Abstract
Rationale
A rise in cytosolic Ca2+ concentration ([Ca2+]cyt) in pulmonary arterial smooth muscle cells (PASMC) is an important stimulus for pulmonary vasoconstriction and vascular remodeling. Increased resting [Ca2+]cyt and enhanced Ca2+ influx have been implicated in PASMC from patients with idiopathic pulmonary arterial hypertension (IPAH).
Objective
We examined whether the extracellular Ca2+-sensing receptor (CaSR) is involved in the enhanced Ca2+ influx and proliferation in IPAH-PASMC and whether blockade of CaSR inhibits experimental pulmonary hypertension.
Methods and Results
In normal PASMC superfused with Ca2+-free solution, addition of 2.2 mM Ca2+ to the perfusate had little effect on [Ca2+]cyt. In IPAH-PASMC, however, restoration of extracellular Ca2+ induced a significant increase in [Ca2+]cyt. Extracellular application of spermine also markedly raised [Ca2+]cyt in IPAH-PASMC, but not in normal PASMC. The calcimimetic R568 enhanced, whereas the calcilytic NPS 2143 attenuated, the extracellular Ca2+-induced [Ca2+]cyt rise in IPAH-PASMC. Furthermore, the protein expression level of CaSR in IPAH-PASMC was greater than in normal PASMC; knockdown of CaSR in IPAH-PASMC with siRNA attenuated the extracellular Ca2+-mediated [Ca2+]cyt increase and inhibited IPAH-PASMC proliferation. Using animal models of pulmonary hypertension, our data showed that CaSR expression and function were both enhanced in PASMC, whereas intraperitoneal injection of the calcilytic NPS 2143 prevented the development of pulmonary hypertension and right ventricular hypertrophy in rats injected with monocrotaline and mice exposed to hypoxia.
Conclusions
The extracellular Ca2+-induced increase in [Ca2+]cyt due to upregulated CaSR is a novel pathogenic mechanism contributing to the augmented Ca2+ influx and excessive PASMC proliferation in patients and animals with pulmonary arterial hypertension.
Keywords: Pulmonary artery, smooth muscle cell, proliferation, G protein-coupled receptor, pulmonary hypertension, receptors
INTRODUCTION
Idiopathic pulmonary arterial hypertension (IPAH) is a fatal and progressive disease with unidentified etiological causes. Pulmonary vascular remodeling and sustained pulmonary vasoconstriction are two major causes for the elevated pulmonary vascular resistance and pulmonary arterial pressure in patients with IPAH. A central aspect of pulmonary vascular remodeling is intimal and medial hypertrophy caused by enhanced proliferation and inhibited apoptosis of pulmonary arterial smooth muscle cells (PASMC)1. An increase in cytosolic free Ca2+ concentration ([Ca2+]cyt) in PASMC is a major trigger for pulmonary vasoconstriction and an important stimulus for PASMC proliferation leading to pulmonary vascular remodeling2-4.
In PASMC, [Ca2+]cyt can be increased by Ca2+ release from the intracellular stores and Ca2+ influx through plasmalemmal Ca2+ channels1, 5. PASMC express various Ca2+-permeable channels including voltage-dependent Ca2+ channels (VDCC), receptor-operated Ca2+ channels (ROC), and store-operated Ca2+ channels (SOC). VDCC are activated by membrane depolarization. ROC channels are mainly opened by vasoconstrictors (e.g., endothelin-1 and serotonin) via an intracellular second messenger, diacylglycerol (DAG), and by growth factors (e.g., epidermal growth factor and platelet-derived growth factor). Activation of ROC by interaction with ligands greatly contributes to the increase in [Ca2+]cyt due to receptor-operated Ca2+ entry (ROCE) in PASMC. Upon activation of membrane receptors and subsequent increase in inositol 1,4,5-trisphosphate (IP3) synthesis, SOC are opened by the depletion of Ca2+ from the sarcoplasmic reticulum (SR), an important intracellular store in PASMC, to elicit store-operated Ca2+ entry (SOCE) or capacitative Ca2+ entry (CCE). SOCE is an important mechanism involved in maintaining a sustained elevation of [Ca2+]cyt and refilling Ca2+ into the SR.
We have previously demonstrated that the resting [Ca2+]cyt is increased, while SOCE and ROCE are both enhanced in PASMC isolated from IPAH patients in comparison to PASMC isolated from normal subjects and patients without pulmonary hypertension6, 7. We have also shown that increased Ca2+ influx during PASMC proliferation is due largely to the enhancement of SOCE; downregulation and blockade of SOC significantly inhibits PASMC proliferation. These results indicate that SOCE plays an important role in regulating cell proliferation in vascular smooth muscle cells2-4, 8. Furthermore, expression and activity of ROC and SOC are both upregulated in PASMC isolated from patients with IPAH and animals with hypoxia-induced pulmonary hypertension2, 9-11.
Opening of ROC and SOC is caused initially by activation of various membrane receptors including G protein-coupled receptors (GPCR) and receptor tyrosine kinases (RTK). The extracellular Ca2+-sensing receptor (CaSR) is a member of GPCR subfamily C (also known as GPRC2A)12-14 which was originally identified in the parathyroid glands and is activated by its ligands including Ca2+, Gd3+, Mg2+, polyamines, antibiotics and amino acids12, 15. The CaSR is involved in multiple cellular processes of the parathyroid glands in response to changes in serum Ca2+ concentrations such as proliferation, differentiation, and apoptosis12, 13, 15. In addition to parathyroid glands, the CaSR is also expressed in kidney, bone, smooth muscle, endothelium, gastrointestinal tract and brain13, 16-19. The physiological and pathological significance of CaSR in the development and progression of pulmonary arterial hypertension, however, remains unknown.
In this study, we examined whether CaSR was involved in the enhanced Ca2+ signaling and augmented proliferation in PASMC from patients with IPAH and whether inhibition of CaSR attenuates IPAH-PASMC proliferation and prevents the development of experimental pulmonary hypertension in animal models.
METHODS
Preparation of human and animal PASMC
Human PASMC were isolated from normal control subjects and patients with IPAH and chronic thromboembolic pulmonary hypertension (CTEPH).8, 20, 21 Approval to use the human lung tissues and cells was granted by the UIC Institutional Review Board. Human PASMC were cultured in Medium 199 supplemented with 10% fetal bovine serum at 37°C. The cells at passages 5-8 were used for the experiments. In some experiments, we also used freshly-dissociated PASMC from rats22 and PASMC from mice.23
[Ca2+]cyt measurement
[Ca2+]cyt was measured in PASMC using fura-2 and a Nikon digital fluorescent imaging system24. Cells were loaded with 4 μM fura-2 acetoxymethyl ester (fura-2/AM) for 60 min at 25°C and [Ca2+]cyt was measured using a ratiometric method at 32°C.
Western blot
Solubilized protein isolated from PASMC, pulmonary arteries and lung tissues was loaded on an 8% acrylamide gel, transferred to an Immobilon-P transfer membrane (Millipore, Bedford, MA), and immunoblotted with anti-CaSR monoclonal antibody (MA1-934, 1:200; Thermo Scientific, Rockford, IL). To isolate the fraction of cellular membrane, the cell lysate was centrifuged at 100,000×g and then the pellet was re-suspended to use for western blot analysis. Signals were detected using a Super Signal West Pico Chemiluminescent Substrate (Thermo Scientific). The protein levels were normalized to β-tubulin (sc-9104, 1:200; Santa Cruz Biotechnology) and expressed in arbitrary units.
Transfection of cDNA and small interfering RNA (siRNA)
PASMC were transiently transfected with vector cDNA (2 μg, pcDNA3.1(+)), human CaSR cDNA (2 μg), control siRNA (50 nM, sc-37007; Santa Cruz Biotechnology), or CaSR siRNA (50 nM, s2440; Applied Biosystems, Austin, TX) using an Amaxa Basic Nucleofector kit for primary smooth muscle cells (Lonza). [Ca2+]cyt measurements and western blot using cDNA- and siRNA-transfected cells were preformed 48-72 h after electroporation.
Proliferation assay
Proliferation of PASMC was determined using an automated cell counter (TC10; Bio-Rad Laboratories, Hercules, CA). At 48 h after subculture, PASMC were counted and re-plated (0 h) into 8-well multidishes (Nunclon Δ, 10.5 cm2 of culture area per well; Thermo Scientific) with 1×105 cells/well (0.95×104 cells/cm2).
Preparation of MCT-PH rats and HPH mice
All experiments were approved by the Ethics/Animal Care Committee of University of Illinois at Chicago. For MCT-PH rat experiments, male Sprague-Dawley rats (190-200 g) were treated with single subcutaneous (s.c.) injection of vehicle (dimethyl sulfoxide, DMSO) or 60 mg/kg MCT. For NPS 2143-treated group, rats were intraperitoneally injected (i.p.) with NPS 2143 at a dose of 4.5 mg/kg per day (from day 1 to 10). Fourteen days after injection, rats were anesthetized with ketamine/xylazine and then RVSP was measured using an MPVS Ultra system (Millar Instruments). For HPH mouse experiments, male mice (8-week-old C57BL/6) were exposed to hypoxia (10% O2) in a ventilated chamber to develop pulmonary hypertension. For NPS 2143-treated group, mice were injected (i.p.) with NPS 2143 (1.0 mg/kg per day; from day 1 to 22). Four weeks after exposure to normobaric hypoxia, mice were anesthetized with ketamine/xylazine, and then RVSP was measured by right heart catheterization.
Reverse transcription-polymerase chain reaction (RT-PCR)
The extraction of total RNA from rat PASMC and the reverse transcription were performed using TRIzol Reagent (Invitrogen) and High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA), respectively. For semi-quantitative analysis of mRNA, Platinum PCR SuperMix (Invitrogen) and specific primers for rat CaSR and GAPDH were used. Quantitative real-time PCR analysis was performed based on the SYBR assay (SYBR Green Master Mix; Roche Applied Science, Indianapolis, IN) using gene-specific primers for rat CaSR and GAPDH on a Bio-Rad CFX384 Real-Time System C1000 Thermal Cycler system (Bio-Rad Laboratories, Hercules, CA).
Immunohistochemical and Hematoxylin-Eosin (H&E) staining
Immunohistochemistry and H&E staining were performed using formalin-fixed and paraffin-embedded sections (3 μm) from left lung lobes of rats. The tissue sections were treated with PBS containing 5% normal goat serum and either CaSR (1:100 dilution) or SM α-actin (1:100; EMD Millipore) antibody for 12 h at 4°C. After washing repeatedly in PBS, the sections were covered with PBS containing Alexa Fluor 488-labeled (1:500 dilution) or Cy3-labeled (1:500) secondary antibody for 1 h at room temperature and then rinsed with PBS. Then sections were mounted in VECTASHIELD hard-set mounting medium with DAPI (1.5 μg/ml). To measure the external diameter of pulmonary arteries stained with H&E, the microscopic images were analyzed using ImageScope software (Ver.11).
Drugs
Pharmacological reagents were obtained from Sigma-Aldrich except for KB-R7943, NPS 2143, and R568 (Tocris, Ellisville, MO). All hydrophobic compounds were dissolved in DMSO at the concentration of 10 or 100 mM as a stock solution.
Statistical analysis
Composite data are shown as the mean±S.E. The statistical significance between two groups was determined by Student’s t-test. The statistical significance among groups was determined by Scheffé’s test after one-way analysis of variance. Significant difference is expressed as *P<0.05 or **, ##P<0.01.
RESULTS
Extracellular Ca2+ induces a significant increase in [Ca2+]cyt in IPAH-PASMC but not in normal PASMC
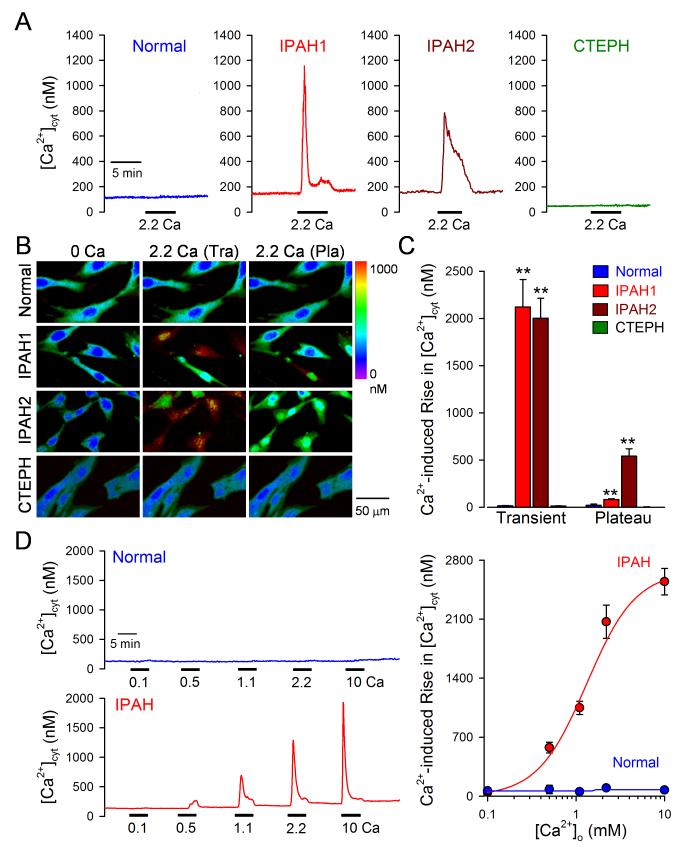
We first examined and compared the effects of extracellular Ca2+ restoration on changes in [Ca2+]cyt in PASMC from normal subjects, IPAH patients and CTEPH patients. In normal PASMC superfused with Ca2+-free solution (plus 1 mM EGTA; for 10 min), restoration of extracellular Ca2+ (2.2 mM) into the bath solution had no effect on [Ca2+]cyt (Fig. 1A-C). Even after long exposure (30 and 60 min) of normal PASMC to Ca2+-free solution, there was no change in [Ca2+]cyt after restoration of extracellular Ca2+. In IPAH-PASMC superfused with Ca2+-free solution (for 10 min), however, restoration of extracellular Ca2+ resulted in a significant increase in [Ca2+]cyt in 96% of the cells tested. The extracellular Ca2+-induced increase in [Ca2+]cyt in IPAH-PASMC was independent of the exposure time (5 to 30 min) to Ca2+-free solution. Interestingly, as shown in Figure 1A-C (IPAH1 vs. IPAH2), the kinetics of the extracellular Ca2+-induced increases in [Ca2+]cyt were different in IPAH-PASMC. Approximately 48% of IPAH-PASMC tested (IPAH1) exhibited a rapid transient increase in [Ca2+]cyt, while more than 50% of the cells (IPAH2) had a sustained plateau phase of [Ca2+]cyt increase after the initial transient (Fig. 1A middle panels and C). In PASMC isolated from CTEPH patients superfused with Ca2+-free solution (for 10 min), restoration of extracellular Ca2+ had no effect on [Ca2+]cyt (Fig. 1A-C).
Figure 1. Enhancement of extracellular Ca2+-induced increase in [Ca2+]cyt in PASMC from IPAH patients.
A-C. Representative records (A), pseudo-color images (B), and summarized data (means±SE, C) showing extracellular Ca2+-mediated changes in [Ca2+]cyt in PASMC from normal subjects (n=45 cells), IPAH patients (n=160 cells), and CTEPH patients (n=27 cells). Two kinetically different responses of [Ca2+]cyt to extracellular Ca2+ in PASMC from two IPAH patients are shown in the middle panels. D. Representative traces of [Ca2+]cyt changes in response to extracellular application of 0.1, 0.5, 1.1, 2.2, and 10 mM Ca2+ (left panels) and the dose-response curves (right panel) in normal PASMC (upper panel) and IPAH-PASMC (lower panel). The EC50 for extracellular Ca2+-induced [Ca2+]cyt increase in IPAH-PASMC is 1.22 mM.
The extracellular Ca2+-induced [Ca2+]cyt increase in IPAH-PASMC was dependent on Ca2+ concentration (Fig. 1D); the EC50 was approximately 1.22 mM (n=57 to 183 cells) which resulted an increase of [Ca2+]cyt by 1,250 nM (Fig. 1D right panel). In contrast, we were unable to detect a significant increase in [Ca2+]cyt in normal PASMC with restoration of 10 mM extracellular Ca2+ (Fig. 1D).
Protein expression of CaSR in IPAH-PASMC is greater than in normal PASMC
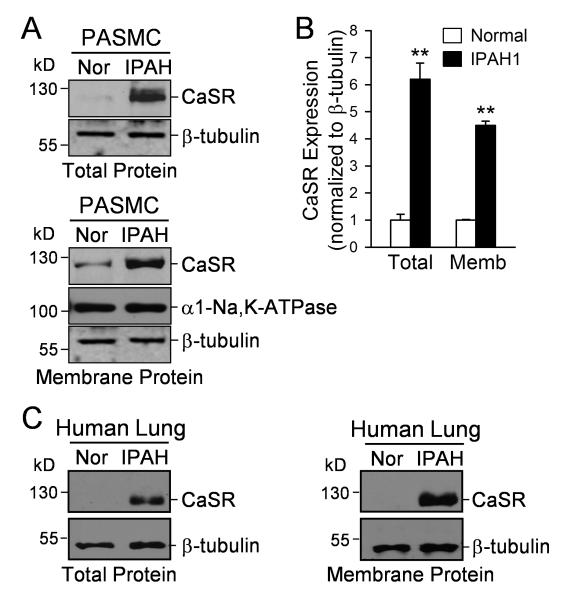
To investigate the potential mechanism of extracellular Ca2+-mediated increase in [Ca2+]cyt, we compared the protein expression level of CaSR in normal and IPAH PASMC. As shown in Figure 2, the protein expression of CaSR in IPAH-PASMC (Fig. 2A and B) and lung tissues from IPAH patients (Fig. 2C and Online Fig. I) was significantly higher than in normal PASMC and lung tissues in both total protein lysates and in the membrane bound fraction (n=5, P<0.01). These data indicate that upregulation of CaSR may be involved in the enhanced Ca2+-induced [Ca2+]cyt increase in IPAH-PASMC.
Figure 2. Upregulation of CaSR in PASMC from IPAH patients.
A. Western blot analysis on CaSR in total proteins (upper panels) and membrane proteins (lower panels) isolated from normal PASMC (Nor) and IPAH-PASMC (IPAH). B. Summarized data (means±SE, lower panel) showing CaSR protein levels that were normalized to the β-tubulin level. **P<0.01 vs. normal PASMC. C. Western blot analysis on CaSR in total and membrane proteins isolated from lung tissues of normal subjects and IPAH patients.
The extracellular Ca2+-induced [Ca2+]cyt rise in IPAH-PASMC is not due simply to Ca2+ leakage
To rule out the possibility that the extracellular Ca2+-induced [Ca2+]cyt increase in IPAH-PASMC was caused by membrane leakage to Ca2+, we first compared the resting [Ca2+]cyt in normal and IPAH-PASMC. In cells superfused with 2.2 mM Ca2+-containing solution, the resting [Ca2+]cyt in IPAH-PASMC (n=370 cells) was significantly higher than in normal PASMC (n=96 cells). Removal of extracellular Ca2+ negligibly affected the resting [Ca2+]cyt in normal PASMC, but significantly decreased the resting [Ca2+]cyt in IPAH-PASMC (Online Fig. IIA). To examine whether IPAH-PASMC had leaky membranes, we incubated normal and IPAH PASMC with trypan blue (TB). No blue (TB-stained) cells were detected in normal and IPAH PASMC incubated in TB-containing solution, whereas following treatment of the cells with 10 μM ionomycin (for 10 min), a Ca2+ ionophore, all normal and IPAH PASMC were TB-stained; there was no difference between normal and IPAH PASMC (Online Fig. IIB). Furthermore, we were unable to detect any fluorescent signals in normal and IPAH PASMC incubated with the membrane-impermeable fura-2 (Online Fig. IIC). All these experiments indicate that the extracellular Ca2+-induced [Ca2+]cyt increase in IPAH-PASMC was not due to Ca2+ leakage through the plasma membrane.
Effects of specific CaSR modulators on the extracellular Ca2+-induced [Ca2+]cyt increase in normal and IPAH PASMC
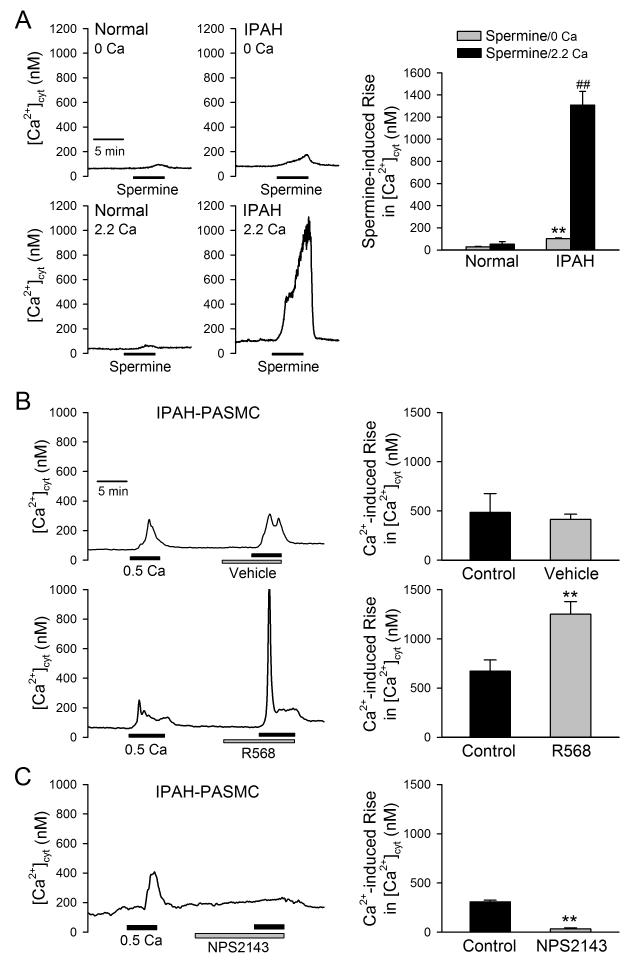
To further confirm that the upregulated CaSR in IPAH-PASMC is involved in the enhanced extracellular Ca2+-induced [Ca2+]cyt rise, we examined whether the polyamine spermine and the calcimimetic R568 induced the same effect on [Ca2+]cyt as did restoration of extracellular Ca2+, and whether the calcilytic NPS 2143 inhibited the extracellular Ca2+-induced [Ca2+]cyt increase. As shown in Figure 3A, extracellular application of 3 mM spermine, a CaSR agonist, induced a slight increase in [Ca2+]cyt (52±24 nM, n=13) in normal PASMC in the presence of 2.2 mM extracellular Ca2+. In IPAH-PASMC, however, extracellular application of spermine caused a huge [Ca2+]cyt increase (1,308±123 nM, n=49; P<0.01 vs. normal PASMC) in the presence of 2.2 mM extracellular Ca2+. In IPAH-PASMC, short-time treatment with the positive allosteric modulator of CaSR, R568 (1 μM), significantly enhanced the extracellular Ca2+-mediated increase in [Ca2+]cyt (Fig. 3B), whereas the negative allosteric modulator of CaSR, NPS 2143 (10 μM), significantly inhibited extracellular Ca2+-mediated increase in [Ca2+]cyt (Fig. 3C). Collectively, these results demonstrate that the upregulated CaSR (Fig. 2) is involved in the enhancement of the extracellular Ca2+-induced [Ca2+]cyt increase in PASMC from IPAH patients.
Figure 3. Effects of CaSR modulators on [Ca2+]cyt in IPAH-PASMC.
A. Representative traces (left panels) and summarized data (means±SE, right panel) showing the effect of spermine (3 mM), a CaSR agonist, on [Ca2+]cyt in the absence (0Ca) and presence (2.2Ca) of extracellular Ca2+ (2.2 mM) in normal (n=30) and IPAH PASMC (n=52). **P<0.01 vs. normal PASMC (spermine/0Ca); ##P<0.01 vs. normal PASMC (spermine/2.2Ca). B and C. Representative records of [Ca2+]cyt changes (left panels) and summarized data (means means±SE, right panels) of the stimulatory effect of R568 (1 μM, n= 63, B), a positive allosteric modulator of CaSR, and the inhibitory effect of NPS-2143 (10 μM, n=120, C), a negative allosteric modulator of CaSR, on the 0.5-mM extracellular Ca2+-induced [Ca2+]cyt increases in IPAH-PASMC. **P<0.01 vs. Control IPAH-PASMC (without treatment with vehicle, R568 or NPS 2143).
Extracellular Ca2+-induced [Ca2+]cyt increase in IPAH-PASMC is dependent of phospholipase C (PLC) and the inositol-2,4,5-trisphosphate receptor (IP3R)
To examine the potential signaling pathway involved in the CaSR-mediated increase in [Ca2+]cyt in IPAH-PASMC, we performed pharmacological experiments on the extracellular Ca2+-mediated increase in [Ca2+]cyt. As shown in Figure 4, short-time pretreatment of IPAH-PASMC with the inhibitor of phospholipase C (PLC), U73122 (1 μM), or the specific blocker of IP3R, xestospongin C (3 μM), significantly inhibited the extracellular Ca2+-induced increase in [Ca2+]cyt (Online Fig. IIIA and C). The inactive form of U73122, U73343 (1 μM), however, had no effect on the extracellular Ca2+-induced increase in [Ca2+]cyt (Online Fig. IIIB). Pretreatment of IPAH-PASMC with the blocker of VDCC, diltizaem (10 μM), or the inhibitor of Na+/Ca2+ exchanger, KB-R7943 (10 μM), however, had no effect on the extracellular Ca2+-induced increase in [Ca2+]cyt (Online Fig. IIID and E). These results indicate that activation of PLC and IP3R is involved in the CaSR-mediated [Ca2+]cyt increase in IPAH-PASMC, while VDCC and the reverse mode of Na+/Ca2+ exchanger are not involved in the Ca2R-mediated Ca2+ influx or inward transportation.
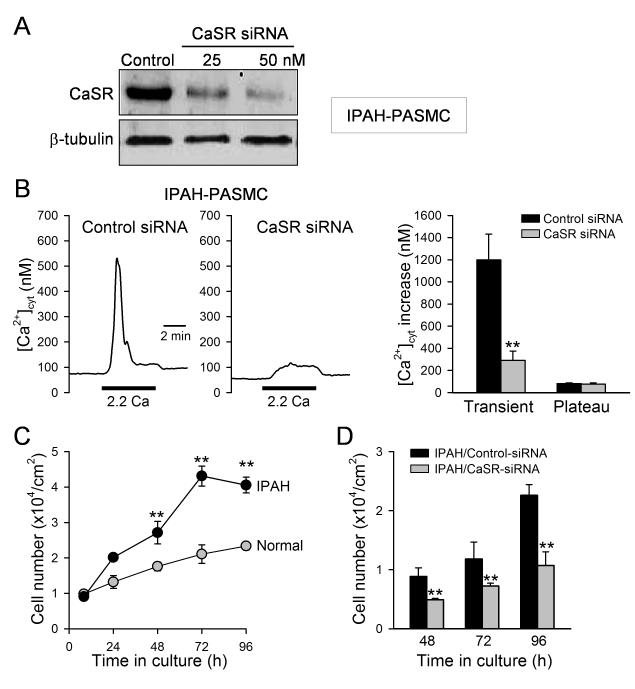
Figure 4. Downregulation of CaSR with siRNA attenuates extracellular Ca2+-induced [Ca2+]cyt increases in IPAH-PASMC and inhibits IPAH-PASMC proliferation.
A. Western blot analysis on CaSR in IPAH-PASMC treated with a control (or scrambled) siRNA (Control) and siRNA for CaSR (at 25 and 50 nM, n=3 for each group). B. Representative records of [Ca2+]cyt changes (left panels) and summarized data (means±SE, right panel) showing the extracellular Ca2+-induced [Ca2+]cyt increases in IPAH-PASMC transfected with control siRNA (n=57) and CaSR-siRNA (n=57). **P<0.01 vs. control-siRNA. C. Summarized data (means±SE) showing the total cell numbers of normal PASMC (open circles) and IPAH-PASMC (solid circles) after cultured in growth media for 8, 24, 48, 72, and 96 hrs. The growth curves for normal and IPAH PASMC are significantly different (P<0.001, n=3 experiments). D. Summarized data (means±SE, n=3 experiments) showing inhibitory effects of CaSR-siRNA on the cell proliferation in IPAH-PASMCs. **P<0.01 vs. control-siRNA.
Downregulation of CaSR in IPAH-PASMC inhibits extracellular Ca2+-induced [Ca2+]cyt increase and attenuates cell proliferation
To obtain direct evidence for the involvement of CaSR in extracellular Ca2+-induced [Ca2+]cyt increase and cell proliferation, we used siRNA to knockdown CaSR expression and examined whether CaSR was necessary for the extracellular Ca2+-induced [Ca2+]cyt increase in IPAH-PASMC. Treatment of IPAH-PASMC with 50 nM siRNA significantly decreased protein level of CaSR (Fig. 4A) and markedly inhibited the extracellular Ca2+-induced increase in [Ca2+]cyt (Fig. 4B). In comparison to normal PASMC, the proliferate rate of IPAH-PASMC, determined by a change in cell number, was much faster (Fig. 4C), while downregulation of CaSR in IPAH-PASMC by transiently transfecting 50 nM siRNA for CaSR significantly attenuated cell proliferation (Fig. 4D). These experiments provide compelling evidence that CaSR is necessary for the augmented extracellular Ca2+-induced [Ca2+]cyt increase and enhanced cell proliferation in IPAH-PASMC.
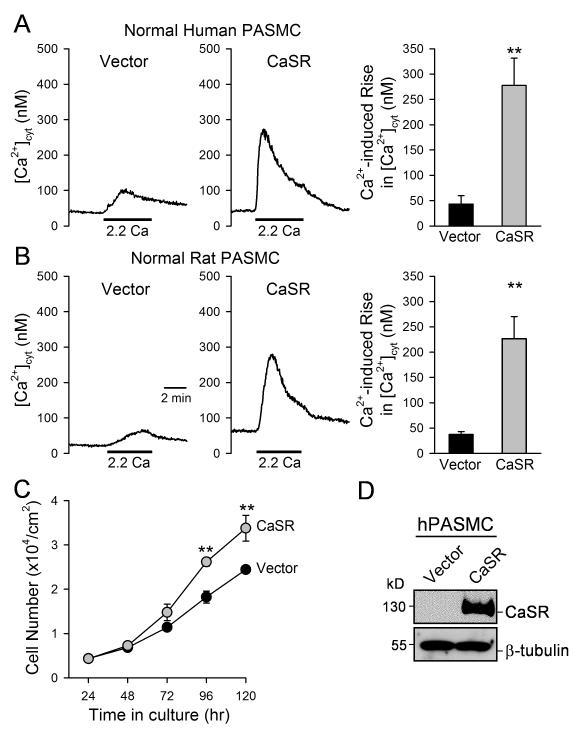
Overexpression of CaSR in normal PASMC augments the extracellular Ca2+-induced [Ca2+]cyt increase and enhances cell proliferation
Extracellular application of either 2.2 mM Ca2+ or 3 mM spermine failed to induce a significant increase in [Ca2+]cyt in normal PASMC because of a low protein expression level of CaSR (see Figs. 2 and 3). We examined whether CaSR was sufficient to mediate extracellular Ca2+-induced [Ca2+]cyt increase in both human and rat PASMC by transiently transfecting the human CaSR into normal PASMC. As shown in Figure 5, overexpression of CaSR (with 2 μg of the human CaSR cDNA) in normal (human and rat) PASMC significantly augmented the extracellular Ca2+-mediated increase in [Ca2+]cyt (Fig. 5A and B) and enhanced cell proliferation (Fig. 5C) in comparison to normal PASMC transiently transfected with an empty vector. Taken together with the data showed earlier (Fig. 4), these results indicate that a) CaSR is sufficient to mediate extracellular Ca2+-induced [Ca2+]cyt increase and cell proliferation in normal PASMC, and b) CaSR is necessary for the augmented extracellular Ca2+-induced [Ca2+]cyt increase and enhanced cell proliferation in IPAH-PASMC.
Figure 5. Overexpression of CaSR in normal PASMC enhances extracellular Ca2+-induced [Ca2+]cyt increase and promotes cell proliferation.
A and B. Representative records of [Ca2+]cyt changes (left panels) and summarized data (means±SE, right panels) extracellular Ca2+-induced [Ca2+]cyt increases in human (A) and rat (B) PASMC transfected with an empty vector (Vector, n=12) and the human CaSR cDNA (CaSR, n=8). C. Summarized data (means±SE) showing the total numbers of normal human PASMC transfected with the vector (solid circles) and CaSR gene (grey circles) after incubation in growth media for 24, 48, 72, 96, and 120 hrs. The growth curves for vector control PASMC and IPAH PASMC are significantly different (P<0.01, n=3 experiments). **P<0.01 vs. vector. D. Western blot analysis on CaSR in human PASMC transfected with an empty vector (Vector) or CaSR.
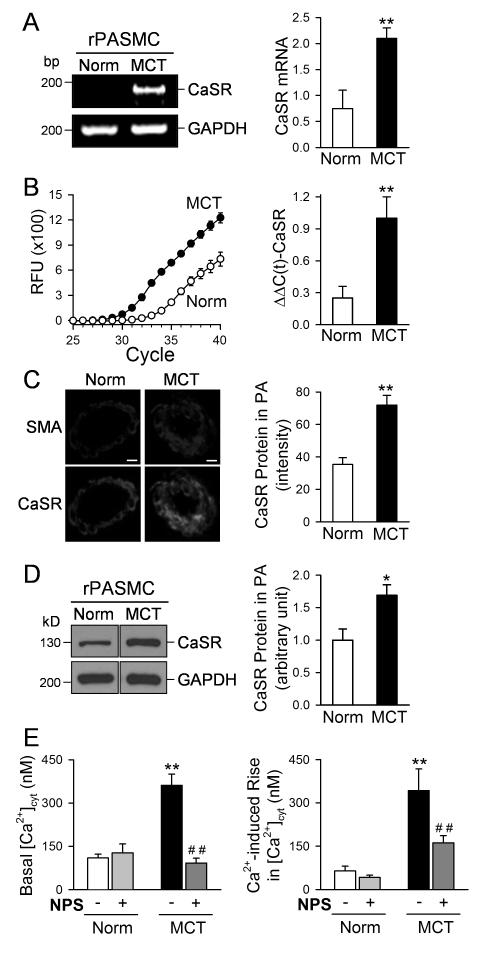
CaSR is functionally upregulated in PASMC from animal models of experimental pulmonary hypertension and blockade of CaSR prevents the development of pulmonary hypertension
The presented in vitro experimental data show that the upregulated CaSR and augmented extracellular Ca2+-mediated [Ca2+]cyt increase in PASMC are involved in the enhanced PASMC proliferation in patients with IPAH. To investigate whether CaSR can be a target for treatment of pulmonary arterial hypertension, we used the rat model of monocrotaline (MCT)-induced pulmonary hypertension (MCT-PH) and the mouse model of hypoxia-induced pulmonary hypertension (HPH) to test the potential therapeutic effect of the calcilytic NPS 2143. We first examined and compared the mRNA and protein expression level of CaSR in PASMC from control and MCT-treated rats. As shown in Figure 6A and B, the mRNA level of CaSR in PASMC isolated from rats (rPASMC) with MCT-PH was much greater than in PASMC isolated from normotensive control rats injected with vehicle. The immunohistochemistry and immunoblotting experimental data indicate that the protein expression level of CaSR in the small pulmonary artery (Fig. 6C) and PASMC (Fig. 6D) of MCT-rats was significantly higher than in the small pulmonary artery and PASMC of control rats. Furthermore, the basal or resting [Ca2+]cyt and the extracellular Ca2+-induced increase in [Ca2+]cyt were both enhanced in freshly-dissociated PASMC from MCT-PH rats compared with freshly-dissociated PASMC from normotensive control rats (Fig. 6E). Treatment with NPS 2143 not only decreased the basal [Ca2+]cyt, but also inhibited the extracellular Ca2+-induced increase in [Ca2+]cyt in PASMC isolated from MCT-PH rats (Fig. 6E). These results imply that upregulation of CaSR and subsequent enhancement of extracellular Ca2+-induced [Ca2+]cyt increase in PASMC contribute to the development of pulmonary hypertension in rats injected with MCT.
Figure 6. Upregulation of CaSR in PASMC from rats with MCT-induced pulmonary hypertension.
A and B. Regular (A) and real-timer (B) RT-PCR analyses on CaSR in PASMC isolated from normal rats (Norm, n=6) and rats with MCT-induced pulmonary hypertension (MCT, n=5). **P<0.01 vs. Norm. C. Immunohistochemistry data showing CaSR expression level (green fluorescence intensity) in normal and MCT-treated rats. D. Western blot analysis on CaSR in PASMC isolated from normal and MCT-PH rats. *P<0.05, **P<0.01 vs. Norm. E. Summarized data (means±SE) showing the basal [Ca2+]cyt (left panel, n=12) and the amplitude of extracellular Ca2+-induced [Ca2+]cyt increases (right panel, n=11) in PASMC freshly isolated from normal rats and MCT-injected rats that are treated with vehicle (−NPS) or 10 μM NPS 2143 (+NPS), a synthetic calcilytic. **P<0.01 vs. Norm (−/+NPS).
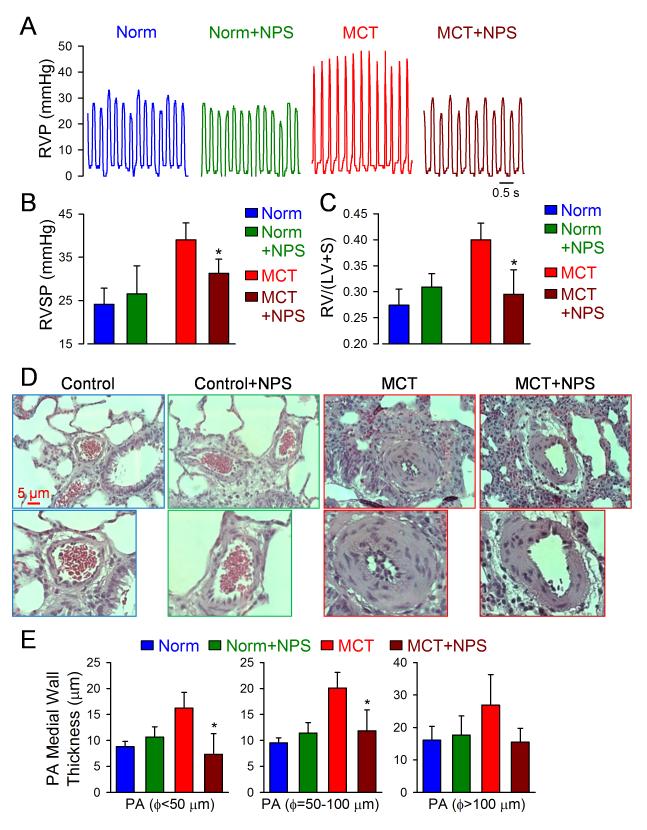
To test the in vivo therapeutic effect of the CaSR antagonist, we examined and compared the right ventricular systolic pressure (RVSP), the Fulton index [i.e., the ratio of right ventricle/left ventricle+septum, RV/(LV+S)] and muscularization of distal pulmonary arteries in normotensive control (Norm) rats and MCT-injected rats with and without treatment with NPS 2143, a CaSR antagonist. Injection of MCT (60 mg/kg) in rats significantly increased RVSP and caused right ventricular (RV) hypertrophy compared with the normotensive control (Norm) rats injected with vehicle (DMSO) (Fig. 7A-C). Intraperitoneal injection of NPS 2143 (4.5 mg/kg per day) had little effect on RVSP and RV/(LV+S) ratio in Norm rats, but significantly attenuated the increase in RVSP and the Fulton index in MCT-PH rats (Fig. 7A-C). There were no significant changes in heart rate in Norm rats with (412±17 bpm, n=6) or without (411±21 bpm, n=6) NPS treatment and MCT-injected rats with (414±23 bpm, n=6) or without (413±21 bpm, n=6) NPS treatment. The MCT-induced increases in RVSP and RV hypertrophy were associated with significant pulmonary vascular remodeling; the vascular medial wall thickness of small pulmonary arteries with the outer diameter less than 100 μm was significantly greater in MCT-injected rats than in Norm rats (Fig. 7D and E). Treatment with the CaSR antagonist (NPS 2143) significantly inhibited the muscularization of small pulmonary arteries (Fig. 7D and E). The in vivo animal experiments are consistent with the in vitro experiments using normal and IPAH PASMC.
Figure 7. Blockade of CaSR by NPS 2143 inhibits the development of pulmonary vascular remodeling and pulmonary hypertension in rats injected with MCT.
A and B. Representative record of right ventricular pressure (RVP, A) and summarized data (means±SE) showing the peak value of right ventricular systolic pressure (RVSP, B) in normal control rats (Norm, n=6) and MCT-injected rats (MCT, n=6) that are treated with vehicle (−NPS) or NPS 2143 (+NPS, 4.5 mg/kg once a day). C. Averaged Fulton index [RV/(LV+S) ratio, means±SE] showing that RV hypertrophy is significantly inhibited in MCT-rats treated with NPS. *P<0.05 vs. MCT along. D and E. Representative H&E images of small pulmonary arteries (D) and summarized data of the medial thickness of pulmonary arteries with a diameter (Ø) less than 50 μm, between 50 and 100 μm and greater than 100 μm (E) in normal control rats (Norm, n=6) and MCT-injected rats (MCT, n=6) that are treated with vehicle or NPS 2143. *P<0.05 vs. MCT-treated rats (red bars).
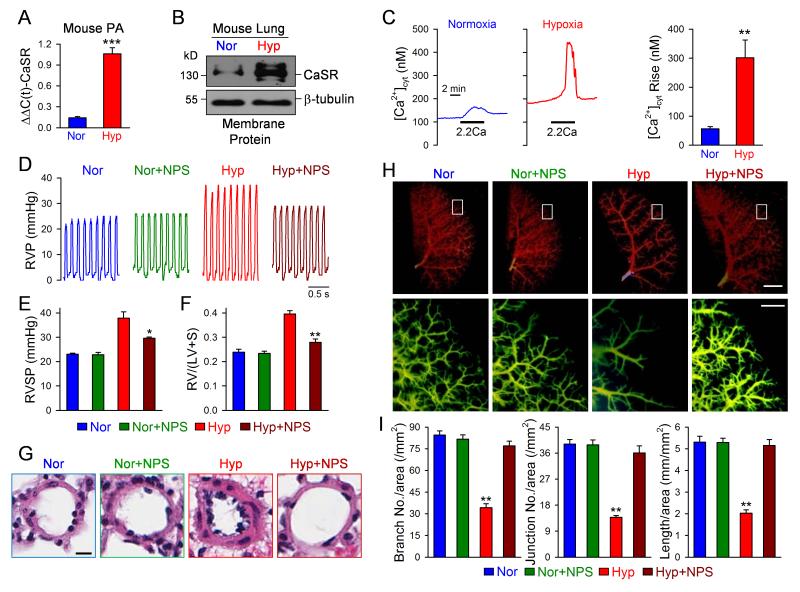
To further validate the pathogenic role of upregulated CaSR in the development of pulmonary hypertension and the therapeutic effect of CaSR antagonists on experimental pulmonary hypertension, we repeated the experiments mentioned above in the HPH mouse model. As shown in Figure 8, the mRNA and protein expression of CaSR was significantly higher in pulmonary arteries and lung tissues in HPH mice than in normoxic control mice (Fig. 8A and B), while the extracellular Ca2+-induced increase in [Ca2+]cyt in freshly-isolated PASMC from HPH mice was significantly enhanced in comparison to cells from normoxic mice (Fig. 8C). These data indicate that CaSR is functionally upregulated in PASMC from HPH mice.
Figure 8. Upregulation of CaSR in PASMC from HPH mice and blockade of CaSR by NPS 2143 inhibits the development of HPH in mice.
A and B. Real-time RT-PCR (A) and Western blot (B) analyses on CaSR in PA and lung tissues isolated from normoxic (Nor) and hypoxic (Hyp) mice. C. Representative record (left panels) and summarized data (means±SE, right panel) showing the extracellular Ca2+-induced increase in [Ca2+]cyt in PASMC isolated from normoxic and hypoxic mice. **P<0.01, ***P<0.001 vs. Nor. D and E. Representative record of right ventricular pressure (RVP, D) and summarized data (means±SE) showing the peak value of right ventricular systolic pressure (RVSP, E) in normoxic (Nor, n=6) and hypoxic (Hyp, n=6) mice that are treated with vehicle or NPS 2143 (+NPS, 1 mg/kg once a day). F. Averaged Fulton index [RV/(LV+S) ratio, means±SE] in normoxic and hypoxic mice treated with or without NPS 2143. *P<0.05 vs. Hyp along. G. Representative H&E images of small pulmonary arteries showing that the medial thickness is significantly increased in Hyp mice and the hypoxia-induced medial hypertrophy is inhibited by NPS 2143. H. Representative angiography of the whole lung (upper panels) and enlarged area of the whole lung (lower panels, indicated by the box in the upper panels) in normoxic and hypoxic mice treated with vehicle or NPS (+NPS). The vertical bars denote 2 mm (upper panels) and 0.5 mm (lower panels). I. Summarized data (means±SE) showing the number of branches, the number of junctions, and the total length of vascular segments per square millimeter (mm2). **P<0.01 vs. other bars.
Intraperitoneal injection of NPS 2143 (1 mg/kg per day from day 1 to 10) had little effect on RVSP and RV/(LV+S) ratio in normoxic control mice (Nor), but significantly inhibited the increase in RVSP and RV/(LV+S) ratio in hypoxic mice (Hyp) (Fig. 8D-F). Furthermore, inhibition of CaSR also significantly inhibited the hypoxia-mediated pulmonary arterial wall thickening (Fig. 8G) and reversed the hypoxia-induced decrease in branch and junction numbers (and total length) of small pulmonary arterial trees (Fig. 8H and I). There were no significant changes in heart rate in normoxc mice with (411±17 bpm, n=6) or without (410±20 bpm, n=6) NPS treatment and hypoxic mice with (412±18 bpm, n=6) or without (413±18 bpm, n=6) NPS treatment.
These data imply that intraperitoneal injection of the CaSR antagonist is an efficient therapeutic approach to inhibit the development and progression of pulmonary vascular remodeling and right ventricular hypertrophy in animal models with experimental pulmonary hypertension induced by injection of monocrotaline and exposure to hypoxia. The observations from this study strongly indicate that increased expression and function of CaSR may play a pathogenic role in the development of pulmonary vascular remodeling and antagonists of CaSR, or calcilytics, may have great therapeutic potential for patients with pulmonary arterial hypertension.
DISCUSSION
In this study, we found that a) extracellular application of Ca2+ (0.5-10 mM) and spermine (3 mM) induced a large increase in [Ca2+]cyt in IPAH-PASMC but not in normal PASMC; b) the protein expression level of CaSR in IPAH-PASMC was greater than in normal PASMC; c) downregulation of CaSR in IPAH-PASMC (with siRNA) inhibited the extracellular Ca2+-induced increase in [Ca2+]cyt and attenuated cell proliferation, while overexpression of CaSR in normal PASMC augmented the extracellular Ca2+-induced rise in [Ca2+]cyt and enhanced cell proliferation; d) the expression and function of CaSR were also increased in PASMC from rats with MCT-PH and mice with HPH, and intraperitoneal injection of a CaSR antagonist (NPS 2143) significantly inhibited pulmonary vascular remodeling and attenuated the development and progression of the experimental pulmonary hypertension. Collectively, the observations from this study indicate that i) functionally upregulated CaSR and subsequently augmented extracellular Ca2+-induced rise in [Ca2+]cyt in PASMC contribute to the enhanced Ca2+ signaling and excessive cell proliferation in IPAH patients; and ii) blockade of the upregulated CaSR with calcilytics may be a novel therapeutic approach for pulmonary arterial hypertension.
[Ca2+]cyt plays an important role in the regulation of contraction, proliferation, and migration of PASMC. An increase in [Ca2+]cyt in PASMC is a major trigger for pulmonary vasoconstriction and an important stimulus for PASMC proliferation and pulmonary vascular remodeling under pathological conditions. Elevation of [Ca2+]cyt in PASMC results from Ca2+ release from intracellular stores and Ca2+ influx through plasmalemmal Ca2+ channels1, 5. We previously showed that the resting [Ca2+]cyt was increased, while ROCE and SOCE were enhanced in PASMC from IPAH patients compared to PASMC from normal subjects and patients without pulmonary hypertension6, 7.
CaSR is a GPCR (belongs to the Family C GPCR) with 1,085 amino acids, which is present constitutively in a homodimeric configuration formed by covalent and non-covalent linkages25-27 and is able to form heterodimers with the metabotropic glutamate receptors (mGLuR1 and mGluR5)27. One of the hallmarks of CaSR is the cysteine-rich large N-terminal extracellular domain (ECD, approximately 600 amino acids). The region between alanine 116 and proline 136 in the ECD is important for maintaining CaSR in an inactive conformation28 and is associated with the activating mutations or single nucleotide polymorphism (SNPs) identified in the human CaSR gene. The ligands or activators of CaSR include polyvalent cations (e.g., Ca2+, Mg2+, Gd3+), polypeptides (e.g., amyloid-β peptide), polyamines (e.g., spermine, spermidine, putrescine), aminoglycoside antibiotics (e.g., neomycin, kanamycin), and amino acids (e.g., phenylalanine, tyrosine, tryptophan, glutamate). In addition, there are synthetic CaSR activators, or calcimimetics (e.g., NPS-R-568, NPS-R-467), and CaSR antagonists, or calcilytics (e.g., NPS 2143) that affect CaSR function12-14, 29-31. The cysteine-rich domain in the ECD also sensitizes the receptor to redox changes and hypoxia/hyperoxia. In Sprague-Dawley rats, treatment with the CaSR activator or the calcimimetic R-568 attenuates aortic wall thickening induced by uremia.32 Our data from this study, however, indicate that the extracellular Ca2+-mediated [Ca2+]cyt increase in IPAH-PASMC was potentiated by NPS-R-568, an allosteric agonist of CaSR, and inhibited by NPS 2143, an allosteric antagonist of CaSR. Extracellular application of spermine significantly increased [Ca2+]cyt in IPAH-PASMC superfused in Ca2+-containing solution. These data imply that multiple ligands can activate the upregulated CaSR in IPAH-PASMC leading to cell proliferation, contraction and migration via Ca2+ signaling and other signal transduction cascades.
Extracellular Ca2+ binding to CaSR is a highly cooperative process. The EC50 for extracellular Ca2+-induced [Ca2+]cyt increase in IPAH-PASMC is approximately 1.2 mM (Fig. 1D), while the EC50 has been reported to be 1.7 mM in parathyroid cells33, 1.5 mM in cardiomyocytes34, and 5.6 mM in bronchial epithelial cells35. However, in reconstituted systems with CaSR clones isolated from parathyroid glands, the EC50 for CaSR activation (or the Ca2+ sensitivity of CaSR) is 3.0-3.5 mM12, 13, 15, 36-38. The lower EC50 for Ca2+-induced [Ca2+]cyt rise in IPAH-PASMC and native vascular smooth muscle cells (compared to the EC50 for the recombinant CaSR) is presumably due to the high degree of cooperativity in the interaction between the upregulated CaSR (e.g., in IPAH-PASMC) and ligands. CaSR is always exposed to various co-agonists physiologically (e.g., Mg2+, polyamines and amino acids) and other activators pathologically (e.g., antibiotics, heavy metals and amyloid-β peptide). Therefore, the sensitivity of CaSR to extracellular Ca2+ is expected to be higher in pathologically conditions than in physiological conditions.
As mentioned earlier, the CaSR senses extracellular Ca2+ concentration and transduces it to intracellular space though multiple signal pathways 12-14, 18. CaSR interacts directly with G proteins (Gqα and G11α). Activation of CaSR by extracellular Ca2+ (or calcimimetics) induces increases in [Ca2+]cyt through phospholipace C (PLC)-mediated hydrolysis of phosphatidylinositol-4,5-bisphosphate (PIP2) to inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 binds to the IP3 receptor (IP3R) on the SR membrane and induces Ca2+ release from the SR to the cytosol. Depletion (or reduction) of Ca2+ from the intracellular stores (i.e., SR) via activated IP3R (or opened Ca2+ release channels) then causes SOCE through SOC in the plasma membrane. Furthermore, DAG causes ROCE by activating ROC in the plasma membrane. The IP3-mediated Ca2+ mobilization, the store depletion-mediated SOCE and the DAG-mediated ROCE all contribute to the increase in [Ca2+]cyt upon activation of CaSR in the plasma membrane by extracellular Ca2+ and CaSR activators. In IPAH-PASMC, the extracellular Ca2+-mediated [Ca2+]cyt increases were significantly attenuated by the PLC inhibitor (U73122) and the IP3R blocker (xestospongin C), but not affected by the voltage-dependent Ca2+ channel blocker (diltiazem) and the Na+/Ca2+ exchanger inhibitor (KB-R7943) (Online Fig. III). These data further confirm the important role of the PLC-IP3 signaling cascade in CaSR-mediated increase [Ca2+]cyt and its proliferative effect on PASMC isolated from patients with IPAH. Most membrane receptors, including many GPCRs, become desensitized with prolonged exposure to agonists. However, CaSR desensitizes very slowly39, indicating that CaSR signals for long periods of time through the regulation of intracellular signaling cascades and other signal transduction pathways.
In addition to increasing [Ca2+]cyt, extracellular Ca2+-mediated activation of CaSR has been linked to several signal transduction cascades. CaSR activates the mitogen-activated protein kinase (MAPK) cascade, e.g. extracellular signal-regulated kinase 1/2 (ERK1/2), p38, and c-jun N-terminal kinase (JNK), potentially through the interaction with filamin A scaffolding protein and caveolin-1 in cholesterol-rich invaginations in the plasma membrane known as caveolae16, 17, 40-44. In IPAH-PASMC, the number of caveoli and the protein expression level of caveolin-1/-2 are both increased, which are associated with increased SOCE45. Downregulation of caveolin-1 with siRNA and treatment with methyl-β-cyclodextrin (MβCD) significantly diminish the number of caveoli on the surface membrane of IPAH-PASMC, significantly attenuate SOCE and markedly inhibit cell proliferation6, 45. It is unknown, however, whether upregulated CaSR in IPAH-PASMC is exclusively or predominantly localized in caveoli and functionally interacts with upregulated TRPC3/C6 channels7, 8.
CaSR is widely distributed in the parathyroid glands15, kidney46, bone47, gastrointestinal tract48, skin49, brain50, immune cells51, and heart34. In addition, CaSR is expressed in vascular smooth muscle cells from rat subcutaneous artery52, aorta53, pulmonary artery16, 17, gerbil spiral modiolar artery54, human renal artery55, aorta55, 56, and internal mammary artery57, as well as in endothelial cells from rat mesenteric artery, porcine coronary artery58, and human aorta19. Activation of CaSR in vascular smooth muscle cells increases [Ca2+] and induces vasoconstriction17, 54, 56 cyt ; therefore, CaSR is involved in regulating myogenic tone, peripheral vascular resistance52, and arterial blood pressure59, 60. Recent observations demonstrate that the CaSR in vascular smooth muscle cells contributes to regulating cell proliferation and apoptosis through the MEK1/ERK1/2 and PLC signaling pathways17, 53, 55, and expression of CaSR is involved in the regulation of mouse lung development61. Our study indicated that in normal human PASMC, CaSR protein was expressed at a low level and extracellular application of Ca2+ (from 0.5 to 10 mM) failed to cause a significant increase in [Ca2+]cyt (Fig. 1D), while extracellular application of spermine caused a small increase in [Ca2+]cyt (Fig. 3A). These observations imply that CaSR is expressed at very low level in normal human PASMC and is not a major contributor to the regulation of pulmonary vascular tone under physiological conditions.
In patients with IPAH and animals with experimental pulmonary hypertension, CaSR is functionally upregulated in PASMC and thus becomes an important GPCR involved in the initiation and progression of pulmonary vascular remodeling and pulmonary hypertension. Knockdown of CaSR with siRNA in PASMC from IPAH patients not only diminished extracellular Ca2+-induced increase in [Ca2+]cyt, but also inhibited cell proliferation (Fig. 4). Overexpression of CaSR in PASMC from normal subjects conferred an extracellular Ca2+-induced increase in [Ca2+]cyt and enhanced cell proliferation (Fig. 5). These experimental data provide compelling evidence that CaSR is necessary and sufficient for the augmented Ca2+ signaling and excessive PASMC proliferation in IPAH patients. In this scenario, Ca2+ is an extracellular ligand and an intracellular signaling element involved in the development and progression of sustained pulmonary vasoconstriction and vascular remodeling in patients with IPAH. The pathogenic role of upregulated CaSR in pulmonary hypertension is further confirmed by the therapeutic effect of CaSR antagonists on experimental pulmonary hypertension in rats injected with MCT and in mice exposed to chronic hypoxia.
The gain-of-function (or activating) mutations or single-nucleotide polymorphisms (SNP) in the human CaSR gene causes autosomal dominant hypocalcemia62 and Bartter’s syndrome type V12, 63. CaSR antagonists (calcilytics), i.e. negative allosteric modulators that indirectly stimulate parathyroid hormone secretion through a decrease in CaSR activity, are potential drug candidates for the treatment of osteoporosis and other bone metabolism diseases64, 65. Using two well-established animal models of pulmonary hypertension (MCT-injected rats and chronic hypoxic mice), we found that increased RVSP and pulmonary vascular medial hypertrophy, as well as right ventricular hypertrophy [determined by the Fulton index, RV/(LV+S)] were associated with upregulated CaSR expression and enhanced extracellular Ca2+-induced [Ca2+]cyt rise (and basal [Ca2+]cyt) in PASMC (Figs. 6-8). Intraperitoneal injection of the CaSR antagonist, NPS 2143 (4.5 mg/kg once a day), had little effect on the pulmonary hemodynamics and the Fulton index in control rats or normoxic mice, but significantly decreased RVSP, RV/(LV+S) ratio and small pulmonary vascular wall thickening in rats with MCT-PH (Fig. 7) and mice with HPH (Fig. 8). These results strongly suggest that CaSR is involved in the development of experimental pulmonary hypertension and a potential target for developing therapeutic approach for pulmonary arterial hypertension.
In conclusion, upregulated expression of CaSR in PASMC and augmented function of CaSR through intracellular Ca2+ signaling (and other signal transduction cascades) are new pathogenic mechanisms involved in the initiation and progression of pulmonary vascular remodeling in patients with pulmonary arterial hypertension. Pharmacological blockade of CaSR in the pulmonary vasculature by synthetic calcilytics and downregulation of CaSR by siRNA (and/or specific microRNA) may be a novel therapeutic approach for IPAH patients who do not respond to the conventional drug therapy.
Supplementary Material
Online Figure I. Upregulated CaSR in pulmonary arteries from IPAH patients. A. Immunohistochemistry data showing CaSR expression level (green fluorescence intensity) in pulmonary arteries of normal subjects and IPAH patients. The lung tissue sections were stained with antibodies against CaSR (green) and smooth muscle α-actin (SM-α-Actin, red) and with DAPI (blue). The overlay images are shown in the bottom. B. Summarized data (means±SE, n=3 for each group) showing CaSR expression level (green fluorescence intensity) in pulmonary arteries of normal subjects (Norm) and IPAH patients. **P<0.01 vs. Normal.
Online Figure II. Extracellular Ca2+-induced increase [Ca2+]cyt in IPAH-PASMCs is not due to Ca2+ leakage. A. Representative traces showing change in resting [Ca2+]cyt before and during application of Ca2+-free solution in normal and IPAH PASMC. Summarized data (right panel, n=96-370 cells) showing the resting [Ca2+] in normal and IPAH PASMC superfused with 2.2 mM Ca2+ cyt - containing solution (black bars) or Ca2+-free solution (grey bars). **P<0.001 vs. normal PASMC. B. Representative images (left panels) of trypan (TB) blue staining (0.4%, 1 min) before (−) and during (+) treatment with 10 μM ionomycin for 10 min in normal (upper panels) and IPAH (lower panels) PASMC. Summarized data (right panel) showing no correlation between the extracellular Ca2+-induced increase in [Ca2+] and the Ca2+ cyt leakage through the plasma membrane. C. Representative images showing [Ca2+]cyt (or Fura-2 fluorescence intensity) in normal (left panels) and IPAH (right panels) PASMC in the absence and presence of 10 μM CPA, an inhibitor of Ca2+-ATPase in the SR. The cells were loaded with the membrane-permeable fura-2/AM (4 μM, upper panels) or the membrane-impermeable fura-2 (4 and 40 μM, middle and lower panels). Data were obtained from 21 to 64 cells.
Online Figure III. Inhibition of PLC and IP3R attenuates extracellular Ca2+-induced [Ca2+]cyt increases in IPAH-PASMC Representative records of [Ca2+]cyt changes (left panels), pseudo images (middle panels) and summarized data (means±SE, right panels) showing extracellular Ca2+-induced increase in [Ca2+]cyt before and during treatment with 1 μM U73122 (a PLC inhibitor; n=57, A), 1 μM U73343 (an inactive form of U73122; n=45, B), 3 μM xestospongin C (an IP3R blocker; n=34, C), 10 μM diltiazem (a VDCC blocker; n=46, D), and KB-R7943 (an Na+/Ca2+ exchanger inhibitor; n=22, E) in IPAH-PASMC. **P<0.01 vs. control.
Novelty and Significance.
What is Known?
Pulmonary vascular remodeling and sustained pulmonary vasoconstriction contribute to the development of idiopathic pulmonary arterial hypertension (IPAH).
Increased cytosolic free Ca2+ concentration ([Ca2+]cyt) stimulates pulmonary arterial smooth muscle (PASMC) proliferation leading to pulmonary vascular remodeling.
Ca2+-sensing receptor (CaSR) is a G protein coupled receptor important for multiple cellular processes of the parathyroid glands such as proliferation, differentiation, and apoptosis.
What New Information Does This Article Contribute?
CaSR is functionally upregulated in IPAH-PASMC contributing to enhanced Ca2+ signaling and excessive cell proliferation in IPAH patients.
Blockade of the CaSR with an antagonist inhibits the development of pulmonary hypertension in animal models.
Targeting the CaSR may be a novel therapeutic approach for IPAH patients.
Idiopathic pulmonary arterial hypertension (IPAH) is a rare, progressive and fatal disease that predominantly affects women. The pathogenic mechanisms involved in the pulmonary vascular abnormalities (e.g., arterial remodeling and sustained vasoconstriction) in IPAH patients remain unclear. An increase in cytosolic Ca2+ concentration ([Ca2+]cyt) in pulmonary arterial smooth muscle cells (PASMC) is a major trigger for pulmonary vasoconstriction and an important stimulus for PASMC migration and proliferation (which subsequently cause pulmonary vascular wall thickening leading to the increase in pulmonary vascular resistance). In this study, we report that a unique G protein-coupled receptor (GPCR), Ca2+-sensing receptor (CaSR), is significantly upregulated in PASMC isolated from patients with IPAH and animals with experimental pulmonary hypertension. The upregulated CaSR is necessary for the enhanced extracellular Ca2+-induced increase in [Ca2+]cyt and the augmented cell proliferation in IPAH-PASMC. Pharmacological blockade of CaSR with a calcilytic, NPS 2143, markedly inhibits the extracellular Ca2+-induced rise in [Ca2+]cyt and attenuates the development of experimental pulmonary hypertension in animal models. These data indicate that functionally upregulated CaSR in PASMC may play an important role in causing sustained pulmonary vasoconstriction and excessive pulmonary vascular remodeling in IPAH patients. Targeting CaSR in PASMC may help develop novel therapeutic approach for pulmonary hypertension.
Acknowledgments
SOURCES OF FUNDING This work was supported, in part, by grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health (HL066012 and HL098053 to JX-JY).
Non-standard Abbreviations
- [Ca2+]cyt
Cytosolic free Ca2+ concentration
- PASMC
Pulmonary arterial smooth muscle cell
- IPAH
Idiopathic pulmonary arterial hypertension
- CaSR
Ca2+-sensing receptor
- VDCC
Voltage-dependent Ca2+ channels
- ROC
Receptor-operated Ca2+ channels
- SOC
Store-operated Ca2+ channels
- DAG
Diacylglycerol
- ROCE
Receptor-operated Ca2+ entry
- IP3
Inositol 1,4,5-trisphosphate
- SR
Sarcoplasmic reticulum
- SOCE
Store-operated Ca2+ entry
- CCE
Capacitative Ca2+ entry
- GPCR
G protein-coupled receptor
- RTK
Receptor tyrosine kinase
- CTEPH
Chronic thromboembolic pulmonary hypertension
- MCT
Monocrotaline
- HPH
Hypoxia-induced pulmonary hypertension
- RVSP
Right ventricular systolic pressure
Footnotes
DISCLOSURES None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hassoun PM, Mouthon L, Barberá JA, Eddahibi S, Flores SC, Grimminger F, Jones PL, Maitland ML, Michelakis ED, Morrell NW, Newman JH, Rabinovitch M, Schermuly R, Stenmark KR, Voelkel NF, Yuan JX, Humbert M. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol. 2009;54:S10–19. doi: 10.1016/j.jacc.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Golovina VA, Platoshyn O, Bailey CL, Wang J, Limsuwan A, Sweeney M, Rubin LJ, Yuan JX. Upregulated trp and enhanced capacitative ca2+ entry in human pulmonary artery myocytes during proliferation. Am J Physiol Heart Circ Physiol. 2001;280:H746–755. doi: 10.1152/ajpheart.2001.280.2.H746. [DOI] [PubMed] [Google Scholar]
- 3.Sweeney M, Yu Y, Platoshyn O, Zhang S, McDaniel SS, Yuan JX. Inhibition of endogenous trp1 decreases capacitative ca2+ entry and attenuates pulmonary artery smooth muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol. 2002;283:L144–155. doi: 10.1152/ajplung.00412.2001. [DOI] [PubMed] [Google Scholar]
- 4.Yu Y, Sweeney M, Zhang S, Platoshyn O, Landsberg J, Rothman A, Yuan JX. Pdgf stimulates pulmonary vascular smooth muscle cell proliferation by upregulating trpc6 expression. Am J Physiol Cell Physiol. 2003;284:C316–330. doi: 10.1152/ajpcell.00125.2002. [DOI] [PubMed] [Google Scholar]
- 5.Firth AL, Remillard CV, Yuan JX. Trp channels in hypertension. Biochim Biophys Acta. 2007;1772:895–906. doi: 10.1016/j.bbadis.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang S, Patel HH, Murray F, Remillard CV, Schach C, Thistlethwaite PA, Insel PA, Yuan JX. Pulmonary artery smooth muscle cells from normal subjects and ipah patients show divergent camp-mediated effects on trpc expression and capacitative ca2+ entry. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1202–1210. doi: 10.1152/ajplung.00214.2006. [DOI] [PubMed] [Google Scholar]
- 7.Yu Y, Keller SH, Remillard CV, Safrina O, Nicholson A, Zhang SL, Jiang W, Vangala N, Landsberg JW, Wang JY, Thistlethwaite PA, Channick RN, Robbins IM, Loyd JE, Ghofrani HA, Grimminger F, Schermuly RT, Cahalan MD, Rubin LJ, Yuan JX. A functional single-nucleotide polymorphism in the trpc6 gene promoter associated with idiopathic pulmonary arterial hypertension. Circulation. 2009;119:2313–2322. doi: 10.1161/CIRCULATIONAHA.108.782458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu Y, Fantozzi I, Remillard CV, Landsberg JW, Kunichika N, Platoshyn O, Tigno DD, Thistlethwaite PA, Rubin LJ, Yuan JX. Enhanced expression of transient receptor potential channels in idiopathic pulmonary arterial hypertension. Proc Natl Acad Sci U S A. 2004;101:13861–13866. doi: 10.1073/pnas.0405908101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin MJ, Leung GP, Zhang WM, Yang XR, Yip KP, Tse CM, Sham JS. Chronic hypoxia-induced upregulation of store-operated and receptor-operated ca2+ channels in pulmonary arterial smooth muscle cells: A novel mechanism of hypoxic pulmonary hypertension. Circ Res. 2004;95:496–505. doi: 10.1161/01.RES.0000138952.16382.ad. [DOI] [PubMed] [Google Scholar]
- 10.Song MY, Makino A, Yuan JX. Stim2 contributes to enhanced store-operated ca2+ entry in pulmonary artery smooth muscle cells from patients with idiopathic pulmonary arterial hypertension. Pulm Circ. 2011;1:84–94. doi: 10.4103/2045-8932.78106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuchs B, Dietrich A, Gudermann T, Kalwa H, Grimminger F, Weissmann N. The role of classical transient receptor potential channels in the regulation of hypoxic pulmonary vasoconstriction. Adv Exp Med Biol. 2010;661:187–200. doi: 10.1007/978-1-60761-500-2_12. [DOI] [PubMed] [Google Scholar]
- 12.Hofer AM, Brown EM. Extracellular calcium sensing and signalling. Nat Rev Mol Cell Biol. 2003;4:530–538. doi: 10.1038/nrm1154. [DOI] [PubMed] [Google Scholar]
- 13.Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev. 2001;81:239–297. doi: 10.1152/physrev.2001.81.1.239. [DOI] [PubMed] [Google Scholar]
- 14.Magno AL, Ward BK, Ratajczak T. The calcium-sensing receptor: A molecular perspective. Endocrine Reviews. 2011;32:3–30. doi: 10.1210/er.2009-0043. [DOI] [PubMed] [Google Scholar]
- 15.Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger MA, Lytton J, Hebert SC. Cloning and characterization of an extracellular ca2+-sensing receptor from bovine parathyroid. Nature. 1993;366:575–580. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- 16.Li GW, Wang QS, Hao JH, Xing WJ, Guo J, Li HZ, Bai SZ, Li HX, Zhang WH, Yang BF, Yang GD, Wu LY, Wang R, Xu CQ. The functional expression of extracellular calcium-sensing receptor in rat pulmonary artery smooth muscle cells. J Biomed Sci. 2011;18:16. doi: 10.1186/1423-0127-18-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li GW, Xing WJ, Bai SZ, Hao JH, Guo J, Li HZ, Li HX, Zhang WH, Yang BF, Wu LY, Wang R, Yang GD, Xu CQ. The calcium-sensing receptor mediates hypoxia-induced proliferation of rat pulmonary artery smooth muscle cells through mek1/erk1,2 and pi3k pathways. Basic Clin Pharmacol Toxicol. 2011;108:185–193. doi: 10.1111/j.1742-7843.2010.00639.x. [DOI] [PubMed] [Google Scholar]
- 18.Riccardi D, Finney BA, Wilkinson WJ, Kemp PJ. Novel regulatory aspects of the extracellular ca2+-sensing receptor, car. Pflugers Arch. 2009;458:1007–1022. doi: 10.1007/s00424-009-0681-z. [DOI] [PubMed] [Google Scholar]
- 19.Ziegelstein RC, Xiong Y, He C, Hu Q. Expression of a functional extracellular calcium-sensing receptor in human aortic endothelial cells. Biochem Biophys Res Commun. 2006;342:153–163. doi: 10.1016/j.bbrc.2006.01.135. [DOI] [PubMed] [Google Scholar]
- 20.Yuan JX, Aldinger AM, Juhaszova M, Wang J, Conte JV, Jr., Gaine SP, Orens JB, Rubin LJ. Dysfunctional voltage-gated k+ channels in pulmonary artery smooth muscle cells of patients with primary pulmonary hypertension. Circulation. 1998;98:1400–1406. doi: 10.1161/01.cir.98.14.1400. [DOI] [PubMed] [Google Scholar]
- 21.Ogawa A, Firth AL, Yao W, Madani MM, Kerr KM, Auger WR, Jamieson SW, Thistlethwaite PA, Yuan JX. Inhibition of mtor attenuates store-operated ca2+ entry in cells from endarterectomized tissues of patients with chronic thromboembolic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2009;297:L666–676. doi: 10.1152/ajplung.90548.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan XJ, Goldman WF, Tod ML, Rubin LJ, Blaustein MP. Ionic currents in rat pulmonary and mesenteric arterial myocytes in primary culture and subculture. Am J Physiol. 1993;264:L107–115. doi: 10.1152/ajplung.1993.264.2.L107. [DOI] [PubMed] [Google Scholar]
- 23.Marshall CMA, Verhoeven AJ, Marshall BE. Pulmonary artery nadph-oxidase is activated in hypoxic pulmonary vasoconstriction. Am. J. Respir. Cell Mol Biol. 1996;15:633–644. doi: 10.1165/ajrcmb.15.5.8918370. [DOI] [PubMed] [Google Scholar]
- 24.Yamamura A, Yamamura H, Zeifman A, Yuan JX-J. Activity of ca2+-activated cl− channels contributes to regulating receptor- and store-operated ca2+ entry in human pulmonary artery smooth muscle cells. Pulm Circ. 2011;1:269–279. doi: 10.4103/2045-8932.83447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bai M, Trivedi S, Brown EM. Dimerization of the extracellular calcium-sensing receptor (car) on the cell surface of car-transfected hek293 cells. J Biol Chem. 1998;273:23605–23610. doi: 10.1074/jbc.273.36.23605. [DOI] [PubMed] [Google Scholar]
- 26.Bai M, Trivedi S, Kifor O, Quinn SJ, Brown EM. Intermolecular interactions between dimeric calcium-sensing receptor monomers are important for its normal function. Proc Natl Acad Sci U S A. 1999;96:2834–2839. doi: 10.1073/pnas.96.6.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gama L, Wilt SG, Breitwieser GE. Heterodimerization of calcium sensing receptors with metabotropic glutamate receptors in neurons. J Biol Chem. 2001;276:39053–39059. doi: 10.1074/jbc.M105662200. [DOI] [PubMed] [Google Scholar]
- 28.Jensen AA, Spalding TA, Burstein ES, Sheppard PO, O’Hara PJ, Brann MR, Krogsgaard-Larsen P, Brauner-Osborne H. Functional importance of the ala(116)-pro(136) region in the calcium-sensing receptor. Constitutive activity and inverse agonism in a family c g-protein-coupled receptor. J Biol Chem. 2000;275:29547–29555. doi: 10.1074/jbc.M910023199. [DOI] [PubMed] [Google Scholar]
- 29.Brown EM, Butters R, Katz C, Kifor O. Neomycin mimics the effects of high extracellular calcium concentrations on parathyroid function in dispersed bovine parathyroid cells. Endocrinology. 1991;128:3047–3054. doi: 10.1210/endo-128-6-3047. [DOI] [PubMed] [Google Scholar]
- 30.Conigrave AD, Quinn SJ, Brown EM. L-amino acid sensing by the extracellular ca2+-sensing receptor. Proc Natl Acad Sci U S A. 2000;97:4814–4819. doi: 10.1073/pnas.97.9.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gowen M, Stroup GB, Dodds RA, James IE, Votta BJ, Smith BR, Bhatnagar PK, Lago AM, Callahan JF, DelMar EG, Miller MA, Nemeth EF, Fox J. Antagonizing the parathyroid calcium receptor stimulates parathyroid hormone secretion and bone formation in osteopenic rats. J Clin Invest. 2000;105:1595–1604. doi: 10.1172/JCI9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koleganoval NPG, Ritz E, Schmitt CP, Gross ML. A calcimimetic (r-568), but not calcitriol, prevents vascular remodeling in uremia. Kidney Int. 2009;75:60–71. doi: 10.1038/ki.2008.490. [DOI] [PubMed] [Google Scholar]
- 33.Nemeth EF, Steffey ME, Hammerland LG, Hung BC, Van Wagenen BC, DelMar EG, Balandrin MF. Calcimimetics with potent and selective activity on the parathyroid calcium receptor. Proc Natl Acad Sci U S A. 1998;95:4040–4045. doi: 10.1073/pnas.95.7.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang R, Xu C, Zhao W, Zhang J, Cao K, Yang B, Wu L. Calcium and polyamine regulated calcium-sensing receptors in cardiac tissues. European Journal of Biochemistry. 2003;270:2680–2688. doi: 10.1046/j.1432-1033.2003.03645.x. [DOI] [PubMed] [Google Scholar]
- 35.Milara J, Mata M, Serrano A, Peiró T, Morcillo EJ, Cortijo J. Extracellular calcium-sensing receptor mediates human bronchial epithelial wound repair. Biochemical Pharmacology. 2010;80:236–246. doi: 10.1016/j.bcp.2010.03.035. [DOI] [PubMed] [Google Scholar]
- 36.Hofer AM, Curci S, Doble MA, Brown EM, Soybel DI. Intercellular communication mediated by the extracellular calcium-sensing receptor. Nat Cell Biol. 2000;2:392–398. doi: 10.1038/35017020. [DOI] [PubMed] [Google Scholar]
- 37.Miedlich S, Gama L, Breitwieser GE. Calcium sensing receptor activation by a calcimimetic suggests a link between cooperativity and intracellular calcium oscillations. J Biol Chem. 2002;277:49691–49699. doi: 10.1074/jbc.M205578200. [DOI] [PubMed] [Google Scholar]
- 38.Quinn SJ, Ye CP, Diaz R, Kifor O, Bai M, Vassilev P, Brown E. The ca2+-sensing receptor: A target for polyamines. Am J Physiol. 1997;273:C1315–1323. doi: 10.1152/ajpcell.1997.273.4.C1315. [DOI] [PubMed] [Google Scholar]
- 39.Gama L, Breitwieser GE. A carboxyl-terminal domain controls the cooperativity for extracellular ca2+ activation of the human calcium sensing receptor. A study with receptor-green fluorescent protein fusions. J Biol Chem. 1998;273:29712–29718. doi: 10.1074/jbc.273.45.29712. [DOI] [PubMed] [Google Scholar]
- 40.Ward DT. Calcium receptor-mediated intracellular signalling. Cell Calcium. 2004;35:217–228. doi: 10.1016/j.ceca.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 41.Arthur JM, Lawrence MS, Payne CR, Rane MJ, McLeish KR. The calcium-sensing receptor stimulates jnk in mdck cells. Biochem Biophys Res Commun. 2000;275:538–541. doi: 10.1006/bbrc.2000.3226. [DOI] [PubMed] [Google Scholar]
- 42.de Jesus Ferreira MC, Helies-Toussaint C, Imbert-Teboul M, Bailly C, Verbavatz JM, Bellanger AC, Chabardes D. Co-expression of a ca2+-inhibitable adenylyl cyclase and of a ca2+-sensing receptor in the cortical thick ascending limb cell of the rat kidney. Inhibition of hormone-dependent camp accumulation by extracellular ca2+ J Biol Chem. 1998;273:15192–15202. doi: 10.1074/jbc.273.24.15192. [DOI] [PubMed] [Google Scholar]
- 43.Kifor O, MacLeod RJ, Diaz R, Bai M, Yamaguchi T, Yao T, Kifor I, Brown EM. Regulation of map kinase by calcium-sensing receptor in bovine parathyroid and car-transfected hek293 cells. Am J Physiol Renal Physiol. 2001;280:F291–302. doi: 10.1152/ajprenal.2001.280.2.F291. [DOI] [PubMed] [Google Scholar]
- 44.McNeil SE, Hobson SA, Nipper V, Rodland KD. Functional calcium-sensing receptors in rat fibroblasts are required for activation of src kinase and mitogen-activated protein kinase in response to extracellular calcium. J Biol Chem. 1998;273:1114–1120. doi: 10.1074/jbc.273.2.1114. [DOI] [PubMed] [Google Scholar]
- 45.Patel HH, Zhang S, Murray F, Suda RY, Head BP, Yokoyama U, Swaney JS, Niesman IR, Schermuly RT, Pullamsetti SS, Thistlethwaite PA, Miyanohara A, Farquhar MG, Yuan JX, Insel PA. Increased smooth muscle cell expression of caveolin-1 and caveolae contribute to the pathophysiology of idiopathic pulmonary arterial hypertension. FASEB J. 2007;21:2970–2979. doi: 10.1096/fj.07-8424com. [DOI] [PubMed] [Google Scholar]
- 46.Riccardi D, Brown EM. Physiology and pathophysiology of the calcium-sensing receptor in the kidney. Am J Physiol Renal Physiol. 2010;298:F485–499. doi: 10.1152/ajprenal.00608.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dvorak MM, Riccardi D. Ca2+ as an extracellular signal in bone. Cell Calcium. 2004;35:249–255. doi: 10.1016/j.ceca.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 48.Geibel JP, Hebert SC. The functions and roles of the extracellular ca2+-sensing receptor along the gastrointestinal tract. Annu Rev Physiol. 2009;71:205–217. doi: 10.1146/annurev.physiol.010908.163128. [DOI] [PubMed] [Google Scholar]
- 49.Tu CL, Oda Y, Komuves L, Bikle DD. The role of the calcium-sensing receptor in epidermal differentiation. Cell Calcium. 2004;35:265–273. doi: 10.1016/j.ceca.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 50.Bandyopadhyay S, Tfelt-Hansen J, Chattopadhyay N. Diverse roles of extracellular calcium-sensing receptor in the central nervous system. Journal of Neuroscience Research. 2010;88:2073–2082. doi: 10.1002/jnr.22391. [DOI] [PubMed] [Google Scholar]
- 51.Olszak IT, Poznansky MC, Evans RH, Olson D, Kos C, Pollak MR, Brown EM, Scadden DT. Extracellular calcium elicits a chemokinetic response from monocytes in vitro and in vivo. J Clin Invest. 2000;105:1299–1305. doi: 10.1172/JCI9799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohanian J, Gatfield KM, Ward DT, Ohanian V. Evidence for a functional calcium-sensing receptor that modulates myogenic tone in rat subcutaneous small arteries. Am J Physiol Heart Circ Physiol. 2005;288:H1756–1762. doi: 10.1152/ajpheart.00739.2004. [DOI] [PubMed] [Google Scholar]
- 53.Smajilovic S, Hansen JL, Christoffersen TE, Lewin E, Sheikh SP, Terwilliger EF, Brown EM, Haunso S, Tfelt-Hansen J. Extracellular calcium sensing in rat aortic vascular smooth muscle cells. Biochem Biophys Res Commun. 2006;348:1215–1223. doi: 10.1016/j.bbrc.2006.07.192. [DOI] [PubMed] [Google Scholar]
- 54.Wonneberger K, Scofield MA, Wangemann P. Evidence for a calcium-sensing receptor in the vascular smooth muscle cells of the spiral modiolar artery. Journal of Membrane Biology. 2000;175:203–212. doi: 10.1007/s00232001068. [DOI] [PubMed] [Google Scholar]
- 55.Molostvov G, James S, Fletcher S, Bennett J, Lehnert H, Bland R, Zehnder D. Extracellular calcium-sensing receptor is functionally expressed in human artery. Am J Physiol Renal Physiol. 2007;293:F946–955. doi: 10.1152/ajprenal.00474.2006. [DOI] [PubMed] [Google Scholar]
- 56.Chow JY, Estrema C, Orneles T, Dong X, Barrett KE, Dong H. Calcium-sensing receptor modulates extracellular ca2+ entry via trpc-encoded receptor-operated channels in human aortic smooth muscle cells. Am J Physiol Cell Physiol. 2011;301:C461–468. doi: 10.1152/ajpcell.00389.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alam MU, Kirton JP, Wilkinson FL, Towers E, Sinha S, Rouhi M, Vizard TN, Sage AP, Martin D, Ward DT, Alexander MY, Riccardi D, Canfield AE. Calcification is associated with loss of functional calcium-sensing receptor in vascular smooth muscle cells. Cardiovasc Res. 2009;81:260–268. doi: 10.1093/cvr/cvn279. [DOI] [PubMed] [Google Scholar]
- 58.Weston AH, Absi M, Ward DT, Ohanian J, Dodd RH, Dauban P, Petrel C, Ruat M, Edwards G. Evidence in favor of a calcium-sensing receptor in arterial endothelial cells: Studies with calindol and calhex 231. Circ Res. 2005;97:391–398. doi: 10.1161/01.RES.0000178787.59594.a0. [DOI] [PubMed] [Google Scholar]
- 59.Fryer RM, Segreti JA, Widomski DL, Franklin PH, Banfor PN, Koch KA, Nakane M, Wu-Wong JR, Cox BF, Reinhart GA. Systemic activation of the calcium sensing receptor produces acute effects on vascular tone and circulatory function in uremic and normal rats: Focus on central versus peripheral control of vascular tone and blood pressure by cinacalcet. J Pharmacol Exp Ther. 2007;323:217–226. doi: 10.1124/jpet.107.123901. [DOI] [PubMed] [Google Scholar]
- 60.Smajilovic S, Yano S, Jabbari R, Tfelt-Hansen J. The calcium-sensing receptor and calcimimetics in blood pressure modulation. Br J Pharmacol. 2011;164:884–893. doi: 10.1111/j.1476-5381.2011.01317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Finney BA, del Moral PM, Wilkinson WJ, Cayzac S, Cole M, Warburton D, Kemp PJ, Riccardi D. Regulation of mouse lung development by the extracellular calcium-sensing receptor, car. J Physiol. 2008;586:6007–6019. doi: 10.1113/jphysiol.2008.161687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pollak MR, Brown EM, Estep HL, McLaine PN, Kifor O, Park J, Hebert SC, Seidman CE, Seidman JG. Autosomal dominant hypocalcaemia caused by a Ca2+-sensing receptor gene mutation. Nat Genet. 1994;8:303–307. doi: 10.1038/ng1194-303. [DOI] [PubMed] [Google Scholar]
- 63.Brown EM. Clinical lessons from the calcium-sensing receptor. Nat Clin Pract Endocrinol Metab. 2007;3:122–133. doi: 10.1038/ncpendmet0388. [DOI] [PubMed] [Google Scholar]
- 64.Brauner-Osborne H, Wellendorph P, Jensen AA. Structure, pharmacology and therapeutic prospects of family c g-protein coupled receptors. Curr Drug Targets. 2007;8:169–184. doi: 10.2174/138945007779315614. [DOI] [PubMed] [Google Scholar]
- 65.Nemeth EF. Calcimimetic and calcilytic drugs: Just for parathyroid cells? Cell Calcium. 2004;35:283–289. doi: 10.1016/j.ceca.2003.10.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Figure I. Upregulated CaSR in pulmonary arteries from IPAH patients. A. Immunohistochemistry data showing CaSR expression level (green fluorescence intensity) in pulmonary arteries of normal subjects and IPAH patients. The lung tissue sections were stained with antibodies against CaSR (green) and smooth muscle α-actin (SM-α-Actin, red) and with DAPI (blue). The overlay images are shown in the bottom. B. Summarized data (means±SE, n=3 for each group) showing CaSR expression level (green fluorescence intensity) in pulmonary arteries of normal subjects (Norm) and IPAH patients. **P<0.01 vs. Normal.
Online Figure II. Extracellular Ca2+-induced increase [Ca2+]cyt in IPAH-PASMCs is not due to Ca2+ leakage. A. Representative traces showing change in resting [Ca2+]cyt before and during application of Ca2+-free solution in normal and IPAH PASMC. Summarized data (right panel, n=96-370 cells) showing the resting [Ca2+] in normal and IPAH PASMC superfused with 2.2 mM Ca2+ cyt - containing solution (black bars) or Ca2+-free solution (grey bars). **P<0.001 vs. normal PASMC. B. Representative images (left panels) of trypan (TB) blue staining (0.4%, 1 min) before (−) and during (+) treatment with 10 μM ionomycin for 10 min in normal (upper panels) and IPAH (lower panels) PASMC. Summarized data (right panel) showing no correlation between the extracellular Ca2+-induced increase in [Ca2+] and the Ca2+ cyt leakage through the plasma membrane. C. Representative images showing [Ca2+]cyt (or Fura-2 fluorescence intensity) in normal (left panels) and IPAH (right panels) PASMC in the absence and presence of 10 μM CPA, an inhibitor of Ca2+-ATPase in the SR. The cells were loaded with the membrane-permeable fura-2/AM (4 μM, upper panels) or the membrane-impermeable fura-2 (4 and 40 μM, middle and lower panels). Data were obtained from 21 to 64 cells.
Online Figure III. Inhibition of PLC and IP3R attenuates extracellular Ca2+-induced [Ca2+]cyt increases in IPAH-PASMC Representative records of [Ca2+]cyt changes (left panels), pseudo images (middle panels) and summarized data (means±SE, right panels) showing extracellular Ca2+-induced increase in [Ca2+]cyt before and during treatment with 1 μM U73122 (a PLC inhibitor; n=57, A), 1 μM U73343 (an inactive form of U73122; n=45, B), 3 μM xestospongin C (an IP3R blocker; n=34, C), 10 μM diltiazem (a VDCC blocker; n=46, D), and KB-R7943 (an Na+/Ca2+ exchanger inhibitor; n=22, E) in IPAH-PASMC. **P<0.01 vs. control.