Abstract
Objective
In rodents, diets exceeding nutritional requirements (i.e. high-energy diets; HED) impair hippocampal-dependent memory. Our research suggests that the effects likely involve HED-induced increases in liver lipids. In this experiment, we provided rats with diet choices and tested whether voluntary consumption of a HED impairs spatial memory, whether differences in initial weight gain predict memory deficits, and whether increases in liver lipids are associated with the memory deficits.
Design & Methods
Adult male Sprague-Dawley rats were given a control diet or cafeteria-style HED for 8 weeks. Weight gain during the first 5 days on the diet was used to divide rats into a HED-Lean group and HED-Obese group. Spatial water maze memory was tested 8 weeks later and postmortem liver lipid concentrations were quantified.
Results
Compared with the HED-Lean and control rats, the HED-Obese rats had impaired spatial memory and met the human diagnostic criterion of non-alcoholic fatty liver disease (> 5% liver lipids relative to liver weight). Moreover, liver lipids were correlated with memory deficits.
Conclusions
These findings show that voluntary consumption of a HED impairs memory, that initial weight gain predicts fatty liver and memory deficits, and that fatty liver may contribute to the memory-impairing effects of obesity.
Introduction
There has been an alarming increase in the prevalence of obesity over the past several decades. Currently, more than 60% of the adult population in the United States is considered to be overweight or obese (1) and the overconsumption of high fat and high sugar foods is a major contributing factor to this epidemic. Evidence suggests that overconsumption of high-energy foods negatively impacts brain function. For instance, in humans, obesity is associated with impaired cognition (2, 3). In rodents, the consumption of a high-energy diet (HED) impairs hippocampal-dependent learning and memory (4–7).
We recently showed that the memory impairing effects of consuming a pelleted 60% fructose diet are associated with elevated liver lipids (7). Approximately 70% of overweight and obese individuals meet the diagnostic criteria for non-alcoholic fatty liver disease (NAFLD, 8). NAFLD is the most common form of liver disease and is prevalent in 15–30% of the normal population (9). Increases in liver lipid concentrations in rats cause metabolic dysfunctions such as insulin resistance (10), oxidative stress (11), and alterations in lipid homeostasis (12). Several lines of evidence suggest that elevated plasma lipids (4, 13) and insulin resistance (5, 14) contribute to the memory-impairing effects of HEDs and obesity. Thus, fatty liver induced metabolic disturbances may contribute to hippocampal-dependent memory deficits.
A limitation of previous studies examining the effects of effects of HEDs on memory is that the diets contained the fat and/or sugars in a single pellet and nutritional alternatives were not provided (e.g., 4). Therefore, the investigator, rather than the rodent, determined the macronutrient composition of the calories consumed. Consequently, the physiological relevance of these diets to human voluntary food intake is questionable. It is well established that rodents show a variable response to high energy diets such that only a subset of rats who consume a high energy diet will become obese (15–20) As a result, we hypothesized that voluntary consumption of a HED would impair memory only in those rats that become obese. To test this, we used a tertile split to divide rats based on percent change in body mass during the first 5 days on the choice diet. This split was performed on the basis of previous findings showing that after 5 days on the choice diet rats in the upper tertile will become obese 4–6 weeks later; those in the bottom tertile will not (15). This would also allow us to determine whether any memory deficits could be predicted.
Based on the evidence reviewed above, we tested: 1) whether voluntary consumption of a HED impairs hippocampal-dependent memory, 2) whether individual differences in weight gain responses to the diet predict memory deficits, and 3) whether elevated liver lipid concentrations are associated with memory deficits. To do so, adult male rats were maintained on a standard laboratory diet or placed on a HED with choices of sucrose, fat, standard chow and tap water (21). After 8 weeks, hippocampal-dependent learning and memory were tested in the spatial water maze. Postmortem fat pad masses and liver lipid concentrations were quantified.
Methods and Procedures
Animals
Ninety-seven adult male Sprague Dawley rats (Charles River, Wilmington, MA), aged 52–53 days upon arrival and were acclimated to the facility for 7 days. The rats were housed singly in OptiRat® ventilated cages with metal floor inserts (Animal Care Systems, Centennial, CO). All procedures were in accordance with the Georgia State University Institutional Animal Care and Use Committee and PHS policy.
Diets
The rats were weighed on the day of arrival and 7 days later. They were then matched on the percent change in body mass and absolute body mass and were placed on either the control diet or the HED. The HED group (n=71) was given Purina 5001 rodent chow (3.01 kcal/g; Gray Summit, MO), a glass petri dish containing lard (9.0 kcal/g; Armour; Omaha, NE), one bottle of tap water, and one bottle containing a 32% sucrose solution (3.75kcal/g of sucrose) ad libitum (21). The control group (n=26) was provided with Purina 5001 rodent chow, two bottles of tap water ad libitum, and an empty petri dish was placed in the cage. Body mass and chow, lard, tap water, and sucrose intake were measured at regular intervals. Chow intake was corrected for spillage. Fresh lard was provided every other day and fresh chow and water were provided every 3 days throughout the duration of the study.
Tail Blood Collection
In order to quantify in vivo plasma TG concentrations, tail blood was collected 1 day before and then 49 days after beginning the diet. The 49 day time-point, which occurred 1 week before behavioral training, was selected to be close in time to the behavioral training without potentially allowing the stressfulness of the manipulation to affect behavior. For the 2 days prior to both blood collections, the rats were handled by placing them into a folded towel and stroking the exposed tail for 2 minutes. To collect blood, the rats were placed in the towel, the bottom of the tail was cleaned with betadine, and a small incision was made approximately 15 mm from the tip. The tail was stroked gently and the blood was collected into heparin-treated tubes (StatSampler, Westwood, MA) and placed on ice. Samples were then centrifuged and stored at −80°C for later analysis.
Spatial Water Maze
After 8 weeks on the control or HED, rats were trained in a hippocampal-dependent spatial version of the water maze (22). The rats were trained over 3 days to use extra-maze cues to learn the location of a submerged platform (11.5 cm) in a circular pool (1.35m wide and 0.46m deep) filled with water (18–22°C). For the purpose of analysis, the pool was divided into four virtual quadrants with the quadrant containing the platform designated as the target quadrant. On the first day of training, the rats were placed on the submerged platform for 30 seconds before the beginning of the first training trial. For each trial, the rat was placed in the pool facing the wall in the middle of one of three randomly chosen non-target quadrants. If the rat did not locate the platform in 60 sec, the experimenter guided the rat gently to the platform. At the end of each trial the rat was allowed to remain on the platform for 15 sec and then placed in an empty cage under a heat lamp for a 30 sec inter-trial interval. During this time, fecal matter was removed from the pool and the water was stirred. Each rat was given 8 acquisition trials on the first and second day of training and 4 trials on the third day. Forty-eight hours following the last training trial, a memory test was administered with the platform removed from the pool. All rats were placed in the pool from the same novel location and allowed 20 sec to swim in the pool.
A camera mounted above the pool recorded behavior for later analysis. The latency to reach the platform for each trial was used as a measure of acquisition. The time spent in the target quadrant, the latency to the target quadrant, the latency to the platform location, the average proximity to the previous platform location, and the path length across the 20 sec probe test were used as memory measures. In addition, swim speed also was measured.
Post-mortem Measures
Forty-eight hours following the memory probe, the rats were fasted for 4 hours and then anesthetized (5% isoflurane; 95% oxygen) prior to decapitation. Trunk blood was collected for measurement of terminal plasma TG concentrations. The liver was removed and rapidly frozen for future measurement of hepatic lipids. To confirm obesity, white adipose tissue depots [epididymal (EWAT), inguinal (IWAT), retroperitoneal and perirenal (RWAT)] were collected and weighed. Plasma TG concentrations were measured using a commercially available colorimetric kit (Sigma, St. Louis, MO). The assay was conducted according to the manufacturer’s recommendations. Hepatic lipids were extracted via the Folch method (23).
Data Analysis
To test the hypothesis that voluntary consumption of a HED would only impair memory in those rats that become obese and to determine whether any memory deficits could be predicted, a tertile split was used to divide HED rats based on the percent change in body mass during the first 5 days on the diet. Rats in the bottom tertile (i.e. those with the least percent change in body mass) were defined as HED-Lean (n=18). The rats in the top tertile (i.e. those that had the largest percent change in body mass) were labeled HED-Obese (n=17). Thirty-five rats from the middle tertile were removed from the study and excluded from further analysis.
The spatial water maze data were analyzed for behavioral outliers by computing z-scores for each group and excluding those rats that had a z-score greater than 2 on two or more behavioral measures. As a result, four control, one HED-Lean, and two HED-Obese rats were removed from the subsequent analyses. In addition, 2 HED-Lean rats were excluded because the probe recordings could not be analyzed. Lastly, one HED-Obese rat was removed from all analyses due to a brain abnormality (i.e. enlarged ventricles, no apparent hippocampus, and underdeveloped cortex). Thus, the final number of rats in each group was Control n=22, HED-Lean=15, and HED-Obese=15.
All statistical analyses were performed using Statistical Package for Social Sciences (SPSS) version 18. A between subjects t-test was used to analyze the differences in the percent change in body mass and caloric consumption during the first 5 days of diet consumption in control versus HED rats before the tertile split. One-way ANOVAs and post-hoc Tukey-HSD tests were computed to analyze differences between control, HED-Lean and HED-Obese in caloric consumption, percent change in body mass, fat pad mass, hepatic lipid concentrations, terminal plasma TG concentrations, time spent in the target quadrant, and proximity to the platform. Given that the latency to the target quadrant was not normally distributed, a Kruskal-Wallis and Mann-Whitney U post-hoc tests were used to analyze these data. Only the rats that reached the platform location were included in the analysis of latency to the platform location and path length; therefore, Kruskal-Wallis and Mann-Whitney U post-hoc tests also were used to analyze these measures. A t-test was used to compare lard and sucrose consumption between the HED-Lean and HED-Obese groups. A 3 × 2 mixed ANOVA was used to analyze diet-induced changes in TG concentrations over time, with diet groups as the between measure and time as the repeated measure. Lastly, Pearson r correlations were computed to determine the relation between food intake and memory measures and between liver lipids and memory measures. All analyses were significant when p<.05
Results
Body mass & food consumption first 5 days
During the first 5 days of the diet, the HED significantly increased total daily caloric consumption [t (95) =18.869, p<0.05; Table 1]. Compared with the control group, the HED rats consumed fewer calories from chow [t (95) =19.163, p<0.05; Table 1]. The increase in total calories consumed in the HED rats did not lead to a significant increase in the percent change in body mass during the first 5 days on the diet (Control: x̄ = 10.25±0.59%; HED: x̄ = 12.80±0.95%). When a tertile division was used to divide the rats into a HED-Lean group (bottom 33%) and HED-Obese group (top 33%) based on percent change in body mass during the first 5 days, there was a significant effect of diet on percent change in body mass [F(2,49)=73.485, p<0.05; Table 1]. HED-Lean rats gained less weight than controls and the HED-Obese group gained more weight than both the control and HED-Lean groups (both p<0.05).
Table 1. Body mass and food intake.
Mean ± SEM percent change in body mass after the first 5 days and 8 weeks on the high energy diet (HED) for the control, HED-Lean, and HED-Obese. Mean ± SEM for the food intake data is for the first 5 days and for the 8 week diet duration.
| Control | HED-Lean | HED-Obese | |
|---|---|---|---|
| % change in body mass | |||
| After first 5 days | 10.88 ± 0.61 a | 6.38 ± 0.59 b | 17.16 ± 0.48c |
| After 8 weeks | 68.07 ± 4.48 a | 60.74 ± 4.04 a | 109.79 ± 7.75 b |
| Chow intake (kcal) | |||
| For first 5 days | 83.71 ± 1.50 a | 36.64 ± 1.48 b | 50.82 ± 2.06 c |
| For 8 weeks | 84.34 ± 1.86 a | 31.71 ± 0.62 b | 41.92 ± 1.68 c |
| Lard intake (kcal) | |||
| For first 5 days | 27.18 ± 4.44 a | 45.30 ± 4.17 b | |
| For 8 weeks | 29.84 ± 2.32 a | 41.12 ± 3.29 b | |
| Sugar intake (kcal) | |||
| For first 5 days | 57.22 ± 3.26 a | 45.55 ± 3.11 b | |
| For 8 weeks | 50.32 ± 2.19 | 49.12 ± 3.23 | |
| Total intake (kcal) | |||
| For first 5 days | 83.71 ± 1.50 a | 121.04 ± 2.84 b | 141.67 ± 2.63 c |
| For 8 weeks | 84.34 ± 1.86 a | 111.87 ± 1.97 b | 132.16 ± 2.57 c |
Data with different superscripts indicate a significant difference p<0.05.
During the first 5 days of the diet, there was a significant effect of the HED on total caloric intake [F (2, 49) =187.480, p<0.05; Table 1] and caloric intake from chow [F (2, 49) =223.413, p<0.05; Table 1], with the HED-Lean and HED-Obese groups consuming significantly more total calories per day but fewer calories from chow than control rats (all p<0.05). Compared with the HED-Obese group, HED-Lean rats consumed significantly fewer calories from lard [t (28) =2.977, p<0.05; Table 1], but significantly more calories from sucrose [t (28) =2.5900, p<0.05].
Body mass & food consumption 8 weeks
A similar effect of diet on consumption patterns remained after 8 weeks of diet exposure. That is, the HED-Lean and HED-Obese groups consumed less calories from chow [F (2, 49) =318.489, p<0.05; Table 1], but more total calories than controls [F (2, 49) =136.085, p<0.05]. The HED-Lean group still ingested significantly fewer calories from lard than the HED-Obese rats [t (28) =2.800, p=0.05]; however, there were no longer any differences between these groups in the calories consumed from sucrose [t(28)=0.308, p>0.05]. Weight gain after 8 weeks on the diet did not completely parallel weight gain during the first 5 days (p<0.05; Table 1). HED-Obese rats still had a higher percent change in body mass than the control and HED-Lean rats [F(2,37)=21.268, p<0.05]; however, the control and HED-Lean rats no longer differed significantly in percent change in body mass (p=0.587).
Fat pad masses
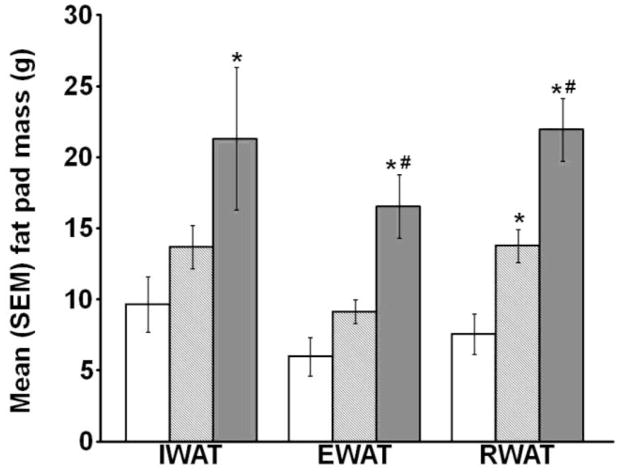
There was a significant effect of diet on IWAT [F(2,18)=3.703, p<0.05], EWAT [F(2,18)=12.641, p<0.05], and RWAT [F(2,18)=18.685, p<0.05; Fig. 1]. HED-Obese rats had heavier IWAT mass than controls and significantly elevated EWAT and RWAT masses compared with both the control and HED-Lean groups (all p<0.05). The HED-Lean rats had significantly heavier RWAT fat pads than controls (p<0.05); however, IWAT and EWAT did not significantly differ between the HED-Lean and control rats.
Fig. 1.
Mean (+/−) SEM IWAT, EWAT, and RWAT fat pad mass (* p<0.05 vs. Control, # p<0.05 vs. HED-Lean).
Spatial water maze
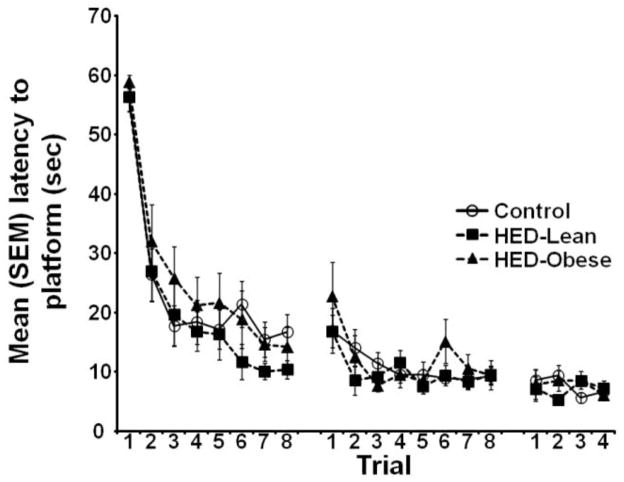
During acquisition of the water maze task, there was no significant effect of diet [F (2, 19) =1.812, p=0.174] or a significant diet by trial interaction [F (2, 38) =0.593, p=0.977]. There was, however, a significant effect of trial [F (2, 19) =44.743, p<0.05; Fig. 2], such that the latency to reach the platform significantly decreased across trials in all groups.
Fig. 2.
Mean (+/−) SEM latency to reach the escape platform during 3 days of water maze training. There were no significant effects of diet on acquisition.
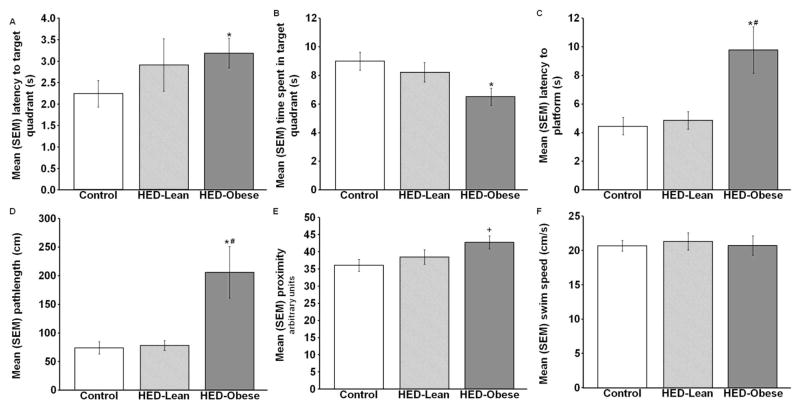
By contrast, on the probe test 48 hours later the HED significantly affected the latency to the target quadrant, the time spent in the target quadrant [F(2,49)=3.811, p<0.05], the latency to reach the platform location [χ2(2, N=33)=8.122, p<0.05], and the distance traveled to reach the platform (i.e. pathlength) [χ2(2,N=33)=7.739, p<0.05; Fig. 3A–D]. More specifically, compared with control and HED-Lean rats, the HED-Obese rats took significantly longer to reach the platform location [U=19.00, p<0.05 vs., control; U=14.00, p<0.05 vs. HED-Lean] and had significantly longer path lengths [U=20.00, p<0.05 vs. control; U=16.00, p<0.05 vs. HED-Lean] Compared with the control group, the HED-Obese group had longer latencies to reach the target quadrant [U=74.00, p<0.05] and spent significantly less time in the target quadrant (p<0.05). There was a tendency for the diet to effect the proximity to the platform [F(2,49)=3.117, p=0.053; Fig. 3E] and post-hoc analysis revealed that the HED-Obese group swam significantly farther away from the platform location over the 20 sec test than the control group (p<0.05). There were no significant differences among the groups in swim speed [F (2, 49) =0.098, p=0.907; Fig. 3F].
Fig. 3.
Mean (+/−) SEM A) latency to the target quadrant, B) time spent in the target quadrant, C) latency to the platform location, D) pathlength, E) proximity to the platform, and F) swim speed during the 20 second memory probe (* p<0.05 vs. Control, # p<0.05 vs. HED-Lean, + p<0.05 vs. Control; main effect p=0.053).
Plasma TG and hepatic lipid concentration
There was a significant effect of the HED on hepatic lipid concentrations [F (2, 37) =8.115; p<0.05; Fig. 4A]. The HED only significantly increased hepatic lipid concentrations in HED-Obese rats.
Fig. 4.
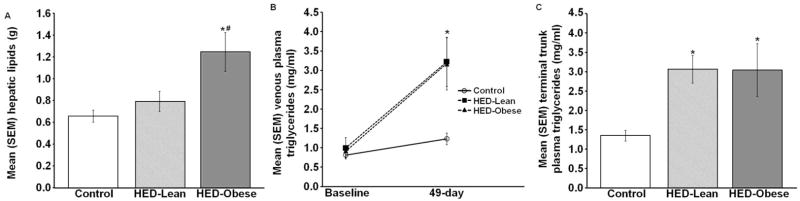
Mean (+/−) SEM A) hepatic lipid concentration, B) plasma TG concentrations 1 day prior and 49 days after the consumption of the control or HED, and C) terminal plasma TG concentrations after 8 weeks (* p<0.05 vs. Control, # p<0.05 vs. HED-Lean).
There was a significant interaction between the HED and time across the 8 week diet duration on plasma TG concentrations [F (2, 24) =9.242, p<0.05; Fig. 4B]. After 49 days on the diet, both the HED-Lean and HED-Obese groups had significantly higher plasma TG concentrations than the control group (both p<0.05). In addition, there was a significant effect of diet on terminal plasma TG concentrations [F (2, 23) =10.345, p<.05; Fig. 4C], such that the TG concentrations for both the HED-Lean and HED-Obese groups were significantly elevated compared with the control group (both p<0.05).
Correlations
Intake patterns during the first 5 days and over the 8 week duration also correlated significantly with memory, such that total caloric intake correlated significantly with most of the memory measures (all p<0.05; Table 2). More precisely, total caloric intake during the first 5 days and over the 8 weeks correlated positively with latency to reach the target quadrant, platform proximity and latency to the platform location and negatively correlated with the amount of time spent in the target quadrant. Lard intake during the first 5 days and over the 8 week duration correlated positively with pathlength (both p< 0.05), whereas sugar intake did not correlate with any of the memory measures at either time point (Table 2).
Table 2. Correlations between food intake and memory.
Pearson r correlations between calorie intake for the first 5 days and for 8 weeks, and multiple measures of memory in the spatial water maze.
| Time in target quadrant | Latency to target quadrant | Proximity | Pathlength | Latency to platform location | |
|---|---|---|---|---|---|
| Lard intake (kcal) | |||||
| For first 5 days | n.s. | n.s. | n.s. | r(19)=.468 p=0.043 |
n.s. |
| For 8 weeks | n.s. | n.s. | n.s. | r(19)=.470 p=0.040 |
n.s. |
| Sugar intake (kcal) | |||||
| For first 5 days | n.s. | n.s. | n.s. | n.s. | n.s. |
| For 8 weeks | n.s. | n.s. | n.s. | n.s. | n.s. |
| Total intake (kcal) | |||||
| For first 5 days | r(52)= −.303 p=0.029 |
n.s. | r(52)=.294 p=0.035 |
r(33)=.511 p=0.002 |
r(33)=.490 p=0.004 |
| For 8 weeks | r(52)= −.307 p=0.027 |
n.s. | r(52)=.294 p=0.034 |
r(33)=.565 p=0.001 |
r(33)=.519 p=0.001 |
Non-significant correlations are labeled n.s.
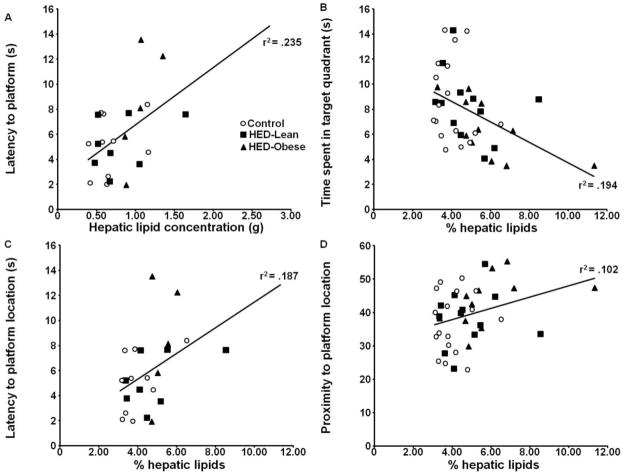
Elevations in liver lipids were significantly correlated with memory deficits (Fig 5A–D). The percentage of liver lipids correlated positively with latency to reach the target quadrant [r (40) =.353, p<0.05], platform proximity [r (40) =.319, p<0.05] and latency to the platform location [r (23) =.432, p<0.05] and negatively with the amount of time spent in the target quadrant [r (40) =−.440, p<0.05].
Fig. 5.
Scatterplots illustrating the correlation between liver lipids and A) latency to the target quadrant, B) time spent in the target quadrant, C) proximity to the platform location, and D) latency to the platform location.
Discussion
To the best of our knowledge, this is the first study to show that voluntary consumption of a HED impairs hippocampal-dependent memory and that individual differences in weight gain responses to the diet during the first 5 days on the diet predict liver lipid concentrations and memory deficits 8 weeks later. Specifically, rats that gain the most weight during the first 5 days of HED consumption are those that will 8 weeks later have the largest increase in fat pad masses and liver lipid content, and will manifest memory deficits. Memory was impaired only in the HED-Obese group that also had significant elevations in liver lipids. Moreover, liver lipids were significantly correlated with memory deficits.
HED-induced memory deficits were not likely due to ill health or other variables affecting performance (e.g., motoric changes, navigational problems), because the HED-Obese rats were not impaired during the training days and their swim speed was not altered on the memory test. The finding that there was no effect of the HED on the acquisition of the spatial water maze task suggests that the rats were able to learn and retain the location of the platform for short periods of time. Moreover, the fact that the deficits were observed exclusively on the retention test given 48 h after training suggests that the HED specifically impaired long-term storage and/or retrieval.
Our findings are consistent with previous research showing that HEDs impair hippocampal-dependent memory (4–6, 24, 25). The present data extend these previous findings by showing that these deficits occur in a model where rats determine the amount of high-energy foods and total calories that are consumed and nutritious alternatives are available. In addition to being relevant to human consumption patterns, this strategy allowed us to discover that weight gain during the first 5 days on the diet predicts the memory deficits. The voluntary consumption approach also is beneficial in that it also can unmask other effects of HEDs. For instance, voluntary consumption of a 50% high fat diet blunts stress-induced increases in adrenocorticotropic hormone and corticosterone levels, but involuntary consumption of the same diet does not (26).
There are likely several variables that cause some rats to be resistant to diet-induced increases in weight and obesity. A genetic component is supported by evidence showing that resistance to the obesity-inducing effects of high-energy diets is transmitted across generations (16). These genetic differences likely influence a number of variables, including sensitivity to the rewarding aspects of food and food cues (27), preference for dietary fat (20), motivation to eat (19), concentrations of hormones and adipokines (16–18), responsiveness to satiety cues (18), and metabolic processes (15–17).
The present findings showed that, compared to control rats, HED-Lean rats had increased caloric intake and fat mass, but did not have increased body mass. In rats, voluntary consumption of a high fat/high sugar diet increases activity levels and energy expenditure (28). This raises the possibility that HED-Lean rats had elevated activity levels and/or increased thermogenesis that prevented increases in body mass and that these increases in energy expenditure could not compensate for the even larger caloric intake observed in HED-Obese rats. This nutrient partitioning away from lean mass and toward adipose mass has been known for decades (e.g., 29) and can occur with a choice lard only (no sucrose) diet, with the choice diet animals significantly overeating and having greater total body fat but equivalent body mass (28).
An important benefit of employing the tertile split to characterize the different HED phenotypes is that it helps identify which of the many effects of over-nutrition contribute to the memory deficits. For instance, the HED significantly elevated plasma TG concentrations to similar levels in the HED-Lean and HED-Obese groups, but only the HED-Obese group had impaired memory. Therefore, the present findings indicate that elevated plasma TG concentrations are not sufficient to produce memory deficits, and perhaps do not contribute to the deficits. More importantly, the findings suggest that excess liver lipids are associated with the memory-impairing effects of HEDs because liver lipids correlated with memory deficits and only the HED-Obese group had significantly elevated hepatic lipid concentrations and met the clinical diagnostic criterion for NAFLD in humans (greater than 5% lipid concentrations in wet weight, 30, Control: x̄ = 4.05 ±0.22%; HED-Lean: x̄ = 4.87±0.43%; HED-Obese: x̄ =5.90±0.63%). This interpretation is consistent with evidence of memory impairments in self-reports from patients with NAFLD (31). Importantly, fatty liver, rather than obesity may be involved in the negative impact of HEDs on the brain because high fructose diets produce fatty liver (7, 32) and impair hippocampal-dependent memory (4, 7), but do not cause significant weight gain (7, 33).
The present study did not identify the mechanisms through which HEDs and fatty liver impair hippocampal-dependent memory. High fat diets and fatty liver induce metabolic and endocrine dysfunction, such as alterations in glucose tolerance (28), insulin and leptin responsiveness (10, 14, 28, 34, 35) and hypothalamic-pituitary-adrenal responsiveness (35). Moreover, HEDs and NAFLD can be associated with peripheral (36) and central inflammation (37) and oxidative stress (38). All of these alterations have been shown to negatively impact memory and thus could mediate the deleterious effects of fatty liver on cognitive function.
In summary, the current study demonstrates that weight gain during the first 5 days of a HED predicts liver lipid accumulation and hippocampal-dependent memory deficits 8 weeks later. To the best of our knowledge, this is the first study to demonstrate that voluntary consumption of a HED, at concentrations determined by the rats, impairs memory and that the memory-impairing effects of HED can be predicted. The present finding that excess liver lipids may contribute to the memory deficits is significant given that NAFLD is estimated to be prevalent in 30% of the population and is the most common form of liver disease (9). Finally, hippocampal lesions increase food intake (39, 40), which raises the possibility that HED-induced hippocampal dysfunction could lead to over-nutrition. Therefore, our findings that voluntary consumption of a HED impairs hippocampal-dependent memory may have important implications for both cognition and for the development and maintenance of obesity and fatty liver.
Acknowledgments
We want to thank Richard Austin Filitor, Courtney Gifford, and Jenine Ampudia for their assistance. This work was supported by a seed grant from the Georgia State University Brains and Behavior Program to MBP and NIH R01 DK35254 to TJB. Competing interests: the authors have no competing interests.
References
- 1.Obesity data and statitics: Obesity trends among US adults between 1985–2009. [cited 2011 February 11]; Available from: http://www.cdc.gov/obesity/data/trends.html.
- 2.Elias MF, Elias PK, Sullivan LM, Wolf PA, D’Agostino RB. Lower cognitive function in the presence of obesity and hypertension: the Framingham heart study. Int J Obes Relat Metab Disord. 2003;27:260–268. doi: 10.1038/sj.ijo.802225. [DOI] [PubMed] [Google Scholar]
- 3.Jeong SK, Nam HS, Son MH, Son EJ, Cho KH. Interactive effect of obesity indexes on cognition. Dement Geriatr Cogn Disord. 2005;19:91–96. doi: 10.1159/000082659. [DOI] [PubMed] [Google Scholar]
- 4.Ross AP, Bartness TJ, Mielke JG, Parent MB. A high fructose diet impairs spatial memory in male rats. Neurobiol Learn Mem. 2009;92:410–416. doi: 10.1016/j.nlm.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stranahan AM, Norman ED, Lee K, et al. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus. 2008;18:1085–1088. doi: 10.1002/hipo.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanoski SE, Davidson TL. Different patterns of memory impairments accompany short- and longer-term maintenance on a high-energy diet. J Exp Psychol Anim Behav Process. 2010;36:313–319. doi: 10.1037/a0017228. [DOI] [PubMed] [Google Scholar]
- 7.Ross AP, Bruggeman EC, Kasumu AW, Mielke JG, Parent MB. Non-alcoholic fatty liver disease impairs hippocampal-dependent memory in male rats. Physiol Behav. 2012;106:133–141. doi: 10.1016/j.physbeh.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis. Med Clin North Am. 1996;80:1147–1166. doi: 10.1016/s0025-7125(05)70483-1. [DOI] [PubMed] [Google Scholar]
- 9.Lazo M, Clark JM. The epidemiology of nonalcoholic fatty liver disease: a global perspective. Semin Liver Dis. 2008;28:339–350. doi: 10.1055/s-0028-1091978. [DOI] [PubMed] [Google Scholar]
- 10.Samuel VT, Liu ZX, Qu X, et al. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. 2004;279:32345–32353. doi: 10.1074/jbc.M313478200. [DOI] [PubMed] [Google Scholar]
- 11.Ruiz-Ramirez A, Chavez-Salgado M, Peneda-Flores JA, Zapata E, Masso F, El-Hafidi M. High-sucrose diet increases ROS generation, FFA accumulation, UCP2 level, and proton leak in liver mitochondria. Am J Physiol Endocrinol Metab. 2011;301:E1198–E11207. doi: 10.1152/ajpendo.00631.2010. [DOI] [PubMed] [Google Scholar]
- 12.Ai ZL, Zhu CH, Min M, et al. The role of hepatic liver X receptor alpha- and sterol regulatory element binding protein-1c-mediated lipid disorder in the pathogenesis of non-alcoholic steatohepatitis in rats. J Int Med Res. 2011;39:1219–1229. doi: 10.1177/147323001103900410. [DOI] [PubMed] [Google Scholar]
- 13.Farr SA, Yamada KA, Butterfield DA, et al. Obesity and hypertriglyceridemia produce cognitive impairment. Endocrinology. 2008;149:2628–2636. doi: 10.1210/en.2007-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mielke JG, Taghibiglou C, Liu L, et al. A biochemical and functional characterization of diet-induced brain insulin resistance. J Neurochem. 2005;93:1568–1578. doi: 10.1111/j.1471-4159.2005.03155.x. [DOI] [PubMed] [Google Scholar]
- 15.Dourmashkin JT, Chang CQ, Hill JO, Gayles EC, Fried SK, Leibowitz SF. Model for predicting and phenotyping at normal weight the long-term propensity for obesity in Sprague-Dawley rats. Physiol Behav. 2006;87:666–678. doi: 10.1016/j.physbeh.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Levin BE, Dunn-Meynell AA, Balkan B, Keesey RE. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am J Physiol. 1997;273:R725–R730. doi: 10.1152/ajpregu.1997.273.2.R725. [DOI] [PubMed] [Google Scholar]
- 17.Pagliassotti MJ, Knobel SM, Shahrokhi KA, Manzo AM, Hill JO. Time course of adaptation to a high-fat diet in obesity-resistant and obesity-prone rats. Am J Physiol. 1994;267:R659–R664. doi: 10.1152/ajpregu.1994.267.3.R659. [DOI] [PubMed] [Google Scholar]
- 18.Perez-Echarri N, Perez-Matute P, Martinez JA, Marti A, Moreno-Aliaga MJ. Serum and gene expression levels of leptin and adiponectin in rats susceptible or resistant to diet-induced obesity. J Physiol Biochem. 2005;61:333–342. doi: 10.1007/BF03167050. [DOI] [PubMed] [Google Scholar]
- 19.Pickering C, Alsio J, Hulting AL, Schioth HB. Withdrawal from free-choice high-fat high-sugar diet induces craving only in obesity-prone animals. Psychopharmacology (Berl) 2009;204:431–443. doi: 10.1007/s00213-009-1474-y. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Alexander JT, Zheng P, Yu HJ, Dourmashkin J, Leibowitz SF. Behavioral and endocrine traits of obesity-prone and obesity-resistant rats on macronutrient diets. Am J Physiol. 1998;276:E1057–E1066. doi: 10.1152/ajpendo.1998.274.6.E1057. [DOI] [PubMed] [Google Scholar]
- 21.Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress: feedforward and feedback effects of chronic stress. Endocrinology. 2004;145:3754–3762. doi: 10.1210/en.2004-0305. [DOI] [PubMed] [Google Scholar]
- 22.Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 23.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 24.Jurdak N, Lichtenstein AH, Kanarek RB. Diet-induced obesity and spatial cognition in young male rats. Nutr Neurosci. 2008;11:48–54. doi: 10.1179/147683008X301333. [DOI] [PubMed] [Google Scholar]
- 25.McNay EC, Ong CT, McCrimmon RJ, Cresswell J, Bogan JS, Sherwin RS. Hippocampal memory processes are modulated by insulin and high-fat-induced insulin resistance. Neurobiol Learn Mem. 2010;93:546–553. doi: 10.1016/j.nlm.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.la Fleur SE, Houshyar H, Roy M, Dallman MF. Choice of lard, but not total lard calories, damps adrenocorticotropin responses to restraint. Endocrinology. 2005;146:2193–2199. doi: 10.1210/en.2004-1603. [DOI] [PubMed] [Google Scholar]
- 27.Rada P, Bocarsly ME, Barson JR, Hoebel BG, Leibowitz SF. Reduced accumbens dopamine in Sprague-Dawley rats prone to overeating a fat-rich diet. Physiol Behav. 2010;101:394–400. doi: 10.1016/j.physbeh.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris RB, Apolzan JW. Changes in glucose tolerance and leptin responsiveness of rats offered a choice of lard, sucrose, and chow. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1327–R1339. doi: 10.1152/ajpregu.00477.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oscai LB, Brown MM, Miller WC. Effect of dietary fat on food intake, growth and body composition in rats. Growth. 1984;48:415–424. [PubMed] [Google Scholar]
- 30.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 31.Newton JL. Systemic symptoms in non-alcoholic fatty liver disease. Dig Dis. 2010;28:214–219. doi: 10.1159/000282089. [DOI] [PubMed] [Google Scholar]
- 32.Kawasaki T, Igarashi K, Koeda T, et al. Rats fed fructose-enriched diets have characteristics of nonalcoholic hepatic steatosis. J Nutr. 2009;139:2067–2071. doi: 10.3945/jn.109.105858. [DOI] [PubMed] [Google Scholar]
- 33.Ackerman Z, Oron-Herman M, Grozovki M, et al. Fructose-induced fatty liver disease: hepatic effects of blood pressure and plasma triglyceride reduction. Hypertension. 2005;45:1012–1018. doi: 10.1161/01.HYP.0000164570.20420.67. [DOI] [PubMed] [Google Scholar]
- 34.Marchesini G, Brizi M, Morselli-Labate AM, et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450–455. doi: 10.1016/s0002-9343(99)00271-5. [DOI] [PubMed] [Google Scholar]
- 35.Tannenbaum BM, Brindley DN, Tannenbaum GS, Dallman MF, McArthur MD, Meaney MJ. High-fat feeding alters both basal and stress-induced hypothalamic-pituitary-adrenal activity in the rat. Am J Physiol. 1997;273:E1168–E1177. doi: 10.1152/ajpendo.1997.273.6.E1168. [DOI] [PubMed] [Google Scholar]
- 36.Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836–1846. doi: 10.1002/hep.24001. [DOI] [PubMed] [Google Scholar]
- 37.Pistell PJ, Morrison CD, Gupta S, et al. Cognitive impairment following high fat diet consumption is associated with brain inflammation. J Neuroimmunol. 2010;219:25–32. doi: 10.1016/j.jneuroim.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Milne GL. Oxidative stress and inflammatory mechanisms in obesity, diabetes, and the metabolic syndrome. CRC Press; Boca Raton: 2008. The role of oxidative stress in diseases associated with overweight and obesity; pp. 33–46. [Google Scholar]
- 39.Davidson TL, Chan K, Jarrard LE, Kanoski SE, Clegg DJ, Benoit SC. Contributions of the hippocampus and medial prefrontal cortex to energy and body weight regulation. Hippocampus. 2009;19:235–252. doi: 10.1002/hipo.20499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henderson YO, Smith GP, Parent MB. Hippocampal neurons inhibit meal onset. Hippocampus. 2012 doi: 10.1002/hipo.22062. (in press) [DOI] [PubMed] [Google Scholar]