Abstract
Drug use and relapse involve learned associations between drug-associated environmental cues and drug effects. Extinction procedures in the clinic can suppress conditioned responses to drug cues, but the extinguished responses typically reemerge after exposure to the drug itself (reinstatement), the drug-associated environment (renewal), or the passage of time (spontaneous recovery). We describe a memory retrieval-extinction procedure that decreases conditioned drug effects and drug seeking in rat models of relapse, and drug craving in abstinent heroin addicts. In rats, daily retrieval of drug-associated memories 10 minutes or 1 hour but not 6 hours before extinction sessions attenuated drug-induced reinstatement, spontaneous recovery, and renewal of conditioned drug effects and drug seeking. In heroin addicts, retrieval of drug-associated memories 10 minutes before extinction sessions attenuated cue-induced heroin craving 1, 30, and 180 days later. The memory retrieval-extinction procedure is a promising nonpharmacological method for decreasing drug craving and relapse during abstinence.
Conditioning plays a major role in drug addiction, and responses to drug-associated cues persist during prolonged abstinence (1, 2). These findings led to the development of cue-exposure therapies to extinguish the craving-and relapse-provoking effects of drug cues (1, 3). However, cue-exposure therapy in clinical settings does not usually prevent relapse when former drug addicts return to their previous drug environments (4). Animal learning studies predict that extinction responding is susceptible to renewal, reinstatement, and spontaneous recovery. Respectively, these terms refer to resumption of original learned responses after change of environmental context, acute exposure to the unconditioned stimulus (such as food or drug), or passage of time (5).
More recently, preclinical investigators have been able to decrease behavioral effects of drug-associated cues by pharmacologically interfering with reconsolidation of drug-cue memories (6–9). Reconsolidation refers to a time-dependent process in which consolidated memory items are rendered transiently unstable shortly after their reactivation (10–12). However, with the exception of the beta-adrenoceptor antagonist propranolol (13, 14), which is approved for human use, the other pharmacological compounds used in these studies are not suitable for human use (15–18). Consequently, the results from rat reconsolidation studies have not yet “translated” to clinical use in addiction treatment.
A nonpharmacological alternative may be possible: the “memory retrieval-extinction” behavioral procedure to interfere with reconsolidation of fear cues in rats and humans (19, 20). Reinstatement, renewal, and spontaneous recovery of fear responding are prevented by acute exposure to cues previously paired with foot-shock (a retrieval manipulation) if that exposure is followed 10 min or 1 hour later (but not 6 hours later) by repeated exposure to the same cues in extinction sessions. Thus, extinction experience within the timeframe of the “reconsolidation window” after cued retrieval of the fear memories mimicked the behavioral effect of a pharmacological manipulation on suppression of fear conditioning (19, 20).
We used an extinction-reinstatement procedure in rats [an animal model of drug relapse (21)] and a cue-induced–craving procedure in humans (1) to assess whether the memory retrieval-extinction procedure can decrease drug- and cue-induced drug preference and relapse in rats and cue-induced drug craving in humans.
We first assessed the effect of the memory retrieval-extinction manipulation on drug-priming–induced reinstatement of drug conditioned place preference (CPP) and spontaneous recovery of drug CPP (details are available in the supplementary material). In the Pavlovian CPP version of the reinstatement model, CPP is induced by a drug, extinguished, and then reinstated by priming injections of the drug (21); like other conditioned responses (22), the extinguished CPP response can reemerge after the passage of time (spontaneous recovery).
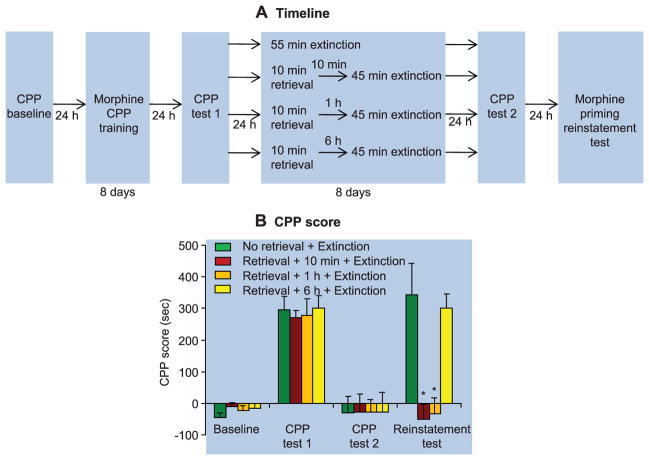
In experiment 1 (cocaine CPP) (fig. S1A) and experiment 2 (morphine CPP) (Fig. 1A), we used four groups of rats: (i) no memory retrieval + extinction; (ii) memory retrieval + 10-min delay + extinction; (iii) memory retrieval + 1-hour delay + extinction; and (iv) memory retrieval + 6-hour delay + extinction. We analyzed the data with the between-subjects factor of group and the within-subjects factor of CPP test (test 2, after extinction; test 3, drug-induced reinstatement or spontaneous recovery test). Brief (10 min) cued retrieval of the drug memories 10 min or 1 hour but not 6 hours before the longer 45-min daily extinction sessions impaired drug-priming–induced reinstatement of drug CPP for cocaine (table S1, statistical results, and fig. S1) and morphine (group × test interaction, F3,25 = 9.5, P < 0.01) (Fig. 1). In experiment 3, we used an identical experimental procedure to demonstrate that the memory retrieval-extinction manipulation also impaired spontaneous recovery of cocaine CPP (fig. S2).
Fig. 1.
In rats, retrieval of drug-cue memories 10 min or 1 hour before extinction sessions prevented drug-priming–induced reinstatement of morphine CPP. (A) During CPP training, rats learned to associate one environmental context with the effect of morphine injections (10 mg/kg, subcutaneous) and to associate another context with saline injections. Next, all rats were tested for their place preference (CPP test 1). Twenty-four hours later, rats were divided into four groups and given different memory retrieval-extinction manipulations: 55-min extinction training (in one group), or 10-min memory retrieval + 45 min extinction training (in the other three groups—with either a 10-min, 1-hour, or 6-hour delay between memory retrieval and extinction training). All rats were tested for reinstatement of morphine CPP induced by a priming injection of morphine (5 mg/kg, subcutaneous). (B) Effect of the experimental manipulations on the CPP score. Data are mean ± SEM of preference score in seconds (time spent in the morphine-paired chamber minus time spent in the saline-paired chamber) during the CPP tests. Asterisk indicates different from the “no memory retrieval” condition; P < 0.05; n = 9 to 11 rats per experimental condition.
We next assessed the effect of the memory retrieval-extinction manipulation on drug-priming–induced reinstatement of the drug self-administration behavior, spontaneous recovery, and context-induced reinstatement of drug seeking [a renewal manipulation (23)]. These experiments used the operant self-administration version of the reinstatement model, in which animals are trained to respond for drug infusions, given daily extinction sessions until operant responding ceases, and then tested for reinstatement of (nonreinforced) pressing on the drug-associated device (such as a lever or nosepoke operandum) after acute noncontingent exposure to drug-priming injections or exposure to drug-associated cues (24, 25). A selective increase in nonreinforced responding on the device previously associated with drug infusions (but not on the inactive device) is interpreted to indicate relapse to drug seeking (26). Like drug CPP, the extinguished drug-reinforced conditioned response undergoes spontaneous recovery after the completion of extinction training (27).
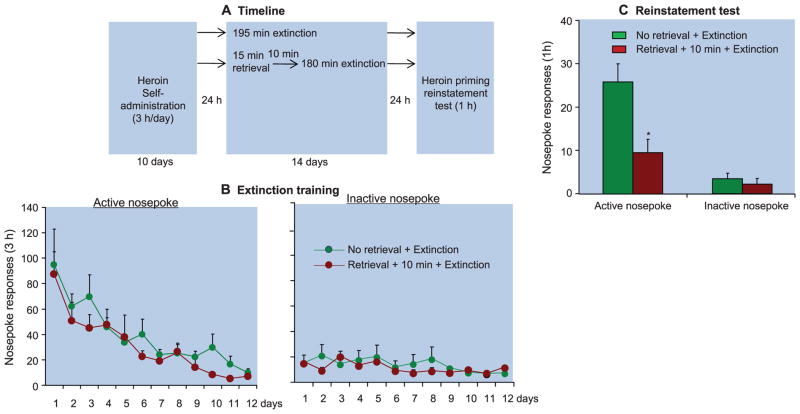
In experiments 4 and 5, we used rats that had been trained to self-administer cocaine or heroin to demonstrate the inhibitory effect of the memory retrieval-extinction manipulation on drug-priming–induced reinstatement (Fig. 2 and fig. S3). The statistical analysis included the between-subjects factor of group (no memory retrieval + extinction, memory retrieval + 10-min delay + extinction, and memory retrieval + 6-hour delay + extinction for cocaine; or no memory retrieval + extinction and memory retrieval + 10-min delay + extinction for heroin) and the within-subjects factor of reinstatement condition (last extinction session, reinstatement test session). Brief (15 min) cued retrieval of the drug memories 10 min but not 6 hours before the long 180-min daily extinction sessions impaired drug-priming–induced reinstatement of cocaine (fig. S3) or heroin (Fig. 2) seeking. There were significant group × reinstatement condition interactions for both cocaine (table S2) and heroin (F1,10 = 6.9, P < 0.05). No group differences were seen in responding on the inactive nosepoke operandum (P > 0.1). Additionally, the memory retrieval-extinction manipulation accelerated extinction responding in the cocaine-trained rats (table S2 and fig. S3) but not the heroin-trained rats (P > 0.05) (Fig. 2).
Fig. 2.
In rats, retrieval of drug-cue memories 10 min before extinction sessions attenuated heroin-priming–induced reinstatement of drug seeking. (A) Timeline of the experimental procedure. Rats were trained to self-administer intravenous heroin during three 1-hour daily sessions over 10 days. Twenty-four hours later, the rats were divided into two groups and given different memory retrieval-extinction manipulations: 195-min extinction training or 15-min memory retrieval + 180-min extinction training, with 10 min between memory retrieval and extinction training. The rats were then tested for reinstatement of nosepoke responding after noncontingent priming injections of heroin (0.25 mg/kg, subcutaneous). (B and C) Number of responses (mean ± SEM) on the active and inactive nosepoke devices during the extinction sessions and the heroin-priming test. Asterisk indicates different from the “no memory retrieval” condition; P < 0.05; n = 6 to 7 rats per experimental condition.
In experiments 6 and 7, we used rats that had been trained to self-administer cocaine to demonstrate the inhibitory effect of the memory retrieval-extinction manipulation on spontaneous recovery (experiment 6) and renewal (context-induced reinstatement; experiment 7) of cocaine seeking (figs. S4 and S5). In the renewal experiment, the rats were trained to self-administer cocaine in a distinct context (context A). Then, the operant responding was extinguished in a different, nondrug context (context B). During the subsequent tests, reinstatement of cocaine seeking was assessed after exposure to context A (28). Brief (15 min) cued retrieval of the cocaine memories 10 min before the long 180-min daily extinction sessions impaired spontaneous recovery (fig. S4) and renewal (fig. S5) of cocaine seeking. The statistical analyses, which included the between-subjects factor of group (no memory retrieval + extinction and memory retrieval + 10-min delay + extinction) and the within-subjects factor of test condition (last extinction session, spontaneous recovery, or renewal test session), showed significant interactions between group × test condition (table S2). No group differences were seen in responding on the inactive nosepoke operandum (P > 0.1). Additionally, the memory retrieval-extinction manipulation modestly accelerated extinction responding in experiment 6 (fig. S4) but not experiment 7 (fig. S5).
In experiment 8, we assessed the effect of the memory retrieval-extinction manipulation on the protein expression of protein kinase Mζ (PKMζ) in medial prefrontal cortex (mPFC; infralimbic and prelimbic subregions) and amygdala (basolateral and central subregions). PKMζ is a constitutively active atypical isoenzyme of protein kinase C that mediates long-term maintenance of aversive and appetitive memories (29, 30), including drug-associated memories (31, 32). We found that extinction training alone increased PKMζ expression in infralimbic (but not prelimbic) cortex and decreased PKMζ expression in basolateral (but not central) amygdala (fig. S6). Furthermore, the memory retrieval-extinction manipulation with a 10-min but not 6-hour delay potentiated extinction-induced increases in PKMζ expression in infralimbic cortex and extinction-induced decreases in PKMζ expression in basolateral amygdala (table S2 and fig. S6). There was no group effect on the levels of β-actin (a control protein).
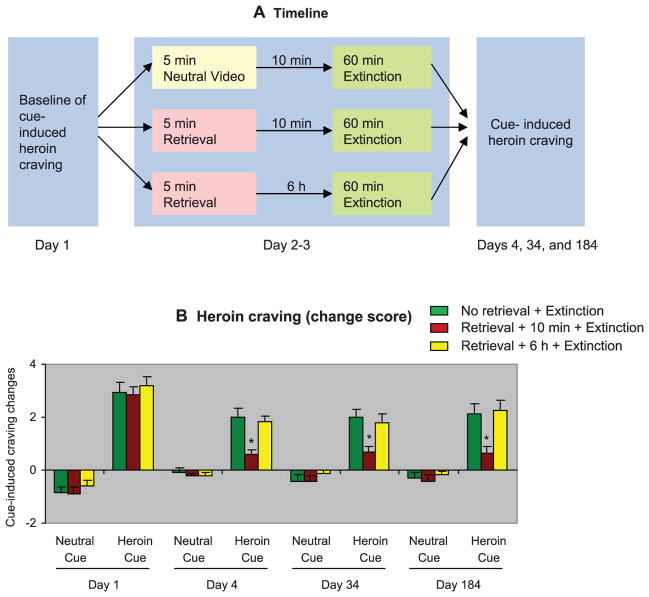
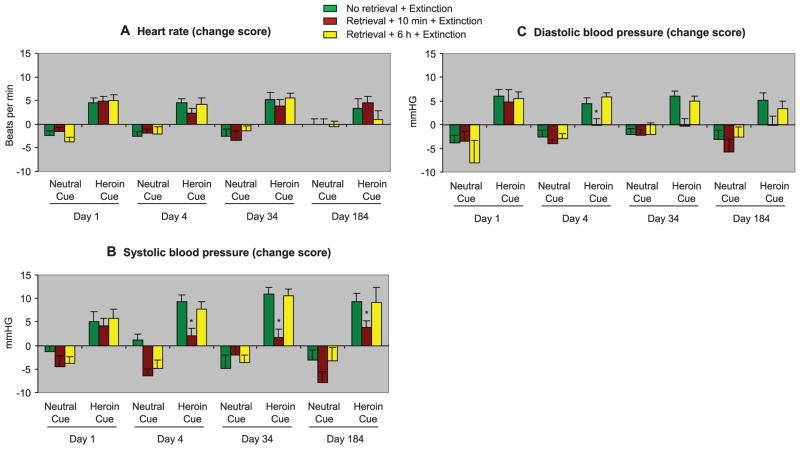
Next, we assessed the clinical relevance of the memory retrieval-extinction procedure in in-patient detoxified heroin addicts. Heroin craving was assessed by using a visual analog scale (VAS) on which the participants had to rate their current craving for heroin, before and immediately after exposure to a neutral cue and a heroin cue. Neutral and heroin cues were both 5-min videotapes. Heart rate (HR) and blood pressure were monitored before and after cue exposure as additional measures of cue reactivity. The heroin addicts were assigned to three groups: (i) no memory retrieval + extinction, (ii) memory retrieval + 10-min delay + extinction, or (iii) memory retrieval + 6-hour delay + extinction. The memory retrieval-extinction manipulation with a 10-min but not 6-hour delay inhibited both cue-induced craving (Fig. 3) and cue-induced increases in blood pressure, but not heart rate (Fig. 4). Craving reactivity to cues was assessed by using change scores from preexposure baseline (33).
Fig. 3.
In humans, retrieval of drug-cue memories 10 min before extinction sessions caused long-lasting attenuation of cue-induced heroin craving. (A) Timeline of the experimental procedure. Neutral- and heroin-cue–induced drug craving (see supplementary materials) in abstinent heroin addicts was measured with VAS on day 1. Twenty-four hours later, the participants were divided into three groups and given different memory retrieval-extinction manipulations for 2 consecutive days: neutral cue exposure + 10-min delay + 60-min extinction training (in one group), or 5-min heroin-cue exposure (memory retrieval) + 60-min extinction training (in the other two groups—with 10 min or 6 hours between memory retrieval and extinction training). During the extinction sessions, the participants were given four consecutive sessions of repeated exposures to three different heroin-related cues (supplementary material). Measures of subjective craving and sympathetic activation (heart rate and blood pressure) were obtained after the extinction sessions. Cue-induced heroin craving was assessed again on days 4, 34, and 184 by using a procedure identical to that used on day 1. (B) Cue-induced heroin craving (mean ± SEM) on day 1 (baseline), days 4, 34, and 184 (1, 30, and 180 days after the memory retrieval-extinction sessions). Asterisk indicates different from “no memory retrieval” group; P < 0.05; n = 22 human subjects per group for day 1, 4, and 34; n = 16 to 18 human subjects per group for day 184.
Fig. 4.
In humans, retrieval of drug-cue memories 10 min before the extinction sessions caused long-lasting attenuation of cue-induced increases in systolic and diastolic blood pressure but not heart rate. The experimental procedure was identical to that described in Fig. 3. (A) Heart rate. (B) Systolic blood pressure. (C) Diastolic blood pressure. Asterisk indicates different from “no memory retrieval” group; P < 0.05, n = 22 human subjects per group for day 1, 4, and 34; n = 16 to 18 human subjects per group for day 184.
For cue-induced craving, the statistical analysis [SAS PROC MIXED, Satterthwaite method for denominator degrees of freedom that takes into account missing cells in repeated-measures analysis of variance (ANOVA)], which included baseline (day 1) as the covariate, the between-subjects factor of group, and the within-subjects factors of test day (posttreatment tests on days 4, 34, and 184) and cue type (neutral cue and heroin cue), showed a significant interaction between group × cue type (F2,23.75 = 9.0, P < 0.01). For systolic and diastolic blood pressure, the analyses showed a trend toward an interaction between group × cue type (F2,343.25 = 2.38, P = 0.094 and F2,374.46 = 2.42, P = 0.09, respectively). No group differences were observed for heart rate.
In 2009, Monfils and colleagues introduced a memory retrieval-extinction procedure whose application to both rats (19) and humans (20) led to long-lasting blockade of shock-conditioned fear responses. Here, we introduce an appetitive-conditioning version of the memory retrieval-extinction procedure whose application caused long-lasting attenuation of conditioned drug effects and drug seeking (in rats) and drug craving (in detoxified heroin users). The behavioral effects of our procedure were also associated with changes in the expression of the memory-maintenance–related molecule PKMζ in infralimbic cortex and basolateral amygdala. As in the fear-conditioning studies, a key determinant of effectiveness was the interval between the shorter memory-retrieval sessions and the longer extinction sessions or the interval between short reexposure to the drug-associated cues (memory retrieval) and subsequent longer nonreinforced reexposure to the same cues (extinction).
The development of the memory retrieval-extinction fear-conditioning procedure was inspired by theoretical accounts of memory retrieval and reconsolidation (34) and studies of pharmacological manipulations of reconsolidation of fear memories (11, 35). The latter body of work has since been extended to appetitive memories (6), including memories of drug-associated cues (7, 36). In those studies, investigators inferred that memory reconsolidation was disrupted on the basis of findings that post-retrieval systemic or intracranial injections of pharmacological agents within a specific time interval (up to 2 hours after retrieval)—often termed a “reconsolidation window”—disrupted the expression of responses to aversive or appetitive cues (6, 12, 37). Accordingly, results from studies of memory retrieval-extinction manipulations have been taken to reflect interference with reconsolidation (19, 20, 38). The findings that the memory retrieval-extinction manipulation is ineffective when the extinction sessions are given at delays that are longer than the reconsolidation window (19, 20, 38) supports this interpretation. The retrieval-extinction manipulation also blocks shock-induced reinstatement, spontaneous recovery, and renewal of conditioned-fear expression [phenomena that are otherwise reliably observed after extinction training (5)] in some studies (19, 20, 38), but not others (39, 40). On the basis of the above findings, a plausible interpretation of our data is that the memory retrieval-extinction manipulation interfered with reconsolidation of memories for drug cues. This hypothesis is supported by two sets of observations. First, across the different experiments in both rats and humans, the memory retrieval-extinction manipulation was effective only within the time window of reconsolidation. Second, in the CPP experiments the memory retrieval-extinction manipulation completely blocked drug-priming–induced reinstatement and spontaneous recovery.
However, a reconsolidation account of the data should be made with some caution in the case of the drug self-administration experiments. In those experiments, nosepoke responding was significantly lower during the last extinction session than during the tests for drug-priming–induced reinstatement, spontaneous recovery, and renewal in the 10-min or 1-hour memory retrieval-extinction condition. Additionally, the effect of the memory retrieval-extinction manipulation on nose-poke responding during extinction training—a behavior induced in part by exposure to the drug-associated cues (26)—was modest and inconsistent across experiments. Together, these observations suggest that our memory retrieval-extinction manipulation only weakened the memories of the drug cues (or decreased their motivational effects) rather than completely preventing the expression of the conditioned response, as would have been predicted by a reconsolidation account of the data.
What might account for the attenuation but not blockade of drug seeking in the self-administration experiments? One possibility is that the memory retrieval-extinction manipulation preferentially disrupted reconsolidation of stimulus-response Pavlovian-based memories that mediate drug CPP in rats and cue-induced drug craving in humans, while having less impact on reconsolidation of response-outcome operant-based memories that play a role in reinstatement of drug seeking in the drug self-administration procedure. Operant drug seeking is controlled by a complex interplay between operant and Pavlovian conditioning processes (7, 26), and there is evidence that reconsolidation of operant memories is more difficult to disrupt than reconsolidation of Pavlovian memories (41).
Another issue to consider in interpreting the present data is that the memory-retrieval manipulations were performed under extinction conditions, and therefore, a given manipulation could have affected reconsolidation of cue memories, consolidation of extinction memory, or both (6). Our retrieval manipulation in the self-administration experiments was 15 min of daily non-reinforced operant responding in the presence of the drug-associated cues. Thus, an alternative interpretation could be that intermittent exposure to extinction training within the consolidation window of extinction memory may have strengthened the extinction memory, rendering the original appetitive memory less susceptible to reinstatement, spontaneous recovery, or renewal. Indeed, results from fear-conditioning studies demonstrate that pharmacological manipulations that promote consolidation of extinction memory, decrease reinstatement, spontaneous recovery, and renewal of fear memories (42–44).
It is also possible that our memory retrieval-extinction manipulation both facilitated extinction consolidation and disrupted reconsolidation. Two lines of evidence from published reports support this hypothesis. The first is our recent finding that post-training PKMζ activity in basolateral amygdala is critical for memories of morphine reward and morphine withdrawal aversion but not extinction memory, whereas PKMζ activity in infralimbic cortex is critical for extinction memory but not reward or withdrawal memories (31). The second is that plasticity in basolateral amygdala is critical for reconsolidation of memories for both aversive and appetitive cues (6, 7) and for the effects of the retrieval-extinction manipulation on fear memories (38), whereas infra-limbic plasticity is critical for maintenance of aversive and appetitive extinction memories (44, 45). In the experiments reported here, we found that repeated cocaine-cue retrieval 10 min before daily extinction sessions potentiated the opposite effects of extinction training alone on PKMζ in the infralimbic cortex (increased expression) versus basolateral amygdala (decreased expression) (fig. S6). These findings are consistent with a “dual” effect of the memory retrieval-extinction manipulation on both consolidation of extinction memory and reconsolidation of cue memories.
Investigators have identified several ways to disrupt cue-memory reconsolidation or strengthen extinction learning (7, 46). However, their potential as preventive treatments for addiction is limited because they often rely on pharmacological agents that are either not approved for human use or that can cause problematic side effects. We used established animal models of drug relapse and a standard human laboratory procedure for drug-induced craving to assess a purely behavioral procedure to decrease the motivational effects of drug cues during abstinence. The memory retrieval-extinction procedure decreased cue-induced drug craving and (extrapolating from our rat data) perhaps could reduce the likelihood of cue-induced relapse during prolonged abstinence periods. If our procedure weakens the original drug-cue memories rather than solely facilitating extinction, it may overcome the contextual renewal problems that have limited the clinical effectiveness of traditional extinction procedures (4), although this possibility needs empirical evaluation in human addicts. Last, although the cellular mechanisms and brain circuits underlying the long-lasting effects of the retrieval-extinction procedure on drug relapse and craving remain to be elucidated, our data point to a role for PKMζ activity in the infralimbic cortex and basolateral amygdala.
Supplementary Material
Acknowledgments
This work was supported in part by the National Basic Research Program of China (No 2009CB522000 and 2011CB707800) and the Natural Science Foundation of China (No 91132716 and 31070958). The preparation of the manuscript was also supported in part by the Intramural Research Program of the National Institute on Drug Abuse.
Footnotes
The authors declare that they do not have any conflicts of interest related to the data presented in this manuscript.
References and Notes
- 1.O’Brien CP, Ehrman RN, Ternes JW. In: Behavioral Analysis of Drug Dependence. Goldberg S, Stolerman I, editors. Academic Press; Orlando, FL: 1986. pp. 329–356. [Google Scholar]
- 2.Stewart J, de Wit H, Eikelboom R. Psychol Rev. 1984;91:251. [PubMed] [Google Scholar]
- 3.Marlatt GA. Addict Behav. 1990;15:395. doi: 10.1016/0306-4603(90)90048-3. [DOI] [PubMed] [Google Scholar]
- 4.Conklin CA, Tiffany ST. Addiction. 2002;97:155. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- 5.Bouton ME. Biol Psychiatry. 2002;52:976. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- 6.Tronson NC, Taylor JR. Nat Rev Neurosci. 2007;8:262. doi: 10.1038/nrn2090. [DOI] [PubMed] [Google Scholar]
- 7.Milton AL, Everitt BJ. Eur J Neurosci. 2010;31:2308. doi: 10.1111/j.1460-9568.2010.07249.x. [DOI] [PubMed] [Google Scholar]
- 8.Lee JL, Di Ciano P, Thomas KL, Everitt BJ. Neuron. 2005;47:795. doi: 10.1016/j.neuron.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Miller CA, Marshall JF. Eur J Neurosci. 2005;21:1385. doi: 10.1111/j.1460-9568.2005.03974.x. [DOI] [PubMed] [Google Scholar]
- 10.Nader K, Schafe GE, Le Doux JE. Nature. 2000;406:722. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- 11.Dudai Y. Curr Opin Neurobiol. 2006;16:174. doi: 10.1016/j.conb.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Alberini CM. Trends Neurosci. 2005;28:51. doi: 10.1016/j.tins.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Milton AL, Lee JL, Everitt BJ. Learn Mem. 2008;15:88. doi: 10.1101/lm.825008. [DOI] [PubMed] [Google Scholar]
- 14.Wouda JA, et al. Front Behav Neurosci. 2010;4:179. doi: 10.3389/fnbeh.2010.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li FQ, et al. J Neurosci. 2010;30:10351. doi: 10.1523/JNEUROSCI.2112-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanchez H, Quinn JJ, Torregrossa MM, Taylor JR. J Neurosci. 2010;30:4401. doi: 10.1523/JNEUROSCI.3149-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milekic MH, Brown SD, Castellini C, Alberini CM. J Neurosci. 2006;26:3010. doi: 10.1523/JNEUROSCI.4818-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JL, Milton AL, Everitt BJ. J Neurosci. 2006;26:5881. doi: 10.1523/JNEUROSCI.0323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monfils MH, Cowansage KK, Klann E, LeDoux JE. Science. 2009;324:951. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schiller D, et al. Nature. 2010;463:49. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. Psychopharmacology (Berl) 2003;168:3. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- 22.Bouton ME, Swartzentruber D. Clin Psychol Rev. 1991;11:123. [Google Scholar]
- 23.Crombag HS, Bossert JM, Koya E, Shaham Y. Philos Trans R Soc London Ser B Biol Sci. 2008;363:3233. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.See RE. Eur J Pharmacol. 2005;526:140. doi: 10.1016/j.ejphar.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 25.de Wit H, Stewart J. Psychopharmacology (Berl) 1981;75:134. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- 26.Shalev U, Grimm JW, Shaham Y. Pharmacol Rev. 2002;54:1. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- 27.Shaham Y, Adamson LK, Grocki S, Corrigall WA. Psychopharmacology (Berl) 1997;130:396. doi: 10.1007/s002130050256. [DOI] [PubMed] [Google Scholar]
- 28.Crombag HS, Shaham Y. Behav Neurosci. 2002;116:169. doi: 10.1037//0735-7044.116.1.169. [DOI] [PubMed] [Google Scholar]
- 29.Sacktor TC. Nat Rev Neurosci. 2011;12:9. doi: 10.1038/nrn2949. [DOI] [PubMed] [Google Scholar]
- 30.Shema R, Sacktor TC, Dudai Y. Science. 2007;317:951. doi: 10.1126/science.1144334. [DOI] [PubMed] [Google Scholar]
- 31.He YY, et al. Neuropsychopharmacology. 2011;36:1972. doi: 10.1038/npp.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li YQ, et al. J Neurosci. 2011;31:5436. doi: 10.1523/JNEUROSCI.5884-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sinha R, Fuse T, Aubin LR, O’Malley SS. Psychopharmacology (Berl) 2000;152:140. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- 34.Eisenberg M, Kobilo T, Berman DE, Dudai Y. Science. 2003;301:1102. doi: 10.1126/science.1086881. [DOI] [PubMed] [Google Scholar]
- 35.Nader K, Schafe GE, LeDoux JE. Nat Rev Neurosci. 2000;1:216. doi: 10.1038/35044580. [DOI] [PubMed] [Google Scholar]
- 36.Diergaarde L, Schoffelmeer AN, De Vries TJ. Eur J Pharmacol. 2008;585:453. doi: 10.1016/j.ejphar.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 37.Nader K, Hardt O. Nat Rev Neurosci. 2009;10:224. doi: 10.1038/nrn2590. [DOI] [PubMed] [Google Scholar]
- 38.Clem RL, Huganir RL. Science. 2010;330:1108. doi: 10.1126/science.1195298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soeter M, Kindt M. Learn Mem. 2011;18:357. doi: 10.1101/lm.2148511. [DOI] [PubMed] [Google Scholar]
- 40.Chan WY, Leung HT, Westbrook RF, McNally GP. Learn Mem. 2010;17:512. doi: 10.1101/lm.1912510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hernandez PJ, Kelley AE. Learn Mem. 2004;11:748. doi: 10.1101/lm.84904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Graham BM, Richardson R. Behav Neurosci. 2010;124:337. doi: 10.1037/a0019582. [DOI] [PubMed] [Google Scholar]
- 43.Davis M, Ressler K, Rothbaum BO, Richardson R. Biol Psychiatry. 2006;60:369. doi: 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- 44.Quirk GJ, Mueller D. Neuropsychopharmacology. 2008;33:56. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peters J, Kalivas PW, Quirk GJ. Learn Mem. 2009;16:279. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taylor JR, Olausson P, Quinn JJ, Torregrossa MM. Neuropharmacology. 2009;56(suppl 1):186. doi: 10.1016/j.neuropharm.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.