Abstract
Abstract: To determine effects of developmental exposure to brominated flame retardants (BFRs), weak thyroid hormone disruptors, on white matter development, white matter-specific global gene expression analysis was performed using microdissection techniques and microarrays in male rats exposed maternally to decabromodiphenyl ether (DBDE), one of the representative BFRs, at 10, 100 or 1000 ppm. Based on previous gene expression profiles of developmental hypothyroidism and DBDE-exposed cases, vimentin+ immature astrocytes and ret proto-oncogene (Ret)+ oligodendrocytes were immunohistochemically examined after developmental exposure to representative BFRs, i.e., DBDE, 1,2,5,6,9,10-hexabromocyclododecane (HBCD; 100, 1000 or 10,000 ppm) and tetrabromobisphenol A (TBBPA; 100, 1000 or 10,000 ppm). Vimentin+ and Ret+ cell populations increased at ≥ 100 ppm and ≥ 10 ppm DBDE, respectively. Vimentin+ and Ret+ cells increased at ≥ 1000 ppm HBCD, with no effect of TBBPA. The highest dose of DBDE and HBCD revealed subtle fluctuations in serum thyroid-related hormone concentrations. Thus, DBDE and HBCD may exert direct effects on glial cell development at ≥ middle doses. At high doses, hypothyroidism may additionally be an inducing mechanism, although its contribution is rather minor.
Keywords: BFRs, glial development, vimentin, Ret, hypothyroidism, rat
Introduction
Thyroid hormones (THs) are essential for normal fetal and neonatal brain development, control neuronal and glial proliferation in definitive brain regions and regulate neuronal migration and differentiation1,2,3. Experimentally, developmental hypothyroidism leads to growth retardation, neurological defects and impaired performance in a variety of behavioral learning actions4,5. Rat offspring exposed maternally to anti-thyroid agents show brain growth impairment associated with neuronal mismigration and white matter hypoplasia involving limited axonal myelination and decreased oligodendroglial distribution2,6. The outcome of this type of impairment is permanent, resulting in apparent structural and functional abnormalities.
Some environmental chemicals are thought to potentiate a TH-disrupting effect that may lead to abnormal brain development7. To evaluate the impact of developmentally exposed TH-disrupting chemicals on brain growth, we have established morphometric analysis methods of brain development in terms of neuronal migration and white matter development using a rat developmental hypothyroidism model8. We also have recently reported the molecules showing fluctuations in expression by microdissected region-specific microarray analysis and following immunohistochemical analysis in each of the hippocampal cornu ammonis and white matter after developmental hypothyroidism in rats9,10,11. With regard to molecules in the white matter responding to developmental hypothyroidism, we found vimentin+ immature astrocytes and ret proto-oncogene (Ret)+ oligodendrocytes in the cingulum11.
Brominated flame retardants (BFRs), some of which are environmental contaminants used in plastics, textiles, electronic circuitry and other materials to prevent fires, are known to be weak TH disruptors. Therefore, there is a growing concern regarding the developmental neurotoxicity of these chemicals12. Among the variety of BFRs, decabromodiphenyl ethers (DBDE), 1,2,5,6,9,10-hexabromocyclododecane (HBCD) and tetrabromobisphenol A (TBBPA) are the most widely used BFRs throughout the world and have been detected in human blood and breast milk13. DBDE, HBCD and TBBPA have been investigated in relation to hypothyroidism and neurotoxic effects. Among these chemicals, developmental exposure to DBDE resulted in a decrease in serum TH levels at the adult stage in mice and rats14, 15, showing in vivo evidence of neurotoxicity involving spontaneous locomotor behavior and synaptogenesis14, 16, 17. Regarding HBCD, developmental exposure showed impairment in learning and memory and aberrant spontaneous behavior18. Also, HBCD inhibited the uptake of neurotransmitters, particularly dopamine and glutamate, into synaptosomes19. In the case of TBBPA, the possibility of hypothyroidism and neurotoxicity has been suggested to be low. In a two-generation reproductive toxicity study, TBBPA did not induce effects on neurodevelopmental end points20. On the other hand, a one-generation reproductive study of TBBPA showed neurobehavioral effects in offspring21, and in vitro studies showed antagonistic activity on TH receptors and inhibition of synaptic neurotransmitter uptake19, 22.
We have recently reported the effects of developmental exposure to DBDE, TBBPA and HBCD on white matter development by histomorphometric assessment using rats in association with thyroid parameters23, 24. Our results suggested that maternal exposure to DBDE or HBCD through diet caused irreversible white matter hypoplasia at the highest doses in offspring as examined in males, as well as the induction of mild developmental hypothyroidism as judged by fluctuations in the serum concentrations of thyroid-related hormones at the end of developmental exposure23, 24. On the other hand, we have also found white matter hypoplasia at the middle dose with DBDE without accompanying fluctuations in serum TH concentrations, suggesting a direct effect on the brain24. In another study, we also found that neuronal development was affected by all of these BFRs, with DBDE and TBBPA appearing to have direct effects on the brain25.
In the present study, to elucidate whether TH-disrupting chemicals, such as BFRs, affect hypothyroidism-related white matter development after developmental exposure, we performed cerebral white matter-specific global gene expression analysis using microarrays in developmentally DBDE-exposed rat offspring and compared this with the profiles in the developmental hypothyroidism model using anti-thyroid agents as previously reported11. Molecules showing commonly altered expression in animals between DBDE and anti-thyroid agents were analyzed for immunohistochemical distribution in the cerebral white matter using the same previously published study samples of DBDE, TBBPA and HBCD23, 24. DBDE study samples also were analyzed for the immunohistochemical distribution of the other candidate molecules obtained from DBDE microarray analysis.
Materials and Methods
Chemicals and animals
DBDE (CAS No. 1163-19-5, purity: >98%) was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). TBBPA (CAS No. 79-94-7, purity: >98%) and HBCD (CAS No. 3194-55-6, purity: >95%) were purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Pregnant CD® (SD) IGS rats were purchased from Charles River Laboratories Japan, Inc. (Yokohama, Japan) at gestational day (GD) 3 (the day when vaginal plugs were observed was designated as GD 0). Animals were individually housed in polycarbonate cages (SK-Clean, 41.5 cm × 26 cm × 17.5 cm; CLEA Japan, Inc., Tokyo, Japan) with wood chip bedding (Sankyo Labo Service Corp., Tokyo, Japan) and maintained in a climate-controlled animal room (24 ± 1°C, relative humidity: 55 ± 5%) with a 12-h light/dark cycle. A soy-free diet (Oriental Yeast Co., Ltd., Tokyo, Japan) was chosen as the basal diet for maternal animals to eliminate possible phytoestrogen effects26. Animals received food and water ad libitum throughout the experimental period, including a 1-week acclimation period.
Experimental design
Exposure studies of DBDE, HBCD and TBBPA were individually performed, and dams were randomly divided into four groups including untreated controls23, 24. The highest dose of each chemical was determined with a preliminary dose-finding study by estimating the dose range that causes changes in thyroid weights and histopathological findings of thyroid glands in dams but does not affect pregnancy, implantation or delivery. For DBDE, 8 dams per group were provided with the soy-free diet containing 0 (control), 10, 100 or 1000 ppm of DBDE from GD 10 to postnatal day (PND) 20 (PND 0: the day of delivery). For TBBPA or HBCD, 8 dams per group in the TBBPA study and 10 dams per group in the HBCD study were provided with the soy-free diet containing 0 (control), 100, 1000 or 10,000 ppm of the compound from GD 10 to PND 20. In all studies, litters were culled randomly on PND 2, leaving 4 male and 4 female offspring. On PND 20, 20 male and 20 female offspring (at least one male and one female per dam) per group were euthanized and subjected to prepubertal necropsy.
All animals used in the present study were killed by exsanguination from the abdominal aorta under deep anesthesia. The protocols were reviewed in terms of animal welfare and approved by the Animal Care and Use Committee of the National Institute of Health Sciences, Japan.
Preparation of tissue specimens and microdissection
For microarray analysis in developmentally DBDE-exposed animals, the whole brain of male offspring was immediately removed at prepubertal necropsy on PND 20 (n = 4/group, 1 pup/litter) and fixed with methacarn solution for 2 h at 4°C27. Coronal brain slices taken at –3.5 mm from the bregma were dehydrated and embedded in paraffin. Embedded tissues were stored at 4°C until tissue sectioning for microdissection.
According to the method described previously11, regions of the corpus callosum (CC) and external capsule in 20-µm-thick serial sections were subjected to laser microbeam microdissection (Leica Microsystems GmbH). Forty sections from each animal were used for microdissection, and microdissected samples were individually stored in 1.5 ml tubes at –80°C until total RNA extraction.
RNA preparation, amplification and microarray analysis
Total RNA extraction from microdissected regions, quantitation of RNA yield and RNA amplification were performed using methods described elsewhere28.
For microarray analysis, second-round-amplified biotin-labeled antisense RNAs were subjected to hybridization with a GeneChip® Rat Genome 230 2.0 Array (Affymetrix, Inc., Santa Clara, CA, USA).
Gene selection and normalization of expression data were performed using GeneSpring® software 7.2 (Silicon Genetics, Redwood City, CA, USA). Per chip normalization was performed according to a method described elsewhere28. Genes with expression changes of at least 2-fold in magnitude caused by DBDE exposure as compared with those of untreated controls were selected. Using these gene expression data, we further selected genes showing commonly fluctuated expressions with previously reported genes responding to developmental hypothyroidism in an experimental induction model using anti-thyroid agents consisting of groups of 3 or 12 ppm propylthiouracil and 200 ppm methimazole11.
Immunohistochemistry
To evaluate the immunohistochemical distribution of molecules showing commonly altered expression between DBDE and anti-thyroid agents, brains of male pups obtained at PND 20 were fixed in Bouin’s solution at room temperature overnight in all BFRs studies of DBDE, TBBPA and HBCD. Five animals (1 pup/litter) for each group were used for immunohistochemistry using antibodies against vimentin (mouse monoclonal antibody, 1:200, Millipore Corporation, Billerica, MA, USA) and Ret (rabbit polyclonal antibody, 1:50, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). Furthermore, to evaluate the immunohistochemical distribution of molecules whose transcript levels fluctuated in microarray analysis due to DBDE exposure, antibodies against neuregulin 1 (Nrg1, also named as heregulin; mouse monoclonal antibody, 1:40, Exalpha Biologicals, Inc., Watertown, MA, USA), Crk (mouse monoclonal antibody, 1:2000, BD Biosciences, Franklin Lakes, NJ, USA) and Claudin 11 (Cld11, also named as oligodendrocyte specific protein; rabbit polyclonal antibody, 1:200, Novus Biologicals, LLC, Littleton, CO, USA) were used in the DBDE study.
For antigen retrieval, the sections were heated in 10 mM citrate buffer by microwave for 10 min before incubation with anti-vimentin and anti-Nrg1 antibodies. Immunodetection was carried out using a VECTASTAIN® Elite ABC kit (Vector Laboratories Inc., Burlingame, CA, USA) with 3,3’-diaminobenzidine/H2O2 for the chromogen as described elsewhere32. Sections were then counterstained with hematoxylin and coverslipped for microscopic examination. Non-immunized sera were substituted for the primary antibody as negative controls for immunoreactivity.
Morphometry of immunolocalized cells
The number of immunoreactive cells was quantitatively measured by vimentin and Ret expression in white matter at the cingulum of the bilateral sides by blind trial for the treatment conditions according to the method and equipment described previously11. The values were normalized and expressed as those in the unit area (mm2).
To evaluate immunoreactivity of Nrg1, Crk and Cld11 in the white matter, staining intensity was scored as 0 (none), 1 (minimal), 2 (slight), 3 (moderate) or 4 (strong) by observation at 40-fold magnification.
Statistical analysis
Data for offspring were analyzed using the litter as the experimental unit. Numerical data were assessed by one-way analysis of variance or the Kruskal-Wallis test following Bartlett’s test. Statistically significant differences were analyzed by Dunnett’s multiple comparison test for comparison with the untreated control group. For grading immunohistochemical findings, scores of Nrg1, Crk and Cld11 expression were compared between the untreated control group and each DBDE-exposed group using the Mann-Whitney’s U-test.
Results
Microarray analysis in developmentally DBDE-exposed rats
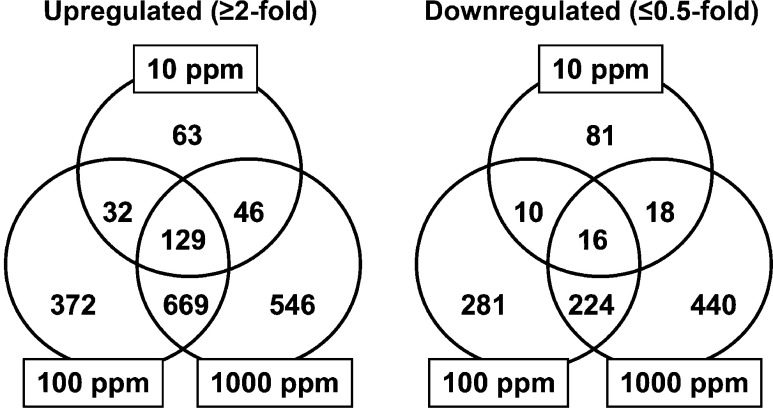
Figure 1 shows a Venn diagram of genes showing altered expression in microdissected cerebral white matter in combination or individually in each DBDE exposure group. Numerous genes were found to be up- or downregulated commonly in DBDE-exposed groups. One hundred forty-five genes (129 genes upregulated; 16 genes downregulated) were identified showing altered expression commonly among all DBDE groups, and 893 genes (669 genes upregulated; 224 genes downregulated) were identified as showing altered expression commonly between 100 and 1000 ppm DBDE groups (Fig. 1 and Supplementary Tables 1
, 2
, 3
, 4
: on-line only). Twelve genes (11 genes upregulated; 1 gene downregulated) were found to be brain development-related among those that commonly fluctuated in expression between all DBDE groups, and 70 genes (52 genes upregulated; 18 genes downregulated) were also identified as those related to brain development in a group of genes that commonly fluctuated between 100 and 1000 ppm DBDE groups (Supplementary Table 5
: on-line only).
Fig. 1.
Venn diagram of genes with altered expression in microarray analysis in response to maternal exposure to DBDE. (Left) Upregulated genes (≥ 2-fold). (Right) Downregulated genes (≤ 0.5-fold).
Comparison of the microarray data between the studies of DBDE and anti-thyroid agents
The global gene-expression profile of the cerebral white matter in the DBDE study was compared with that of the study using anti-thyroid agents11. Figure 2 shows a Venn diagram of genes with commonly altered expression between the studies of DBDE and anti-thyroid agents. Among upregulated genes, 4 genes were detected as those showing common fluctuation in all DBDE and anti-thyroid agents groups (Table 1). Forty-two genes were found to be upregulated commonly in 100 and 1000 ppm DBDE and all anti-thyroid agents groups. Thirty-three genes were upregulated commonly in 1000 ppm DBDE and all anti-thyroid agents groups. Downregulated genes were not observed commonly in all DBDE exposure groups and all anti-thyroid agents groups. Two downregulated genes were commonly observed in 100 and 1000 ppm DBDE and all anti-thyroid agents groups. Also, another two downregulated genes were commonly observed in 1000 ppm DBDE and all anti-thyroid agents groups.
Fig. 2.
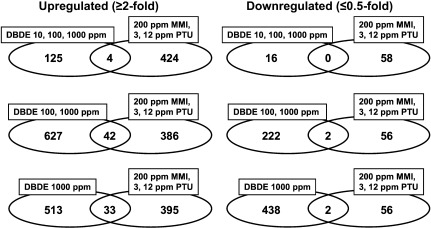
Venn diagram of genes with altered expression in microarray analysis between the studies of DBDE and anti-thyroid agents, propylthiouracil (PTU) and methimazole (MMI). (Left) Upregulated genes (≥ 2-fold). (Right) Downregulated genes (≤ 0.5-fold).
Table 1. List of Genes with Commonly Altered Expression Between the Studies of DBDE and Anti-thyroid Agents (≥ 2-fold, ≤ 0.5-fold).
Vimentin and Ret in the cerebral white matter of BFR-exposed animals
Microarray analysis showed that vimentin and Ret commonly upregulated transcript levels between anti-thyroid agents and DBDE (Table 1). The immunohistochemical distributions of vimentin+ or Ret+ cells were found to be increased in the cingulum in our previous study of developmental hypothyroidism by maternal exposure to anti-thyroid agents11. We, therefore, evaluated the cellular distribution of these molecules in the white matter of BFR-exposed animals.
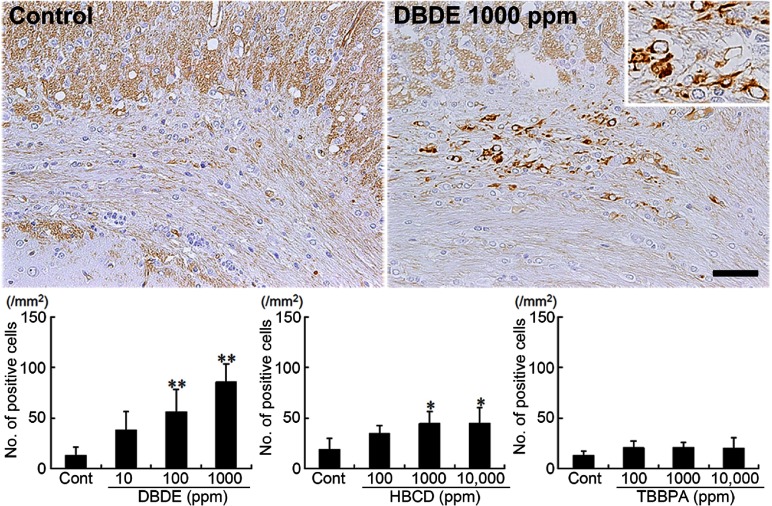
Vimentin+ cells were scarcely distributed in the white matter tissue of untreated control animals (Fig. 3). With DBDE, the distribution of vimentin+ cells was mainly observed in the cingulum and increased dose dependently with statistical significance at 100 and 1000 ppm as compared with the untreated controls (Fig. 3). In HBCD-exposed animals, vimentin+ cells showed a similar distribution to the DBDE-exposed animals, showing a statistically significant increased distribution at 1000 and 10,000 ppm as compared with the untreated controls (Fig. 3). TBBPA-exposed animals did not show a significant change in the number of positive cells compared with the untreated control group at all doses (Fig. 3).
Fig. 3.
Immunohistochemical distributions of vimentin+ cells in the white matter tissue of BFR-exposed offspring. Untreated control animal (left) and 1000 ppm DBDE-exposed animal (right). 200× magnification (inset: 400× magnification). Bar = 50 μm. Graph shows the mean number of positive cells within the cingulum at 100× magnification (n = 5/group). Values are expressed as means + SD. * P < 0.05 vs. untreated controls, ** P < 0.01 vs. untreated controls.
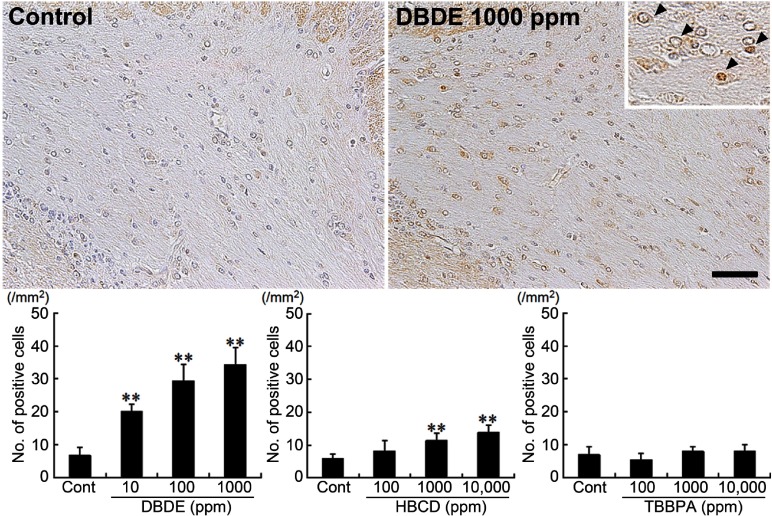
With regard to Ret, immunoreactive cells were sparsely observed in oligodendrocytes of the white matter (Fig. 4). DBDE exposure showed that distribution of Ret+ cells was mainly observed in the cingulum and increased dose dependently with statistical significance in the number of positive cells in all dose groups as compared with untreated controls (Fig. 4). In HBCD-exposed animals, Ret+ cells also increased, showing statistical significance at 1000 and 10,000 ppm groups as compared with untreated controls (Fig. 4). TBBPA-exposed animals did not show a significant change in the number of positive cells compared with the untreated control group at all doses (Fig. 4).
Fig. 4.
Immunohistochemical distributions of Ret+ cells in the white matter tissue of BFR-exposed offspring. Untreated control animal (left), 1000 ppm DBDE-exposed animal (right). 200× magnification (inset: 400× magnification, Ret+ cells are indicated with arrowheads). Bar = 50 μm. Graph shows the mean number of positive cells within the cingulum at 100× magnification (n = 5/group). Values are expressed as means + SD. ** P < 0.01 vs. untreated controls.
Immunohistochemical staining scores of molecules in the cerebral white matter of DBDE-exposed animals
Among genes selected from microarray analysis, we additionally examined immunohistochemical staining scores in molecules showing diffuse immunoreactivity in the white matter without specific cellular localization in DBDE-exposed animals. Immunoreactivity of Nrg1, Crk and Cld11 in the cerebral white matter was examined. Cld11 was found to show common downregulation between DBDE (100 and 1000 ppm) and all anti-thyroid agents (Table 1). Nrg1 and Crk showed upregulation at 100 and 1000 ppm DBDE (Supplementary Table 5). Because the 100 and 1000 ppm DBDE groups showed white matter hypoplasia targeting oligodendrocytes by morphological analysis, these molecules were selected24.
Nrg1, Crk and Cld11 showed diffuse immunoreactivity in the white matter suggestive of the myelin sheaths (Fig. 5A–C). The immunoreactivity of Nrg1 or Crk showed a statistically significant increase at 1000 ppm as compared with untreated controls (Fig. 5A and B). The immunoreactivity of Cld11 showed a significant decrease at 10 ppm DBDE as compared with untreated controls. While statistically nonsignificant, 100 and 1000 ppm DBDE also tended to decrease the intensity scores of Cld11 (Fig. 5C).
Fig. 5.
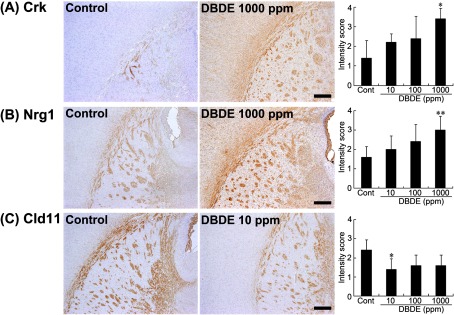
Immunohistochemical distributions of Crk, Nrg1 and Cld11 in the white matter tissue of DBDE-exposed offspring. (A) Crk immunoreactivity in the myelin sheath of the external capsule, internal capsule and fimbria of the hippocampus. Untreated control animal (left), 1000 ppm DBDE-exposed animal (right). 40× magnification. Bar = 250 μm. Graph shows the mean intensity score of immunoreactivity at 40× magnification (n = 5/group). Values are expressed as means + SD. * P < 0.05 vs. untreated controls. (B) Nrg1 immunoreactivity in the myelin sheath of the external capsule, internal capsule and fimbria of the hippocampus. Untreated control animal (left), 1000 ppm DBDE-exposed animal (right). 40× magnification. Bar = 250 μm. Graph shows the mean intensity score of immunoreactivity at 40× magnification (n = 5/group). Values are expressed as means + SD. ** P < 0.01 vs. untreated controls. (C) Cld11 immunoreactivity in the myelin sheath of the external capsule, internal capsule and fimbria of the hippocampus. Untreated control animal (left), 10 ppm DBDE-exposed animal (right). 40× magnification. Bar = 250 μm. Graph shows the mean intensity score of immunoreactivity at 40× magnification (n = 5/group). Values are expressed as means + SD. * P < 0.05 vs. untreated controls.
Discussion
In our previous study24, maternal exposure to DBDE induced white matter hypoplasia targeting oligodendrocytes beginning at 100 ppm in rat offspring accompanied by mild hypothyroidism at least at 1000 ppm. Using the same study samples, in the present study, we obtained a global gene expression profile of DBDE-responding genes in the cerebral white matter tissue consisting of the CC and external capsule collected using a microdissection technique. We further compared this expression profile with that responding to developmental hypothyroidism in rats using anti-thyroid agents11. As a result, there was a population of genes with commonly fluctuating expression between hypothyroidism and DBDE exposure, suggesting a similar mechanism to developmental hypothyroidism of action on the cerebral white matter by DBDE, while the gene expression changes observed only after DBDE exposure might be the direct brain effect of DBDE. Among genes showing commonly altered expression between hypothyroidism and DBDE exposure, vimentin and Ret were found to show an increased immunoreactive cell distribution after developmental hypothyroidism11.
In the present study, increased distributions of vimentin+ cells were observed in the cingulum of offspring maternally exposed to DBDE or HBCD at both middle and high doses on PND 20. These changes were well in accordance with the development of white matter hypoplasia detected at PND 77 by quantitative histomorphometric analysis in these cases, while a hypoplastic change was evident only at the high dose in HBCD-exposed cases23, 24. On the other hand, TBBPA-exposed offspring did not show changes in the number of vimentin+ cells in the present study, in accordance with the lack of the development of white matter hypoplasia as reported in our previous study23. We previously found a similar immunohistochemical localization of vimentin and glial fibrillary acidic protein (GFAP) in the cingulum after developmental hypothyroidism11. Vimentin is expressed in immature astrocytes during development29, and GFAP is expressed in both immature and mature astrocytes30. Therefore, vimentin+ cells that appeared in response to DBDE or HBCD exposure may be immature astrocytes as seen in developmental hypothyroidism. While the reason for the increase in vimentin+ cells in the cingulum as a result of exposure to DBDE or HBCD in the present study is not clear, we previously reported frequent induction of subcortical band heterotopia in the corpus callosum, manifested by the appearance of aberrant cortical tissue in this anatomical area, in hypothyroid animals8. While the anatomical location of this heterotopic tissue was close to the cingulum accumulating immature astrocytes in the hypothyroid cases, suggestive of an etiological relation between the two, we did not observe heterotopic tissue with DBDE and HBCD. As another possibility, the increased immature astrocytes may simply be a reactive change in response to reduced oligodendrocytes as a result of exposure to DBDE or HBCD23, 24.
In the present study, we found an increase of Ret+ oligodendrocytes in the cingulum of offspring at all doses of DBDE and the middle and high doses of HBCD on PND 20, while TBBPA-exposed cases did not show any distribution changes of Ret+ oligodendrocytes. Ret is a receptor protein-tyrosine kinase of glial cell line-derived neurotrophic factor (GDNF)31. It induces cell death in the absence of its ligand32. Because we did not find an increase in GDNF transcript levels in DBDE-exposed cases in microarray analysis in the present study, facilitation of apoptosis due to the increase of ligand-free Ret may be responsible for induction of white matter hypoplasia in DBDE and HBCD cases, as suggested in our previous hypothyroidism cases11.
In the present study, Cld11 was found to be commonly downregulated in the white matter in the microarray analysis between developmental hypothyroidism and DBDE exposure. We, therefore, examined the immunohistochemical staining intensity of Cld11 in DBDE-exposed offspring. Cld11 is a four-transmembrane protein expressed in oligodendrocytes33. In vitro study has shown that Cld11 overexpression results in induction of oligodendrocyte proliferation34. This result indicates that the decrease in Cld11 immunoreactivity in the white matter may cause white matter hypoplasia in DBDE-exposed offspring24.
Among genes showing altered expression by microarray analysis in DBDE study, Crk and Nrg1 showed an increase in immunoreactive intensity in the white matter of DBDE-exposed offspring at 1000 ppm in the present study. Crk is an adaptor molecule associated with the tyrosine phosphorylated Dab1 in the reelin pathway regulating the migration and maturation of newborn granule cells in the hippocampal dentate gyrus35. Nrg1 is a trophic factor that regulates neuronal migration, axonal pathfinding, neurotransmission and synaptic plasticity in the central nervous system36. The increased intensity of both Crk and Nrg1 only at 1000 ppm DBDE suggested that these molecules could be considered to be less sensitive biomarkers for detection of DBDE-induced effects than vimentin and Ret. Also, immunohistochemical expression of these molecules could not be measured quantitatively.
Both thyrotoxic effects14, 15 and developmental neurobehavioral effects14, 16 have been reported in mice and rats after exposure to DBDE. In our previous study, DBDE at 1000 ppm was suggested to cause developmental hypothyroidism in the same samples as used in the present study 24. Zhang et al.37 recently reported the tissue distribution, including the brain, of DBDE and its debrominated metabolites in suckling rat pups after prenatal and/or postnatal exposure, suggesting a possibility of direct effects of DBDE on the glial population changes. In the present study, we observed an increase in vimentin+ immature astrocytes after exposure to DBDE beginning at 100 ppm (7.0–22.8 mg/kg body weight/day) and an increase in Ret+ oligodendrocytes after exposure to DBDE beginning at 10 ppm (0.7–2.4 mg/kg body weight/day) on PND 20. We also observed that only around 6% of total genes showing altered expression due to DBDE exposure commonly fluctuated with developmental hypothyroidism due to exposure to anti-thyroid agents. These results suggest primarily a direct effect of DBDE on glial population changes accompanied by subtle hypothyroidism-related effects at 1000 ppm. The European food safety authority (EFSA) reported that average adult consumers were exposed to between 0.35 and 2.82 ng/kg body weight/day of DBDE via diet and between 0.045 and 7 ng/kg body weight/day via house dust38. Breast-fed infants were exposed to between 1.44 and 19.95 ng/kg body weight/day of DBDE via human milk38. In the present study, the treatment doses of DBDE were approximately ×106 higher than the estimated daily intake level. In our previous study, effects on neural development were also observed after exposure to DBDE at 100 ppm and higher on PND 2025. There is a study suggesting a disruption of TH-mediated transcription by DBDE due to interference with the thyroid receptor-DNA binding domain39. These results suggest the potential of DBDE to suppress TH action in the brain; however, the effect may appear at extremely high doses as compared with human exposure levels.
We have previously shown a decrease in serum triiodothyronine (T3) concentration and increase in serum thyroid-stimulating hormone concentration caused by HBCD at 10,000 ppm on PND 20 in the same samples as used in the present study; however, the magnitude of changes was rather mild23. As a hypothyroidism-related effect, we have previously reported reduction of the oligodendrocyte distribution at 10,000 ppm (803.2–2231.3 mg/kg body weight/day)23. We also observed effects on neuronal development caused by HBCD, at least at 10,000 ppm, similar to the effect on the number of oligodendrocytes in relation to developmental hypothyroidism25. In the present study, however, we detected increases in vimentin+ immature astrocytes and Ret+ oligodendrocytes at 1000 ppm (80.7–212.9 mg/kg body weight/day) and higher on PND 20, suggesting possible direct action on the developing brain. The EFSA reported that average adult consumers were exposed to between 0.09 and 0.99 ng/kg body weight/day of HBCD via diet and between 2.4 and 6 ng/kg body weight/day via house dust and that breast-fed infants were exposed to between 0.90 and 213 ng/kg body weight/day of HBCD via human milk40. In the present study, the treatment doses of HBCD were approximately ×107 higher than the estimated daily intake level. There are studies that have shown a direct action of HBCD on TH receptors or competing potential of HBCD with thyroxin (T4) for binding to transthyretin, a T4 plasma transporter protein41, 42. Therefore, there is a possibility that HBCD affects brain development directly, although the effect may appear at extremely high doses as compared with human exposure levels.
With regard to TBBPA, we have shown no obvious thyrotoxic changes in the offspring except for a non-dose-related decrease in serum T3 concentrations at 100 and 1000 ppm on PND 20 using the same samples as used in the present study23. While a direct effect on neuronal development was suggested in our previous study25, we did not detect any changes in the number of vimentin+ immature astrocytes and Ret+ oligodendrocytes as a result of developmental TBBPA exposure in the present study.
In conclusion, by means of microarray analysis of DBDE-exposed offspring, we found increased expression of vimentin and Ret in the cerebral white matter as observed with cases of developmental hypothyroidism11. By immunohistochemical analysis of these molecules in offspring developmentally exposed to BFRs, we found increases in vimentin+ immature astrocytes at ≥ 100 ppm for DBDE and ≥ 1000 ppm for HBCD and in Ret+ oligodendrocytes at ≥ 10 ppm for DBDE and ≥ 1000 ppm for HBCD. TBBPA did not show an effect on either vimentin+ or Ret+ cells. A direct effect of DBDE and HBCD on glial cell development may be considered under the present experimental conditions. At the highest dose of DBDE as well as that of HBCD, hypothyroidism may additionally be an inducing mechanism, although its contribution is rather minor. The observed brain effects occurred at levels extremely higher than the human exposure level of these compounds. The results from this study suggest that vimentin+ and Ret+ cells in the cerebral white matter may be used for assessment of the effects of developmental neurotoxicants mimicking TH-disrupting chemicals.
Acknowledgments
We thank Miss Tomomi Morikawa and Miss Ayako Kaneko for their technical assistance in conducting the animal study. This work was supported in part by Health and Labour Sciences Research Grants (Research on Risk of Chemical Substances) from the Ministry of Health, Labour and Welfare of Japan. All authors disclose that there are no conflicts of interest that could inappropriately influence the outcome of the present study.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (by-nc-nd) License <http://creativecommons.org/licenses/by-nc-nd/3.0/>.
References
- 1.Porterfield SP. Thyroidal dysfunction and environmental chemicals-Potential impact on brain development. Environ. Health Perspect. 108: 433–438 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schoonover CM, Seibel MM, Jolson DM, Stack MJ, Rahman RJ, Jones SA, Mariash CN, and Anderson GW. Thyroid hormone regulates oligodedrocyte accumulation in developing rat brain white matter tracts. Endocrinology. 145: 5013–5020 2004. [DOI] [PubMed] [Google Scholar]
- 3.Montero-Pedrazuela A, Venero C, Lavado-Autric R, Fernández-Lamo I, García-Verdugo JM, Bernal J, and Guadaño-Ferraz A. Modulation of adult hippocampal neurogenesis by thyroid hormones: implications in depressive-like behavior. Mol Psychiatry. 11: 361–371 2006. [DOI] [PubMed] [Google Scholar]
- 4.Comer CP, and Norton S. Effects of perinatal methimazole exposure on a developmental test battery for neurobehavioral toxicity in rats. Toxicol Appl Pharmacol. 63: 133–141 1982. [DOI] [PubMed] [Google Scholar]
- 5.Akaike M, Kato N, Ohno H, and Kobayashi T. Hyperactivity and spatial maze learning impairment of adult rats with temporary neonatal hypothyroidism. Neurotoxicol Teratol. 13: 317–322 1991. [DOI] [PubMed] [Google Scholar]
- 6.Lavado-Autric R, Ausó E, García-Velasco JV, Arufe Mdel C, Escobar del Rey F, Berbel P, and Morreale de Escobar G. Early maternal hypothyroxinemia alters histogenesis and cerebral cortex cytoarchitecture of the progeny. J Clin Invest. 111: 1073–1082 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bansal R, and Zoeller RT. Polychlorinated biphenyls (Aroclor 1254) do not uniformly produce agonist actions on thyroid hormone responses in the developing rat brain. Endocrinology. 149: 4001–4008 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shibutani M, Woo GH, Fujimoto H, Saegusa Y, Takahashi M, Inoue K, Hirose M, and Nishikawa A. Assessment of developmental effects of hypothyroidism in rats from in utero and lactation exposure to anti-thyroid agents. Reprod Toxicol. 28: 297–307 2009. [DOI] [PubMed] [Google Scholar]
- 9.Saegusa Y, Woo GH, Fujimoto H, Inoue K, Takahashi M, Hirose M, Igarashi K, Kanno J, Mitsumori K, Nishikawa A, and Shibutani M. Gene expression profiling and cellular distribution of molecules with altered expression in the hippocampal CA1 region after developmental exposure to anti-thyroid agents in rats. J Vet Med Sci. 72: 187–195 2010. [DOI] [PubMed] [Google Scholar]
- 10.Saegusa Y, Woo GH, Fujimoto H, Kemmochi S, Shimamoto K, Hirose M, Mitsumori K, Nishikawa A, and Shibutani M. Sustained production of Reelin-expressing interneurons in the hippocampal dentate hilus after developmental exposure to anti-thyroid agents in rats. Reprod Toxicol. 29: 407–414 2010. [DOI] [PubMed] [Google Scholar]
- 11.Fujimoto H, Woo GH, Inoue K, Igarashi K, Kanno J, Hirose M, Nishikawa A, and Shibutani M. Increased cellular distribution of vimentin and Ret in the cingulum induced by developmental hypothyroidism in rat offspring maternally exposed to anti-thyroid agents. Reprod Toxicol. 34: 93–100 2012. [DOI] [PubMed] [Google Scholar]
- 12.de Wit CA. An overview of brominated flame retardants in the environment. Chemosphere. 46: 583–624 2002. [DOI] [PubMed] [Google Scholar]
- 13.Birnbaum LS, and Staskal DF. Brominated flame retardants: cause for concern? Environ Health Perspect. 112: 9–17 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rice DC, Reeve EA, Herlihy A, Zoeller RT, Thompson WD, and Markowski VP. Developmental delays and locomotor activity in the C57BL6/J mouse following neonatal exposure to the fully-brominated PBDE, decabromodiphenyl ether. Neurotoxicol Teratol. 29: 511–520 2007. [DOI] [PubMed] [Google Scholar]
- 15.Kim TH, Lee YJ, Lee E, Kim MS, Kwack SJ, Kim KB, Chung KK, Kang TS, Han SY, Lee J, Lee BM, and Kim HS. Effects of gestational exposure to decabromodiphenyl ether on reproductive parameters, thyroid hormone levels, and neuronal development in Sprague-Dawley rats offspring. J Toxicol Environ Health A. 72: 1296–1303 2009. [DOI] [PubMed] [Google Scholar]
- 16.Viberg H, Fredriksson A, and Eriksson P. Changes in spontaneous behaviour and altered response to nicotine in the adult rat, after neonatal exposure to the brominated flame retardant, decabromodiphenyl ether (PBDE 209). Neurotoxicology. 28: 136–142 2007. [DOI] [PubMed] [Google Scholar]
- 17.Viberg H. Neonatal ontogeny and neurotoxic effect of decabrominated diphenyl ether (PBDE 209) on levels of synaptophysin and tau. Int J Dev Neurosci. 27: 423–429 2009. [DOI] [PubMed] [Google Scholar]
- 18.Eriksson P, Fischer C, Wallin M, Jakobsson E, and Fredriksson A. Impaired behaviour, learning and memory, in adult mice neonatally exposed to hexabromocyclododecane (HBCDD). Environ Toxicol Pharmacol. 21: 317–322 2006. [DOI] [PubMed] [Google Scholar]
- 19.Mariussen E, and Fonnum F. The effect of brominated flame retardants on neurotransmitter uptake into rat brain synaptosomes and vesicles. Neurochem Int. 43: 533–542 2003. [DOI] [PubMed] [Google Scholar]
- 20.EU-Report. European Union Risk Assessment Report on: 2,2’,6,6’-tetrabromo-4,4’-isopropylene diphenol (tetrabromobisphenol-A), CAS No. 79-94-7, EINECS No. 201-236-9. 2005. [Google Scholar]
- 21.Lilienthal H, Verwer CM, van der Ven LT, Piersma AH, and Vos JG. Exposure to tetrabromobisphenol A (TBBPA) in Wistar rats: neurobehavioral effects in offspring from a one-generation reproduction study. Toxicology. 246: 45–54 2008. [DOI] [PubMed] [Google Scholar]
- 22.Darnerud PO. Toxic effects of brominated flame retardants in man and in wildlife. Environ Int. 29: 841–853 2003. [DOI] [PubMed] [Google Scholar]
- 23.Saegusa Y, Fujimoto H, Woo GH, Inoue K, Takahashi M, Mitsumori K, Hirose M, Nishikawa A, and Shibutani M. Developmental toxicity of brominated flame retardants, tetrabromobisphenol A and 1,2,5,6,9,10-hexabromocyclododecane, in rat offspring after maternal exposure from mid-gestation through lactation. Reprod Toxicol. 28: 456–467 2009. [DOI] [PubMed] [Google Scholar]
- 24.Fujimoto H, Woo GH, Inoue K, Takahashi M, Hirose M, Nishikawa A, and Shibutani M. Impaired oligodendroglial development by decabromodiphenyl ether in rat offspring after maternal exposure from mid-gestation through lactation. Reprod Toxicol. 31: 86–94 2011. [DOI] [PubMed] [Google Scholar]
- 25.Saegusa Y, Fujimoto H, Woo GH, Ohishi T, Wang L, Mitsumori K, Nishikawa A, and Shibutani M. Transient aberration of neuronal development in the hippocampal dentate gyrus after developmental exposure to brominated flame retardants in rats. Arch Toxicol. 86: 1431–1442 2012. [DOI] [PubMed] [Google Scholar]
- 26.Masutomi N, Shibutani M, Takagi H, Uneyama C, and Hirose M. Dietary influence on the impact of ethinylestradiol-induced alterations in the endocrine/reproductive system with perinatal maternal exposure. Reprod Toxicol. 18: 23–33 2004. [DOI] [PubMed] [Google Scholar]
- 27.Shibutani M, Uneyama C, Miyazaki K, Toyoda K, and Hirose M. Methacarn fixation: a novel tool for analysis of gene expressions in paraffin-embedded tissue specimens. Lab Invest. 80: 199–208 2000. [DOI] [PubMed] [Google Scholar]
- 28.Woo GH, Takahashi M, Inoue K, Fujimoto H, Igarashi K, Kanno J, Hirose M, Nishikawa A, and Shibutani M. Cellular distributions of molecules with altered expression specific to thyroid proliferative lesions developing in a rat thyroid carcinogenesis model. Cancer Sci. 100: 617–625 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alonso G. Proliferation of progenitor cells in the adult rat brain correlates with the presence of vimentin-expressing astrocytes. Glia. 34: 253–266 2001. [DOI] [PubMed] [Google Scholar]
- 30.Eddleston M, and Mucke L. Molecular profile of reactive astrocytes—implications for their role in neurologic disease. Neuroscience. 54: 15–36 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sariola H, and Saarma M. Novel functions and signalling pathways for GDNF. J Cell Sci. 116: 3855–3862 2003. [DOI] [PubMed] [Google Scholar]
- 32.Bordeaux MC, Forcet C, Granger L, Corset V, Bidaud C, Billaud M, Bredesen DE, Edery P, and Mehlen P. The RET proto-oncogene induces apoptosis: a novel mechanism for Hirschsprung disease. EMBO J. 19: 4056–4063 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bronstein JM, Micevych PE, and Chen K. Oligodendrocyte-specific protein (OSP) is a major component of CNS myelin. J Neurosci Res. 50: 713–720 1997. [DOI] [PubMed] [Google Scholar]
- 34.Tiwari-Woodruff SK, Buznikov AG, Vu TQ, Micevych PE, Chen K, Kornblum HI, and Bronstein JM. OSP/claudin-11 forms a complex with a novel member of the tetraspanin super family and beta1 integrin and regulates proliferation and migration of oligodendrocytes. J Cell Biol. 153: 295–305 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang Y, Magdaleno S, Hopkins R, Slaughter C, Curran T, and Keshvara L. Tyrosine phosphorylated Disabled 1 recruits Crk family adapter proteins. Biochem Biophys Res Commun. 318: 204–212 2004. [DOI] [PubMed] [Google Scholar]
- 36.Mei L, and Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci. 9: 437–452 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang W, Cai Y, Sheng G, Chen D, and Fu J. Tissue distribution of decabrominated diphenyl ether (BDE-209) and its metabolites in sucking rat pups after prenatal and/or postnatal exposure. Toxicology. 283: 49–54 2011. [DOI] [PubMed] [Google Scholar]
- 38.EFSA Panel on Contaminants in the Food Chain. Scientific Opinion on Polybrominated Diphenyl Ethers (PBDEs) in Food. EFSA Journal. 9: 2156 [274 pp]. 2011. .
- 39.Ibhazehiebo K, Iwasaki T, Kimura-Kuroda J, Miyazaki W, Shimokawa N, and Koibuchi N. Disruption of thyroid hormone receptor-mediated transcription and thyroid hormone-induced Purkinje cell dendrite arborization by polybrominated diphenyl ethers. Environ Health Perspect. 119: 168–175 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.EFSA Panel on Contaminants in the Food Chain. Scientific Opinion on Hexabromocyclododecanes (HBCDDs) in Food. EFSA Journal. 9: 2296 [118 pp]. 2011. .
- 41.Yamada-Okabe T, Sakai H, Kashima Y, and Yamada-Okabe H. Modulation at a cellular level of the thyroid hormone receptor-mediated gene expression by 1,2,5,6,9,10-hexabromocyclododecane (HBCD), 4,4’-diiodobiphenyl (DIB), and nitrofen (NIP). Toxicol Lett. 155: 127–133 2005. [DOI] [PubMed] [Google Scholar]
- 42.Hamers T, Kamstra JH, Sonneveld E, Murk AJ, Kester MH, Andersson PL, Legler J, and Brouwer A. In vitro profiling of the endocrine-disrupting potency of brominated flame retardants. Toxicol Sci. 92: 157–173 2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.