Summary
Microbial drug persistence is a widespread phenomenon in which a sub-population of microorganisms is able to survive antimicrobial treatment without acquiring resistance-conferring genetic changes. Microbial persisters can cause recurrent or intractable infections, and like resistant mutants, they carry an increasing clinical burden. In contrast to heritable drug resistance, however, the biology of persistence is only beginning to be unraveled. Persisters have traditionally been thought of as metabolically dormant, non-dividing cells. However, as discussed in this review, increasing evidence suggests that persistence is in fact an actively maintained state, triggered and enabled by a network of intracellular stress-responses that can accelerate processes of adaptive evolution. Beyond shedding light on the basis of persistence, these findings raise the possibility that persisters behave as an evolutionary reservoir from which resistant organisms can emerge. As persistence and its consequences come into clearer focus, clinically relevant eradication strategies are urgently needed.
Introduction
It was not long after the introduction of antibiotics that the emergence of resistant organisms was first reported (Abraham and Chain, 1940). Around the same time, a similar phenomenon began frustrating physicians. “Penicillin has undoubtedly saved lives and limbs of patients suffering from staphylococcal infections, but it has not usually cured the disease,” wrote Joseph Bigger, an army physician who noticed that antibiotic treatment often failed to completely sterilize soldiers’ wounds, resulting in recurrent infections after therapy (Bigger, 1944).
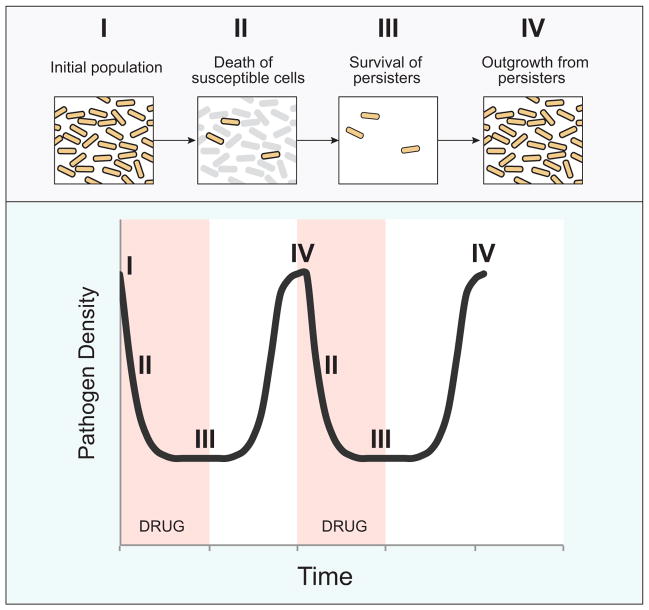
This phenomenon, in which a small sub-population of microbes survives the lethal effects of a drug, is referred to as “persistence.” Persistence is distinct from resistance in that, unlike resistant mutants, persister populations do not expand in the presence of the toxic compound, and population growth resumes only once the drug has been removed. Furthermore, upon re-treatment, the recrudescent organisms are as drug-sensitive as the initial population, suggesting that unlike resistance, persistence is a non-heritable phenotype (Figure 1).
Figure 1. Drug persistence and recurrent infection.<.
br>Schematic model of killing and persistence kinetics during antimicrobial therapy. Treatment of an initial population of pathogens (I) causes killing of the majority of cells (II), but fails to eradicate a small subset of persisters (III). When antibiotic pressure is removed, persisters resume growth, resulting in recurrent infection of the host (IV). Re-treatment results in similar killing kinetics.
Many human pathogens cause recurrent infections despite appropriate therapy and in the absence of apparent genetic resistance, and can thus be considered persistent. Despite their clinical importance, the protracted nature of these infections, as well as the lack of suitable animal models have hampered the study of this phenomenon. Nevertheless, models of persistence in vitro, particularly, the study of non-inherited drug tolerance in bacterial cultures, biofilms and microfluidic devices, has helped shed some light on the biological basis of the persister phenotype.
In this review, we discuss the increasing clinical burden of microbial persistence, and summarize the current understanding of the mechanisms underlying this phenomenon. In particular, we outline the emerging role of stress responses in potentiating persister survival, and highlight the notion that persistence is an actively maintained state rather than solely a consequence of passive growth rate reduction. Importantly, the stress processes enabling persistence are also implicated in the accelerated acquisition of heritable resistance. Thus, we propose that persisters may serve as an evolutionary reservoir from which resistance might emerge, and review the clinical evidence lending credence to this idea. Finally, we discuss potential therapeutic strategies for the prevention or treatment of persistent infections.
Part I: Drug persistence in human disease
Persistence is widespread and has been described in bacteria, fungi, parasites, and even cancerous human cell populations (Table 1). Persistence contributes to the pathogenesis of several notable human infections that require protracted treatment and relapse after therapy. Furthermore, advances in medical technology are allowing patients to survive longer with reduced host defenses or in otherwise compromised states. In the absence of appropriate host immunity, pathogens that survive drug treatment are more likely to thrive. As a consequence, the prevalence of persistent infections in which antimicrobial therapy fails to provide a cure is rising.
Table 1.
Human pathogens associated with persistent infection.
| Pathogen | Duration of Treatmenta | Number of drugsb | Persistence characteristics | Hosts susceptible to persistent infection |
|---|---|---|---|---|
|
| ||||
| Bacteria | ||||
| Mycobacteria | ||||
| Mycobacterium tuberculosis | months | 4 | slow clearance | All, particularly HIV |
| Non-tuberculous Mycobacteria | months–years | 3–4 | slow clearance, recurrence | All, particularly bronchiectasis, HIV |
| Other bacteria | Other bacteria | |||
| Staphylococcus aureus | weeks–lifelong | 1–2 | biofilm, recurrence | Implanted material |
| Escherichia coli | days–weeks | 1 | biofilm | Urinary catheters |
| Pseudomonas aeruginosa | days–weeks | 1–2 | recurrence | CF, immunocompromized |
| Burkholderia pseudomallei | weeks | 1–2 | recurrence | All |
| Burkholderia cenocepacia | days - weeks | 1–2 | recurrence | CF |
|
| ||||
| Fungi | Fungi | |||
| Candida species | days–lifelong | 1 | slow clearance, recurrence | Cancer, immunocompromized, HIV |
|
| ||||
| Parasites | Parasites | |||
| Plasmodium falciparum | days | 1–2 | recrudescence (artemisinins) | All |
| Babesia species | weeks months | 1–2 | recurrence | Immune-deficient |
|
| ||||
| Mammalian cells | ||||
| Tumor cells | months | varies | recurrence | All |
Range of treatment duration;
typical number of drugs required to achieve apparent eradication of organism; CF, Cystic fibrosis.
Persistence in bacterial infections
Among of the best-characterized persistent pathogens are Mycobacteria, slow growing aerobic bacteria that include Mycobacterium tuberculosis (Mtb) and non-tuberculous Mycobacteria (NTM). Infections with these organisms require treatment with unusually protracted courses of combinations of antibiotics. Mtb therapy can last up to 12 months, and NTM, such as Mycobacterium avium complex (MAC), can require up to 2 years of treatment. Furthermore, despite apparent microbiologic cure, relapse rates of mycobacterial infections are high, underscoring the clinical importance of persistent sub-populations that are undetectable using current diagnostic technology (McCune et al., 1956). Consistent with these observations, detailed studies of infection in mice revealed that treatment of Mtb infections reduces organism titers, but fails to sterilize the animal (McCune and Tompsett, 1956).
A variety of other bacteria cause persistent infections in humans. Recurrence of Staphylococcus aureus skin, soft tissue and bloodstream infections, for instance, is common, despite protracted treatment courses (Chong et al., 2013). S. aureus is also the frequent cause of infections of foreign devices implanted into humans, including catheters and prosthetic joints. Despite appropriate therapy, infections on these devices recur due to the presence of persistent biofilms. Recurrent infection is also common in the lungs of patients with cystic fibrosis (CF). Though CF lungs are colonized by hundreds of bacterial species, Pseudomonas aeruginosa is the most common cause of recurrent pneumonia in these patients (Zhao et al., 2012). Antibiotic treatment of pneumonia in CF patients is the mainstay of therapy, however, antibiotics rarely if ever successfully sterilize the airway. In fact, recent evidence indicates that antibiotics have minimal impact on the microbial community structure in these patients, suggesting the existence of antimicrobial tolerance within this bacterial population (Fodor et al., 2012). A relative of Pseudomonas, Burkholderia pseudomallei, causes meliodosis, a severe systemic infection that can recur months to years after apparent clearance by appropriate antibiotic treatment. Whole-genome sequencing of recrudescent isolates demonstrated nearly clonal organisms, indicating that antimicrobial therapy failed to eradicate the initial infecting population (Hayden et al., 2012).
Persistence in other pathogens
Persistence can also complicate therapy of non-bacterial infections. Biofilm-associated infections with fungi, for example, are highly recalcitrant to routine antifungal therapy. Even in the absence of genetic resistance, management of C. albicans infection can frequently require prolonged courses of drugs or the removal of an infected implanted device.
A form of drug tolerance analogous to persistence, referred to as dormancy, has been described for the blood stage of Plasmodium species, the protozoal parasites that cause malaria. In dormancy, much as in persistence, a small subpopulation of parasites survives drug treatment and can cause recurrent infection after cessation of therapy. This phenomenon has been described for a wide variety of antimalarial drugs, including mefloquine, atovaquone, and most recently with artemisinins, the most commonly used therapy for malaria worldwide (LaCrue et al., 2011; Nakazawa et al., 1995; Nakazawa et al., 2002; Teuscher et al., 2010; Thapar et al., 2005; Witkowski et al., 2010). Similarly, failure of antiprotozoal drugs to clear bloodstream parasites has also been described for Babesia, an emerging tickborne infection that causes a malaria-like illness (Krause et al., 2008). Importantly, as has been observed for other classes of persisters, the bulk recrudescent parasites appear to remain susceptible to the initial drugs used in treatment.
The impact of persistence in compromised host states
Although drug persistence is common in microbes and can interfere with treatment, in most cases, antimicrobial therapy succeeds. It is generally accepted that if drugs successfully kill the overwhelming majority of an infecting population, intrinsic host defenses “mop up” the remainder. Advances in medical technology, however, have resulted in the increased prevalence of compromised host states. In these individuals, persisters that would normally be cleared may linger, causing recurrent or intractable infections.
In addition to increasing susceptibility to infection, a number of immunocompromised states are also associated with an increased incidence of persistent and recurrent infections. Infection with HIV and its concomitant T cell immunodeficiency state is associated with increased severity and recrudescence of tuberculosis in co-infected individuals (Perriens et al., 1995). In solid organ or hematopoetic stem cell transplant recipients, who are chronically immunosuppressed to prevent transplant rejection, infectious complications are a major cause of mortality. Patients with cancer exhibit impaired mucosal barriers leading to the development of oral candidiasis that can be kept at bay but not eradicated using local antifungal therapy. Evaluation of isolates of C. albicans and C. glabrata from such patients demonstrated the presence of drug tolerant populations, and found that patients with prolonged fungal carriage had significantly higher levels of persisters (Lafleur et al., 2010). Similarly, infection with the protozoan parasite Babesia microti, which responds well to therapy in normal hosts, recrudesces in immunocompromised individuals despite protracted courses of seemingly appropriate therapy (Krause et al., 2008).
In addition to these impairments in host immunity, compromised host states related to the implantation of cardiac devices, prosthetic joints, devices interacting with the central nervous system and other foreign bodies are increasing as the population ages. Treatment of microbial biofilms that form and infect these devices is ineffective and often requires dangerous and costly removal of the implant due to the persistence of a small nucleus of surviving microbes.
Human cancer persisters
Non-inherited drug tolerance may extend beyond the realm of infectious organisms. A recent study of non-small cell lung tumors identified a sub-population of reversibly drug tolerant cancer cells that survived therapy with tyrosine kinase inhibitors (Sharma et al., 2010). Interestingly, this tumor cell line was able to maintain growth in the presence of continued chemotherapy. Furthermore, the drug tolerant phenotype was mediated through a signal transduction cascade resulting in epigenetic modifications within the cell, rather then genetic mutations. Inhibition of histone deacetylase prevented the tolerant state, and the epigenetic modifications involved were suggested to occur as part of a cellular stress response program.
These observations underscore the clinical importance of persistence in human disease, and indicate that persister formation is a core survival strategy in both prokaryotic and eukaryotic organisms.
Part II: Mechanisms of drug persistence
The rising clinical burden of persistence stresses the need to better understand the mechanisms underlying persister formation and maintenance. Despite the paucity of appropriate animal models, the study of persistence in cultures and biofilms, cell-fate mapping experiments using microfluidic devices, as well as a deepened understanding of antimicrobial toxicity pathways have helped shed light on the basis of persistence in pathogens and model organisms. Bacterial persistence has been found to arise both spontaneously and be environmentally induced. These distinct persister classes are discussed below, as well as the mechanisms thought to allow their generation and survival.
Spontaneous persistence
The existence of a small fraction of persisters among growing, isogenic microbes has been hypothesized to reflect a population-level strategy of survival in a rapidly changing environment. In this “bet-hedging” view, phenotypic switching of a few organisms to a dormant or protected state occurs spontaneously and continuously in any growing microbial population regardless of the presence of drug (Kussell et al., 2005).
It has been hypothesized that stochastic variation at the level of gene expression may engender such pre-exisiting phenotypic heterogeneity (Balaban et al., 2004). In particular, toxin/antitoxin (TA) systems, two-gene operons that encode a cellular poison and its antidote and are common throughout the bacterial kingdom, have been implicated in the control of persistence. When in excess, the toxin components typically increase the frequency of bacterial persisters in culture. For instance, in E. coli, over-expression of the toxin HipA, or a mutation in this gene that desensitizes HipA to its antitoxin HipB, leads to a 10 to 10,000 fold increase in the frequency of persistence to ampicillin and ciprofloxacin (Falla and Chopra, 1998; Moyed and Bertrand, 1983). Similarly, over-expression of the toxin components of the RelE/B, MazF/E, YafQ/DinJ, MqsR/A, HigB/A, CcdA/B TA systems enhances persistence to several antibiotics (Maisonneuve et al., 2011; Tripathi et al., 2012). While ablation of any single toxin gene typically does not dramatically affect persister formation, deletion of multiple TA loci does cause an appreciable drop in the number of persisters (Maisonneuve et al., 2011), suggesting that although redundant, TA systems play a role in spontaneous persistence.
Variation in the expression of a number of other genes, however, can also affect persistence or the ability of bacteria to grow in the presence of drug. These include global regulators, genes involved in metabolism and stress response components (De Groote et al., 2009; Dhar and McKinney, 2010; Dorr et al., 2009; Hansen et al., 2008; Murakami et al., 2005; Spoering et al., 2006; Wakamoto et al., 2013). In a recent example, Mycobacterial growth in the presence of the antibiotic isoniazid (INH) was directly correlated to stochastic variation in the pulsatile expression of KatG, a catalase-peroxidase required to process and activate the drug. Specifically, slow pulsing cells, which processed less drug, survived longer than fast pulsing bacteria (Wakamoto et al., 2013).
Genes affecting survival under stress may be particularly prone to epigenetic plasticity. Although this question has not been widely addressed, large-scale transcriptional analysis of a clonal population of P. falciparum found that stochasticity is an intrinsic property of the expression of gene families involved in host-parasite interactions, suggesting that variability in these genes might improve the persistence of an infecting population (Rovira-Graells et al., 2012). In addition to stochastic gene expression variation, phenotypic heterogeneity in drug sensitivity may result from built-in deterministic mechanisms. For instance, asymmetric growth of M. smegmatis gives rise to daughter cells of different sizes and with different elongation rates, and the slower-growing progeny are less susceptible to multiple drugs (Aldridge et al., 2012).
Environmentally-induced persistence
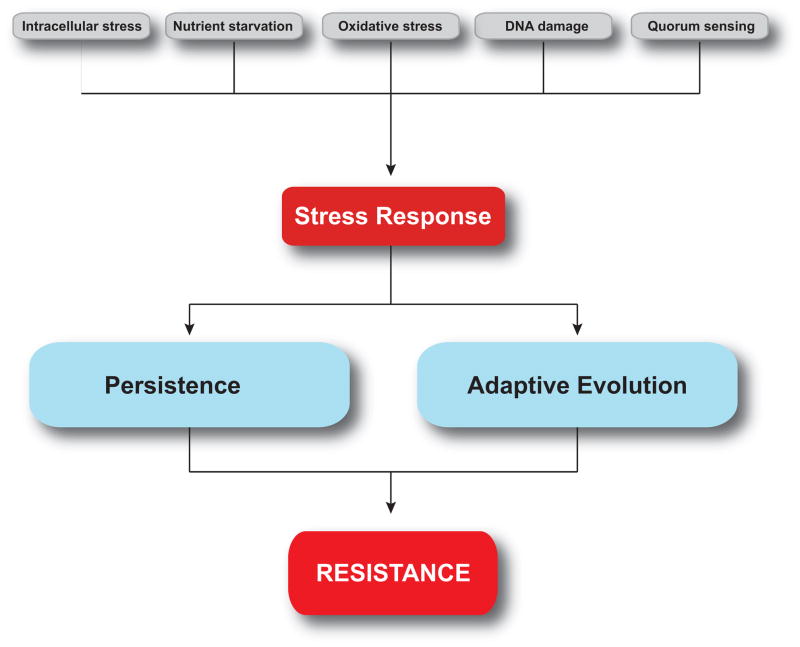
A number of environmental signals can modulate the level of persistence in an isogenic microbial population. This phenomenon has been best studied in bacterial cultures, where a variety of noxious cues have been found to increase the frequency of persistence (Figure 2).
Figure 2. Stress responses link persistence, adaptive evolution and resistance.<.
br>In this model, microbial responses to endogenous or exogenous stresses promote survival as well as genetic plasticity. Persistent organisms undergo rapid adaptive evolution, and can function as a reservoir for the elaboration of drug resistance.
Heat shock, for example increases the survival of both A. baumannii and P. aeruginosa in the presence of aminoglycosides or beta-lactams, respectively (Cardoso et al., 2010; Murakami et al., 2005). Oxidative stress and DNA damage caused by treatment with paraquat, hydrogen peroxide or sub-lethal doses of antibiotics promotes persistence to fluoroquinolone antibiotics in both E. coli and P. aeruginosa (Dorr et al., 2009; Moker et al., 2010; Vega et al., 2012; Wu et al., 2012). Similarly, bacterial envelope stress has been associated with enhanced persister formation (Murakami et al., 2005; Poole, 2012), as has signaling mediated by soluble quorum sensing (QS) molecules including phenazine pyocyanin (PYO) in P.aeruginosa, peptide alarmones in S. mutans, and indole, a stationary phase QS molecule secreted and sensed by a wide range of bacteria (Kayama et al., 2009; Leung and Levesque, 2012; Moker et al., 2010; Vega et al., 2012). Nutrient starvation and diauxic carbon source transitions can also induce of persistence (Amato et al., 2013; Betts et al., 2002; Fung et al., 2010; Nguyen et al., 2011). In fungi, environmental conditions have similarly been suggested to affect drug susceptibility (Kucharikova et al., 2011; Pettit et al., 2010). Biofilm-encased or stationary phase cells, which are both deprived of certain nutrients and may be subjected to QS signaling, exhibit persistence at levels typically orders of magnitude higher than that of logarithmically growing organisms (Keren et al., 2004; LaFleur et al., 2006).
Importantly, data suggest that in some cases, bacterial persistence that appears spontaneous may in fact have been environmentally induced. Specifically, maintenance of E. coli cultures in log phase by repeated re-inoculation leads to the loss of a detectable persister population. These data suggest that persisters found in actively growing, low stress populations may in fact represent left-over cells from high-stress stationary phase inoculums rather then actively growing bacteria that have switched phenotypes spontaneously (Keren et al., 2004).
Together, these data underscore the role of environmental parameters such as nutrient availability, population density and oxidative stress in the modulation of the persistence levels in a population.
Stress response pathways enable drug persistence
Whether persistence is acquired spontaneously or environmentally induced, there is increasing evidence, particularly in bacteria, for the active involvement of various and interconnected intracellular stress responses (Poole, 2012).
In bacteria, both spontaneous and stress-induced persistence have been shown to depend on the stringent response (SR). The SR, which can be triggered by a range of cues, depends on RelA and SpoT-mediated synthesis of (p)ppGpp, an alarmone that broadly modulates gene expression to promote cell survival (Potrykus and Cashel, 2008). In E. coli HipA7 mutants, which express a gain-of-function mutation in the HipA toxin and exhibit elevated spontaneous persister frequencies (Balaban et al., 2004), simultenously deleting RelA and SpoT eliminates persistence (Korch et al., 2003). The broad toxic effects of HipA on nucleic acid and protein synthesis (Korch and Hill, 2006) may be responsible for triggering the SR. In addition, genetic knockout of RelA and SpoT in both P. aeruginosa and E. coli diminishes starvation-induced persistence, biofilm drug tolerance, and persistence in vivo (Fung et al., 2010; Nguyen et al., 2011). Recently, E. coli persistence induced by diauxic carbon source transitioning has also been found to require the SR (Amato et al., 2013). Starvation-induced drug tolerance in M. tuberculosis has been suggested to depend on RelMtb, a RelA homolog (Betts et al., 2002). Interestingly, a gain-of-function mutation in RelA causing permanent activation of the SR generated a high-persistence phenotype in a primary isolate of S. aureus identified in human infection (Gao et al., 2010).
Activation of the bacterial SOS response to DNA damage, much like the SR, has been associated with induction or maintenance of persistence. E. coli mutants lacking RecA, RecB or LexA, key SOS response genes, are more susceptible to quinolones, and in the presence of these drugs, the frequency of both spontaneous and induced persistence is reduced by 10 to 100 fold (Dorr et al., 2009; Fung et al., 2010; Wu et al., 2012). Conversely, constitutive expression of SOS response genes enhances drug tolerance (Dorr et al., 2009). Thus persistence to quinolones is aided and partially depends on the cell’s ability to repair DNA damage.
A number of other stress response pathways have been implicated in persister formation. These include the oxidative stress response, which depends on OxyR and SoxS, as well as the phage-shock response, both of which are involved in indole-induced persistence in E. coli (Grant and Hung, 2013; Vega et al., 2012; Wu et al., 2012). Genes regulating the heat shock response have been reported to play a role in tolerance to aminoglycosides (Cardoso et al., 2010), and the alternative sigma factor RpoS, which coordinates the general stress response, is important in QS-induced persistence in P. aeruginosa (Kayama et al., 2009; Murakami et al., 2005).
Taken together, these data suggest that centralized stress responses are at the core of the persister phenotype. Both environmental factors as well as stochastic endogenous processes can give rise to stress, and thus contribute to the generation of multi-drug tolerant persisters.
Mechanisms of survival
Most stress responses lead directly or indirectly to a slowing or stalling of bacterial growth and division. This slowed or arrested cellular growth has been suggested as the major factor underlying drug persistence, as the ability of antibiotics to kill bacteria is generally proportional to their growth rate (Eng et al., 1991). Reduced growth rates have indeed been correlated with increased drug persistence both in vitro (Aldridge et al., 2012; Balaban et al., 2004; Keren et al., 2004; Shah et al., 2006) and in vivo, when bacterial growth tapers over the course of an infection due to immune pressure or lack of nutrients (Levin and Rozen, 2006). The reduced rates of DNA replication, translation, cell wall synthesis, and metabolism directly targeted by antibiotics have been assumed to account for the relative drug tolerance of dormant bacteria. Although stalled biosynthesis likely promotes persistence, its effects are difficult to untangle from that of accompanying stress response processes, and it is becoming clear that many active cellular processes occurring in parallel to growth rate reduction are central for cellular survival in a toxic environment.
For instance, a number of active intracellular detoxification mechanisms that can be triggered by stress play an important role in persistence. Multidrug efflux pumps are up-regulated in response to various cues including oxidative stress and quorum sensing (Hirakawa et al., 2005), and can contribute to both in vitro and in vivo persistence. For example, paraquat-induced persistence in E. coli in vitro is dependent on AcrAB-TolC multi-drug efflux system (Wu et al., 2012). Similarly, drug tolerance in a Mycobacterial infection model was reported to depend on drug efflux pumps induced by macrophage-mediated oxidative stress (Adams et al., 2011). In some cases, persisters exhibit a reversible defect in drug uptake (Allison et al., 2011b). Beyond active efflux, detoxification can take other forms. For example, bacterial exposure to antibiotics can enhance their synthesis of nitric oxide (NO), which chemically modifies certain drugs and inactivates them (Gusarov et al., 2009; Shatalin et al., 2011). In Mycobacteria, stress can also promote alternative metabolic pathways that produce lower levels of ROS, thus elevating the threshold for antibiotic-mediated death (Baek et al., 2011). Finally, generation of reactive oxygen species (ROS) following antibiotic exposure has been proposed to contribute to the lethal effects of antimicrobials (Dwyer et al., 2007; Foti et al., 2012; Kohanski et al., 2007; Kohanski et al., 2008; Wang and Zhao, 2009). Although the role of ROS in antibiotic-mediated bacterial killing has recently become a matter of debate (Keren et al., 2013; Liu and Imlay, 2013), active mechanisms of oxidative stress relief have been found to promote drug tolerance. For example, activation of catalase and superoxide dismutase (SOD), antioxidant enzymes, promotes stress-induced drug persistence and biofilm-associated persistence in bacteria and fungi (Belenky and Collins, 2011; Nguyen et al., 2011; Shatalin et al., 2011; Van Acker et al., 2013).
In addition to detoxification, stress can promote active microbial mechanisms of physical sequestration. For instance, bacterial surface adhesiveness and the subsequent formation of protective biofilms can be enhanced in response to sublethal antibiotic treatment and other stresses (He et al., 2012; Hoffman et al., 2005). Although tolerance mechanisms in biofilms are still a subject of intense investigation, it is clear that mechanisms other than dormancy are at play (Hoiby et al., 2010).
Interestingly, persistence and cellular growth may not always be incompatible. Although spontaneous E. coli persisters have been shown to be growth arrested (Balaban et al., 2004), a microfluidics-based study following the fate of individual Mycobacteria during antibiotic treatment found that the apparent stability of persister numbers was in fact due to a dynamic state of balanced death and division, rather then generally arrested growth (Wakamoto et al., 2013).
Together, these recent studies challenge the prevailing view that persistence is solely linked to passive dormancy, and suggest that these cells are engaged in a host of active processes that allow survival (Adams et al., 2011; Nguyen et al., 2011; Wakamoto et al., 2013).
Part III: Persistence-enabling stress responses accelerate acquisition of resistance
The discovery that cellular activity and division can occur during persistence raises the possibility that persisters function as an intermediate state in the elaboration of heritable drug resistance. Residual cell division combined with active survival mechanisms could support or even enhance the development of heritable genetic changes, as many of the same stress response programs important for the generation and survival of persisters can also accelerate genome-wide mutagenesis and horizontal gene transfer. Thus, persisters may represent a pool of adaptively evolving organisms from which resistant mutants can emerge (Figure 2).
Stress responses promote adaptive mutation
Although in theory, low rates of cell division in persisters under drug pressure may suffice to give rise to resistance-conferring mutations, stress responses might vastly accelerate this process. Recently, exogenous stress has been shown to promote genome instability, or “adaptive mutation”, leading to fast-tracked population diversification.
Recent systems-level analyses have uncovered that overlapping stress responses (SR and SOS) function as upstream hubs of a program of adaptive mutagenesis in E. coli, S. aureus and P. aeruginosa (Al Mamun et al., 2012; Cirz et al., 2007; Cirz et al., 2006). For example, activation of the SR by starvation stress increases basal mutation rates in E. coli by activation of the error prone DinB polymerase (Petrosino et al., 2009). Similarly, mutagenic polymerases are induced in S. aureus and P. aeruginosa following induction of the SOS response (Cirz et al., 2007; Cirz et al., 2006).
In addition to the activity of microbial stress response systems, bactericidal antibiotic therapy alone has been shown to increase rates of mutagenesis. Treatment of bacteria with sublethal doses of bactericidal antibiotics results in genome-wide mutations by stimulating ROS production, and through RpoS-mediated activation of the error-prone polymerase PolIV (Gutierrez et al., 2013; Kohanski et al., 2010; Nair et al., 2013). Selection of such resistance mutations by antibiotics can occur at drug concentrations orders of magnitude below the inhibitory level of susceptible bacteria (Gullberg et al., 2011).
Interestingly, a link between ROS-specific mutagenesis by sublethal antibiotics and microbial stress response pathways has been recently demonstrated. In E. coli lacking the SR (both RelA and SpoT), treatment of cultures with sublethal antibiotics failed to generate adaptive resistance, a phenomenon that was linked to reduced rates of oxidative stress in the SR null organism (Nguyen et al., 2011). This suggests that an intact SR pathway is required for antibiotic stress caused adaptive mutation to develop.
The structure of bacterial communities may also play an important role in the occurrence of adaptive mutations (Zhang et al., 2011). Higher mutation rates have been found in biofilm-associated P. aeruginosa (Driffield et al., 2008) and S. aureus (Ryder et al., 2012) than in planktonic cultures, and these increased rates appear to be linked to higher levels of oxidative stress in the biofilm community. Thus, features consistent with persister formation – exposure to bactericidal antibiotics, activation of stress response pathways, and biofilm association – create a suitable environment for adaptive mutation to occur within this subpopulation.
Stress responses promote horizontal gene transfer
A second major mechanism by which antibiotic resistance is elaborated is by sharing resistance determinants on mobile genetic elements. Stress response pathways, particularly SOS, have also been found to promote horizontal gene transfer in bacteria. Induction of the SOS response by the quinolone class of antibiotics increases horizontal gene transfer frequency in E. coli and Vibrio cholera (Beaber et al., 2004), specifically promoting the sharing of integrative conjugative elements that result in resistance to aminoglycosides, lincosamides, and antifolate antibiotics.
In organisms that lack a traditional SOS system, alternative stress response pathways have been found to promote gene transfer. In Streptococcus pneumoniae, which lacks an SOS system, competence induction by antibiotics has been linked to activation of quorum sensing signals as a response to the management of misfolded proteins caused by aminoglycoside treatment (Prudhomme et al., 2006; Stevens et al., 2011). Higher rates of phage transduction, which can disseminate antibiotic resistance determinants, have also been reported with treatment of some antibiotics (Zhang et al., 2000). Interestingly, environmental stress from sources other than antibiotics have similarly been shown to increase rates of horizontal gene transfer in bacterial communities (Stecher et al., 2012). Biofilm formation, in addition to increasing rates of adaptive mutation, can also promote horizontal gene transfer in S. aureus (Savage et al., 2013). Thus, stress response pathways and microbial community structures that favor development of persisters can also potentiate horizontal gene transfer.
Part IV: Is persistence linked to drug resistance in vivo?
Just as our understanding of persisters in vivo remains limited, characterization of how drug resistance emerges in patients remains largely obscure. The increasing use of whole-genome sequencing of microbes is beginning to shed light on pathogen dynamics during persistent infection.
For example, longitudinal whole-genome sequencing of S. aureus isolates in a patient with persistent infection demonstrated the accumulation of a small number of point mutations over several months, associated with antibiotic resistance, that would not have been detected by standard laboratory practice (Mwangi et al., 2007). This dynamic structure of bacterial populations was further explored using whole-genome sequencing of a CF pneumonia outbreak strain, Burkholderia dolosa, in multiple consecutive patients. In this longitudinal analysis, near continual parallel adaptive evolution occurred among isolates in very similar genetic loci involved in antibiotic resistance and oxygen tolerance across all patients (Lieberman et al., 2011). Similarly, whole-genome sequencing of recrudescent infection with Burkholderia pseudomallei established that infection recurrences did not result from re-infection, but rather from re-growth of persisting organisms. The study further identified in these isolates a number of antibiotic resistance conferring mutations. Although these antibiotic-resistant mutants may have been present in the initial infecting population and subsequently selected for, the nature of the genetic changes, and the detection of specific mutations typically associated with recurrent isolates but not with environmental strains suggested that evolution towards resistance had occurred in the host (Hayden et al., 2012).
Whole-genome sequencing of populations of microbes, rather than just individual clones, has also disclosed significantly greater community complexity than previously appreciated. This is particularly important in the context of antibiotic resistance. In vitro, populations of bacteria under antibiotic pressure have been found to be composed of mixed subpopulations of susceptible and resistant organisms. A small number of altruistic, resistant organisms sensing environmental stress can lead to a population-wide drug tolerant state by secreting indole (Lee et al., 2010). In an in vivo example, whole-genome sequencing of Mtb populations in sputum from patients who experienced recrudescent infection, including analysis of very low abundance variants in the population, revealed a remarkable amount of co-existing genetic diversity and the frequent emergence of resistant strains at low frequencies (Sun et al., 2012).
The links between dormancy in Plasmodium species and the development of resistance to antimalarial compounds in vivo remain unclear. Resistance to artemisinin in Plasmodium falciparum has recently emerged and is likely to spread more widely (Miotto et al., 2013). Artemisinin resistance was originally identified by delay in clearance of parasites from the bloodstream (Amaratunga et al., 2012; Dondorp et al., 2009). A recent single nucleotide polymorphism analysis of parasites with delayed clearance identified four associated polymorphisms, including mutations associated with a DNA damage tolerance pathway (Takala-Harrison et al., 2013). Mathematical modeling experiments have suggested that reduced artemesinin susceptibility is related to reduced ring stage susceptibility, the life stage associated with dormancy (Saralamba et al., 2011). Modeling has also suggested that recrudescence is an important clinical feature for the dissemination of de novo drug resistance (White et al., 2009). Though these associations provoke interest in a potential link between dormancy and artemesinin resistance, more research is needed to draw meaningful conclusions.
Part V: Towards therapeutic eradication of persisters
As discussed above, not only do microbial persisters thwart our efforts to treat infection, but they may also act as reservoirs of actively and adaptively evolving organisms. Although a causal relationship between persistence and resistance remains to be definitively established, the possibility that persisters may give rise to resistant mutants further underscores the importance of developing methods to eradicate such phenotypically drug-refractory subpopulations. As our mechanistic understanding of persistence deepens, new strategies to target these cells specifically or to enhance their susceptibility to killing by existing antimicrobials are being devised (Allison et al., 2011a).
One possibility is to target the stress responses that coordinate the persistent phenotype and the generation of resistance. Although work in this area is still in its infancy, a few promising studies have begun to explore this approach (Cirz and Romesberg, 2007). For example, inhibition of the SOS response either by deletion of RecA or overexpression of an uncleavable mutant of the LexA repressor potentiates killing by quinolones, aminoglycosides and beta-lactams (Kohanski et al., 2007; Lu and Collins, 2009). Supplementing antibiotic therapy with engineered bacteriophage constitutively expressing an uncleavable LexA repressor improved survival in a murine model of acute E. coli infection, and even improved killing of genetically drug-resistant mutants.
Furthermore, combination therapy with these bacteriophage substantially reduced the rate of antibiotic-induced mutation and resistance emergence in sub-lethally treated bacteria (Lu and Collins, 2009). Small molecule screens have identified RecA inhibitors that might similarly enhance antibiotic activity and prevent adaptive mutation in bacterial pathogens (Wigle et al., 2009). Similarly, preventing the de-repression of the SOS response or ablating SOS-induced alternative polymerases prevents UV-induced adaptive mutation in S. aureus (Cirz et al., 2007).
Interestingly, bacteriophage engineered to interfere with the oxidative stress response by overexpressing SoxR also potentiates antibiotic therapy (Lu and Collins, 2009). Other findings suggest that drugs targeting the stringent response (Wexselblatt et al., 2012) or interfering with envelope repair pathways (Lee et al., 2009) may also help prevent persister formation or enhance bactericidal antibiotic activity.
Another strategy is to interfere with persister survival mechanisms. Therapies could target residual metabolic activity in persisting organisms, for example. Indeed, inhibition of the mycobacterial proteasome or ATP synthase has been shown to promote death of non-growing M. tuberculosis (Andries et al., 2005; Gandotra et al., 2007; Lin et al., 2009). Because of the protective role biofilms play in drug- and immune-related persistence, the processes required for their formation are also being investigated for potential targets. Small molecule screens have been designed to find candidate biofilm inhibitors (Cegelski et al., 2009; Junker and Clardy, 2007). Among others, one group discovered compounds that prevent the biogenesis of pili and curli, extracellular bacterial fibers that mediate substrate attachment in the initial phases of biofilm formation (Aberg and Almqvist, 2007). Although treatment susceptibility was not assessed, these compounds effectively reduced the virulence of uropathogenic E. coli in a murine infection model (Cegelski et al., 2009). Therapeutic strategies to disaggregate existing biofilms are also being considered (Boles and Horswill, 2008; Lu and Collins, 2007). Detoxification mechanisms such as efflux pumps are being investigated as new antibiotic targets or as adjuvants to existing therapies (Fernandez and Hancock, 2012). Persisters can also be antagonized by potentiating antibiotic toxicity though the enhancement of drug uptake. For example, aminoglycoside uptake by persisters and the efficacy of aminoglycoside treatment of catheter-associated E. coli infection in mice can be increased by co-administration of specific metabolites that help power the trans-membrane proton motive force (Allison et al., 2011b; Lu and Collins, 2009). Finally, the use of adjuvants that maximize antibiotic-induced intracellular ROS production is also being explored (Brynildsen et al., 2013; Grant et al., 2012).
Drug screens have traditionally focused on identifying compounds lethal to microbes in stress-free in vitro culture environments. While this has been a successful way to identify drug targets, it is becoming clear that many microbial processes not required for idealized life in vitro are essential for survival over the course of infection and treatment in humans (Barczak and Hung, 2009). Such processes, which include both persistence and virulence, are intimately linked to dynamic and complex networks of stress responses that orchestrate phenotypic adaptation. Learning more about these processes and their role in infection is likely to yield a rich trove of potential new drug targets, and hopefully lead to more effective therapies against persistent, relapsing, and resistant organisms.
Conclusion and future directions
Persistent and recurrent infections in humans are common, and their frequency is rising. Despite this, our understanding of antimicrobial treatment failure remains limited. Though insight into microbial drug tolerance has been gained using in vitro systems, the persister phenotype remains only partially characterized. Going forward, innovative approaches to persister isolation and molecular definition are urgently needed to more deeply explore mechanisms involved in induction, maintenance, and exit from the persister state. Further, animal models that faithfully recapitulate human infection and treatment courses should be devised to corroborate culture-based data. Lastly, to explore persistence and resistance dynamics in vivo, longitudinal studies of microbial population size, diversity, and evolution occurring within individual patients during the course of infection and treatment should be initiated. Although indirect evidence suggests that the persister state may promote the emergence of resistance, no definitive experiment has established this to be the case. An integrative approach that compares the results of culture systems, animal models, and natural history cohorts of primary human infection will facilitate the elucidation of persistence mechanisms, help to determine the magnitude of the impact of persistence to human treatment failure and drug resistance, and usher in new approaches to the treatment and eradication of infectious diseases.
Acknowledgments
We thank Drs. Kara Lassen, Ahmad S. Khalil, Peter Belenky and Rebecca S. Shapiro for critical input and reading the manuscript. This work was supported by funding from the Wyss Institute Clinical Fellowship program and the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aberg V, Almqvist F. Pilicides-small molecules targeting bacterial virulence. Org Biomol Chem. 2007;5:1827–1834. doi: 10.1039/b702397a. [DOI] [PubMed] [Google Scholar]
- Abraham EP, Chain E. An Enzyme from Bacteria able to Destroy Penicillin. Nature. 1940;146:837–837. [PubMed] [Google Scholar]
- Adams KN, Takaki K, Connolly LE, Wiedenhoft H, Winglee K, Humbert O, Edelstein PH, Cosma CL, Ramakrishnan L. Drug tolerance in replicating mycobacteria mediated by a macrophage-induced efflux mechanism. Cell. 2011;145:39–53. doi: 10.1016/j.cell.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Mamun AA, Lombardo MJ, Shee C, Lisewski AM, Gonzalez C, Lin D, Nehring RB, Saint-Ruf C, Gibson JL, Frisch RL, et al. Identity and function of a large gene network underlying mutagenic repair of DNA breaks. Science. 2012;338:1344–1348. doi: 10.1126/science.1226683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge BB, Fernandez-Suarez M, Heller D, Ambravaneswaran V, Irimia D, Toner M, Fortune SM. Asymmetry and aging of mycobacterial cells lead to variable growth and antibiotic susceptibility. Science. 2012;335:100–104. doi: 10.1126/science.1216166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison KR, Brynildsen MP, Collins JJ. Heterogeneous bacterial persisters and engineering approaches to eliminate them. Curr Opin Microbiol. 2011a;14:593–598. doi: 10.1016/j.mib.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison KR, Brynildsen MP, Collins JJ. Metabolite-enabled eradication of bacterial persisters by aminoglycosides. Nature. 2011b;473:216–220. doi: 10.1038/nature10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaratunga C, Sreng S, Suon S, Phelps ES, Stepniewska K, Lim P, Zhou C, Mao S, Anderson JM, Lindegardh N, et al. Artemisinin-resistant Plasmodium falciparum in Pursat province, western Cambodia: a parasite clearance rate study. Lancet Infect Dis. 2012;12:851–858. doi: 10.1016/S1473-3099(12)70181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato SM, Orman MA, Brynildsen MP. Metabolic control of persister formation in Escherichia coli. Cell. 2013;50:1–13. doi: 10.1016/j.molcel.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Andries K, Verhasselt P, Guillemont J, Gohlmann HW, Neefs JM, Winkler H, Van Gestel J, Timmerman P, Zhu M, Lee E, et al. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science. 2005;307:223–227. doi: 10.1126/science.1106753. [DOI] [PubMed] [Google Scholar]
- Baek SH, Li AH, Sassetti CM. Metabolic regulation of mycobacterial growth and antibiotic sensitivity. PLoS Biol. 2011;9:e1001065. doi: 10.1371/journal.pbio.1001065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban NQ, Merrin J, Chait R, Kowalik L, Leibler S. Bacterial persistence as a phenotypic switch. Science. 2004;305:1622–1625. doi: 10.1126/science.1099390. [DOI] [PubMed] [Google Scholar]
- Barczak AK, Hung DT. Productive steps toward an antimicrobial targeting virulence. Curr Opin Microbiol. 2009;12:490–496. doi: 10.1016/j.mib.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaber JW, Hochhut B, Waldor MK. SOS response promotes horizontal dissemination of antibiotic resistance genes. Nature. 2004;427:72–74. doi: 10.1038/nature02241. [DOI] [PubMed] [Google Scholar]
- Belenky P, Collins JJ. Microbiology. Antioxidant strategies to tolerate antibiotics. Science. 2011;334:915–916. doi: 10.1126/science.1214823. [DOI] [PubMed] [Google Scholar]
- Betts JC, Lukey PT, Robb LC, McAdam RA, Duncan K. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol Microbiol. 2002;43:717–731. doi: 10.1046/j.1365-2958.2002.02779.x. [DOI] [PubMed] [Google Scholar]
- Bigger JW. Treatment of staphylococcal infections with penicillin by intermittent sterilisation. The Lancet 1944 [Google Scholar]
- Boles BR, Horswill AR. Agr-mediated dispersal of Staphylococcus aureus biofilms. PLoS Pathog. 2008;4:e1000052. doi: 10.1371/journal.ppat.1000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brynildsen MP, Winkler JA, Spina CS, Macdonald IC, Collins JJ. Potentiating antibacterial activity by predictably enhancing endogenous microbial ROS production. Nat Biotechnol. 2013;31:160–165. doi: 10.1038/nbt.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso K, Gandra RF, Wisniewski ES, Osaku CA, Kadowaki MK, Felipach-Neto V, Haus LF, de Simao RC. DnaK and GroEL are induced in response to antibiotic and heat shock in Acinetobacter baumannii. J Med Microbiol. 2010;59:1061–1068. doi: 10.1099/jmm.0.020339-0. [DOI] [PubMed] [Google Scholar]
- Cegelski L, Pinkner JS, Hammer ND, Cusumano CK, Hung CS, Chorell E, Aberg V, Walker JN, Seed PC, Almqvist F, et al. Small-molecule inhibitors target Escherichia coli amyloid biogenesis and biofilm formation. Nat Chem Biol. 2009;5:913–919. doi: 10.1038/nchembio.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong YP, Park SJ, Kim HS, Kim ES, Kim MN, Park KH, Kim SH, Lee SO, Choi SH, Jeong JY, et al. Persistent Staphylococcus aureus Bacteremia: A Prospective Analysis of Risk Factors, Outcomes, and Microbiologic and Genotypic Characteristics of Isolates. Medicine (Baltimore) 2013;92:98–108. doi: 10.1097/MD.0b013e318289ff1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirz RT, Jones MB, Gingles NA, Minogue TD, Jarrahi B, Peterson SN, Romesberg FE. Complete and SOS-mediated response of Staphylococcus aureus to the antibiotic ciprofloxacin. J Bacteriol. 2007;189:531–539. doi: 10.1128/JB.01464-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirz RT, O’Neill BM, Hammond JA, Head SR, Romesberg FE. Defining the Pseudomonas aeruginosa SOS response and its role in the global response to the antibiotic ciprofloxacin. J Bacteriol. 2006;188:7101–7110. doi: 10.1128/JB.00807-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirz RT, Romesberg FE. Controlling mutation: intervening in evolution as a therapeutic strategy. Crit Rev Biochem Mol Biol. 2007;42:341–354. doi: 10.1080/10409230701597741. [DOI] [PubMed] [Google Scholar]
- De Groote VN, Verstraeten N, Fauvart M, Kint CI, Verbeeck AM, Beullens S, Cornelis P, Michiels J. Novel persistence genes in Pseudomonas aeruginosa identified by high-throughput screening. FEMS Microbiol Lett. 2009;297:73–79. doi: 10.1111/j.1574-6968.2009.01657.x. [DOI] [PubMed] [Google Scholar]
- Dhar N, McKinney JD. Mycobacterium tuberculosis persistence mutants identified by screening in isoniazid-treated mice. Proc Natl Acad Sci U S A. 2010;107:12275–12280. doi: 10.1073/pnas.1003219107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorr T, Lewis K, Vulic M. SOS response induces persistence to fluoroquinolones in Escherichia coli. PLoS Genet. 2009;5:e1000760. doi: 10.1371/journal.pgen.1000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driffield K, Miller K, Bostock JM, O’Neill AJ, Chopra I. Increased mutability of Pseudomonas aeruginosa in biofilms. J Antimicrob Chemother. 2008;61:1053–1056. doi: 10.1093/jac/dkn044. [DOI] [PubMed] [Google Scholar]
- Dwyer DJ, Kohanski MA, Hayete B, Collins JJ. Gyrase inhibitors induce an oxidative damage cellular death pathway in Escherichia coli. Mol Syst Biol. 2007;3:91. doi: 10.1038/msb4100135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng RH, Padberg FT, Smith SM, Tan EN, Cherubin CE. Bactericidal effects of antibiotics on slowly growing and nongrowing bacteria. Antimicrob Agents Chemother. 1991;35:1824–1828. doi: 10.1128/aac.35.9.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falla TJ, Chopra I. Joint tolerance to beta-lactam and fluoroquinolone antibiotics in Escherichia coli results from overexpression of hipA. Antimicrob Agents Chemother. 1998;42:3282–3284. doi: 10.1128/aac.42.12.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez L, Hancock RE. Adaptive and mutational resistance: role of porins and efflux pumps in drug resistance. Clin Microbiol Rev. 2012;25:661–681. doi: 10.1128/CMR.00043-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor AA, Klem ER, Gilpin DF, Elborn JS, Boucher RC, Tunney MM, Wolfgang MC. The adult cystic fibrosis airway microbiota is stable over time and infection type, and highly resilient to antibiotic treatment of exacerbations. PLoS One. 2012;7:e45001. doi: 10.1371/journal.pone.0045001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti JJ, Devadoss B, Winkler JA, Collins JJ, Walker GC. Oxidation of the guanine nucleotide pool underlies cell death by bactericidal antibiotics. Science. 2012;336:315–319. doi: 10.1126/science.1219192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung DK, Chan EW, Chin ML, Chan RC. Delineation of a bacterial starvation stress response network which can mediate antibiotic tolerance development. Antimicrob Agents Chemother. 2010;54:1082–1093. doi: 10.1128/AAC.01218-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandotra S, Schnappinger D, Monteleone M, Hillen W, Ehrt S. In vivo gene silencing identifies the Mycobacterium tuberculosis proteasome as essential for the bacteria to persist in mice. Nat Med. 2007;13:1515–1520. doi: 10.1038/nm1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Chua K, Davies JK, Newton HJ, Seemann T, Harrison PF, Holmes NE, Rhee HW, Hong JI, Hartland EL, et al. Two novel point mutations in clinical Staphylococcus aureus reduce linezolid susceptibility and switch on the stringent response to promote persistent infection. PLoS Pathog. 2010;6:e1000944. doi: 10.1371/journal.ppat.1000944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant SS, Hung DT. Persistent bacterial infections, antibiotic tolerance, and the oxidative stress response. Virulence. 2013:4. doi: 10.4161/viru.23987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant SS, Kaufmann BB, Chand NS, Haseley N, Hung DT. Eradication of bacterial persisters with antibiotic-generated hydroxyl radicals. Proc Natl Acad Sci U S A. 2012;109:12147–12152. doi: 10.1073/pnas.1203735109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullberg E, Cao S, Berg OG, Ilback C, Sandegren L, Hughes D, Andersson DI. Selection of resistant bacteria at very low antibiotic concentrations. PLoS Pathog. 2011;7:e1002158. doi: 10.1371/journal.ppat.1002158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusarov I, Shatalin K, Starodubtseva M, Nudler E. Endogenous nitric oxide protects bacteria against a wide spectrum of antibiotics. Science. 2009;325:1380–1384. doi: 10.1126/science.1175439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez A, Laureti L, Crussard S, Abida H, Rodriguez-Rojas A, Blazquez J, Baharoglu Z, Mazel D, Darfeuille F, Vogel J, et al. beta-lactam antibiotics promote bacterial mutagenesis via an RpoS-mediated reduction in replication fidelity. Nat Commun. 2013;4:1610. doi: 10.1038/ncomms2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen S, Lewis K, Vulic M. Role of global regulators and nucleotide metabolism in antibiotic tolerance in Escherichia coli. Antimicrob Agents Chemother. 2008;52:2718–2726. doi: 10.1128/AAC.00144-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden HS, Lim R, Brittnacher MJ, Sims EH, Ramage ER, Fong C, Wu Z, Crist E, Chang J, Zhou Y, et al. Evolution of Burkholderia pseudomallei in recurrent melioidosis. PLoS One. 2012;7:e36507. doi: 10.1371/journal.pone.0036507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Cooper JN, Mishra A, Raskin DM. Stringent response regulation of biofilm formation in Vibrio cholerae. J Bacteriol. 2012;194:2962–2972. doi: 10.1128/JB.00014-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa H, Inazumi Y, Masaki T, Hirata T, Yamaguchi A. Indole induces the expression of multidrug exporter genes in Escherichia coli. Mol Microbiol. 2005;55:1113–1126. doi: 10.1111/j.1365-2958.2004.04449.x. [DOI] [PubMed] [Google Scholar]
- Hoffman LR, D’Argenio DA, MacCoss MJ, Zhang Z, Jones RA, Miller SI. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature. 2005;436:1171–1175. doi: 10.1038/nature03912. [DOI] [PubMed] [Google Scholar]
- Hoiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents. 2010;35:322–332. doi: 10.1016/j.ijantimicag.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Junker LM, Clardy J. High-throughput screens for small-molecule inhibitors of Pseudomonas aeruginosa biofilm development. Antimicrob Agents Chemother. 2007;51:3582–3590. doi: 10.1128/AAC.00506-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayama S, Murakami K, Ono T, Ushimaru M, Yamamoto A, Hirota K, Miyake Y. The role of rpoS gene and quorum-sensing system in ofloxacin tolerance in Pseudomonas aeruginosa. FEMS Microbiol Lett. 2009;298:184–192. doi: 10.1111/j.1574-6968.2009.01717.x. [DOI] [PubMed] [Google Scholar]
- Keren I, Kaldalu N, Spoering A, Wang Y, Lewis K. Persister cells and tolerance to antimicrobials. FEMS Microbiol Lett. 2004;230:13–18. doi: 10.1016/S0378-1097(03)00856-5. [DOI] [PubMed] [Google Scholar]
- Keren I, Wu Y, Inocencio J, Mulcahy LR, Lewis K. Killing by bactericidal antibiotics does not depend on reactive oxygen species. Science. 2013;339:1213–1216. doi: 10.1126/science.1232688. [DOI] [PubMed] [Google Scholar]
- Kohanski MA, DePristo MA, Collins JJ. Sublethal antibiotic treatment leads to multidrug resistance via radical-induced mutagenesis. Mol Cell. 2010;37:311–320. doi: 10.1016/j.molcel.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. A common mechanism of cellular death induced by bactericidal antibiotics. Cell. 2007;130:797–810. doi: 10.1016/j.cell.2007.06.049. [DOI] [PubMed] [Google Scholar]
- Kohanski MA, Dwyer DJ, Wierzbowski J, Cottarel G, Collins JJ. Mistranslation of membrane proteins and two-component system activation trigger antibiotic-mediated cell death. Cell. 2008;135:679–690. doi: 10.1016/j.cell.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korch SB, Henderson TA, Hill TM. Characterization of the hipA7 allele of Escherichia coli and evidence that high persistence is governed by (p)ppGpp synthesis. Mol Microbiol. 2003;50:1199–1213. doi: 10.1046/j.1365-2958.2003.03779.x. [DOI] [PubMed] [Google Scholar]
- Korch SB, Hill TM. Ectopic overexpression of wild-type and mutant hipA genes in Escherichia coli: effects on macromolecular synthesis and persister formation. J Bacteriol. 2006;188:3826–3836. doi: 10.1128/JB.01740-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause PJ, Gewurz BE, Hill D, Marty FM, Vannier E, Foppa IM, Furman RR, Neuhaus E, Skowron G, Gupta S, et al. Persistent and relapsing babesiosis in immunocompromised patients. Clin Infect Dis. 2008;46:370–376. doi: 10.1086/525852. [DOI] [PubMed] [Google Scholar]
- Kucharikova S, Tournu H, Lagrou K, Van Dijck P, Bujdakova H. Detailed comparison of Candida albicans and Candida glabrata biofilms under different conditions and their susceptibility to caspofungin and anidulafungin. J Med Microbiol. 2011;60:1261–1269. doi: 10.1099/jmm.0.032037-0. [DOI] [PubMed] [Google Scholar]
- Kussell E, Kishony R, Balaban NQ, Leibler S. Bacterial persistence: a model of survival in changing environments. Genetics. 2005;169:1807–1814. doi: 10.1534/genetics.104.035352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaCrue AN, Scheel M, Kennedy K, Kumar N, Kyle DE. Effects of artesunate on parasite recrudescence and dormancy in the rodent malaria model Plasmodium vinckei. PLoS One. 2011;6:e26689. doi: 10.1371/journal.pone.0026689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFleur MD, Kumamoto CA, Lewis K. Candida albicans biofilms produce antifungal-tolerant persister cells. Antimicrob Agents Chemother. 2006;50:3839–3846. doi: 10.1128/AAC.00684-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafleur MD, Qi Q, Lewis K. Patients with long-term oral carriage harbor high-persister mutants of Candida albicans. Antimicrob Agents Chemother. 2010;54:39–44. doi: 10.1128/AAC.00860-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HH, Molla MN, Cantor CR, Collins JJ. Bacterial charity work leads to population-wide resistance. Nature. 2010;467:82–85. doi: 10.1038/nature09354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Hinz A, Bauerle E, Angermeyer A, Juhaszova K, Kaneko Y, Singh PK, Manoil C. Targeting a bacterial stress response to enhance antibiotic action. Proc Natl Acad Sci U S A. 2009;106:14570–14575. doi: 10.1073/pnas.0903619106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung V, Levesque CM. A stress-inducible quorum-sensing peptide mediates the formation of persister cells with noninherited multidrug tolerance. J Bacteriol. 2012;194:2265–2274. doi: 10.1128/JB.06707-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin BR, Rozen DE. Non-inherited antibiotic resistance. Nat Rev Microbiol. 2006;4:556–562. doi: 10.1038/nrmicro1445. [DOI] [PubMed] [Google Scholar]
- Lieberman TD, Michel JB, Aingaran M, Potter-Bynoe G, Roux D, Davis MR, Jr, Skurnik D, Leiby N, LiPuma JJ, Goldberg JB, et al. Parallel bacterial evolution within multiple patients identifies candidate pathogenicity genes. Nat Genet. 2011;43:1275–1280. doi: 10.1038/ng.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G, Li D, de Carvalho LP, Deng H, Tao H, Vogt G, Wu K, Schneider J, Chidawanyika T, Warren JD, et al. Inhibitors selective for mycobacterial versus human proteasomes. Nature. 2009;461:621–626. doi: 10.1038/nature08357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Imlay JA. Cell death from antibiotics without the involvement of reactive oxygen species. Science. 2013;339:1210–1213. doi: 10.1126/science.1232751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu TK, Collins JJ. Dispersing biofilms with engineered enzymatic bacteriophage. Proc Natl Acad Sci U S A. 2007;104:11197–11202. doi: 10.1073/pnas.0704624104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu TK, Collins JJ. Engineered bacteriophage targeting gene networks as adjuvants for antibiotic therapy. Proc Natl Acad Sci U S A. 2009;106:4629–4634. doi: 10.1073/pnas.0800442106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonneuve E, Shakespeare LJ, Jorgensen MG, Gerdes K. Bacterial persistence by RNA endonucleases. Proc Natl Acad Sci U S A. 2011;108:13206–13211. doi: 10.1073/pnas.1100186108. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- McCune RM, Jr, McDermott W, Tompsett R. The fate of Mycobacterium tuberculosis in mouse tissues as determined by the microbial enumeration technique. II. The conversion of tuberculous infection to the latent state by the administration of pyrazinamide and a companion drug. J Exp Med. 1956;104:763–802. doi: 10.1084/jem.104.5.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCune RM, Jr, Tompsett R. Fate of Mycobacterium tuberculosis in mouse tissues as determined by the microbial enumeration technique. I. The persistence of drug-susceptible tubercle bacilli in the tissues despite prolonged antimicrobial therapy. J Exp Med. 1956;104:737–762. doi: 10.1084/jem.104.5.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miotto O, Almagro-Garcia J, Manske M, Macinnis B, Campino S, Rockett KA, Amaratunga C, Lim P, Suon S, Sreng S, et al. Multiple populations of artemisinin-resistant Plasmodium falciparum in Cambodia. Nat Genet. 2013 doi: 10.1038/ng.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moker N, Dean CR, Tao J. Pseudomonas aeruginosa increases formation of multidrug-tolerant persister cells in response to quorum-sensing signaling molecules. J Bacteriol. 2010;192:1946–1955. doi: 10.1128/JB.01231-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyed HS, Bertrand KP. hipA, a newly recognized gene of Escherichia coli K-12 that affects frequency of persistence after inhibition of murein synthesis. J Bacteriol. 1983;155:768–775. doi: 10.1128/jb.155.2.768-775.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K, Ono T, Viducic D, Kayama S, Mori M, Hirota K, Nemoto K, Miyake Y. Role for rpoS gene of Pseudomonas aeruginosa in antibiotic tolerance. FEMS Microbiol Lett. 2005;242:161–167. doi: 10.1016/j.femsle.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Mwangi MM, Wu SW, Zhou Y, Sieradzki K, de Lencastre H, Richardson P, Bruce D, Rubin E, Myers E, Siggia ED, et al. Tracking the in vivo evolution of multidrug resistance in Staphylococcus aureus by whole-genome sequencing. Proc Natl Acad Sci U S A. 2007;104:9451–9456. doi: 10.1073/pnas.0609839104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair CG, Chao C, Ryall B, Williams HD. Sub-lethal concentrations of antibiotics increase mutation frequency in the cystic fibrosis pathogen Pseudomonas aeruginosa. Lett Appl Microbiol. 2013;56:149–154. doi: 10.1111/lam.12032. [DOI] [PubMed] [Google Scholar]
- Nakazawa S, Kanbara H, Aikawa M. Plasmodium falciparum: recrudescence of parasites in culture. Exp Parasitol. 1995;81:556–563. doi: 10.1006/expr.1995.1149. [DOI] [PubMed] [Google Scholar]
- Nakazawa S, Maoka T, Uemura H, Ito Y, Kanbara H. Malaria parasites giving rise to recrudescence in vitro. Antimicrob Agents Chemother. 2002;46:958–965. doi: 10.1128/AAC.46.4.958-965.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen D, Joshi-Datar A, Lepine F, Bauerle E, Olakanmi O, Beer K, McKay G, Siehnel R, Schafhauser J, Wang Y, et al. Active starvation responses mediate antibiotic tolerance in biofilms and nutrient-limited bacteria. Science. 2011;334:982–986. doi: 10.1126/science.1211037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perriens JH, St Louis ME, Mukadi YB, Brown C, Prignot J, Pouthier F, Portaels F, Willame JC, Mandala JK, Kaboto M, et al. Pulmonary tuberculosis in HIV-infected patients in Zaire. A controlled trial of treatment for either 6 or 12 months. N Engl J Med. 1995;332:779–784. doi: 10.1056/NEJM199503233321204. [DOI] [PubMed] [Google Scholar]
- Petrosino JF, Galhardo RS, Morales LD, Rosenberg SM. Stress-induced beta-lactam antibiotic resistance mutation and sequences of stationary-phase mutations in the Escherichia coli chromosome. J Bacteriol. 2009;191:5881–5889. doi: 10.1128/JB.00732-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit RK, Repp KK, Hazen KC. Temperature affects the susceptibility of Cryptococcus neoformans biofilms to antifungal agents. Med Mycol. 2010;48:421–426. doi: 10.1080/13693780903136879. [DOI] [PubMed] [Google Scholar]
- Poole K. Stress responses as determinants of antimicrobial resistance in Gram-negative bacteria. Trends Microbiol. 2012;20:227–234. doi: 10.1016/j.tim.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Potrykus K, Cashel M. (p)ppGpp: still magical? Annu Rev Microbiol. 2008;62:35–51. doi: 10.1146/annurev.micro.62.081307.162903. [DOI] [PubMed] [Google Scholar]
- Prudhomme M, Attaiech L, Sanchez G, Martin B, Claverys JP. Antibiotic stress induces genetic transformability in the human pathogen Streptococcus pneumoniae. Science. 2006;313:89–92. doi: 10.1126/science.1127912. [DOI] [PubMed] [Google Scholar]
- Rovira-Graells N, Gupta AP, Planet E, Crowley VM, Mok S, Ribas de Pouplana L, Preiser PR, Bozdech Z, Cortes A. Transcriptional variation in the malaria parasite Plasmodium falciparum. Genome Res. 2012;22:925–938. doi: 10.1101/gr.129692.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder VJ, Chopra I, O’Neill AJ. Increased mutability of staphylococci in biofilms as a consequence of oxidative stress. PLoS One. 2012;7:e47695. doi: 10.1371/journal.pone.0047695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saralamba S, Pan-Ngum W, Maude RJ, Lee SJ, Tarning J, Lindegardh N, Chotivanich K, Nosten F, Day NP, Socheat D, et al. Intrahost modeling of artemisinin resistance in Plasmodium falciparum. Proc Natl Acad Sci U S A. 2011;108:397–402. doi: 10.1073/pnas.1006113108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage VJ, Chopra I, O’Neill AJ. Staphylococcus aureus biofilms promote horizontal transfer of antibiotic resistance. Antimicrob Agents Chemother. 2013 doi: 10.1128/AAC.02008-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah D, Zhang Z, Khodursky A, Kaldalu N, Kurg K, Lewis K. Persisters: a distinct physiological state of E. coli. BMC Microbiol. 2006;6:53. doi: 10.1186/1471-2180-6-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SV, Lee DY, Li B, Quinlan MP, Takahashi F, Maheswaran S, McDermott U, Azizian N, Zou L, Fischbach MA, et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell. 2010;141:69–80. doi: 10.1016/j.cell.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatalin K, Shatalina E, Mironov A, Nudler E. H2S: a universal defense against antibiotics in bacteria. Science. 2011;334:986–990. doi: 10.1126/science.1209855. [DOI] [PubMed] [Google Scholar]
- Spoering AL, Vulic M, Lewis K. GlpD and PlsB participate in persister cell formation in Escherichia coli. J Bacteriol. 2006;188:5136–5144. doi: 10.1128/JB.00369-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecher B, Denzler R, Maier L, Bernet F, Sanders MJ, Pickard DJ, Barthel M, Westendorf AM, Krogfelt KA, Walker AW, et al. Gut inflammation can boost horizontal gene transfer between pathogenic and commensal Enterobacteriaceae. Proc Natl Acad Sci U S A. 2012;109:1269–1274. doi: 10.1073/pnas.1113246109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens KE, Chang D, Zwack EE, Sebert ME. Competence in Streptococcus pneumoniae is regulated by the rate of ribosomal decoding errors. MBio. 2011:2. doi: 10.1128/mBio.00071-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G, Luo T, Yang C, Dong X, Li J, Zhu Y, Zheng H, Tian W, Wang S, Barry CE, 3rd, et al. Dynamic population changes in Mycobacterium tuberculosis during acquisition and fixation of drug resistance in patients. J Infect Dis. 2012;206:1724–1733. doi: 10.1093/infdis/jis601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takala-Harrison S, Clark TG, Jacob CG, Cummings MP, Miotto O, Dondorp AM, Fukuda MM, Nosten F, Noedl H, Imwong M, et al. Genetic loci associated with delayed clearance of Plasmodium falciparum following artemisinin treatment in Southeast Asia. Proc Natl Acad Sci U S A. 2013;110:240–245. doi: 10.1073/pnas.1211205110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuscher F, Gatton ML, Chen N, Peters J, Kyle DE, Cheng Q. Artemisinin-induced dormancy in plasmodium falciparum: duration, recovery rates, and implications in treatment failure. J Infect Dis. 2010;202:1362–1368. doi: 10.1086/656476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapar MM, Gil JP, Bjorkman A. In vitro recrudescence of Plasmodium falciparum parasites suppressed to dormant state by atovaquone alone and in combination with proguanil. Trans R Soc Trop Med Hyg. 2005;99:62–70. doi: 10.1016/j.trstmh.2004.01.016. [DOI] [PubMed] [Google Scholar]
- Tripathi A, Dewan PC, Barua B, Varadarajan R. Additional role for the ccd operon of F-plasmid as a transmissible persistence factor. Proc Natl Acad Sci U S A. 2012;109:12497–12502. doi: 10.1073/pnas.1121217109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Acker H, Sass A, Bazzini S, De Roy K, Udine C, Messiaen T, Riccardi G, Boon N, Nelis HJ, Mahenthiralingam E, et al. Biofilm-Grown Burkholderia cepacia Complex Cells Survive Antibiotic Treatment by Avoiding Production of Reactive Oxygen Species. PLoS One. 2013;8:e58943. doi: 10.1371/journal.pone.0058943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega NM, Allison KR, Khalil AS, Collins JJ. Signaling-mediated bacterial persister formation. Nat Chem Biol. 2012;8:431–433. doi: 10.1038/nchembio.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakamoto Y, Dhar N, Chait R, Schneider K, Signorino-Gelo F, Leibler S, McKinney JD. Dynamic persistence of antibiotic-stressed mycobacteria. Science. 2013;339:91–95. doi: 10.1126/science.1229858. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhao X. Contribution of oxidative damage to antimicrobial lethality. Antimicrob Agents Chemother. 2009;53:1395–1402. doi: 10.1128/AAC.01087-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexselblatt E, Oppenheimer-Shaanan Y, Kaspy I, London N, Schueler-Furman O, Yavin E, Glaser G, Katzhendler J, Ben-Yehuda S. Relacin, a novel antibacterial agent targeting the Stringent Response. PLoS Pathog. 2012;8:e1002925. doi: 10.1371/journal.ppat.1002925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NJ, Pongtavornpinyo W, Maude RJ, Saralamba S, Aguas R, Stepniewska K, Lee SJ, Dondorp AM, White LJ, Day NP. Hyperparasitaemia and low dosing are an important source of anti-malarial drug resistance. Malar J. 2009;8:253. doi: 10.1186/1475-2875-8-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigle TJ, Sexton JZ, Gromova AV, Hadimani MB, Hughes MA, Smith GR, Yeh LA, Singleton SF. Inhibitors of RecA activity discovered by high-throughput screening: cell-permeable small molecules attenuate the SOS response in Escherichia coli. J Biomol Screen. 2009;14:1092–1101. doi: 10.1177/1087057109342126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkowski B, Lelievre J, Barragan MJ, Laurent V, Su XZ, Berry A, Benoit-Vical F. Increased tolerance to artemisinin in Plasmodium falciparum is mediated by a quiescence mechanism. Antimicrob Agents Chemother. 2010;54:1872–1877. doi: 10.1128/AAC.01636-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Vulic M, Keren I, Lewis K. Role of oxidative stress in persister tolerance. Antimicrob Agents Chemother. 2012;56:4922–4926. doi: 10.1128/AAC.00921-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Lambert G, Liao D, Kim H, Robin K, Tung CK, Pourmand N, Austin RH. Acceleration of emergence of bacterial antibiotic resistance in connected microenvironments. Science. 2011;333:1764–1767. doi: 10.1126/science.1208747. [DOI] [PubMed] [Google Scholar]
- Zhang X, McDaniel AD, Wolf LE, Keusch GT, Waldor MK, Acheson DW. Quinolone antibiotics induce Shiga toxin-encoding bacteriophages, toxin production, and death in mice. J Infect Dis. 2000;181:664–670. doi: 10.1086/315239. [DOI] [PubMed] [Google Scholar]
- Zhao J, Schloss PD, Kalikin LM, Carmody LA, Foster BK, Petrosino JF, Cavalcoli JD, VanDevanter DR, Murray S, Li JZ, et al. Decade-long bacterial community dynamics in cystic fibrosis airways. Proc Natl Acad Sci U S A. 2012;109:5809–5814. doi: 10.1073/pnas.1120577109. [DOI] [PMC free article] [PubMed] [Google Scholar]