Abstract
It has been hypothesized that, in the developing rodent hippocampus, mossy fiber terminals release GABA together with glutamate. Here, we used transgenic glutamic acid decarboxylase-67 (GAD67)-GFP expressing mice and multi-label immunohistochemistry to address whether glutamatergic and GABAergic markers are colocalized. We demonstrate that in the dentate gyrus, interneurons positive for GABA/GAD are sparsely distributed along the edge of the hilus, in a different pattern than the densely packed granule cells. Co-staining for synaptophysin and vesicular glutamate transporter1 (VGLUT1) in postnatal day 14 brain sections from both mice and rats identified mossy fiber terminals as a group of large (2 – 5μm in diameter) VGLUT1-positive excitatory presynaptic terminals in the stratum lucidum of area CA3a/b. Furthermore, co-staining for synaptophysin and vesicular GABA transporter (VGAT) revealed a group of small-sized (~0.5μm in diameter) inhibitory presynaptic terminals in the same area where identified mossy fiber terminals were present. The two types of terminals appeared to be mutually exclusive, and showed no colocalization. Thus, our results do not support the hypothesis that GABA is released as a neurotransmitter from mossy fiber terminals during development.
Keywords: granule cell, vesicular glutamate transporter, vesicular GABA transporter, immunofluorescence, synaptic button
1. Introduction
Mossy fibers, the axons from hippocampal dentate granule cells, terminate onto area CA3 pyramidal neurons, in a terminal field called the stratum lucidum (sl). These terminals are large, approximately 3 – 6μm in diameter in adult rats (Amaral and Witter 2000). As they are extensions arising from hippocampal principal cells, mossy fibers have long been thought to release glutamate as their primary neurotransmitter.
A hypothesis positing that mossy fiber terminals co-release GABA together with glutamate in the developing rodent brain (Gutiérrez, 2003; Gutiérrez, 2005; Gutiérrez and Heinemann, 2006; Jaffe and Gutiérrez 2007) has attracted considerable attention, since excitatory glutamatergic and inhibitory GABAergic transmission oppose each other in their induced neuronal actions. Electrophysiological recordings (Gutiérrez et al., 2003) demonstrated that in sections from neonatal rats up to postnatal day 22 (P22), stimulation of the granule cell layer in the presence of ionotropic glutamate receptor antagonists, resulted in bicuculline-sensitive monosynaptic IPSPs recorded in area CA3 pyramidal cells. It has been hypothesized that these IPSPs are a result of GABA release from mossy fiber terminals onto CA3 pyramidal cells (Gutiérrez et al., 2003).
Conversely, other physiological experiments have lent credence to the hypothesis that mossy fibers exclusively release glutamate during development. Uchigashima et al. (2007) reported electrophysiological data in developing mice and rats (P14 – P20) demonstrating that strong stimulation of the dentate granule cell layer in the presence of ionotropic glutamate receptor antagonists, elicited picrotoxin-sensitive monosynaptic GABAergic IPSCs from CA3 neurons. Blocking mossy fiber-CA3 transmission abolished almost all postsynaptic CA3 responses elicited by weak stimulation of granule cell layer. These findings suggest that monosynaptic IPSCs previously reported might be evoked by co-stimulation of nearby inhibitory interneurons (Uchigashima et al. (2007; Gutiérrez, 2009). It was suggested that the source of co-stimulated interneurons might be mossy fiber associated interneurons (Uchigashima et al., 2007). These mossy fiber associated interneurons are located in sl of area CA3 and generate axonal collaterals to the hilus of the dentate gyrus (Vida and Frotscher, 2000; Losonczy et al., 2004).
Anatomical evidences supporting glutamate-GABA co-release hypothesis from previous studies are controversial. Immunohistochemical staining demonstrated immunoreactivity of GABA and glutamic acid decarboxylase-67 (GAD67, an isoform for the GABA synthesizing enzyme usually detected in GABAergic cell bodies and neuropiles) in granule cells and mossy fiber terminals from developing rats (Ramirez and Gutierrez, 2001; Gutiérrez et al., 2003; Maqueda et al., 2003; Uchigashima et al., 2007; Gutiérrez, 2009) and mice (Uchigashima et al., 2007). Conversely, GAD65, which preferentially resides in axonal terminals (Kaufman, et al., 1991; Esclapez, et al., 1994; Castaneda et al., 2005) has not been shown in granule cells or mossy fiber terminals (Gutiérrez et al., 2003; Maqueda et al., 2003). While it has been suggested that the GABAergic phenotype of granule cells/mossy fiber terminals is transient, disappearing by adulthood (Gutiérrez, 2005, 2009), other studies reported that GAD and/or GABA were normally detected in granule cells and/or mossy fiber terminals from normal adult animals including the mouse (Sloviter et al., 1996), rat (Sloviter et al., 1996; Lehmann et al., 1996; Bergersen et al., 2003; Zander et al., 2010) and monkey/human (Sandler and Smith, 1991; Lehmann et al., 1996). To classify a synaptic terminal as GABAergic the existence of vesicular GABA transporter (VGAT) is more convincing than labeling with other markers. A high expression level of mRNA for VGAT has been detected in granule cells from developing rats (Gutiérrez et al., 2003; Gómez-Lira 2005; Zander et al., 2010) and adult rats (Zander et al., 2010). Although VGAT protein has been shown in mossy fiber terminals in developing (Safiulina et al., 2006) and normal adult rats (Zander et al., 2010), other studies reported a lack of VGAT staining (see Gutiérrez, 2009 for a review; Uchigashima et al., 2007). The inconsistency in anatomical findings from previous studies might be at least partially due to the lack of specific neuronal or synaptic markers to identify granule cells/mossy fiber terminals.
Using specific neuronal (GAD67-GFP to highlight GABAergic inhibitory neurons combined with EAAC1 to label hippocampal principal neurons including granule cells) and synaptic markers [type 1 vesicular glutamate transporter (VGLUT1) in conjunction with synaptophysin to label mossy fiber terminals], we re-examined if GABA/GAD positive structures are indeed granule cells or mossy fiber terminals. To further investigate whether GABA co-localizes with glutamate in developing mossy fiber terminals, we performed a series of double immunofluorescent staining experiments in fixed hippocampal sections from P14 rodents. We used both developing mice and rats in order to eliminate species dependence as the possible source of the reported experimental discrepancy. Using transgenic GAD67-GFP expressing mice (Tamamaki et al., 2003), we demonstrate that GAD67-GFP expressing interneurons are a discrete cellular population from dentate granule neurons. Large mossy fiber terminals were specifically positive for VGLUT1 in both mice and rats and could not be co-labeled with markers for GABAergic synapses, thus supporting the idea that mossy fiber terminals do not contain GABA during rodent development.
2. Results
2.1. Immunoblot detection of GABA and other synaptic markers
To ensure that the primary antibodies are specific for their targets we performed a dot blot for GABA and Western blots for the other primary antibodies. tThe sampling dot of the GABA conjugate could be clearly seen, whereas the dots of the other amino acids including glutamate, aspartate and glutamine were not recognized (Fig. S1a).
Western blots conducted on whole brain lysates from P14 mice, comfirmed that each antibody recognized a single protein band at the appropriate molecular weight of the target proteins (Fig. S1b). The synaptophysin antibody recognized a band between 37 to 40 kD, while the GAD65/67, VGLUT1 and VGAT antibodies recognized band between 50 to 60 kD, respectively.
2.2. Identity of granule cells using P14 GAD67-GFP mice and rats
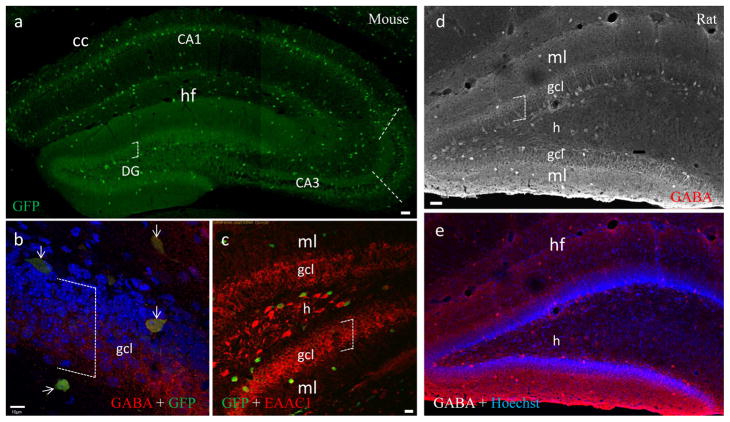
To determine whether granule cells are GABAergic at an early postnatal stage, we first evaluated the distribution pattern of inhibitory neurons in the hippocampus using P14 GAD67-GFP expressing mice. Since the transgenic mice were engineered with GFP tagged to the locus encoding GAD67, all GAD67 expressing inhibitory neurons are presumed to be GFP positive (Tamamaki et al., 2003). In general, GFP positive neurons were distributed throughout the hippocampus (Fig. 1a). A majority of them were immunoreactive to GABA (Fig. 1b, arrows). In both areas CA1 and CA3, they were distributed predominantly in strata radiatum (sr) and oriens (so). In the dentate gyrus, these neurons were distributed along the outer boundaries of the hilus, which is sandwiched between the supra- and infrapyramidal blades of the granule cell layers (gcl) (Fig. 1a,), similar to the distribution pattern demonstrated in adults of the same transgenic mice (Tamamaki et al., 2003).
Fig. 1.
GABAergic hippocampal neurons identified in sections from a P14 mouse (a–c) and rat (d–e). a, Composite of a stack of confocal photomicrographs showing the distribution of GAD-GFP expressing neurons from a GAD67-GFP transgenic mouse. Subregion CA3a/b (between dashed lines) is highlighted where mossy fiber terminals are distributed in the stratum lucidum, from which subsequent (b & c) figures for synaptic labeling were taken. cc, corpus callosum; DG, dentate gyrus; hf, hippocampal fissure. Open boxes of dashed lines indicate the dorsaventral extent of gcl (a–d). b, GAD-GFP expressing neurons co-labeled with GABA (arrows). Nuclei were counterstained with the DNA dye Hoechst (blue). Dense inhibitory synapses were also labeled with GABA (red), perisomaticly distributed throughout the granual cell layer (gcl). c, GAD-GFP expressing neurons are independent from dentate granule cells labeled with EAAC1 (red), a marker for hippocampal principal neurons. h, hilus of DG; ml, molecular layer. d, A converted grayscale projection of a stack of confocal photomicrographs showing the distribution of GABA-positive neurons in rat DG. e, Same image of d with Hoechst counterstaining. Scale bar: 50μm in a, d and e; 10μm in b; 20μm in c.
We then co-stained sections from GAD67-GFP mice with antibodies to type 1 excitatory amino acid carrier (EAAC1), a marker for hippocampal principal neurons including granule cells (Shashidharan et al., 1997; He et al., 2000). Consistent with previous findings (He et al., 2000), our EAAC1 staining demonstrated a gradient in fluorescent intensity from CA3 pyramidal neurons (most intensive) to dentate granule cells (moderate). In the hilus, a small number of EAAC1-positive cells could be identified (Fig. 1c). Within the granule cell layer populated by a numerous number of EAAC1-positive neurons, only a very few GAD67-GFP interneurons could be found (Fig. 1c). Taken together, GAD67-GFP-positive GABAergic interneurons are comparatively rare and belong to an independent and discrete neuronal population separate from the densely packed granule cells (Fig. 1a–c, open boxes).
In order to provide comparison to previous electrophysiological work in rats, we performed GABA immunoflourescent staining to demonstrate GABAergic interneurons in sections from P14 rats. The distribution pattern of GABA-positive hippocampal interneurons in developing rats is similar to that of GAD67-GFP expressing interneurons in the transgenic P14 mice. That is, in rat dentate gyrus, like that in mouse, GABA-positive interneurons are distributed predominantly in the hilus, directly adjacent to gcl(Fig. 1d and e).
2.3. Identity of mossy fiber terminals using P14 mice and rats
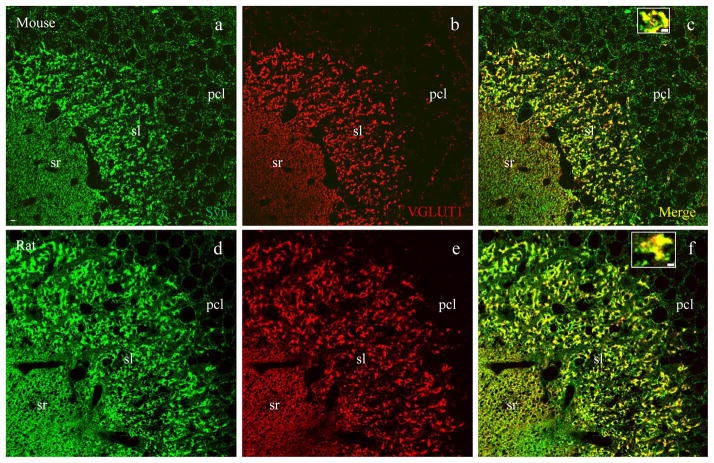
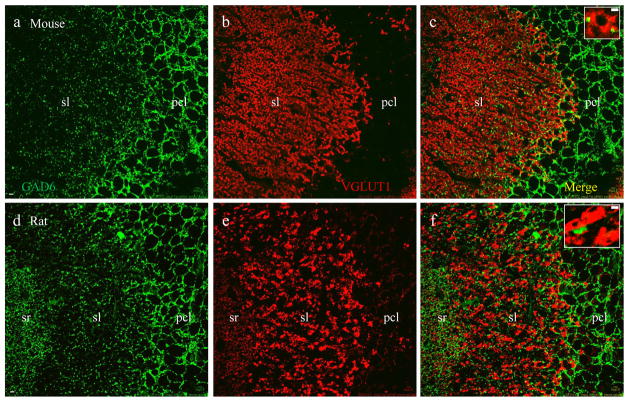
To determine the identity of mossy fiber terminals, we carried out double immunofluorescent staining using synaptophysin (a pan presynaptic marker) and VGLUT1 (a presynaptic marker for excitatory terminals). Axons from dentate granule cells course through the hilus and subregion CA3c, synapsing onto subregion CA3a/b pyramidal neurons (Amaral and Witter, 2000). Therefore, we targeted subregion CA3a/b (Fig. 1a, dotted lines) where mossy fiber terminals are densely packed in the stratum lucidum (sl), forming large excitatory synapses apposing spines on the apical dendrites of CA3 pyramidal neurons (Amaral and Witter, 2000). Synaptophysin labeling (Figs. 2 – 4, a and d, and also insets in c and f, green) revealed two major groups of presynaptic terminals in CA3a/b: large-sized (2 – 5μm in diameter) mossy fiber terminals in sl and small-sized terminals (~0.5μm) in sr, so and pcl. The presynaptic excitatory marker VGLUT1 labeled three groups of synapses in sl, sr (Fig. 2b, red) and so (see also Figs. 5 and 6), with almost no staining in pcl. In sl, large mossy fiber terminals demonstrated intense VGLUT1 labeling (Fig. 2b and e) and perfect colocalization with synaptophysin (Fig. 2c and f including insets, yellow), leaving small terminals positive for synaptophysin only (Fig. 2 insets, green), scattered among co-stained mossy fiber terminals.
Fig. 2.
Vesicular glutamate transporter 1 (VGLUT1) is a presynaptic marker for excitatory mossy fiber terminals. Confocal photomicrographs taken in are CA3a/b subregion as highlighted in Fig. 1a (dashed lines) from a mouse (a–c) and rat (d–f). a and d, both excitatory and inhibitory synapses labeled with synaptophysin (green), a pan-presynaptic marker. b and e, excitatory synapses labeled with VGLUT1 (red). c and f, merged images showing that all VGLUT1-positive synapses are co-labeled with synaptophysin (yellow). Single terminals from sl were highlighted at higher magnification in insets. Note that large-sized (2–5μm in diameter) mossy fiber terminals (yellow) are exclusively present in sl, where small-sized (around 0.5μm) VGLUT1-negative terminals including inhibitory synapses (green) are scattered throughout the region. Scale bar: 5μm in a–f, 2μm in insets.
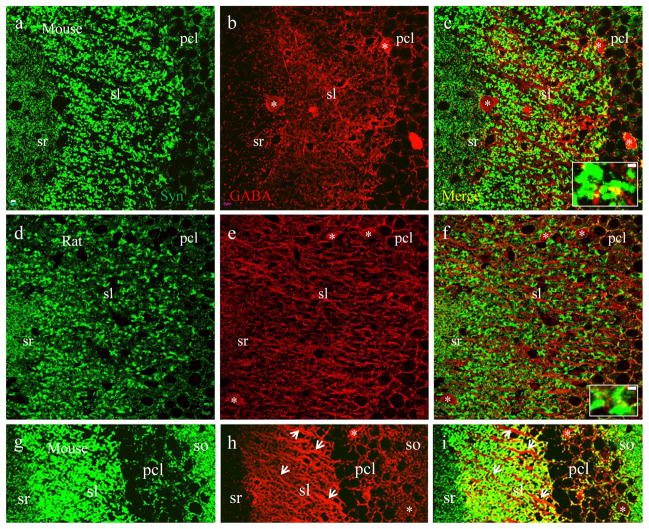
Fig. 4.
Mossy fiber terminals are not GABA-positive. Confocal images taken in subregion CA3a/b from a mouse (a–c) and rat (d–f). a and c, excitatory and inhibitory synapses labeled with synaptophysin (green). b and e, inhibitory synapses labeled with GABA (red). c and f, merged images showing that all GABA-labeled inhibitory synapses are synaptophysin positive (yellow). Single terminals from sl were highlighted at higher magnification in insets. g-i, projection of a stack of confocal microphotographs from mice illustrating the relationship between GABA-positive (red) neuronal processes (arrowheads) and mossy fiber terminals (green) in sl. Astericks (*) indicate somata of GABAergic neurons. Scale bar: 5μm in a–i, 2μm in insets.
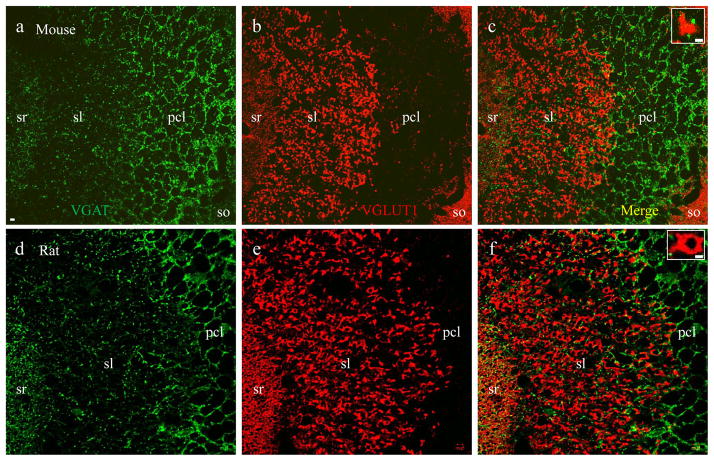
Fig. 5.
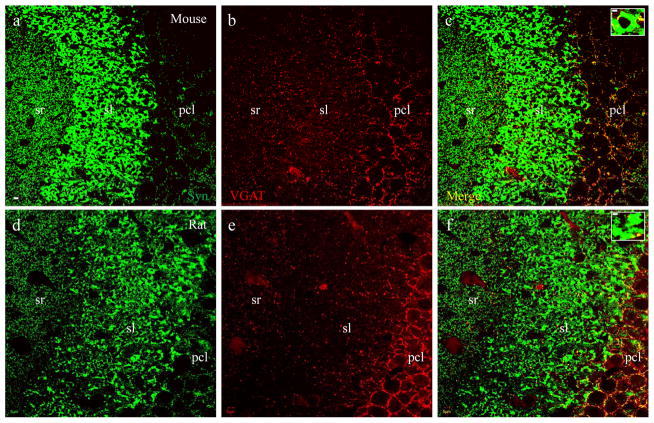
VGLUT1-labeled mossy fiber terminals are independent from VGAT-positive inhibitory synapses. Confocal photomicrographs taken in subregion CA3a/b from a mouse (a–c) and rat (d–f). a and d, small inhibitory synapses labeled with VGAT (green). b and e, large excitatory mossy fiber synapses labeled with VGLUT1 (red). c and f, merged images showing that VGLUT1-positive mossy fiber terminals are discrete and not co-labeled with VGAT. In the sl small-sized VGAT-positive inhibitory synapses (green) are scattered among large-sized VGLUT1-positive mossy fiber terminals (red). Single terminals from sl were highlighted at higher magnification in insets. Scale bar: 5μm in a–f, 2μm in insets.
Fig. 6.
VGLUT1-labeled mossy fiber terminals are independent from GAD-positive inhibitory synapses. Confocal images taken in subregion CA3a/b from a mouse (a–c) and rat (d–f). a and d, inhibitory synapses are labeled with GAD65/67 (green). b and e, excitatory synapses labeled with VGLUT1 (red). c and f, merged images showing that VGLUT1-positive mossy fiber terminals are not co-labeled with GAD immunoreactivity. In the sl, small GAD-positive inhibitory synapses (green) are scattered among large VGLUT1-positive mossy fiber terminals (red). Single terminals from sl were highlighted at higher magnification in insets. Scale bar: 5μm in a–f, 2μm in insets.
The synaptophysin-VGLUT1 (Fig. 2) co-staining pattern is different and disparate from co-staining of synaptophysin with either VGAT, a presynaptic marker for GABAergic synapses (Fig 3) or GABA (a marker for GABAergic cell bodies, neuropiles and presynaptic terminals (Fig 4). VGAT labeled small-sized (~ 0.5μm) terminals which were distributed perisomaticly in pcl. VGAT staining also demonstrated scattered small terminals throughout sl, sr (Fig. 3b and e) and so (refer to Fig. 5a and c). VGAT-positive terminals also demonstrated overlying colocalization with synaptophysin (Fig. 3c and f including insets, yellow), leaving large mossy fiber terminals in sl immunoreactive to synaptophysin only (Fig. 3 insets, green). Similarly, GABA intensively labeled small presynaptic terminals distributed perisomaticly in pcl. GABA also stained out small terminals scattered in sl, sr and so (Fig. 4b and e). GABA-positive terminals were co-labeled with synaptophysin (Fig. 4c and f including insets, yellow), leaving large mossy fiber terminals labeled only by synaptophysin (Fig. 4 insets, green). Since GABA immunoreactivity was also present on some cell bodies (Fig. 4 astericks) and dendritic/axonal segments in sl (Fig. 4h–i, arrowheads), scattered GABA-positive terminals were not as clear as VGAT staining. Furthermore, a potential false colocalization would be displayed if VGLUT1-positive mossy fiber terminals are distributed directly adjacent to GABA-positive dendritic/axonal segments (Fig. 4i).
Fig. 3.
Mossy fiber terminals are not labeled with vesicular GABA transporter (VGAT). Confocal photomicrographs taken in CA3a/b subregion from a mouse (a–c) and rat (d–f). a and d, both excitatory and inhibitory synapses are labeled with synaptophysin (green). b and e, small inhibitory synapses labeled with VGAT (red), a presynaptic marker for GABAergic and glycinergic synapses. c and f, merged images showing all VGAT-labeled synapses are synaptophysin positive (yellow). Single terminals from sl were highlighted at higher magnification in insets. Note that small-sized inhibitory synapses (yellow) are scattered among large-sized mossy fiber terminals (green) in sl. Scale bar: 5μm in a–f, 2μm in insets.
To determine whether a specific portion of VGLUT1-labeled mossy fiber terminals were also positive for GABAergic presynaptic markers, we conducted double staining of VGLUT1 with VGAT in sections from P14 mice (Fig 5a–c) and rats (Fig 5d–e). VGLUT1 (Fig. 5a and d, red) and VGAT (Fig. 5b and e, green) labeled completely discrete groups of terminals. Among large VGLUT1-positive mossy fiber terminals (red) in sl, small-sized VGAT-positive inhibitory terminals (green) were scattered but did not colocalize (Fig. 5c and f including insets). The independent and discrete distribution pattern was confirmed using double labeling of VGLUT1 with an antibody that recognizes both isoforms of glutamic acid decarboxylase (GAD65/67), the key GABA synthesizing enzyme for converting glutamate to GABA in mice (Fig. 6a–c) and rats (Fig. 6d–f), including insets. In similar fashion to GABA staining, GAD also labeled some cell bodies and dendritic/axonal segments.
3. Discussion
Here, we used transgenic mice together with double immunofluorescent staining and confocal laser scanning microscopy to determine whether GABA and glutamate co-localize in developing mossy fiber terminals. First, we demonstrated that GAD67-GFP expressing inhibitory interneurons in the dentate gyrus belong to an independent neuronal population separate from granule cells. Next we identified a group of large-sized mossy fiber terminals in the hippocampus of both P14 mice and rats. These terminals measured 2–5μm in diameter, similar to the size range previously reported in adult rats (Amaral and Witter, 2000). These presynaptic terminals are densely packed in the stratum lucidum of subregion CA3a/b, forming excitatory synapses with spines along proximal apical dendrites of CA3 pyramidal neurons. Serial comparisons demonstrated that they are solely positive to VGLUT1 and not labeled by GABAergic markers including GABA, GAD and VGAT. The present study supports the classical notion that both granule cells and their mossy fiber terminals are glutamatergic (Amaral and Witter, 2000), but not GABAergic.
Previous physiological data including minimal stimulation studies suggest co-release of GABA and glutamate from mossy fiber terminals (Walker et al., 2001; Safiulina et al, 2009). However, in such experiments co-stimulation of interneurons cannot be ruled out. As the authors of those and similar studies concede, the evidence for co-release is indirect (Safiulina et al. 2009; Walker et al. 2001; Walker et al. 2002; Gutierrez 2005; Gutierrez 2009; Zander et al. 2010), and exists alongside a compelling report to the contrary (Uchigashima et al. 2007). Conclusive proof of co-release would require simultaneous paired cell recordings between a dentate granule cell and a CA3 pyramidal neuron (Walker et al. 2001; Walker et al. 2002; Gutierrez 2005; Gutierrez 2009). Interestingly, optogenetic methods might be employed to stimulate a single granule cell without activating nearby interneurons allowing for examination of monosynaptic GABAergic responses in CA3 pyramidal neurons.
Some inhibitory interneurons reside within or very near the granule cell layer (Freund and Buzsaki, 1996), although they are rare as compared to the numerous densely packed granule cells. This implies that separate and non overlapping neuronal markers specific for granule cells or GABAergic interneurons are necessary to identify cells in this area. As previously reported, GAD67 immunoperoxidase staining demonstrated a large number of GAD67-positive neurons in the granule cell layer of developing rats (Gutierrez et al., 2003) and adult rats after seizures (Ramirez and Gutierrez, 2001) compared to normal adult rats. However without granule cell identification in the same sections, it is unclear if those GAD67-stained neurons are GABAergic interneurons originally present within granule cell layer or whether they are granule cells that may also be expressing GABA. Accordingly, immunofluorescent staining using glutamate in conjunction with GAD67 or GABA suggesting co-localization in “granule cells” (Guetirrez, 2009) are also suspect since glutamate is naturally present in both glutamatergic and GABAergic neurons.
Interestingly, GABA or GAD67 immunohistochemistry does not only label inhibitory presynaptic terminals, it may also reveal GABAergic cell bodies and dendritic/axonal segments (Fig. 4). Therefore to address if putative GABA- or GAD67-stained synaptic structures could be truncated dendrites or axons, further co-staining with other presynaptic markers will be required. Uchigashima et al (2007) reported co-localization of VGLUT1 with GAD65/67 or GABA but not with VGAT in calbindin-labeled mossy fiber terminals and non-terminal portions of mossy fibers. However, calbindin is primarily used as an inhibitory neuronal marker and thus would also label GABAergic dendritic/axonal segments.
A previous study using post-embedding immunogold electron microscopy reported three kinds of “co-localization” in mossy fiber terminals from normal adult rats: VGAT with VGLUT1, VGAT with VGLUT2 and VGLUT1 with VGLUT2 (Zander et al., 2010). VGLUT2 has indeed been previously documented in the hippocampus, but only in the dentate granule cell layer and CA2 pyramidal cell layer (Kaneko et al., 2002; Boulland et al., 2009), and not in the mossy fibers. Regarding VGLUT1 and VGLUT2, we did not find the overlapping distributions reported by Zander et al2010, but found instead a non-overlapping distribution similar to that reported by Kaneko et al. (2002), with VGLUT1 predominantly along dendritic branches and VGLUT2 surrounding principal cell bodies. Last, while we also detected VGLUT2/VGAT co-localization with the present protocol, this co-localization was perisomatic, not dendritic, and present exclusively in DG and CA2, and not in mossy fiber terminals as Boulland et al (2009) reported.
To date, the GABA transporter protein VGAT is the ideal marker for GABAergic terminals because it labels only GABAergic synaptic vesicles in presynaptic terminals. Furthermore, to classify a GABAergic terminal, VGAT must be identified in its presynaptic vesicles. VGAT mRNA has been detected in granule cell bodies from developing and adult rats (Gutiérrez et al., 2003; Gómez-Lira et al., 2005; Zander et al., 2010) but this result remains controversial (Uchigashima et al., 2007). Nevertheless, mRNA expression in granule cell bodies does not guarantee VGAT protein translation in mossy fiber terminals. A lack of correlation between mRNA expression and protein translation is common due to mRNA decay (Isken and Maquat, 2007) as well as other mechanisms (Greenbaum et al., 2003). The present study showed that VGAT-positive GABAergic terminals were scattered among VGLUT1-positive mossy fiber terminals throughout sl in subregion CA3a/b, with no overlap or co-localization between the two populations of presynaptic terminals. It could be argued that lack of immunostaining cannot be taken as a firm evidence for the absence of an antigen and that the present staining protocol may not be sensitive enough to detect GAD, GABA or VGAT in mossy fiber terminals. This is unlikely because we showed intense staining of GAD/GABA/VGAT-positive terminals perisomaticly surounding principal neurons in CA3a/b (Fig. 3–6), CA1 and dentate gyrus. The adjacently distributed VGAT- or GAD-stained inhibitory terminals in sl of the same sections can be taken as an internal positive control for VGLUT1-stained mossy fiber terminals, which were negative for VGAT and GAD labeling. Most importantly, we illustrated a considerable size difference between large-sized (2–5μm) mossy fiber terminals and small-sized (approximately 0.5μm) GABAergic terminals, demonstrating that they belong to completely different neuronal populations. Therefore, our data strongly suggests that during postnatal development (P14) of both mice and rats, mossy fiber terminals do not contain GABA.
In the present study, we showed a large bundle of neuronal processes with GABA labeling in sl of CA3ab (Fig. 4h–i). These GABA-positive processes are not mossy fibers since they could not be tracked back to CA3c subregion or the hilus and could not be labeled with VGLUT1. They may be dendritic arbors and/or axonal branches of local inhibitory neurons, including mossy fiber associated interneurons that issue dense neuropiles to the area of mossy fiber termination (Vida and Frotscher, 2000; Losonczy et al., 2004). These mossy fiber associated interneurons may be responsible for monosynaptic IPSPs recorded in CA3 pyramidal neurons induced from stimulation in the granule cell layer (Uchigashima et al., 2007). However, it is unknown if the interneuron source of co-stimulation includes any hilar interneurons as shown in both mouse and rat pups (present study) or adult mice (Tamamaki et al., 2003), since both axo-axonic cells (Han 1993) and dendritic inhibitory cells (Sik et al., 1997) reside in the hilus and may establish axon collaterals to area CA3 and thus impact pyramidal cell activities.
4. Experimental Procedure
4.1. Animals
A total of 18 P14 rodents [3 rats, 6 GAD-GFP heterozygous mice (Tamamaki et al., 2003) and 9 wild type mice] were used. Until sacrifice, mouse and rat pups were kept with their mothers who had access to food and water ad libitum and housed under a 12hr light/12hr dark cycle. The procedures and protocols for all animal studies were approved by the Children’s Hospital of Philadelphia and UPENN Institutional Animal Care and Use Committees in accordance with international guidelines on the ethical use of animals (National Research Council, 1996).
4.2. Tissue preparation
P14 mice and rats were deeply anesthetized with 5% chloral hydrate. Intra-cardiac perfusion was made with saline and 4% paraformaldehyde in 0.1M phosphate buffer (pH 7.4). Brains were removed and post-fixed in the same fixative for 1hr at room temperature (RT). For GABA immunohistochemical staining, 0.125% glutaraldehyde was added to the fixative. Frontal sections through the hippocampus were cut at 50μm thickness with a Leica VT 1000s vibratome. To minimize the number of animals sacrificed, the sections were collected in series for different immunolabeling settings with an interval of 300μm between two adjacent sections within an identical series.
4.3. Immunohistochemistry
To identify specific proteins in the hippocampus, double immunofluorescent staining was performed on each brain section. Two primary antibodies raised from different hosts were chosen for double staining (rabbit anti-EAAC1, 1:1000, α-Diagnostic Inc, San Antonio, TX 78244 USA; rabbit anti-GABA, 1:2000, Sigma-Aldrich, St. Louis, MO 63178; mouse anti-GAD-65/67, 1:50, Developmental Study of Hybridoma Bank, Iowa City, IA 52242; mouse anti-GFP, 1:500, Sigma-Aldrich; rabbit anti-GFP, 1:1000, Abcam, Cambridge, MA 02139; mouse anti-synaptophysin, 1:1000, Sigma-Aldrich; mouse anti-VGAT, 1:250, Synaptic Systems, 37079 Goettingen, Germany; rabbit anti-VGAT, 1:250, Synaptic Systems; rabbit anti-VGLUT1, 1:2000, Synaptic Systems). Visualization was carried out with Alexa Fluor®-488 (AF488, green) or -594 (AF594, red) conjugated secondary antibodies (Molecular Probes, 1:200). In some cases, primary antibody-incubated sections were treated with biotinylated secondary antibodies (Vector Lab, 1:250) and then visualized with streptavidin-Cy3 (Sigma, 1:250, red). The incubation time for primary antibodies was 90 minutes at room temperature (RT) and then overnight at 4°C. For secondary antibodies and streptavidin-Cy3, the incubation time was 90 minutes at RT. All the immunolabeled sections were counterstained with Hoechst 33342, a DNA dye for nuclear staining (blue). Finally, the sections were mounted on glass slides and coverslipped with aqueous mounting medium. Each immunolabeling condition was repeated at least twice with different animals to confirm the labeling pattern.
We co-stained VGLUT1, VGAT, GABA and GAD65/67 with synaptophysin to confirm their presynaptic location (although GABA and GAD67 may show other neuronal components as well, as discussed later). Synaptophysin, a synaptic vesicular protein is present in all types of presynaptic terminals (Wiedenmann and Franke, 1985) and has been widely used as a pan presynaptic label (or positive control) for glutamatergic, GABAergic as well as other types of synapses. Synaptophysin with VGLUT1 was used to identify glutamatergic (excitatory) terminals, while synaptophysin with VGAT or GABA were used to identify GABAergic (inhibitory) axonal terminals (or synaptic buttons).
4.4. Confocal imaging
Confocal images were acquired with Olympus Fluoview 1000 System. The Z-step size was set at 0.5μm. A sequential imaging module was used to prevent signal leakage from the green channel to the red and vice versa. For synaptic staining, a 63x immersion lens was used to acquire punctate images. A single confocal image was used to illustrate each set of co-staining throughout the present study, except for Fig. 1a, d–e and 4g–i where a stack of confocal images were projected.
4.5. Dot blot
To verify whether the key synaptic markers used in the present study are specific, amino acid dot blots and protein Western blots were performed. Amino acids were conjugated to BSA via glutaraldehyde, according to Storm-Matisen et al (1983). The conjugates were diluted at 1:100 with PBS. Two microliters of each diluted conjugate was blotted directly onto the nitrocellulose membrane. The membrane was naturally dried for 1hr and the blots were subsequently blocked with 5% BSA for 1hr. It was then incubated with the polyclonal GABA antibody (1:10000) and IR-680 conjugated goat anti-rabbit IgG (1:10000) for 1hr, respectively. Images were acquired using Odyssey Infrared Imaging System.
4.6. Western Blot
The whole brain from each P14 mouse was collected into a glass homogenizer. It was subsequently homogenized in 1.5ml of lysis buffer containing 50 mm Tris, pH 7.4, 1.0 mm EDTA, 150 mm NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, and 1% protease inhibitor cocktail (Sigma P2714-1BTL). The brain lysate was sonicated for 10 s and chilled on ice for 20 min before centrifugation at 12,000 × g for 20 min at 4°C. The supernatants were collected, the concentration was determined, and equal amounts of protein were run in SDS-PAGE in 6× sample buffer (300 mm Tris-HCl, pH 6.8, 12% SDS, 0.6% bromophenol blue, 30% glycerol). After transfer to nitrocellulose membrane and blocking in 5% nonfat dried milk for 1 h at room temperature, the membranes were incubated with the primary antibodies overnight at 4°C: mouse anti-synaptophysin (1:1000 in nonfat milk); mouse anti-GAD65/67 (1:50), rabbit anti-VGLUT1 (1:2000) and rabbit anti-VGAT (1:500). After washing, the blots were incubated with IR680-conjugated goat anti-mouse or -rabbit IgG (1:10000 in skim milk) for 60min at RT. Images were acquired using Odyssey Infrared Imaging System.
5. Conclusion
In summary, we never found overlapping populations and demonstrate discrete and independent populations of inhibitory and excitatory terminals. Thus, our data indicate that mossy fiber terminals are glutamatergic throughout rodent development and do not co-release GABA.
Acknowledgments
The authors thank Dr. Michael Schell for his invaluable suggestions on their manuscript. This work was supported by grants from the National Institutes of Health (HD059288 and NS069629 to A.S.C.).
References
- Amaral DG, Witter MP. Hippocampal formation. In: Paxinos G, editor. The rat nervous system. 2. Academic Press; New York: 2000. pp. 443–493. [Google Scholar]
- Bergersen L, Ruiz A, Bjaalie JG, Kullmann DM, Gundersen V. GABA and GABAA receptors at hippocampal mossy fibre synapses. Eur J Neurosci. 2003;18:931–941. doi: 10.1046/j.1460-9568.2003.02828.x. [DOI] [PubMed] [Google Scholar]
- Boulland JL, Jenstad M, Boekel AJ, Wouterlood FG, Edwards RH, Storm-Mathisen J, Chaudhry FA. Vesicular glutamate and GABA transporters sort to distinct sets of vesicles in a population of presynaptic terminals. Cereb Cortex. 2009;19:241–248. doi: 10.1093/cercor/bhn077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaneda MT, Emilio R, Sanabria G, Hernandez S, Ayala A, Reyna TA, Wu JY, Colom LV. Glutamic acid decarboxylase isoforms are differentially distributed in the septal region of the rat. Neurosci Res. 2005;52:107–119. doi: 10.1016/j.neures.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Esclapez M, Tillakaratne NJ, Kaufman DL, Tobin AJ, Houser CR. Comparative localization of two forms of glutamate acid decarboxylase and their mRNAs in rat brain supports the conception of functional differences between the forms. J Neurosci. 1994;14:1835–1855. doi: 10.1523/JNEUROSCI.14-03-01834.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Gómez-Lira G, Lamas M, Romo-Parra H, Gutiérrez R. Programmed and induced phenotype of the hippocampal granule cells. J Neurosci. 2005;25:6939–6946. doi: 10.1523/JNEUROSCI.1674-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum D, Colangelo C, Williams K, Gerstein M. Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biology. 2003;4:117. doi: 10.1186/gb-2003-4-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez R. The GABAergic phenotype of the “glutamatergic” granule cells of the dentate gyrus. Prog Neurobiol. 2003;71:337–358. doi: 10.1016/j.pneurobio.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Gutiérrez R. The dual glutamatergic–GABAergic phenotype of hippocampal granule cells. Trends Neurosci. 2005;28:297–303. doi: 10.1016/j.tins.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Gutiérrez R. The Dual Glutamatergic/GABAergic Phenotype of Hippocampal Granule Cells. In: Gutiérrez R, editor. Co-Existence and Co-Release of Classical Neurotransmitters. Springer Science + Business Media LLC; New York: 2009. pp. 118–201. [Google Scholar]
- Gutiérrez R, Heinemann U. Co-existence of GABA and Glu in the hippocampal granule cells: implications for epilepsy. Curr Top Med Chem. 2006;6:975–978. doi: 10.2174/156802606777323692. [DOI] [PubMed] [Google Scholar]
- Gutiérrez R, Romo-Parra H, Maqueda J, Vivar C, Ramirez M, Morales MA, Lamas M. Plasticity of the GABAergic phenotype of the “glutamatergic” granule cells of the rat dentate gyrus. J Neurosci. 2003;23:5594–5598. doi: 10.1523/JNEUROSCI.23-13-05594.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han ZS, Buhl EH, Lörinczi Z, Somogyi P. A high degree of spatial selectivity in the axonal and dendritic domains of physiologically identified local-circuit neurons in the dentate gyrus of the rat hippocampus. Eur J Neurosci. 1993;5:395–410. doi: 10.1111/j.1460-9568.1993.tb00507.x. [DOI] [PubMed] [Google Scholar]
- He Y, Janssen WG, Rothstein JD, Morrison JH. Differential synaptic localization of the glutamate transporter EAAC1 and glutamate receptor subunit GluR2 in the rat hippocampus. J Comp Neurol. 2000;418:255–269. [PubMed] [Google Scholar]
- Isken O, Maquat LE. Quality control of eukaryotic mRNA:safeguarding cells from abnormal mRNA function. Genes Dev. 2007;21:1833–3856. doi: 10.1101/gad.1566807. [DOI] [PubMed] [Google Scholar]
- Jaffe DB, Gutiérrez R. Mossy fiber synaptic transmission: communication from the dentate gyrus to area CA3. Prog Brain Res. 2007;163:109–32. doi: 10.1016/S0079-6123(07)63006-4. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Fujiyama F, Hioki H. Immunohistochemical localization of candidates for vesicular glutamate transporters in the rat brain. J Comp Neurol. 2002;444:39–62. doi: 10.1002/cne.10129. [DOI] [PubMed] [Google Scholar]
- Kaufman DL, Houser CR, Tobin AJ. Two forms of the gamma-aminobutyric acid synthetic enzyme glutamate decarboxylase have distinct intraneuronal distributions and cofactor interactions. J Neurochem. 1991;56:720–723. doi: 10.1111/j.1471-4159.1991.tb08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann H, Ebert U, Löscher W. Immunocytochemical localization of GABA immunoreactivity in dentate granule cells of normal and kindled rats. Neurosci Lett. 1996;212:41–44. doi: 10.1016/0304-3940(96)12777-4. [DOI] [PubMed] [Google Scholar]
- Losonczy A, Biro AA, Nusser Z. Persistently active cannabinoid receptors mute a subpopulation of hippocampal interneurons. Proc Natl Acad Sci USA. 2004;101:1362–1367. doi: 10.1073/pnas.0304752101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maqueda J, Ramirez M, Lamas M, Gutiérrez R. Glutamic acid decarboxylase (GAD)67, but not GAD65, is constitutively expressed during development and transiently overexpressed by activity in the granule cells of the rat. Neurosci Lett. 2003;353:69–71. doi: 10.1016/j.neulet.2003.08.077. [DOI] [PubMed] [Google Scholar]
- Ramírez M, Gutiérrez R. Activity-dependent expression of GAD67 in the granule cells of the rat hippocampus. Brain Res. 2001;917:139–146. doi: 10.1016/s0006-8993(01)02794-9. [DOI] [PubMed] [Google Scholar]
- Safiulina VF, Fattorini G, Conti F, Cherubini E. GABAergic signaling at mossy fiber synapses in neonatal rat hippocampus. J Neurosci. 2006;26:597–608. doi: 10.1523/JNEUROSCI.4493-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler R, Smith AD. Coexistence of GABA and glutamate in mossy fiber terminals of the primate hippocampus: an ultrastructural study. J Comp Neurol. 1991;303:177–192. doi: 10.1002/cne.903030202. [DOI] [PubMed] [Google Scholar]
- Shashidharan P, Huntley GW, Murray JM, Buku A, Moran T, Walsh MJ, Morrison JH, Plaitakis A. Immunohistochemical localization of the neuron-specific glutamate transporter EAAC1 (EAAT3) in rat brain and spinal cord revealed by a novel monoclonal antibody. Brain Res. 1997;773:139–148. doi: 10.1016/s0006-8993(97)00921-9. [DOI] [PubMed] [Google Scholar]
- Sik A, Penttonen M, Buzsáki G. Interneurons in the hippocampal dentate gyrus: an in vivo intracellular study. Eur J Neurosci. 1997;9:573–588. doi: 10.1111/j.1460-9568.1997.tb01634.x. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Dichter MA, Rachinsky TL, Dean E, Goodman JH, Sollas AL, Martin DL. Basal expression and induction of glutamate decarboxylase and GABA in excitatory granule cells of the rat and monkey hippocampal dentate gyrus. J Comp Neurol. 1996;373:593–618. doi: 10.1002/(SICI)1096-9861(19960930)373:4<593::AID-CNE8>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Storm-Mathisen J, Leknes AK, Bore AT, Vaaland JL, Edminson P, Haug FM, Ottersen OP. First visualization of glutamate and GABA in neurones by immunocytochemistry. Nature. 1983;301:517–20. doi: 10.1038/301517a0. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol. 2003;467:60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- Uchigashima M, Fukaya M, Watanabe M, Kamiya H. Evidence against GABA release from glutamatergic mossy fiber terminals in the developing hippocampus. J Neurosci. 2007;27:8088–8100. doi: 10.1523/JNEUROSCI.0702-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vida I, Frotscher M. A hippocampal interneuron associated with the mossy fiber system. Proc Natl Acad Sci USA. 2000;97:1275–1280. doi: 10.1073/pnas.97.3.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MC, Ruiz A, Kullmann DM. Monosynaptic GABAergic Signaling from Dentate to CA3 with a Pharmacological and Physiological Profile Typical of Mossy Fiber Synapses. Neuron. 2001;29:703–715. doi: 10.1016/s0896-6273(01)00245-8. [DOI] [PubMed] [Google Scholar]
- Walker MC, Ruiz A, Kullmann DM. Do mossy fibers release GABA? Epilepsia. 2002;43:196–202. doi: 10.1046/j.1528-1157.43.s.5.6.x. [DOI] [PubMed] [Google Scholar]
- Wiedenmann B, Franke WW. Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell. 1985;41:1017–1028. doi: 10.1016/s0092-8674(85)80082-9. [DOI] [PubMed] [Google Scholar]
- Zander JF, Munster-Wandowski A, Brunk I, Pahner I, Gomez-Lira G, Heinemann U, Gutiérrez R, Laube G, Ahnert-Hilger G. Synaptic and Vesicular Coexistence of VGLUT and VGAT in Selected Excitatory and Inhibitory Synapses. J Neurosci. 2010;30:7634–7645. doi: 10.1523/JNEUROSCI.0141-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]