Abstract
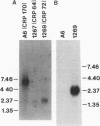
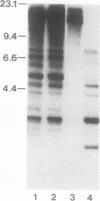
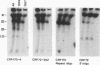
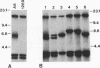
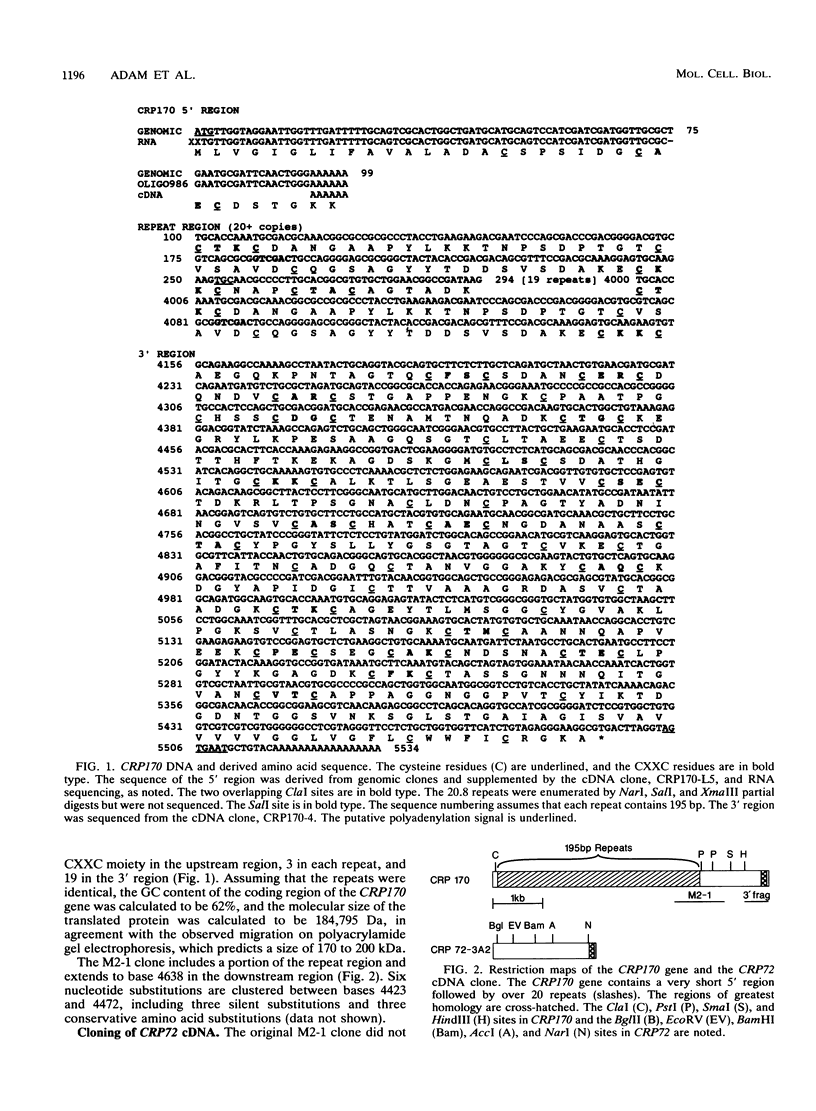
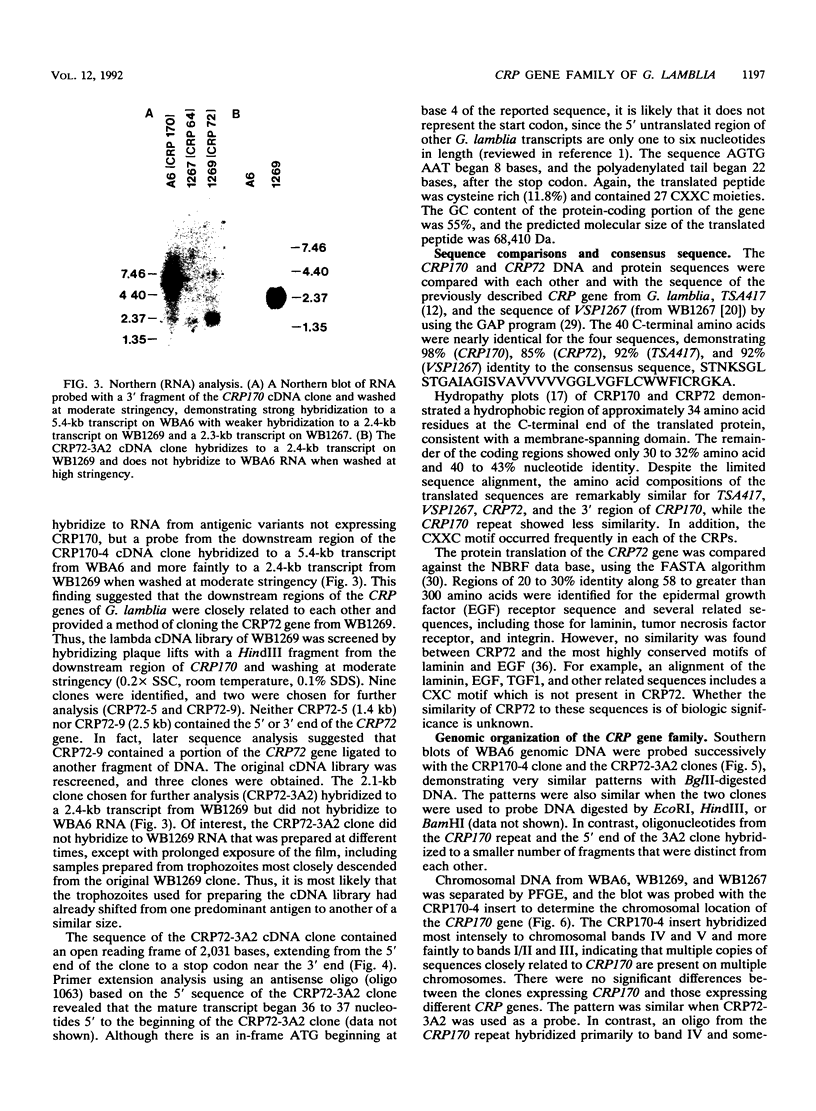
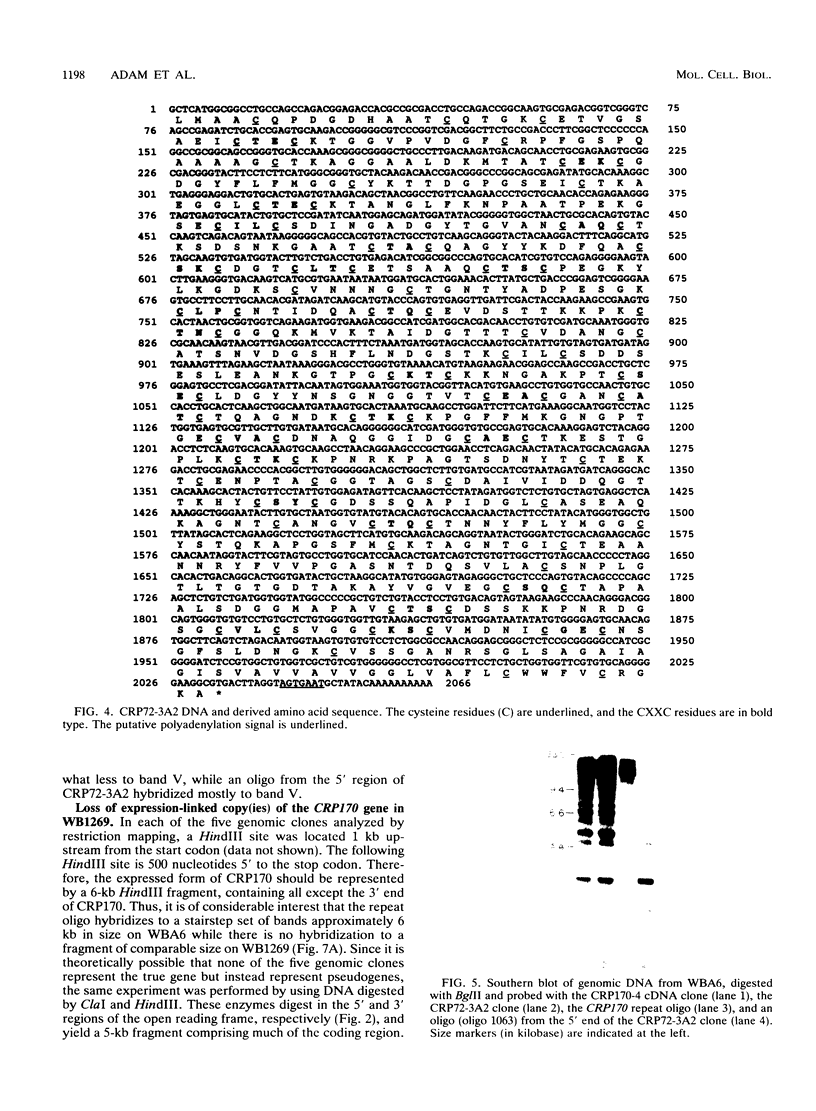
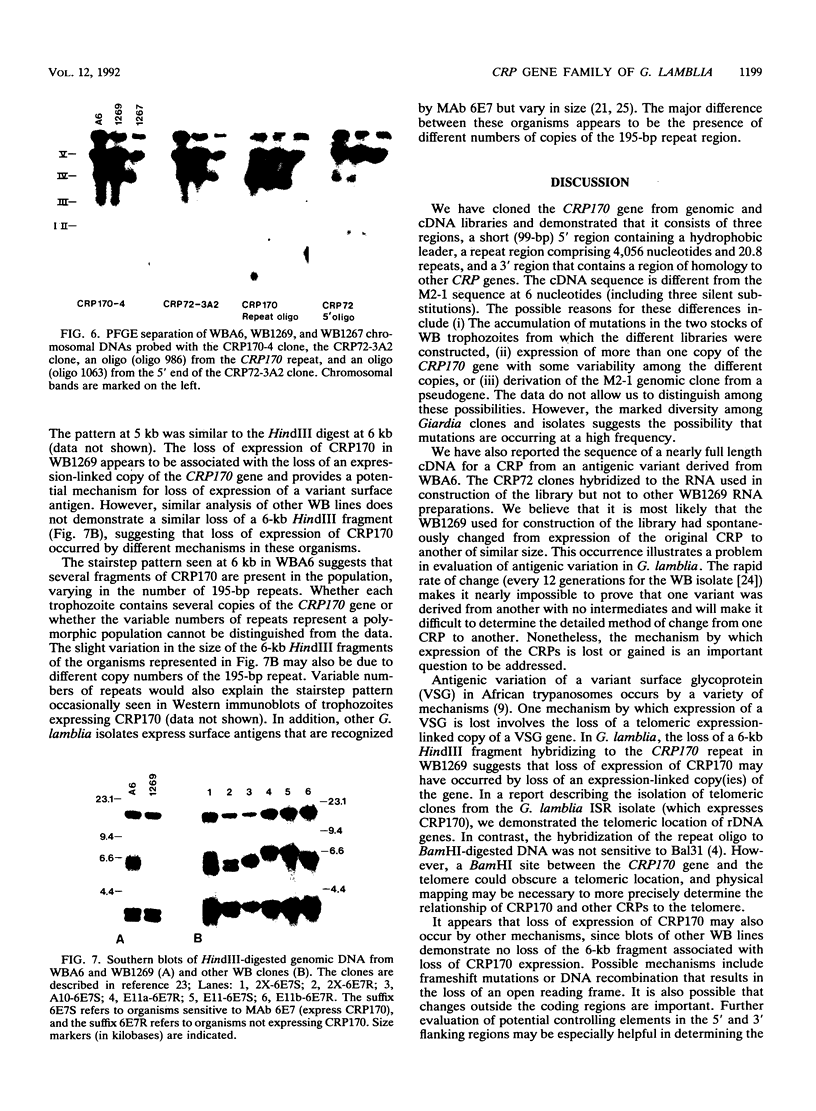
Giardia lamblia trophozoites demonstrate variable expression of a repertoire of cysteine-rich surface antigens in vitro and in vivo. The size of the repertoire has been estimated at 20 to 184, and specific variants can be detected after approximately 12 generations of in vitro growth for the WB isolate. In earlier studies, we cloned a portion of the gene for a 170-kDa surface antigen (CRP170) and demonstrated by DNA sequencing that it was cysteine rich (12%) and contained 2.6 copies of a tandemly repeated 195-bp pair sequence. The clone hybridized to multiple bands on a Southern blot of G. lamblia DNA in a pattern that was variable among the cloned lines but did not correlate with expression of CRP170. We have now cloned a nearly full length cDNA as well as genomic clones for CRP170 from the WBA6 cloned isolate. In addition, we have isolated a cDNA clone from the WB1269 line (expressing CRP72), an antigenic variant which was derived from WBA6. Sequence analysis of the CRP170 and CRP72 genes revealed marked C-terminal amino acid homology, suggesting a conserved functional role such as membrane anchoring. The CRP170 repeat oligonucleotide hybridized to a stairstep of bands approximately 6 kb in size on HindIII-digested WBA6 DNA representing the expressed copy(ies) of CRP170. In contrast, there was no hybridization to a fragment of similar size in WB1269, suggesting that WB1269 trophozoites have lost the expressed copy of the CRP170 gene.
Full text
PDF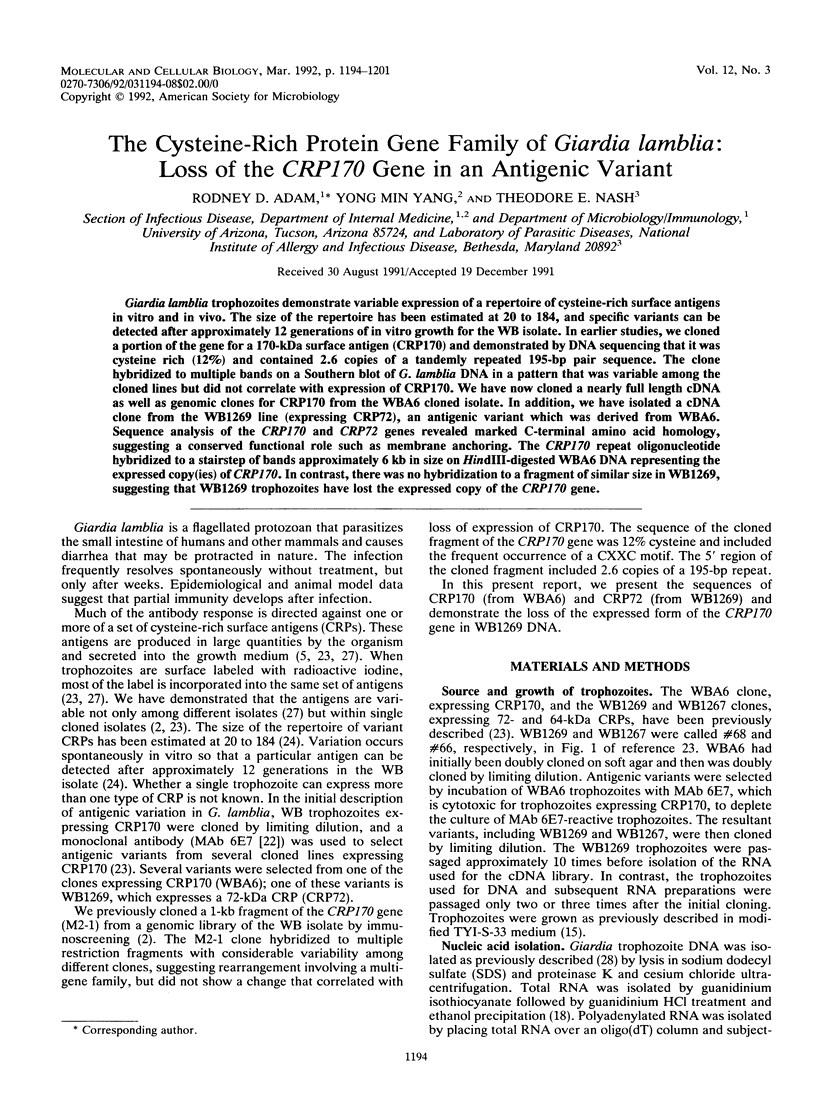



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam R. D., Aggarwal A., Lal A. A., de La Cruz V. F., McCutchan T., Nash T. E. Antigenic variation of a cysteine-rich protein in Giardia lamblia. J Exp Med. 1988 Jan 1;167(1):109–118. doi: 10.1084/jem.167.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam R. D., Nash T. E., Wellems T. E. Telomeric location of Giardia rDNA genes. Mol Cell Biol. 1991 Jun;11(6):3326–3330. doi: 10.1128/mcb.11.6.3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam R. D., Nash T. E., Wellems T. E. The Giardia lamblia trophozoite contains sets of closely related chromosomes. Nucleic Acids Res. 1988 May 25;16(10):4555–4567. doi: 10.1093/nar/16.10.4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam R. D. The biology of Giardia spp. Microbiol Rev. 1991 Dec;55(4):706–732. doi: 10.1128/mr.55.4.706-732.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal A., Merritt J. W., Jr, Nash T. E. Cysteine-rich variant surface proteins of Giardia lamblia. Mol Biochem Parasitol. 1989 Jan 1;32(1):39–47. doi: 10.1016/0166-6851(89)90127-8. [DOI] [PubMed] [Google Scholar]
- Aggarwal A., Nash T. E. Antigenic variation of Giardia lamblia in vivo. Infect Immun. 1988 Jun;56(6):1420–1423. doi: 10.1128/iai.56.6.1420-1423.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carle G. F., Olson M. V. Separation of chromosomal DNA molecules from yeast by orthogonal-field-alternation gel electrophoresis. Nucleic Acids Res. 1984 Jul 25;12(14):5647–5664. doi: 10.1093/nar/12.14.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gillin F. D., Hagblom P., Harwood J., Aley S. B., Reiner D. S., McCaffery M., So M., Guiney D. G. Isolation and expression of the gene for a major surface protein of Giardia lamblia. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4463–4467. doi: 10.1073/pnas.87.12.4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Keister D. B. Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans R Soc Trop Med Hyg. 1983;77(4):487–488. doi: 10.1016/0035-9203(83)90120-7. [DOI] [PubMed] [Google Scholar]
- Kirk-Mason K. E., Turner M. J., Chakraborty P. R. Evidence for unusually short tubulin mRNA leaders and characterization of tubulin genes in Giardia lamblia. Mol Biochem Parasitol. 1989 Aug;36(1):87–99. doi: 10.1016/0166-6851(89)90204-1. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- MacDonald R. J., Swift G. H., Przybyla A. E., Chirgwin J. M. Isolation of RNA using guanidinium salts. Methods Enzymol. 1987;152:219–227. doi: 10.1016/0076-6879(87)52023-7. [DOI] [PubMed] [Google Scholar]
- MacDonald R. J., Swift G. H., Przybyla A. E., Chirgwin J. M. Isolation of RNA using guanidinium salts. Methods Enzymol. 1987;152:219–227. doi: 10.1016/0076-6879(87)52023-7. [DOI] [PubMed] [Google Scholar]
- Mann B. J., Torian B. E., Vedvick T. S., Petri W. A., Jr Sequence of a cysteine-rich galactose-specific lectin of Entamoeba histolytica. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3248–3252. doi: 10.1073/pnas.88.8.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowatt M. R., Aggarwal A., Nash T. E. Carboxy-terminal sequence conservation among variant-specific surface proteins of Giardia lamblia. Mol Biochem Parasitol. 1991 Dec;49(2):215–227. doi: 10.1016/0166-6851(91)90065-e. [DOI] [PubMed] [Google Scholar]
- Nash T. E., Aggarwal A., Adam R. D., Conrad J. T., Merritt J. W., Jr Antigenic variation in Giardia lamblia. J Immunol. 1988 Jul 15;141(2):636–641. [PubMed] [Google Scholar]
- Nash T. E., Aggarwal A. Cytotoxicity of monoclonal antibodies to a subset of Giardia isolates. J Immunol. 1986 Apr 1;136(7):2628–2632. [PubMed] [Google Scholar]
- Nash T. E., Banks S. M., Alling D. W., Merritt J. W., Jr, Conrad J. T. Frequency of variant antigens in Giardia lamblia. Exp Parasitol. 1990 Nov;71(4):415–421. doi: 10.1016/0014-4894(90)90067-m. [DOI] [PubMed] [Google Scholar]
- Nash T. E., Conrad J. T., Merritt J. W., Jr Variant specific epitopes of Giardia lamblia. Mol Biochem Parasitol. 1990 Aug;42(1):125–132. doi: 10.1016/0166-6851(90)90120-b. [DOI] [PubMed] [Google Scholar]
- Nash T. E., Herrington D. A., Levine M. M., Conrad J. T., Merritt J. W., Jr Antigenic variation of Giardia lamblia in experimental human infections. J Immunol. 1990 Jun 1;144(11):4362–4369. [PubMed] [Google Scholar]
- Nash T. E., Keister D. B. Differences in excretory-secretory products and surface antigens among 19 isolates of Giardia. J Infect Dis. 1985 Dec;152(6):1166–1171. doi: 10.1093/infdis/152.6.1166. [DOI] [PubMed] [Google Scholar]
- Nash T. E., McCutchan T., Keister D., Dame J. B., Conrad J. D., Gillin F. D. Restriction-endonuclease analysis of DNA from 15 Giardia isolates obtained from humans and animals. J Infect Dis. 1985 Jul;152(1):64–73. doi: 10.1093/infdis/152.1.64. [DOI] [PubMed] [Google Scholar]
- Needleman S. B., Wunsch C. D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970 Mar;48(3):443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peattie D. A., Alonso R. A., Hein A., Caulfield J. P. Ultrastructural localization of giardins to the edges of disk microribbons of Giarida lamblia and the nucleotide and deduced protein sequence of alpha giardin. J Cell Biol. 1989 Nov;109(5):2323–2335. doi: 10.1083/jcb.109.5.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prat A., Katinka M., Caron F., Meyer E. Nucleotide sequence of the Paramecium primaurelia G surface protein. A huge protein with a highly periodic structure. J Mol Biol. 1986 May 5;189(1):47–60. doi: 10.1016/0022-2836(86)90380-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M., Kato S., Kohno K., Martin G. R., Yamada Y. Sequence of the cDNA encoding the laminin B1 chain reveals a multidomain protein containing cysteine-rich repeats. Proc Natl Acad Sci U S A. 1987 Feb;84(4):935–939. doi: 10.1073/pnas.84.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannich E., Ebert F., Horstmann R. D. Primary structure of the 170-kDa surface lectin of pathogenic Entamoeba histolytica. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1849–1853. doi: 10.1073/pnas.88.5.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]