Abstract
Preterm birth can be caused by intrauterine infection and maternal/fetal inflammatory responses. Maternal inflammation (chorioamnionitis) is often followed by a systemic fetal inflammatory response characterized by elevated levels of pro-inflammatory cytokines in the fetal circulation. The inflammation signal is likely transmitted across the blood-brain barrier, and initiates a neuroinflammatory response. Microglial activation has a central role in this process, and triggers excitotoxic, inflammatory, and oxidative damage in the developing brain. Neuroinflammation can persist over a period of time and sensitize the brain to subinjurious insults in early and chronic phases, but may offer relative tolerance in the intermediate period through activation of endogenous anti-inflammatory, protective, and repair mechanisms. Neuroinflammatory injury not only destroys what exists, but also changes what develops.
Keywords: infection, inflammation, anti-inflammation, neuroinflammation, fetus, brain, injury, protection, repair
Systemic inflammatory conditions outside the fetal brain can induce injury in the fetal brain and modulate its response to various insults. In this paper, we review the evidence for the relationship between the fetal inflammatory response and brain injury, and pathomechanisms by which this injury can occur, with main emphasis on brain damage in preterm newborns. We also shed light on the concept of altered brain development and response to injury in the setting of fetal inflammation, and the potential for protection and repair. We acknowledge that our overview is selective rather than all-inclusive. More comprehensive discussion of recent progress in the field of fetal inflammation and perinatal brain injury is available elsewhere.1–4
Intrauterine Infection, Inflammation, and Preterm Birth
The fetus, placenta, and fetal membranes co-exist in close proximity to the microbial flora in the lower genitourinary tract. For more than four decades, microbial infections were suspected to play a role in the etiology of preterm labor, and the complications and outcomes of prematurity.5–8 Recent summaries of this scenario are available in the current obstetric literature.9–11
Most recently, a large multicenter cohort of around 1500 extremely low-gestational- age newborns (ELGANs, <28 weeks gestation) was recruited. Extensive data were collected on placental histology and bacteriology, neonatal biomarkers, and neonatal brain, lung, and eye outcomes over a 2-year follow-up period.12–18 Placental microbiology studies revealed significantly higher rates of bacterial colonization in pregnancies that progressed to spontaneous preterm labor, compared with pregnancies delivered for preeclampsia as an initiator for their preterm delivery.17 In particular, colonization of placental tissues with 2 or more microorganisms was associated with a significantly increased risk for preterm labor as an initiator of delivery compared with colonization with one or no microorganism.18
Bacteria and bacterial products activate toll-like receptors on the surface of inflammatory cells in the decidua and placental membranes, resulting in the release of proinflammatory chemokines and cytokines from these cells and the initiation of a local inflammatory reaction in the placenta.19,20 Chorioamnionitis is the histological condition of (maternal) neutrophil infiltration of the chorion and amnion. Umbilical vasculitis is the (fetal) neutrophil response in umbilical and chorionic plate vessels.13,21 Activation of inflammatory cells in the placenta and the spread of proinflammatory molecules appear to propagate chorioamnionitis and fetal vasculitis.22 Viral infections can also act as initiators of histologic chorioamnionitis and fetal vasculitis.23 These data add to the growing evidence linking placental and fetal infection and inflammation to preterm labor and preterm birth.11
Not all prematurity is equal with regard to infectious/inflammatory characteristics. A clinically helpful classification of pregnancy conditions that lead to preterm delivery prior to 28 weeks of gestation divides disorders of pregnancy into 2 groups.16 The first includes placental histology patterns associated with aberrations of placentation such as decidual hemorrhages or fibrin deposition, placental infarcts, increased syncytial knots, thrombosis of fetal stem vessels, and a lack of microorganisms and markers of inflammation. This pattern is associated with preeclampsia and fetal indication/intrauterine growth restriction, leading to preterm delivery. The second group includes placental patterns associated with intrauterine infection/inflammation, such as inflammation of the chorionic plate or of the fetal membranes, fetal stem vessels, or umbilical cord vasculitis, or placental microbe recovery. This pattern is associated with spontaneous preterm labor, prelabor premature rupture of membranes, placental abruption and cervical insufficiency. Keeping initiators of preterm delivery separate is important for our scenario because they might carry different risk information for neonatal24 and longterm neurocognitive outcomes.25
Intrauterine Infection, Inflammation, and Preterm Brain Injury
Infants with postmortem bacteremia are much more likely to have histologic white matter damage than infants whose blood cultures were sterile.26 Because no microbes were identified in these babies’ brains, the authors of this seminal study postulated more than three decades ago that a circulating, noninfectious product of inflammation or endotoxin from bacteremia might lead to brain damage. Since then, evidence has accumulated in support of the concept that infection distant from the brain and exposure to endotoxins and/or inflammatory cytokines can damage the developing fetal brain.1,2,27–44
The proinflammatory response in the placenta involves the activation of a complex cytokine network at the chorio-decidual interface, including mediators that transmit inflammatory signals between maternal and fetal gestational tissues.45 Chorioamnionitis with funisitis is associated with umbilical cord endothelial cell activation, with upregulation and shedding of cell adhesion molecules,46,47 and with a systemic elevation of interleukin-6 levels.48,49 This rapid innate immune response can activate fetal leukocytes,50,51 which is, in turn, associated with neonatal white matter damage depicted on magnetic resonance images.52 We speculate that fetal white cell activation53 and the interaction between the innate and adaptive components of the immune system54 play a prominent role in fetal/neonatal white matter damage.
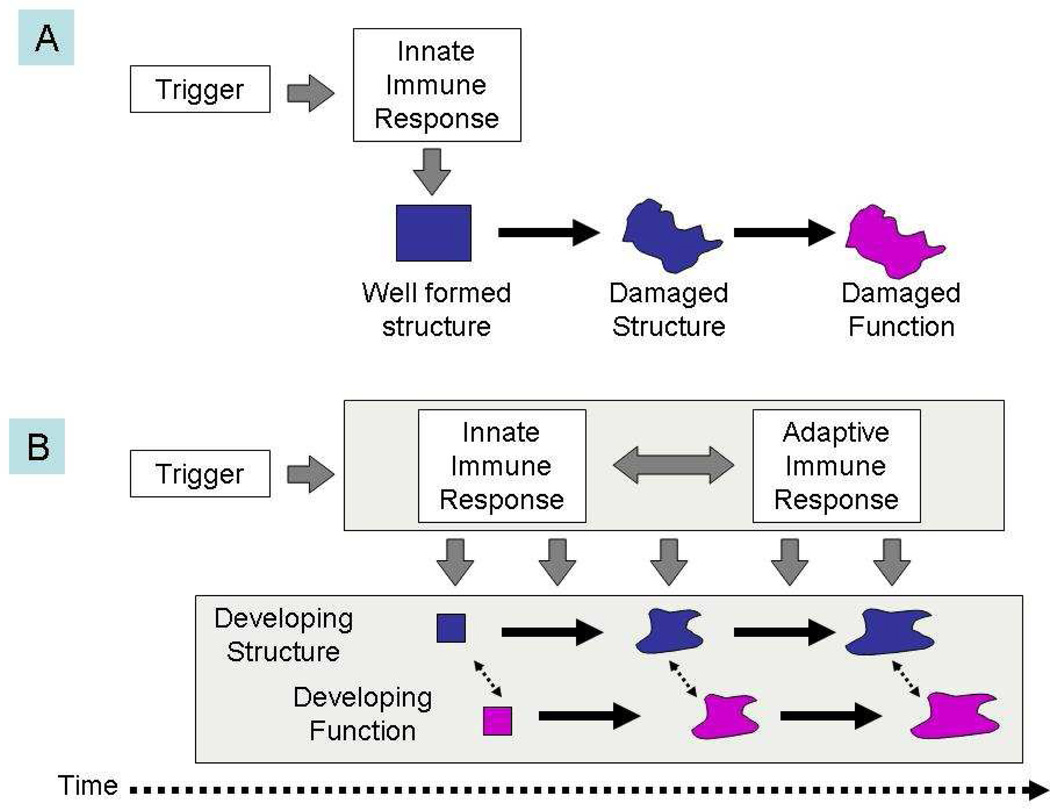
One important inference based on these assumptions is that neuroinflammation as a pathogenetic mediator is not a single hit in time, but a process extended over a period of time (Figure).55 An ongoing process involving T-cell-related immune mechanisms, together with cross-links to other systemic systems (eg, the coagulation56 and complement systems)57, 58 is a much more plausible scenario (depicted as scenario A in the Figure) than a single-hit model (scenario B) for the fetal systemic inflammatory response as an initiator of the multiorgan system dysfunctions seen in exposed fetuses.10,59
Figure 1.
The traditional scenario of perinatal brain injury (A) postulates that a single and rather short insult damages existing structure and leads to altered function. We propose the alternative view (B), which postulates that after an initial trigger has occurred, an ongoing interaction between innate and adaptive immune processes adversely affects the development of brain structure and function over an extended period of time.
It is often purported that cerebral hypoperfusion as a consequence of systemic hypoperfusion and shock is one pathomechanism by which the fetal brain can be damaged by in the setting of systemic inflammation. However, this concept is not well-supported by studies of systemic hypotension as a risk factor for neonatal brain damage.60,61 Experimental models of antenatal systemic exposure of the ovine fetus to inflammation also do not suggest that circulatory insufficiency contributes to the associated damage seen in the developing white matter.62–64 Taken together, these data suggest that inflammatory processes in and of themselves play an important role in perinatal brain damage causation in preterm newborns, whether circulatory disturbances are present or not.
From System to Brain
An intact blood-brain barrier separates the brain parenchyma from circulatory molecules that may alter the homeostasis of the central nervous system. Tight junctions in barrier interfaces in the developing brain are impermeable to even small lipid insoluble molecules.65–67 Nevertheless, there are multiple putative mechanisms by which the brain can sense inflammatory signals in the systemic circulation.67,68
Circumventricular organs represent areas in the brain that are devoid of the blood-brain barrier. Here, large molecules such as peptides, cytokines, and bacterial products in the circulation can come in direct contact with cellular elements in the central nervous system.69 These areas possess a population of antigen-presenting cells and macrophages that can interface with many of these circulating molecules and activate a local inflammatory response comparable to the activation of the innate immune response system elsewhere in the body. Toll-like receptors for lipopolysaccharide and receptors for interleukin-1 have been identified on the surface of these cells in the circumventricular organs.70,71 Activation of these receptors triggers a nuclear factor NF-kB-mediated cascade that releases in a paracrine fashion a number of cytokines and chemokines that can propagate the inflammatory signal to neighboring cells in the central nervous system.72
Alternatively, direct access into the central nervous system by inflammatory molecules and/or activated white blood cells can be facilitated by breakdown of the blood-brain barrier in the setting of hypoxia-ischemia/reperfusion injury,73 or through leaking of the blood-brain barrier in the setting of peripheral inflammatory pain signaling through the vagal nerve.74 Cytokines also may gain access into the brain through carrier-mediated transporter mechanisms across the blood-brain barrier. These mechanisms however appear to be of limited capacity and are rapidly saturated.75 Another mechanism for inflammatory signaling across the blood-brain barrier involves a role for the blood-brain barrier itself as a sensor organ for the central nervous system.68 When cytokines in the circulation bind to their receptors on the luminal side of endothelial and other cells associated with the blood-brain barrier, prostaglandins are released from the basal side of the blood-brain barrier and spread as a paracrine inflammatory signal throughout the adjacent brain parenchyma.
The exact contribution of any of these mechanisms in the setting of fetal inflammation remains unknown. To our knowledge, only one group of colleagues has embarked upon this endeavor.76–78 They have most recently summarized their results (together with a most interesting hypothetical expansion toward neurologic disorders in adulthood), suggesting “that breakdown of normal blood-brain barrier function resulting in a short-lasting influx of blood borne molecules, in particular plasma proteins, may cause local damage such as reduction of brain white matter observed in some newborn babies, but may also be the mechanism behind some neurodegenerative diseases related to underlying brain damage and long-term changes in barrier properties”.79
Microglia, Oligodendrocytes, and Neurons
Activation of microglia is one major characteristic of the neuroinflammatory response in the central nervous system.34 Microglia are the only non-neural cells in the brain expressing toll-like receptor 4, the lipopolysaccharide receptor, and must be present in co-culture with oligodendrocytes for lipopolysaccharide-induced oligodendrocyte death to occur.80 Microglial activation is considered crucial for excitotoxic, inflammatory, and free radical injury to cells in the developing central nervous system.81
Immature oligodendrocytes have been proposed also to be a main target of these injury mechanisms in the premature brain.82–84 These cells may be particularly more vulnerable to developmental injury than their mature counterparts, a finding that helps explain the vulnerability of the premature brain to various insults.85 A diffuse encephalopathy involving elements of the fetal grey matter, such as axonal tracts, neural progenitor cells, and neurons, is likely to be the underlying scenario of cognitive disability in premature infants in the setting of perinatal infection/inflammation.86,87
Inflammation as Modulator of Other Insults
In addition to direct injury mediated by the processes outlined above, the activation of the neuroinflammatory response can also sensitize the brain to the damaging effects of other insults, even when these insults do not cause much injury in and of themselves. For example, a degree of hypoxia-ischemia insufficient to result in overt brain injury caused massive brain injury when the insult was preceded by exposure to lipopolysaccharide in an immature rat model.88
Inflammation, however, can also induce a resistance to damage from insults to the immature brain.89 This preconditioning effect appears to depend upon the timing of the exposure to the inflammatory stimulus in relation to the onset of the injurious insult to the immature brain. While enhanced vulnerability of the developing brain to hypoxic-ischemic insult was observed in 7-day-old rats both in the acute (4- to 6-hour) and the chronic (72-hour) phase after lipopolysaccharide administration, lipopolysaccharide reduced brain injury by 78% when administered 24 hours before the insult.90 Similar preconditioning effects are present in other brain injury models as well.20,89,91–94 These issues further illustrate the concept that perinatal brain damage is more like a dynamic process over an extended period of time rather than a sudden, short-lasting insult (Figure). Multiple cellular and molecular mechanisms are activated along the perinatal time continuum, each with its own particular influence on the various cellular and histological components of the developing central nervous system.20
Protection
Neuroinflammation induces secondary anti-inflammatory cascades.95 Negative feedback mechanisms slow down the progression of inflammation and protect the central nervous system from the various injurious effects of neuroinflammation, thereby preventing extensive brain damage subsequent to inflammation.96 At least part of such mechanisms appear to be orchestrated by what has been termed “neuro-immune crosstalk,” the information exchange between immune and brain cells.97
One important additional level of complexity in this scenario is provided by the hypothalamic-pituitary-adrenal axis, which might play a role as a major anti-inflammatory system in the central nervous system in the setting of systemic inflammation.72 Endotoxemia induces a surge in plasma ACTH and cortisol levels,98 and prostaglandin synthesis can mediate the response of the hypothalamus to systemic inflammation.99 This hypothalamic-pituitary-adrenal activation has protective anti-inflammatory effects on the central nervous system in the setting of inflammation. For example, profound neurodegeneration was found after intracerebral lipopolysaccharide injection in animals pretreated with the glucocorticoid receptor inhibitor Mifepristone (RU486) compared with controls.96 The extent to which the hypothalamic-pituitary-adrenal axis is involved in the anti-inflammatory effects in the setting of fetal inflammation in humans is still unclear. However, the relative adrenal insufficiency of sick preterm newborns100 might help explain their vulnerability to inflammatory brain damage.
A detailed review of potential anti-inflammatory and immunomodulatory strategies to actively protect the preterm brain is provided elsewhere.101,102 However, there is currently no established neonatal intervention that could supplement the only antenatal protective intervention (ie, exposure to glucocorticoid).
In addition to activating anti-inflammatory feedback loops, neuroinflammation can activate some of the endogenous neuroprotective mechanisms that might help decrease the extent of damage subsequent to inflammatory insult. For example, a number of bioactive lipid messengers are formed and released in the local milieu at the site of brain injury and ongoing neurodegeneration.103 Neuroprotectin D1 is a messenger with anti-apoptotic properties that can inhibit upstream signaling of the apoptotic cascade.104 Interleukin-1-beta enhances NPD1 synthesis,105 and may offer cytoprotection to cells adjacent to the site of ongoing neuroinflammation.
Repair
Neuroinflammation can also activate endogenous repair mechanism. Mutual interactions between cytokines and neurotrophic factors characterize normal and disease states.106 For example, the pro-inflammatory cytokines tumor-necrosis-factor and interleukin-6, usually considered damage initiators, can lead to the production of neurotrophins, such as brain-derived neurotrophic factor107 and nerve growth factor,106 respectively, by astrocytes in defined brain regions. Other recent studies suggest a role for macrophages in axonal regeneration,108 and for immune-based regulation of hippocampal neurogenesis (in adults).109 In essence, inflammation probably not only initiates damage but also provides regulatory help in limiting the damage. One possible inference in this context is that a window might be present to modulate fetal inflammatory responses subsequent to the onset of inflammation, with the goal of protecting and repairing the preterm brain after the onset of preterm brain injury.82,110
Summary and Conclusion
The fetal inflammatory response to intrauterine infection plays a crucial role the pathogenesis of preterm birth and preterm brain injury. A neuroinflammatory response in the fetal central nervous system secondary to fetal infection and/or systemic inflammation is a likely pathomechanism of damage to the developing brain. The concurrent activation of anti-inflammatory mechanisms can provide negative feedback loops and induce neuroprotective and perhaps even repair mechanisms in the developing brain. A major concept that deserves further development is that brain injury associated with fetal/neonatal inflammation is not a static, one-point-in-time event, but rather a progression of cellular and molecular cascades in the fetal brain that evolves over time (Figure). This is illustrated by the time-dependent dual effect of inflammation on the central nervous system, either to sensitize the developing brain to subinjurious insults, or to offer a preconditioning neuroprotective effect to various insults. A related evolving concept is that brain injury in the setting of systemic inflammation is not only a destruction of what exists, but also a change of what develops. A better understanding of the different faces of perinatal neuroinflammation will help design interventions to improve neurodevelopmental outcomes after preterm birth.
Acknowledgments
The authors wish to acknowledge support from the Susan B. Saltonstall Fund, the R. Saltonstall Charitable Foundation, and the European Union (LSHM-CT-2006-036534).
Supported by grants from the National Institutes of Health (5R13NS040925-09), the Cerebral Palsy International Research Foundation, the Kennedy Krieger Institute, and the Child Neurology Society.
Footnotes
Presented at the Neurobiology of Disease in Children Conference: Symposium on Injury to the Preterm Brain and Cerebral Palsy, in conjunction with the 37th Annual Meeting of the Child Neurology Society, Santa Clara, California, November 5, 2008.
References
- 1.Dammann O, Leviton A. Inflammatory brain damage in preterm newborns--dry numbers, wet lab, and causal inferences. Early Hum Dev. 2004;79:1–15. doi: 10.1016/j.earlhumdev.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 2.Dammann O, O'Shea TM. Cytokines and perinatal brain damage. Clin Perinatol. 2008;35:643–663, v. doi: 10.1016/j.clp.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edwards AD, Tan S. Perinatal infections, prematurity and brain injury. Curr Opin Pediatr. 2006;18:119–124. doi: 10.1097/01.mop.0000193290.02270.30. [DOI] [PubMed] [Google Scholar]
- 4.Kadhim HJ, Duchateau J, Sebire G. Cytokines and brain injury: invited review. J Intensive Care Med. 2008;23:236–249. doi: 10.1177/0885066608318458. [DOI] [PubMed] [Google Scholar]
- 5.Cheynier JM. Premature delivery: with reference to certain etiological data and the role of cervico-vaginal infections. Rev Fr Gynecol Obstet. 1974;69:479–486. [PubMed] [Google Scholar]
- 6.Erdmann G. Listeriosis and premature birth. Dtsc Med Wochenschr. 1953;78:813–815. doi: 10.1055/s-0028-1131371. [DOI] [PubMed] [Google Scholar]
- 7.Henderson M. Significant bacteriuria and duration of pregnancy. Arch Environ Health. 1964;8:527–530. doi: 10.1080/00039896.1964.10663712. [DOI] [PubMed] [Google Scholar]
- 8.Mackay EV. Premature labour and still birth resulting from ante-natal infection with Listeria monocytogenes. Med J Aust. 1962;49:852–854. [PubMed] [Google Scholar]
- 9.Goldenberg RL, Culhane JF, Johnson DC. Maternal infection and adverse fetal and neonatal outcomes. Clin Perinatol. 2005 Sep;32(3):523–559. doi: 10.1016/j.clp.2005.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gotsch F, Romero R, Kusanovic JP, et al. The fetal inflammatory response syndrome. Clinical Obstet Gynecol. 2007;50:652–683. doi: 10.1097/GRF.0b013e31811ebef6. [DOI] [PubMed] [Google Scholar]
- 11.Romero R, Espinoza J, Goncalves LF, et al. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25:21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dammann O, Phillips TM, Allred EN, et al. Mediators of fetal inflammation in extremely low gestational age newborns. Cytokine. 2001;13:234–239. doi: 10.1006/cyto.2000.0820. [DOI] [PubMed] [Google Scholar]
- 13.Hecht JL, Allred EN, Kliman HJ, et al. Histological characteristics of singleton placentas delivered before the 28th week of gestation. Pathology. 2008;40:372–376. doi: 10.1080/00313020802035865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hecht JL, Onderdonk A, Delaney M, et al. Characterization of chorioamnionitis in 2nd-trimester C-section placentas and correlation with microorganism recovery from subamniotic tissues. Ped Dev Pathol. 2008;11:15–22. doi: 10.2350/07-06-0285.1. [DOI] [PubMed] [Google Scholar]
- 15.Laughon M, Bose C, Allred E, et al. Factors associated with treatment for hypotension in extremely low gestational age newborns during the first postnatal week. Pediatrics. 2007;119:273–280. doi: 10.1542/peds.2006-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McElrath TF, Hecht JL, Dammann O, et al. Pregnancy disorders that lead to delivery before the 28th week of gestation: an epidemiologic approach to classification. Am J Epidemiol. 2008;168:980–989. doi: 10.1093/aje/kwn202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Onderdonk AB, Delaney ML, DuBois AM, et al. Extremely Low Gestational Age Newborns Study. I. Detection of bacteria in placental tissues obtained from extremely low gestational age neonates. Am J Obstet Gynecol. 2008;198:110.e111–110.e117. doi: 10.1016/j.ajog.2007.05.044. [DOI] [PubMed] [Google Scholar]
- 18.Onderdonk AB, Hecht JL, McElrath TF, et al. Colonization of second-trimester placenta parenchyma. Am J Obstet Gynecol. 2008;199:52.e51–52.e10. doi: 10.1016/j.ajog.2007.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abrahams VM, Bole-Aldo P, Kim YM, et al. Divergent trophoblast responses to bacterial products mediated by TLRs. J Immunol. 2004;173:4286–4296. doi: 10.4049/jimmunol.173.7.4286. [DOI] [PubMed] [Google Scholar]
- 20.Mallard C, Hagberg H. Inflammation-induced preconditioning in the immature brain. Semin Fetal Neonatal Med. 2007;12:280–286. doi: 10.1016/j.siny.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 21.Dammann O, Allred EN, Leviton A, et al. Fetal vasculitis in preterm newborns: interrelationships, modifiers, and antecedents. Placenta. 2004;25:788–796. doi: 10.1016/j.placenta.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Redline RW. Placental inflammation. Semin Neonatol. 2004;9:265–274. doi: 10.1016/j.siny.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Srinivas SK, Ma Y, Sammel MD, et al. Placental inflammation and viral infection are implicated in second trimester pregnancy loss. Am J Obstet Gynecol. 2006;195:797–802. doi: 10.1016/j.ajog.2006.05.049. [DOI] [PubMed] [Google Scholar]
- 24.Ancel PY, Marret S, Larroque B, et al. Are maternal hypertension and small-for-gestational age risk factors for severe intraventricular hemorrhage and cystic periventricular leukomalacia? Results of the EPIPAGE cohort study. Am J Obstet Gynecol. 2005;193:178–184. doi: 10.1016/j.ajog.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 25.Dammann O, Allred EN, Veelken N. Increased risk of spastic diplegia among very low birth weight children after preterm labor or prelabor rupture of membranes. J Pediatr. 1998;132(3 Pt 1):531–535. doi: 10.1016/s0022-3476(98)70035-6. [DOI] [PubMed] [Google Scholar]
- 26.Leviton A, Gilles F, Neff R, Yaney P. Multivariate analysis of risk of perinatal telencephalic leucoencephalopathy. Am J Epidemiol. 1976;104:621–626. doi: 10.1093/oxfordjournals.aje.a112340. [DOI] [PubMed] [Google Scholar]
- 27.Adinolfi M. Infectious diseases in pregnancy, cytokines and neurological impairment: an hypothesis. Dev Med Child Neurol. 1993;35:549–553. [PubMed] [Google Scholar]
- 28.Badawi N, Kurinczuk JJ, Keogh JM, et al. Intrapartum risk factors for newborn encephalopathy: the Western Australian case-control study. BMJ. 1998;317:1554–1558. doi: 10.1136/bmj.317.7172.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blume HK, Li CI, Loch CM, Koepsell TD. Intrapartum fever and chorioamnionitis as risks for encephalopathy in term newborns: a case-control study. Dev Med Child Neurol. 2008;50:19–24. doi: 10.1111/j.1469-8749.2007.02007.x. [DOI] [PubMed] [Google Scholar]
- 30.Dammann O, Drescher J, Veelken N. Maternal fever at birth and non-verbal intelligence at age 9 years in preterm infants. Dev Med Child Neurol. 2003;45:148–151. doi: 10.1017/s001216220300029x. [DOI] [PubMed] [Google Scholar]
- 31.Dammann O, Leviton A. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr Res. 1997;42:1–8. doi: 10.1203/00006450-199707000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Dammann O, Leviton A. Infection remote from the brain, neonatal white matter damage, and cerebral palsy in the preterm infant. Semin Pediatr Neurol. 1998;5:190–201. doi: 10.1016/s1071-9091(98)80034-x. [DOI] [PubMed] [Google Scholar]
- 33.Gilles FH, Averill DR, Jr, Kerr CS. Neonatal endotoxin encephalopathy. Ann Neurol. 1977;2:49–56. doi: 10.1002/ana.410020108. [DOI] [PubMed] [Google Scholar]
- 34.Hagberg H, Mallard C. Effect of inflammation on central nervous system development and vulnerability. Current Opin Neurol. 2005;18:117–123. doi: 10.1097/01.wco.0000162851.44897.8f. [DOI] [PubMed] [Google Scholar]
- 35.Leviton A. Preterm birth and cerebral palsy: is tumor necrosis factor the missing link? Developmental medicine and child neurology. 1993 Jun;35(6):553–558. doi: 10.1111/j.1469-8749.1993.tb11688.x. [DOI] [PubMed] [Google Scholar]
- 36.Nelson KB, Ellenberg JH. Predictors of low and very low birth weight and the relation of these to cerebral palsy. JAMA. 1985;254:1473–1479. [PubMed] [Google Scholar]
- 37.Ornoy A, Altshuler G. Maternal endotoxemia, fetal anomalies, and central nervous system damage: a rat model of a human problem. Am J Obstet Gynecol. 1976;124:196–204. doi: 10.1016/s0002-9378(16)33298-7. [DOI] [PubMed] [Google Scholar]
- 38.Silverstein FS, Barks JD, Hagan P, Liu XH, Ivacko J, Szaflarski J. Cytokines and perinatal brain injury. Neurochem Int. 1997;30:375–383. doi: 10.1016/s0197-0186(96)00072-1. [DOI] [PubMed] [Google Scholar]
- 39.Stoll BJ, Hansen NI, Adams-Chapman I, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292:2357–2365. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 40.Verma U, Tejani N, Klein S, et al. Obstetric antecedents of intraventricular hemorrhage and periventricular leukomalacia in the low-birth-weight neonate. Am J Obstet Gynecol. 1997;176:275–281. doi: 10.1016/s0002-9378(97)70485-x. [DOI] [PubMed] [Google Scholar]
- 41.Wu YW. Systematic review of chorioamnionitis and cerebral palsy. Ment Retard Dev Disabil Res Rev. 2002;8:25–29. doi: 10.1002/mrdd.10003. [DOI] [PubMed] [Google Scholar]
- 42.Wu YW, Colford JM., Jr Chorioamnionitis as a risk factor for cerebral palsy: A meta-analysis. JAMA. 2000;284:1417–1424. doi: 10.1001/jama.284.11.1417. [DOI] [PubMed] [Google Scholar]
- 43.Wu YW, Escobar GJ, Grether JK, et al. Chorioamnionitis and cerebral palsy in term and near-term infants. JAMA. 2003;290:2677–2684. doi: 10.1001/jama.290.20.2677. [DOI] [PubMed] [Google Scholar]
- 44.Yoon BH, Romero R, Yang SH, et al. Interleukin-6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia. Am J Obstet Gynecol. 1996;174:1433–1440. doi: 10.1016/s0002-9378(96)70585-9. [DOI] [PubMed] [Google Scholar]
- 45.Dudley DJ, Trautman MS, Mitchell MD. Inflammatory mediators regulate interleukin-8 production by cultured gestational tissues: evidence for a cytokine network at the chorio-decidual interface. J Clin Endocrinol Metab. 1993;76:404–410. doi: 10.1210/jcem.76.2.8432783. [DOI] [PubMed] [Google Scholar]
- 46.Craven CM, Ward K. Fetal endothelial cells express vascular cell adhesion molecule in the setting of chorioamnionitis. Am J Reprod Immunol. 2000;43:259–263. doi: 10.1111/j.8755-8920.2000.430503.x. [DOI] [PubMed] [Google Scholar]
- 47.D'Alquen D, Kramer BW, Seidenspinner S, et al. Activation of umbilical cord endothelial cells and fetal inflammatory response in preterm infants with chorioamnionitis and funisitis. Pediatr Res. 2005;57:263–269. doi: 10.1203/01.PDR.0000148713.48218.86. [DOI] [PubMed] [Google Scholar]
- 48.Naccasha N, Hinson R, Montag A, et al. Association between funisitis and elevated interleukin-6 in cord blood. Obstet Gynecol. 2001;97:220–224. doi: 10.1016/s0029-7844(00)01149-2. [DOI] [PubMed] [Google Scholar]
- 49.Kim CJ, Yoon BH, Park SS, et al. Acute funisitis of preterm but not term placentas is associated with severe fetal inflammatory response. Hum Pathol. 2001;32:623–629. doi: 10.1053/hupa.2001.24992. [DOI] [PubMed] [Google Scholar]
- 50.Jaswon MS, Linch DC. Priming of neutrophil function in the newborn. Br J Haematol. 2001;113:1078–1079. doi: 10.1046/j.1365-2141.2001.02821-3.x. [DOI] [PubMed] [Google Scholar]
- 51.Yachie A, Takano N, Yokoi T, et al. The capability of neonatal leukocytes to produce IL-6 on stimulation assessed by whole blood culture. Pediatr Res. 1990;27:227–233. doi: 10.1203/00006450-199003000-00005. [DOI] [PubMed] [Google Scholar]
- 52.Duggan PJ, Maalouf EF, Watts TL, et al. Intrauterine T-cell activation and increased proinflammatory cytokine concentrations in preterm infants with cerebral lesions. Lancet. 2001;358:1699–1700. doi: 10.1016/s0140-6736(01)06723-x. [DOI] [PubMed] [Google Scholar]
- 53.Dammann O, Durum S, Leviton A. Do white cells matter in white matter damage? Trends Neurosci. 2001;24:320–324. doi: 10.1016/s0166-2236(00)01811-7. [DOI] [PubMed] [Google Scholar]
- 54.Leviton A, Dammann O, Durum SK. The adaptive immune response in neonatal cerebral white matter damage. Ann Neurol. 2005;58:821–828. doi: 10.1002/ana.20662. [DOI] [PubMed] [Google Scholar]
- 55.Dammann O. Persistent neuro-inflammation in cerebral palsy: a therapeutic window of opportunity? Acta Paediatrica. 2007;96:6–7. doi: 10.1111/j.1651-2227.2007.00097.x. [DOI] [PubMed] [Google Scholar]
- 56.Leviton A, Dammann O. Coagulation, inflammation, and the risk of neonatal white matter damage. Pediatr Res. 2004;55:541–545. doi: 10.1203/01.PDR.0000121197.24154.82. [DOI] [PubMed] [Google Scholar]
- 57.Blatteis CM, Li S, Li Z, et al. Signaling the brain in systemic inflammation: the role of complement. Front Biosci. 2004;9:915–931. doi: 10.2741/1297. [DOI] [PubMed] [Google Scholar]
- 58.Taylor FB., Jr Staging of the pathophysiologic responses of the primate microvasculature to Escherichia coli and endotoxin: examination of the elements of the compensated response and their links to the corresponding uncompensated lethal variants. Crit Care Med. 2001;29(7 Suppl):S78–S89. doi: 10.1097/00003246-200107001-00026. [DOI] [PubMed] [Google Scholar]
- 59.Gomez R, Romero R, Ghezzi F, et al. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;179:194–202. doi: 10.1016/s0002-9378(98)70272-8. [DOI] [PubMed] [Google Scholar]
- 60.Dammann O, Allred EN, Kuban KC, et al. Systemic hypotension and white-matter damage in preterm infants. Dev Med Child Neurol. 2002;44:82–90. doi: 10.1017/s0012162201001724. [DOI] [PubMed] [Google Scholar]
- 61.Limperopoulos C, Bassan H, Kalish LA, et al. Current definitions of hypotension do not predict abnormal cranial ultrasound findings in preterm infants. Pediatrics. 2007;120:966–977. doi: 10.1542/peds.2007-0075. [DOI] [PubMed] [Google Scholar]
- 62.Duncan JR, Cock ML, Suzuki K, et al. Chronic endotoxin exposure causes brain injury in the ovine fetus in the absence of hypoxemia. J Soc Gynecol Investig. 2006;13:87–96. doi: 10.1016/j.jsgi.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 63.Mallard C, Welin AK, Peebles D, et al. White matter injury following systemic endotoxemia or asphyxia in the fetal sheep. Neurochem Res. 2003;28:215–223. doi: 10.1023/a:1022368915400. [DOI] [PubMed] [Google Scholar]
- 64.Peebles DM, Miller S, Newman JP, et al. The effect of systemic administration of lipopolysaccharide on cerebral haemodynamics and oxygenation in the 0.65 gestation ovine fetus in utero. BJOG. 2003;110:735–743. [PubMed] [Google Scholar]
- 65.Ek CJ, Dziegielewska KM, et al. Functional effectiveness of the blood-brain barrier to small water-soluble molecules in developing and adult opossum (Monodelphis domestica) J Comp Neurol. 2006;496:13–26. doi: 10.1002/cne.20885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ek CJ, Habgood MD, Dziegielewska KM, Saunders NR. Structural characteristics and barrier properties of the choroid plexuses in developing brain of the opossum (Monodelphis Domestica) J Comp Neurol. 2003;460:451–464. doi: 10.1002/cne.10661. [DOI] [PubMed] [Google Scholar]
- 67.Ek M, Engblom D, Saha S, et al. Inflammatory response: pathway across the blood-brain barrier. Nature. 2001;410:430–431. doi: 10.1038/35068632. [DOI] [PubMed] [Google Scholar]
- 68.Engblom D, Ek M, Saha S, et al. Prostaglandins as inflammatory messengers across the blood-brain barrier. J Mol Med. 2002;80:5–15. doi: 10.1007/s00109-001-0289-z. [DOI] [PubMed] [Google Scholar]
- 69.Ganong WF. Circumventricular organs: definition and role in the regulation of endocrine and autonomic function. Clin Exp Pharmacol Physiol. 2000;27:422–427. doi: 10.1046/j.1440-1681.2000.03259.x. [DOI] [PubMed] [Google Scholar]
- 70.Hashimoto M, Ishikawa Y, Yokota S, et al. Action site of circulating interleukin-1 on the rabbit brain. Brain Res. 1991;540:217–223. doi: 10.1016/0006-8993(91)90510-3. [DOI] [PubMed] [Google Scholar]
- 71.Laflamme N, Rivest S. Toll-like receptor 4: the missing link of the cerebral innate immune response triggered by circulating gram-negative bacterial cell wall components. FASEB J. 2001;15:155–163. doi: 10.1096/fj.00-0339com. [DOI] [PubMed] [Google Scholar]
- 72.Rivest S. Molecular insights on the cerebral innate immune system. Brain Behav Immun. 2003;17:13–19. doi: 10.1016/s0889-1591(02)00055-7. [DOI] [PubMed] [Google Scholar]
- 73.Krizanac-Bengez L, Mayberg MR, Cunningham E, et al. Loss of shear stress induces leukocyte-mediated cytokine release and blood-brain barrier failure in dynamic in vitro blood-brain barrier model. J Cell Physiol. 2006;206:68–77. doi: 10.1002/jcp.20429. [DOI] [PubMed] [Google Scholar]
- 74.Willis CL, Davis TP. Chronic inflammatory pain and the neurovascular unit: a central role for glia in maintaining BBB integrity? Curr Pharm Des. 2008;14:1625–1643. doi: 10.2174/138161208784705414. [DOI] [PubMed] [Google Scholar]
- 75.Banks WA, Kastin AJ, Broadwell RD. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation. 1995;2:241–248. doi: 10.1159/000097202. [DOI] [PubMed] [Google Scholar]
- 76.Stolp HB, Dziegielewska KM, Ek CJ, et al. Breakdown of the blood-brain barrier to proteins in white matter of the developing brain following systemic inflammation. Cell Tissue Res. 2005;320:369–378. doi: 10.1007/s00441-005-1088-6. [DOI] [PubMed] [Google Scholar]
- 77.Stolp HB, Dziegielewska KM, Ek CJ, et al. Long-term changes in blood-brain barrier permeability and white matter following prolonged systemic inflammation in early development in the rat. Eur J Neurosci. 2005;22:2805–2816. doi: 10.1111/j.1460-9568.2005.04483.x. [DOI] [PubMed] [Google Scholar]
- 78.Stolp HB, Ek CJ, Johansson PA, et al. Effect of minocycline on inflammation-induced damage to the blood-brain barrier and white matter during development. Eur J Neurosci. 2007;26:3465–3474. doi: 10.1111/j.1460-9568.2007.05973.x. [DOI] [PubMed] [Google Scholar]
- 79.Stolp H, Dziegielewska K. Role of developmental inflammation and blood-brain barrier dysfunction in neurodevelopmental and neurodegenerative diseases. Neuropathol Appl Neurobiol. 2008 doi: 10.1111/j.1365-2990.2008.01005.x. [DOI] [PubMed] [Google Scholar]
- 80.Lehnardt S, Lachance C, Patrizi S, et al. The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J Neurosc. 2002;22:2478–2486. doi: 10.1523/JNEUROSCI.22-07-02478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Back SA, Rivkees SA. Emerging concepts in periventricular white matter injury. Semin Perinatol. 2004;28:405–414. doi: 10.1053/j.semperi.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 82.Dammann O, Leviton A. Brain damage in preterm newborns: might enhancement of developmentally regulated endogenous protection open a door for prevention? Pediatrics. 1999;104(3 Pt 1):541–550. doi: 10.1542/peds.104.3.541. [DOI] [PubMed] [Google Scholar]
- 83.Khwaja O, Volpe JJ. Pathogenesis of cerebral white matter injury of prematurity. Arch Dis Child Fetal Neonatal Ed. 2008;93:F153–F161. doi: 10.1136/adc.2006.108837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kinney HC, Back SA. Human oligodendroglial development: relationship to periventricular leukomalacia. Semin Pediatr Neurol. 1998;5:180–189. doi: 10.1016/s1071-9091(98)80033-8. [DOI] [PubMed] [Google Scholar]
- 85.Back SA, Riddle A, McClure MM. Maturation-dependent vulnerability of perinatal white matter in premature birth. Stroke. 2007;38(2 Suppl):724–730. doi: 10.1161/01.STR.0000254729.27386.05. [DOI] [PubMed] [Google Scholar]
- 86.Dammann O, Hagberg H, Leviton A. Is periventricular leukomalacia an axonopathy as well as an oligopathy? Pediatric Res. 2001;49:453–457. doi: 10.1203/00006450-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 87.Leviton A, Gressens P. Neuronal damage accompanies perinatal white-matter damage. Trends Neurosci. 2007;30:473–478. doi: 10.1016/j.tins.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 88.Eklind S, Mallard C, Leverin AL, et al. Bacterial endotoxin sensitizes the immature brain to hypoxic--ischaemic injury. Eur J Neurosci. 2001;13:1101–1106. doi: 10.1046/j.0953-816x.2001.01474.x. [DOI] [PubMed] [Google Scholar]
- 89.Hagberg H, Dammann O, Mallard C, Leviton A. Preconditioning and the developing brain. Semin Perinatol. 2004;28:389–395. doi: 10.1053/j.semperi.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 90.Eklind S, Mallard C, Arvidsson P, Hagberg H. Lipopolysaccharide induces both a primary and a secondary phase of sensitization in the developing rat brain. Pediatr Res. 2005;58:112–116. doi: 10.1203/01.PDR.0000163513.03619.8D. [DOI] [PubMed] [Google Scholar]
- 91.Furuya K, Zhu L, Kawahara N, et al. Differences in infarct evolution between lipopolysaccharide-induced tolerant and nontolerant conditions to focal cerebral ischemia. J Neurosurg. 2005;103:715–723. doi: 10.3171/jns.2005.103.4.0715. [DOI] [PubMed] [Google Scholar]
- 92.Huang CY, Yang HI, Chen SD, et al. Protective effects of lipopolysaccharide preconditioning against nitric oxide neurotoxicity. J Neurosci Res. 2008;86:1277–1289. doi: 10.1002/jnr.21594. [DOI] [PubMed] [Google Scholar]
- 93.Orio M, Kunz A, Kawano T, et al. Lipopolysaccharide induces early tolerance to excitotoxicity via nitric oxide and cGMP. Stroke. 2007;38:2812–2817. doi: 10.1161/STROKEAHA.107.486837. [DOI] [PubMed] [Google Scholar]
- 94.Rosenzweig HL, Lessov NS, Henshall DC, et al. Endotoxin preconditioning prevents cellular inflammatory response during ischemic neuroprotection in mice. Stroke. 2004;35:2576–2581. doi: 10.1161/01.STR.0000143450.04438.ae. [DOI] [PubMed] [Google Scholar]
- 95.Chikanza IC, Grossman AB. Neuroendocrine immune responses to inflammation: the concept of the neuroendocrine immune loop. Baillieres Clin Rheumatol. 1996;10:199–225. doi: 10.1016/s0950-3579(96)80015-x. [DOI] [PubMed] [Google Scholar]
- 96.Nadeau S, Rivest S. Glucocorticoids play a fundamental role in protecting the brain during innate immune response. J Neurosci. 2003;23:5536–5544. doi: 10.1523/JNEUROSCI.23-13-05536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kerschensteiner M, Meinl E, Hohlfeld R. Neuro-immune crosstalk in CNS diseases. Neuroscience. 2008 doi: 10.1016/j.neuroscience.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 98.Givalois L, Dornand J, Mekaouche M, et al. Temporal cascade of plasma level surges in ACTH, corticosterone, and cytokines in endotoxin-challenged rats. Am J Physiol. 1994;267(1 Pt 2):R164–R170. doi: 10.1152/ajpregu.1994.267.1.R164. [DOI] [PubMed] [Google Scholar]
- 99.Rivest S, Lacroix S, Vallieres L, et al. How the blood talks to the brain parenchyma and the paraventricular nucleus of the hypothalamus during systemic inflammatory and infectious stimuli. Proc Soc Exp Biol Med. 2000;223:22–38. doi: 10.1046/j.1525-1373.2000.22304.x. [DOI] [PubMed] [Google Scholar]
- 100.Watterberg KL. Adrenocortical function and dysfunction in the fetus and neonate. Semin Neonatol. 2004;9:13–21. doi: 10.1016/j.siny.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 101.Dammann O, Leviton A. Possible strategies to protect the preterm brain against the fetal inflammatory response. Dev Med Child Neurol. 2001;86:18–20. doi: 10.1111/j.1469-8749.2001.tb04141.x. [DOI] [PubMed] [Google Scholar]
- 102.Wolfberg AJ, Dammann O, Gressens P. Anti-inflammatory and immunomodulatory strategies to protect the perinatal brain. Semin Fetal Neonatal Med. 2007;12:296–302. doi: 10.1016/j.siny.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 103.Bazan NG. The onset of brain injury and neurodegeneration triggers the synthesis of docosanoid neuroprotective signaling. Cell Mol Neurobiol. 2006;26:901–913. doi: 10.1007/s10571-006-9064-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci U S A. 2004;101:8491–8496. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bazan NG. Neuroprotectin D1 (NPD1): a DHA-derived mediator that protects brain and retina against cell injury-induced oxidative stress. Brain Pathol. 2005;15:159–166. doi: 10.1111/j.1750-3639.2005.tb00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Otten U, Marz P, Heese K, et al. Cytokines and neurotrophins interact in normal and diseased states. Ann N Y Acad Sci. 2000;917:322–330. doi: 10.1111/j.1749-6632.2000.tb05398.x. [DOI] [PubMed] [Google Scholar]
- 107.Saha RN, Liu X, Pahan K. Up-regulation of BDNF in astrocytes by TNF-alpha: a case for the neuroprotective role of cytokine. J Neuroimmune Pharmacol. 2006;1:212–222. doi: 10.1007/s11481-006-9020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Benowitz L, Yin Y. Rewiring the injured CNS: lessons from the optic nerve. Exp Neurol. 2008;209:389–398. doi: 10.1016/j.expneurol.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ziv Y, Schwartz M. Immune-based regulation of adult neurogenesis: implications for learning and memory. Brain Behav Immun. 2008;22:167–176. doi: 10.1016/j.bbi.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 110.Crutcher KA, Gendelman HE, Kipnis J, et al. Debate: "is increasing neuroinflammation beneficial for neural repair?". J Neuroimmune Pharmacol. 2006;1:195–211. doi: 10.1007/s11481-006-9021-7. [DOI] [PubMed] [Google Scholar]