Abstract
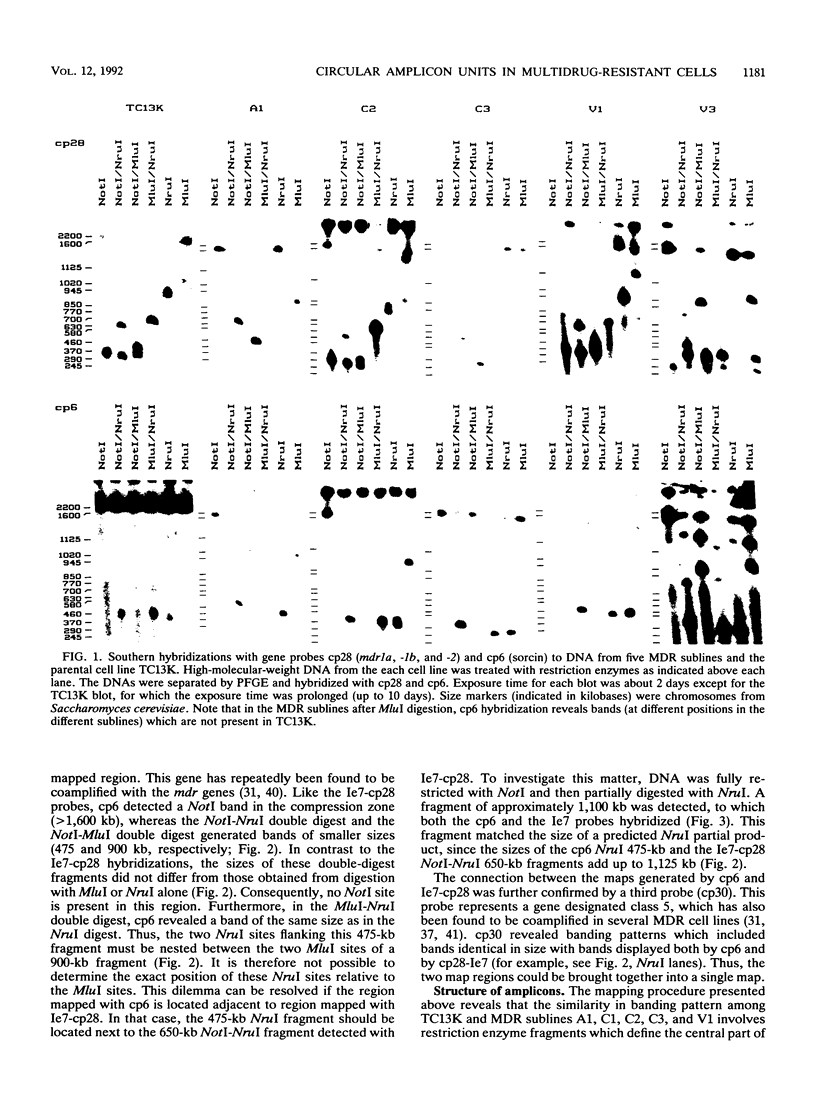
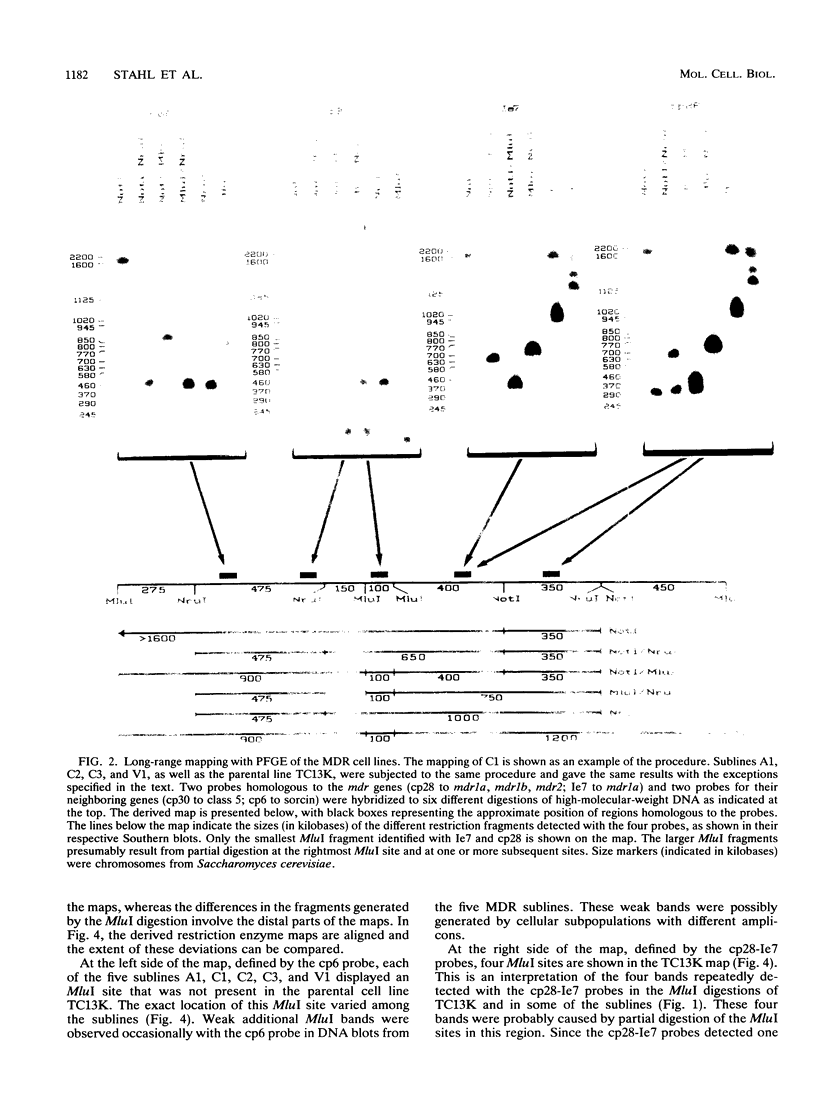
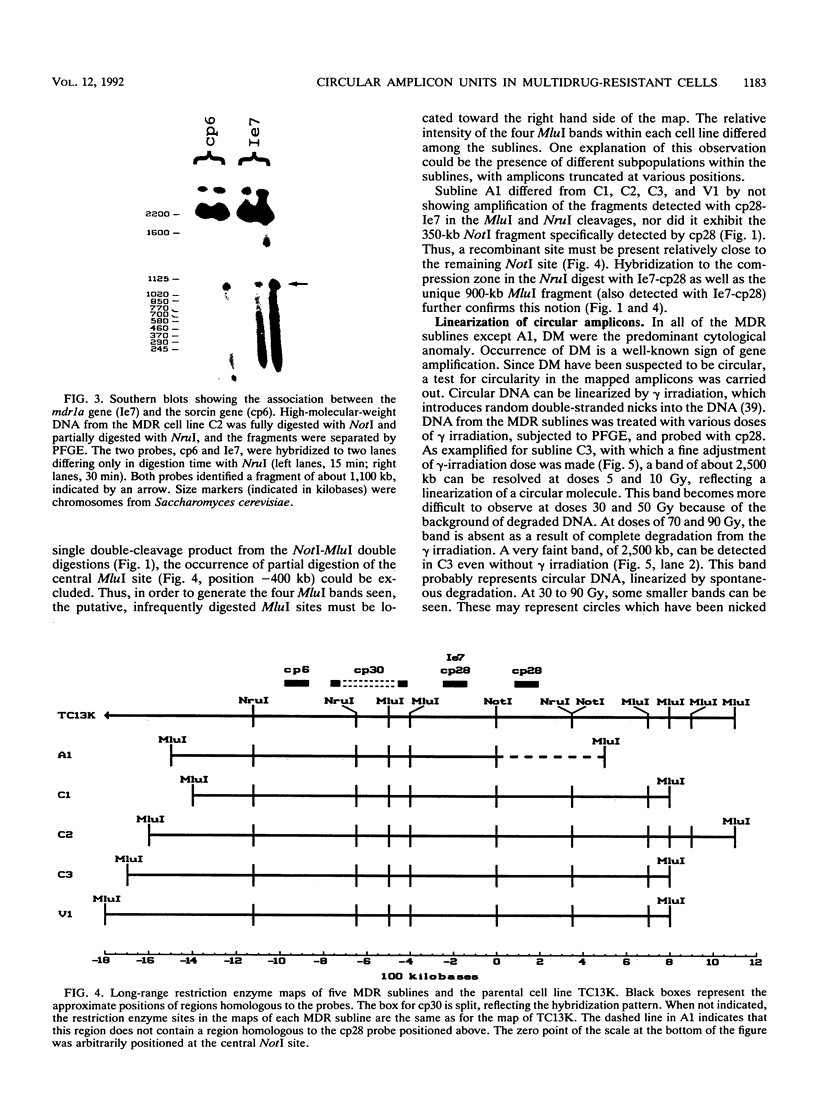
Multidrug resistance (MDR) in tumor cell lines is frequently correlated with amplification of one or more mdr genes. Usually the amplified domain also includes several neighboring genes. Using pulsed-field gel electrophoresis, we have established a restriction map covering approximately 2,200 kb in the drug-sensitive mouse tumor cell line TC13K. The mapped region is located on mouse chromosome 5 and includes the three mdr genes, the gene for the calcium-binding sorcin protein, and a gene with unknown function designated class 5. Long-range maps of the amplified DNA sequences in five of six MDR sublines that had been independently derived from TC13K generally displayed the same pattern as did the parental cell line. All six MDR sublines exhibited numerous double minutes, and one of them displayed a homogeneously staining region in a subpopulation. Large circular molecules, most likely identical to one chromatid of the double minutes, were detected in four of the sublines by linearization with gamma irradiation. The size of the circles was about 2,500 kb, which correlated to a single unit of the amplified domain. We therefore propose that in four independent instances of MDR development, a single unit of about 2,500 kb has been amplified in the form of circular DNA molecules. The restriction enzyme map of the amplified unit is unchanged compared with that of the parental cell line, whereas the joining sites of the circular DNA molecules are not identical but are in the same region.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borst P., Van der Bliek A. M., Van der Velde-Koerts T., Hes E. Structure of amplified DNA, analyzed by pulsed field gradient gel electrophoresis. J Cell Biochem. 1987 Aug;34(4):247–258. doi: 10.1002/jcb.240340404. [DOI] [PubMed] [Google Scholar]
- Carroll S. M., DeRose M. L., Gaudray P., Moore C. M., Needham-Vandevanter D. R., Von Hoff D. D., Wahl G. M. Double minute chromosomes can be produced from precursors derived from a chromosomal deletion. Mol Cell Biol. 1988 Apr;8(4):1525–1533. doi: 10.1128/mcb.8.4.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. J., Chin J. E., Ueda K., Clark D. P., Pastan I., Gottesman M. M., Roninson I. B. Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell. 1986 Nov 7;47(3):381–389. doi: 10.1016/0092-8674(86)90595-7. [DOI] [PubMed] [Google Scholar]
- Chin J. E., Soffir R., Noonan K. E., Choi K., Roninson I. B. Structure and expression of the human MDR (P-glycoprotein) gene family. Mol Cell Biol. 1989 Sep;9(9):3808–3820. doi: 10.1128/mcb.9.9.3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell J. K. Double minutes and homogeneously staining regions: gene amplification in mammalian cells. Annu Rev Genet. 1982;16:21–59. doi: 10.1146/annurev.ge.16.120182.000321. [DOI] [PubMed] [Google Scholar]
- Croop J. M., Raymond M., Haber D., Devault A., Arceci R. J., Gros P., Housman D. E. The three mouse multidrug resistance (mdr) genes are expressed in a tissue-specific manner in normal mouse tissues. Mol Cell Biol. 1989 Mar;9(3):1346–1350. doi: 10.1128/mcb.9.3.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endicott J. A., Juranka P. F., Sarangi F., Gerlach J. H., Deuchars K. L., Ling V. Simultaneous expression of two P-glycoprotein genes in drug-sensitive Chinese hamster ovary cells. Mol Cell Biol. 1987 Nov;7(11):4075–4081. doi: 10.1128/mcb.7.11.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endicott J. A., Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Gerlach J. H., Endicott J. A., Juranka P. F., Henderson G., Sarangi F., Deuchars K. L., Ling V. Homology between P-glycoprotein and a bacterial haemolysin transport protein suggests a model for multidrug resistance. Nature. 1986 Dec 4;324(6096):485–489. doi: 10.1038/324485a0. [DOI] [PubMed] [Google Scholar]
- Gerlach J. H., Kartner N., Bell D. R., Ling V. Multidrug resistance. Cancer Surv. 1986;5(1):25–46. [PubMed] [Google Scholar]
- Goldie J. H., Coldman A. J. The genetic origin of drug resistance in neoplasms: implications for systemic therapy. Cancer Res. 1984 Sep;44(9):3643–3653. [PubMed] [Google Scholar]
- Gros P., Croop J., Housman D. Mammalian multidrug resistance gene: complete cDNA sequence indicates strong homology to bacterial transport proteins. Cell. 1986 Nov 7;47(3):371–380. doi: 10.1016/0092-8674(86)90594-5. [DOI] [PubMed] [Google Scholar]
- Gros P., Raymond M., Bell J., Housman D. Cloning and characterization of a second member of the mouse mdr gene family. Mol Cell Biol. 1988 Jul;8(7):2770–2778. doi: 10.1128/mcb.8.7.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamkalo B. A., Farnham P. J., Johnston R., Schimke R. T. Ultrastructural features of minute chromosomes in a methotrexate-resistant mouse 3T3 cell line. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1126–1130. doi: 10.1073/pnas.82.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin J. L., Milbrandt J. D., Heintz N. H., Azizkhan J. C. DNA sequence amplification in mammalian cells. Int Rev Cytol. 1984;90:31–82. doi: 10.1016/s0074-7696(08)61487-4. [DOI] [PubMed] [Google Scholar]
- Hsu S. I., Lothstein L., Horwitz S. B. Differential overexpression of three mdr gene family members in multidrug-resistant J774.2 mouse cells. Evidence that distinct P-glycoprotein precursors are encoded by unique mdr genes. J Biol Chem. 1989 Jul 15;264(20):12053–12062. [PubMed] [Google Scholar]
- Jongsma A. P., Duijndam W. A., Borst P. DNA content and structure of (double) minutes of a methotrexate-resistant cell line. Histochemistry. 1989;93(1):87–92. doi: 10.1007/BF00266852. [DOI] [PubMed] [Google Scholar]
- Kane S. E., Gottesman M. M. Multidrug resistance in the laboratory and clinic. Cancer Cells. 1989 Sep;1(1):33–36. [PubMed] [Google Scholar]
- Levan A., Levan G. Have double minutes functioning centromeres? Hereditas. 1978;88(1):81–92. doi: 10.1111/j.1601-5223.1978.tb01606.x. [DOI] [PubMed] [Google Scholar]
- Martinsson T., Levan G. Localization of the multidrug resistance-associated 170 kDa P-glycoprotein gene to mouse chromosome 5 and to homogeneously staining regions in multidrug-resistant mouse cells by in situ hybridization. Cytogenet Cell Genet. 1987;45(2):99–101. doi: 10.1159/000132437. [DOI] [PubMed] [Google Scholar]
- Martinsson T., Tenning P., Lundh L., Levan G. Methotrexate resistance and double minutes in a cell line from the SEWA mouse ascites tumor. Hereditas. 1982;97(1):123–137. doi: 10.1111/j.1601-5223.1982.tb00720.x. [DOI] [PubMed] [Google Scholar]
- Moscow J. A., Cowan K. H. Multidrug resistance. J Natl Cancer Inst. 1988 Mar 2;80(1):14–20. doi: 10.1093/jnci/80.1.14. [DOI] [PubMed] [Google Scholar]
- Ng W. F., Sarangi F., Zastawny R. L., Veinot-Drebot L., Ling V. Identification of members of the P-glycoprotein multigene family. Mol Cell Biol. 1989 Mar;9(3):1224–1232. doi: 10.1128/mcb.9.3.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond M., Rose E., Housman D. E., Gros P. Physical mapping, amplification, and overexpression of the mouse mdr gene family in multidrug-resistant cells. Mol Cell Biol. 1990 Apr;10(4):1642–1651. doi: 10.1128/mcb.10.4.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz J. C., Choi K. H., von Hoff D. D., Roninson I. B., Wahl G. M. Autonomously replicating episomes contain mdr1 genes in a multidrug-resistant human cell line. Mol Cell Biol. 1989 Jan;9(1):109–115. doi: 10.1128/mcb.9.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen S., Hittelman W. N., Teeter L. D., Kuo M. T. Model for the formation of double minutes from prematurely condensed chromosomes of replicating micronuclei in drug-treated Chinese hamster ovary cells undergoing DNA amplification. Cancer Res. 1989 Dec 1;49(23):6731–6737. [PubMed] [Google Scholar]
- Stark G. R., Debatisse M., Giulotto E., Wahl G. M. Recent progress in understanding mechanisms of mammalian DNA amplification. Cell. 1989 Jun 16;57(6):901–908. doi: 10.1016/0092-8674(89)90328-0. [DOI] [PubMed] [Google Scholar]
- Stark G. R., Wahl G. M. Gene amplification. Annu Rev Biochem. 1984;53:447–491. doi: 10.1146/annurev.bi.53.070184.002311. [DOI] [PubMed] [Google Scholar]
- Stähl F., Sandberg P., Martinsson T., Skoog J., Dahllöf B., Wettergren Y., Bjursell G., Levan G. Isolation of selectively amplified DNA sequences from multidrug-resistant SEWA cells. Hereditas. 1987;106(1):97–105. doi: 10.1111/j.1601-5223.1987.tb00241.x. [DOI] [PubMed] [Google Scholar]
- Ståhl F., Martinsson T., Dahllöf B., Levan G. Amplification and overexpression of the P-glycoprotein genes and differential amplification of three other genes in SEWA murine multidrug-resistant cells. Hereditas. 1988;108(2):251–258. doi: 10.1111/j.1601-5223.1988.tb00310.x. [DOI] [PubMed] [Google Scholar]
- Van der Bliek A. M., Baas F., Ten Houte de Lange T., Kooiman P. M., Van der Velde-Koerts T., Borst P. The human mdr3 gene encodes a novel P-glycoprotein homologue and gives rise to alternatively spliced mRNAs in liver. EMBO J. 1987 Nov;6(11):3325–3331. doi: 10.1002/j.1460-2075.1987.tb02653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Bliek A. M., Baas F., Van der Velde-Koerts T., Biedler J. L., Meyers M. B., Ozols R. F., Hamilton T. C., Joenje H., Borst P. Genes amplified and overexpressed in human multidrug-resistant cell lines. Cancer Res. 1988 Nov 1;48(21):5927–5932. [PubMed] [Google Scholar]
- Van der Bliek A. M., Meyers M. B., Biedler J. L., Hes E., Borst P. A 22-kd protein (sorcin/V19) encoded by an amplified gene in multidrug-resistant cells, is homologous to the calcium-binding light chain of calpain. EMBO J. 1986 Dec 1;5(12):3201–3208. doi: 10.1002/j.1460-2075.1986.tb04630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Bliek A. M., Van der Velde-Koerts T., Ling V., Borst P. Overexpression and amplification of five genes in a multidrug-resistant Chinese hamster ovary cell line. Mol Cell Biol. 1986 May;6(5):1671–1678. doi: 10.1128/mcb.6.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M. The importance of circular DNA in mammalian gene amplification. Cancer Res. 1989 Mar 15;49(6):1333–1340. [PubMed] [Google Scholar]
- de Bruijn M. H., Van der Bliek A. M., Biedler J. L., Borst P. Differential amplification and disproportionate expression of five genes in three multidrug-resistant Chinese hamster lung cell lines. Mol Cell Biol. 1986 Dec;6(12):4717–4722. doi: 10.1128/mcb.6.12.4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bliek A. M., Borst P. Multidrug resistance. Adv Cancer Res. 1989;52:165–203. doi: 10.1016/s0065-230x(08)60213-4. [DOI] [PubMed] [Google Scholar]
- van der Bliek A. M., Lincke C. R., Borst P. Circular DNA of 3T6R50 double minute chromosomes. Nucleic Acids Res. 1988 Jun 10;16(11):4841–4851. doi: 10.1093/nar/16.11.4841. [DOI] [PMC free article] [PubMed] [Google Scholar]