Abstract
Chemotaxis signals between hepatic stellate cells (HSC) and sinusoidal endothelial cells (SEC) maintain hepatic vascular homeostasis and integrity and also regulate changes in sinusoidal structure in response to liver injury. Our prior studies have demonstrated that the bidirectional chemotactic signaling molecules EphrinB2 and EphB4 are expressed in HSC. The aim of our present study was to explore whether and how the EphrinB2/EphB4 system in HSC could promote SEC recruitment, which is essential for sinusoidal structure and remodeling. Stimulation of human HSC (hHSC) with chimeric agonists (2 μg/ml) of either EphrinB2 or EphB4 (EphrinB2 Fc or EphB4 Fc, respectively) significantly increased VEGF mRNA levels in hHSC as assessed by quantitative PCR, with respective small interfering RNAs for EphrinB2 and EphB4 inhibiting this increase (P < 0.05, n = 3). EphrinB2 agonist-induced increase in VEGF mRNA levels in hHSC was associated with increased phosphorylation of Erk and was significantly blocked by U0126 (20 μM), an inhibitor of MEK, which is a kinase upstream from Erk (P < 0.05, n = 3). The EphB4 agonist also significantly increased human VEGF promoter activity (P < 0.05, n = 3) as assessed by promoter reporter luciferase assay in transfected LX2-HSC. This was associated with upregulation of the vasculoprotective transcription factor, Kruppel-like factor 2 (KLF2). In Boyden chamber assays, conditioned media from hHSC stimulated with agonists of EphrinB2 or EphB4 increased SEC chemotaxis in a VEGF-dependent manner, compared with control groups that included basal media with agonists of EphrinB2, EphB4, or HSC-conditioned media from HSC in absence of agonist stimulation (P < 0.05, n = 3). EphB4 expression was detected in situ within liver sinusoidal vessels of rats after carbon tetrachloride-induced liver injury. In summary, activation of the EphrinB2/EphB4 signaling pathway in HSC promotes chemotaxis of SEC through a pathway that involves Erk, KLF2, and VEGF. These studies identify EphrinB2 or EphB4 as a key intermediary that links HSC signal transduction pathways with angiogenesis and sinusoidal remodeling.
Keywords: hepatic stellate cell, sinusoidal endothelial cell, VEGF, ephrins
hepatic stellate cells (HSC) are liver-specific pericytes (3, 30), which signal with adjacent sinusoidal endothelial cells (SEC) to regulate vascular homeostasis and sinusoidal structural changes that occur in response to liver injury and chronic liver disease (1, 19, 34). HSC embrace SEC with their long cytoplasmic processes and extensions that ideally position HSC for paracrine signaling with SEC. Indeed, multiple molecules are implicated in HSC-SEC cross talk, especially vascular endothelial growth factor A (VEGF), which is a key molecule released by HSC that promotes SEC migration, recruitment, and angiogenesis (19, 26, 38). Although failure of such signaling results in severe vascular defects (20, 22, 23), the molecular mechanisms that mediate HSC-SEC cross talk mechanisms such as VEGF secretion are not fully defined.
Eph receptor tyrosine kinases constitute a large family of transmembrane proteins with a single cytoplasmic kinase domain that is activated in response to binding of ephrin ligands to the extracellular globular domain of the receptor (11). Based on their ligand-binding characteristics, Ephs have been subdivided into Eph A and Eph B receptors, although significant redundancy and cross talk exists between subclasses (28). Several Eph/ephrin molecules are expressed in blood vessels, and targeted inactivation of genes encoding EphrinB2 and EphB4 in mice has demonstrated their essential role for angiogenesis and vascular remodeling (2, 15, 40). Our prior studies (31) have identified the ephrin family of proteins as key regulators of HSC motility downstream from platelet derived growth factor (PDGF) stimulation. However, effects of this class of molecules on release of angiogenic factors from HSC has not been explored. Therefore, these proteins appear to be interesting candidates for studying SEC and HSC cross talk.
In this study, we interrogate the role of ephrins in SEC recruitment signaling. Especially since vascular endothelial growth factor (VEGF) is a canonical SEC chemotactic agent secreted by HSC, we hypothesized that ephrin activation in HSC may promote recruitment of SEC through HSC secretion of VEGF. Indeed, we demonstrate, using complementary mechanistic approaches, that ephrin-Eph interactions regulate VEGF gene transcription in HSC and that this in turn promotes SEC recruitment. Additionally, we provide insights into several key signal intermediaries that determine this process both in cytosol and in nucleus. We anticipate that these results should expand our understanding of the dynamic interplay of SEC and HSC within the hepatic sinusoids.
MATERIALS AND METHODS
Cell culture.
All tissue culture reagents were obtained from GIBCO (Rockville, MD). Isolated primary human HSC (ScienCell Research Laboratories) and primary human SEC (ScienCell Research Laboratories) were used between passages 2 and 6 (31). HSC and SEC were cultured in defined medium obtained from the vendor and supplemented with 10% fetal bovine serum, penicillin (100 IU/ml), and streptomycin (100 μg/ml).
EphB4/EphrinB2 chimeras.
EphB4 and EphrinB2 chimeras were purchased from R & D Biosystems (Minneapolis, MN). EphrinB2 and EphB4 chimeras consisted of the extracellular domain of the respective ephrin protein fused to the Fc portion of human IgG and were utilized to activate EphB4 and EphrinB2, respectively (14, 31).
Quantitative real-time PCR.
Total RNA was extracted from HSC by using the RNeasy kit according to the manufacturer's instruction (Qiagen, Valencia, CA). Total RNA (1 μg) was used for the cDNA synthesis by using random hexamer primer of the SuperScript III First-Strand Synthesis System (Invitrogen, Carlsbad, CA). For TaqMan-based real-time PCR analysis, 25 ng of each cDNA was added to the Taqman Universal PCR Master Mix along with 900 nM of each primer and 200 nM of probe according to the manufacturer's instruction (Applied Biosystems, Carlsbad, CA). Real-time fluorescence monitoring was performed with the Applied Biosystems 7500 Real-time PCR System instrument. Amplification of human GAPDH and eukaryotic 18S rRNA was used in the same reaction of all samples as an internal control. Gene-specific mRNA was subsequently normalized to GAPDH mRNA. Levels of VEGF A mRNA were expressed as fold difference of EphrinB2 Fc- or EphB4 Fc-treated cells compared with vehicle-treated cells. For KLF2 mRNA levels, SYBRgreen was used for quantitative real-time PCR with GAPDH mRNA serving as normalization control.
Gene silencing.
Small interfering RNA (siRNA) targeting human EphrinB2, EphB4, and a scrambled control were obtained from Qiagen (Valencia, CA). Cells were transfected with siRNA by use of oligofectamine (Invitrogen). Conditions and concentrations required for specificity of knockdown with high transfection efficiency were individually established. In some experiments, reconstitution of functional EphrinB2 signaling was achieved by addition of the agonistic recombinant EphrinB2 Fc chimera.
VEGF ELISA.
VEGF-A concentration in supernatants of vehicle, EphrinB2-FC (2 μg/ml), and EphB4-FC (2 μg/ml)-stimulated HSC were measured by use of a hVEGF Quantitative kit (R & D Biosystems) as per the manufacturer's instructions. Data were calculated as picograms of VEGF per microgram of cellular protein.
Luciferase promoter assay.
LX2 cells, an immortalized human HSC line (18), were transfected with human VEGF-promoter-luciferase reporter constructs (pVEGF) and 0.01 μg Renilla luciferase reporter vector to control for transfection efficiency (pRL-TK) by use of Lipofectamine 2000 (Invitrogen). At 10 h later, culture medium was changed and cells were cultured for an additional 12–14 h in presence or absence of stimulation by Fc chimeras of EphrinB2 or EphB4. In a separate set of experiments, LX2 cells were cotransfected with human pVEGF and either EphrinB2/EphB4 siRNA or scrambled (control) siRNA. Luciferase assays were conducted by using a dual luciferase kit (Promega) as described previously (8, 9).
Immunoblotting.
Cells were homogenized in a lysis buffer (50 mM Tris·HCl, 0.1 mM EGTA, 0.1 mM EDTA, 1% NP-40, 0.1% deoxycholic acid, 0.1% SDS, pH 7.5). Protein quantification of samples was performed with the Lowry assay. Detergent-soluble protein lysates were separated by SDS-PAGE on a 10% acrylamide gel, and proteins were transferred onto nitrocellulose membrane. The membranes were washed in Tris-buffered saline with 0.05% Tween, blocked in 5% nonfat dry milk, and incubated with p44/42 MAPK, phospho-p44/42 MAPK antibody (Ab) (Thr202/Tyr204), Akt, and phospho-Akt Ab (Ser473 and Thr308) (Cell Signaling, Santa Cruz, CA). Membrane was reprobed for actin to confirm equal protein loading and transfer between lanes.
Boyden chamber assay.
Cell migration assays were performed by using a Boyden chamber (Becton Dickinson, Heidelberg, Germany) with 12-μm-pore-size polycarbonated filters coated with type I collagen (50 μg/ml). Cells suspended in serum free medium were seeded to the upper wells (20 × 103 cells/well) and lower chambers were filled with 26 μl of EphrinB2-Fc, EphB4-Fc, or vehicle-treated HSC cell culture supernatant (conditioned media). In another set of experiments, cell culture supernatants from vehicle, EphrinB2-Fc or EphB4-Fc-stimulated HSC, preincubated with either VEGF-A neutralizing antibody (10 μg/ml) (R & D Biosystems) or isotype IgG control (10 μg/ml), were used to assess VEGF dependency of the effect. After 4 h of incubation at 37°C the polycarbonated filter was removed and migrated cells on the lower surface were stained with HEMA-3. Cells passed through the filter were quantified from random microscopic fields. Each assay was carried out in triplicate with six replicates of each group per assay. Results are expressed as the mean number of migrated cells ± SD.
Confocal immunofluorescence microscopy.
Liver tissues from CCl4-treated rats were harvested and embedded in optimal cutting temperature compound. Then 5-μm sections were cut and mounted on slides. Samples were blocked with 10% goat serum in PBS for 1 h and then incubated with goat anti-mouse EphB4 (1:50), rabbit monoclonal PDGFR-β antibody (1:80), and von Willebrand factor (vWF) antibody [1:400; vWF is detected in situ on SEC after liver injury (33)] overnight at 4°C degrees, followed by incubation for 1 h with Alexa Fluor 488-conjugated donkey anti-goat (1:250) and Alexa Fluor 546-conjugated goat anti-rabbit (1:250) secondary antibodies, respectively. The slides were then counterstained with Toto3, and confocal microscopy was performed by LSM 5 Pascal (Carl Zeiss, Thornwood, NY).
Statistical analysis.
Experiments were performed in triplicate with a minimum of three independent experiments. Data are depicted as means ± SE. Comparisons were performed via Student's t-test or one-way analysis of variance when comparing more than two sample groups, with statistical significance set at P < 0.05.
RESULTS
EphrinB2 and EphB4 activation stimulates VEGF secretion by HSC.
HSC interact with SEC through bidirectional paracrine signaling to form angiogenic vascular networks in liver. We had previously shown that PDGF signaling through EphrinB2 regulates HSC motility and vascular coverage (31); however, effects on SEC were not explored. Since VEGF signaling is critical for endothelial cell sprouting, proliferation, migration, permeability, and survival signaling (10), we looked at the ability of HSC to produce VEGF in response to EphrinB2/EphB4 stimulation. VEGF mRNA levels were increased after stimulation with EphrinB2/EphB4 Fc chimera as measured by quantitative real-time PCR (Fig. 1A). The stimulation of VEGF-A mRNA induced by EphrinB2/EphB4 Fc occurred in a time-dependent manner (Fig. 1A). Additionally, cycloheximide, an inhibitor of protein translational, blocked EphB4 Fc-induced increases in VEGF mRNA, indicating that the pathway of regulation involved de novo protein translation (Fig. 1B). To further corroborate these results, we silenced EphrinB2 and EphB4 with their respective siRNA, both reagents that we have previously validated with regard to their specificity and magnitude of knockdown (31). Interestingly, VEGF mRNA levels were significantly decreased by 40% in presence of either EphrinB2 siRNA or EphB4 siRNA, indicating that inhibition of endogenous cell-to-cell activation of EphrinB2/EphB4 signaling is important for maintaining basal VEGF mRNA production (Fig. 1C). Furthermore, this siRNA-induced decrease in VEGF A mRNA expression could be reconstituted and amplified by adding back the relevant Fc chimeras in an exogenous manner (Fig. 1C). A similar and statistically significant effect, albeit quantitatively smaller, was also observed by VEGF ELISA performed from supernatants of EphrinB2-Fc-stimulated HSC (vehicle: 373.2 pg/μg ± 2.5 pg/μg vs. EphrinB2 FC: 413.6 pg/μg ± 6.7 pg/μg, n = 3 separate experiments; P < 0.05 between the groups). Thus these studies demonstrate that EphrinB2/EphB4 activation increases VEGF mRNA levels in HSC.
Fig. 1.
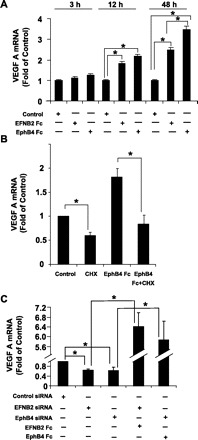
EphrinB2 and EphB4 increase VEGF mRNA levels in human hepatic stellate cells (HSC). HSC were treated with EphrinB2 chimera (2 μg/ml), EphB4 chimera (2 μg/ml), and/or respective small interfering RNA (siRNA) for 3, 12, and 48 h, respectively, and the total RNA was extracted for VEGF mRNA estimation by quantitative RT-PCR analysis. A: there was a significant increase in VEGF mRNA levels in both EphrinB2- and EphB4-treated groups compared with their respective time controls at 12 and 48 h (n = 3 separate experiments, each in triplicate, *P ≤ 0.05). B: cyclohexamide (10 μg/ml) blocked EphB4 Fc-induced VEGF A mRNA production (n = 3 separate experiments, each in triplicate, *P ≤ 0.05). C: basal VEGF mRNA levels were attenuated by incubating HSC with EphrinB2 or EphB4 siRNA (n = 3 separate experiment, each in triplicate, *P ≤ 0.05). Stimulation of siRNA-transfected cells with EphrinB2 and EphB4 chimeras reversed the siRNA-mediated inhibitory effect on VEGF mRNA levels.
Ephrins activate VEGF transcription by regulating its promoter activity.
To explore the potential mechanisms of VEGF transcription regulation by EphrinB2 and EphB4, we studied the activity of a human VEGF reporter luciferase construct (pVEGF) in presence or absence of ephrin Fc chimeras in LX2 cells. Interestingly, the EphB4 Fc chimeric agonist was more potent than the EphrinB2 Fc and significantly increased VEGF promoter activity by 2.3-fold (Fig. 2A). To substantiate the role of EphB4 on VEGF gene transcription, we performed reporter assays in cells cotransfected with human VEGF promoter and either EphrinB2 siRNA or EphB4 siRNA or scrambled (control) siRNA. Relative luciferase activity in cells cotransfected with either EphrinB2 or EphB4 siRNA was significantly decreased by 40–50% compared with cells cotransfected with scrambled siRNA, consistent with the effects of these siRNA VEGF mRNA levels shown in Fig. 1 and reflective of the effects of endogenous cell-cell EphrinB2/EphB4 interactions on VEGF transcription (Fig. 2B). This decrease in VEGF promoter activity could again be rescued by the respective Fc chimeras, which increased the relative luciferase activity with EphB4 Fc, again showing a more prominent effect than EphrinB2 Fc (Fig. 2B). Lastly, EphrinB2/EphB4-stimulated HSC showed an increase in mRNA levels of the vasculoprotective transcription factor KLF2 (27) compared with the vehicle-treated group as assessed by quantitative real-time PCR (Fig. 2C). In summary, these results indicate a significant effect of EphrinB2 and EphB4 on VEGF gene transcription.
Fig. 2.

EphB4 activates VEGF promoter. A: LX2 cells cotransfected with Renilla control and full-length human VEGF reporter luciferase construct (pVEGF) showed an increased luciferase activity upon stimulation with both EphrinB2 (2 μg/ml) and EphB4 (2 μg/ml) chimeras, with EphB4 stimulation achieving a statistically significant effect (n = 3 separate experiments, each in triplicate, *P < 0.05). B: knockdown of EphrinB2 or EphB4 with their respective siRNA resulted in a 40–50% reduction in luciferase activity compared with control siRNA, whereas stimulation of siRNA-knockdown cells with activating chimera reconstituted basal promoter activity (n = 3 separate experiments, each in triplicate, *P < 0.05). C: stimulation of LX2 cells by EphB4 or EphrinB2 chimeras significantly increased KLF2 mRNA levels as assessed by quantitative real-time PCR (n = 3 separate experiments, each in triplicate, *P < 0.05).
EphrinB2/EphB4 regulate VEGF mRNA levels via Erk signaling.
We next sought to identify key cytosolic signaling intermediates that may regulate EphrinB2/EphB4 stimulation of the VEGF promoter. We focused our attention on Erk and Akt (Ser473 and Thr308), both key signaling intermediaries implicated in VEGF production (12, 17, 24, 36). Western blot analysis showed that EphrinB2/EphB4 stimulation in HSC signals intracellularly via Erk phosphorylation (although increasing doses of chimera did not have an incremental intracellular response) (Fig. 3A). Interestingly, the positive control PDGF stimulated both Erk and Akt, whereas EphrinB2/EphB4 activation specifically activated Erk but not Akt (Fig. 3B). The specificity of Erk phosphorylation by EphrinB2/EphB4 was further examined by using the MEK inhibitor U0126. EphrinB2 induced Erk phosphorylation was significantly reduced in cells stimulated with U1026 (Fig. 3B). Lastly, VEGF mRNA levels were analyzed in EphrinB2/EphB4-stimulated HSC pretreated with U0126. VEGF mRNA levels were significantly reduced after pretreatment of cells with U0126, indicating that EphrinB2/EphB4 stimulates VEGF release from HSC through an Erk-dependent but Akt-independent pathway (Fig. 3C).
Fig. 3.
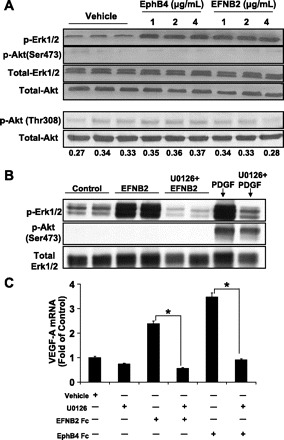
Ephrin-induced VEGF-A upregulation requires Erk phosphorylation. A: EphrinB2 (EFNB2) and EphB4 stimulation of HSC increased Erk phosphorylation compared with vehicle, whereas Akt phosphorylation (pAKT-Ser473 and pAKT-Thr308) was unchanged (0–4 μg/ml; n = 3 separate experiments; pThr308-to-total Akt ratio from densitometric analysis is shown below the Western blot). B: U0126 (10 μm) attenuated the increase in Erk phosphorylation as assessed by Western blot. Total Erk served as a protein loading control. PDGF stimulation was used as a positive control for Erk and Akt activation. C: U0126 (10 μm) decreased VEGF mRNA levels in EphrinB2- and EphB4-stimulated cells compared with vehicle (*P < 0.05).
EphrinB2/EphB4 stimulation of HSC promotes SEC migration through a VEGF-dependent mechanism.
SEC migration is a key step in angiogenesis and therefore to ascertain whether EphrinB2/EphB4 stimulation of HSC was sufficient to mediate HSC release of chemotactic molecules that regulate SEC migration, HSC were stimulated with EphrinB2 or EphB4 Fc chimera and conditioned medium was collected to ascertain chemotactic effects of the conditioned media on SEC by use of a modified Boyden chamber. Indeed, conditioned media from EphrinB2 Fc-stimulated HSC enhanced SEC chemotaxis compared with its relevant control groups, including non-chimera-stimulated conditioned media and nonconditioned chimera-containing media (Fig. 4A; *P < 0.05 for EphrinB2-Fc vs. conditioned media). Preincubation of the condition media with either VEGF-A-neutralizing antibody (10 μg/ml) significantly reduced (∼50%) the ability of SEC to migrate in response to EphrinB2 Fc compared with an isotype IgG control-treated group (Fig. 4B). The neutralizing antibody also significantly reduced migration by >50% in response to EphB4 Fc as well (data not shown). Thus these studies highlight the role of EphrinB2/EphB4 stimulation of HSC as a mechanism to promote SEC chemotaxis through VEGF-A production.
Fig. 4.

Conditioned media from EphrinB2/EphB4-stimulated HSC increases sinusoidal endothelial cell (SEC) migration through VEGF-A production. A: conditioned medium (CM) from HSC stimulated with EphrinB2-Fc, EphB4-Fc, or vehicle for 48 h was used to assess the migratory effect on SEC via Boyden chamber. Ave No., average number; HHSEC, human hepatic sinusoidal endothelial cells; Ab, antibody. Media from both EphrinB2- and EphB4-treated groups (4th and 6th bars) increased SEC migration compared with the media from the vehicle-treated HSC (2nd bar) and compared with groups where basal medium (BM) containing EphrinB2 or EphB4 was used as a chemokine (3rd and 5th bars) (n = 3 separate experiment, *P < 0.05). B: conditioned media from vehicle or EphrinB2-Fc-treated HSC when preincubated with VEGF-A neutralizing antibody (10 μg/ml) significantly decreased (∼50% reduction) the ability of SEC to migrate compared with the respective groups in which the conditioned medium was preincubated with isotype IgG control (n = 3 separate experiments, *P < 0.05).
Lastly, since EphrinB2/EphB4 activation and signaling require cell-to-cell contact because both the ligand and its cognate receptor are cell membrane bound and the ligand and receptor both signal bidirectionally, we looked at the expression of EphB4 in rats with CCl4-induced liver injury, since the process of liver fibrosis involves robust angiogenic responses (13) requiring cross talk between EC and HSC for sinusoidal remodeling. Although the immunofluorescent signal was very low in vehicle-treated rats (data not shown), CCl4-treated rats evidenced prominent expression of EphB4 within the sinusoids with close proximity of both EC and HSC (Fig. 5), thereby providing in situ positioning of ephrins within the hepatic sinusoids.
Fig. 5.
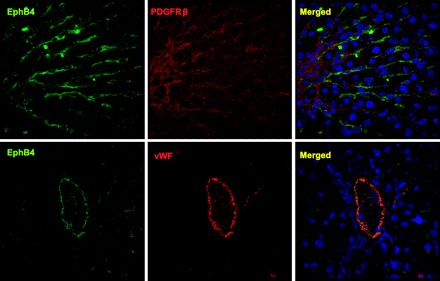
EphB4 is localized within vascular structures during hepatic remodeling in cirrhosis. Liver tissues of rats administered either vehicle or CCl4 were fixed and coimmunostained for either EphB4 and an HSC marker, PDGFRβ or EphB4, and a marker of SEC after liver injury, von Willebrand factor (vWF). Top: EphB4-positive sinusoidal cells are seen in close proximity to PDGFRβ-expressing myofibroblasts. Bottom: colocalization of EphB4 with vWF within a vessel in the cirrhotic liver samples.
DISCUSSION
Hepatic microcirculation, by virtue of its sinusoidal configuration, modulates hepatic blood flow and facilitates transport of nutrients to parenchymal liver cells. The structural proximity and communication between HSC and SEC within the sinusoids is essential for maintaining sinusoidal integrity and function. Paracrine signaling between these two predominant cell populations in the sinusoids also plays a significant role in settings of chronic liver diseases (10, 31) wherein remodeling and distortion of the sinusoidal architecture occurs in coordination with derangements of vascular growth factor production such as VEGF (7, 25, 29). Although the ability of HSC to secrete VEGF (4) and their angiogenic effects (26) are well known, the pathways that modulate VEGF production from HSC are not as well defined. In this regard, the present studies identify a novel observation, expanded with mechanistic details, whereby the ephrin signaling pathway regulates HSC-derived VEGF production and ensuing SEC recruitment.
Ephrins have been shown to play an important role in regulation of vascular assembly and homeostasis (6). Indeed, they provide molecular distinction to endothelium when it is developing into artery or vein and also play an important role in angiogenic interaction between embryonic vessels (35, 40). Our prior studies (31) showed that the ephrin pathway could regulate HSC whereby HSC acquire an angiogenic phenotype in response to PDGF through an ephrin-dependent pathway. The present studies tie together these two lines of work in HSC and SEC by demonstrating that the ephrin pathway in HSC can promote SEC recruitment via release of substances that are chemoattractive for SEC such as VEGF. Indeed, HSC have been implicated in the secretion of angiogenic factors such as angiopoietin and VEGF (37). We observed that EphrinB2/EphB4 chimeras increased VEGF mRNA production from HSC along with an increase in VEGF promoter activity and that this effect could be specifically blocked by silencing with EphB4 and EphrinB2 siRNA. Interestingly, members of the ephrin family have been observed to be upregulated in hepatitis C virus-associated cirrhosis (32) and cancer (16), both conditions that may require increased vascularity to maintain a conducive environment for the disease process to progress and consistent with the immunofluorescent analysis performed in this study using rats exposed to CCl4.
Our study also highlights some interesting transcriptional mechanisms by which EphB4/EphrinB2 stimulation leads to VEGF secretion. For example, EphB4/EphrinB2 stimulation also led to the upregulation of KLF2, a member of the zinc finger-containing family of transcription factor (5). A previous study by Wu et al. (41) has shown that KLF2−/− mouse embryos show a defect in vascular maturation due to the failure of the mural cells to migrate to the arterial wall. Other work has also implicated KLF2 as a key modulator of vascular integrity (21, 27), making it a logical transcriptional molecule to regulate HSC and SEC cross talk requisite for homeostasis as well as remodeling.
An important signaling pathway by which ephrins can stimulate VEGF secretion from HSC might involve Erk phosphorylation as we have observed in our study. Phosphorylation of Erk, a key cell survival and proliferation protein, has been shown previously by other to regulate VEGF-A expression through HIF-1α phosphorylation (39). We observed that EphrinB2 stimulated phosphorylation of Erk. Furthermore, the MEK inhibitor U0126 blocked the phosphorylation secondary to EphrinB2 treatment, showing the specificity of the signaling pathway. Since VEGF mRNA expression was also attenuated by U0126, these studies indicate that Erk phosphorylation may be an important step in ephrin-dependent VEGF production and highlight the important role of Erk in vascular integrity and function.
Given that SEC motility was enhanced by supernatant from HSC treated with EphB4 or EphrinB2 chimera, it can be speculated that HSC might play an important role in recruitment of SEC through VEGF production upon stimulation with ephrins. This mutual interaction between HSC and SEC through VEGF and PDGF (31) production, respectively, would be essential for juxtaposing these cells in the hepatic sinusoids, thereby maintaining vascular homeostasis in the liver and in turn for the changes involved in sinusoidal vascular remodeling. One question that emerges from these data is the autocrine as opposed to paracrine stimulation of ephrins in HSC. Indeed, EphrinB2 and EphB4 bind and signal bidirectionally, a process that may provide redundancy of function. Indeed, in our studies some of the mechanistic readouts were more prominent in response to stimulation of EphrinB2-Fc whereas others were more prominent in response to EphB4-Fc. For example, EphrinB2-FC revealed a greater stimulatory effect in studies focused on the protein level such as chemotaxis and ELISA, whereas EphB4-FC revealed quantitatively greater effects on studies focused on transcription studies. This observation highlights that although signaling through these receptors is bidirectional, there may be quantitative and qualitative differences in the way that signals are transduced between the two receptors. In vivo, we anticipate that ephrin stimulation may occur through cell-cell interactions between adjacent stellate cells as well as between adjacent EC and HSC (Fig. 6). Indeed, the immunofluorescence analysis of liver from animals with experimental cirrhosis depicts close proximity of EphB4 with both SEC and HSC.
Fig. 6.
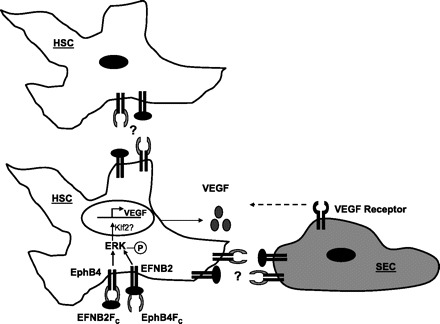
Proposed mechanism. Stimulation of either EphB4 receptor by EphrinB2 or EphrinB2 by EphB4 results in increased Erk phosphorylation (circled P) that leads to increased KLF2 and VEGF promoter activity. HSC-derived VEGF, in turn, provides a chemotactic stimulus for SEC migration and recruitment. In an in vivo setting EphrinB2/EphB4 stimulation may occur by cell to cell contact between HSC or between HSC and SEC.
In summary, EphrinB2 and EphB4 regulate VEGF production from HSC via Erk phosphorylation and is associated with KLF2 upregulation. We anticipate that this pathway importantly regulates HSC and SEC cross talk that regulates sinusoidal function in health and disease.
GRANTS
This work was supported by National Institutes of Health Grants R01 DK 59615 and R01 HL 86990 (V. H. Shah), P30 DK 084567, Clinical Core. Amitava Das was supported through the Pilot Feasibility Award by Mayo Clinic Centre on Cell Signaling in Gastroenterology.
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1. Adams DH, Burra P, Hubscher SG, Elias E, Newman W. Endothelial activation and circulating vascular adhesion molecules in alcoholic liver disease. Hepatology 19: 588–594, 1994 [DOI] [PubMed] [Google Scholar]
- 2. Adams RH, Wilkinson GA, Weiss C, Diella F, Gale NW, Deutsch U, Risau W, Klein R. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev 13: 295–306, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Allt G, Lawrenson JG. Pericytes: cell biology and pathology. Cells Tissues Organs 169: 1–11, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Ankoma-Sey V, Wang Y, Dai Z. Hypoxic stimulation of vascular endothelial growth factor expression in activated rat hepatic stellate cells. Hepatology 31: 141–148, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Atkins GB, Jain MK. Role of Kruppel-like transcription factors in endothelial biology. Circ Res 100: 1686–1695, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Augustin HG, Reiss Y. EphB receptors and ephrinB ligands: regulators of vascular assembly and homeostasis. Cell Tissue Res 314: 25–31, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Corpechot C, Barbu V, Wendum D, Kinnman N, Rey C, Poupon R, Housset C, Rosmorduc O. Hypoxia-induced VEGF and collagen I expressions are associated with angiogenesis and fibrogenesis in experimental cirrhosis. Hepatology 35: 1010–1021, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Das A, Fernandez-Zapico ME, Cao S, Yao J, Fiorucci S, Hebbel RP, Urrutia R, Shah VH. Disruption of an SP2/KLF6 repression complex by SHP is required for farnesoid X receptor-induced endothelial cell migration. J Biol Chem 281: 39105–39113, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Das A, Yaqoob U, Mehta D, Shah VH. FXR promotes endothelial cell motility through coordinated regulation of FAK and MMP-9. Arterioscler Thromb Vasc Biol 29: 562–570, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Deleve LD, Wang X, Guo Y. Sinusoidal endothelial cells prevent rat stellate cell activation and promote reversion to quiescence. Hepatology 48: 920–930, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dodelet VC, Pasquale EB. Eph receptors and ephrin ligands: embryogenesis to tumorigenesis. Oncogene 19: 5614–5619, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Dong G, Chen Z, Li ZY, Yeh NT, Bancroft CC, Van Waes C. Hepatocyte growth factor/scatter factor-induced activation of MEK and PI3K signal pathways contributes to expression of proangiogenic cytokines interleukin-8 and vascular endothelial growth factor in head and neck squamous cell carcinoma. Cancer Res 61: 5911–5918, 2001 [PubMed] [Google Scholar]
- 13. El-Assal ON, Yamanoi A, Soda Y, Yamaguchi M, Igarashi M, Yamamoto A, Nabika T, Nagasue N. Clinical significance of microvessel density and vascular endothelial growth factor expression in hepatocellular carcinoma and surrounding liver: possible involvement of vascular endothelial growth factor in the angiogenesis of cirrhotic liver. Hepatology 27: 1554–1562, 1998 [DOI] [PubMed] [Google Scholar]
- 14. Foo SS, Turner CJ, Adams S, Compagni A, Aubyn D, Kogata N, Lindblom P, Shani M, Zicha D, Adams RH. Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell 124: 161–173, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Gerety SS, Wang HU, Chen ZF, Anderson DJ. Symmetrical mutant phenotypes of the receptor EphB4 and its specific transmembrane ligand ephrin-B2 in cardiovascular development. Mol Cell 4: 403–414, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Hirai H, Maru Y, Hagiwara K, Nishida J, Takaku F. A novel putative tyrosine kinase receptor encoded by the eph gene. Science 238: 1717–1720, 1987 [DOI] [PubMed] [Google Scholar]
- 17. Kosmidou I, Xagorari A, Roussos C, Papapetropoulos A. Reactive oxygen species stimulate VEGF production from C2C12 skeletal myotubes through a PI3K/Akt pathway. Am J Physiol Lung Cell Mol Physiol 280: L585–L592, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Lee JS, Kang Decker N, Chatterjee S, Yao J, Friedman S, Shah V. Mechanisms of nitric oxide interplay with Rho GTPase family members in modulation of actin membrane dynamics in pericytes and fibroblasts. Am J Pathol 166: 1861–1870, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee JS, Semela D, Iredale J, Shah VH. Sinusoidal remodeling and angiogenesis: a new function for the liver-specific pericyte? Hepatology 45: 817–825, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Leveen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev 8: 1875–1887, 1994 [DOI] [PubMed] [Google Scholar]
- 21. Lin Z, Kumar A, SenBanerjee S, Staniszewski K, Parmar K, Vaughan DE, Gimbrone MA, Jr, Balasubramanian V, Garcia-Cardena G, Jain MK. Kruppel-like factor 2 (KLF2) regulates endothelial thrombotic function. Circ Res 96: e48–57, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 277: 242–245, 1997 [DOI] [PubMed] [Google Scholar]
- 23. Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, Rosenfeldt HM, Nava VE, Chae SS, Lee MJ, Liu CH, Hla T, Spiegel S, Proia RL. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest 106: 951–961, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mazure NM, Chen EY, Laderoute KR, Giaccia AJ. Induction of vascular endothelial growth factor by hypoxia is modulated by a phosphatidylinositol 3-kinase/Akt signaling pathway in Ha-ras-transformed cells through a hypoxia inducible factor-1 transcriptional element. Blood 90: 3322–3331, 1997 [PubMed] [Google Scholar]
- 25. Medina J, Arroyo AG, Sanchez-Madrid F, Moreno-Otero R. Angiogenesis in chronic inflammatory liver disease. Hepatology 39: 1185–1195, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Olaso E, Salado C, Egilegor E, Gutierrez V, Santisteban A, Sancho-Bru P, Friedman SL, Vidal-Vanaclocha F. Proangiogenic role of tumor-activated hepatic stellate cells in experimental melanoma metastasis Hepatology 37: 674–685, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Parmar KM, Larman HB, Dai G, Zhang Y, Wang ET, Moorthy SN, Kratz JR, Lin Z, Jain MK, Gimbrone MA, Jr, Garcia-Cardena G. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J Clin Invest 116: 49–58, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pasquale EB. The Eph family of receptors. Curr Opin Cell Biol 9: 608–615, 1997 [DOI] [PubMed] [Google Scholar]
- 29. Rosmorduc O, Wendum D, Corpechot C, Galy B, Sebbagh N, Raleigh J, Housset C, Poupon R. Hepatocellular hypoxia-induced vascular endothelial growth factor expression and angiogenesis in experimental biliary cirrhosis. Am J Pathol 155: 1065–1073, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sato M, Suzuki S, Senoo H. Hepatic stellate cells: unique characteristics in cell biology and phenotype. Cell Struct Funct 28: 105–112, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Semela D, Das A, Langer D, Kang N, Leof E, Shah V. Platelet-derived growth factor signaling through ephrin-b2 regulates hepatic vascular structure and function. Gastroenterology 135: 671–679, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shackel NA, McGuinness PH, Abbott CA, Gorrell MD, McCaughan GW. Insights into the pathobiology of hepatitis C virus-associated cirrhosis: analysis of intrahepatic differential gene expression. Am J Pathol 160: 641–654, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shah V, Haddad FG, Garcia-Cardena G, Frangos JA, Mennone A, Groszmann RJ, Sessa WC. Liver sinusoidal endothelial cells are responsible for nitric oxide modulation of resistance in the hepatic sinusoids. J Clin Invest 100: 2923–2930, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Soriano P. Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes Dev 8: 1888–1896, 1994 [DOI] [PubMed] [Google Scholar]
- 35. Steinle JJ, Meininger CJ, Forough R, Wu G, Wu MH, Granger HJ. Eph B4 receptor signaling mediates endothelial cell migration and proliferation via the phosphatidylinositol 3-kinase pathway. J Biol Chem 277: 43830–43835, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Takahashi A, Kureishi Y, Yang J, Luo Z, Guo K, Mukhopadhyay D, Ivashchenko Y, Branellec D, Walsh K. Myogenic Akt signaling regulates blood vessel recruitment during myofiber growth. Mol Cell Biol 22: 4803–4814, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Taura K, De Minicis S, Seki E, Hatano E, Iwaisako K, Osterreicher CH, Kodama Y, Miura K, Ikai I, Uemoto S, Brenner DA. Hepatic stellate cells secrete angiopoietin 1 that induces angiogenesis in liver fibrosis. Gastroenterology 135: 1729–1738, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Tugues S, Fernandez-Varo G, Munoz-Luque J, Ros J, Arroyo V, Rodes J, Friedman SL, Carmeliet P, Jimenez W, Morales-Ruiz M. Antiangiogenic treatment with sunitinib ameliorates inflammatory infiltrate, fibrosis, and portal pressure in cirrhotic rats. Hepatology 46: 1919–1926, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Wang FS, Wang CJ, Chen YJ, Chang PR, Huang YT, Sun YC, Huang HC, Yang YJ, Yang KD. Ras induction of superoxide activates Erk-dependent angiogenic transcription factor HIF-1alpha and VEGF-A expression in shock wave-stimulated osteoblasts. J Biol Chem 279: 10331–10337, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell 93: 741–753, 1998 [DOI] [PubMed] [Google Scholar]
- 41. Wu J, Bohanan CS, Neumann JC, Lingrel JB. KLF2 transcription factor modulates blood vessel maturation through smooth muscle cell migration. J Biol Chem 283: 3942–3950, 2008 [DOI] [PubMed] [Google Scholar]


