Abstract
Dynorphin, an endogenous opioid peptide, mediates progesterone-negative feedback on gonadotropin-releasing hormone (GnRH) neurons in other species. The role of dynorphin in humans is unclear. The objective of this study was to determine if dynorphin fibers have close contacts with GnRH neurons in humans. Dual-label immunocytochemistry was performed on postmortem human hypothalamic tissue. The majority of GnRH neurons, 87.5%, had close contacts with dynorphin fibers and multiple close contacts were common, 62.5%. There were no regional differences between the hypothalamus and preoptic area in the distribution of close contacts. More close contacts were identified on the GnRH dendrites compared to the cell bodies (P < .001), but this difference was not significant when corrected for length. In conclusion, dynorphin fibers form close contacts with GnRH neurons in humans. This neuroanatomical evidence may suggest that dynorphin has effects on GnRH regulation in humans as seen in other species.
Keywords: GnRH neurons, dynorphin, endogenous opioid peptides, close contacts
Introduction
The female menstrual cycle is controlled by complex interactions involving the hypothalamus, anterior pituitary gland, and ovaries.1 Progesterone, in particular, inhibits pulsatile gonadotropin-releasing hormone (GnRH) release during the luteal phase.2 3 The progesterone receptor antagonist, RU-486, completely blocks the effects of progesterone on pulsatile GnRH secretion,3 suggesting the classic nuclear progesterone receptor was involved. However, GnRH neurons in ovine brain lack progesterone receptors.4 Therefore, progesterone-negative feedback may involve other cells that contain progesterone receptors, which then project that influence to the GnRH neurons.5 6 There is significant evidence that the endogenous opioid peptides (EOP) are important mediators of progesterone-negative feedback on GnRH neurons.6 7
The EOPs are a family of neuropeptides that include dynorphin, endorphins, and enkephalins.8 It is well established that dynorphin6 and β-endorphin7 have effects on reproductive function. Studies in sheep have demonstrated that dynorphin is likely the EOP responsible for mediating the progesterone-negative feedback on GnRH neurons during the luteal phase.5 6 9 Further understanding of the role of dynorphin in the control of the female menstrual cycle may lead to a foundation on which to build novel treatments for a variety of reproductive problems including amenorrhea and infertility. The objective of the current study was to determine whether dynorphin fibers have close contacts with GnRH neurons in the human brain.
Materials and Methods
Institutional Review Board approval was obtained from the University of Cincinnati to carry out the experiments described. We used dual-label immunocytochemistry to examine the relationship between immunoreactive GnRH neurons and dynorphin fibers in human hypothalamic tissue. Fixed blocks of postmortem hypothalamic tissue were obtained from the New York Brain Bank at Columbia University. Consent was previously obtained for autopsy and use of brain tissue and clinical records for research purposes. The subjects were healthy females (n = 3) with no known neurological disease (Table 1). Brain tissue was collected by the New York Brain Bank at Columbia. Status of the menstrual cycle or use of exogenous hormones was not reported at the time of death. Brain tissue blocks were dissected, postfixed in formalin, and stored at 4°C. Upon receipt, the tissue blocks were removed from formalin and placed in 0.1 mol/L phosphate buffer containing 0.9% sodium chloride (PBS) at 4°C for 10 days. Tissue blocks were then placed in 0.1 mol/L phosphate buffer (PB) with 20% sucrose for 3 hours at 4°C. After blocks were infiltrated with sucrose, 30-µm sections were cut in series of 6 using a Leitz freezing microtome. Tissue sections were placed in cryopreservative solution and stored at 4°C until processing for immunocytochemistry.
Table 1.
Identification Number, Age, and Postmortem Interval (PMI) of Subjectsa
| Subject | Age (years) | Cold PMI | Frozen PMI |
|---|---|---|---|
| T-133 | 33 | 3 hours 40 minutes | 34 hours 50 minutes |
| T-180 | 52 | 2 hours 25 minutes | 6 hours 7 minutes |
| T-111 | 33 | 8 hours | 11 hours 25 minutes |
a Cold PMI represents patient’s reported time of death to the time the subject was brought into the cold room. Frozen PMI represents the patient’s time of death to time tissue was processed.
Tissue sections were removed from the cryopreservative solution and gently placed in tissue processing cups to minimize tissue fragmentation. The sections were rinsed in PB with 0.01% sodium azide for 30 minutes and then sections were rinsed 6 times in PB for 10 minutes at room temperature. All subsequent washes were performed for 5 minutes at room temperature, unless otherwise noted. The tissue sections were subjected to an antigen-retrieval procedure consisting of heating the tissue for 20 minutes at 90°C in 1% sodium citrate buffer (Vector Laboratories, Burlingame, CA) in PB. The solution containing the sections was removed from heat and allowed to cool at room temperature for 20 minutes. Sections were then washed 4 times in PBS. Sections were incubated in PBS with 1% sodium borohydride for 30 minutes and then washed in PBS 4 times. Sections were incubated in PBS with 1% hydrogen peroxide for 10 minutes and then washed in PBS 4 times. Sections were incubated in PBS containing 4% normal goat serum and 0.4% Triton X-100 for 60 minutes to minimize nonspecific binding of the antibody.
For detection of dynorphin, sections were incubated in rabbit polyclonal antibody against dynorphin-A (1:160 000; dynorphin A1–17; Peninsula Laboratories, Torrance, CA) diluted in PBS containing 0.4% Triton X-100 and 4% normal goat serum for 16 hours at room temperature. This antiserum is specific to dynorphin A 1-17 and has less than 0.1% cross-reactivity with β-endorphin, Leu-enkephalin, or dynorphin B.10 After incubation with the primary antibody, sections were rinsed and then incubated with biotinylated goat anti-rabbit immunoglobulins (IgG), (1:200; Vector Laboratories), in PBS containing 0.4% Triton X-100 for 1 hour. Sections were washed in PBS 4 times and then incubated with avidin-biotin-complex (1:250; ABC-elite; Vector Laboratories) in PBS for 1 hour. Tissue sections were washed again in PBS 4 times. Immunoreactivity was visualized by exposure of sections to a chromagen solution containing 0.02% 3,3′-diaminobenzidine tetrahydrochloride (DAB), 2% Nickel sulfate, and 0.015% hydrogen peroxide in PB to produce a blue–black reaction product. After completion of the procedure for detection of dynorphin, sections were washed 3 times in PB and then incubated in PBS with 1% hydrogen peroxide for 10 minutes. The sections were then washed in PBS 4 times. For detection of GnRH, the sections were then incubated in LR-5 anti-GnRH (1:40,000; gift of Dr R Benoit), in PBS containing 0.4% Triton X-100 and 4% normal goat serum for 16 hours at room temperature. Tissue was washed 4 times in PBS and then incubated in biotinylated goat anti-rabbit IgG (1:200), in PBS containing 0.4% Triton X-100 for 1 hour. Tissue sections were rinsed in PBS 4 times and then incubated in ABC-elite (1:250), in PBS for 1 hour. Sections were washed in PBS 4 times. Immunoreactivity was visualized by incubation with DAB diluted in PB containing 0.015% hydrogen peroxide for 10 minutes, which produced a brown reaction product. Sections were then rinsed in PB 4 times and gently mounted on slides with 0.3% gelatin in PB and allowed to dry for 4 hours. Slides were dehydrated in a series of increasing concentrations of alcohol and then were rinsed in CitraSolv for 5 minutes 3 times. Finally, slides were coverslipped using DPX (Fluka, Milwaukee, WI).
Controls for determining specificity of immunoreactive reactions included preabsorption of GnRH and dynorphin primary antibodies for 24 hours at 4°C with their respective peptides. Preabsorption of the GnRH and dynorphin antibodies was performed with nanomolar concentrations of purified mammalian GnRH (Accurate Chemical, Westbury, NY) and dynorphin-A 1-17 (American Peptide, Sunnyvale, CA) peptides, respectively. Additional controls for dual-labeling included omission of the dynorphin antibody, omission of the GnRH antibody, and omission of both of the primary antibodies from the staining protocol. Both the preabsorption and the omission controls resulted in elimination of all specific fiber and cell body staining for dynorphin and GnRH, respectively.
Data Analysis
Gonadotropin-releasing hormone neurons were identified by the presence of dense, brown colored, reaction product that filled their cell body and dendrites. Dynorphin immunoreactivity was identified by the presence of dense blue–black fibers with beaded varicosities. Immunoreactive signals were observed using a brightfield microscope (Nikon Optiphot microscope, Nikon Corporation, Tokyo, Japan). Every sixth coronal section through the preoptic area, and hypothalamus was analyzed. Only GnRH neurons identified with intact cell bodies and dendrites were evaluated. The numbers of close contacts between dynorphin-containing fibers and GnRH cell bodies and dendrites were recorded for each subject. Close contacts were defined as direct appositions between dynorphin fibers and GnRH neurons in the same focal plane under ×40 magnification. The sections were evaluated by 2 examiners, blinded to the subject’s identification number. Images of labeled cells and fibers were captured using a Magnifire CCD camera (Optronics, Goleta, CA) attached to a Leica microscope (Leica, Deerfield, IL). Images were not altered in any way except for minor adjustments in brightness and contrast. The SSPS program (Chicago, IL) was used for statistical analysis of descriptive data and 2-tailed t tests. Data are presented as mean ± standard deviation and statistical significance was assessed at P < .05.
Results
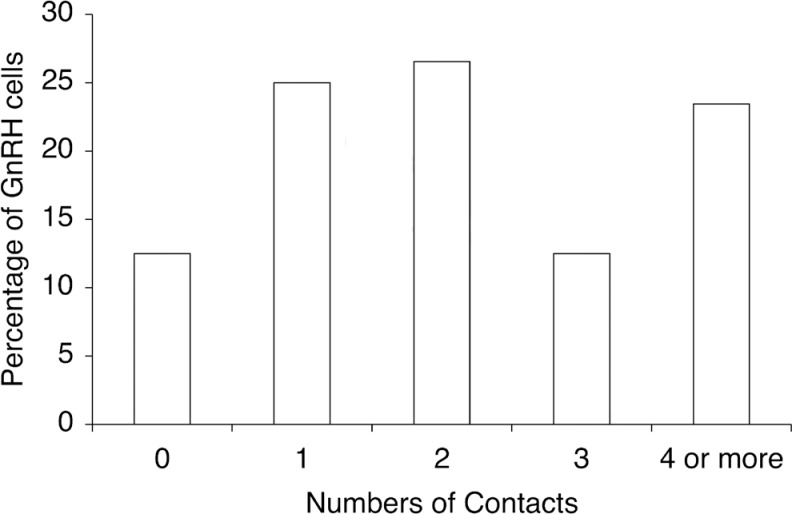
Age and postmortem intervals for each subject are listed in Table 1. As expected, GnRH cell bodies and dendrites were observed in the preoptic area, anterior, and mediobasal hypothalamus. The GnRH neurons with intact cell bodies and dendrites were identified in the 3 female subjects. These cells were equally distributed in the preoptic area, anterior hypothalamus, and mediobasal hypothalamus. The GnRH neurons displayed typical fusiform morphology (Figure 1A-C) and size of both cell bodies (12.5 ± 0.3 µm mean width, 26.8 ± 0.7 µm mean length) and dendrites (82.9 ± 7.5 µm mean length, range 10 to 330 µm). Dynorphin fibers were identified in the preoptic area, anterior, and mediobasal hypothalamus and exhibited close contacts with GnRH cell bodies and dendrites (Figure 1A-C). Dynorphin close contacts were identified on 87.5% of the GnRH cells analyzed (Table 2). GnRH neurons received an average of 2.3 ± 1.8 contacts/cell (Table 2). The percentage of GnRH neurons that had close contacts with dynorphin and the average number of close contacts for each subject is listed in Table 2. The majority, 62.5%, of GnRH neurons were observed to possess >1 close contacts with dynorphin fibers (Figure 2). The distribution of these close contacts did not differ among neuroanatomical regions including the preoptic area, anterior hypothalamus, or mediobasal hypothalamus, or in the number of close contacts (data not shown). The mean number of close contacts identified on the GnRH dendrites (1.69 ± 1.53) was greater than the cell bodies (0.59 ± 1.08; P < .001) but this difference was not significant when corrected for length (0.028 ± 0.03 contacts/µm, 0.023 ± 0.04 contacts/µm respectively; P = .15). Examples of dynorphin fibers and preabsorbed immunocytochemical control for dynorphin staining with elimination of specific staining in the preoptic area (POA) are demonstrated in Figure 3A and B, respectively. The preabsorbed controls for GnRH cell bodies and the elimination controls for both dynorphin and GnRH demonstrated no specific staining (data not shown).
Figure 1.
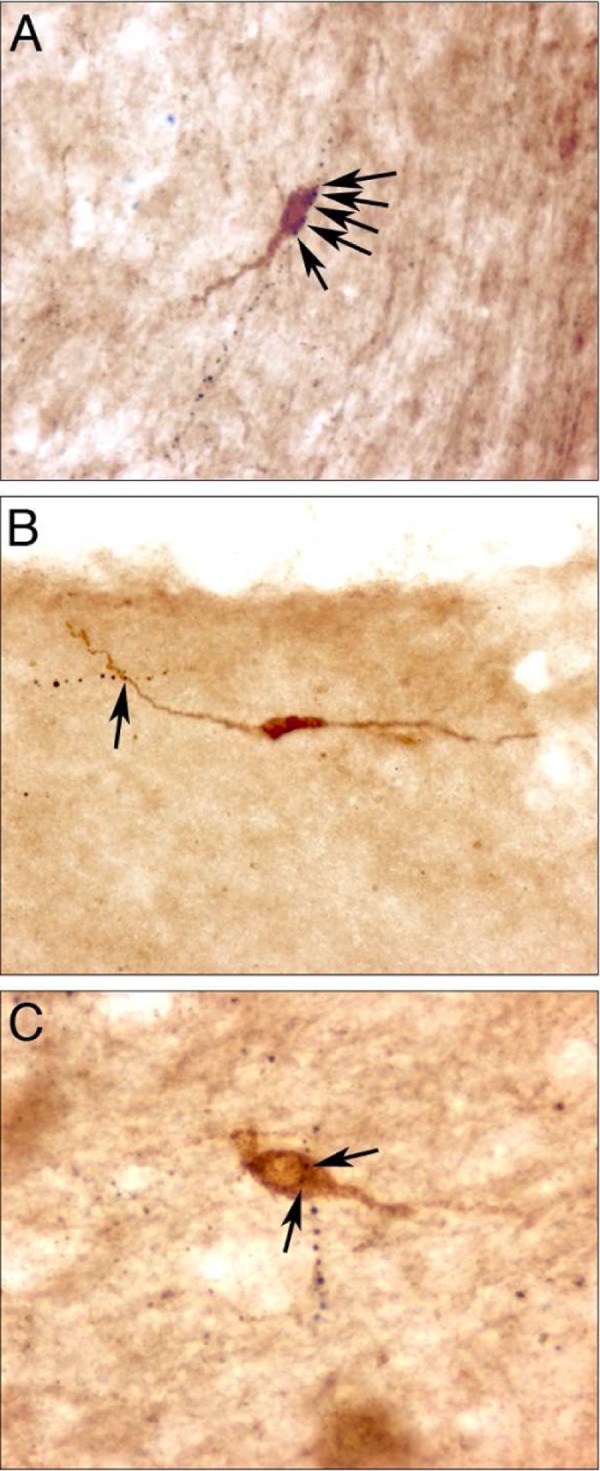
Immunocytochemical identification of gonadotropin-releasing hormone (GnRH) neurons (brown). Dynorphin fibers appear as black-beaded varicosities. Dynorphin fibers are observed in close contacts with GnRH neuron dendrite (A) and cell bodies (B-C; black arrows). A and B were acquired at ×20 magnification. C was acquired at ×40 magnification.
Table 2.
Summary of GnRH Neurons With Close Contacts From Dynorphin Fibers for Each of the 3 Female Subjects
| Subject | Percent of GnRH Cells With Dynorphin Close Contacts | Mean Number of Contacts Per Cell ± SD |
|---|---|---|
| T-133 | 85.2% | 2.0 ± 1.5 |
| T-180 | 84.2% | 2.7 ± 2.1 |
| T-111 | 94.4% | 2.2 ± 1.9 |
| Total | 87.5% | 2.3 ± 1.8 |
Abbreviations: GnRH, gonadotropin-releasing hormone; SD, standard deviation.
Figure 2.
Percentages of gonadotropin-releasing hormone (GnRH) cells with none, one, or multiple close contacts with dynorphin immunoreactive fibers. Most of the GnRH neurons 40/64 (62.5%) examined received multiple close contacts from dynorphin immunoreactive fibers.
Figure 3.
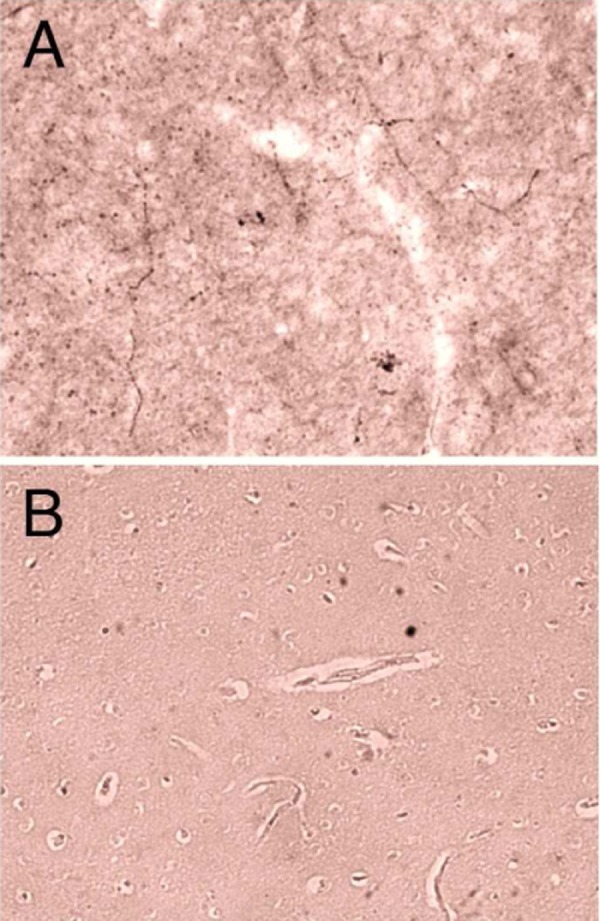
Example of immunocytochemical control for dynorphin staining in the human preoptic area (POA; A). Adjacent control section of the POA incubated with preabsorbed dynorphin antibody (B). Images acquired at ×20 magnification.
Discussion
This study in humans revealed that dynorphin immunoreactive fibers have close contacts with GnRH neurons in the female hypothalamus. This neuroanatomical evidence supports the hypothesis that dynorphin is involved in the regulation of GnRH neurons in humans as seen in other species.
The morphology and distribution of GnRH cell bodies and fibers we observed in this study was consistent with other reports of GnRH neuron distribution in humans.11 There were a greater number of dynorphin close contacts identified on the dendrite portion of the GnRH neurons compared to the cell bodies. However, the mean dendrite length identified in this study was notably longer than the mean cell body length. When corrected for unit length, there was no difference between the cell bodies and dendrites in the number of close contacts identified from dynorphin fibers. The majority of GnRH neurons identified in this study received multiple contacts from dynorphin fibers. Studies in sheep demonstrated that close contacts seen under light microscopy were actual synaptic contacts by electron microscopy.6 Future studies should clarify whether dynorphin neurons contain progesterone receptors in humans as seen in other species including sheep.5
The distribution of dynorphin fibers in this study was consistent with a previously reported distribution of dynorphin in the human.12 Studies have demonstrated that GnRH neurons have close contacts with other endogenous opioid peptides in humans. The anatomic relationship between β-endorphin and GnRH neurons was examined in postmortem human hypothalamic tissue.11 Close contacts from β-endorphin were seen in 71% of GnRH neurons in the mediobasal hypothalamus and median eminence but were less common in the preoptic area. The majority of these GnRH neurons received 1 to 3 close contacts.11 Close contacts from leu-enkephalin were identified on 44% of GnRH neurons, with the majority of these close contacts located in the mediobasal hypothalamus and median eminence, with fewer contacts observed in GnRH neurons in the preoptic area and anterior hypothalamus.13 In contrast, in our study, no regional differences were observed between the preoptic area and hypothalamus in relation to the percentage of GnRH neurons that received close contacts from dynorphin immunoreactive fibers. This suggests that the various endogenous opioid peptides may have differential roles in the control of reproductive neuroendocrine function. Although studies suggest that dynorphin mediates progesterone’s negative feedback on GnRH neurons, endorphin is thought to play a more important role in the generation of the preovulatory surge of GnRH.6
It is difficult to make direct comparisons between our study of dynorphin and previous studies with β-endorphin and leu-enkephalin. The subjects from previous studies included both females and males. The authors reported no sexual dimorphic differences between the female and male subjects. However, the endogenous opioid peptides have been shown to be sexually dimorphic in hypothalamic nuclei of other species.14 15 There may have been differences in the postmortem delay as well as differences in the tissue fixation process itself that led to different findings between the studies. Interestingly, the percentage of GnRH neurons that received β-endorphin or leu-enkephalin close contacts was lower than the percentage of GnRH neurons observed to have close contacts with dynorphin in this study.
It is fairly well accepted that endogenous opioid peptides are important in human menstrual cycle regulation.7 16 The frequency and amplitude of luteinizing hormone (LH) pulses were increased in normal cycling women treated with a nonspecific opioid antagonist, naloxone, during the luteal phase of the menstrual cycle.17 18 In contrast, no effect of naloxone was observed during the early follicular phase of the cycle,18 in postmenopausal women,19 or in women with surgical menopause.20 Interestingly, the effects of naloxone enhancing gonadotropin release were reinstated with progesterone replacement.19 20 This suggests that endogenous opioid peptides may inhibit LH release during the luteal phase, a time of the menstrual cycle when circulating concentrations of progesterone are elevated. There are few studies on the more specific role of dynorphin in the negative feedback of progesterone on GnRH neurons in humans. The sheep estrous cycle, like the human menstrual cycle, includes a true luteal phase dominated by the inhibitory feedback of progesterone on GnRH secretion. This makes sheep an ideal model to study the regulation of GnRH neurons by dynorphin.
There is substantial evidence in sheep that dynorphin plays a critical role in conveying the inhibitory influence of progesterone upon GnRH neurons. Greater than 95% of dynorphin cells in the sheep hypothalamus contain nuclear progesterone receptors.5 Progesterone increases concentrations of dynorphin in the cerebrospinal fluid collected from the third ventricle and dynorphin gene expression is decreased by ovariectomy.9 Progesterone replacement in ovariectomized ewes increases preprodynorphin messenger RNA (mRNA) in the preoptic area and anterior hypothalamus.9 Dynorphin fibers have close contacts with GnRH neurons in sheep and synaptic inputs of dynorphin containing terminals onto GnRH neurons have also been demonstrated in sheep.6 Pharmacological studies provide evidence for dynorphin regulation of GnRH release. Dynorphin binds preferentially to the κ opioid receptor.21 Studies demonstrated that microinjections of the specific antagonists to the κ opioid receptor increases the frequency of LH release.6 In other words, blocking the dynorphin receptor ultimately blocks the effects of progesterone on GnRH neurons. There are ongoing studies to determine whether ovine GnRH neurons contain dynorphin receptors or whether dynorphin acts through other mechanisms. In the rat, there is evidence to suggest the EOPs influence GnRH neurons via presynaptic inhibition of norepinephrine.22
In addition to the role of EOPs on the regulation of reproductive physiology, several studies have implicated a possible role for EOPs in pathological syndromes of the reproductive system including polycystic ovarian syndrome,23 amenorrhea,24 and infertility.24 Elevated levels of β-endorphin were seen in a group of obese women with polycystic ovarian syndrome compared to control women with regular menstrual cycles.23 Treatment of hypothalamic amenorrhea patients with naltrexone, a nonspecific EOP antagonist, restored normal ovulation and women with infertility achieved pregnancies.24 This suggests that, in those patients, high levels of EOPs suppress GnRH release leading to amenorrhea. The study also found that the EOP antagonist was not effective in restoring ovulation in women with primary amenorrhea due to Kallmann’s syndrome.24 This syndrome is associated with an absence of functional GnRH neurons, primary amenorrhea, and anosmia and is additional evidence along with data in sheep25 that dynorphin acts at the level of GnRH neurons and not at the level of the pituitary.24 Considering the potential role of dynorphin in normal and pathological syndromes of the reproductive system, it is interesting to speculate that specific agonists and antagonists to dynorphin and other endogenous opioid peptides may be useful pharmaceuticals in the future. There is ongoing research to develop highly selective dynorphin A analogues as potential new medications.26
Conclusion
Dynorphin is an endogenous opioid peptide that has close contacts with GnRH neurons in the female hypothalamus. This neuroanatomical evidence in humans, as well as numerous studies in other species, may suggest a role for dynorphin in the regulation of GnRH neurons. Further understanding of the role of the endogenous opioid peptides including dynorphin in the control of human reproductive physiology and its potential involvement in reproductive disorders may lay a foundation for novel treatment options.
Acknowledgments
We gratefully acknowledge the following sources of support: NIH T32 HD40135 to SKD, NIH RO1 HD039916 to MNL. We also acknowledge The New York Brain Bank at Columbia University for supplying the human hypothalamic tissue and Dr Robert Benoit (Mont. Gen. Hosp.) for GnRH antibody (LR-5).
References
- 1. Fritz MA, Speroff L. The endocrinology of the menstrual cycle: the interactions of folliculogenesis and neuroendocrine mechanisms. Fertil Steril. 1982;38(5):509–529 [DOI] [PubMed] [Google Scholar]
- 2. Brann DW, Mahesh VB. Regulation of gonadotropin secretion by steroid hormones. Front Neuroendocrinol. 1991;12(2):165–207 [Google Scholar]
- 3. Skinner DC, Evans NP, Delaleu B, Goodman RL, Bouchard P, Caraty A. The negative feedback actions of progesterone on gonadotropin-releasing hormone secretion are transduced by the classical progesterone receptor. Proc Natl Acad Sci. 1998;95(18):10978–10983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Skinner DC, Caraty A, Allingham R. Unmasking the progesterone receptor in the preoptic area and hypothalamus of the ewe: no colocalization with gonadotropin-releasing neurons. Endocrinology. 2001;142(2):573–579 [DOI] [PubMed] [Google Scholar]
- 5. Foradori CD, Coolen LM, Fitzgerald ME, Skinner DC, Goodman RL, Lehman MN. Colocalization of progesterone receptors in parvicellular dynorphin neurons of the ovine preoptic area and hypothalamus. Endocrinology. 2002;143(11):4366–4374 [DOI] [PubMed] [Google Scholar]
- 6. Goodman RL, Coolen LM, Anderson GM, et al. Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology. 2004;145(6):2959–2967 [DOI] [PubMed] [Google Scholar]
- 7. Ferin M. Endogenous opioid peptides and the menstrual cycle. Trends Neurosci. 1984;7(6):194–196 [Google Scholar]
- 8. Moore MR, Black PM. Neuropeptides. Neurosurg Rev. 1991;14(2):97–110 [DOI] [PubMed] [Google Scholar]
- 9. Foradori CD, Goodman RL, Adams VL, Valent M, Lehman MN. Progesterone increases dynorphin a concentrations in cerebrospinal fluid and preprodynorphin messenger ribonucleic acid levels in a subset of dynorphin neurons in the sheep. Endocrinology. 2005;146(4):1835–1842 [DOI] [PubMed] [Google Scholar]
- 10. Tan-No K, Takahashi H, Nakagawasai O, et al. Pronociceptive role of dynorphins in uninjured animals:N-ethylmaleimide-induced nociceptive behavior mediated through inhibition of dynorphin degradation. Pain. 2005;113(3):301–309 [DOI] [PubMed] [Google Scholar]
- 11. Dudas B, Merchenthaler I. Close anatomical associations between β-endorphin and luteinizing hormone-releasing hormone neuronal systems in the human diencephalon. Neuroscience. 2004;124(1):221–229 [DOI] [PubMed] [Google Scholar]
- 12. Abe J, Okamura H, Kitamura T, et al. Immunocytochemical demonstration of dynorphin (PH-8P)-like immunoreactive elements in the human hypothalamus. J Comp Neurol. 1988;276(4):508–513 [DOI] [PubMed] [Google Scholar]
- 13. Dudas B, Merchenthaler I. Topography and Associations of Leu-Enkephalin and luteinizing hormone-releasing hormone neuronal systems in the human diencephalon. J Clin Endocrinol Metab. 2003;88(4):1842–1848 [DOI] [PubMed] [Google Scholar]
- 14. Watson RE, Jr, Hoffmann GE, Wiegand SJ. Sexually dimorphic opioid distribution in the preoptic area: manipulation by gonadal steroids. Brain Res. 1986;398(1):157–163 [DOI] [PubMed] [Google Scholar]
- 15. Simerly RB, McCall LD, Watson SJ. Distribution of opioid peptides in the preoptic region: immunohistochemical evidence for a steroid-sensitive enkephalin sexual dimorphism. J Comp Neurol. 1988;276(3):442–459 [DOI] [PubMed] [Google Scholar]
- 16. Steele PA, Judd SJ. Role of endogenous opioids in reducing the frequency of pulsatile luteinizing hormone secretion induced by progesterone in normal women. Clin Endocrin. 1986;25(6):669–674 [DOI] [PubMed] [Google Scholar]
- 17. Ropert JF, Quigley ME, Yen SSC. Endogenous opiates modulate pulsatile luteinizing hormone release in humans. J Clin Endocrinol Metab. 1981;52(3):583–585 [DOI] [PubMed] [Google Scholar]
- 18. Quigley ME, Yen SSC. Role of endogenous opiates on LH secretion during the menstrual cycle. J Clin Endocrinol Metabol. 1980;51(1):179–181 [DOI] [PubMed] [Google Scholar]
- 19. Casper RF, Alapin-Rubillovitz S. Progestins increase endogenous opioid peptide activity in postmenopausal women. J Clin Endocrinol Metabol. 1985;60(1):34–36 [DOI] [PubMed] [Google Scholar]
- 20. Shoupe D, Montz FJ, Lobo RA. The effects of estrogen and progestin on endogenous opioid activity in oophorectomized women. J Clin Endocrinol Metab. 1985;60(1):178–183 [DOI] [PubMed] [Google Scholar]
- 21. Chavkin C, James IF, Goldstein A. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science. 1982;215(4531):413–415 [DOI] [PubMed] [Google Scholar]
- 22. Kalra SP, Kalra PS. Opioid-adrenergic-steroid connection in regulation of luteinizing hormone secretion in the rat. Neuroendocrinology. 1984;38(5):418–426 [DOI] [PubMed] [Google Scholar]
- 23. Aleem FA, McIntash T. Elevated plasma levels of β-endorphin in a group of women with polycystic ovarian disease. Fertil Steril. 1984;42(5):686–689 [PubMed] [Google Scholar]
- 24. Wildt L, Leyendecker G, Sir-Petermann T, Waibel-Treber S. Treatment with naltrexone in hypothalamic ovarian failure: induction of ovulation and pregnancy. Hum Reprod. 1993;8(3):350–358 [DOI] [PubMed] [Google Scholar]
- 25. Whisnant CS, Havern RL, Goodman RL. Endogenous opioid suppression of luteinizing hormone pulse frequency and amplitude in the ewe: hypothalamic sites of action. Neuroendocrinology. 1991;54(6):587–593 [DOI] [PubMed] [Google Scholar]
- 26. Lung FD, Chen CH, Liu JH. Development of highly potent and selective dynorphin a analogues as new medicines. J Pept Res. 2005;66(5):263–276 [DOI] [PubMed] [Google Scholar]