Summary
Therapeutic targeting of the epidermal growth factor receptor (EGFR), which is highly overexpressed and correlated with poor prognosis in colorectal and head and neck squamous cell carcinoma (SCCHN), has shown clinical efficacy using the blocking mAbs, cetuximab or panitumumab, but only in 10% to 20% of patients. Clinical responsiveness is correlated with certain Fcγ receptor genotypes, suggesting immune activity may contribute to therapeutic efficacy. In addition, cetuximab-resistant tumor cells exhibit ubiquitination and degradation of EGFR, which would increase its processing as a tumor antigen for cytotoxic T lymphocyte (CTL) lysis. Thus, T cell-based immunotherapy might enhance the antitumor efficacy of EGFR-specific mAbs, but CTL epitopes are poorly defined. To permit combinatorial EGFR-targeted immunotherapy, we identified a novel immunogenic wild-type sequence peptide, EGFR853 – 861 and modified its anchor sequence to enhance HLA-A*0201 binding and stimulation of cross-reactive anti-wild–type EGFR853 – 861-specific CTL. Cross-reactivity was also observed with HER2861 – 869. EGFR853 – 861-specific CTL recognition of SCCHN cells was increased by incubation of tumor cells with cetuximab, which led to EGFR degradation. In addition, EGFR853 – 861-specific CTLs were elevated in the circulation of SCCHN patients as compared with healthy control peripheral blood mononuclear cells. Thus, a novel, immunogenic EGFR-encoded CTL epitope may be incorporated into vaccines and would be useful for combinatorial immunotherapy with EGFR-specific mAbs in cancer patients.
Keywords: EGFR, CTL, head and neck cancer
Elevated epidermal growth factor receptor (EGFR) expression in colorectal,1 and head and neck squamous cell carcinomas (SCCHNs, Ref. (2) is a powerful indicator of poor prognosis,3 suggesting that this tumor antigen may be a promising immunotherapeutic target. Indeed, clinically efficacious targeting of EGFR using the blocking mAbs cetuximab,4,5 or panitumumab,6,7 has been achieved. However, clinical responses are seen in only 10% to 20% of patients, do not correlate with level of EGFR expression,8,9 but correlate with Fcγ receptor genotypes. Several studies have demonstrated the ability of cetuximab to mediate antibody-mediated cellular cytotoxicity against SCCHN,10 esophageal,11 breast,12 and nonsmall cell lung carcinoma cells.13 In colorectal carcinoma, clinical response to single agent cetuximab therapy correlated with Fcγ receptor polymorphisms expressed by patients’ lymphocytes, supporting an immune mechanism in clinical activity.14 In addition, some cetuximab-treated tumors acquire resistance, due to internalization and degradation of EGFR.15,16 This form of tumor cell escape from cetuximab treatment could increase EGFR-derived peptide presentation to EGFR-specific cytotoxic T lymphocytes (CTLs), as proteasomal degradation is the first step in the HLA class 1 antigen processing pathway for transmembrane proteins.17,18 Thus, novel combination approaches may increase the number of SCCHN patient responses and avoid treatment escape by tumor cells, through combination EGFR-specific mAb and T cell-based immunotherapeutic strategies.
In breast carcinoma, the anti-HER2–specific mAb, trastuzumab (Herceptin), increases tumor cell lysis in combination with HER2-specific CTL.19,20 Although there is significant homology between EGFR (HER1) and HER2, an analogous effect has not been shown with SCCHN. In part this may be due to a lack of identification of EGFR-specific CTL epitope(s) and the resulting paucity of T-cell immunotherapeutic trials targeting EGFR. However, due to recent widespread use of the clinically efficacious EGFR-specific mAbs,1,8,21 increasing cetuximab resistance is being observed. HER2 upregulation is also observed in aero-digestive tract malignancies as an escape mechanism from anti-EGFR therapies.16 This presents a compelling need for more efficacious combinatorial immunotherapeutic approaches, to expand the number of patients experiencing clinical responses and to obviate tumor escape from mAb therapy.
MATERIALS AND METHODS
Cell Lines
The HLA-A*0201+SCCHN cell lines SCC-4, PCI-13, and PCI-30,22,23 overexpress EGFR and have been published elsewhere.23 All tumor cell lines were cultured in RPMI 1640 medium [Invitrogen Life Technologies HBSS (Hank’s balanced salt solution)] supplemented with 10% fetal calf serum, L-arginine (116 mg/L), L-asparagine (36 mg/L), and L-glutamine (216 mg/L). The T2 mutant cells that lack genes encoding the transporter associated with antigen processing (TAP)1/2,24 were grown in AIM-V serum-free media and cleaned using a Ficoll-Paque PLUS (GE Healthcare Bio-Science AB; SE-751 84 Uppsala, Sweden) gradient every 30 days.
Cytokines
GM-CSF, IFN-γ, IL-1β, IL-4, IL-6, PGE2, TNF-α, were purchased from R&D Systems (Minneapolis, MN).
Antibodies
The following antibodies were used: HLA-A, HLA-B, HLA-C–specific mAb W6/32,25 HLA-A2 and HLA-A68–specific mAb BB7.2,26 HLA-A–specific mAb LGIII.147.4,27 and HLA-DR–specific mAb L243.28 The humanized mouse anti-human EGFR chimeric IgG1 mAb, cetuximab (Erbitux, ImClone Systems Incorporated, New York, NY and Bristol-Myers Squibb Company, Princeton, NJ), and rituximab, a humanized mouse anti-human chimeric CD20-specific IgG1 isotype mAb control mAb (Roche, Basel, Switzerland) were purchased. An irrelevant specificity control human IgG2 mAb was purchased from BD Biosciences (Rochester, NY).
Western Blot Analysis
Western blot was performed as described previously.29 A 1:200 dilution of the EGFR antibody (rabbit polyclonal IgG mAb sc-03; Santa Cruz Biotechnology, Inc) and a 1:10,000 dilution of anti-rabbit antibody IgG-HRP: W401B (Promega) were used for protein detection. Beta-actin expression was determined with a 1:4000 dilution of an anti-β–actin antibody (Sigma-Aldrich) and used as a loading control.
Peptides
Peptides derived from EGFR (EGFR853 – 861, ITDF-GLAKL), HIV-1 polymerase (HIV-POL476 – 484, ILKEPV-HGV), HER2 (HER2861 – 869, ITDFGLARL), or influenza A (Flu58 – 66, GILGFVFTL) were synthesized using standard F-moc chemistry by the University of Pittsburgh Peptide Synthesis Facility, and were >90% pure by analytical high-pressure liquid chromatography and by mass spectrometry. Lyophilized peptides were stored in 100% dimethylsulfoxide at a concentration of 2 mg/mL at −20°C until use.
HLA-A*0201 Stabilization Assay
The HLA-A*0201 stabilization assay was performed by incubating T2-cell line with varying peptides and concentrations, as described elsewhere.30 In brief, 5 × 105 T2 cells were incubated in the presence of 10-fold dilutions of peptide in AIM-V medium for 18 hours at 37°C. Surface HLA-A*0201 molecule expression were detected using the anti-HLA-A, anti-HLA-B, anti-HLA-C (W6/32), or anti-HLA-A2 mAb (BB7.2). After incubation with a fluorescein isothiocyanate-conjugated 2° mAb, T2 cells were analyzed by flow cytometry using a FACScan (BD Biosciences, San Diego, CA) as described previously.30
In Vitro Stimulation of EGFR-specific CD8+ T Cells
Peripheral blood mononuclear cells (PBMC) from 5 healthy donors (HD) or 1 SCCHN patient were isolated by density centrifugation on Ficoll-Paque PLUS (GE Health-care Bio-Science) and used to prepare mature DC as described previously,31 with minor modifications. PBMC were resuspended at 5 × 107/mL in AIM-V medium (Invitrogen) and were incubated for 90 minutes in 75 cm2 tissue culture flasks or 6-well plates (37°C in 5% CO2). Nonadherent (T cell-enriched) cells were gently washed off with Hank’s balanced salt solution and subsequently frozen. The plastic adherent cells were cultured in AIM-V medium supplemented with 1000 U/mL rhGM-CSF and 1000 U/mL rhIL-4. Six days later, the culture medium was removed, and the immature DC were cultured in AIM-V supplemented with 1000 U/mL rhGM-CSF and 1000 U/mL rhIL-4, 10 ng/mL rhIL-6, 10 ng/mL recombinant human tumor necrosis factor (TNFα), and 10 ng/mL IL-1β and 1 μg/mL PGE-2. Mature DC were harvested on day 8, centrifuged, frozen, or used to stimulate autologous T cells. The stimulator cells were resuspended in AIM-V at 106/mL supplemented with each peptide (10 μg/mL) and incubated for 4 hours at 37°C. The peptide-pulsed DC were then irradiated (50 Gy) and washed and resuspended in culture medium (Iscove’s medium supplemented with 10% human serum, L-arginine, L-asparagine, and L-glutamine). Auto-logous CD8+ T cells were negatively isolated from PBMC with immunomagnetic beads (Miltenyi Biotech, Germany) and added at 1 × 106 cells/mL to 1 × 105 peptide-pulsed DC in a final volume of 2 mL of culture medium (24-well tissue culture plate). The cells were cultured for 48 hours at 37°C with IL-2 (20 U/mL) and IL-7 (5 ng/mL). On day 7 and weekly thereafter, lymphocytes were restimulated with autologous irradiated DC pulsed with peptide in culture medium supplemented with 20 U/mL IL-2 and 5 ng/mL IL-7. The stimulated CD8+ T cells were analyzed for specificity in IFN-γ enzyme-linked immunosorbent spot (ELISPOT) and 51Cr release assays at day 21 and then every 7 days thereafter. CTLs were found to be antigen specific after 2 to 3 rounds of in vitro stimulation (IVS), and data presented are representative of IVS from 2 individual HD and 1 SCCHN patient.
CTL Recognition Assays
The recognition of APCs pulsed with peptides and tumor cells were assessed by ELISPOT assays specific for hu-IFN-γ. For the ELISPOT assays, multiscreen HTS plates (Millipore, Bedford, MA) were coated with 10 μg/mL of either mAb antihuman IFN (1-D1K; Mabtech, Stockholm, Sweden) in phosphate-buffered saline (PBS) (Life Technologies, Inc) overnight at 4°C. Unbound mAb was removed by 4 successive washings with PBS. After the plates were blocked with Iscove’s modified Dulbecco’s medium with 10% human serum (1 h at 37°C), CD8+ T cells were seeded in triplicate (5 × 104 for bulk CD8+ T cells and 103 for CD8+ T cell clones) in multiscreen HTS plates. Nonirradiated T2 cells (5 × 104) or head and neck tumor cell lines (5 × 104) were added. Synthetic peptides were then added at indicated concentrations into ELISPOT assays after APCs were seeded. Control wells contained unstimulated T cells, T cells in the presence of unloaded T2 cells, L or tumor cells alone. Culture medium was AIM-V at a final volume of 200 μL/well. After incubation at 37°C in 5% CO2 for either 40 hours (IFN-γ ELISPOT assays), cells were removed by washings with PBS-0.05% Tween 20 (PBS-T). Captured cytokine was detected at sites of its secretion by incubation for 4 hours with either biotinylated mAb anti-hIFN-γ (7-B6-1; Mabtech) at 2 μg/mL in PBS-0.5% bovine serum albumin. Plates were washed 6 times with PBS-T, phosphate buffered saline and avidin-peroxidase complex, diluted 1:100 (Vectastain Elite Kit: Vector, Burlingame, CA), was added for 1 hour at room temperature. Unbound complex was removed by 3 successive washings with PBS-T and 3 with PBS alone. Peroxidase staining was performed with 3,3,5′-tetramethylbenzidine (Vector Laboratories) for 4 minutes and stopped by rinsing the plates under running tap water. Spots were enumerated using computer-assisted video image analysis software.
Flow Cytometry Analysis Using HLA-A2-Peptide Tetramers
The PE-labeled HLA-A*0201-EGFR853 – 861 and HLA-A*0201 EGFR854L tetramers were obtained from the Tetramer Facility of the National Institute of Allergy and Infectious Disease (Atlanta, GA). Specificity was confirmed by staining of the CTL line specific for each peptide after IVS and the lack of staining of irrelevant (MAGE-3271–279 specific) CTL,30 or HLA-A2− PBMC obtained from normal donors. Three-color flow cytometry assays (FACScan, BD Biosciences) were performed with fluorescent labeled anti-CD3 and fluorescein isothiocya-nate-anti-CD8 Abs (Beckman Coulter) and PE-tetramer. Flow cytometry was performed on a CyAn flow cytometer (Dako, Ft. Collins, CO) machine, and data analyzed using Summit V4.3 software. Generally, 100,000 events per sample were collected after gating on lymphocytes by forward-scatter and side-scatter. CTL clones were sorted using PE-labeled HLA-A2 EGFR853 – 861 peptide-loaded tetramers. Clones were plated in 96-well U-bottom plates and 50 μL/well of cytokine stimulation mixture was added. IVS was performed using 106 allogeneic PBLs/mL with the concentration of 5 × 106 per plate and irradiated with 50 Gy, IL-2 at 200 IU/mL and PHA-L at 1 μg/mL. The clones were restimulated every 7 to 10 days with 50 μL of cytokine stimulation mixture.
Data Analysis
T-cell reactivity as measured by the ELISPOT assay was considered positive if test wells were significantly greater than background wells when using a 2-tailed permutation test at P≤0.05. Tetramer frequencies were transformed by computing the log (base 10) of the reciprocal frequency (number of T cells per single tetramer-positive cell. A 1-tailed 1 sample t test was used to assess the within-subject increase in tetramer-positive cells resulting from IVS. Changes in tetramer frequencies with IVS of SCCHN patients were then compared with normal controls with the 2-sided t test.
RESULTS
Identification and Immunogenicity of EGFR853 – 861
The human EGFR sequence was scanned for sequences homologous with known HER-2 peptides which bind HLA-A*0201 and have demonstrated immunogenicity. This analysis led to the identification of EGFR853 – 861 (ITDF-GLAKL), which varies from the HER2861 – 869 sequence at position 868. In conjunction, using a publicly available algorithm for prediction of HLA peptide-binding motifs (www.syfpeithi.de), we screened the EGFR protein sequence for suitable HLA-A*0201-binding peptides, and identified EGFR853 – 861. The algorithm score of 25 by the EG FR853 – 861 peptide suggested the potential for HLA-A*0201 binding, according to the www.syfpeithi.de threshold of ≥24,32,33 which was tested in a T2 stabilization assay (Fig. 1).
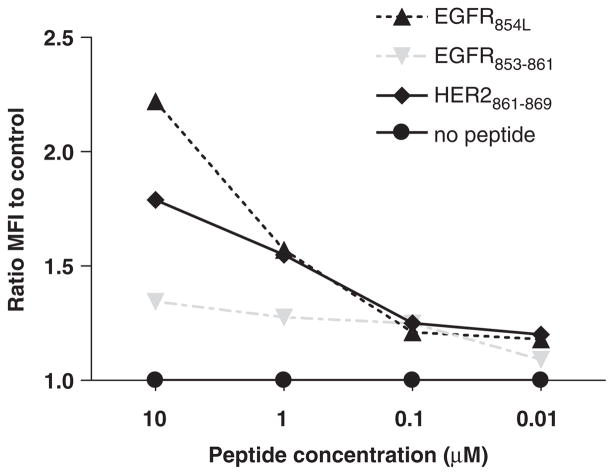
FIGURE 1.
HLA-A*0201 stabilization assay comparing peptides derived from epidermal growth factor receptor (EGFR). Stabilization of cell surface HLA-A*0201 molecules was determined after incubation of T2 cells with EGFR or flu peptides at various concentrations (ranging from 10 to 0.01 μg/mL) as described in Materials and Methods. Flow cytometry staining was performed by incubating 5 × 105 loaded cells with anti-HLA class 1 mAb W6/32, then fluorescein isothiocyanate-labeled secondary mAb for 30 minutes each at 4°C. Mean fluorescence intensity detected by flow cytometry is shown, reflecting results representative of 3 similar experiments.
To improve HLA-A*0201 binding and immunogenicity, the anchor residue of the EGFR853 – 861 peptide was modified at position 2, from the encoded threonine (T) to a leucine (L), to conform more closely to the canonical HLA-A*0201 motif,34–36 and to maximize cross-reactivity with the parental, wild-type (wt) peptide. As shown in Figure 1, the modified EGFR854L peptide was found to stabilize HLA-A*0201 molecules significantly better than the parental, wt EGFR853 – 861 peptide, and comparable to the influenza matrix58 – 66 (Flu58 – 66) peptide. The modified EGFR854L peptide in the same prediction algorithm received a score of 31 whereas the Flu58 – 66 received a score of 30. These results indicate a general correlation between peptide score and HLA-A*0201 binding using the T2 stabilization assay.
IVS of wt EGFR853 – 861 and Modified EGFR854L-specific CTL
Owing to the potential for discrepancy between HLA-binding prediction score and immunogenicity,37,38 we investigated the significance of the wt EGFR853 – 861 peptide. IVS was performed using the wt EGFR853 – 861 or modified EGFR854L peptide to induce CTL, using CD8+ PBMC from 5 HLA-A*0201+ HD and 1 SCCHN patient. The resulting EGFR-specific CTLs were tested for EGFR853 – 861 specificity and HLA class 1 antigen restriction using peptide-pulsed T2 cells. All experiments were derived from CTL from 2 of the HD or the SCCHN patient-derived CTL.
As shown in Figure 2A, CTL generated against the wt EGFR853 – 861 peptide recognized T2 cells pulsed with either the wt or the modified (EGFR854L) peptide, but not T2 cells alone or T2 cells incubated with the HIV Pol476 – 484 peptide (10 μg/mL at 37°C). This CTL recognition of T2 cells, which only express HLA-A2 molecules, was blocked by incubation with the anti-HLA-A, anti-HLA-B, anti-HLA-C mAb (W6/32), and with the anti-HLA-A mAb (LGIII.147.4, Ref. (27).
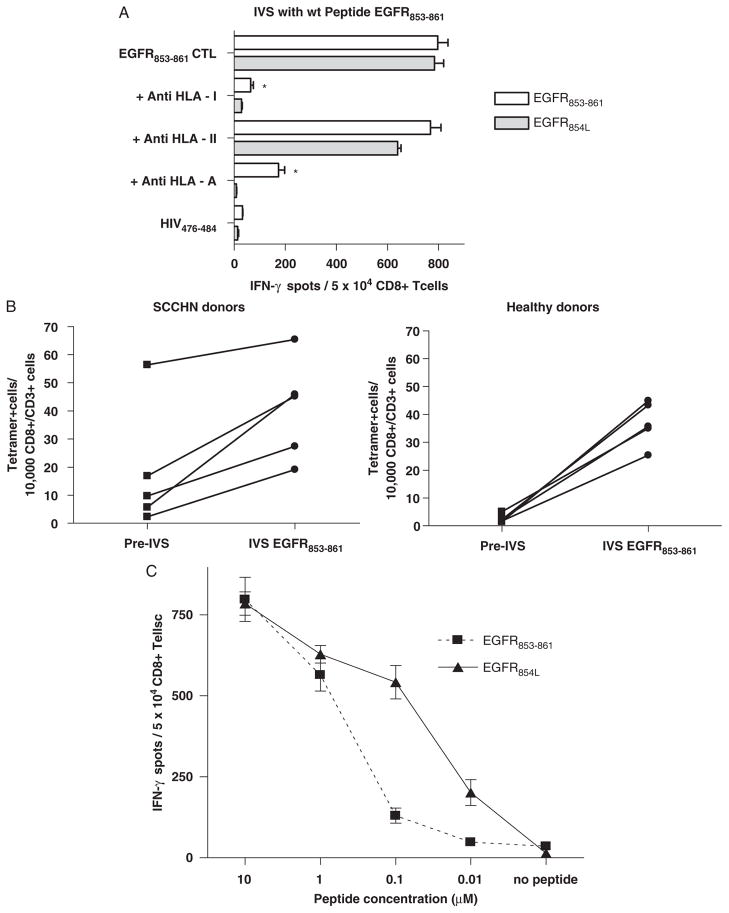
FIGURE 2.
A, Cross-reactivity of CTL derived by IVS using wild-type EGFR853 – 861 peptide. CD8+ T cells from HLA-A*0201+ HD or SCCHN patients were stimulated using IVS with DC loaded with EGFR853 – 861. Reactivity of CTL against peptide-pulsed target cells was tested using IFN-γ ELISPOT assays. This recognition was blocked by HLA class 1 and HLA-A–specific mAb, but not an HLA class 2-specific mAb. Cross-reactivity of EGFR854L peptide and EGFR853 – 861 peptide-specific CTL was observed. Each bar represents the mean spot number of triplicate experiments ± SD with 104 CTL per well. Background IFN-γ secretion was measured in response to T2 cells pulsed with HIV peptide or T2 cells alone. Background IFN-γ release was quantified using T2 plus HIV-1–derived peptide. Each data point represents the mean spot number of triplicate determinations ± SD with 104 CTL per well. B, Comparison of EGFR853 – 861-specific tetramer+ cells in PBMC from SCCHN patients or HD after 1-week IVS. IVS was performed using autologous DC from 5 HLA-A*0201+ SCCHN patients or 5 HLA-A*0201+ HD loaded with wt EGFR853 – 861 (10 μg/mL at 37°C for 4 h). Autologous CD8+ T cells were negatively isolated from PBMC with immunomagnetic beads (Miltenyi Biotech, Germany) and added at 1 × 106 cells/ml to 1 × 105 peptide-pulsed DC in a final volume of 2 mL of culture medium (24-well tissue culture plate). The cells were cultured for 7 days at 37°C with IL-2 (20 U/mL) and IL-7 (5 ng/mL). On day 7 lymphocytes were harvested and tested for the specificity of PE-labeled HLA-A*0201-EGFR853 – 861 tetramer. Tetramer staining of EGFR853 – 861-specific CD8+ T cells was performed before and after IVS and statistically compared. Higher EGFR853 – 861 tetramer+ T cells were observed at baseline (pre-IVS) in the 5 SCCHN patient PBMC versus the 5 HD (P<0.05). IVS induced significantly higher levels of EGFR853 – 861 tetramer+ T cells in both groups (*P = 0.013 and **P = 0.0002, respectively). Interestingly, a stronger induction of EGFR853 – 861 tetramer+ T cells was observed during the 1-week IVS in HD versus SCCHN PBMC (***P = 0.014). C, EGFR853 – 861-specific CTL recognition of EGFR853 – 861 and EGFR854L peptides. Reactivity of EGFR853 – 861 peptide-specific CTL from a HD was tested against T2 cells incubated with decreasing concentrations of EGFR853 – 861 or EGFR854L (ranging from 10 to 0.01 μM). Each data point represents the mean spot number of triplicate experiments ± SD with 1 × 104 CTL per well. CTL indicates cytotoxic T lymphocyte; EGFR, epidermal growth factor receptor; ELISPOT, enzyme-linked immunosorbent spot; HD, healthy donors; IVS, in vitro stimulation; SCCHN, head and neck squamous cell carcinoma.
To compare the immunogenicity of the wt EGFR853 – 861 between SCCHN patients and HD, we performed a 1-week IVS procedure using autologous DC loaded with wt EGFR853 – 861 (10 μg/mL at 37°C for 4 h). Tetramer staining of EGFR853 – 861-specific CD8+ T cells before and after IVS was performed (Fig. 2B) and statistically compared. Higher EGFR853 – 861 tetramer+ T-cell frequencies were observed at baseline (pre-IVS) in 5 HLA-A*0201+ SCCHN patient PBMC versus 5 HD (P<0.05), and IVS induced significantly higher levels of EGFR853 – 861 tetramer+ T cells in both groups (P = 0.013 and 0.0002, respectively). Interestingly, a stronger induction of EGFR853 – 861 tetramer+ T cells was observed during the 1-week IVS in HD versus SCCHN PBMC (P = 0.014).
EGFR853 – 861-specific CTL recognition of T2 cells pulsed with EGFR854L (10 μg/mL at 37°C, Fig. 2) indicated that EGFR853 – 861-specific CTLs were cross-reactive with the optimized EGFR854L peptide. To compare the ability of the wt or the modified peptide EGFR peptide to stimulate EGFR853 – 861-specific CTL, a peptide titration experiment was performed with 10-fold dilutions of wt EGFR853 – 861 or the modified EGFR854L peptide, using wt EGFR853 – 861-specific CTL (Fig. 2C). Recognition of T2 cells incubated with the EGFR854L peptide was detectable at approximately 20-fold lower concentrations than CTL recognition of T2 cells incubated with wt EGFR853 – 861 peptide, indicating improved immunogenicity of the EGFR854L peptide, likely due to enhanced HLA-A*0201 binding and stabilization.35
CTL cross-reactivity at high doses (≥1 μg/mL) led to comparable levels of CTL activation in this assay, though in pilot studies the wt EGFR853 – 861 peptide expanded CTL to a frequency of 2-fold lower tetramer+ T cells in IVS cultures than did the modified EGFR854L peptide. To evaluate whether the converse was true, IVS was performed using the EGFR854L peptide. CTL reactivity, specificity, and HLA restriction were tested, indicating cross-reactivity of EGFR854L-specific CTL against wt EGFR853 – 861 peptide-specific CTL (Fig. 3). In addition, CTL generated by IVS with either the wt or modified (EGFR854L) peptide were stained with HLA-A*0201-EGFR853 – 861 tetramer by flow cytometry. Tetramer frequencies of CTL cultures were similar to reactive spot numbers in IFN-γ ELISPOT assays (data not shown).
FIGURE 3.
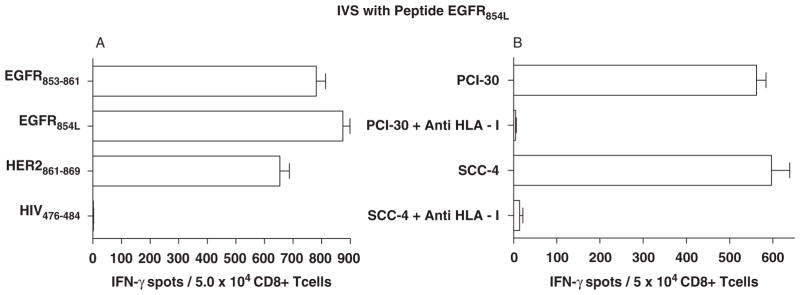
Cross-reactivity of CTL derived from HLA-A*0201+ SCCHN patient PBMC after IVS with either EGFR853 – 861 or EGFR854L. A, EGFR854L peptide-stimulated CD8+ T cells from an HLA-A*0201+ healthy donor or SCCHN patient were tested in IFN-γ ELISPOT assays using T2 cells incubated with EGFR853 – 861, EGFR854L, or the homologous peptide, HER2861 – 869. Cross-reactivity of EGFR854L peptide-specific CTL was observed against peptide-pulsed target cells, which was blocked by anti-HLA-A mAb, but not an anti-HLA class 2-specific mAb (Fig. 2). No reactivity of these CTL was observed against HIV-1 Pol control peptide. Each bar represents the mean spot number of triplicate determinations ± SD with 5 × 104 CTL per well. B, EGFR854L-specific CTL generated by IVS are cross-reactive with wt EGFR853 – 861-expressing SCCHN cells. ELISPOT assays were performed using EGFR854L-specific CTL and recognition of SCCHN cells PCI-30 and SCC4 was observed in an HLA class 1-specific fashion. CTL indicates cytotoxic T lymphocyte; EGFR, epidermal growth factor receptor; ELISPOT, enzyme-linked immunosorbent spot; IVS, in vitro stimulation; SCCHN, head and neck squamous cell carcinoma; wt, wild-type.
EGFR853 – 861 Peptide-specific CTL Recognize Naturally Processed Peptide on SCCHN Cells
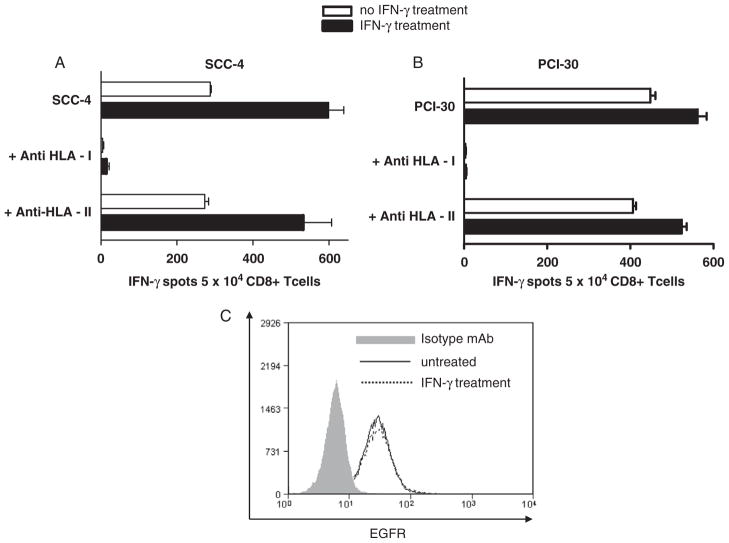
To determine whether the wt EGFR853 – 861 peptide-specific CTL recognized naturally processed peptide expressed by SCCHN cells, IFN-γ ELISPOT (Fig. 4) or 51Cr release assays were performed. As shown in Figure 4A, EGFR853 – 861-specific CTL recognized 3 HLA-A*0201+ SCCHN cell lines, PCI-30, SCC-4, and PCI-13 (not shown). This recognition was HLA-A restricted, as it was blocked by the HLA-A mAb LGIII.147.4 but not the HLA-DR–specific mAb, L243. No reactivity was observed with HLA-A*0201− SCCHN cells (not shown). Incubation of the SCCHN cells with IFN-γ (100 IU at 37°C for 72 h) significantly enhanced their recognition by EGFR853 – 861-specific CTL.30 All SCCHN cell lines tested for CTL reactivity expressed similar levels of EGFR by flow cytometry using the mAb cetuximab, which was not upregulated after IFN-γ treatment (4B).
FIGURE 4.
CTL recognition of naturally processed EGFR853 – 861 on SCCHN cells. EGFR853 – 861 CTL recognition of naturally processed peptide expressed by SCCHN cell lines (A) SCC-4 and (B) PCI-30 using IFN-γ ELISPOT assays was tested using IFN-γ ELISPOT assays. IFN-γ release was blocked by an anti-HLA class 1 mAb. No reactivity was observed using HLA-A*0201− SCCHN cells (not shown). Incubation of SCCHN cells with IFN-γ (100 IU/mL for 72 h at 37°C) increased CTL recognition, but not EGFR expression (Fig. 4C). CTL indicates cytotoxic T lymphocyte; EGFR, epidermal growth factor receptor; ELISPOT, enzyme-linked immunosorbent spot; SCCHN, head and neck squamous cell carcinoma.
EGFR-specific Tetramer+ T-cell Frequencies in HLA-A*0201+ SCCHN Patients
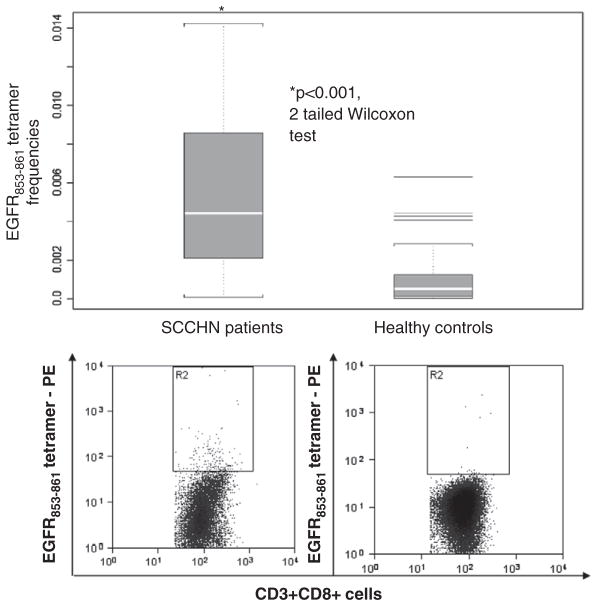
To determine the clinical significance of this novel CTL epitope, we tested whether EGFR853 – 861 precursor CTL were present at elevated levels in the circulation of tumor-bearing SCCHN patients’ PBMC (n = 24) before oncologic therapy. HLA-A*0201:EGFR853 – 861 specific tetramer staining of CD3+CD8+ T cells was performed (Fig. 5) and compared with tetramer frequencies in PBMC from HLA-A*0201+ HD. Tetramer frequencies measured by flow cytometry showed a significantly higher level of EGFR853 – 861-specific precursor CTL in the SCCHN patient PBMC than in healthy controls (P<0.001, 2-tailed Wilcoxon test).
FIGURE 5.
Frequency of EGFR853 – 861-specific T cells in the circulation of SCCHN patients. EGFR-specific tetramer+ T-cell frequencies were elevated in HLA-A*0201+ SCCHN patients (n = 24) as compared with HLA-A*0201+ healthy donors (n = 24). EGFR853 – 861 tetramer staining of CD3+CD8+ cells were stained and quantified as shown. Quantification mean ± SD and statistical analysis (2-tailed Wilcoxon test, P<0.001) compared relative frequencies between PBMC from SCCHN patients and healthy donors. EGFR indicates epidermal growth factor receptor; SCCHN, head and neck squamous cell carcinoma.
Cetuximab Treatment of SCCHN Cells Leads to EGFR Degradation and Enhanced EGFR853 – 861-specific CTL Recognition
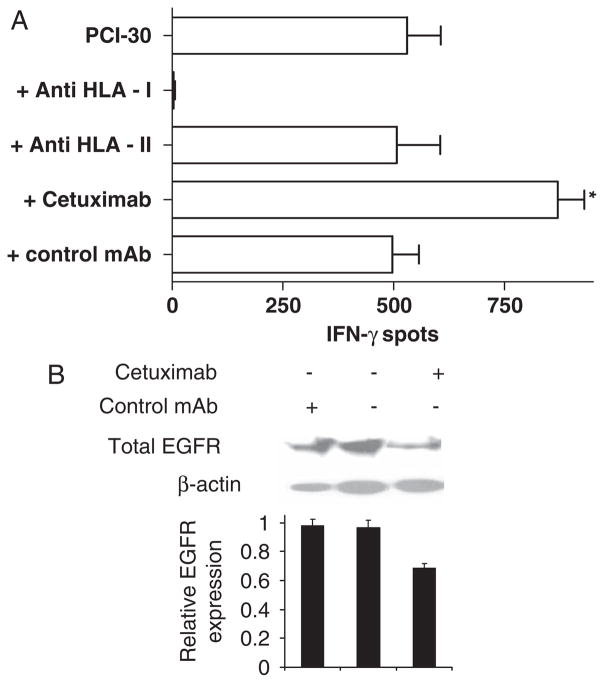
Treatment of tumor cells by EGFR-specific mAb induces resistance through internalization and degradation of EGFR from the cell surface.15 Thus, we investigated whether cetuximab treatment of SCCHN cells caused internalization and degradation of EGFR from the cell surface, resulting in elevated levels of HLA-A*0201-EGFR853 – 861 peptide complexes, as measured by CTL recognition. To test whether EGFR-specific mAb treatment of SCCHN cells enhanced EGFR853 – 861-specific CTL recognition, PCI-30 SCCHN cells were treated with cetuximab (10 μg/mL for 18 h at 37°C), isotype matched control IgG1 mAb, or media alone, then tested for EGFR853 – 861-specific CTL recognition in IFN-γ ELISPOT assays (Fig. 6A). EGFR853 – 861 peptide-specific CTL recognition of PCI-30 cells was significantly enhanced after incubation with cetuximab (P≤0.05) but not with an irrelevant specificity mAb. In addition, we found that EGFR protein levels were reduced in PCI-30 cells were treated for 2 or 6 hours with cetuximab (10 μg/mL at 37°C), as shown in Figure 6B.
FIGURE 6.
Enhancement of EGFR853 – 861 peptide-specificity CD8+ T cells and EGFR degradation after cetuximab treatment of SCCHN cells. A, EGFR853 – 861 peptide-specificity CD8+ T cells recognized PCI-30 SCCHN cells after incubation with cetuximab or a control mAb (10 μg/mL for 18 h at 37°C) was tested in an IFN-γ ELISPOT assay. IFN-γ release was blocked by an anti-HLA-A, anti-HLA-B, anti-HLA-C–specific mAb (W6/32), but not an HLA class 2-specific mAb (L243). CTL recognition was augmented by incubation of the SCCHN cells with cetuximab, but not by control mAb IgG1 treatment. *P≤0.05, comparing CTL reactivity (50 × 104 T cells) against PCI-30 cells incubated with cetuximab relative to PCI-30 cells incubated with a control IgG1 mAb (2-tailed permutation test). B, Degradation of EGFR in SCCHN cells using western blot after 6 hours of treatment with cetuximab. SCCHN cells were incubated with no mAb, with cetuximab or control mAb (10 μg/mL) in serum-free media (AIM-V) for 6 hours at 37°C. The cells were then lysed and equal amounts of cellular protein were subjected to western blot analysis for total EGFR. The level of β-actin was used as a reference for cell lysate protein loaded in the western blot analysis, and densitometric comparisons presented graphically. CTL indicates cytotoxic T lymphocyte; EGFR, epidermal growth factor receptor; ELISPOT, enzyme-linked immunosorbent spot; SCCHN, head and neck squamous cell carcinoma.
DISCUSSION
EGFR inhibition using blocking mAbs has become a clinically efficacious therapeutic approach for solid malignancies, but response rates are low (≤20%), and no reliable biomarker of response has been identified.8 Clinical responses to EGFR inhibitors in cancer patients are not reliably correlated with level of expression of EGFR,8 and one cetuximab-based clinical trial suggested clinical response correlated with inversely with level of EGFR expression.39 Increasing therapeutic resistance has been found due to widespread use of the EGFR-specific mAbs, cetuximab and panitumumab, compelling the effort to develop new treatments to counteract tumor escape from this type of immunotherapy and expand the number of candidates achieving a clinical response. Owing to the enhanced EGFR internalization and proteasomal degradation due to cetuximab treatment,15,16 the site where HLA class 1 antigen processing begins, CTL-based approaches become more appealing in combination with mAb-based approaches. Thus, combinatorial EGFR-targeted therapy may successfully exploit this potential escape mechanism by treated tumor cells. Our data indicate a potential benefit of combinatorial immunotherapy targeting EGFR through CTL and mAb approaches (Fig. 6). Such an approach has the potential for improving clinical efficacy in previously untreated patients and avoiding or reducing treatment resistance in a subset of patients undergoing escape from EGFR mAb immunotherapy.
Our identification of a novel EGFR-derived CTL epitope, and an optimized peptide with enhanced immunogenicity, provides a means for incorporation of T-cell–based immunotherapy targeting EGFR. The potentially promising application of EGFR-targeted vaccine approaches to multiple cancer types is reinforced by the cross-reactivity of CTL derived from IVS using the wt or optimized peptide, as well as a HER2-encoded peptide (Fig. 2). As HER2 upregulation has been observed in aerodigestive tract patients treated with EGFR inhibitors, this cross-reactivity may expand the candidates for immunotherapy with the EGFR853 – 861 peptide or variant, to include HER2+ breast carcinoma patients or EGFR-inhibitor–treated patients who develop treatment resistance through HER2 upregulation. This is likely due to the substitution of threonine for leucine, to conform more closely to the HLA-A*0201-binding anchor motif at position 2.34–36 In addition, our SCCHN patient data document the presence of increased frequencies of EGFR-specific T cells are present in the circulation of SCCHN patients. We performed phenotypic analyses which demonstrated a shift to central and effector memory differentiation in this CTL repertoire (not shown). Further work will focus on the prognostic value of these CTL, their phenotypic attributes, relation to disease status and correlation with clinical responses to therapy. Our preliminary data also suggest that cetuximab therapy may induce cross-presentation of EGFR853 – 861 (Lopez et al, unpublished data), providing a mechanism for this observation and possibly contributing to cetuximab or panitumumab’s antitumor efficacy.
The finding that a stronger induction of EGFR853 – 861 tetramer+ T cells was observed during the 1-week IVS in HD versus SCCHN PBMC (P = 0.014) may be related to the fact that higher EGFR853 – 861 tetramer+ T cells were observed at baseline (pre-IVS) in 5 HLA-A*0201+ SCCHN patient PBMC versus 5 HD (P<0.05). Although IVS induced significantly higher levels of EGFR853 – 861 tetramer+ T cells in both groups (P = 0.013 and 0.0002, respectively), the higher induction of tetramer+ T cells in HD may be due to the lack of tolerance induction restricting expansion of EGFR-specific CTL after exposure of EGFR in the context of cancer patients in vivo. Further work, beyond the scope of this report, will investigate in detail potential approaches for enhancing levels of EGFR-specific CTL in SCCHN patients with modified peptides and IVS stimulation conditions.
Cetuximab clinical efficacy is low as monotherapy but increases with concomitant radiation therapy, thus the potential exist that radiation may increase expression of the APM components and thus CTL recognition and lysis of tumor cells in the microenvironment. Low APM component expression has been shown as a mechanism of immune escape in SCCHN and colorectal carcinoma, a likely barrier to T-cell–based vaccination approaches. However, stimulation of CTL may provide the necessary cytokines (TNF-α, IFN-γ), which are known to increase expression of the APM component pathways. Indeed, responsiveness to cetuximab mAb immunotherapy was correlated in a phase 2 clinical trial (UPCI #05-003, manuscript submitted) with levels of IFN-inducible cytokines and chemokines. Thus, the potential for cross-priming of CTL specific for EGFR and other TA in dying tumor cells rises the potential for expansion of a polyclonal TA-specific CTL response. Further studies will investigate a panel of EGFR epitopes, epitope spreading, and non-EGFR tumor antigens in combination with cetuximab antitumor effects.
Interestingly, the novel EGFR peptide epitope was expressed by all HLA-A*0201+ SCCHN cells tested, suggesting it might be a candidate for incorporation into cancer vaccine approaches. The significantly higher frequencies of EGFR853 – 861-specific T cells in the circulation of SCCHN patients versus HD also suggests that immunity to EGFR may occur during tumorigenesis and would provide a circulating repertoire of CTL for expansion through vaccination. Validation of these findings in cetuximab-treated patients would provide additional rationale for combinatorial immune-based strategies.
Indeed multiepitope vaccine strategies have long been favored in combinatorial immunotherapy using TA-specific mAb immunotherapy. This approach may be both feasible and efficacious, providing avoidance of therapeutic escape and expanding candidates for clinical response. Prospective clinical trials are warranted and necessary to validate this approach.
Footnotes
All authors have declared that there are no financial conflicts of interest in regard to this work.
References
- 1.Cassidy J. Cetuximab for the treatment of patients with colorectal cancer. Nat Clin Pract Oncol. 2008;5:310–311. doi: 10.1038/ncponc1130. [DOI] [PubMed] [Google Scholar]
- 2.Grandis JR, Tweardy DJ. Elevated levels of transforming growth factor alpha and epidermal growth factor receptor messenger RNA are early markers of carcinogenesis in head and neck cancer. Cancer Res. 1993;53:3579–3584. [PubMed] [Google Scholar]
- 3.Ang KK, Berkey BA, Tu X, et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002;62:7350–7356. [PubMed] [Google Scholar]
- 4.Baselga J, Pfister D, Cooper MR, et al. Phase I studies of anti-epidermal growth factor receptor chimeric antibody C225 alone and in combination with cisplatin. J Clin Oncol. 2000;18:904–914. doi: 10.1200/JCO.2000.18.4.904. [DOI] [PubMed] [Google Scholar]
- 5.Mendelsohn J, Baselga J. The EGF receptor family as targets for cancer therapy. Oncogene. 2000;19:6550–6565. doi: 10.1038/sj.onc.1204082. [DOI] [PubMed] [Google Scholar]
- 6.Lynch DH, Yang XD. Therapeutic potential of ABX-EGF: a fully human anti-epidermal growth factor receptor monoclonal antibody for cancer treatment. Semin Oncol. 2002;29 (1 suppl 4):47–50. doi: 10.1053/sonc.2002.31522. [DOI] [PubMed] [Google Scholar]
- 7.Yang XD, Jia XC, Corvalan JR, et al. Development of ABX-EGF, a fully human anti-EGF receptor monoclonal antibody, for cancer therapy. Crit Rev Oncol Hematol. 2001;38:17–23. doi: 10.1016/s1040-8428(00)00134-7. [DOI] [PubMed] [Google Scholar]
- 8.Kim S, Grandis JR, Rinaldo A, et al. Emerging perspectives in epidermal growth factor receptor targeting in head and neck cancer. Head Neck. 2008;30:667–674. doi: 10.1002/hed.20859. [DOI] [PubMed] [Google Scholar]
- 9.Valentini AM, Pirrelli M, Caruso ML. EGFR-targeted therapy in colorectal cancer: does immunohistochemistry deserve a role in predicting the response to cetuximab? Curr Opin Mol Ther. 2008;10:124–131. [PubMed] [Google Scholar]
- 10.Lopez-Albaitero A, Ferris RL. Immune activation by epidermal growth factor receptor specific monoclonal antibody therapy for head and neck cancer. Arch Otolaryngol Head Neck Surg. 2007;133:1277–1281. doi: 10.1001/archotol.133.12.1277. [DOI] [PubMed] [Google Scholar]
- 11.Kawaguchi Y, Kono K, Mimura K, et al. Cetuximab induce antibody-dependent cellular cytotoxicity against EGFR-expressing esophageal squamous cell carcinoma. Int J Cancer. 2007;120:781–787. doi: 10.1002/ijc.22370. [DOI] [PubMed] [Google Scholar]
- 12.Roda JM, Joshi T, Butcher JP, et al. The activation of natural killer cell effector functions by cetuximab-coated, epidermal growth factor receptor positive tumor cells is enhanced by cytokines. Clin Cancer Res. 2007;13:6419–6428. doi: 10.1158/1078-0432.CCR-07-0865. [DOI] [PubMed] [Google Scholar]
- 13.Kurai J, Chikumi H, Hashimoto K, et al. Antibody-dependent cellular cytotoxicity mediated by cetuximab against lung cancer cell lines. Clin Cancer Res. 2007;13:1552–1561. doi: 10.1158/1078-0432.CCR-06-1726. [DOI] [PubMed] [Google Scholar]
- 14.Zhang W, Gordon M, Schultheis AM, et al. FCGR2A and FCGR3A polymorphisms associated with clinical outcome of epidermal growth factor receptor expressing metastatic colorectal cancer patients treated with single-agent cetuximab. J Clin Oncol. 2007;25:3712–3718. doi: 10.1200/JCO.2006.08.8021. [DOI] [PubMed] [Google Scholar]
- 15.Lu Y, Li X, Liang K, et al. Epidermal growth factor receptor (EGFR) ubiquitination as a mechanism of acquired resistance escaping treatment by the anti-EGFR monoclonal antibody cetuximab. Cancer Res. 2007;67:8240–8247. doi: 10.1158/0008-5472.CAN-07-0589. [DOI] [PubMed] [Google Scholar]
- 16.Wheeler DL, Huang S, Kruser TJ, et al. Mechanisms of acquired resistance to cetuximab: role of HER (ErbB) family members. Oncogene. 2008;27:3944–3956. doi: 10.1038/onc.2008.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferris RL, Buck C, Hammond SA, et al. Class I-restricted presentation of an HIV-1 gp41 epitope containing an N-linked glycosylation site. Implications for the mechanism of processing of viral envelope proteins. J Immunol. 1996;156:834–840. [PubMed] [Google Scholar]
- 18.Ferris RL, Hall C, Sipsas NV, et al. Processing of HIV-1 envelope glycoprotein for class I-restricted recognition: dependence on TAP1/2 and mechanisms for cytosolic localization. J Immunol. 1999;162:1324–1332. [PubMed] [Google Scholar]
- 19.Emens LA, Reilly RT, Jaffee EM. Augmenting the potency of breast cancer vaccines: combined modality immunotherapy. Breast Dis. 2004;20:13–24. doi: 10.3233/bd-2004-20103. [DOI] [PubMed] [Google Scholar]
- 20.Mittendorf EA, Storrer CE, Shriver CD, et al. Investigating the combination of trastuzumab and HER2/neu peptide vaccines for the treatment of breast cancer. Ann Surg Oncol. 2006;13:1085–1098. doi: 10.1245/ASO.2006.03.069. [DOI] [PubMed] [Google Scholar]
- 21.Wirth LJ, Posner MR, Tishler RB, et al. Phase I study of panitumumab+ chemoradiotherapy (CRT) for head and neck cancer (HNC) J Clin Oncol. 2008;26(suppl):6007. Abstract. [Google Scholar]
- 22.Heo DS, Snyderman C, Gollin SM, et al. Biology, cytogenetics, and sensitivity to immunological effector cells of new head and neck squamous cell carcinoma lines. Cancer Res. 1989;49:5167–5175. [PubMed] [Google Scholar]
- 23.Lin CJ, Grandis JR, Carey TE, et al. Head and neck squamous cell carcinoma cell lines: established models and rationale for selection. Head Neck. 2007;29:163–188. doi: 10.1002/hed.20478. [DOI] [PubMed] [Google Scholar]
- 24.Salter RD, Cresswell P. Impaired assembly and transport of HLA-A and -B antigens in a mutant TxB cell hybrid. Embo J. 1986;5:943–949. doi: 10.1002/j.1460-2075.1986.tb04307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parham PBC, Bodmer WF. Use of a monoclonal antibody (W6/32) in structural studies of HLA-A,B,C, antigens. J Immunol. 1979;123:342–349. [PubMed] [Google Scholar]
- 26.Parham PBW. Monoclonal antibody to a human histocompatibility alloantigen, HLA-A2. Nature. 1978;276:397–399. doi: 10.1038/276397a0. [DOI] [PubMed] [Google Scholar]
- 27.Wang X, Liang B, Rebmann V, et al. Specificity and functional characteristics of anti-HLA-A mAbs LGIII-147.4.1 and LGIII-220.6.2. Tissue Antigens. 2003;62:139–148. doi: 10.1034/j.1399-0039.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- 28.Goldman JM, Hibbin J, Kearney L, et al. HLA-DR monoclonal antibodies inhibit the proliferation of normal and chronic granulocytic leukaemia myeloid progenitor cells. Br J Haematol. 1982;52:411–420. doi: 10.1111/j.1365-2141.1982.tb03910.x. [DOI] [PubMed] [Google Scholar]
- 29.Vitale M, Rezzani R, Rodella L, et al. HLA class I antigen and transporter associated with antigen processing (TAP1 and TAP2) down-regulation in high-grade primary breast carcinoma lesions. Cancer Res. 1998;58:737–742. [PubMed] [Google Scholar]
- 30.Lopez-Albaitero A, Nayak JV, Ogino T, et al. Role of antigen-processing machinery in the in vitro resistance of squamous cell carcinoma of the head and neck cells to recognition by CTL. J Immunol. 2006;176:3402–3409. doi: 10.4049/jimmunol.176.6.3402. [DOI] [PubMed] [Google Scholar]
- 31.Jonuleit H, Kuhn U, Muller G, et al. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997;27:3135–3142. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- 32.Gomez-Nunez M, Pinilla-Ibarz J, Dao T, et al. Peptide binding motif predictive algorithms correspond with experimental binding of leukemia vaccine candidate peptides to HLA-A*0201 molecules. Leuk Res. 2006;30:1293–1298. doi: 10.1016/j.leukres.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 33.Rammensee H, Bachmann J, Emmerich NP, et al. SYF-PEITHI: database for MHC ligands and peptide motifs. Immunogenetics. 1999;50:213–219. doi: 10.1007/s002510050595. [DOI] [PubMed] [Google Scholar]
- 34.Falk K, Rotzschke O, Stevanovic S, et al. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature. 1991;351:290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- 35.Petersen TR, Buus S, Brunak S, et al. Identification and design of p53-derived HLA-A2-binding peptides with increased CTL immunogenicity. Scand J Immunol. 2001;53:357–364. doi: 10.1046/j.1365-3083.2001.00887.x. [DOI] [PubMed] [Google Scholar]
- 36.Rotzschke O, Falk K. Naturally-occurring peptide antigens derived from the MHC class-I-restricted processing pathway. Immunol Today. 1991;12:447–455. doi: 10.1016/0167-5699(91)90018-O. [DOI] [PubMed] [Google Scholar]
- 37.Deng Y, Yewdell JW, Eisenlohr LC, et al. MHC affinity, peptide liberation, T cell repertoire, and immunodominance all contribute to the paucity of MHC class I-restricted peptides recognized by antiviral CTL. J Immunol. 1997;158:1507–1515. [PubMed] [Google Scholar]
- 38.Morgan CL, Ruprai AK, Solache A, et al. The influence of exogenous peptide on beta2-microglobulin exchange in the HLA complex: analysis in real-time. Immunogenetics. 1998;48:98–107. doi: 10.1007/s002510050409. [DOI] [PubMed] [Google Scholar]
- 39.Burtness B, Goldwasser MA, Flood W, et al. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an Eastern Cooperative Oncology Group study. J Clin Oncol. 2005;23:8646–8654. doi: 10.1200/JCO.2005.02.4646. [DOI] [PubMed] [Google Scholar]