.
Prevention of ACF and Cancer of the Large Intestine
The change in dietary habits from traditional Japanese to Western style has been paralleled by an increase in colon and breast cancer in Japan, providing evidence for the greater importance of environment of food intake as opposed to genetic background as a cause of cancer14. In an epidemiologic study, the risk of cancer in the large intestine was decreased by soybean consumption15. Many articles have been published concerning physiological properties of soybean that might contribute to suppression of the development of large intestinal cancer or ACF from the perspectives of vegetable fiber16, isoflavones17,18,19, saponin20 and high molecular weight fractions of water soluble material21, 22 in soybeans, but there are also components that are clearly not effective18,19.
Effects of miso, salt, kojic acid and biochanin A on the development of ACF
A study was designed to investigate the effects of fermented miso, pure NaCl, crude salts, kojic acid and biochanin A by the induction of ACF with AOM, a carcinogen for large intestinal cancer23,24,25 (Table 2).
Table 2. Effects of Dietary Miso, NaCl and Other Agents on Azoxymethane (AOM)-induced Aberrant Crypt Foci (ACF) in the F344 Rat Colon23,24,25.
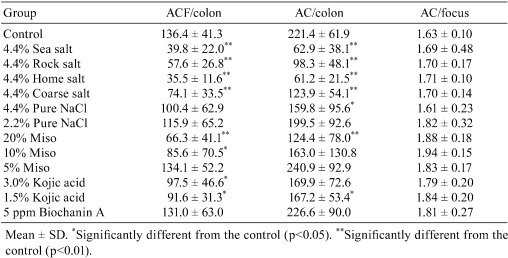
Six-week-old male F344/DuCrj rats were fed different kinds of diets, and after one week, 15 mg/kg/body weight of AOM (Sigma) was injected once per week for 3 weeks with continued feeding for 2 weeks. All rats were fed a commercial MF diet (Oriental Yeast, Tokyo, Japan) alone or with 5%, 10%, 20% dried red miso; 2.2% or 4.4% NaCl (Wako Pure Chemical, Tokyo, Japan), an NaCl content equivalent to 10% and 20% miso, respectively; 4.4% sea salt (Ryoen, Tokyo, Japan), coarse salt (Diasalt, Tokyo), rock salt (Tomen, Tokyo, Japan) or table salt (JT, Tokyo, Japan); 1.5% or 3.0% of kojic acid (Nagase Seikagaku, Tokyo, Japan); or 5 ppm of biochanin A (Sigma). After animals were autopsied, the colons were removed and fixed in 10% buffered formalin and stained with 0.5% methylene blue. By viewing the stained colons with a microscope at a magnification of 20-30 times, number of aberrant crypt foci (ACFs), total number of aberrant crypts (ACs) per colon and number of ACs per focus were counted.
All rats treated with AOM showed a 100% incidence of ACFs. Numbers of ACFs per animal were significantly decreased as compared with controls on MF except in the groups given 4.4% pure NaCl, 2.2% pure NaCl, 5% miso or biochanin A. Total AC numbers were significantly reduced except in the groups receiving 2.2% pure NaCl, 3% kojic acid and biochanin A. ACs per focus increased significantly in the 10% and 20% miso groups.
ACFs decreased as the concentration of miso increased but not as the concentration of pure NaCl increased. They also decreased in the groups of crude salts and 3.0% kojic acid but not as in the groups given 1.5% kojic acid and biochanin A. Kojic acid exists in miso during fermentation period but not in the final product. (Mohri & Fujinami, personal communication), and therefore, the suppressive effect of miso on ACFs did not appear to be caused by kojic acid or a similar substance.
This study showed that pure NaCl did not have a suppressive effect on ACFs but that crude salt did. BrdU labeling indices and/or sizes of germinal regions were measured in some groups, but there was no correlation between ACF and AC induction. Impurities of purified salt used in this study were less than 0.01% but crude salt contained calcium and magnesium, and these substances were reported to suppress cancer of the large intestine and ACF induction26,27,28,29,30,31 and to prevent from DNA synthesis32, 33. It is also reported that seren34, copper and iron35 had the same effect of suppression of ACFs. Therefore, the suppressive effect of AOM on ACFs was considered to be caused by the minerals in miso, such as calcium and magnesium. This suggests that because of the large intake of minerals in natural salt used in regular Japanese food, the occurrence of large intestinal cancer in Japan is relatively small.
Effects of long-term fermented miso on ACFs
A study was designed to investigate the effect on ACFs induced by AOM depending on different durations of fermentation of miso36. All rats treated with AOM developed ACFs. The number of ACFs per animal were, 87.8 ± 28.9 in the MF group, 84.9 ± 52.1 in the 2.2% NaCl group, 85.5 ± 19.3 in the 3- to 4-day fermented miso group, 83.6 ± 20.8 in the 120-day fermented miso group and 65.1 ± 18.4 in the 180-day fermented miso group. The number of ACFs in the 180-day miso group decreased significantly compared with those of the MF group and 3- to 4-day fermented miso group, and 120-day fermented miso group. Total AC number was largest in the 120-day fermented miso group and increased significantly compared with the 180-day fermented miso group. In this study, 180-day fermented miso exhibited the most suppression of ACFs induced by AOM, and the effect was considered to be caused by components of soybean, minerals and other substances produced during fermentation of miso described before.
Effect on large intestinal adenocarcinomas
Five-week-old male F344/DuCrj rats were maintained on MF diet supplemented with 10% each of dried miso prepared from two different fermentation stages including 3- to 4-day and 180-day fermented miso. Twenty-four weeks after administration of the carcinogen AOM, rats were autopsied, and their colons were investigated. There was no significant difference among the groups in averaged number of ACFs per colon, total number of ACs and number of ACs per ACF37, 38 (Table 3).
Table 3. Decrease by 180-day Fermented Miso in Size of Colon Carcinomas Induced by AOM in F344 Rats37.
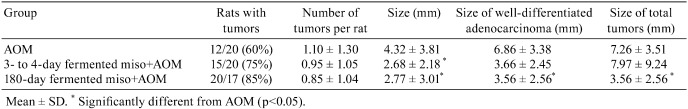
Each rat that died on the 103rd, 149th and 163rd day after the first day of AOM injection in the miso groups had signet ring cell carcinoma in the duodenum. Total colon tumors incidence and number of colon tumors per rat were not significantly different (Table 3). By pathological observation, signet ring cell carcinomas in the large intestine were not observed in the 180-day fermented miso group. The sizes of the well-differentiated type of adenocarcinomas and total adenocarcinomas (well-differentiated type and signet ring cell) in the 180-day and 3- to 4-day fermented miso groups were significantly smaller in the MF+AOM group. Number of crypts, number of PCNA-positive cells and the highest position and width of the germinal region of the PCNA-positive index decreased significantly in the 180-day fermented miso group compared with the MF+AOM group. In this study, dietary administration of 180-day fermented miso inhibited the development of AOM-induced ACFs in the rat colon, but 10% miso did not have a suppressive effect on ACFs induced by DMH39 in mice.
Effects of soybean products on colon carcinogenesis
Epidemiological study showed that intake of miso soup decreased large intestinal cancer40 or increased it41. Kono et al. reported that taking two cups of miso soup per day reduced the number of S-type colon cancers 42. Watanabe et al. reported that consumption of tofu and soybean decreased the number of rectal tumors43, and Poole et al. reported a decrease in large intestinal tumors44 and Hu et al. reported a decrease in colon tumors in men consuming soybean products and miso in China45. Le et al. reported that intake of soybean or soybean products in Hawaii reduced occurrence of large intestinal cancer in women but not in men46. Tuyns et al. reported soybean to be clearly protective against colon and rectal cancers on the basis of a case control study in Belgium15.
In the study by Davies et al., F344 rats were fed soybean with a high content of isoflavones and a diet supplemented with high fat and less calcium one week before and 31 weeks after AOM administration. The soybean group did not show a decrease in the occurrence of large intestinal tumors47. Min mice were fed a Western-type diet (high fat and less vegetable fibers and calcium) with a higher amount of isoflavones, but the study did not show a preventive effect on large intestinal cancer48. Rao et al.49 reported an increase in noninvasive tumor numbers and adenocarcinomas based on a study of AOM-treated rats fed casein-based feed with genistein. McIntosh et al.50 reported a larger increase in tumor number with soy protein than casein induced by DMH in rats. Gee et al.19 reported no suppression of ACFs in the large intestinal tumor by genistein using a DMH model. Hahhak et al.51 and Thiagarajan et al.52 observed a protective effect of soy and soy products against ACF induction. Pereira et al.53 also found that purified genistein inhibited induction of ACFs. Gotoh et al. demonstrated that administration of biochanin A, a genistein precursor, inhibited the development of mammary tumors54, but we reported that biochanin A did not reduce colonic ACF development in rats treated with AOM25. It is considered that isoflavones have a preventive effect on breast cancer related to hormones. The epidemiological relationship between large intestinal cancer and isoflavones remains to be clarified.
Previous study showed that long-term fermentation miso, such as for 180 days significantly decreased in the PCNA-positive index. It is hypothesized that calcium is a regulator of cell proliferation in the colon and that dietary intake of calcium inhibits experimental carcinogenesis55. Therefore, during fermentation of miso, a substance that suppresses cell proliferation is generated and may work as a factor in preventing colonic cancer.
Prevention of Lung Cancer
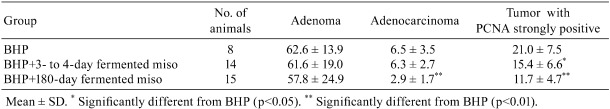
Among Japanese, the incidence of lung cancer has become the highest, exceeding that of stomach cancer. Being a serious problem, it is an urgent matter to develop preventive methods for lung cancer. Epidemiological studies in Okinawa56 and Singapore57 have indicated that soybean products might have inhibitory effects on lung cancer, and Shiraki et al.58, 59 investigated such effects by using short-term and long-term for fermented miso, such as miso 3 to 4 days, and 180 days, respectively. Six-week-old male Slc:Wister rats were treated with di-isopropanol-nitrosamine (BHP) through drinking water with a concentration of 200 mg/liter for 10 weeks. All rats were fed on a commercial MF diet (Oriental Yeast, Tokyo, Japan) alone or with added miso. Dry red miso after 3–4 days or 180 days of fermentation was supplemented into MF at a ratio of 10% by weight. The diet was supplied with normal tap water ad libitum for 12 weeks after treatment with BHP, and the rats were autopsied 22 weeks after treatment. Macroscopically, all animals had many nodules in the lung. The number of nodules in the 180-day fermented miso group was significantly decreased as compared with the other groups, but the tumor size was not significantly different among the groups. All rats had hyperplasia, adenomas and adenocarcinomas, and papillomas or squamous cell carcinomas were evident in 13%, 27% and 29% of the rats in the BHP, BHP+3- to 4-day fermentation and BHP+180-day fermentation groups but the incidence rate did not show a significant difference. The numbers of adenocarcinomas in the BHP+180-day fermentation group and PCNA strongly positive tumors in the miso groups were significantly decreased as compared with the group with BHP alone. Tumor size decreased significantly in the BHP+180-day fermented miso. It was obvious that 180-day fermented miso reduced the numbers of lung tumors (Table 4).
Table 4. Inhibition by 180-day Fermented Miso of Induction of Pulmonary Tumors by N-nitrosobis (2-hydroxypropyl) amine (BHP) in Wistar Rats58.
Koo et al.60 reported a significant inverse association between tofu/soy intake and lung cancer in nonsmokers in Hong Kong after adjustment for age, numbers of live births, and schooling. Swanson et al.61 reported a dose-dependent inverse relationship with tofu and risk of lung cancer in Yunnan Province, China. On the other hand, Ozawa et al.62 reported that among females, a high intake of miso soup significantly and almost dose-dependently increased the risk of lung cancer. However, consumption of miso soup seems to be associated with a Japanese-style diet and increased salt intake, but the significance of these findings is unclear.
Prevention of Breast Cancer
Yamamoto et al.63 reported that consuming a cup of miso three times a day reduced the occurrence of breast cancer but that tofu, natto, soybean and fried bean curd did not have such an effect and women in menopause had less breast cancer when a large amount of isoflavones was consumed.
Baggott el al. reported that when dimethylbenz[a]-anthracene (DMBA) was administrated to Sprague-Dawley (SD) rats, number of mammary tumors occurred, up to 4.1 per rat, but when the rats were fed a diet containing 25% miso, the number of tumors decreased to be 2.95 per rat, and the time until occurrence with miso was longer than that with only the carcinogen64. Gotoh et al.54, 65 investigated the induction of mammary tumors by administration of soybean, miso, and biochanin A in SD rats treated with 40 mg/kg of N-nitroso-N-methylurea (MNU). The time until incidence of a tumor was longer in the miso and biochanin groups than in the group treated with MNU alone. The number of tumors was 2.2 in the MNU group, but in the miso group, it decreased significantly to 1.2 and so did the numbers of tumors in 10% soybean and biochanin A groups. They also investigated the incidence of tumors with miso and tamoxifen, a medicine used for breast cancer treatment. The number of tumors per rat induced by MNU was only 4.5, but in the miso and tamoxifen groups, the numbers were 2.4 and 1.4, respectively, and the number decreased significantly to 0.2 when miso and tamoxifen were fed together. This investigation clearly indicated that administration of miso in the diet can reduce the occurrence of mammary tumors as well as tamoxifen at the beginning of carcinogen treatment and that it can also suppress the multiplicity of tumors induced by a carcinogen
Prevention of Hepatic Tumor
When C3H male mice were bred for 1 year under normal conditions, 89% of the mice had naturally occurring liver cancer. When B6C3F1 mice were irradiated with 252Cf neutrons, which are the same neutrons as in an atomic bomb of the Hiroshima type, at 2 Gy 13 month later, 62% of the male mice and 29% of female had liver cancer. However, these mice were fed diets containing 10% miso (Miso Central Institute, Tokyo, Japan), and the frequencies of cancer for C3H mice, male B6C3F1 mice and female B6C3F1 mice decreased to 32%, 13%, 13% respectively66.
Ogundigie et al. demonstrated the same effect of miso. A strong carcinogen for hepatocytes, such as diethylnitrosamine (DEN), was injected in 15-day-old B6C3F1 mice, and when they were 4 weeks old, the mice were irradiated with 2Gy of 252Cf neutrons; when they became 40 weeks old, the mice were autopsied for investigation. Various kinds of preventive materials including miso were applied to investigate the occurrence of hepatic tumors. All mice had hepatomas, and the control group without any preventive material had 46 tumors/mouse. But with miso, the number of tumors decreased significantly to 32.5/mouse. When 10 ppm and 20 ppm of biochanin A were applied, the number of tumors in the 10 ppm group was 40.1/mouse, but the number in the 20 ppm group decreased significantly to 32.5/mouse67. Obviously, both results indicated that administration of miso inhibited the development of hepatomas occurring naturally or by administration of 252Cf neutrons or a carcinogen in experimental animals.
Kurosawa et al.68 reported that consumption of miso soup among women without a history of liver diseases showed a significant inverse association with hepatocellular carcinoma. Also Sharp et al.69 suggested that consumption of soy foods, especially miso soup, is associated with reduced risk of hepatocellular carcinoma among a-bomb survivors.
Prevention of Gastric Tumors
Hirayama reported that consumption of miso soup on a daily basis might reduce the risk of stomach cancer70. But there is a report indicating that since miso contains 10-12% salt, it would be expected that the NaCl would greatly increase the likelihood of development of stomach cancer71. This chapter concerns experimental data indicating that consumption of salt through miso is different from consumption of salt itself.
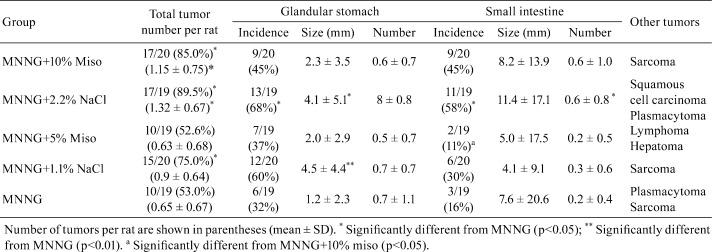
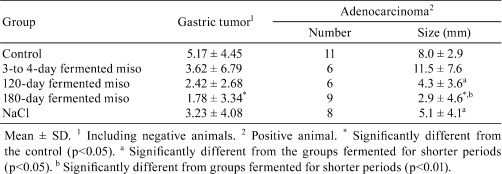
Male CD:Crj rats were treated N-methyl-N’-nitro-N-nitrosoguanidine (MNNG) in drinking water for four months and simultaneously maintained on diet supplemented with 10% dry red miso, 5% red dry miso, 2.2% NaCl (equivalent amount to 10% dry miso) or 1.1% NaCl (equivalent to 5% miso) or a control MF feed. As a control, instead of MNNG, tap water was given72, 73. Generally, the incidence of gastric tumors in the NaCl groups was higher than that of the miso groups. In particular, the incidence of gastric tumors with MNNG+2.2% NaCl and the sizes of gastric tumors with MNNG+2.2% NaCl and MNNG+1.1% NaCl and of adenocarcinomas with MNNG+1.1% NaCl were significantly increased as compared with MNNG+MF (Table 5).
Table 5. Incidences of Gastric Tumor in CD(SD) Rats Treated with N-methyl-N’-nitro-N-nitorosoguanidine (MNNG) and Miso or NaCl72.
Furthermore, when a similar experiment was conducted with 50% salt-reduced miso, the incidence of gastric tumors was the same as with regular miso containing 2% NaCl74. Asahara et al.75 and Rajendran et al.76 reported that according to the Umu test, which is used as an indicator of mutagenicity, the strains of yeast, lactic acid bacteria and other molds in miso can remove or detoxicate carcinogens such as trp-p-2, a pyrolysis product of foods. Yanagihara et al.77 reported that by using digestive tract cells, a group of isoflavone-like substances in miso, such as biochanin A and genistein, prevented the growth of gastric tumor cells by stimulating death of cells by apoptosis.
To further confirm the importance of the fermentation process, a study was conducted to examine the effects of miso at various fermentation stages on MNNG induction of rat glandular stomach neoplasia. Male CD:Crj(SD) rats were maintained on MF diet supplemented with 10% each of dried miso prepared with three different fermentation stages, 3 to 4 days, 120 days and 180 days, or 1.1% NaCl (Wako, special grade) was as a control during administration of the carcinogen78 (Table 6). There was no significant difference among groups in the total number of tumors, number of tumors per rat or incidence of gastric tumors, duodenum tumors and other tumors. Tumor size of in the 180-day fermented miso group was decreased significantly compared with controls (p<0.01). Tumor size in the 3-to 4-day fermented miso group was significantly greater than in the 120-day fermented miso (p<0.05) and 180-day fermented miso groups (p<0.01). Thus there is a high possibility that during the fermentation period of miso, substances that inhibit growth of tumors might be produced.
Table 6. Reduction by 180-day Fermented Miso of Gastric Tumor Number and Size in CD Rats Treated with MNNG78.
During fermentation of miso, two reactions occur: one is enzymatic by koji (rice starter with Aspergillus oryzae), and the other is through fermentation by microorganisms such as yeasts and lactic acid bacteria. By enzymatic reactions, soybean and rice undergo hydrolysis of proteins, saccharification of starches and decomposition of oils, generating low molecular peptides, amino acids, reduced sugars, fatty acids and glycerol. Some proportion of these nutrients is consumed by salt-tolerant microorganisms, and as by-products of fermentation, alcohol, organic acids and esters are synthesized, which are flavor components of miso. In addition, Maillard materials are produced by a nonenzymatic reaction, contributing to the color of miso2. Fujinami et al. reported that the contents of glutamine and formol nitrogen increase during miso fermentation79, and Higashi et al. described production of amylase and acid protease80. However, analysis of these compounds remains for future investigation.
Protection Against Hypertension
It was often considered that excess intake of miso led to lifestyle-related disease even the salt in miso increased the blood pressure81,82,83,84,85,86,87. As described before, unlike the cases of salt alone, the salt in miso reduced stomach cancer72, and miso did not cause an increase in gastric tumors. So the effects of salt in miso on blood pressure were studied88.
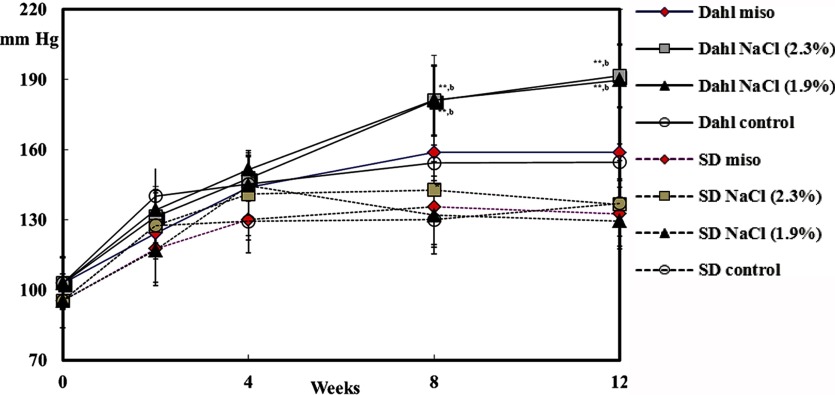
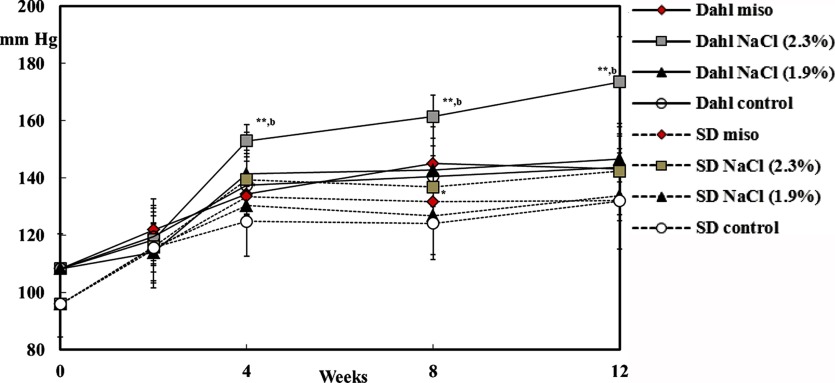
Six-week-old salt-sensitive Dahl rats, similar to SD male or female rats (original strain of salt-sensitive Dahl rat), were maintained on a commercial diet of MF alone, which contains 0.3% salt, MF supplemented with 10% dry red miso (180-day fermented miso), MF supplemented with 2.3% salt, because 10% dry miso contains 2.3% NaCl, or MF supplemented with 1.9% salt for 12 weeks. The systolic blood pressure in the Dahl male rat being administrated 2.3% NaCl was significantly increased for 8 and 12 weeks but not in the SD rat (Fig. 2). However, the blood pressure in the rats fed a diet with miso or commercial control diet (MF) was not increased. Even though miso contains 2.3% NaCl, the blood pressure was as stable as in the rats fed a commercial diet containing 0.3% salt. The systolic blood pressure of the Dahl female rat was increased by the salt diet but not by the miso diet (Fig. 3). The blood pressure of the female rat was not increased by the 1.9% salt diet but that of the male was increased. The male rat was more sensitive to salt than the female, as in humans. As a result, the blood pressure of the salt-sensitive rat was not increased by miso diet containing 2.3% NaCl, which indicates that miso diet does not to increase blood pressure in salt-sensitive people. Recently, Yoshinaga et al. showed that drinking miso soup for a long term attenuated salt-induced hypertension in Dahl salt-sensitive rats given salt equivalent to the content of miso soup, and the results were associated with an attenuation of kidney damage89. Salt in miso works in a different manner from NaCl alone on its own in the case of gastric tumors and blood pressure. The exact mechanisms for the antihypertensive action remain to be elucidated. Current hypertension guidelines recommend that salt intake should be lower than 6 g/day. Considering the results concerning of blood pressure between salt in miso and salt along, it is better to refrain from excessive intake of salt, but it may be recommended to consume miso soup to prevent lifestyle-related diseases including hypertension.
Fig. 2.
Systolic blood pressure in male rats88. **Significantly different from same strain control (p<0.01) . b Significantly different from same strain miso (p<0.01).
Fig. 3.
Systolic blood pressure in female rats88. *Significantly different from same strain control (p<0.05). **Significantly different from same strain control (p<0.01). b Significantly different from same strain miso (p<0.01).
Regarding epidemiological data, He et al. demonstrated that dietary soybean protein reduced blood pressure in a randomized, double-blind, controlled trial in Chinese adults85, 86. Several small clinical trials have reported inconsistent findings regarding the effect of soybean protein on blood pressure87, 90, 91. Washburn and colleagues91 compared the effect of 20 g of soybean protein containing 34 mg of phytoestrogens given in either 1 dose or 2 doses on that of 20 g of complex carbohydrates on cardiovascular disease risk factors and menopausal symptoms among 51 women in a randomized controlled trial. They observed a significant reduction in diastolic blood pressure in the twice-daily soybean protein diet compared with the carbohydrate control diet. Burke and colleagues90 examined the effects of soybean protein on 24-hour ambulatory blood pressure among 41 treated hypertensive patients in a randomized controlled trial. He et al. also documented that the decrease in blood pressure reduction associated with soybean dietary protein was greater than that of the carbohydrates control86. Conventionally, higher intake of complex carbohydrate has been recommended as a replacement for saturated fat to reduce cardiovascular risk92. A soy-based diet attenuated the development of hypertension both in female and male SHR rats93. These findings suggested that higher intake of soybean protein may play an important role in prevention and treatment of hypertension.
Nakamura et al.94 conducted a double-blind, randomized placebo-controlled study to evaluate the effects on blood pressure among Japanese of a 6-week diet containing approximately 25% to 20% low-sodium soy sauce and miso (an approximately 10% decrease in total dietary salt intake). Significant changes in blood pressure were not observed in the experiment. However, it was noted that a 6.4 mm Hg net decrease in diastolic blood pressure resulted in no significant change in systolic blood pressure for those aged 40 and older in the low-sodium intake group.
They considered that a 6-week trial of low-sodium soy sauce and miso resulted in a reduction in urinary salt of 0.7 g/day in the intervention group, and the net difference in urinary salt excretion between the intervention group and the controls was 1.4 g/day. For example, when 13 g dietary salt is consumed on a daily basis, the estimated salt intake from soy sauce will change from 3.3 g to 2.4 g and that from miso will change from 1.6 to 1.3 if they are replaced with low-sodium types, and thus one can achieve a 1.2 g reduction in daily dietary salt intake. They concluded that an approximately 10% reduction in dietary salt intake affected diastolic blood pressure in middle aged or older people and possibly had beneficial effects on blood pressure in those with hypertension. Dietary reduction of NaCl in soy sauce may be used to reduce diastolic blood pressure rather than miso.
On the other hand, Kanda et al. performed a study on the association of lifestyle parameters with the future risk of hypertension in normotensive subjects. They administered a baseline questionnaire and performed a four-year follow-up of 445 normotensive Japanese between the ages of 35 and 89 years. Among the 60- to 69-year-old subjects, changes in blood pressure during the four years were negatively correlated with boiled rice intake in men and with Japanese tea intake in women95. Multiple logistic regression analysis revealed that two or more bowls of miso soup per day was protective against hypertension during the follow-up (p<0.05). These results indicate that the nature and amount of food intake is important in the prevention of hypertension in the elderly. It also prevents the increase in blood pressure in men and animals when compared with rats fed on the equivalent of NaCl concentration without miso.
For protection from hypertension, Fujita reported96 that taking miso soup together with protein and potassium-rich vegetable as spinach, “wakame”, or seaweed, calcium rich food such as dried sardine and dried bonito; or magnesium such as seaweeds and soybean, prevented increases in blood pressure even in salt-sensitive individual. Anderson et al.97 reported dietary sources of sodium in China, Japan, the United Kingdom, and the United States from the INTERMAP study and that the sodium intakes per day (systolic blood pressure mm) in these countries were 3,990 mg (121.3), 4,651 mg (117.2), 3,406 mg (120.4) and 3,660 mg (118.6), respectively. This data showed that sodium intake was the highest in Japanese but that the Japanese also had the lowest blood pressure. It seems that sodium intake was higher but that blood pressure was low individuals lived longer. Alderman98 described that Yamamono Indians have a very low sodium intake and rather short life expectancy, while the Japanese, with much higher sodium intakes, have the longest life expectancy among all national groups. Kagan et al.99 reported that no relation was found between salt intake and the incidence of stroke in Hawaiian Japanese men. In general, excessive sodium intake is a key factor in the epidemic of perhypertension/hypertension and is linked to increased risk of cardiovascular diseases. Kokubo et al.100 found a significant inverse association between soy and isoflavone intake with the risk of incidence for cerebral and myocardial infarction in women. Recently, we observed that the incidence of and life span after a brain stroke in an NaCl group of SHRSP rats was significantly increased as compared with a group fed miso containing the same sodium content101. So we considered that sodium in miso might behave differently compare with NaCl alone.
Conclusion
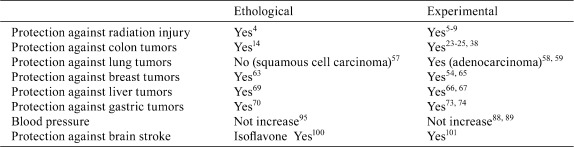
The most commonly occurring cancers in Japan used to be in the stomach. However, with recent change in dietary habits from a traditional Japanese to Western style, cancers of the large intestine, breast and prostate are increasing, while stomach cancer is becoming less common. This indicates that the importance of diet and traditional food such as miso soup should be reevaluated not only in Japan but across the world. Since miso has multiple functions, as discussed in previous sections, with preventive effects that help to maintain health (Table 7), it deserves further studies to elucidate the mechanisms of action of materials produced during fermentation. Analysis of effective biological compounds in miso promises to be a fruitful avenue for future investigation.
Table 7. Summary of Biological Functions of Miso.
Acknowledgments
This work was supported in part by a Grant-in-Aid from the Central Miso Institute. The author thanks Dr M.A. Moore and Ms Yuri Miyasaka for the critical review of this manuscript.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (by-nc-nd) License <http://creativecommons.org/licenses/by-nc-nd/3.0/>.
References
- 1.Miso Health Promotion Committee www:miso.or.jp/miso-e/index/html
- 2.Shurtleff W, and Aoyagi A. The Book of Miso. Autumn Press. 1976 [Google Scholar]
- 3.Ito A, Gotoh T, and Fujimoto T. Chemoprevention of cancer by miso and isoflavones. J Toxicol Pathol. 11: 79–84 1998. [Google Scholar]
- 4.Akizuki T. Health Condition and Diet. Kurie press, 1980. (in Japanese). [Google Scholar]
- 5.Watanabe H, Takahashi T, Ishimoto T, and Ito A. The Effect of miso diet on small intestinal damage in mice irradiated by X-ray. Miso Tech Sci. 39: 29–32 1991; (in Japanese). [Google Scholar]
- 6.Watanabe H, and Ito A. Radioprotective effects of miso in digestive tract and preventive effect of miso on tumors. Daizu Geppou. November, 11-17. 1996. (in Japanese).
- 7.Ohara M, Lu H, Shiraki K, Ishimura Y, Uesaka T, Katoh O, and Watanabe H. Radioprotective effects of miso (fermented soy bean paste) against radiation in B6C3F1 mice: increased small intestinal crypt survival, crypt lengths and prolongation of average time to death. Hiroshima J Med Sci. 50: 83–86 2001. [PubMed] [Google Scholar]
- 8.Ohara M, Lu H, Hamda H, Nishioka T, Kageyama N, Ishimura Y, Shiraki K, Uesaka T, Katoh O, Watanabe H, and Kawano K. Radioprotective effects of long-term fermented miso against irradiation in B6C3F1 mice. Miso Tech Sci. 50: 21–27 2002; (in Japanese). [Google Scholar]
- 9.Kawano K, Matsuda S, Kashiwabara S, Kashimoto N, Kageyama N, Hashimoto K, Nishioka T, Uesaka T, Katoh O, and Watanabe H. Effect of each fractions extracted from miso on the protection against radiation damage and aberrant crypt foci. Miso Tech Sci. 51: 429–434 2003; (in Japanese). [Google Scholar]
- 10.Houchen CW, George RJ, Strumoski MA, and Cohen SM. FGF-2 enhances intestinal stem cell survival and its expression is induced after radiation injury. Amer J Physiol. 276: G249–G258 1999. [DOI] [PubMed] [Google Scholar]
- 11.Takahama Y, Ochiya T, Tanooka H, Yamamoto H, Sakamoto H, Nakano H, and Terada M. Adenovirus-mediated transfer of HST-1/FGF-4 gene protects mice from lethal irradiation. Oncogene. 18: 5943–5947 1999. [DOI] [PubMed] [Google Scholar]
- 12.Farrell CL, Bready JV, Rex KL, Chen JN, DiPalma CR, Whitcomn KL, Yin S, Hill DC, Wiemann B, Starnes CO, Havill AM, Lu Z-N, Aukerman SL, Aukerman L, Pierce GF, Thomason A, Potten CS, Ulich TR, and Lacey DL. Keratinocyte growth factor protects mice from chemotherapy and radiation-induced gastrointestinal injury and mortality. Cancer Res. 58: 933–939 1998. [PubMed] [Google Scholar]
- 13.Watanabe H, Shiono T, Kawamura D, Kawano K, Kajimura J, Kashimoto N, and Kamiya K. Radioprotective effects of melanoidine against irradiation in B6C3F1 mice. J Nagasaki Med Ass. 81: 296–298 2006. (in Japanese). [Google Scholar]
- 14.Tajima K, Hirose K, Nakagawa N, Kuriishi T, and Tominaga S. Urban-rural difference in the trend of colorectal cancer mortality with special reference to the subsites of colon cancer in Japan. Jpn J Cancer Res. 76: 717–728 1985. [PubMed] [Google Scholar]
- 15.Tuyns AJ, Kaaks R, and Haelterman M. Colorectal cancer and the consumption of foods: a case-control study in Belgium. Nutr Cancer. 11: 189–204 1988. [DOI] [PubMed] [Google Scholar]
- 16.Barnes DS, Clapp NK, Scott DA, Oberst DL, and Berry SG. Effects of wheat, rice corn, and soybean bran on 1,2-dimethylhydrazine-induced large bowel tumorigenesis in F344 rats. Nutr Cancer. 5: 1–9 1983. [DOI] [PubMed] [Google Scholar]
- 17.Messine M, and Bennink M. Soyfoods, isoflavones and risk of colon cancer: a review of the in vitro and in vivo data. Ballieres Clin Encocrinol Metab. 12: 707–728 1998. [DOI] [PubMed] [Google Scholar]
- 18.Davies MJ, Bowey EA, Adlercreutz H, Rowland IR, and Rumsby PC. Effects of soy or rye supplementation of high-fat diets on colon tumour development in azoxymethane-treated rats. Carcinogenesis. 20: 927–931 1999. [DOI] [PubMed] [Google Scholar]
- 19.Gee JM, Noteborn HPJM, Polley ACJ, and Johnson IT. Increased induction of abbeant crypt foci by 1,2-dimethlhyrdazine in rats fed diets containing purified genistein or genistein-rich soya protein. Carcinogenesis. 21: 2255–2259 2000. [DOI] [PubMed] [Google Scholar]
- 20.Koratkar R, and Rao AV. Effect of soya bean saponines on azoxymethane-induced preneoplastic lesions in the colon of mice. Nutr Cancer. 27: 206–209 1997. [DOI] [PubMed] [Google Scholar]
- 21.Azuma N, Suda H, Iwasaki H, Kanamori R, and Iwami K. Soy bean crude refuse alleviates experimental tumorigenesis in rat colon. Biosci Biotechnol Biochem. 63: 2256–2258 1999. [DOI] [PubMed] [Google Scholar]
- 22.Azuma N, Machida K, Saeki T, Kanamoto R, and Iwami K. Prevention effect of soybean resistant proteins against experimental tumorigenesis in rat colon. J Nutr Sci Vitaminol. 46: 23–29 2000. [DOI] [PubMed] [Google Scholar]
- 23.Masaoka Y, Watanabe H, Katoh O, and Dohi K. Effects of miso and NaCl on the development of colonic aberrant crypt foci induced by azoxymethane in F344 rats. Nutr Cancer. 32: 25–28 1998. [DOI] [PubMed] [Google Scholar]
- 24.Masaoka Y, Katoh O, and Watanabe H. Inhibitory effects of crude salts on the induction and development of colonic aberrant crypt foci in F344 rats given azoxymethane. Nur Cancer. 37: 78–81 2000. [DOI] [PubMed] [Google Scholar]
- 25.Masaoka Y, Hamada H, Nishioka T, Lu H, Ohara M, Ishimura Y, Shiraki K, Katoh O, and Watanabe H. Inhibition effects of aberrant crypt foci (ACF) induced by azoxymethane (AOM) on miso and the related substances in F344 rats. Miso Tech Sci. 48: 393–398 2000. (in Japanese). [Google Scholar]
- 26.Wargovich MJ, Lynch PM, and Leven B. Modulating effects of calcium in animal models of colon carcinogenesis and short-term studies in subjects at increased risk for colon cancer. Am J Clin Nutr. 54: 202S–205S 1991. [DOI] [PubMed] [Google Scholar]
- 27.Newmark HL, and Lipkin M. Calcium, vitamin D and colon cancer. Cancer Res. 52: 2067s–2070s 1992. [PubMed] [Google Scholar]
- 28.Viñas-Salas J, Biendicho-Felis C, Miguelsanz-Garcia S, and Perez-Holanda S. Calcium inhibits colon carcinogenesis in an experimental model in the rat. Eur J Cancer. 34: 1941–1945 1998. [DOI] [PubMed] [Google Scholar]
- 29.Baron JA, Beach M, Mandel JS, van Stolk RU, Haile RW, Sandler RS, Rothstein R, Summers RW, Snover DC, Beck GJ, Bond JH, and Greenberg ER. Calcium supplements for the prevention of colorectal adenomas. Calcium Polyp Prevention Study Group. N Engl J Med. 340: 101–107 1999. [DOI] [PubMed] [Google Scholar]
- 30.Marcus PM, and Newcomb PA. The association of calcium and vitamin D, and colon and rectal cancer in Wisconsin women. Int J Epidemiol. 27: 788–793 1998. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka T, Shinoda T, Yoshimi N, Niwa K, Iwata H, and Mori H. Inhibitory effect of magnesium hydroxide on methylazoxymethanol acetate-induced large bowel carcinogenesis in male F344 rats. Carcinogenesis. 10: 613–616 1989. [DOI] [PubMed] [Google Scholar]
- 32.Mori H, Tanaka T, Sugie S, Yoshimi N, Kawamori T, Hirose Y, and Ohnishi M. Chemoprevention by naturally occurring and synthetic agents in oral, liver, and large bowel carcinogenesis. J Cell Biochem. 27(Suppl): 35–41 1997. [PubMed] [Google Scholar]
- 33.Li H, Kramer PM, Lubet RA, Steele VE, Kelloff GJ, and Pereira MA. Effect of calcium on azoxymethane-induced aberrant crypt foci and cell proliferation in the colon of rats. Cancer Lett. 124: 39–46 1998. [DOI] [PubMed] [Google Scholar]
- 34.Feng Y, Finley JW, Davis CD, Becker WK, Fretland AJ, and Hein DW. Dietary selenium reduces the formation of aberrant crypts in rats administered 3,2’-dimethyl-4-aminobiphenyl. Toxicol Appl Pharmacol. 157: 36–42 1999. [DOI] [PubMed] [Google Scholar]
- 35.Davis CD, and Feng Y. Dietary copper, manganese and iron affect the formation of aberrant crypts in colon of rats administered 3,2’-dimethyl-4-aminobiphenyl. J Nutr. 129: 1060–1067 1999. [DOI] [PubMed] [Google Scholar]
- 36.Ohara M, Lu H, Shiraki K, Ishimura Y, Uesaka T, Katoh O, and Watanabe H. Prevention by long-term fermented miso of induction of colonic aberrant crypt foci by azoxymethane in F344 rats. Oncol Rep. 9: 69–73 2002. [PubMed] [Google Scholar]
- 37.Ohuchi Y, Myojin Y, Shimamoto F, Kashimoto N, Kamiya K, and Watanabe H. Decrease in size of azoxymethane induced colon carcinoma in F344 rats by 180-day fermented miso. Oncol Rep. 14: 1559–1564 2005. [PubMed] [Google Scholar]
- 38.Ohuchi Y, Myoujin Y, Kashimoto N, Kajimura J, Kamiya K, and Watanabe H. Prevention by 180-day fermented miso of colon carcinoma induced by azoxymethane in F344 rat. Miso Tech Sci. 54: 139–148 2006. (in Japanese). [Google Scholar]
- 39.Ishimoto T, Watanabe T, Okamoto T, Matsuda M, and Ito A. Effect of miso diet on large intestinal tumorigenesis in mice. Miso Tech Sci. 40: 447–451 1992. . [Google Scholar]
- 40.Nishi M, Yoshida K, Hirata K, and Miyake H. Eating habits and colorectal cancer. Oncol Rep. 4: 995-998. 1997. (in Japanese). [DOI] [PubMed]
- 41.Hoshiyama Y, Sekine T, and Sasabe T. A case-control study of colorectal cancer and its relation to diet, cigarettes, and alcohol consumption in Saitama Prefecture, Japan. Tohoku J Exp Med. 171: 153–165 1993. [DOI] [PubMed] [Google Scholar]
- 42.Kono S, Shinchi K, Ikeda N, Yanai F, and Imanishi K. Physical activity, dietary habits and adenomatous polyps of the sigmoid colon: a study of self-defense officials in Japan. J Clin Epidemiol. 44: 1255–1261 1991. [DOI] [PubMed] [Google Scholar]
- 43.Watanabe Y, Tada M, Kawamoto K, Uozumi N, Kajiwara Y, Hayashi K, Yamaguchi K, Murakami K, Misaki F, Akasaka T, and Kawai K. A case control study of cancer of the rectum and colon. Nihon Shokakibyo Gakkai zasshi. 81: 185–193 1984. (in Japanese). [PubMed] [Google Scholar]
- 44.Poole C. A case-control study of diet and colon cancer. Harvard School of Public Health, Boston MA. 1989 [Google Scholar]
- 45.Hu JF, Liu YY, Yu YK, Zhao TZ, Liu SD, and Wang QQ. Diet and cancer of the colon and rectum: a case-control study in China. Int J Epidemiol. 20: 362–367 1991. [DOI] [PubMed] [Google Scholar]
- 46.Le Marchand L, Hankin JH, Wilkens LR, Kolonel LN, Englyst HN, and Lyu LC. Dietary fiber and colorectal cancer risk. Epidemiology. 8: 658–665 1997. [DOI] [PubMed] [Google Scholar]
- 47.Davies MJ, Bowey EA, Adlercreutz H, Rowland IR, and Rumsby PC. Effects of soy or rye supplementation of high-fat diets on colon tumor development in azoxymethane-treated rats. Carcinogenesis. 20: 927–931 1999. [DOI] [PubMed] [Google Scholar]
- 48.Sørensen IK, Kristiansen E, Mortensen A, Nicolaisen GM, Wijnands JA, van Kranen HJ, and van Kreijl CF. The effect of soy isoflavones on the development of intestinal neoplasia in ApcMin mouse. Cancer Lett. 130: 217–225 1998. [DOI] [PubMed] [Google Scholar]
- 49.Rao CV, Wang CX, Simi B, Lubet R, Kelloff G, Steele V, and Reddy BS. Enhancement of experimental colon cancer by genistein. Cancer Res. 57: 3717–3722 1997. [PubMed] [Google Scholar]
- 50.McIntosh GH, Regester GO, Le Leu RK, Royle PJ, and Smithers GW. Dairy proteins protect against dimethylhydrazine-induced intestinal cancers in rats. J Nutr. 125: 809–816 1995. [DOI] [PubMed] [Google Scholar]
- 51.Hakkak R, Korourian S, Ronis MJ, Johnston JM, and Badger TM. Soy protein isolate consumption protects against azoxymethane-induced colon tumors in male rats. Cancer Lett. 166: 27–32 2001. [DOI] [PubMed] [Google Scholar]
- 52.Thiagarajan DG, Bennink MR, Bourquin LD, and Kavas FA. Prevention of precancerous colonic lesions in rats by soy flakes, soy flour, genistein, and calcium. Am J Clin Nutr. 68(Suppl): 1394S–1399S 1998. [DOI] [PubMed] [Google Scholar]
- 53.Pereira MA, Barnes LH, Rassman VL, Kelloff GV, and Steele VE. Use of azoxymethane-induced foci of aberrant crypts in rat colon to identify potential cancer chemopreventive agents. Carcinogenesis. 15: 1049–1054 1994. [DOI] [PubMed] [Google Scholar]
- 54.Gotoh T, Yamada K, Yin H, Ito A, Kataoka T, and Dohi K. Chemoprevention of N-nitroso-N-methylurea-induced rat mammary carcinogenesis by soy foods or biochanin A. Jpn J Cancer Res. 89: 137–142 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Appleton GVN, Davies PW, Bristol JB, and Williamson RCN. Inhibition of intestinal carcinogenesis by dietary supplementation with calcium. Br J Surg. 74: 523–525 1987. [DOI] [PubMed] [Google Scholar]
- 56.Wakai K, Ohno Y, Genka K, Ohmine K, Kawamura T, Tamakoshi A, Lin Y, Nakayama T, Aoki K, and Fukuma S. Risk modification in lung cancer by a dietary intake of preserved foods and soyfoods: findings from a case-control study in Okinawa, Japan. Lung Cancer. 25: 147–159 1999. [DOI] [PubMed] [Google Scholar]
- 57.Seow A, Poh WT, Teh M, Eng P, Wang YT, Tan WC, Chia KS, Yu MC, and Lee HP. Diet, reproductive factors and lung cancer risk among Chinese women in Singapore: evidence for a protective effect of soy in nonsmokers. Int J Cancer. 97: 365–371 2002. [DOI] [PubMed] [Google Scholar]
- 58.Shiraki K, Une K, Yano R, Otani A, Mineoka A, and Watanabe H. Inhibition by long-term fermented miso of induction of pulmonary adenocarcinoma by diisopropanolnitrosamine in Wistar rats. Hiroshima J Med Sci. 52: 9–13 2003. [PubMed] [Google Scholar]
- 59.Shiraki K, Kashiwabara S, Kashimoto N, Une K, Yano R, Otani S, Mineoka A, Hashimoto K, Kageyama N, Nishioka T, Kominami Y, Sasaki A, Shiraishi M, Uesaka T, Katoh O, and Watanabe H. Inhibition by long-term fermented miso of induction of pulmonary adenocarcinoma by diisopropanolnitrosamine (BHP) in wister rats. Miso Tech Sci. 51: 391–397 2003. (in Japanese). [PubMed] [Google Scholar]
- 60.Koo LC, Ho JH, Matsuki H, Shimizu H, Mori T, and Tominaga S. A comparison of the prevalence of respiratory illnesses among nonsmoking mothers and their children in Japan and Hong Kong. Am Rev Respir Dis. 138: 290–295 1988. [DOI] [PubMed] [Google Scholar]
- 61.Swanson CA, Mao BL, Li JY, Lubin JH, Yao SX, Wang JZ, Cai SK, Hou Y, Luo QS, and Blot WJ. Dietary determinants of lung-cancer risk: results from a case-control study in Yunnan Province, China. Int J Cancer. 50: 876–880 1992. [DOI] [PubMed] [Google Scholar]
- 62.Ozasa K, Watanabe Y, Ito Y, Suzuki K, Tamakoshi A, Seki N, Takaaki T, Wakai K, Ando M, Ohno Y, and for the JACC Study group. Dietary habits and risk of lung cancer death in a large-scale cohort study (JACC Study) in Japan by sex and smorking habit. Jpn J Cancer Res. 92: 1259–1269 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamamoto S, Sobue T, Kobayashi M, Sasaki S, and Tsugane S. Soy, isoflavones, and breast cancer risk in Japan. J Natl Cancer Inst. 95: 906–913 2003. [DOI] [PubMed] [Google Scholar]
- 64.Baggott JE, Ha T, Vaughn WH, Juliana M, Hardin JM, and Grubbs CJ. Effect of miso (Japanese Soybean paste) and NaCl on DMBA-induced rat mammary tumors. Nutr Cancer. 14: 103–109 1990. [DOI] [PubMed] [Google Scholar]
- 65.Gotoh T, Yamada K, Ito A, Yin H, Kataoka T, and Dohi K. Chemoprevention of N-nitroso-N-methylurea rat mammary cancer by miso and tamoxifen, alone and in combination. Jpn J Cancer Res. 89: 487–495 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ito A, Watanabe H, and Basaran N. Effects of soy products in reducing risk of spontaneous and neutron-induced liver tumors in mice. Int J Oncol. 2: 773–776 1993. [DOI] [PubMed] [Google Scholar]
- 67.Ogundigie P, Roy G, Kanin G, Goto T, and Ito A. Effect of biochanin A or teststerone on liver tumors induced by a combined treatment of DEN and fission neutron in BCF1 mice. Oncol Rep. 2: 271–275 1995. [DOI] [PubMed] [Google Scholar]
- 68.Kurozawa Y, Ogimoto I, Shibata A, Nose T, Yoshimura T, Suzuki H, Sakata R, Fujita Y, Ichikawa S, Iwai N, Fukuda K, and Tamakoshi A. Dietary habits and risk of death due to hepatocellular carcinoma in a large scale cohort study in Japan. Univariate analysis of JACC study data. Kurume Med J. 51: 141–149 2004. [DOI] [PubMed] [Google Scholar]
- 69.Sharp GB, Lagarde F, Mizuno T, Sauvaget C, Fukuhara T, Allen N, Suzuki G, and Tokuoka S. Relationship of hepatocellular carcinoma to soya food consumption: A cohort-based case-control study in Japan. Int J Cancer. 115: 290–295 2005. [DOI] [PubMed] [Google Scholar]
- 70.Hirayama T. Relationship of soybean paste soup intake to gastric cancer risk. Nutr Cancer. 3: 223–233 1982. [DOI] [PubMed] [Google Scholar]
- 71.Tsugane S, Sasazuki S, Kobayashi M, and Sasaki S. JPHC Study Group Salt and salted food intake and subsequent risk of gastric cancer among middle-aged Japanese men and women. Br J Cancer. 90: 128–134 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Watanabe H, Uesaka T, Kido S, Ishimura Y, Shiraki K, Kuramoto K, Hirata S, Shoji S, Katoh O, and Fujimoto N. Influence of concomitant Miso or NaCl treatment on induction of gastric tumors by N-methyl-N’-nitro-N-nitorosoguanidine in rats. Oncol Rep. 6: 989–993 1999. [DOI] [PubMed] [Google Scholar]
- 73.Watanabe H, Ishimoto T, Okamoto T, Takahashi T, and Ito A. Effects of miso diet or sodium chloride diet on gastric tumorigenesis in rats. Miso Tech Sci. 40: 324–329 1992. (in Japanese). [Google Scholar]
- 74.Watanabe H, Hamada H, Nishioka T, Uesaka T, Huimei L, Ohara M, Shoji S, and Katoh O. Reduction of gastric tumors by administration with reduced salt miso diet during N-methyl-N’-nitro-N-nitorosoguanidine (MNNG) treatment in rats. Miso Tech Sci. 48: 22–26 2000. (in Japanese). [Google Scholar]
- 75.Asahara N, Zhang XB, and Ohta Y. Anti-mutagenic and mutagen-binding activation of mutagenic pyrolyzate by microorganisms isolated from Japanese miso. J Sci Food Agric. 58: 395–401 1992. [Google Scholar]
- 76.Rajendran R, and Ohta Y. Binding of heterocyclic amines by lactic acid bacteria from miso, a fermented Japanese food. Can J Microbiol. 44: 109–115 1998. [DOI] [PubMed] [Google Scholar]
- 77.Yanagihara K, Ito A, Tuge T, and Numoto M. Antiproliferation effects of isoflavones on human cancer cell lines established from the gastrointestinal tract. Cancer Res. 53: 5815–5821 1993. [PubMed] [Google Scholar]
- 78.Ohara M, Lu H, Shiraki K, Ishimura Y, Uesaka T, Katoh O, and Watanabe H. Inhibition by long-term fermented miso of induction of gastric tumor by N-methyl-N’-nitro-N-nitrosoguanidine in CD (SD) rats. Oncol Rep. 9: 613–616 2002. [PubMed] [Google Scholar]
- 79. Fujinami H, Mochizuki T, Sagawa I, and Mori M. Change of glutamic acid, glutamine and pyroglutamic acid during fermentation of miso at different temperature on the ripening of miso (I). J Brew Soc Jpn. 78: 466–474 1983. (in Japanese). [Google Scholar]
- 80.Higashi K. Fermentation and Brewing (1), 2002. Korin Press (in Japanese). [Google Scholar]
- 81.Yamagishi K, Iso H, Tanigawa T, Cui R, Kudo M, and Shimamoto T. High sodium intake strengthens the association between angiotensinogen T174M polymorphism and blood pressure levels among lean men and women: a community-based study. Hypertens Res. 27: 53–60 2004. [DOI] [PubMed] [Google Scholar]
- 82.Tamaki J, Kikuchi Y, Yoshita K, Takebayashi T, Chiba N, Tanaka T, Okamura T, Kasagi F, Minai J, and Ueshima H. HIPOP-OHP Research Group Stage of changes for salt intake and urinary salt excretion: Baseline results from the high-risk and population (HIPOP-OHP) study. Hypertens Res. 27: 157–166 2004. [DOI] [PubMed] [Google Scholar]
- 83.Tochikubo O, and Nishijima K. Sodium intake and cardiac sympatho-vagal balance in young men with high blood pressure. Hypertens Res. 27: 393–398 2004. [DOI] [PubMed] [Google Scholar]
- 84.He FJ, and MacGregor GA. Blood pressure—importance of salt intake. Am J Hypertens. 18: 1258–1259 2005. [DOI] [PubMed] [Google Scholar]
- 85.He FJ, Markandu ND, and MacGregor GA. Modest salt reduction lowers blood pressure in isolated systolic hypertension and combined hypertension. Hypertension. 46: 66–70 2005. [DOI] [PubMed] [Google Scholar]
- 86.He J, Gu D, Wu X, Chen J, Duan X, Chen J, and Whelton PK. Effect of soybean protein on blood pressure: a randomized, controlled trial. Ann Intern Med. 143: 1–9 2005. [DOI] [PubMed] [Google Scholar]
- 87.Obarzanek E, Velletri PA, and Cutler JA. Dietary protein and blood pressure. JAMA. 275: 1598–1603 1996. [DOI] [PubMed] [Google Scholar]
- 88.Watanabe H, Kashimoto N, Kajimura J, and Kamiya K. A miso (Japanese soybean paste) diet conferred greater protection against hypertension than a sodium chloride diet in Dahl salt-sensitive rats. Hypertens Res. 29: 731–738 2006. [DOI] [PubMed] [Google Scholar]
- 89.Yoshinaga M, Toda N, Tamura Y, Terakado S, Ueno M, Otsuka K, Numabe A, Kawabata Y, and Uehara Y. Japanese traditional miso soup attenuates salt-induced hypertension and its organ damage in Dahl salt-sensitive rats. Nutrition. 28: 924–931 2012. [DOI] [PubMed] [Google Scholar]
- 90.Burke V, Hodgson JM, Beilin LJ, Giangiulioi N, Rogers P, and Puddey IB. Dietary protein and soluble fiber reduce ambulatory blood pressure in treated hypertensives. Hypertension. 38: 821–826 2001. [DOI] [PubMed] [Google Scholar]
- 91.Washburn S, Burke GL, Morgan T, and Anthony M. Effect of soy protein supplementation on serum lipoproteins, blood pressure, and menopausal symptoms in perimenopausal women. Menopause. 6: 7–13 1999. [PubMed] [Google Scholar]
- 92.Hu FB, Stampfer MJ, Manson JE, Rimm E, Colditz GA, Rosner BA, Hennekens CH, and Willett WC. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med. 337: 1491–1499 1997. [DOI] [PubMed] [Google Scholar]
- 93.Nevala R, Vaskonen T, Vehniainen J, Korpela R, and Vapaatalo H. Soy based diet attenuates the development of hypertension when compared to casein based diet in spontaneously hypertensive rat. Life Sci. 66: 115–124 2000. [DOI] [PubMed] [Google Scholar]
- 94.Nakamura M, Aoki N, Yamada T, and Kubo N. Feasibility and effect on blood pressure of 6-week trial of low sodium soy sauce and Miso (fermented soybean paste). Circ J. 67: 530–534 2003. [DOI] [PubMed] [Google Scholar]
- 95.Kanda A, Hoshiyama Y, and Kawaguchi T. Association of lifestyle parameters with the prevention of hypertension in elderly Japanese men and women: a four-year follow-up of normotensive subjects. Asia Pac J Public Health. 11: 77–81 1999. [DOI] [PubMed] [Google Scholar]
- 96.Fujita T. Low salt diet should be reviewed. Miso soup is the best to prevent hypertension by intake of potassium or magnesium. Miso News Letter. 65-71. 1995. (in Japanese).
- 97.Anderson CA, Appel LJ, Okuda N, Brown IJ, Chan Q, Zhao L, Ueshima H, Kesteloot H, Miura K, Curb JD, Yoshita K, Elliott P, Yamamoto ME, and Stamler J. Dietary sources of sodium in China, Japan, the United Kingdom, and the United States, women and men aged 40 to 59 years: the INTERMAP study. J Am Diet Assoc. 110: 736–745 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Alderman MH. Evidence relating dietary sodium to cardiovascular disease. J Am Coll Nutr. 25(3Suppl): 256S–261S 2006. [DOI] [PubMed] [Google Scholar]
- 99.Kagan A, Popper J, Reed DM, MacLean CJ, and Grove JS. Trends in stroke incidence and mortality in Hawaiian Japanese men. Stroke. 25: 1170–1175 1994. [DOI] [PubMed] [Google Scholar]
- 100.Kokubo Y, Iso H, Ishihara J, Okada K, Inoue M, and Tsugane S. JPHC Study Group Association of dietary intake of soy, beans, and isoflavones with risk of cerebral and myocardial infarctions in Japanese populations: the Japan Public Health Center-based (JPHC) study cohort I. Circulation. 116: 2553–2562 2007. [DOI] [PubMed] [Google Scholar]
- 101.Kamiya K, Watanabe H, and Sasatani M. Protection of hypertension and stroke by Japanese soy bean paste (Miso) in SHRSP rats. Rep Central Miso Res Inst. 33: 112–119 2012. (in Japanese). [Google Scholar]