Abstract
Purpose of review
Combination antiretroviral therapy (ART) has turned HIV infection into a complex chronic disease. This article documents cancer risk among HIV-infected persons, reviews immune system effects of HIV infection in relation to cancer risk, discusses implications for cancer prevention, and suggests future research directions.
Recent findings
There has been a shift in the cancer spectrum from AIDS-defining cancers (ADC) to non-ADC, although the burden of ADC remains high. Although a high prevalence of non-HIV cancer risk factors among HIV-infected persons contributes to cancer risk, substantial evidence has accumulated in favor of an independent association between HIV-induced immunodeficiency and elevated risk of many specific cancer types, most of viral cause, although further work is needed to disentangle immunodeficiency and smoking effects for lung cancer, and immunodeficiency and hepatitis virus effects for liver cancer. Relationships between cancer risk and two other immune system hallmarks of HIV infection, chronic inflammation, and immune dysfunction/senescence, remain poorly understood.
Summary
Early, sustained ART is a crucial component of cancer prevention. Continued epidemiologic monitoring is needed to detect possible effects on cancer risk of specific ART classes or medications, long-term exposure to systemic inflammation or immune dysfunction, or earlier or more effective ART.
Keywords: aging, cancer, HIV, immune system, inflammation
INTRODUCTON
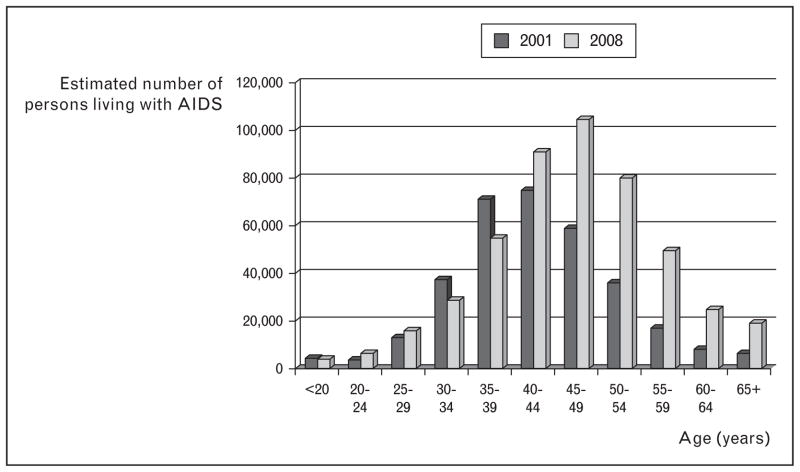
Combination antiretroviral therapy (ART), introduced in the mid-1990s, has turned HIV infection into a complex chronic disease [1]. Consequently, more people are living with HIV [2] to older ages (Fig. 1) [3,4]; by 2015, more than half of people living with HIV/AIDS (PLWHA) in the USA will be more than 50 years of age [5]. In this article, we document cancer risk among PLWHA in high-income countries, review immune system effects of HIV infection in relation to cancer risk, discuss implications for cancer prevention, and suggest future research directions.
FIGURE 1.
Age distribution of persons living with AIDS, USA, 2001 and 2008. We show persons living with AIDS because data were available for all 50 states and the District of Columbia for both 2001 and 2008. For persons living with HIV (with or without an AIDS diagnosis), data were available for 40 states in 2008, but only 33 states in 2001, such that the 2 years were not strictly comparable. Adapted with permission from [3] and [4].
EPIDEMIOLOGY OF CANCER IN PEOPLE LIVING WITH HIV/AIDS
In the ART era, the spectrum of cancer diagnoses among PLWHA has shifted from AIDS-defining cancers (ADC), primarily Kaposi sarcoma and non-Hodgkin lymphoma, to non-AIDS-defining cancers (NADC). We explore this shift in detail in Table 1 [6–8,9▪▪,10,11,12▪,13,14▪,15–17,18▪▪], which presents, by cancer type, the estimated number of incident cancer diagnoses among PLWHA in the USA in 2001–2005, and how this number changed from 1991–1995 to 2001–2005 [18▪▪]. In Table 1, we also present, by cancer type, relative risk estimates for PLWHA compared with the general population or an uninfected comparison group [6–8,9▪▪,10,11,12▪,13,14▪,15–17]. To create this table, we relied heavily on two important studies by Shiels et al. [6,18▪▪].
Table 1.
Estimated number of incident cancer cases among persons living with HIV in 2001–2005 in the USA, ratio of incident cases in 2001–2005 compared with 1991–1995, and relative risk among persons living with HIV compared with uninfected persons, by cancer type
| Cancer category | Cancer type | Estimated number of cases (%) in 2001–2005a | 2001–2005/1991–1995b | Relative risk (95% confidence interval)
|
|
|---|---|---|---|---|---|
| Meta-analysis [6]c | Recent additional studiesd | ||||
| Kaposi sarcoma-associated herpes virus | Kaposi sarcoma | 3827 (15.3) | 0.18 | – | 55 (9.1–2244) [7]; 112 (95–133) [8]; 197 (139–279) [9▪▪]; 210 (100–442) [10]; 790 (640–980) [11]; 1584 (1486–1687) [12▪] |
|
| |||||
| Epstein–Barr virus | Non-Hodgkin lymphoma | 5968 (23.9) | 0.47 | – | 6.5 (5.4–7.7) [11]; 8.0 (6.8–9.4) [10]; 11 (4.2–37) [7]; 15 (14–16) [12▪]; 16 (13–19) [9▪▪]; 17 (14–20) [8] |
| Hodgkin lymphoma | 1143 (4.6) | 2.1 | 11 (8.8–15) | 4.9 (3.6–6.6) [10]; 11 (10–13) [12▪]; 20 (13–31) [9▪▪] | |
|
| |||||
| Human papillomavirus | Cervix | 530 (2.1) | 1.6 | – | 2.9 (1.8–4.4) [11]; 5 (4.0–6.2) [12▪]; 10 (6.5–16) [8] |
| Anus | 1885 (7.6) | 7.6 | 28 (21–35) | 15 (10–22) [10]; 19 (2.6–823) [7]; 20 (6.6–42) (non-MSM men) [13]; 25 (9.1–48) (women) [13]; 32 (29–36) [12▪]; 61 (37–101) [9▪▪]; 79 (58–102) (MSM) [13] | |
| Vulva and vagina | 124 (0.5) | 8.3 | 9.4 (4.9–18) | 6.7 (3.9–11) [12▪] | |
| Penis | 75 (0.3) | 4.9 | 6.8 (4.2–11) | 5.0 (2.5–8.9) [12▪] | |
| Oral cavity and pharynx | 677 (2.7) | 2.8 | 1.9 (1.4–2.6) (oropharynx); 2.0 (1.1–3.6) (head and neck); 2.2 (1.0–4.7) (lip, oral and pharynx); 4.1 (2.1–7.9) (nasopharynx) | 1.8 (1.5–2.0) [12▪]; 1.9 (1.3–2.9) [9▪▪]; 2.3 (1.6–3.2) (tongue) [12▪]; 3.6 (1.7–6.8) (lip) [12▪] | |
|
| |||||
| Human papillomavirus suspected | Larynx | 415 (1.7) | 4.5 | 1.5 (1.1–2.0) | 3.0 (2.3–3.7) [12▪] |
| Esophagus | 328 (1.3) | 6.2 | 1.5 (0.99–2.3) | 1.4 (0.9–2.0) [12▪] | |
| Eye | – | – | 3.1 (1.6–5.9) | – | |
| Nonmelanoma skine | – | – | 3.5 (1.8–6.8) | – | |
|
| |||||
| Hepatitis B/C virus | Liver | 780 (3.1) | 5.0 | 5.6 (4.0–7.7) | 2.6 (1.7–4.0) [9▪▪]; 2.8 (2.2–3.5) [10]; 4.4 (3.6–5.2) [12▪] |
|
| |||||
| Helicobacter pylori-related | Stomach | 190 (0.8) | 2.4 | 1.7 (1.2–2.5) | 1.2 (0.8–1.7) [12▪] |
|
| |||||
| Common epithelial | Lung | 2630 (10.5) | 2.2 | 2.6 (2.1–3.1) | 1.7 (1.5–2.0) [14▪]; 1.8 (1.4–2.4) [9▪▪]; 2.0 (0.92–4.2) [15]; 2.4 (1.6–3.5) [16]; 2.6 (2.4–2.8) [12▪]; 3.4 (0.49–148) [17]; 6.0 (0.47–321) [7] |
| Colorectal | 687 (2.8) | 4.1 | 0.81 (0.48–1.4) (colon); 1.1 (0.69–1.7) (colorectal); 1.5 (0.54–4.2) (rectum) | 0.9 (0.6–1.3) [9▪▪]; 0.9 (0.8–1.1) [12▪] | |
| Female breast | 613 (2.5) | 9.4 | 0.74 (0.56–0.97) | 0.7 (0.5–0.8) [12▪] | |
| Prostate | 1171 (4.7) | 8.7 | 0.69 (0.55–0.86) | 0.5 (0.5–0.6) [12▪]; 0.8 (0.6–0.9) [9▪▪]; 1.0 (0.9–1.1) [10]; 1.1 (0.44–2.5) [7] | |
| Ovary | 46 (0.2) | 2.8 | 1.4 (0.78–2.4) | 1.0 (0.5–1.9) [12▪] | |
| Pancreas | 344 (1.4) | 7.5 | 1.0 (0.74–1.4) | 1.0 (0.7–1.4) [12▪] | |
| Uterine corpus | 96 (0.4) | 6.7 | 1.5 (0.68–3.4) | 0.5 (0.2–1.0) [12▪] | |
| Bladder | 97 (0.4) | 2.3 | 1.1 (0.72–1.7) | 0.9 (0.6–1.3) [12▪] | |
| Kidney | 358 (1.4) | 3.2 | 1.7 (1.3–2.2) | 0.7 (0.5–1.0) [12▪] | |
|
| |||||
| Other | Melanoma | 319 (1.3) | 3.5 | 1.2 (0.88–1.6) | 1.1 (0.8–1.4) [12▪]; 1.7 (1.3–2.3) [10]; 1.8 (1.3–2.6) [9▪▪]; 1.9 (0.25–12) [7] |
| Soft tissue | 129 (0.5) | 3.4 | – | 1.4 (0.9–2.2) [12▪] | |
| Testis | 128 (0.5) | 0.92 | 1.4 (1.1–1.9) | 0.7 (0.5–1.1) [12▪] | |
| Brain | 73 (0.3) | 1.4 | 1.8 (1.2–2.7) | 0.6 (0.3–1.0) [12▪] | |
| Thyroid | 145 (0.6) | 4.2 | 1.1 (0.56–2.3) | 0.7 (0.4–1.0) [12▪] | |
| Myeloma | 251 (1.0) | 2.5 | 2.6 (1.5–4.5) | 0.7 (0.4–1.1) [12▪] | |
| Leukemia | 215 (0.9) | 1.9 | 2.6 (1.9–3.5) | 1.7 (0.8–3.3) (lymphocytic) [12▪]; 2.1 (1.5–2.9) (myeloid/monocytic) [12▪] | |
|
| |||||
| AIDS-defining cancers (Kaposi sarcoma, non-Hodgkin lymphoma, cervical cancer) | All | 10 325 (41.4) | 0.30 | – | – |
|
| |||||
| Non-AIDS-defining cancers | All | 14 036 (56.3) | 3.2 | 2.0 (1.8–2.2) | 1.6 (1.5–1.7) [10]; 1.6 (1.6–1.7) [12▪] |
|
| |||||
| Total | 24 944 | ||||
Adapted with permission from [18▪▪]. For each non-AIDS-defining cancer (NADC), we applied the Shiels et al. estimates for persons living with AIDS or HIV only in 34 US states in 2004–2007 to their estimates for persons living with AIDS in the USA as a whole in 2001–2005 to obtain extrapolated estimates for all persons living with HIV (with or without an AIDS diagnosis) in the USA in 2001–2005. Note the caveat that these extrapolations rely on the assumption that HIV patients in the 34 states are representative of HIV patients in the entire USA with respect to cancer risk. It was not necessary to perform this extrapolation for AIDS-defining cancers (ADC) because these only occur among AIDS patients by definition. Percentages of all ADC and all NADC add to 97.7% because 2.3% of cancer were ‘poorly specified malignancies’ and could not be classified as ADC or NADC. Some rare NADC are not listed in the table.
Adapted with permission from [18▪▪]. Ratio of the number of new cancer diagnoses among persons living with AIDS in 2001–2005 compared with 1991–1995. This ratio is restricted to persons living with AIDS. Note that this ratio reflects both changes in the crude cancer incidence rates and the increase in the size of the population living with AIDS between 1991–1995 and 2001–2005.
Adapted with permission from [6]. This meta-analysis calculated NADC-type-specific summary standardized incidence ratios across 18 studies of persons living with HIV/AIDS compared with the general population. The studies spanned the pre-ART and ART eras and included persons living with AIDS and HIV only.
Restricted to recent studies, not included in the Shiels et al. [6] meta-analysis, with data from the ART era. The relative risks presented in the table were adjusted for demographic variables, but not for non-HIV risk factors such as smoking. Relative risks are comparisons between an HIV-infected population and the general population or an HIV-uninfected comparison group.
This result should be interpreted with caution as it may reflect ascertainment of rare nonmelanoma skin cancers, as opposed to the common basal cell and squamous cell carcinomas, which are often not ascertained by cancer registries. Furthermore, Kaposi sarcoma can be misclassified as nonmelanoma skin cancer.
The decrease in ADC diagnoses was driven by the sharp decline in Kaposi sarcoma and non-Hodgkin lymphoma incidence rates since the early 1990s [11,12▪,18▪▪,19▪,20–22], corresponding with the introduction of ART. Nevertheless, even in 2001–2005, non-Hodgkin lymphoma accounted for 24% and Kaposi sarcoma accounted for 15% of cancer diagnoses (Table 1). In the ART era, relative risk (RR) estimates range from 55 to 1584 for Kaposi sarcoma and from 6.5 to 17 for non-Hodgkin lymphoma [7,8,9▪▪,10,11,12▪].
The increase in NADC diagnoses was driven by the aging of the HIV-infected population (as well as by the increasing number of PLWHA) [18▪▪]. Because incidence rates for most types of cancer increase exponentially with age [23], the number of diagnoses for these cancer types increased simply because PLWHA are aging. Thus, between 1991–1995 and 2001–2005, NADC diagnoses tripled even though the overall age-standardized, sex-standardized and race-standardized NADC incidence rate declined by about 13% among PLWHA [18▪▪]. In 2001–2005, the five leading NADC diagnosed among PLWHA were cancers of the lung and anus, Hodgkin lymphoma, and cancers of the prostate and liver (Table 1) [18▪▪].
Cancer types with a known or suspected viral cause had elevated RRs (Table 1) [6,24,25]. In contrast, with the notable exception of lung cancer and some other smoking-related cancers, RRs for most nonviral-related epithelial cancers were null or below null (prostate, breast). In spite of their low RRs, cancers of the prostate and breast were among the cancer types with the greatest rise in crude incidence (Table 1), due to their high baseline risks and strong associations with age.
The elevated risk for a given cancer type in PLWHA may be caused by immune system effects of HIV infection, by high prevalence of non-HIV cancer risk factors, or by combined immune system and non-HIV risk factor effects. The high prevalence of infection from human papillomavirus (HPV) [26–28], hepatitis C virus (HCV) [29], hepatitis B virus (HBV) [29], and Kaposi sarcoma-associated herpesvirus [30], as well as of smoking [31] and alcohol consumption [32], are well documented. Here, we focus on immune system effects of HIV infection, including progress in disentangling immune system effects from non-HIV risk factor effects.
IMMUNE SYSTEM AND CANCER
The immune system protects against cancer by clearing or suppressing oncogenic virus infections and through general cancer immunosurveillance, a process in which innate and adaptive immunity interact to recognize and destroy cancer cells [33]. Thus, immunosuppressive conditions, including inherited immunodeficiency disorders, post-transplant immunosuppression, and HIV infection, are associated with increased risk of specific cancer types, most prominently lymphomas or other cancers caused by viruses [34].
Chronic immune activation and inflammation, through stimulation of cell proliferation, generation of genotoxic reactive oxygen and nitrogen species, production of procarcinogenic cytokines and growth factors, and possibly other mechanisms, also promote cancer development [35,36]. Such chronic inflammation is typically localized to a specific tissue and may be caused by viruses (e.g., HCV or HBV and liver cancer), other infectious agents (e.g., Helicobacter pylori and gastric cancer), autoimmune disorders (e.g., inflammatory bowel disease and colorectal cancer), or toxic exposures (e.g., inflammation caused by smoking or other irritants and lung cancer) [35,36,37▪].
IMMUNE SYSTEM EFFECTS OF HIV INFECTION
The three immune system hallmarks of HIV infection are immunodeficiency, chronic immune activation/inflammation, and immune dysfunction/senescence. HIV infection is characterized by a paradoxical coexistence of immunodeficiency, driven by infection and depletion of CD4 cells, and systemic chronic activation of both the innate and adaptive immune systems with resultant chronic inflammation [38,39▪▪,40▪]. Mechanisms underlying chronic immune activation appear to include the immune response to HIV infection itself, direct HIV gene product activation of lymphocytes and macrophages and production of proinflammatory cytokines, immunodeficiency-induced reactivation and replication of other viruses, particularly cytomegalovirus and Epstein–Barr virus, and translocation of intestinal bacterial flora across the gut wall precipitated by massive depletion of CD4 cells in the intestinal mucosa [38,39▪▪,40▪].
Chronic immune activation leads to progressive exhaustion of immune resources associated with thymic involution, impaired hematopoiesis, lymphatic tissue fibrosis, sustained T-cell turnover and apoptosis, decline in T-cell renewal, a deficit of naive T cells, and an excess of differentiated, functionally defective memory T cells with shortened telomeres and limited diversity [38,40▪,41▪]. This immune dysfunction mimics the process of aging-associated immunosenescence [38,40▪,41▪], which has been postulated to be causally related to the increased cancer risk associated with aging [42,43].
Immune activation and inflammation may persist in persons on ART, albeit at lower levels than among untreated patients [39▪▪,41▪,44▪▪]. Thus, inflammatory markers, including interleukin 6 (IL-6), C-reactive protein (CRP), and D-dimer, remain elevated among persons on ART [41▪,44▪▪,45▪,46▪], although a recent study comparing demographically similar HIV-infected and uninfected veterans of similar comorbidity status found elevated IL-6 and D-dimer only among HIV-infected veterans with HIV RNA 500 copies/ml or more or CD4 cell count less than 200 cells/μl, and found elevated soluble CD14 (a biomarker of monocyte activation) only among HIV-infected veterans with CD4 cell count less than 200 cells/μl [47▪]. Cellular immune dysfunction markers, including low CD4 and CD8 naive : memory cell ratios, low activated CD8 cell percentage, and low CD4 : CD8 cell ratio, also persist in patients on ART, especially those who initiated ART at lower CD4 counts and older patients [44▪▪,48,49▪].
HIV IMMUNE EFFECTS AND CANCER RISK
A recent meta-analysis of studies of cancer risk among PLWHA compared with the general population found the standardized incidence ratio (SIR) for all NADC combined to be 3.7 among persons with AIDS, but only 1.2 among HIV-infected persons without AIDS [6], implicating more profound immune system defects in NADC risk. This result is consistent with any of the three immune system hallmarks of HIV infection contributing to elevated cancer risk. However, to date, research has focused on the role of immunodeficiency, with substantial evidence accumulating in favor of an association with elevated risk of a number of specific cancer types, independent of non-HIV risk factors.
The most compelling evidence comes from a seminal meta-analysis in which the pattern of cancer risk was similar between PLWHA and organ transplant patients [24]. Both populations exhibited elevated SIRs for all cancer types known or suspected to be of viral cause, as well as cancers of the lung, stomach, and kidney, melanoma, multiple myeloma, and leukemia. These populations probably share few cancer risk factors apart from immunodeficiency, the main immune system defect they have in common. However, it should be noted that although immunosuppressive therapy for transplant recipients is not associated with immune activation, a recent study suggested that long-term immunosuppressive therapy leads to T-cell senescence [50].
Additional compelling evidence for an effect of immunodeficiency is provided by the well established strong, inverse relationships between CD4 count and risk for Kaposi sarcoma and non-Hodgkin lymphoma, respectively [21,22,51,52]. Early studies were inconsistent regarding the relationship between CD4 count and NADC risk, probably due to use of insensitive, static CD4 measures, such as CD4 count at AIDS diagnosis. However, recent studies have noted inverse associations between current (i.e., time updated) CD4 count and risk of NADC (grouped) [53,54▪▪,55▪▪,56▪▪,57,58], virus-related NADC (grouped) and nonvirus-related epithelial NADC (grouped) [56▪▪], Hodgkin lymphoma [9▪▪,56▪▪,59▪,60], melanoma [9▪▪], and anal [9▪▪,56▪▪], lung, [9▪▪,56▪▪,60], cervical [60], oral cavity/pharynx [9▪▪], liver [9▪▪,60,61,62▪], and colorectal cancers [9▪▪]. Two studies found no association with NADC (grouped) [63,64▪▪].
A key outstanding question is whether current immunodeficiency versus duration of immunodeficiency is more closely associated with increased cancer risk. One study found longer exposure to CD4 cell count less than 200 cells/μl, but not lower current CD4, to be independently associated with increased NADC (grouped) risk [64▪▪]. A second study found that lower current CD4 count was a better predictor of elevated risk for Kaposi sarcoma, non-Hodgkin lymphoma, Hodgkin lymphoma, and cancers of the lung, liver and cervix than longer exposure to CD4 cell count less than 200, 350, or 500 cells/μl, whereas longer exposure to CD4 cell count less than 200 cells/μl was the best predictor of elevated anal cancer risk [60]. A third study found lower current CD4 count, but not longer exposure to CD4 cell count less than 350 cells/μl, to be associated with increased NADC (grouped) risk [57], whereas a fourth found lower current CD4 count, but not longer exposure to CD4 cell count less than 200 cells/μl, to be associated with increased NADC (grouped) and ADC (grouped) risk, respectively [58]. A fifth study found longer and current exposure to CD4 cell count less than 500 cells/μl to be an equally good predictor of increased NADC (grouped) risk [53]. Finally, a study found that current, but not longer, exposure to CD4 cell count less than 500 cells/μl was associated with increased liver cancer risk [62▪]. For some cancer types, particularly Hodgkin lymphoma [59▪], low current CD4 count may be the result of preclinical disease, as opposed to the cause of disease.
The relationships between CD4 count and risk of specific NADC are generally weaker and more subtle than the relationships for ADC [9▪▪,52,53,55▪▪,58,60]. Furthermore, because CD4 count and markers of inflammation and immune dysfunction are intercorrelated, it is possible that CD4 count is a marker of risk, but not the sole mediator of risk. In general, the association between lower CD4 count and increased NADC risk has been found to be independent of time-updated HIV RNA level [53,57,58,59▪,60,64▪▪], a rough proxy for immune activation. However, higher current HIV RNA level was found to be independently associated with increased risk of non-Hodgkin lymphoma [53,60] and Kaposi sarcoma [60] and longer exposure to high HIV RNA was observed to be independently associated with increased risk of non-Hodgkin lymphoma [53] and anal cancer [60].
The association between lower CD4 count and risk of NADC (grouped) appears to be independent of non-HIV risk factors, persisting after adjustment for smoking, alcohol abuse, HBV coinfection, and/or HCV coinfection [53,54▪▪,56▪▪,58,64▪▪], with the caveat that adjustment was often imperfect due to missing data for adjustment variables.
The weight of evidence also suggests that the inverse association between CD4 count and risk of HPV-related cancers is mostly independent of non-HIV risk factors. As mentioned above, associations have been observed between lower current CD4 count and increased risk of several HPV-related cancers (anal, cervical, oral cavity/pharynx). In addition, lower CD4 count at AIDS onset [65] or at baseline [13], longer duration of low CD4 count [60,64▪▪], and lower nadir CD4 [66] were found to be associated with increased anal cancer risk. HIV adversely impacts the natural history of HPV infection, including increased HPV cervical viral load and persistence [26], which are inversely associated with CD4 count [26], as is prevalence of precancerous cervical [26] and anal lesions [67]. The anal cancer incidence rate among PLWHA rose between the pre-ART and early ART eras [13,18▪▪,65,66], perhaps because longer survival allowed enough time for progression of precancerous lesions to invasive cancer [65], but appears to have plateaued in the later ART era [13,18▪▪]. Longer duration of HIV infection was observed to be associated with increased anal cancer risk [66]. We should note that for oral cavity/pharynx cancer, in one study the elevated risk among PLWHA compared with uninfected persons was no longer significant after adjusting for smoking and alcohol/drug abuse [9▪▪].
The relationships between HIV infection/lower CD4 count and lung and liver cancer risk are less clear. For lung cancer, in most [14▪,15,16,68–70], but not all [9▪▪], comparisons, incidence in PLWHA compared with uninfected persons remained elevated after adjusting for smoking. In addition, in three studies, the inverse association between current CD4 count and lung cancer risk among PLWHA persisted after adjustment for smoking [9▪▪,56▪▪,60]. However, no association between CD4 count and lung cancer risk, smoking adjusted or not, was observed in four other studies [64▪▪,68,69,71▪]. Immunodeficiency-induced recurrent pneumonia and the associated inflammation may contribute to lung carcinogenesis in HIV-infected persons [72].
With respect to liver cancer, HIV accelerates the natural history of HCV [73,74] and HBV [75] infection. In some studies, the inverse association between current CD4 count and liver cancer risk persisted after adjustment for alcohol abuse/dependence [9▪▪] and hepatitis coinfection [60,62▪]. However, other studies found no increased risk of liver cancer in HIV-infected compared with uninfected veterans after adjusting for HCV infection and alcohol abuse/dependence [76], no difference in liver cancer incidence between HIV/HCV-coinfected versus HCV-monoinfected patients [77], and no association between duration of exposure to low CD4 count and liver cancer risk [64▪▪].
CANCER PREVENTION
The two pillars of cancer prevention among PLWHA are restoration of immune function and reduction in the prevalence of non-HIV cancer risk factors. Here, we focus on immune function.
Early and uninterrupted antiretroviral therapy
Department of Health and Human Services guidelines recommend ART initiation for all PLWHA regardless of CD4 count, but with a lower strength of recommendation for patients with CD4 cell count more than 500 cells/μl [78]. Guidelines are mainly based on large observational cohort studies that found early ART initiation to reduce risk of death and/or AIDS [78]. Specific cancer types, individual or grouped, have not yet been analyzed as separate endpoints in such studies. Our working hypothesis is that early ART initiation directly reduces the risk of a number of specific cancer types.
For these benefits to be realized, early entry of PLWHA into medical care is essential. However, in the USA, one-fifth of PLWHA are unaware of their infection and one-third develop AIDS within a year of their diagnosis [2]; in North America in 2007, more than half had CD4 cell count 350 cells/μl or less at first presentation to medical care [79▪]. In the USA, during 2003–2009, entry into medical care after diagnosis was delayed for 28% of PLWHA [80].
With these shortcomings, it should not be surprising that the ADC burden among PLWHA, closely linked to CD4 count, remains unacceptably high, and that the standardized incidence rate for all NADC combined declined only about 13% between 1991–1995 and 2001–2005 [18▪▪]. Public health efforts to facilitate early diagnosis of HIV infection, linkage to and retention in care, and adherence to ART – all cornerstones of the US National HIV/AIDS Strategy [81] – are crucial in reducing the cancer burden among PLWHA.
Improved antiretroviral therapy
ART does not fully restore immunologic health, especially when it is initiated late [78,82,83]. Normal CD4 counts are often not achieved with long-term therapy [82,83], and chronic inflammation and immune dysfunction persist. This suggests the need for improved ART regimens [40▪,83].
Evidence from in-vitro and in-vivo model systems indicates that antiretroviral agents may have antitumor activity independent of their antiviral effect [84], including protease inhibitors [85], nucleoside reverse transcriptase inhibitors (NRTIs) [86–88], and non-NRTIs [89]. There is also concern that some antiretroviral agents may increase cancer risk, including NRTIs [90,91] and CCR5 antagonists [92]. The NRTI zidovudine has been classified as possibly carcinogenic in humans [90]. Epidemiologic data are limited, although in two studies ART class was unrelated to overall NADC risk [54▪▪,93].
RESEARCH DIRECTIONS
Although the evidence is substantial that HIV-induced immunodeficiency, as measured by CD4 count, is independently associated with elevated risk of specific cancer types, most of viral cause, relationships between HIV-induced chronic inflammation and immune dysfunction/senescence and cancer risk remain poorly understood. Research challenges to understanding these relationships, which are likely to differ across cancer types, include intercorrelation among immunodeficiency, inflammation, and immune dysfunction biomarkers, as well as achieving sufficient sample size for all but the most common cancer types. A limitation of much previous research has been the need to use grouped cancer categories to attain reasonable statistical power.
With respect to immunodeficiency, we do not yet understand the role of duration of low CD4 count versus current CD4 count, latency, the shape of dose–response relationships, or reversibility of effects. Inflammation and immune dysfunction bio-markers are beginning to be studied in relation to mortality and other outcomes, but not yet in relation to cancer risk. In PLWHA, higher levels of the inflammatory markers IL-6, CRP, and D-dimer have been found to be strongly associated with increased mortality risk, independent of CD4 count [44▪▪,94]; higher levels of D-dimer were found to be independently associated with elevated cardiovascular disease risk [95]; and higher levels of soluble tumor necrosis factor receptor-1, an inflammation marker, and soluble CD27 and soluble CD40 ligand, both immune activation markers, were found to be independently associated with increased risk of the composite outcome of ADC or death [96▪▪]. In the later study, ADC was not evaluated separately as an outcome. There is a wealth of soluble and cellular biomarkers of inflammation and immune dysfunction awaiting to be studied in relation to risk of specific cancer types among PLWHA [44▪▪].
Further research also is needed to determine whether specific ART classes or medications are associated with cancer risk. It is particularly important to identify medications that might increase cancer risk because of the lifelong commitment to ART. Finding that an ART medication is associated with reduced risk of a specific cancer type could inform treatment decisions. For example, if a medication specifically protected against anal cancer, its use might be preferred for patients with persistent anal HPV infection.
Finally, many carcinogenic processes have latency periods of decades. Given that we are less than 2 decades into the ART era, additional cancer types associated with increased risk may emerge. Of particular concern is the possibility that long-term exposure to HIV-induced systemic inflammation (or immune dysfunction) may eventually lead to increased risk for common epithelial cancers (e.g., colorectal, breast, prostate) for which inflammation is already known or suspected to be an etiologic factor [35,97–99]. On a more optimistic note, earlier and more effective ART on a population level may result in reduced incidence of cancer types that currently exhibit elevated incidence among PLWHA. For these reasons, epidemiologic monitoring of cancer incidence among PLWHA should continue.
CONCLUSION
ART and the aging of PLWHA have resulted in a shift in the cancer spectrum from ADC to NADC, although the burden of ADC remains high. HIV effects on the immune system and a high prevalence of non-HIV cancer risk factors contribute independently to elevated risk of many cancer types, most of viral cause. Although HIV-induced immunodeficiency is the main immune system factor implicated in the elevated cancer risk, research is needed on the roles of chronic inflammation and immune dysfunction. Research also is needed on the long-term cancer effects of ART and specific ART classes and medications. Finally, epidemiologic monitoring of long-term trends in cancer incidence among HIV-infected persons should continue.
KEY POINTS.
The spectrum of cancer diagnoses among HIV-infected persons has shifted from ADC to NADC, although the burden of ADC remains high.
Although a high prevalence of non-HIV cancer risk factors, including oncogenic virus infection and smoking, contributes to cancer risk, substantial evidence has accumulated in favor of an independent association between HIV-induced immunodeficiency and elevated risk of many specific cancer types, most of viral cause.
Relationships between cancer risk and the other two immune system hallmarks of HIV infection, chronic inflammation and immune dysfunction/senescence, remain poorly understood.
Early, sustained ART is a crucial component of cancer prevention.
Continued epidemiologic monitoring is needed to detect possible effects on cancer risk of specific ART classes or medications, long-term exposure to systemic inflammation or immune dysfunction, or earlier or more effective ART.
Acknowledgments
This work was funded by grants from the National Institute on Alcohol Abuse and Alcoholism (U10-AA3566, U24-AA020794, U01-AA020790), the National Institute of Mental Health (5T32-MH020031, P30-MH062294), and the National Institute of Allergy and Infectious Diseases (K01-AI071725). The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 590–591).
- 1.Justice AC. HIV and aging: time for a new paradigm. Curr HIV/AIDS Rep. 2010;7:69–76. doi: 10.1007/s11904-010-0041-9. [DOI] [PubMed] [Google Scholar]
- 2.Center for Disease Control and Prevention. HIV surveillance – United States, 1981–2008. MMWR. 2011;60:689–693. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. [Accessed 1 March 2012];HIV surveillance report, 2009. 2011 21 http://www.cdc.gov/hiv/topics/surveillance/resources/reports/ [Google Scholar]
- 4.Centers for Disease Control and Prevention. HIV/AIDS Surveillance Report, 2005. Vol. 17. Atlanta: United States Department of Health and Human Services, Center for Disease Control and Prevention; 2007. [Accessed 1 March 2012]. Rev ed. Also available at: http://www.cdc.gov/hiv/surveillance/resources/reports/2005report/ [Google Scholar]
- 5.Effros RB, Fletcher CV, Gebo K, et al. Aging and infectious diseases: workshop on HIV infection and aging: what is known and future research directions. Clin Infect Dis. 2008;47:542–553. doi: 10.1086/590150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shiels MS, Cole SR, Kirk GD, Poole C. A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. J Acquir Immune Defic Syndr. 2009;52:611–622. doi: 10.1097/QAI.0b013e3181b327ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seaberg EC, Wiley D, Martinez-Maza O, et al. Cancer incidence in the multicenter AIDS Cohort Study before and during the HAART era: 1984 to 2007. Cancer. 2010;116:5507–5516. doi: 10.1002/cncr.25530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;148:728–736. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- 9▪▪.Silverberg MJ, Chao C, Leyden WA, et al. HIV infection, immunodeficiency, viral replication, and the risk of cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:2551–2559. doi: 10.1158/1055-9965.EPI-11-0777. This study compared cancer risk in demographically similar PLWHA and uninfected persons enrolled in the Kaiser Permanente California healthcare system. After adjustment for several non-HIV cancer risk factors, elevated RRs were observed for seven of 10 cancer types examined, and lower current CD4 count was associated with increased risk for nine of 10 cancer types examined. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bedimo RJ, McGinnis KA, Dunlap M, et al. Incidence of non-AIDS-defining malignancies in HIV-infected versus noninfected patients in the HAART era: impact of immunosuppression. J Acquir Immune Defic Syndr. 2009;52:203–208. doi: 10.1097/QAI.0b013e3181b033ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123:187–194. doi: 10.1002/ijc.23487. [DOI] [PubMed] [Google Scholar]
- 12▪.Simard EP, Pfeiffer RM, Engels EA. Spectrum of cancer risk late after AIDS onset in the United States. Arch Intern Med. 2010;170:1337–1345. doi: 10.1001/archinternmed.2010.253. This study updated the HIV/AIDS Cancer Match Study, a large cohort of persons living with AIDS in the United States, to study cancer risk in the period 3–10 years after AIDS onset compared with the general population. SIRs remained highly elevated for Kaposi sarcoma and non-Hodgkin lymphoma and were elevated for a number of NADC, most of viral cause. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silverberg MJ, Lau B, Justice AC, et al. Risk of anal cancer in HIV-infected and HIV-uninfected individuals in North America. Clin Infect Dis. 2012;54:1026–1034. doi: 10.1093/cid/cir1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14▪.Sigel K, Wisnivesky J, Gordon K, et al. HIV as an independent risk factor for incident lung cancer. AIDS. 2012;26:1017–1025. doi: 10.1097/QAD.0b013e328352d1ad. This study compared lung cancer incidence in demographically similar HIV-infected and uninfected veterans enrolled in the Veterans Aging Cohort Study. HIV infection was found to be an independent risk factor for lung cancer after controlling for smoking. This was the largest study to date to directly examine the relative contributions of HIV infection and smoking to lung cancer risk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shiels MS, Cole SR, Mehta SH, Kirk GD. Lung cancer incidence and mortality among HIV-infected and HIV-uninfected injection drug users. J Acquir Immune Defic Syndr. 2010;55:510–515. doi: 10.1097/QAI.0b013e3181f53783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engsig FN, Kronborg G, Larsen CS, et al. Lung cancer in HIV patients and their parents: a Danish cohort study. BMC Cancer. 2011;11:272. doi: 10.1186/1471-2407-11-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levine AM, Seaberg EC, Hessol NA, et al. HIV as a risk factor for lung cancer in women: data from the Women’s Interagency HIV Study. J Clin Oncol. 2010;28:1514–1519. doi: 10.1200/JCO.2009.25.6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18▪▪.Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103:753–762. doi: 10.1093/jnci/djr076. This study characterized the changing cancer burden among people living with HIV in the United States by estimating the number of incident cancers, by cancer type, between 1991 and 2005. The spectrum of cancer diagnoses shifted from ADC to NADC, although the ADC burden remained high. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19▪.Simard EP, Pfeiffer RM, Engels EA. Cumulative incidence of cancer among individuals with acquired immunodeficiency syndrome in the United States. Cancer. 2011;117:1089–1096. doi: 10.1002/cncr.25547. This study used competing risk methods to measure cumulative cancer incidence during the first 5 years after AIDS onset according to calendar year of AIDS onset (1980–2006) in the HIV/AIDS Cancer Match Study. Cumulative incidence of ADC declined sharply, whereas cumulative incidence of NADC increased, especially for anal cancer, Hodgkin lymphoma, and liver cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buchacz K, Baker RK, Palella FJ, Jr, et al. AIDS-defining opportunistic illnesses in US patients, 1994–2007: a cohort study. AIDS. 2010;24:1549–1559. doi: 10.1097/QAD.0b013e32833a3967. [DOI] [PubMed] [Google Scholar]
- 21.Franceschi S, Maso LD, Rickenbach M, et al. Kaposi sarcoma incidence in the Swiss HIV Cohort Study before and after highly active antiretroviral therapy. Br J Cancer. 2008;99:800–804. doi: 10.1038/sj.bjc.6604520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polesel J, Clifford GM, Rickenbach M, et al. Non-Hodgkin lymphoma incidence in the Swiss HIV Cohort Study before and after highly active anti-retroviral therapy. AIDS. 2008;22:301–306. doi: 10.1097/QAD.0b013e3282f2705d. [DOI] [PubMed] [Google Scholar]
- 23.Howlader N, Noone AM, Krapcho M, et al., editors. SEER Cancer Statistics Review 1975–2009 (Vintage 2009 Populations) Bethesda, MD: National Cancer Institute; 2012. [Accessed 14 April 2012]. http://seer.cancer.gov/csr/1975_2009_pops09/ [Google Scholar]
- 24.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 25.Silverberg MJ, Chao C, Leyden WA, et al. HIV infection and the risk of cancers with and without a known infectious cause. AIDS. 2009;23:2337–2345. doi: 10.1097/QAD.0b013e3283319184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Vuyst H, Lillo F, Broutet N, Smith JS. HIV, human papillomavirus, and cervical neoplasia and cancer in the era of highly active antiretroviral therapy. Eur J Cancer Prev. 2008;17:545–554. doi: 10.1097/CEJ.0b013e3282f75ea1. [DOI] [PubMed] [Google Scholar]
- 27.Palefsky JM, Rubin M. The epidemiology of anal human papillomavirus and related neoplasia. Obstet Gynecol Clin North Am. 2009;36:187–200. doi: 10.1016/j.ogc.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Fakhry C, D’Souza G, Sugar E, et al. Relationship between prevalent oral and cervical human papillomavirus infections in human immunodeficiency virus-positive and negative women. J Clin Microbiol. 2006;44:4479–4485. doi: 10.1128/JCM.01321-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol. 2006;44:S6–S9. doi: 10.1016/j.jhep.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 30.O’Brien TR, Kedes D, Ganem D, et al. Evidence for concurrent epidemics of human herpesvirus 8 and human immunodeficiency virus type 1 in US homosexual men: rates, risk factors, and relationship to Kaposi’s sarcoma. J Infect Dis. 1999;180:1010–1017. doi: 10.1086/315039. [DOI] [PubMed] [Google Scholar]
- 31.Rahmanian S, Wewers ME, Koletar S, et al. Cigarette smoking in the HIV-infected population. Proc Am Thorac Soc. 2011;8:313–319. doi: 10.1513/pats.201009-058WR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braithwaite RS, Conigliaro J, Roberts MS, et al. Estimating the impact of alcohol consumption on survival for HIV+ individuals. AIDS Care. 2007;19:459–466. doi: 10.1080/09540120601095734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Ann Rev Immunol. 2011;29:235–271. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 34.Morgan GJ, Linet MS, Rabkin CS. Immunologic factors. In: Schottenfeld D, Fraumeni JF Jr, editors. Cancer Epidemiology and Prevention. Oxford: Oxford University Press; 2006. pp. 549–561. [Google Scholar]
- 35.Schottenfeld D, Beebe-Dimmer J. Chronic inflammation: a common and important factor in the pathogenesis of neoplasia. CA Cancer J Clin. 2006;56:69–83. doi: 10.3322/canjclin.56.2.69. [DOI] [PubMed] [Google Scholar]
- 36.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37▪.Pine SR, Mechanic LE, Enewold L, et al. Increased levels of circulating interleukin 6, interleukin 8, C-reactive protein, and risk of lung cancer. J Natl Cancer Inst. 2011;103:1112–1122. doi: 10.1093/jnci/djr216. This study found serum levels of the inflammation markers IL-8 and CRP to predict future lung cancer risk among participants in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Study of the relationship of these biomarkers to lung cancer risk among PLWHA could provide insight into the mechanism by which HIV infection is associated with increased lung cancer risk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Appay V, Sauce D. Immune activation and inflammation in HIV-1 infection: causes and consequences. J Pathol. 2008;214:231–241. doi: 10.1002/path.2276. [DOI] [PubMed] [Google Scholar]
- 39▪▪.Plaeger SF, Collins BS, Musib R, et al. Immune activation in the pathogenesis of treated chronic hiv disease: a workshop summary. AIDS Res Hum Retroviruses. 2012;28:469–477. doi: 10.1089/aid.2011.0213. This article summarized results of a National Institutes of Health-sponsored workshop that addressed the cause of persistent immune activation during treated HIV infection, biomarkers of immune activation, possible therapeutic interventions, and directions for future research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40▪.Desai S, Landay A. Early immune senescence in HIV disease. Curr HIV/AIDS Rep. 2010;7:4–10. doi: 10.1007/s11904-009-0038-4. This article reviews immune activation and the development of early immune senescence in PLWHA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41▪.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–155. doi: 10.1146/annurev-med-042909-093756. This article reviews similarities between HIV-related chronic inflammation and immune dysfunction and changes in the immune system associated with aging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malaguarnera L, Cristaldi E, Malaguarnera M. The role of immunity in elderly cancer. Crit Rev Oncol Hematol. 2010;74:40–60. doi: 10.1016/j.critrevonc.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 43.Fulop T, Kotb R, Fortin CF, et al. Potential role of immunosenescence in cancer development. Ann N Y Acad Sci. 2010;1197:158–165. doi: 10.1111/j.1749-6632.2009.05370.x. [DOI] [PubMed] [Google Scholar]
- 44▪▪.Nixon DE, Landay AL. Biomarkers of immune dysfunction in HIV. Curr Opin HIV AIDS. 2010;5:498–503. doi: 10.1097/COH.0b013e32833ed6f4. This article provides a comprehensive review of soluble and cellular biomarkers of inflammation and immune dysfunction in the context of HIV infection and suggests potential biomarkers for future evaluation in relation to outcomes in HIV patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45▪.Neuhaus J, Jacobs DR, Jr, Baker JV, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201:1788–1795. doi: 10.1086/652749. This study found that CRP, IL-6, and D-dimer remained elevated in PLWHA [a subgroup of participants in the Strategic Management of ART (SMART) trial] compared with HIV-uninfected persons, even after viral suppression with ART. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46▪.Baker JV, Neuhaus J, Duprez D, et al. Changes in inflammatory and coagulation biomarkers: a randomized comparison of immediate versus deferred antiretroviral therapy in patients with HIV infection. J Acquir Immune Defic Syndr. 2011;56:36–43. doi: 10.1097/QAI.0b013e3181f7f61a. This study, among a subgroup of participants in the SMART trial, found that D-dimer, but not IL-6 or CRP, declined significantly after ART initiation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47▪.Armah KA, McGinnis K, Baker J, et al. HIV status, burden of comorbid disease, and biomarkers of inflammation, altered coagulation and monocyte activation. Clin Infect Dis. 2012 doi: 10.1093/cid/cis406. [Epub ahead of print] This study compared immune activation/inflammation biomarkers between demographically-similar HIV-infected and uninfected veterans of similar comorbidity status. Elevated IL-6 and D-dimer were observed only among HIV-infected veterans with unsuppressed HIV RNA and low CD4 counts, and elevated soluble CD14 was observed only among HIV-infected veterans with low CD4 counts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robbins GK, Spritzler JG, Chan ES, et al. Incomplete reconstitution of T cell subsets on combination antiretroviral therapy in the AIDS Clinical Trials Group protocol 384. Clin Infect Dis. 2009;48:350–361. doi: 10.1086/595888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49▪.Appay V, Fastenackels S, Katlama C, et al. Old age and anticytomegalovirus immunity are associated with altered T-cell reconstitution in HIV-1-infected patients. AIDS. 2011;25:1813–1822. doi: 10.1097/QAD.0b013e32834640e6. This study found that decline in naive T-cells is a better marker of premature development of an HIV-induced immunosenescence phenotype than accumulation of CD57+ senescent T cells. [DOI] [PubMed] [Google Scholar]
- 50.Li P, Tian C, Ge N, et al. Premature senescence of T cells in long-term survivors of renal transplantation. Biochem Biophys Res Commun. 2011;407:599–604. doi: 10.1016/j.bbrc.2011.03.079. [DOI] [PubMed] [Google Scholar]
- 51.Biggar RJ, Chaturvedi AK, Goedert JJ, Engels EA. AIDS-related cancer and severity of immunosuppression in persons with AIDS. J Natl Cancer Inst. 2007;99:962–972. doi: 10.1093/jnci/djm010. [DOI] [PubMed] [Google Scholar]
- 52.Clifford GM, Franceschi S. Cancer risk in HIV-infected persons: influence of CD4(+) count. Future Oncol. 2009;5:669–678. doi: 10.2217/fon.09.28. [DOI] [PubMed] [Google Scholar]
- 53.Bruyand M, Thiebaut R, Lawson-Ayayi S, et al. Role of uncontrolled HIV RNA level and immunodeficiency in the occurrence of malignancy in HIV-infected patients during the combination antiretroviral therapy era: Agence Nationale de Recherche sur le Sida (ANRS) CO3 Aquitaine Cohort. Clin Infect Dis. 2009;49:1109–1116. doi: 10.1086/605594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54▪▪.Krishnan S, Schouten JT, Jacobson DL, et al. Incidence of non-AIDS-defining cancer in antiretroviral treatment-naive subjects after antiretroviral treatment initiation: an ACTG longitudinal linked randomized trials analysis. Oncology. 2011;80:42–49. doi: 10.1159/000328032. In the AIDS Clinical Trials Group Longitudinal Linked Randomized Trials cohort, lower current CD4 count, but not initial ART drug class, was found to be associated with increased NADC (grouped) risk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55▪▪.Prosperi MC, Cozzi-Lepri A, Castagna A, et al. Incidence of malignancies in HIV-infected patients and prognostic role of current CD4 cell count: evidence from a large Italian cohort study. Clin Infect Dis. 2010;50:1316–1321. doi: 10.1086/651688. In an Italian cohort, lower current CD4 count was found to be associated with increased risk for ADC (grouped) and for NADC (grouped). The association was stronger for ADC (grouped) [DOI] [PubMed] [Google Scholar]
- 56▪▪.Reekie J, Kosa C, Engsig F, et al. Relationship between current level of immunodeficiency and nonacquired immunodeficiency syndrome-defining malignancies. Cancer. 2010;116:5306–5315. doi: 10.1002/cncr.25311. In the multinational EuroSIDA cohort, lower current CD4 count was found to be associated with increased risk for virus-related NADC (grouped), nonvirus-related epithelial NADC (grouped), anal cancer, lung cancer, and Hodgkin lymphoma. [DOI] [PubMed] [Google Scholar]
- 57.Marin B, Thiebaut R, Bucher HC, et al. Non-AIDS-defining deaths and immunodeficiency in the era of combination antiretroviral therapy. AIDS. 2009;23:1743–1753. doi: 10.1097/QAD.0b013e32832e9b78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Monforte A, Abrams D, Pradier C, et al. HIV-induced immunodeficiency and mortality from AIDS-defining and non-AIDS-defining malignancies. AIDS. 2008;22:2143–2153. doi: 10.1097/QAD.0b013e3283112b77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59▪.Bohlius J, Schmidlin K, Boue F, et al. HIV-1-related Hodgkin lymphoma in the era of combination antiretroviral therapy: incidence and evolution of CD4 T-cell lymphocytes. Blood. 2011;117:6100–6108. doi: 10.1182/blood-2010-08-301531. In the Collaboration of Observational HIV Epidemiological Research Europe cohort collaboration, lower current CD4 count was associated with higher Hodgkin lymphoma risk. However, this might be explained by preclinical Hodgkin lymphoma causing declining CD4 counts. [DOI] [PubMed] [Google Scholar]
- 60.Guiguet M, Boue F, Cadranel J, et al. Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): a prospective cohort study. Lancet Oncol. 2009;10:1152–1159. doi: 10.1016/S1470-2045(09)70282-7. [DOI] [PubMed] [Google Scholar]
- 61.Clifford GM, Rickenbach M, Polesel J, et al. Influence of HIV-related immunodeficiency on the risk of hepatocellular carcinoma. AIDS. 2008;22:2135–2141. doi: 10.1097/QAD.0b013e32831103ad. [DOI] [PubMed] [Google Scholar]
- 62▪.Bruyand M, Dabis F, Vandenhende MA, et al. HIV-induced immune deficiency is associated with a higher risk of hepatocarcinoma, ANRS CO3 Aquitaine Cohort, France, 1998–2008. J Hepatol. 2011;55:1058–1062. doi: 10.1016/j.jhep.2011.02.017. In the French ANRS CO3 Aquitaine Cohort, current CD4 cell count less than 500 cells/μl, but not longer exposure to CD4 cell count less than 500 cells/μl, was associated with increased liver cancer risk. [DOI] [PubMed] [Google Scholar]
- 63.Crum-Cianflone N, Hullsiek KH, Marconi V, et al. Trends in the incidence of cancers among HIV-infected persons and the impact of antiretroviral therapy: a 20-year cohort study. AIDS. 2009;23:41–50. doi: 10.1097/QAD.0b013e328317cc2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64▪▪.Kesselring A, Gras L, Smit C, et al. Immunodeficiency as a risk factor for non-AIDS-defining malignancies in HIV-1-infected patients receiving combination antiretroviral therapy. Clin Infect Dis. 2011;52:1458–1465. doi: 10.1093/cid/cir207. In the AIDS Therapy Evaluation in the Netherlands cohort, longer exposure to CD4 cell count less than 200 cells/μl, but not lower current CD4, was found to be independently associated with increased NADC (grouped) risk. This increased risk was restricted to infection-related NADC (grouped) [DOI] [PubMed] [Google Scholar]
- 65.Chaturvedi AK, Madeleine MM, Biggar RJ, Engels EA. Risk of human papillomavirus-associated cancers among persons with AIDS. J Natl Cancer Inst. 2009;101:1120–1130. doi: 10.1093/jnci/djp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Crum-Cianflone NF, Hullsiek KH, Marconi VC, et al. Anal cancers among HIV-infected persons: HAART is not slowing rising incidence. AIDS. 2010;24:535–543. doi: 10.1097/QAD.0b013e328331f6e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Conley L, Bush T, Darragh TM, et al. Factors associated with prevalent abnormal anal cytology in a large cohort of HIV-infected adults in the United States. J Infect Dis. 2010;202:1567–1576. doi: 10.1086/656775. [DOI] [PubMed] [Google Scholar]
- 68.Engels EA, Brock MV, Chen J, et al. Elevated incidence of lung cancer among HIV-infected individuals. J Clin Oncol. 2006;24:1383–1388. doi: 10.1200/JCO.2005.03.4413. [DOI] [PubMed] [Google Scholar]
- 69.Kirk GD, Merlo C, O’ Driscoll P, et al. HIV infection is associated with an increased risk for lung cancer, independent of smoking. Clin Infect Dis. 2007;45:103–110. doi: 10.1086/518606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chaturvedi AK, Pfeiffer RM, Chang L, et al. Elevated risk of lung cancer among people with AIDS. AIDS. 2007;21:207–213. doi: 10.1097/QAD.0b013e3280118fca. [DOI] [PubMed] [Google Scholar]
- 71▪.Clifford GM, Lise M, Franceschi S, et al. Lung cancer in the Swiss HIV Cohort Study: role of smoking, immunodeficiency and pulmonary infection. Br J Cancer. 2012;106:447–452. doi: 10.1038/bjc.2011.558. In the Swiss HIV Cohort Study, CD4 count was found to be unrelated to lung cancer risk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shebl FM, Engels EA, Goedert JJ, Chaturvedi AK. Pulmonary infections and risk of lung cancer among persons with AIDS. J Acquir Immune Defic Syndr. 2010;55:375–379. doi: 10.1097/QAI.0b013e3181eef4f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hernandez MD, Sherman KE. HIV/hepatitis C coinfection natural history and disease progression. Curr Opin HIV AIDS. 2011;6:478–482. doi: 10.1097/COH.0b013e32834bd365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Operskalski EA, Kovacs A. HIV/HCV co-infection: pathogenesis, clinical complications, treatment, and new therapeutic technologies. Curr HIV/AIDS Rep. 2011;8:12–22. doi: 10.1007/s11904-010-0071-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thio CL. Hepatitis B and human immunodeficiency virus coinfection. Hepatology. 2009;49:S138–S145. doi: 10.1002/hep.22883. [DOI] [PubMed] [Google Scholar]
- 76.McGinnis KA, Fultz SL, Skanderson M, et al. Hepatocellular carcinoma and non-Hodgkin’s lymphoma: the roles of HIV, hepatitis C infection, and alcohol abuse. J Clin Oncol. 2006;24:5005–5009. doi: 10.1200/JCO.2006.05.7984. [DOI] [PubMed] [Google Scholar]
- 77.Kramer JR, Giordano TP, Souchek J, et al. The effect of HIV coinfection on the risk of cirrhosis and hepatocellular carcinoma in U.S. veterans with hepatitis C. Am J Gastroenterol. 2005;100:56–63. doi: 10.1111/j.1572-0241.2005.40670.x. [DOI] [PubMed] [Google Scholar]
- 78.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents. Department of Health and Human Services; 2012. [Accessed 1 April 2012]. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [Google Scholar]
- 79▪.Althoff KN, Gange SJ, Klein MB, et al. Late presentation for human immunodeficiency virus care in the United States and Canada. Clin Infect Dis. 2010;50:1512–1520. doi: 10.1086/652650. In the North American-AIDS Cohort Collaboration on Research and Design in 2007, 54% of PLWHA had CD4 cell count 350 cells/μl or less at first presentation to medical care, suggesting an urgent need for earlier diagnosis and treatment to reduce mortality and morbidity, including cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marks G, Gardner LI, Craw J, Crepaz N. Entry and retention in medical care among HIV-diagnosed persons: a meta-analysis. AIDS. 2010;24:2665–2678. doi: 10.1097/QAD.0b013e32833f4b1b. [DOI] [PubMed] [Google Scholar]
- 81.Morin SF, Kelly JA, Charlebois ED, et al. Responding to the National HIV/AIDS Strategy-setting the research agenda. J Acquir Immune Defic Syndr. 2011;57:175–180. doi: 10.1097/QAI.0b013e318222c0f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Volberding PA, Deeks SG. Antiretroviral therapy and management of HIV infection. Lancet. 2010;376:49–62. doi: 10.1016/S0140-6736(10)60676-9. [DOI] [PubMed] [Google Scholar]
- 83.Deeks SG. Immune dysfunction, inflammation, and accelerated aging in patients on antiretroviral therapy. Top HIV Med. 2009;17:118–123. [PubMed] [Google Scholar]
- 84.Monini P, Toschi E, Sgadari C, et al. The use of HAART for biological tumour therapy. J HIV Ther. 2006;11:53–56. [PubMed] [Google Scholar]
- 85.Monini P, Sgadari C, Toschi E, et al. Antitumour effects of antiretroviral therapy. Nat Rev Cancer. 2004;4:861–875. doi: 10.1038/nrc1479. [DOI] [PubMed] [Google Scholar]
- 86.Brown T, Sigurdson E, Rogatko A, Broccoli D. Telomerase inhibition using azidothymidine in the HT-29 colon cancer cell line. Ann Surg Oncol. 2003;10:910–915. doi: 10.1245/aso.2003.03.032. [DOI] [PubMed] [Google Scholar]
- 87.Ji HJ, Rha SY, Jeung HC, et al. Cyclic induction of senescence with intermittent AZT treatment accelerates both apoptosis and telomere loss. Breast Cancer Res Treat. 2005;93:227–236. doi: 10.1007/s10549-005-5156-0. [DOI] [PubMed] [Google Scholar]
- 88.Kurokawa M, Ghosh SK, Ramos JC, et al. Azidothymidine inhibits NF-kappaB and induces Epstein-Barr virus gene expression in Burkitt lymphoma. Blood. 2005;106:235–240. doi: 10.1182/blood-2004-09-3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Landriscina M, Spadafora C, Cignarelli M, Barone C. Antitumor activity of nonnucleosidic reverse transcriptase inhibitors. Curr Pharm Des. 2007;13:737–747. doi: 10.2174/138161207780249191. [DOI] [PubMed] [Google Scholar]
- 90.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Some antiviral and antineo-plastic drugs, and other pharmaceutical agents: summary of data reported and evaluation. Lyon: International Agency for Research on Cancer; 2000. pp. 73–127. [Google Scholar]
- 91.Olivero OA, Tejera AM, Fernandez JJ, et al. Zidovudine induces S-phase arrest and cell cycle gene expression changes in human cells. Mutagenesis. 2005;20:139–146. doi: 10.1093/mutage/gei019. [DOI] [PubMed] [Google Scholar]
- 92.Gulick RM, Su Z, Flexner C, et al. Phase 2 study of the safety and efficacy of vicriviroc, a CCR5 inhibitor, in HIV-1-Infected, treatment-experienced patients: AIDS clinical trials group 5211. J Infect Dis. 2007;196:304–312. doi: 10.1086/518797. [DOI] [PubMed] [Google Scholar]
- 93.Crum-Cianflone NF, Hullsiek KH, Marconi V, et al. The impact of nelfinavir exposure on cancer development among a large cohort of HIV-infected patients. J Acquir Immune Defic Syndr. 2009;51:305–309. doi: 10.1097/QAI.0b013e3181aa13c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ford ES, Greenwald JH, Richterman AG, et al. Traditional risk factors and D-dimer predict incident cardiovascular disease events in chronic HIV infection. AIDS. 2010;24:1509–1517. doi: 10.1097/QAD.0b013e32833ad914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96▪▪.Kalayjian RC, Machekano RN, Rizk N, et al. Pretreatment levels of soluble cellular receptors and interleukin-6 are associated with HIV disease progression in subjects treated with highly active antiretroviral therapy. J Infect Dis. 2010;201:1796–1805. doi: 10.1086/652750. In a case–control study nested within two AIDS Clinical Trials Group protocols, higher pre-ART levels of soluble tumor necrosis factor receptor-1, soluble CD27, and soluble CD40 ligand were found to be independently associated with increased risk of a composite outcome of ADC or death after ART initiation. ADC was not examined by itself as an outcome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Morris PG, Hudis CA, Giri D, et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res. 2011;4:1021–1029. doi: 10.1158/1940-6207.CAPR-11-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.De Marzo AM, Platz EA, Sutcliffe S, et al. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7:256–269. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cuzick J, Otto F, Baron JA, et al. Aspirin and nonsteroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol. 2009;10:501–507. doi: 10.1016/S1470-2045(09)70035-X. [DOI] [PubMed] [Google Scholar]