Abstract
Some anticancer therapeutic antibodies are designed to act through complement-dependent cytotoxicity (CDC). It has been reported that there are many membrane complement regulatory proteins (mCRPs) that inhibit CDC. In the present study, we examined the expression of two mCRPs, the complement receptor 1-related gene/protein Y (Crry) and the decay-accelerating factor CD55, in three normal rats by immunohistochemistry. Crry and CD55 were detected widely in rat organs and tissues. Crry was found mainly in the urinary, digestive, respiratory, immunohematopoietic, circulatory and neuroendocrine systems. CD55 was found in the urinary, digestive and neuroendocrine systems. However, the two molecules were expressed in separate cells within the same organ. These results suggest that the distribution of mCRPs is related to the strict regulation of CDC activation in these organs and tissues and that the two molecules have a nonoverlapping expression pattern, a fact indicating specific roles in CDC regulation.
Keywords: membrane complement regulatory proteins, Crry, CD55, rat
Many anticancer therapeutic antibodies block the physiological function of their target antigens by neutralization or by inducing complement-dependent cytotoxicity (CDC) or antibody-dependent cell-mediated cytotoxicity, or they are designed to act as drug delivery carriers (missile therapy)1. There are three known activation pathways of CDC—the classical, lectin (MBL/Ficolin), and alternative pathways—of which the classical pathway is the main pathway for antibody-mediated CDC2. It has been reported that there are many systems regulating the classical complement activation pathway. These regulatory systems include membrane complement regulatory proteins (mCRPs), such as complement receptor 1-related gene/protein Y (Crry), decay-accelerating factor CD55, membrane cofactor protein (MCP) CD46, complement receptor 1 (CR1) CD35, and CD59, or soluble complement regulatory proteins, such as factor I, factor H, C1 inhibitor and C4-binding protein3,4.
The molecules that function in the complement regulatory system differ between human and animal species3,5. In the rat, we have previously demonstrated that complement activation induced by the anti-Thy-1.1 antibody was not only regulated by the distribution of the injected antibody to the antigen but also by them CRPs expressed in the anti-Thy-1 glomerulonephritis model6. Based on that result, we considered that analysis of Crry and CD55 expression is important to understand the efficacy and toxicity of therapeutic antibodies with CDC functions. However, there are only a few reports concerning the distribution or functions of these mCRPs in the rat7,8,9,10. Here we examined the expression and tissue distribution of two mCRPs, Crry and CD55, in the normal rat and found that the two molecules are expressed in separate cells. The nonoverlapping expression of the two molecules was thought to be a novel finding related to the regulation mechanism of CDC in the rat.
A total of 3 male Wistar rats aged 6 weeks were purchased from Japan SLC, Inc. (Shizuoka, Japan) and used in this experiment at 7 weeks of age. They were housed in wire cages in an environmentally controlled room (temperature of 23 ± 3°C, relative humidity of 55 ± 20%, ventilation rate of 10–16 times per hour and 12-h/12-h light/dark cycle), and given pelleted chow (CE-2; Clea Japan, Inc., Tokyo, Japan) and tap water ad libitum. Animals were sacrificed by exsanguination under anesthesia for pathological examination. All experiments on the animals were approved by the Ethical Committee for Treatment of Laboratory Animals at Chugai Pharmaceutical Co., Ltd.
At necropsy, the kidneys, bladder, small intestine, large intestine, pancreas, liver, lungs, thymus, mesenteric lymph nodes, spleen, adrenal glands, heart, skeletal muscle and sciatic nerve were removed from each animal. The tissues were processed and embedded in paraffin by the PLP-AMeX method11. Tissue sections were cut at 3–5 μm from the paraffin blocks for immunohistochemical staining.
Immunohistochemistry for Crry and CD55 was carried out in all collected organs. Antibodies against Crry (512, BD PharMingen, San Jose, CA, USA, 0.7 μg/mL) and CD55 (I-19, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA, 8 μg/mL) were used as the primary antibodies and applied to the tissues. Isotype and species-matched antibodies were used as negative controls. Immunohistochemical staining was performed according to the labeled streptavidin-biotin (LSAB) method with a Dako LSAB kit (Dako Denmark A/S, Glostrup, Denmark). Antigen retrieval for both Crry and CD55 by microwave heating in 0.01 M citrate buffer (pH 6.0) at 98˚C in a microwave oven (H2800; Energy Beam Sciences, East Granby, CT, USA) was performed prior to applying the primary antibody. The immunoreaction was visualized by a peroxidase-diaminobenzidine reaction. The sections were counterstained with hematoxylin.
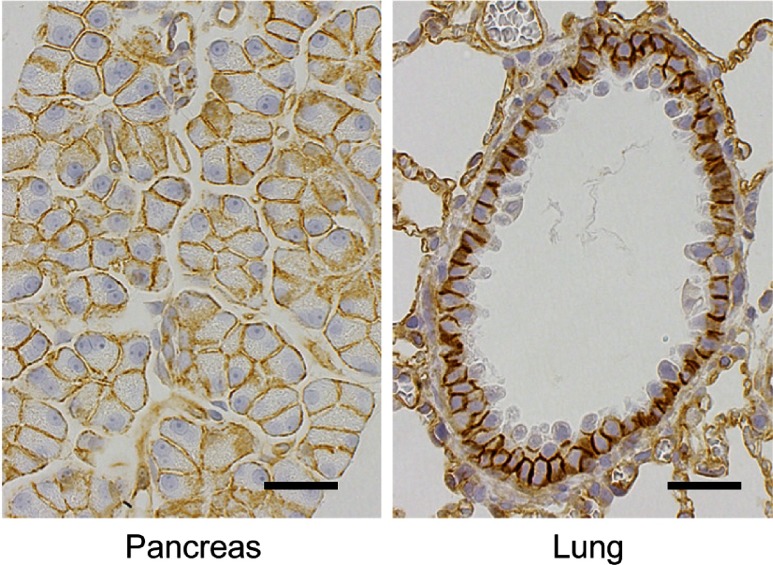
Crry or CD55 was detected widely in rat organs and tissues, but the distribution of the two molecules rarely overlapped (Table 1 and Fig. 1). In most organs, expression of Crry was observed. In the urinary system, renal tubular epithelial cells, transitional epithelial cells in the renal pelvis, mesangial cells of the kidney and transitional epithelial cells, basal and intermediate cells, of the bladder were positive (Table 1 and Fig. 1). In the digestive system, absorptive epithelial cells, goblet cells and stromal cells in the lamina propria of the intestine, and exocrine glandular cells in the pancreas were positive (Table 1 and Fig. 1 and 2). In addition, bronchial epithelial cells of the lung were found positive in the respiratory system (Table 1 and Fig. 2). In the immunohematopoietic system, expression of Crry was observed in lymphocytes of the thymus, mesenteric lymph nodes and GALT (gut-associated lymphoid tissue), megakaryocytes of the spleen and follicular stromal cells of the spleen, mesenteric lymph nodes and GALT (Table 1). In the circulatory system, endothelial cells of multiple organs were also positive (Table 1 and Fig. 2). As for the neuroendocrine system, the medullary and cortical cells of the adrenal gland were positive (Table 1 and Fig. 1). CD55 was found in the urinary and digestive system, such as the kidney, bladder and liver. Positive cells were podocytes of the kidney, transitional epithelial cells, umbrella cells, of the bladder and hepatocytes of the liver (Table 1 and Fig. 1). In the neuroendocrine system, CD55 was observed in medullary cells of the adrenal gland and enteroendocrine cells (basal-granulated cells) of the intestine (Table 1 and Fig. 1). The medullary cells of the adrenal gland expressed both molecules, with high expression of CD55 and much lower expression of Crry (Fig. 1). The tissue elements of the heart, skeletal muscle and sciatic nerve were negative except for the endothelial cells. The staining patterns of Crry and CD55 were consistent between animals. Our result differ slightly from previous reports7,9, which was thought due to differences in the tissue processing and immunohistochemical method.
Table 1. Distribution of mCRPs in Normal Rats.
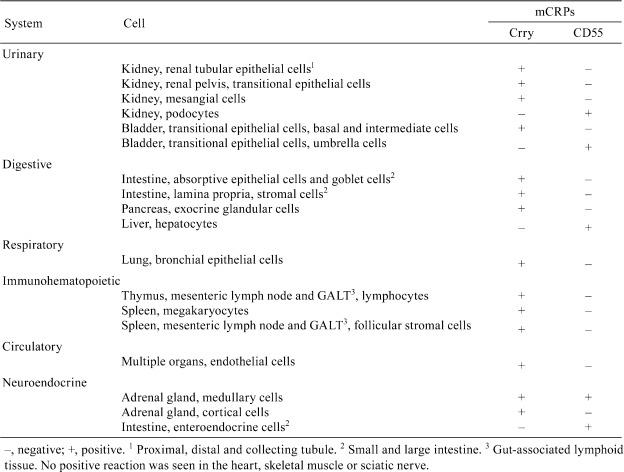
Fig. 1.
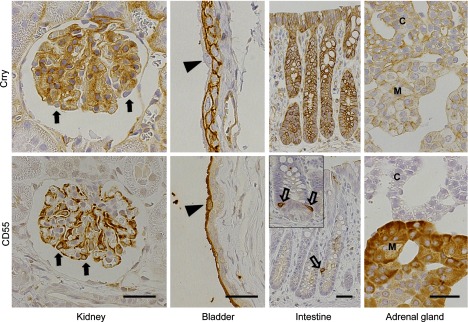
Immunohistochemistry of Crry and CD55 in the kidney, bladder, intestine and adrenal gland of normal rat. In the kidney, mesangial cells are positive for Crry and podocytes are positive for CD55 but negative for Crry (closed arrows). In the bladder, basal and intermediate transitional cells are positive for Crry, and umbrella transitional cells are positive for CD55 but negative for Crry (arrowheads). In the large intestine, absorptive epithelial cells and goblet cells are positive for Crry, and enteroendocrine cells are positive for CD55 (open arrow). CD55-positive enteroendocrine cells are also observed in the small intestine (insert, open arrows). In the adrenal gland, both molecules are expressed: medullary cells are strongly positive for CD55 and weakly positive for Crry, and cortical cells express Crry weakly. M, medulla; C, cortex. Bar = 30 µm.
Fig. 2.
Immunohistochemistry of Crry in the pancreas and lung in normal rat. Positive reaction is observed in exocrine glandular cells in the pancreas and bronchial epithelial cells in the lung. In addition, endothelial cells in the alveoli are also positive. Bar = 30 µm.
mCRPs, particularly Crry, were mainly found in epithelial tissues related to excretion, absorption and digestion: the urinary system, the digestive system and the respiratory system, which all contain tissues that are readily exposed to external pathogens. The complement system can be effective in destroying external pathogens, but unintended activation of complements could cause unnecessary injury. Thus the distribution of mCRPs may be subjected to tight regulation of nonspecific activation in these tissues. To better elucidate the biological meaning of the expression pattern of mCRPs, it may be worth examining the relationship between complement activation and expression of mCRPs.
From the view point of anatomical systems, Crry and CD55 were co-expressed in the same organs; however, expression of Crry or CD55 was distinctly different between cells (Table 1 and Fig. 1). For instance, there was a difference between mesangial cells and podocytes of the kidney, basal, intermediate and umbrella transitional epithelial cells of the bladder, and absorptive epithelial cells, goblet cells and enteroendocrine cells of the intestine (Table 1 and Fig. 1).
These nonoverlapping expression patterns are characteristic and unique, which is indicative of a biological meaning. It is well known that both Crry and CD55 regulate C3 activation. Crry has two main regulating mechanisms: the first mechanism prevents formation of the C3 convertase and accelerates its dissociation (decay-accelerating activity), and the second mechanism controls the ability (mediated by a serine esterase factor I in the presence of protein cofactors) to cleave activated C33,8,12,13. CD55 inhibits activation of C3 and C5 by preventing the formation of new C3 and C5 convertases and also by accelerating the decay of the preformed convertases, the so-called decay-accelerating activity3,10,14. The two molecules have a common function in inhibiting C3 deposition, but the present results show that they have a separate expression pattern, a fact that indicates specific roles in CDC regulation.
It is known that there are differences in mCRPs molecules between species3. Crry is defined as an mCRP in the mouse and rat, although it does not exist in the human3,7,8,12,13. On the other hand, CD55 is known to be expressed in the mouse, rat and human3,9,10. In the human, CD55 and MCP are known as the main mCRPs that regulate C3 activation, and it has been proposed that the biochemical C3 regulatory properties of Crry in the rat are similar 3,5,12,13,14,15,16. We have found in a previous study that antibody-induced CDC is regulated by mCRPs6; therefore, we believe that rodent models are suitable for a meticulous evaluation of the impact of mCRPs on the efficacy and toxicity of therapeutic antibodies. The species difference should be kept in mind when interpreting the results of preclinical experiments, and when considering their relevance to the human, it is important to remember the differences of these molecules and their function between animals and humans.
In conclusion, we examined the expression of the mCRPs Crry and CD55 in the normal rat. Crry or CD55 were detected widely in rat organs and tissues: Crry was found mainly in tissues of the urinary, digestive, respiratory, immunohematopoietic, circulatory and neuroendocrine systems, and CD55 was found in tissues of the urinary, digestive and neuroendocrine systems. However, the two molecules were expressed in separate cells within the same organ. These results suggest that the distribution of mCRPsis related to the strict regulation of CDC activation in these tissues and that the two molecules have a nonoverlapping expression pattern, a fact indicating specific roles in CDC regulation. Moreover, we believe that rodent models are suitable for meticulous evaluation of the impact of mCRPs on the efficacy and toxicity of therapeutic antibodies.
Acknowledgments
We would like to thank Ms. Shino Teruya for technical assistance in tissue preparation and performing immunohistochemical staining.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (by-nc-nd) License <http://creativecommons.org/licenses/by-nc-nd/3.0/>.
References
- 1.Scott AM, Wolchok JD, and Old LJ. Antibody therapy of cancer. Nat Rev Cancer. 12: 278–287 2012. [DOI] [PubMed] [Google Scholar]
- 2.Ricklin D, Hajishengallis G, Yang K, and Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 11: 785–797 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miwa T, and Song WC. Membrane complement regulatory proteins: insight from animal studies and relevance to human diseases. Int Immunopharmacol. 1: 445–459 2001. [DOI] [PubMed] [Google Scholar]
- 4.Fraczek LA, and Martin BK. Transcriptional control of genes for soluble complement cascade regulatory proteins. Mol Immunol. 48: 9–13 2010. [DOI] [PubMed] [Google Scholar]
- 5.Kim DD, and Song WC. Membrane complement regulatory proteins. Clin Immunol. 118: 127–136 2006. [DOI] [PubMed] [Google Scholar]
- 6.Kato C, Kato A, Adachi K, Fujii E, Isobe K, Matsushita T, Watanabe T, and Suzuki M. Anti-Thy-1 antibody-mediated complement-dependent cytotoxicity is regulated by the distribution of antigen, antibody and membrane complement regulatory proteins in rats. J Toxicol Pathol. 26: 41–49 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Funabashi K, Okada N, Matsuo S, Yamamoto T, Morgan BP, and Okada H. Tissue distribution of complement regulatory membrane proteins in rats. Immunology. 81: 444–451 1994. [PMC free article] [PubMed] [Google Scholar]
- 8.Takizawa H, Okada N, and Okada H. Complement inhibitor of rat cell membrane resembling mouse Crry/p65. J Immunol. 152: 3032–3038 1994. [PubMed] [Google Scholar]
- 9.Spiller OB, Hanna SM, and Morgan BP. Tissue distribution of the rat analogue of decay-accelerating factor. Immunology. 97: 374–384 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hinchliffe SJ, Spiller OB, Rushmere NK, and Morgan BP. Molecular cloning and functional characterization of the rat analogue of human decay-accelerating factor (CD55). J Immunol. 161: 5695–5703 1998. [PubMed] [Google Scholar]
- 11.Suzuki M, Katsuyama K, Adachi K, Ogawa Y, Yorozu K, Fujii E, Misawa Y, and Sugimoto T. Combination of fixation using PLP fixative and embedding in paraffin by the AMeX method is useful for histochemical studies in assessment of immunotoxicity. J Toxicol Sci. 27: 165–172 2002. [DOI] [PubMed] [Google Scholar]
- 12.Kim YU, Kinoshita T, Molina H, Hourcade D, Seya T, Wagner LM, and Holers VM. Mouse complement regulatory protein Crry/p65 uses the specific mechanism of both human decay-accelerating factor and membrane cofactor protein. J Exp Med. 181: 151–159 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molina H. The murine complement regulator Crry: new insights into the immunobiology of complement regulation. Cell Mol Life Sci. 59: 220–229 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lublin DM, and Atkinson JP. Decay-accelerating factor: biochemistry, molecular biology, and function. Annu Rev Immunol. 7: 35–58 1989. [DOI] [PubMed] [Google Scholar]
- 15.Liszewski MK, Post TW, and Atkinson JP. Membrane cofactor protein (MCP or CD46): newest member of the regulators of the complement activation gene cluster. Annu Rev Immunol. 9: 431–455 1991. [DOI] [PubMed] [Google Scholar]
- 16.Turnberg D, and Botto M. The regulation of the complement system: insights from genetically-engineered mice. Mol Immunol. 40: 145–153 2003. [DOI] [PubMed] [Google Scholar]