Abstract
Composite restorations in modern restorative dentistry rely on the bond formed in the adhesive-infiltrated acid-etched dentin. The physical characteristics of etched dentin are, therefore, of paramount interest. However, characterization of the acid-etched zone in its natural state is fraught with problems stemming from a variety of sources including its narrow size, the presence of water, heterogeneity, and spatial scale dependency. We have developed a novel homotopic (same location) measurement methodology utilizing scanning acoustic microscopy (SAM). Homotopic measurements with SAM overcome the problems encountered by other characterization/ imaging methods. These measurements provide us with acoustic reflectance at the same location of both the pre- and post-etched dentin in its natural state. We have applied this methodology for in vitro measurements on dentin samples. Fourier spectra from acid-etched dentin showed amplitude reduction and shifts of the central frequency that were location dependent. Through calibration, the acoustic reflectance of acid-etched dentin was found to have complex and non-monotonic frequency dependence. These data suggest that acid-etching of dentin results in a near-surface graded layer of varying thickness and property gradations. The measurement methodology described in this paper can be applied to systematically characterize mechanical properties of heterogeneous soft layers and interfaces in biological materials.
I. Introduction
The acid-etched human dentin surface plays a significant role in modern restorative dentistry. Composite restorations rely on the bond formed in the adhesive-infiltrated acid-etched dentin. The knowledge of the physical characteristics of etched dentin is therefore critical for understanding the bonding mechanism and behavior. However, the characterization of the acid-etched zone presents significant challenges. First, the high spatial heterogeneity induced by etching requires location-specific comparison to the dentin state before acid application. Second, the physical properties are very sensitive to intrusive specimen preparation and experimental conditions such as exposure to drying. Finally, scale-dependent physical properties arising from the acid-etching regime need to be measured using probes that provide various spatial resolutions. To address these problems, we performed homotopic measurements using scanning acoustic microscopy (SAM) to characterize the acid-etched dentin surface. The term homotopic (from the Greek homos, identical, and topos, place) has been introduced recently by the authors [1] to describe the methodology under which measurements of a set of material properties, for example physical and mechanical properties, are performed at the same location of the same sample. In SAM, measurements are performed in an aqueous environment, such that the etched dentin is maintained in its near-natural hydrated state. Because SAM is a non-destructive technique, the dentin surface from the same specimen can be characterized before and after etching. Moreover, homotopic measurements can be performed at different frequencies to reveal the scale-dependent physical characteristics of heterogeneous substrates while avoiding spatial mismatch when comparing measurements obtained before and after etching.
Here we describe the results obtained from SAM measurements of a dentin surface before and after etching. Using SAM, we are able to obtain the acoustic reflectance through precise homotopic measurements at selected locations using two broadband transducers of different central frequencies. Because SAM can be used over large regions at high resolutions, measurements were obtained at microscale resolution over the whole etched dentin sample such that different locations of the same substrate can be directly compared. These measurements show for the first time that different locations of the same substrate have different etching behavior. They also indicate that acid-etching of dentin results in severe alteration of its near-surface composition and microstructure. The reflection coefficients from SAM measurements are found to have a complex frequency dependence, which suggests that acid-etched dentin can be characterized as a near-surface graded layer of varying thickness and property gradations.
In the subsequent discussion, we first give a brief background of the significance of dentin acid-etching, its characterization, and the applicability of SAM. We then describe the specific methodology used for data acquisition and analysis. Finally, we discuss the obtained results.
II. Background
A. Acid-Etched Dentin’s Role in Restorative Dentistry
Since its inception, the problem of bonding composite restorations to dentin has behaved as the Lernaean Hydra. During dentin surface preparation, a layer of debris known as the smear layer is produced that proved to be the culprit for the premature failure of early resin composite restorations [2], [3]. To improve these restorations, a conditioning step before adhesive application was suggested in which the dentin surface is exposed to a phosphoric acid treatment for a short duration of few seconds [4]. Although the phosphoric acid-etching step served its intended purpose of removing the smear layer from the dentin surface, it also completely altered the near-surface of the exposed dentin creating a new and unknown substrate, different from the native tissue. The unexpected outcome of the etching regime was the removal of the minerals from the first few micrometers of dentin, leaving behind a porous collagen scaffold [5], [6]. The resulting interface formed by the subsequent application of the dentin adhesive system was a highly heterogeneous composite of collagen, mineral, and adhesive monomer, known as the hybrid layer [7]. Consequently, researchers in clinical dentistry have been working to understand the effects that acid-etching imposes on dentin and its relationship to bonding efficiency, and to develop alternative conditioning techniques and adhesive formulations that are compatible with the etched substrate.
B. Acid-Etched Dentin Characterization
Several techniques have been used to investigate acid-etched dentin. High-resolution scanning electron microscopy (SEM) and transmission electron microscopy (TEM) observations showed that the near-surface layer of acid-etched inter-tubular dentin was separated into three distinct sub-layers [8]. The first layer was comprised of collapsed disorganized collagen that could potentially inhibit adhesive infiltration [2], [9], [10]. Beneath it, the collagen showed partial structure with sparse instances of residual mineral. The third layer was a partially demineralized zone that transitioned to the deeper native dentin. Attempts have been made to quantify the dimensional changes that occur in dentin during demineralization and to relate them to the mineral density distribution as a function of depth using atomic force microscopy (AFM) and X-ray tomography [5]. This work suggested that the tubule orifices become wider and the mineral density distribution is affected by two different rates of demineralization. The extent of demineralization of the acid-etched zone as well as the effects on the tubule orifice widening has been shown to vary with the conditioner used, the concentration of the conditioner, time of exposure, and technique [11]—[13]. Furthermore, the extent of demineralization has been shown to depend on the substrate, because dentin may be sclerotic or affected by caries [14], [15].
The profile and degree of inter-tubular dentin demineralization under short-term clinically relevant exposure to acid-etching has been most effectively shown using infrared and micro-Raman spectroscopic techniques [16]—[21]. Results from these studies have shown clearly that the adhesives were unable to completely infiltrate the dentin’s etched surface. Roughness and surface recession of acid-etched dentin have been observed using AFM and profilometry [11], [22], [23]. However, surface topography measurements do not provide information regarding the changes in the subsurface alteration of composition and structure.
Mechanical property measurements of etched dentin are scarce in the literature. AFM-based indentation has been used under small deformations to obtain the viscoelastic and elastic values of demineralized dentin [24]. According to their work, the Young’s modulus of elasticity of hydrated demineralized dentin was ~0.2 MPa, whereas that for de-hydrated demineralized dentin was ~2 GPa. Indentation studies are very challenging for substrates such as etched dentin and the interaction of the indent with soft graded substrates is not well defined.
These techniques are imperfect either because they alter the material state during sample preparation before imaging or because the data interpretation is based upon models that ignore the material’s complexity. Although these techniques provide highly localized measurements, they do so over a very limited area. As a result, the information of how the different regions of a substrate are affected by the same treatment is obscured. These techniques also preclude pre- and post-etching comparisons of the same locations.
Although imperfect, these characterization techniques have increased our qualitative understanding of the morphological and ultra-structural characteristics of acid-etched dentin; however, a gap remains in our knowledge of the physical properties of acid-etched dentin. This gap is compounded by the fact that natural materials have varying structure and composition at different spatial scales, which is ultimately responsible for the way material properties are manifested in experimental measurements.
C. Scanning Acoustic Microscopy
Scanning acoustic microscopy appeared in the early 1970s as a new research technique that could provide measurement of mechanical properties of a substrate at spatial resolutions comparable to that of optical instruments [25]. Since then, it has received considerable attention and has been used in numerous applications for imaging as well as quantitative characterization [26], [27]. Applications of SAM to dental tissues have been limited. In the earliest efforts to apply SAM to dental materials, carious enamel lesions were imaged at a spatial resolution of approximately 4 µm [28], [29]. Even though the work was largely qualitative, the sensitivity of SAM in detecting small changes in elastic properties of enamel and the existence of an intimate relationship to the level of demineralization was recognized. The viscoelastic properties of dental hard tissues have been investigated using line-focus and point-focus objectives and it was found that the surface acoustic wave velocities and attenuations showed a variation with frequency, position, and direction for both enamel and dentin [30]. More recently, SAM at 25 to 100 MHz was used for obtaining acoustic impedance images of teeth [31]. At 50 MHz, longitudinal wave velocities in dentin were measured using SAM in reflectance mode and time-of-flight. In mantle dentin near the enamel, the longitudinal wave velocities were found to be 7% to 8% less than in bulk dentin [32]. SAM at 400 MHz was used to infer the mechanical properties of mineralized, partially demineralized, and completely demineralized dentin through a series of calibration curves [33], [34]. The effect of storage media on the acoustic properties of enamel and dentin has been evaluated with SAM at 50 MHz [35]. None of these studies have investigated the frequency-dependent reflectance of acid-etched human dentin.
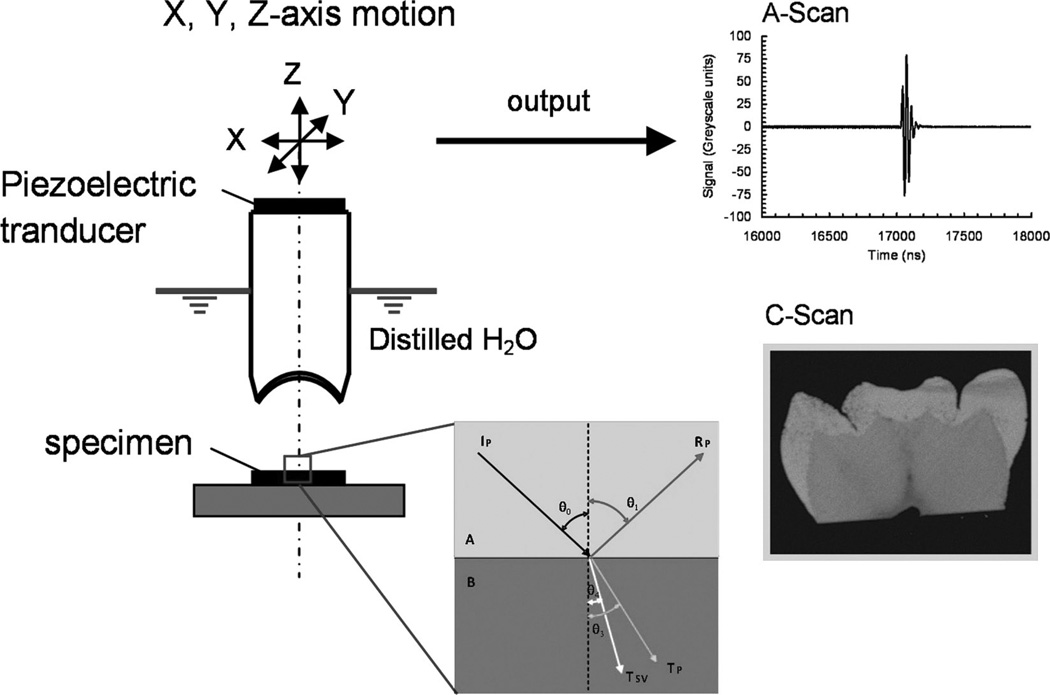
The principle of acoustic microscopy is well known. An acoustic wave-field is generated by a piezoelectric element and focused onto a specimen by a lens. During SAM measurements, the specimen is typically immersed in water (or other suitable coupling fluid). In reflection acoustic microscopy, the ultrasonic field interacts with the near-surface layer of the material in question and part of it reflects back through the coupling medium to the lens. The lens redirects the returned field to the piezoelectric element. The deformation of the piezoelectric element creates a potential difference which is displayed by an oscilloscope as a waveform. Such waveforms are called A-Scans. The element and the lens are located in the same housing, which is termed the ultrasonic objective. In SAM, the objective is coupled with an x-y-z positioning system that enables a raster scan over the specimen surface. As the objective is scanned over the specimen, an A-Scan signal is acquired for each scanned location and selected signal parameters may then be displayed as a grayscale image, termed a C-Scan. A schematic showing the SAM setup used is shown in Fig. 1.
Fig. 1.
Schematic of SAM measurement setup.
The spatial resolution of the generated acoustic field is a function of the operating frequency and the lens dimensions. Consequently, it is possible to successively interrogate finer material volumes by using ultrasonic objectives of increasing frequency. The ability to interrogate different material volumes allows for characterization of complex substrates with varying microstructures. These substrates are known to exhibit dissipative properties that manifest as a dispersion or spreading of acoustic pulses as they propagate through the thickness of a material [36]. A related wave propagation phenomenon is the frequency dependence of reflection coefficients. In this work, we exploit this acoustic wave/substrate interaction phenomenon and the non-destructive capabilities of SAM to investigate the frequency-dependent reflectance of the acoustic signals from acid-etched human dentin.
III. Materials and Methods
A. Dentin Sample Preparation and Storage
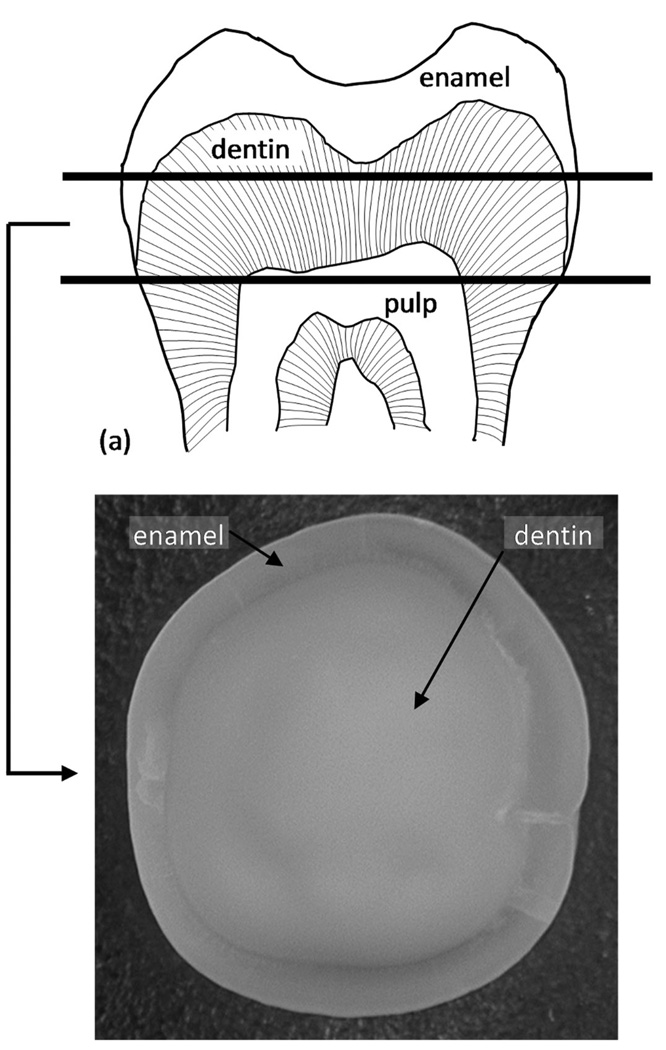
A human unerupted third molar with no visual caries was used under an institutional review board (IRB) approved protocol. The project only involved teeth that were already treatment planned for extraction for other reasons. The only exclusion criteria were teeth that were fractured during extraction. The teeth were not identified by patient number or name. Each patient signed a patient consent form when signing the treatment plan. The specimen preparation proceeded as follows: first the root was sectioned perpendicular to its long axis to create a surface for mounting the tooth on to a metal stub, subsequently, the occlusal crown was removed and, finally a second section at the cervical third, right below the neck of the tooth, was made, resulting in an approximately 5-mm-thick crown segment, as shown in Fig. 2. We note that the pulpal side of the crown segment was not ground to maintain the parallelism of the top and bottom surfaces. The sectioning was performed using a water-cooled low-speed diamond saw (Buehler, Lake Bluff, IL). To remove surface irregularities such as saw marks and the smear layer caused by the diamond saw, the specimen was polished successively by 600- and 1200-grit-size abrasive papers followed by a few passes with a polishing cloth (Buehler). An alternative protocol for removing the smear layer was evaluated which included microtoming an approximately 5-µm section from the surface. Microtoming was evaluated because previous researchers have suggested that polishing has a tendency to alter the surface microstruc-ture by smearing the collagen and mineral [37]. We found that micro-toming generally resulted in some damage of the dentin structure and even more at the vicinity of the dentin-enamel junction (DEJ). In contrast, hand polishing using 600 to 1200 grit paper produced uniform and smooth surfaces for SAM measurement. We also found that the results from SAM were not affected, provided the polishing was done by applying a light pressure and the surfaces are rinsed clean promptly after polishing. For the acid-etching studies, the tooth was etched for 20 s using 35% phosphoric acid (H3PO4). The phosphoric acid was thoroughly rinsed with distilled water. When the specimen was not in use, it was stored in phosphate-buffered saline solution with 0.002% sodium azide, thus keeping it constantly hydrated.
Fig. 2.
(a) Schematic of the vertical section along the long axis of a 3rd molar. The thick horizontal lines indicate the slab that was sectioned for use in this work. (b) Optical image of the 3rd molar slab.
B. SAM Calibration
Elastic property characterization using acoustic microscopy is typically based upon surface acoustic wave velocity measurements. However, there are cases where the aperture of the ultrasonic objective does not favor the generation of leaky Rayleigh waves or any other type of surface waves from the specimen. In such cases, one relies only on the reflected signal amplitude to recover the elastic properties of the material. Numerous researchers have used SAM in pulse-echo mode to directly measure material reflection coefficient and, consequently, acoustic impedance [33], [34], [38]-[41].
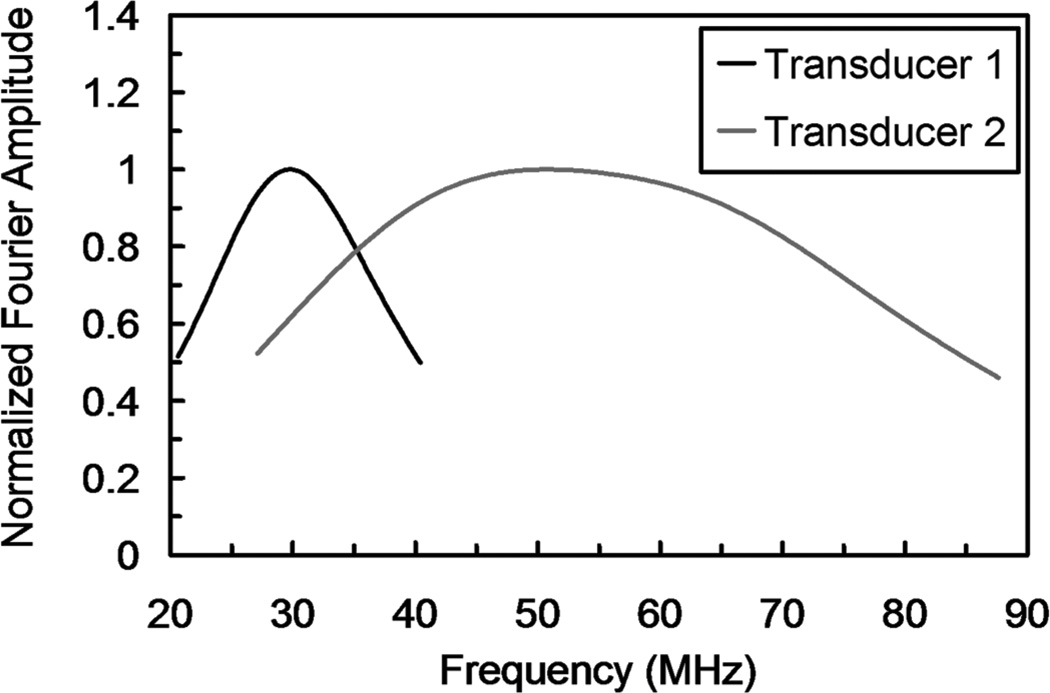
A commercially available SAM (WINSAM 100, Kramer Scientific Instruments GmbH, Herborn, Germany) was used in pulse-echo mode. Two ultrasonic objectives were used, with piezoelectric elements vibrating at resonant frequencies of 30 MHz (KSI PT30-002) and 110 MHz. In the subsequent discussion, we will refer to the KSI PT30-002 objective as Transducer 1 and the 110-MHz objective as Transducer 2. The transducer specifications are given in Table I. To propagate the ultrasonic signal to the specimen, distilled water was used as the coupling fluid. The temperature of the coupling fluid was monitored and was found to be 22 to 24°C. The Fourier amplitude spectra for the two objectives are shown in Fig. 3.
TABLE I.
Nominal Specifications of Ultrasonic Objectives Used.
| Focal length (mm) |
Lateral resolution (µm) |
Half-aperture angle (°) |
Frequency range at —6-dB amplitude (MHz) |
|
|---|---|---|---|---|
| Transducer 1 | 12.7 | 100 | 13.4 | 20.6 to 40.3 |
| Transducer 2 | 8.0 | 50 | 10.0 | 27.1 to 87.5 |
Fig. 3.
Fourier amplitude spectra of the two ultrasonic objectives used in this study.
For soft materials, such as demineralized dentin, the reflected signal amplitude is weak and high amplification settings have to be used to obtain measurable signals. This received signal is influenced by the saturation effects of system electronics at high amplifications. The accuracy of the calculated properties is greatly compromised if the saturation effects are not properly considered. Therefore, calibration relationships were developed based upon a methodology that can distinguish the effect of system electronics from the material response [1]. To obtain the calibration relationships, a set of materials was used that provided a wide range of reflection coefficients.
The reflection coefficients of these materials were independently determined and juxtaposed with the corresponding Fourier amplitudes measured in SAM over the frequencies spanning the —6-dB bandwidth of each objective. The resulting frequency- dependent calibration relationships can be used to obtain the reflection coefficient of an unknown material using the following best-fit relationship:
| (1) |
where V is the measured Fourier amplitude of the unknown substrate, [ineq] is the measured Fourier amplitude of the tungsten target, R is the reflection coefficient of the unknown substrate, RW is the reflection coefficient of the tungsten target, and a1(ω) and a0(ω) are frequency-dependent fitting constants. The applicability of the calibration relationships to predict low-reflection-coefficient (softer) materials that lie outside the range used for calibration was tested. To that end, the reflection coefficients for LDPE and TPX, which are low-moduli polymeric materials, were predicted and compared with those obtained through independent measurements.
We note that in acoustic wave propagation, the reflection coefficient is strictly defined as the complex amplitude corresponding to a reflected plane wave. The focused pressure wave-field that emanates from the ultrasonic objective in SAM can be considered as a superposition of plane waves that are propagating at various directions of incidence. As these plane waves reflect from the surface of the substrate, they are modified by the substrate reflectance function, which in general is a function of both angle and frequency. Thus, the reflection coefficient that is obtained for the unknown substrate through the calibration is the magnitude of an average reflection coefficient over all of the angles of incidence. This reflection coefficient varies from zero to 1, where an interface with reflection coefficient of 1 is a perfect reflector.
C. Homotopic Measurement Protocol
Dentin substrates inherently vary from location to location and respond in a heterogeneous manner to surface treatments. To characterize the heterogeneity of the acid-etching process, the specimen was measured in SAM both before and after acid-etching. Because the etching process could not be performed in situ, it was necessary that the specimen be moved and placed back in SAM. Moreover, because multiple ultrasonic objectives were used, it was necessary for the lower resolution measurement to contain the region measured with the higher resolution. Therefore, for meaningful comparison between the pre- and post-acid-etched states of the specimen, an acquisition protocol and location system was developed that allowed us to perform homotopic measurements while moving the sample from its original position within SAM or using different objectives [42].
The homotopic measurement methodology used in this work does not rely on image matching and processing that is typically associated with methods such as image registration, data fusion, or data integration. The main drawback of these image-based methods is that the matching of regions of interest (ROI) is performed directly on the images post-data acquisition. In these methods, for images with a given pixel size (which are related to the resolution of the imaging modality), the distances between locations in the ROI that have undergone rigid body motions are generally not preserved. Thus the overlap of the ROI, and more importantly the overlap of the material volumes represented by each pixel on the image, is not guaranteed. Clearly, if homotopic measurements are not made and image-based methods are used instead, both the quantitative before and after comparison and the comparison of information at different resolutions, as is required in this study, can be highly erroneous.
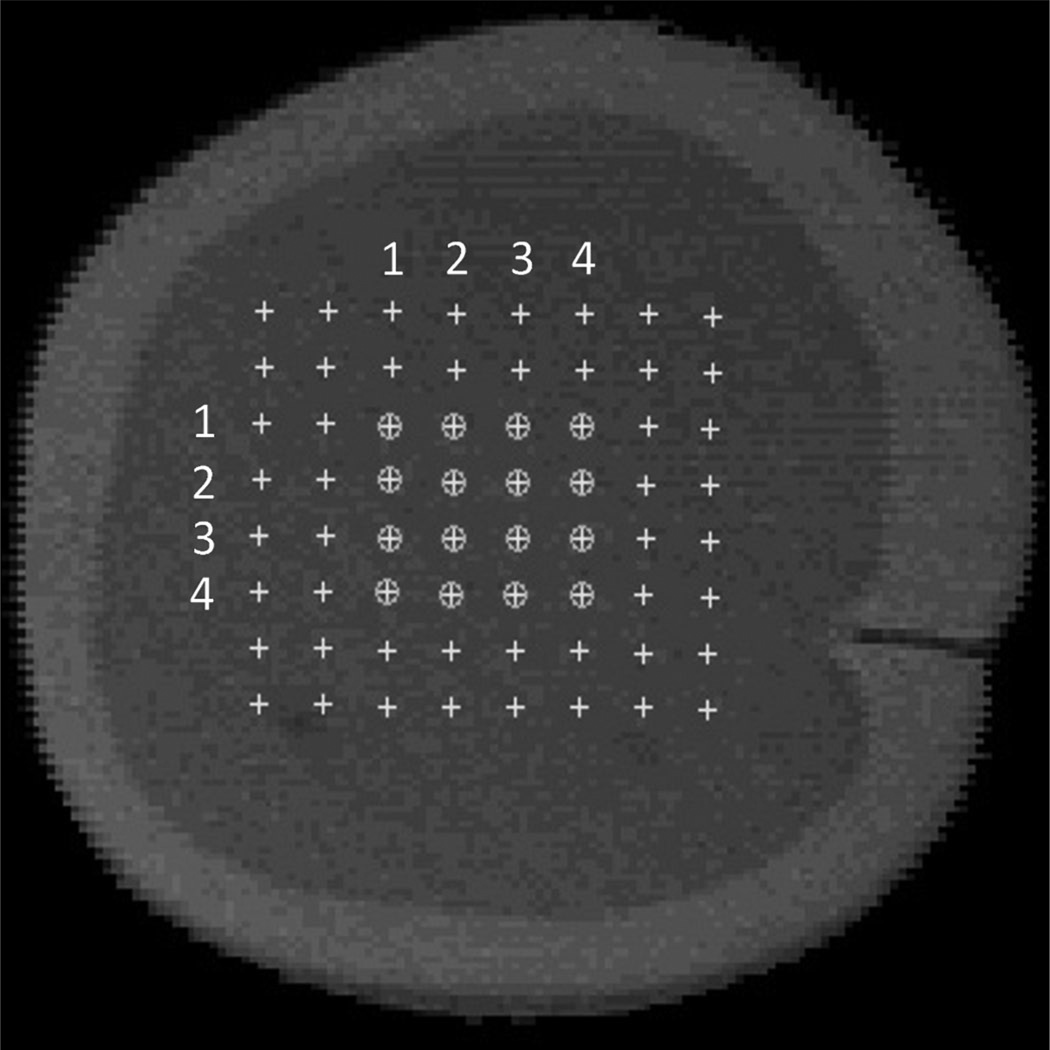
The data acquisition was completed in two phases: 1) a pre-acid-etching phase, and 2) a post-acid-etching phase. Before acid-etching, sixty-four locations of interest were identified over the specimen surface, arranged as shown in Fig. 4 and spaced at 664 µm. At each of the 64 locations, 32 waveforms were acquired. Care was taken so that the acoustic field was focused on all of the selected locations.
Fig. 4.
SAM grayscale image showing the 64 locations spaced at 664 µm where waveforms were acquired with transducer 1 (denoted by cross) and the subset of 16 locations where waveforms were acquired with transducer 1 and 2 (denoted by cross within a circle).
Transducer 1 was then replaced by transducer 2. Out of the 64 locations, the central subset of 16 locations was selected and 32 waveforms were acquired over each of these locations. At the completion of the pre-acid-etching phase, the specimen was removed from SAM and acid-etched as described in Section III-A. After etching, the specimen was placed again in SAM and the same procedure as the pre-acid-etching phase was followed. During this process, images were used as a guide to recover the initially measured locations. Once the point of interest was relocated, the measurement was performed. This is a key distinction from the image-based methods in which the measurements are done first and the attempt to match images performed later. As discussed previously, the image matching could lead to errors. Using our homotopic measurement methodology, it was possible to relocate the point of interest with a precision that is within 10% of the spatial resolution of the ultrasonic objective, as determined by repeated relocation of points of interests.
D. Data Analysts
Following data acquisition, the 32 waveforms acquired at each location were averaged, gated and Fourier transformed. The amplification setting for the data acquired with transducer 1 was 6 dB, whereas for transducer 2 the amplification setting was set to 24 dB. These amplification settings were chosen so that the signal-to-noise ratio was sufficient and the saturation effects caused by the electronics were not excessive. The calibration relationships corresponding to these gain settings were retrieved and by inverting (1), the measured Fourier amplitudes were converted to reflection coefficients over the useful frequency bandwidth of each transducer.
IV. Results and Discussion
A. C-Scans and Waveforms
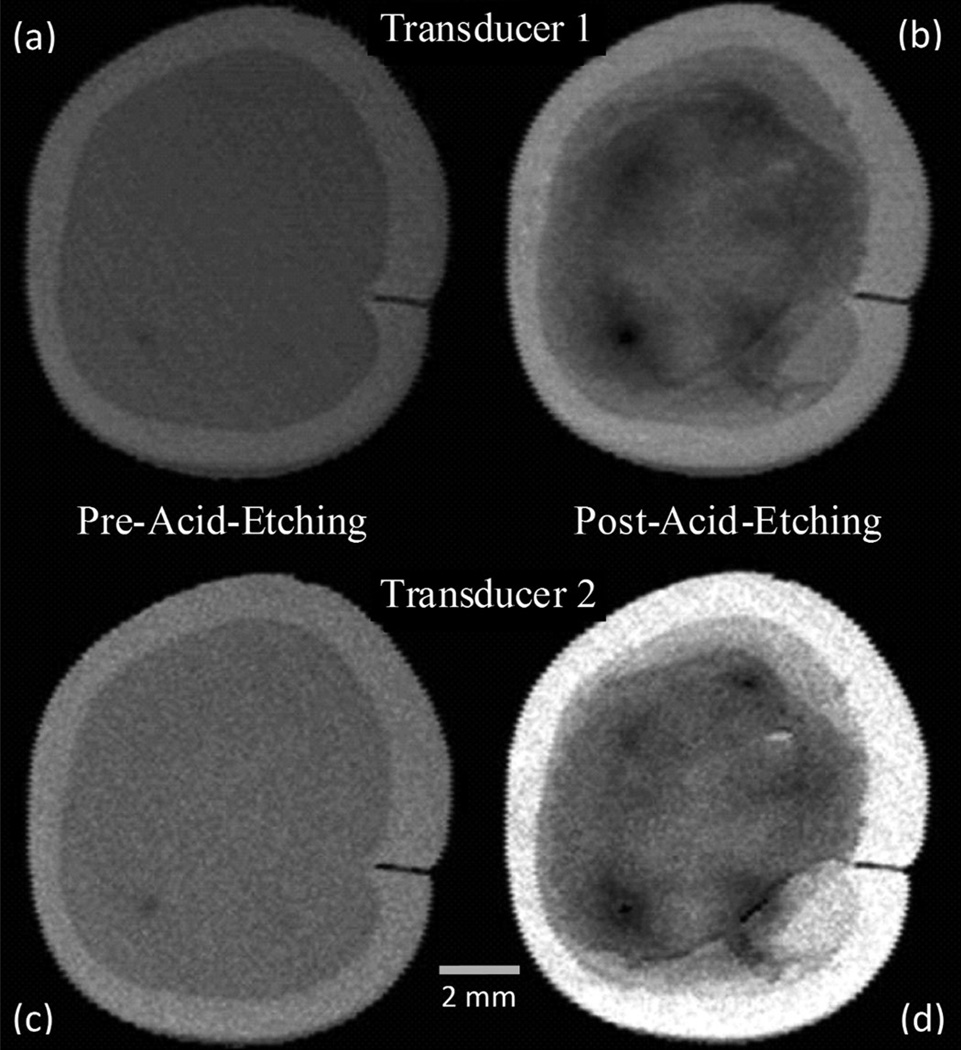
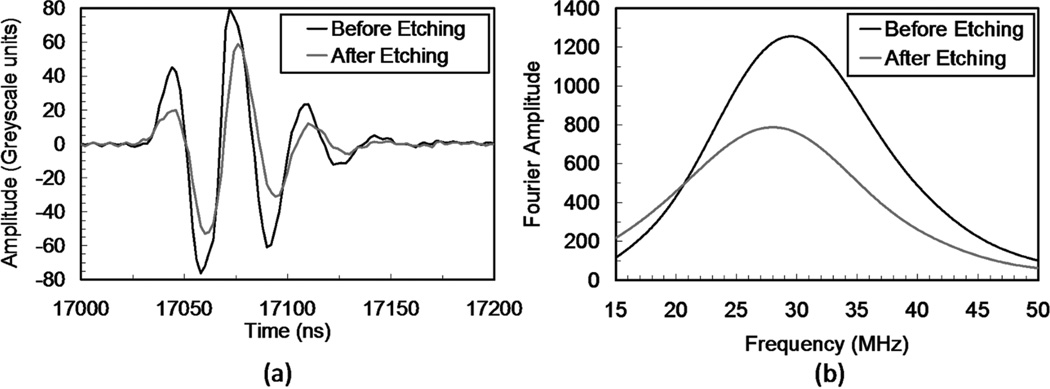
C-Scans for the pre- and post-acid-etching states for both transducers are compared in Fig. 5. The brightness of the images was uniformly increased to highlight the alterations caused by acid-etching. In the images shown in Fig. 5, the outer, lighter ring is the enamel, and the inner, darker region is the dentin. Before acid-etching, the dentin surface was homogeneous in terms of grayscale values. However, stark differences were observed after acid-etching. After etching, different regions on the dentin surface appeared to have varying grayscale values, which indicate a change of the reflectance of the substrate in a location-dependent manner. The A-scan signal amplitudes after etching were significantly lower than before acid-etching at the same locations, as shown in Fig. 6.
Fig. 5.
C-Scan images of dentin substrate using transducer 1 (central frequency 30 MHz) (a) before and (b) after acid-etching, and using transducer 2 (central frequency 50 MHz) (c) before and (d) after acid-etching.
Fig. 6.
(a) Representative reflected signal waveforms and (b) their Fourier amplitude spectra for transducer 1 before and after acid-etching of the dentin substrate.
B. Fourier Amplitude Spectra
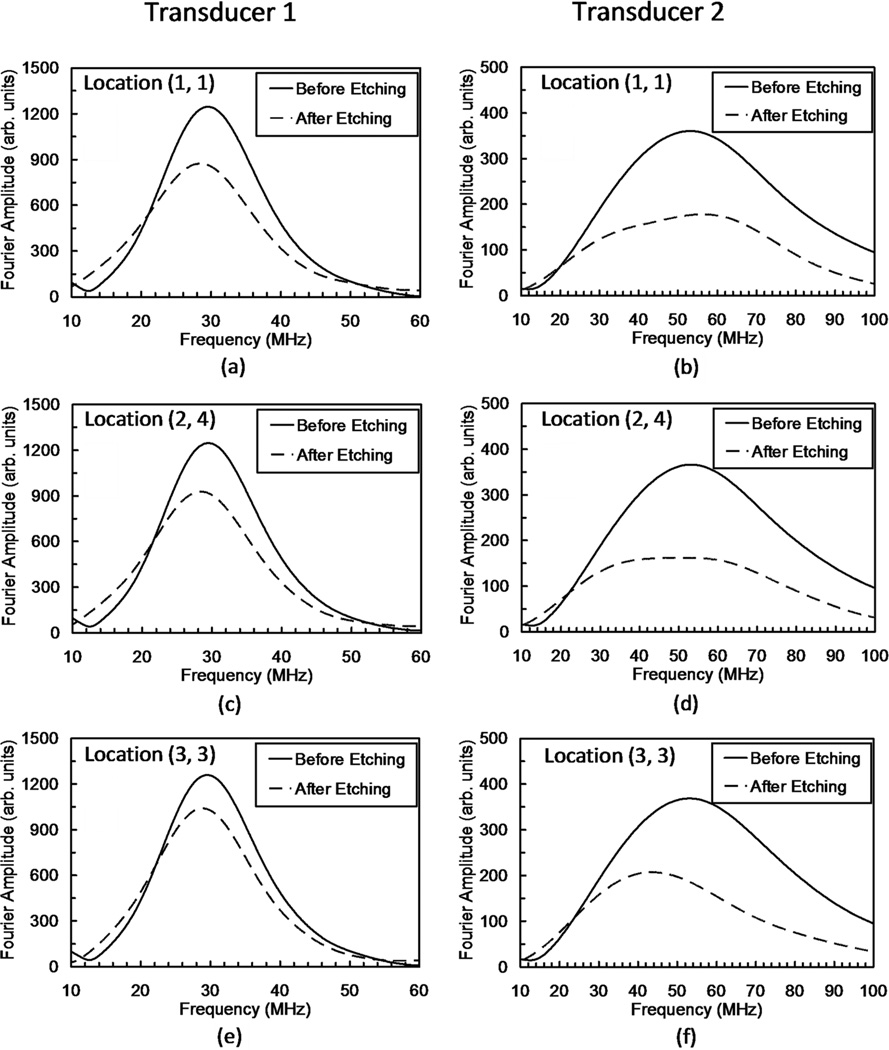
To further understand the differences between locations of the dentin substrate before and after acid-etching, we examined the Fourier amplitude spectra of the subset of the 16 locations where waveforms were obtained from both transducers. Fourier amplitude spectra from three selected locations following the grid-numbering scheme of Fig. 4 are shown in Fig. 7. These three locations were chosen because they are representative of the observed frequency dependence. The Fourier amplitude spectra from transducer 1, before etching, showed minor differences. After etching however, a general reduction in the overall amplitude was observed accompanied by a downshift of the peak frequency that differed depending upon the location. For transducer 2, the Fourier amplitude spectra revealed remarkable differences. Whereas before etching the Fourier amplitudes were almost identical at all locations, after etching, each location showed a different frequency behavior. Although a reduction of the Fourier amplitudes was consistently observed, the frequency shift behavior was highly variable between locations. For example, at location (1,1) the peak frequency was up-shifted, whereas at location (2, 4) the amplitude spectrum exhibited a plateau over a large range of frequencies (35 to 60 MHz). At location (3, 3) a downshift of the peak frequency was noted.
Fig. 7.
Fourier amplitude spectra of reflected signal waveforms for 3 representative locations.
C. Reflection Coefficients
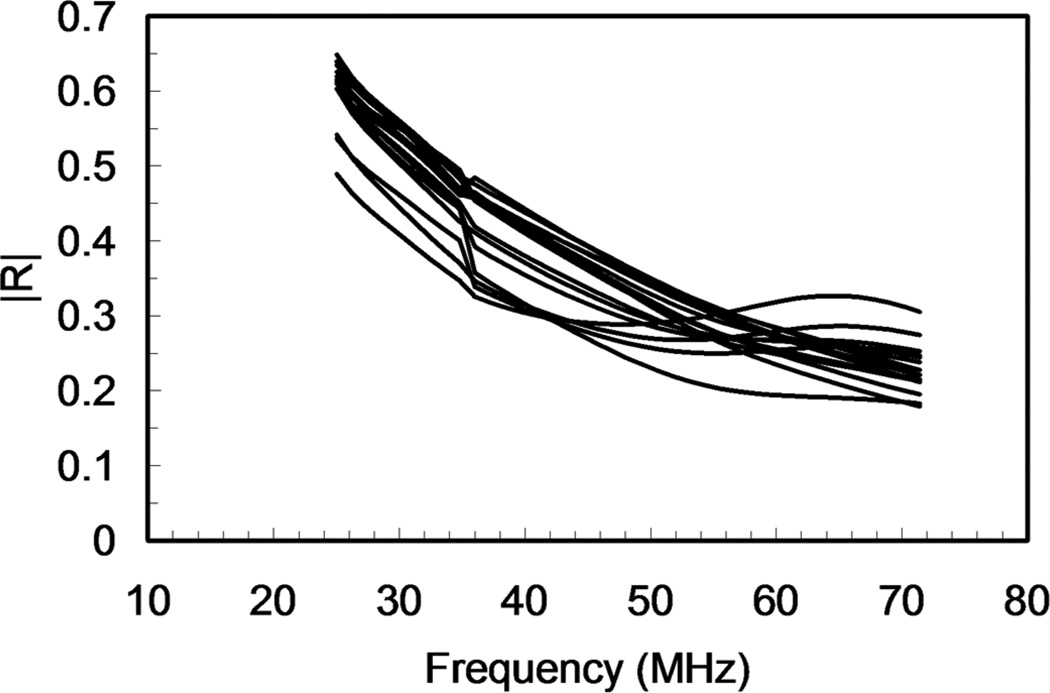
For quantitative comparison, the reflection coefficients as a function of frequency were determined using (1) for the subset of the sixteen locations. Because the useful bandwidths of the transducers overlapped, as shown in Fig. 3, the reflection coefficients for all 16 locations were plotted as a function of frequency for both transducers on the same graph as shown in Fig. 8. As a result, frequency-dependent reflection coefficients for the range of from 25 to 70 MHz were obtained. As shown, all of the locations after etching were generally different in terms of their reflectance. For example, at a frequency of approximately 25 MHz, the reflection coefficient ranged between 0.49 and 0.65. This is a significant difference, especially because the measurement error in the mean reflection coefficient is approximately 0.01, based upon an error analysis of (1) [43]. A similar range of the reflection coefficient amplitude was observed over all of the frequencies of the combined result. Interestingly, in the range between 40 and 70 MHz, several locations showed a non-monotonic behavior in the amplitude of the reflection coefficient.
Fig. 8.
Reflection coefficients versus frequency for the subset of 16 locations post-etching.
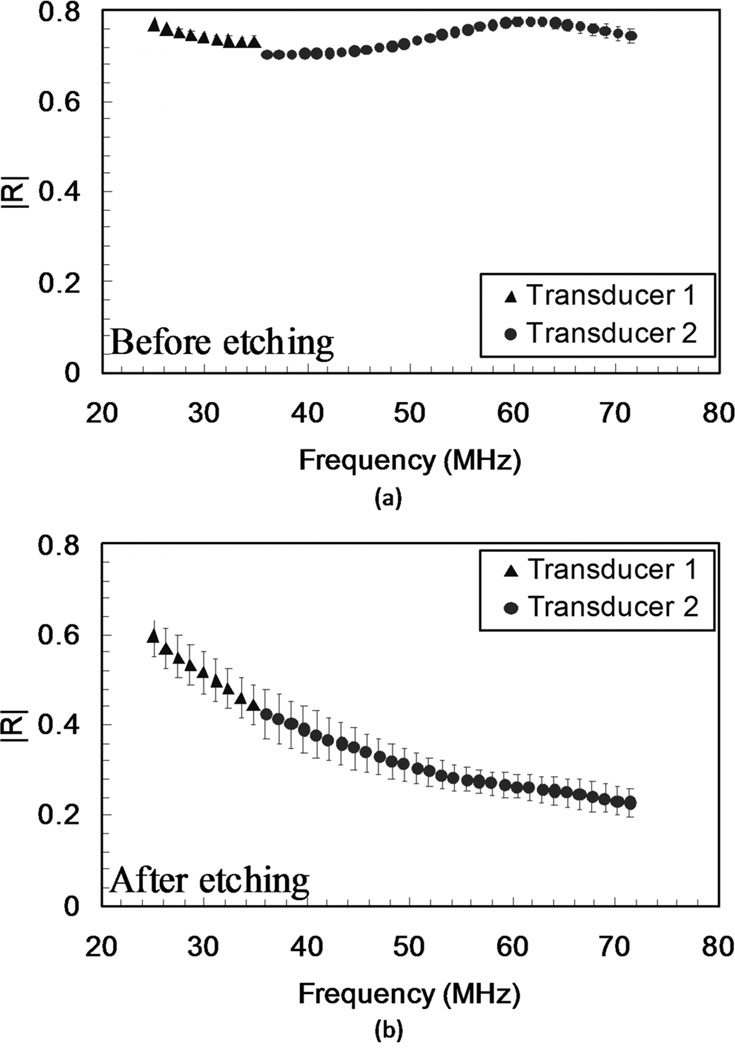
In Fig. 9, we plot the average reflection coefficient of the sixteen locations shown in Fig. 8. Each data point represents the spatial average of the 16 locations and the error bars show their spatial standard deviations. The average reflection coefficients confirm our previous observations. Before etching, the reflection coefficient was bounded between 0.7 and 0.8 over the frequency range 25 to 70 MHz. The standard deviation at each frequency was less than 0.01. However after etching, the average reflection coefficient showed a gradual reduction and ranged between 0.6 and 0.2, and the spatial standard deviation increased to approximately 0.04, which indicates the location-dependent reflectance. It is noteworthy that the average behavior can be very misleading, because it obscures the phenomena that are location-specific.
Fig. 9.
Average reflection coefficients as functions of frequency (a) before etching and (b) after etching for the subset of 16 locations.
Because the reflection process involves interaction with a material volume of dimensions comparable to an acoustic wavelength (wavelength in the coupling fluid for SAM, which is ~20 to 75 µm for our testing), severe attenuation must occur for frequency dependence to be detected.
For example, it has been shown that for viscoelastic polymeric materials, the contribution of attenuation to the reflection coefficients becomes important as the contrast between the elastic reflection coefficients becomes small [44]. Significant attenuation in the reflection can occur for substrates whose near-surface properties vary with depth, as is expected for acid-etched dentin, especially when the layer thickness is comparable to the acoustic wavelength. Attenuation of reflectance can also occur in the presence of roughness [45] or lateral microstructure. These attenuation mechanisms are sensitive to the acoustic wavelength of the incident wave and the characteristic size of the graded layer or the surface roughness. For the frequency (acoustic wavelength) range used in our SAM measurements, we expect that the attenuation and the non-monotonic dependence of reflection coefficients on frequency can be explained by the presence of a near-surface graded layer. Surface roughness is likely to have a small effect, considering that etching-induced mean roughness and surface recession have been found to be less than 1 micrometer, based upon AFM and conventional profilometry of demineralized dentin [11], [22], [23], [46]. Theoretical models of ultrasonic wave propagation in substrates with graded near-surface properties can be used to quantify property gradation as well as layer thickness or surface roughness. The theoretical analysis will be presented in a future publication.
The spatial variability of the frequency-dependent attenuation behavior suggests that depth and extent of etching is location-dependent, such that the result of etching can be described as a layer of varying thickness (up to ~100 µm) and property gradations (from ~2 GPa of demineralized collagen at the top to ~30 GPa of the native dentin at the bottom of the graded layer). Such large spatial variability of etched dentin is likely to be explained by the native local microstructural organization, including the shape, size, and orientation of apatitic crystallites as well as the diameter, orientation, and density of dentinal tubules. For the sample used in this study, the central zone is likely to be further from the pulp chamber than the peripheral regions. In this case, the microstructural organization of the central zone is expected to be different than that of the peripheral regions. Although the effect of the microstructural features before etching is insignificant, the effect could be large after etching because of the likely higher permeability and etch potential of the peripheral region. From a clinical viewpoint, the adhesive is required to infiltrate and bond in this highly variable environment. It is therefore not surprising to find un-infiltrated, exposed collagen below the hybrid layer in composite restorations. The exposed collagen becomes a weak link in the adhesive bonding of composite restorations [47].
V. Conclusion
The physical characteristics of etched dentin are of clinical interest because composite restorations rely upon the bond formed by the etched dentin and adhesive. There are considerable obstacles in characterization of the acid-etched zone in its natural state using traditional imaging methods such as SEM, TEM, and AFM. These techniques either alter the material state during sample preparation before imaging or their data interpretation is based upon models that ignore the material’s complexity. In addition, these techniques do not provide the mechanical properties. In this paper, we have described a novel homotopic (same location) measurement methodology using SAM to characterize acid-etched dentin. We find that SAM overcomes the problems encountered by other imaging/characterization methods because of its non-destructive nature and the ability to perform measurements in an aqueous environment at a range of frequencies so as to reveal the scale-dependent characteristics of the material. Furthermore, the homotopic measurement methodology utilized in this work allows meaningful comparison between two different states of the same substrate, as is the case for acid-etched dentin. The homotopic measurement methodology is essential when comparisons are performed with techniques capable of localized measurements, such as SAM and indentation, or when quantitative multi-scalar characterizations of heterogeneous medium are required. The homotopic measurement methodology differs fundamentally from the image-based methods, such as image registration, data fusion, and data integration.
Using SAM, C-scans and detailed waveforms were acquired from the same dentin sample before and after acid-etching. These measurements provided us with acoustic reflectance at the same location of the pre- and post-etched dentin surface over a range of frequencies. We find that by performing the measurements over the chosen range of frequencies, we can detect the near-surface graded nature of the acid-etched dentin. Acoustic reflectance of acid-etched dentin was found to have significant spatial variation and attenuation compared with native dentin. These results show for the first time that different locations of the same substrate can have vastly different etching behavior. Additionally, the observed frequency dependence suggests that acid-etching of dentin results in a near surface graded layer of varying thickness and property gradations. The attenuation behavior can be analyzed using theoretical methods to potentially quantify property gradation as well as layer thickness.
There are clinical implications of this finding becuase the dental literature suggests that only the first few micrometers of the etched dentin surface are eventually infiltrated by the dentin adhesive. Thus, the dentin-adhesive bonding is a near-surface interaction of the adhesive and the etched adherent, which is mostly demineralized dentin. Our results would then suggest that a soft graded layer is present underneath the composite restoration. The contribution of this layer to the eventual failure of the composite restoration is expected to be significant and should be investigated.
Acknowledgments
This research supported in part by National Institutes of Health/National Institute of Dental and Craniofacial Research (R01DE014392-08 and 3R01DE014392-08S1).
Biographies

Orestes Marangos was born in Nicosia, Cyprus. He received his M.S. degree from University of Missouri-Kansas City in 2004 and his Ph.D. degree from the University of Kansas, in civil engineering in 2010. He is currently working as a postdoctoral researcher at the Bioengineering Research Center at the University of Kansas. His research interests include quantitative scanning acoustic microscopy of biomaterials, ultrasonic wave propagation modeling of graded and laterally inhomogeneous substrates, numerical methods for focused ultrasonic field computations, and the development of methodologies for material property measurements using complementary microscopic techniques. So far, he has published 15 peer-reviewed articles in journals and conference proceedings, more than 30 reviewed abstracts, and more than 10 conference presentations.
Dr. Marangos is a member of the International Association for Dental Research (IADR) and has been a student member of the IEEE, American Society of Civil Engineers (ASCE) and the American Geophysical Union (AGU). He is also a member of Tau Beta Pi (2002) and Chi Epsilon (2008) honor societies.

Anil Misra received his bachelor’s degree in civil engineering from the Indian Institute of Technology, Kanpur, India in 1985, and his M.S. and Ph.D. degrees from the University of Massachusetts at Amherst in 1988 and 1991, respectively. He is currently a Professor in the Civil, Environ-mental and Architectural Engineering Department of the University of Kansas, Lawrence. He also serves as Associate Director of the University of Kansas Bioengineering Research Center (KU-BERC). From 1990 to 2007, he served as a faculty member at the University of Missouri-Kansas City. Dr. Misra has a broad research interest that spans topics covering both basic and applied aspects of mechanics of granular materials, interfaces and biomaterials, including analytical, computational, and experimental micro-mechanics, multi-scale modeling, constitutive behavior, micro-macro correlations, and multi-modal material characterization using high-resolution techniques such as scanning acoustic microscopy. He has co-edited three books; guest edited two journal special issues; and authored more than 200 papers in journals, edited books and conference proceedings. He is an active member of the American Society of Civil Engineers, International Association of Dental Researchers, and was a member of IEEE, the American Society of Mechanical Engineers and American Geophysical Union.

Paulette Spencer received her D.D.S. degree in 1978 from the University of Missouri, Kansas-City(UMKC) School of Dentistry, her M.S. degree inpediatric dentistry in 1980 from the University of Minnesota and her M.S. degree in materials engineering in 1988 from the Rensselaer Polytechnic Institute (RPI), and her Ph.D. in oral biology and physics in 1993 from UMKC. From 1981 to 1984, she was an Assistant Professor of Pediatric Dentistry at the University of Mississippi and an attending dentist at University Hospital, University of Mississippi Medical Center, from 1982 to 1984. From 1988 to 1999, she had a joint appointment as a Professor in the department of Pediatric Dentistry and Oral Biology at the UMKC School of Dentistry. She later became a Hamilton B. G. Robinson Professor at the UMKC School of Dentistry in 2001 and a Curators’ Professor in 2004. She was also the Director of the UMKC Center for Research on Interfacial Structure & Properties (UMKC-CRISP) in 2003. She is currently a Deane E. Ackers Distinguished Professor in the Mechanical Engineering Department, and the founding Director of the Bioengineering Research Center (BERC) at the University of Kansas. Dr. Paulette Spencer has worked in the area of dental materials research for 20 years. Her work has been focused on understanding the fundamental phenomena controlling biological interactions at material interfaces, for the design and development of new restorative materials and the development of non-destructive techniques for material/tissue interface characterization.
Professor Spencer is an active member of several professional societies. She is a fellow of the American Academy of Pediatric Dentistry, the American Academy of Dental Materials, the American Institute for Medical and Biological Engineering, the American College of Dentists, Biomaterials Science and Engineering and the American Association for the Advancement of Science (AAAS).

J. Lawrence Katz received the B.S., M.S. and Ph.D. degrees in 1950, 1951, and 1954, from the Polytechnic Institute of Brooklyn (currently the Polytechnic Institute of New York University) in physics. From 1956 to 1972, he was Professor of Physics at Rensselaer Polytechnic Institute (RPI), Troy, NY. He was a Professor of Biophysics in Biomedical Engineering at RPI from 1972 to 1989 and the founding Chairman of the Department of Biomedical Engineering at RPI from 1974 to 1985. From 1989 to 2002, he was Professor of Biomedical Engineering at Case Western Reserve University (CWRU), Cleveland, OH, where he served as Dean of the School of Engineering from 1989 to 1992. He has held appointments of Visiting Professor of Biomedical Engineering and Oral Biology, University of Miami, Schools of Engineering and Medicine, Coral Gables, FL from 1969 to 1970; the Department of Materials, Queen Mary College, University of London, England, in 1985; and the Laboratoire de Recherches Orthopediques, Faculte de Medecine, Paris, Francé, in 1986. From 2002 to 2007, he was Distinguished Research Professor of Biomedical Engineering and Oral Biology, University of Missouri-Kansas City. He is currently professor Emeritus of Biomedical Engineering at RPI and CWRU. He introduced the concept that to understand and model the structure/property relationships in bone, it is necessary to consider bone as a hierarchical structural/material system from the molecular to the macroscopic level. He has published more than 210 papers in journals, edited books, and conference proceedings and transactions in the field of calcified tissues biomechanics and biomaterials.
Professor Katz is active in many professional societies. He has been President of the Society for Biomaterials from 1978 to 1979 and the Biomedical Engineering Society from 1983 to 1984; he was Chairman of the Biomedical Engineering Division of the ASEE from 1978 to 1979. He is a Founding Fellow of AIMBE and the Society for Biomaterials; he is a Life Fellow of IEEE; a Life Fellow of ASME; and a Fellow of the American Physical Society. He was Associate Editor for Biomaterials and Biomechanics for the Annals of Biomedical Engineering for 10 years and for the Journal of Dental Research for 5 years; he serves on the Editorial Boards of several other journals and continually reviews articles for many journals. He has won a number of significant awards for his research and/or teaching from both American and foreign societies, and from the universities in which he has served. He has been a NSF Science Faculty Fellow from 1959 to 1960 at University College, London; a Guggenheim Fellow in 1978 at Harvard Medical School; and a Senior International (Fogarty) NIH Fellow at Queen Mary College, London, England, in 1985 and the Faculté de Medécine Lariboisiere-Saint-Louis, Paris, France, 1986.
Contributor Information
Orestes Marangos, Civil, Environmental, and Architectural Engineering Department, University of Kansas, Lawrence, KS.
Anil Misra, Civil, Environmental, and Architectural Engineering Department, University of Kansas, Lawrence, KS.
Paulette Spencer, Mechanical Engineering Department, University of Kansas, Lawrence, KS.
J. Lawrence Katz, Email: amisra@ku.edu, Bioengineering Research Center (BERC), University of Kansas, Lawrence, KS.
References
- 1.Marangos O, Misra A, Spencer P, Bohaty B, Katz JL. Physico-mechanical properties determination using microscale homotopic measurements: Application to sound and caries-affectedprimary tooth dentin. Acta Biomater. 2009 May;vol. 5(no. 4):1338–1348. doi: 10.1016/j.actbio.2008.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eick JD. Smear layer-materials surface. Proc. Finn. Dent. Soc. 1992;vol. 88(suppl 1):225–242. [PubMed] [Google Scholar]
- 3.Kramer IRH, McLean JW. Alterations in the staining reaction of dentine resulting from a constituent of a new self-polymerizing resin. Br. Dent. J. 1952;vol. 93:150–153. [Google Scholar]
- 4.Buonocore MG. A simple method of increasing the adhesion of acrylic filling materials to enamel surfaces. J. Dent. Res. 1955 Dec;vol. 34(no. 6):849–853. doi: 10.1177/00220345550340060801. [DOI] [PubMed] [Google Scholar]
- 5.Kinney JH, Balooch M, Haupt DL, Marshall SJ, Marshall GW. Mineral distribution and dimensional changes in human dentin during demineralization. J. Dent. Res. 1995 May;vol. 74(no. 5):1179–1184. doi: 10.1177/00220345950740050601. [DOI] [PubMed] [Google Scholar]
- 6.Pashley DH, Ciucchi B, Sano H, Horner JA. Permeability of dentin to adhesive agents. Quintessence Int. 1993 Sep;vol. 24(no. 9):618–631. [PubMed] [Google Scholar]
- 7.Nakabayashi N, Kojima K, Masuhara E. The promotion of adhesion by the infiltration of monomers into tooth substrates. J. Biomed. Mater. Res. 1982;vol. 16(no. 3):265–273. doi: 10.1002/jbm.820160307. [DOI] [PubMed] [Google Scholar]
- 8.Van Meerbeek B, Dhem A, Goret-Nicaise M, Braem M, Lam-brechts P, Vanherle G. Comparative SEM and TEM examination of the ultrastructure of the resin-dentin interdiffusion zone. J. Dent. Res. 1993 Feb;vol. 72(no. 2):495–501. doi: 10.1177/00220345930720020501. [DOI] [PubMed] [Google Scholar]
- 9.Nakabayashi N, Ashizawa M, Nakamura M. Identification of a resin-dentin hybrid layer in vital human dentin created in vivo: Durable bonding to vital dentin. Quintessence Int. 1992 Feb;vol. 23(no. 2):135–141. [PubMed] [Google Scholar]
- 10.Spencer P, Swafford JR. Unprotected protein at the dentin-adhesive interface. Quintessence Int. 1999 Jul;vol. 30(no. 7):501–507. [PubMed] [Google Scholar]
- 11.Oliveira SS, Marshall SJ, Hilton JF, Marshall GW. Etching kinetics of a self-etching primer. Biomaterials. 2002 Oct;vol. 23(no.20):4105–4112. doi: 10.1016/s0142-9612(02)00149-7. [DOI] [PubMed] [Google Scholar]
- 12.Susin AH, Alves LS, de Melo GP, Lenzi TL. Comparative scanning electron microscopic study of the effect of different dental conditioners on dentin micromorphology. J. Appl. Oral Sci. 2008 Apr;vol. 16(no. 2):100–105. doi: 10.1590/S1678-77572008000200004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Spencer P. Effect of acid etching time and technique on interfacial characteristics of the adhesive-dentin bond using differential staining. Eur. J. Oral Sci. 2004 Jun;vol. 112(no. 3):293–299. doi: 10.1111/j.1600-0722.2004.00127.x. [DOI] [PubMed] [Google Scholar]
- 14.Marshall GW, Chang YJ, Saeki K, Gansky SA, Marshall SJ. Citric acid etching of cervical sclerotic dentin lesions: An AFM study. J. Biomed. Mater. Res. 2000 Mar;vol. 49(no. 3):338–344. doi: 10.1002/(sici)1097-4636(20000305)49:3<338::aid-jbm6>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 15.Marshall GW, Chang YJ, Gansky SA, Marshall SJ. De-mineralization of caries-affected transparent dentin by citric acid: An atomic force microscopy study. Dent. Mater. 2001 Jan;vol. 17(no. 1):45–52. doi: 10.1016/s0109-5641(00)00056-7. [DOI] [PubMed] [Google Scholar]
- 16.Lemor R, Kruger MB, Wieliczka DM, Spencer P, May T. Dentin etch chemistry investigated by Raman and infrared spectroscopy. J. Raman Spectrosc. 2000 Mar;vol. 31(no. 3):171–176. [Google Scholar]
- 17.Santini A, Miletic V. Quantitative micro-Raman assessment of dentine demineralization, adhesive penetration, and degree of conversion of three dentine bonding systems. Eur. J. Oral Sci. 2008 Apr;vol. 116(no. 2):177–183. doi: 10.1111/j.1600-0722.2008.00525.x. [DOI] [PubMed] [Google Scholar]
- 18.Spencer P, Wang Y, Walker MP, Wieliczka DM, Swafford JR. Interfacial chemistry of the dentin/adhesive bond. J. Dent. Res. 2000 Jul;vol. 79(no. 7):1458–1463. doi: 10.1177/00220345000790070501. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki M, Kato H, Wakumoto S. Vibrational analysis by Raman-spectroscopy of the interface between dental adhesive resin and dentin. J. Dent. Res. 1991 Jul;vol. 70(no. 7):1092–1097. doi: 10.1177/00220345910700071501. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Spencer P. Hybridization efficiency of the adhesive/ dentin interface with wet bonding. J. Dent. Res. 2003 Feb;vol. 82(no. 2):141–145. doi: 10.1177/154405910308200213. [DOI] [PubMed] [Google Scholar]
- 21.Wieliczka DM, Kruger MB, Spencer P. Raman imaging of dental adhesive diffusion. Appl. Spectrosc. 1997 Nov;vol. 51(no. 11):1593–1596. [Google Scholar]
- 22.Ma SY, Cai JY, Zhan XW, Wu YZ. Effects of etchant on the nanostructure of dentin: An atomic force microscope study. Scanning. 2009 Jan-Feb;vol. 31(no. 1):28–34. doi: 10.1002/sca.20135. [DOI] [PubMed] [Google Scholar]
- 23.Rosales JI, Marshall GW, Marshall SJ, Watanabe LG, Toledano M, Cabrerizo MA, Osorio R. Acid-etching and hydration influence on dentin roughness and wettability. J. Dent. Res. 1999 Sep;vol. 78(no. 9):1554–1559. doi: 10.1177/00220345990780091001. [DOI] [PubMed] [Google Scholar]
- 24.Balooch M, Wu-Magidi IC, Balazs A, Lundkvist AS, Marshall SJ, Marshall GW, Siekhaus WJ, Kinney JH. Viscoelas-tic properties of demineralized human dentin measured in water with atomic force microscope (AFM)-based indentation. J. Biomed. Mater. Res. 1998 Jun;vol. 40(no. 4):539–544. doi: 10.1002/(sici)1097-4636(19980615)40:4<539::aid-jbm4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 25.Lemons RA, Quate CF. Acoustic microscope-scanning version. Appl. Phys. Lett. 1974;vol. 24(no. 4):163–165. [Google Scholar]
- 26.Briggs GAD. Acoustic Microscopy. Oxford, UK: Clarendon; 1992. [Google Scholar]
- 27.Maev RG. Acoustic Microscopy: Fundamentals and Applications. Weinheim, Germany: Wiley-VCH; 2008. [Google Scholar]
- 28.Peck SD, Briggs GAD. A scanning acoustic microscope study of the small caries lesion in human enamel. Caries Res. 1986 Jul-Aug;vol. 20(no. 4):356–360. doi: 10.1159/000260958. [DOI] [PubMed] [Google Scholar]
- 29.Peck SD, Rowe JM, Briggs GAD. Studies on sound and carious enamel with the quantitative acoustic microscope. J. Dent. Res. 1989 Feb;ol. 68(no. 2):107–112. doi: 10.1177/00220345890680020201. [DOI] [PubMed] [Google Scholar]
- 30.Kushibiki J, Ha KL, Kato H, Chubachi N, Dunn F. Application of acoustic microscopy to dental material characterization, in. Proc. IEEE Ultrasonics Symp. 1987;vol. 1:837–842. [Google Scholar]
- 31.Zheng YP, Maeva EY, Denisov AA, Maev RG. Ultrasound imaging of human teeth using a desktop scanning acoustic microscope. In: Lee H, editor. Acoustical Imaging. vol. 24. New York, NY: Kluwer Academic/Plenum; 2002. pp. 165–171. [Google Scholar]
- 32.Maev RG, Denisova LA, Maeva EY, Denissov AA. New data on histology and physico-mechanical properties of human tooth tissue obtained with acoustic microscopy. Ultrasound Med. Biol. 2002 Jan;vol. 28(no. 1):131–136. doi: 10.1016/s0301-5629(01)00480-x. [DOI] [PubMed] [Google Scholar]
- 33.Katz JL, Bumrerraj S, Dreyfuss J, Wang Y, Spencer P. Micromechanics of the dentin/adhesive interface. J. Biomed. Mater. Res. 2001;vol. 58(no. 4):366–371. doi: 10.1002/jbm.1030. [DOI] [PubMed] [Google Scholar]
- 34.Katz JL, Spencer P, Nomura T, Wagh A, Wang Y. Mi-cromechanical properties of demineralized dentin collagen with and without adhesive infiltration. J. Biomed. Mater. Res. A. 2003;vol. 66(no. 1):120–128. doi: 10.1002/jbm.a.10551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raum K, Kempf K, Hein HJ, Schubert J, Maurer P. Preservation of microelastic properties of dentin and tooth enamel in vitro—A scanning acoustic microscopy study. Dent. Mater. 2007;vol. 23(no. 10):1221–1228. doi: 10.1016/j.dental.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 36.Ophir J, Jaeger P. Spectral shifts of ultrasonic propagation through media with nonlinear dispersive attenuation. Ultrason. Imaging. 1982;vol. 4(no. 3):282–289. doi: 10.1177/016173468200400304. [DOI] [PubMed] [Google Scholar]
- 37.Ho SP, Balooch M, Marshall SJ, Marshall GW. Local properties of a functionally graded interphase between cementum and dentin. J. Biomed. Mater. Res. A. 2004 Sep;vol. 70(no. 3):480–489. doi: 10.1002/jbm.a.30105. [DOI] [PubMed] [Google Scholar]
- 38.Hirsekorn S, Pangraz S, Weides G, Arnold W. Measurement of elastic impedance with high spatial resolution using acoustic microscopy. Appl. Phys. Lett. 1995 Aug;vol. 67(no. 6):745–747. [Google Scholar]
- 39.Hirsekorn S, Pangraz S, Weides G, Arnold W. Measurement of elastic impedance with high spatial resolution using acoustic microscopy. Appl. Phys. Lett. 1996 Sep;vol. 69(no. 14):2138. vol 67, pg 745, 1995. [Google Scholar]
- 40.Prasad M. Mapping impedance microstructures in rocks with acoustic microscopy. Lead. Edge (Tulsa Okla.) 2001 Feb;vol. 20(no. 2):172–179. [Google Scholar]
- 41.Raum K, Jenderka KV, Klemenz A, Brandt J. Multilayer analysis: Quantitative scanning acoustic microscopy for tissue characterization at a microscopic scale. IEEE Trans. Ultrason. Ferro-electr. Freq. Control. 2003 May;vol. 50(no. 5):507–516. [Google Scholar]
- 42.Marangos O. Scanning acoustic microscopy modeling for microme-chanical measurements of complex substrates. Lawrence, KS: Dept. of Civil, Environmental and Architectural Eng., Univ. Kansas; 2010. Ph.D. dissertation. [Google Scholar]
- 43.Taylor JR. An Introduction to Error Analysis: The Study of Uncertainties in Physical Measurements. Sausalito, CA: University Science Books; 1997. [Google Scholar]
- 44.Bourbie T, Nur A. Effects of attenuation on reflections: Experimental test. J. Geophys. Res. 1984 Jul;vol. 89(no. B7):6197–6202. [Google Scholar]
- 45.Nagy PB, Adler L. Surface roughness induced attenuation of reflected and transmitted ultrasoanic waves. J. Acoust. Soc. Am. 1987 Jul;vol. 82(no. 1):193–197. [Google Scholar]
- 46.Toledano M, Osorio R, Perdigao J, Rosales JI, Thompson JY, Cabrerizo-Vilchez MA. Effect of acid etching and collagen removal on dentin wettability and roughness. J. Biomed. Mater. Res. 1999 Nov;vol. 47(no. 2):198–203. doi: 10.1002/(sici)1097-4636(199911)47:2<198::aid-jbm9>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 47.Nishitani Y, Yoshiyama M, Tay FR, Wadgaonkar B, Waller J, Agee K, Pashley DH. Tensile strength of mineralized/demin-eralized human normal and carious dentin. J. Dent. Res. 2005 Nov;vol. 84(no. 11):1075–1078. doi: 10.1177/154405910508401121. [DOI] [PMC free article] [PubMed] [Google Scholar]