Abstract
During the last 2 decades the role of bisphosphonates (BPs) to reduce skeletal-related events from bone metastases in breast cancer has been well defined. Several preclinical studies have strongly suggested that BPs may also provide an anti-cancer effect in early breast cancer. Indeed, the use of adjuvant BPs represents a unique approach that attempts at eradicating occult tumor micro-metastases residing in the bone marrow via targeting the bone microenvironment to render it less favorable for cancer cell growth. Although, this concept has been tested clinically for more than 15 years, no final consensus has been reached as for the routine use of BPs in the adjuvant phase of breast cancer, owing to conflicting results of randomized studies. Nevertheless, accumulating evidence from recent trials has indicated a therapeutic benefit of adjuvant BPs—particularly zoledronic acid—in women with established menopause, with no or perhaps detrimental effects in premenopausal women. Indeed, this hypothesis has opened a new chapter on the role of estrogen-poor microenvironment as a potential pre-requisite for the anti-tumor effects of BPs in the adjuvant phase of breast cancer. In this review, we will emphasize the biological rational of using BPs to target bone microenvironment in patients with early breast cancer and we will explore mechanistic differences; related to bisphosphonates effects in premenopausal versus postmenopausal women and how the endocrine environment would influence the anticancer potential of these compounds.
KEY WORDS : Adjuvant, bisphosphonates (BPs), anti-tumor activity, premeopausal, breast cancer
Introduction
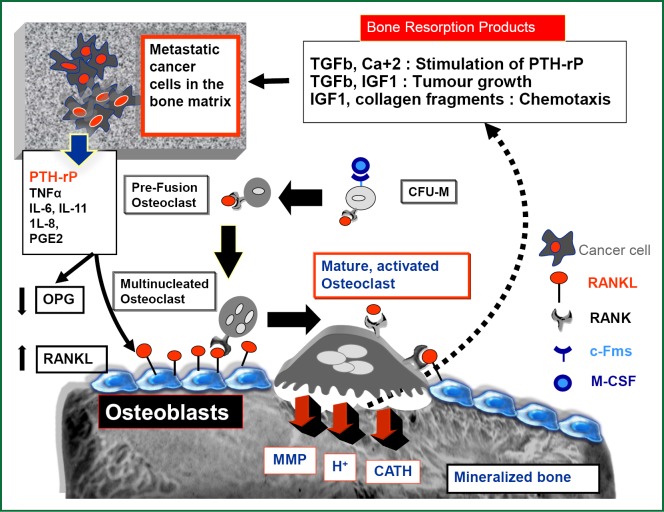
Unlike other tissues, bone is mainly composed of hard-mineralized tissue; hence it is more resistant to invasion and destruction by cancer cells compared to other metastatic sites (1). Osteoclasts have been described as the most efficient cells to induce bone resorption (1,2). Therefore, and in order to grow in bone matrix, the cancer cells must recruit and activate osteoclasts to destroy the bone matrix which is the main cellular mechanism for cancer induced bone destruction (2-4). This would provide the space in which cancer cells can grow and allow them to induce further molecular interactions with the different cytokines released during bone resorption, thus creating a microenvironment that is conducive for tumor invasion “soil and seed hypothesis” (3-6). The details of cross talks between breast cancer cells and bone microenvironment is shown in Figure 1. Identifying osteoclasts as the main cellular component in the development and progression of bone metastasis, has promoted the use of bisphosphonates (BPs), which are potent inhibitors of osteoclastic bone resorption, in the treatment of almost all types of bone metastases (7,8). In clinical practice, four BPs (clodronate, pamidronate, ibandronate, and zoledronic acid) have been widely used to treat breast cancer patients with bone metastases. In placebo controlled studies, these agents could significantly decrease skeletal-related events (SREs) associated with bone metastases in the treated patients, with zoledronic acid (ZA) clearly producing the greatest benefit in these patients (41% reduction in SRE versus placebo and 20% versus pamidronate) (9,10). BPs localize predominantly to skeletal areas of high bone turnover including osteolytic bone metastases. The two negatively charged phosphonate groups give these compounds the ability to bind with a very high affinity to calcium ions within the hydroxyapatite crystals in mineralized bones (11,12) where they are concentrated for a very long half life that may exceed one year (as in case of ZA) (13). BPs are subsequently released from the bone mineral during bone resorption, to be internalized by the activated osteoclasts (11,12). In general all BPs inhibit osteoclast formation and migration, and promote osteoclast apoptosis. BPs also increase production of osteoprogerin (OPG) by osteoblasts (14). OPG is a secreted soluble receptor, that functions as a decoy receptor for RANKL, which is a pivotal molecule for osteoclastic activation. Hence, OPG is considered as a natural inhibitor of osteoclastogenesis, that induces suppression of physiological and pathological bone resorption (5,6). Of note, BPs are cleared rapidly from the blood stream via their avid binding to mineralized bone and by renal filtration of unbound drug (15). As these agents do not readily cross the plasma membrane, the intracellular concentration of BPs in most tissues is very low.
Figure 1.
Molecular basis of bone metastasis in breast cancer: tumor cell-osteoclast cross talks comments. Diagrammatic illustration of Osteoclasts (OC) activation and its interplay with breast cancer cells and bone microenvironment. OC precursors differentiate from the population of monocytes/macrophages (CFU-M), by virtue of their expression of the receptor RANK. When RANKL (expressed by osteoblasts, and stromal cells) binds to this receptor in the presence of M-CSF, which in turn binds to its receptor, c-Fms, OC precursors differentiate and fuse together to form mature, multinucleated bone-resorbing OCs. Activated osteoclasts will then attach to the bone surface and via a proton pump mechanism it secrets hydrogen ions that dissolve bone minerals thus releasing calcium ions into the extracellular space. Osteoclasts also secret proteolytic enzymes like matrix metalloproteinases, collagenases, cathepsins and cysteine proteinases to induce collagen degradation and digestion of the organic matrix. Large amount of TGF-b and IGF II and other cytokines are stored within the mineralized bone matrix, and will be released during the process of OC bone resorption. When breast cancer cells colonize within the bone matrix, they start to secrete PTHrP and other osteolytic cytokines, which stimulate osteoblast production of RANKL while OPG levels are reduced, leading to enhanced osteoclastogenesis and increased bone resorption. Consequently the local milleau will be enriched by growth factors and other products of osteolysis (extracellular Ca++ and collagen fragments) which will induce: 1-stimulation of PTH-rP secretion (via TGF, Ca+ 2), 2-stimulation of tumor growth (via TGF, IGF1) and 3-chemotaxis of circulating tumor cells to arrest in bone matrix (via IGF1, collagen fragments). This evokes further PTH-rP release with worsening osteolysis, in addition to supporting the growth of breast cancer cells within the bone matrix. This reciprocal feedback between tumor cells and the bone microenvironment has been referred to as the “vicious cycle” of bone destruction. PTH-rP, parathyroid hormone related protein; TGFβ, transforming growth factor beta; Ca, calcium; IGF1, insulin growth factor 1; OPG, osteoprotegerin; RANKL, RANK ligand; c-Fms, colony-stimulating factor receptor 1.
Anti-cancer effects of BPs in breast cancer
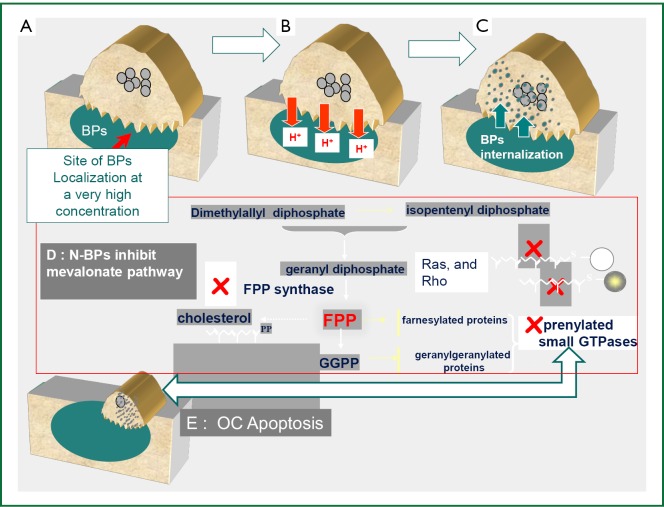
Extensive in vitro and animal data suggests that BPs may act as antitumor agents and can reduce skeletal tumor burden (15,16). However, and in view of their high affinity for bone mineral and very low concentration in other tissues, the evidence for their in vivo antitumor activity outside the bone is less convincing (17-19). BPs exert direct antitumor effects via inhibition of tumor cell adhesion, invasion, and proliferation, in addition to induction of tumor cell apoptosis (15,16). A major molecular target inhibited by nitrogen containing BP (N-BP) like ZA, pamidronate, and ibandronate is farnesyl pyrophosphate synthase (FPPS), a key enzyme in the mevalonate pathway (20,21). This is an important metabolic pathway required for producing steroids, maintaining cell-membrane integrity, regulating cellular metabolism and is also crucial for the prenylation of regulatory proteins involved in many intracellular signaling pathways that control cell proliferation. Inhibition of the mevalonate pathway will ultimately cause osteoclasts to undergo apoptosis (20,21) (Figure 2). The mevalonate pathway is also an important part of the metabolic and proliferative processes in cancer cells. Compared to other BPs, ZA has been shown to be the most potent inhibitor of FPPS activity in cancer cells, which correlates with its highest anti-osteoclastic activity in vitro and in vivo (22). The N-BPs may also act indirectly on tumor cells through anti-angiogenic (23) and immuno-modulatory mechanisms (24-26). The later is especially attributed to their ability to accumulate in macrophages and monocytes which share the same ontogeny with osteoclasts (24). Therapeutic doses of ZA has been shown to modulate monocyte, macrophage and dendritic cell function and improve the γδ T-cell anti- cancer properties (16,22,25,27).
Figure 2.
Nitrogen-containing bisphosphonates anti-osteoclastic and anti-tumor molecular mechanism of action. A. BPs localize with a very high affinity to skeletal areas of high bone turnover including osteolytic bone metastases where they are concentrated underneath the activated osteoclasts; B. BPs are subsequently released from the bone mineral during bone resorption; C. BPs are then internalized by the activated osteoclasts; D. Within the osteoclasts (and also breast cancer cells) the N-BPs inhibit the activity of farnesyl diphosphonate (FPP) synthase, a key enzyme in the mevalonate pathway. FPP is necessary for prenylation of small guanine triphosphatases (GTPases)--such as Ras, and Rho, which are involved in intracellular signaling; E. Inhibition of the mevalonate pathway will ultimately cause osteoclasts to undergo apoptosis. Experimental studies have shown that inhibition of this pathway by BPs, will also results in inhibition of malignant cell growth and survival in cell culture and animal models.
Although the exact mechanism(s) responsible for the observed anti-tumor effects of BPs remains unclear, recent data from animal studies strongly suggested that the main in vivo effect of clinically relevant doses of BPs on breast cancer cells, is mediated via inhibition of osteoclast-mediated bone resorption rather than a direct cytotoxic effect (28). This supports the argument that tumor growth can be effectively inhibited in the clinic by targeting the bone microenvironment and not necessarily via a direct cytotoxic effect against the primary tumor.
Bone microenvironment as a rational target to prevent breast cancer replapse
It has been known for a while that dormant tumor cells (DTCs) in the bone marrow (BM), can provide a major source of late relapse in patients with early breast cancer (EBC). A significant correlation between DTC in bone marrow or circulating tumor cells in the blood stream and poor prognosis has been demonstrated in several studies (29). Indeed, the BM microenvironment can provide an ideal sanctuary site for these cancer cells to evade systemic anticancer therapy (30). Two distinct protective interactions within the bone marrow have been described as an endosteal niche and a vascular niche (31). The endosteal niche allows DTC to interact with osteoblasts, which are critical mediators of stem cell dormancy and survival. The vascular niche facilitates DTC to interact with hematopoietic stem cells. Meads et al. has shown that the hematopoietic stem cell can induce environmentally mediated drug resistance (EM-DR), which protects the tumor cells from the cytotoxic effects of chemotherapy as well as the physiologic mediators of cell death (32). Although, the specific signals responsible for reactivation of DTC are still unclear (33,34), yet it has been postulated that DTC in the BM can be activated by osteoclast-mediated release of bone derived growth factors (34), to form metastases at other osseous and non osseous sites, while serving also as a source of local recurrences (‘tumor self-seeding’ phenomenon) (35).
In several phase II clinical studies, including women with high risk, early-stage breast cancer, both ZA and ibandronate, in combination with standard adjuvant therapy, could effectively reduce DTC number and persistence in bone marrow compared with standard therapy alone (36-39). Although, the prognostic impact of such reduction of DTC has never been addressed in these studies, yet this should definitely bring enthusiasm to incorporate BPs into the adjuvant treatment regimens in EBC, in an attempt to interfere with the unique support that bone micro environment provides to cancer cell survival. Altering the BM microenvironment by adjuvant BPs therapy would--at least theoretically--render it less conducive to cancer cell survival, and therefore may provide a unique mechanism to prevent cancer recurrence in EBC (16,28,34).
The emergence of estrogen poor microenvironment as a pre-requisite to obtain a therapeutic benefit from adjuvant BPs
It is widely accepted that estrogens play a critical role in the maintenance of bone homeostasis and that the osteoclastic activation, in response to estrogen depletion is the main cellular basis of bone resorption in postmenopausal women (40,41). Importantly, it has been hypothesized that increased bone resorption would create a bone microenvironment that might serve as a homing site for DTCs, that would be subsequently associated with increase rate of relapse (42,43). Recently, this notion was indirectly supported in the clinic by some speculations from the MA27 study which was designed to compare anastrazole versus exemestane in post menopausal women with EBC. The study has reported no difference between the 2 aromatase inhibitors in terms of DFS (44). However, in a subsequent exploratory analysis, the authors have shown that patients who had osteoporosis (self reporting) and who received no therapy for their osteoporosis had the highest rate of relapse, compared to those who never had osteoporosis or those who received osteoporosis therapy (45). This strongly supports the hypothesis that an impaired bone micro-environment induced by post menopausal estrogen depletion and aromatase inhibitors (AIs) treatment would provide a fertile “soil” for DTC, and that osteoporosis (as a surrogate marker of estrogen depletion) would negatively affect the treatment outcomes in EBC patients, which can be significantly reversed by anti-bone resorption therapy.
More recently, and in a very good animal model, that mimics the clinical situation in EBC, the group of Sheffield University has unequivocally shown that ZA could prevent breast cancer relapse only in estrogen poor microenvironment (i.e., in the ovariectomized mice), with no benefit at all in non ovariectomized mice (46). This study presented the first direct clue for a differential anti-tumor effect of ZA in the pre versus post-menopausal settings, which directly proves that the anti-cancer effect of adjuvant BPs will be exclusively seen in the post-menopausal setting, and that ZA (and probably other BPs) would mainly act by inhibiting an ovarian suppression-mediated proliferation of tumor cells resident in the BM. Therefore, estrogen poor microenvironment, with its accelerated bone resorption sequences seems to be a prerequisite to obtain a therapeutic anti-tumor benefit from adjuvant BPs (16,46,47).
Interpretation of adjuvant BPs clinical trials in early breast cancer
The first generation of clinical studies testing the anti-tumor role of BPs in early breast cancer evaluated oral clodronate in 3 randomized trials. The long term follow-up data have shown conflicting outcome, with 2 studies (48,49) demonstrating a significant benefit at some follow up periods, while in the 3rd trial the ten-year DFS was significantly lower in the clodronate group compared to the control arm (45% vs. 58%, P=0.01, respectively) (50). A meta-analysis of the three trials has shown that clodronate did not provide any significant benefit in bone metastasis-free survival, or DFS (51). Therefore, no real take home message could have been concluded from these trials.
Later on, the ABCSG-12 and the ZO-FAST trials, have strongly concluded for a therapeutic benefit of adjuvant ZA in women with poor estrogen microenvironment at the time of their breast cancer treatment. The ABCSG12 study (52), included 1,803 premenopausal women with stage I/II breast cancer, who were randomized to receive 3 years of ZA versus observation; added to endocrine therapy (luteinizing hormone-releasing hormone agonist to suppress the ovarian function and anastrozole or tamoxifen). The study demonstrated a 36% reduction in the relative risk of disease progression among those patients taking ZA. Importantly, and unlike the earlier clodronate studies, the therapeutic gain obtained by ZA was maintained at 84 months median follow-up, with a significant benefit in DFS (HR=0.72; P=0.014) and OS (HR=0.63; P= 0.049) (53). The ZO-FAST trial included 1,065 Stage I-IIIa, ER positive postmenopausal patients who were treated with letrozole and were randomized to either immediate or delayed ZA (54). Delayed ZA therapy was administered in case of non-traumatic fracture or crossing a bone loss threshold. At 5 years follow up, a DFS benefit (which was a secondary endpoint) of immediate ZA treatment has been reported (HR=0.66; log-rank P value=0.0375) with a trend for an OS gain (HR=0.69; P value=0.196). Of notice, the patients in the above 2 trials were treated with endocrine therapies known to induce a profound estrogen poor environment and significant bone loss. The patients in the 2 trials have received a small dose of ZA (once/6 months), that was good enough to prevent bone loss in the treated patients (which was a secondary end point for the ABCSG-12 trial and a primary end points for the ZO-FAST trial).
Unfortunately, the 2 studies cannot really answer the question related to the benefit of adjuvant BPs in other adjuvant settings (i.e., in women with estrogen rich microenvironment or in women with ER negative EBC). However, the exclusive benefit of adjuvant ZA in women with estrogen poor environment was subsequently concluded from the Azure trial, which was a randomized phase III study addressing the role of adjuvant ZA (5 years of ZA in a gradual tapering fashion) in chemotherapy treated stage II/III breast cancer. Of notice the Azure study failed to show that adding ZA to chemotherapy improves disease-free survival in the overall patient population (which was its primary endpoint). However, in a pre-specified subgroup analysis, the postmenopausal patients (5 years or more) had an significant DFS benefit with the addition of ZA (Adjusted HR=0.75; 95% CI: 0.59-0.96; P=0.02) (55). The restricted benefit of BPs adjuvant treatment in postmenopausal women was further suggested by 2 subsequent phase III studies: NSABP B-34 (3,323 patients randomized to receive oral clodronate 1,600 mg or placebo daily for 3 years and GAIN trial [3,023 randomized to receive oral ibandronate (50 mg daily for 2 years) or observation] (56,57). In line with AZURE trial, these 2 studies failed to show improvement in DFS, which was their primary end point. Still, again prespecified subgroup analysis suggested that BPs might perform better in patients who are ≥50 years (in NSABP B-34) and ≥60 years (in GAIN), or in other wards those who would have achieved complete ovarian suppression at the time of BPs treatment.
Bisphosphonates in the adjuvant treatment of young breast cancer patients: is it ready for a prime time?
With the exception of the ABCSG 12 and the ZOFAST [and its sister trials Z-FAST and E-ZO-FAST (58,59)], the majority of clinical trials addressing the anti-cancer role of adjuvant BPs in EBC, were designed on “the one size fits all” approach (Table 1) as they included a very heterogeneous patient population in terms of the disease phenotypes, menstrual status, and type of the standard adjuvant treatment given to their patients, which in our opinion was a major reason for their hard to interpret results. Furthermore, the 3 largest studies, AZURE, B34 and GAIN, had used different types of BPs for a variable treatment period (ranging from 2 to 5 years) and adopted different definitions of menopause. This would certainly pose many difficulties towards their combined analysis. Nevertheless, a meta-analysis of these 3 trials together with other 3 trials that specifically evaluated the effects of adjuvant BPs on DFS according to menopausal was recently presented (60). The authors reported no beneficial effect in the entire population of EBC treated by BPs compared to the control arm, with a significant DFS benefit in the subgroup of women with established menopause [HR=0.81 (0.69-0.95)]. However, an alarming conclusion was made in this meta-analysis, which suggested an apparent harm of adjuvant BPs in pre- and perimenopausal women. Importantly, this observation has been previously highlighted by AZURE study in which there was a significant detrimental effect of ZA on the rate of non-skeletal metastases in premenopausal women, that was independent of the ER status of the tumor [HR=1.32 (95% CI: 1.09-1.59)], and that was never discussed by the authors (55). Interestingly an older Finnish trial had also made a similar conclusion, when clodronate was given in the adjuvant setting, where the frequency of non-skeletal recurrences was significantly higher in the clodronate group versus the control group especially in ER negative patients (DFS at 10 years were 25% vs. 58%, P=0.004, respectively). Importantly, in this particular study, the only subgroup where no adverse effect of clodronate was seen, were postmenopausal ER positive patients (50). Of interest, some preclinical studies have also indicated that adjuvant BPs may enhance the development of non-skeletal metastases, if given without a concomitant anticancer drugs (like the situation in the long term BPs treatment in ER negative breast cancer) (19). This particular observation was strongly emphasized as a worrying issue when BPs are to used in the prevention setting (4,50). Till further evidence emerges, this potentially detrimental effect of adjuvant BPs in premenopausal and/or ER negative EBC could be considered due to chance. Still we wish to raise some critical questions in this context: what could be putatively tumor promoting when a high dose of ZA (as adopted in the AZURE) is given in the adjuvant phase of BC in premenopausal women? Is it the estrogen rich microenvironment or is it the ER negative phenotype or both? In fact, there is a lot of potential speculations to explain the lack of response to ZA in estrogen rich microenvironment (61). Of notice estrogen and BPs may interact at the level of BM cancer cell dormancy. The estrogen-rich bone microenvironment appears to better support the survival and expansion of DTC in the endosteal niche. This observation is supported by the findings that estrogen increases the number and activity of endosteal osteoblasts, which are critical mediators of stem cell dormancy and survival (30,62). This may imply that the ability of BPs to decrease DTC is offset by the high level of oestrogen in premenopausal women.
Table 1. The major trials testing the anti-tumor effects of bisphosphonates in women with EBC remarks.
| TRIAL | No. of patients | Age | Post menopausal | ER/PR positive | Chemo therapy | Hormone treatment | HR (DFS) Median FU in mo |
P value | HR (OS) | P value | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of BP | ||||||||||||
| Duration of BP | ||||||||||||
| AZURE ZA (high dose) ×5 years |
3,360 | NA | 45% | 78% | 95.5% | NA, mostly Tam |
adj 0.98 At 60 mo |
0.79 | 0.85 | 0.07 | ||
| ABCSG-12 ZA (/6 mo) ×3 years |
1,803 | 45 | Induced by LHRH agonist goserelin |
≥90% | 5% | LHRH +TAM or Ana | 0.72 At 84 mo |
0.01 | 0.61 | 0.03 | ||
| NSABP 34 Clodronate ×3 years |
3,323 | 50 | NA | 7% | NA | Tam | 0.91 At 90 mo |
0.27 | 0.84 | 0.10 | ||
| GAIN Ibandronate 50 mg/po ×2 years |
3,023 | 49.5 | 51.6% | 77% | 100% | Mostly Tam | 0.94 At 36 mo |
0.59 | 1.04 | 0.8 | ||
| ZOFAST ZA (/6 mo) ×5 years |
1,065 | 57 | 100% 83% Established |
100% | 54% | Letrozole | 0.66 At 60 mo |
0.037 | 0.69 | 0.196 | ||
| ZFAST* ZA (/6 mo) ×5 years |
602 | 60 | 100% | 100% | 47% | Letrozole | NA At 61 mo |
0.628 | NA | |||
| EZOFAST** ZA (/6 mo) ×5 years |
527 | 58 | 100% | 100% | 52% | Letrozole | NA At 12 mo |
NA | ||||
| Powles 2006 Clodronate ×2 years |
1,069 | 52.8 | 50% | ER+: 45% PR+: 22% |
60% | TAM | Bone metastasis HR 0.69 | 0.043 | 0.768 | 0.048 | ||
| Diel 2008*** Clodronate ×2 years |
290 | NA | 62% | 73% | 43% | TAM Goserelin 9% |
NA | NA | ||||
| Saarto 2004 Clodronate ×3 years |
282 | 52 | 50% | 61% (ER) 55% (PR) |
54% | TAM 63% Toremifen 37% |
RR 1.52 At 120 mo |
0.02 | RR 0.33 | 0.12 | ||
*ZFAST, At month 61 DFS events were almost similar in the 2 groups [percentage (95% CI): upfront, 9.8 (6.0-10.3); delayed, 10.5 (6.6-14.4); P=0.6283]. Disease recurrence alone occurred in slightly more delayed group patients compared with the upfront group [16 patients (5.3%) vs. 21 patients (7.0%)]. **EZOFAST, At 12 months, 7 patients (2.8%) in the immediate ZOL group and 5 patients (1.9%) in the delayed ZOL group experienced distant recurrent disease. ***Diel’s Study, significant OS improvement in the clodronate group at a median follow-up of 103±12 months, 79.6% in the clodronate group versus 39.3% in the control group group (P=0.04). The Significant reductions in the incidence of bony and visceral metastases and improvement in of DFS at 36- and 55-month follow-up periods were no longer seen with clodronate at a median follow-up period of 103 months.
Finally, we believe that the altered immune profile in response to ZA that may explain a preferential benefit of this drug in relation to the disease phenotype. As mentioned earlier, standard doses of ZA have been consistently reported to induce selective stimulation of γδ T -cells which exert a beneficial anti-tumor function in vivo (16,22,25,26). Clinically, γδ T-cell expansion and activation has been confirmed in cancer patients after ZA administration . Recently, Benzaid et al. (27) showed that only the ER positive, HER2 negative breast cancer cell lines are sensitive to the immune-mediated attack by γδ T -cells. This may suggest that ER positive phenotypes are more likely to have a therapeutic benefit from adjuvant ZA. It may be assumed that premenopausal women have more ER negative disease (data not shown by the AZURE authors), which is less sensitive to γδ T-cell-mediated cytotoxicity.
Another immunologically significant molecule affected by ZA is OPG, which as mentioned earlier is a potent inhibitor of bone resorption. The ability of OPG to inhibit osteolysis suggests that OPG can have an inhibitory effect on cancer-induced bone disease and metastasis (5,6). Both ZA (in a dose dependant fashion) (14) and estrogen have been reported to increase the serum level of OPG (63-65), which is one of the suggested mechanisms for their anti-resorptive function. Interestingly, OPG may promote tumor cell survival though its ability to enhance angiogenesis and to inhibit TRAIL induced apoptosis (66-68). TRAIL (TNF-related apoptosis-inducing ligand) is an important molecule mediating major antitumor effects of the immune system (66). Importantly, in several cancer types, elevated levels of serum OPG were significantly associated with poor prognosis (69,70). Of note, it has been shown that OPG preferentially protects ER negative breast cancer cell lines from TRAIL-induced apoptosis in vitro (71). Taken together, we speculate that the premenopausal population like those treated in the AZURE trial, could have been exposed to a higher concentration of OPG in skeletal and none skeletal sites, secondary to their elevated estrogen levels and the high dose of ZA. This relative increase of OPG may shift the fine balance involved between the beneficial effects of OPG in skeletal sites, and potentially detrimental effects of inhibiting TRAIL-mediated tumor cell apoptosis and stimulation of angiogenesis (68). Actually, and in line with our assumption, premenopausal women in the AZURE did not have any detrimental effect of ZA on skeletal relapse rate. On the contrary, there was a non significant reduction of skeletal relapse in ZA treated patients compared to the control group [HR=0.86 (95% CI: 0.63-1.16)]. This would again argue for a preferential role of the immune system when a patient is exposed to high dose of ZA during the adjuvant setting: a beneficial effect in ER positive phenotype (more sensitive to γδ T cells cytotoxicity) and a potentially detrimental effect in ER negative phenotype (more protected by OPG induced Trail inhibition). Since ZA dose is critical in regulating OPG, then the positive results observed in the ABCSG-12 and ZOFAST may be also explained by the low level of OPG related to the 6 monthly ZA treatment, being given in an estrogen poor microenvironment, in a pure ER positive population which was not the case in the AZURE.
In conclusion, a number of clinical trials and animal studies have strongly suggested that the benefits of adjuvant bone targeted treatments on risks of recurrence or death in EBC are restricted to women with established menopause (72). We strongly believe that this statement is clinically and biologically correct. However, while we are focusing on ‘the estrogen poor soil’, as a prerequisite for a preferential benefit of adjuvant BPs, the properties of ‘the seed’ may be also valuable or even crucial in this context, where the ER positive and not the ER negative breast cancer phenotype may be expected to derive the maximum benefit of these agents. To this end we would certainly recommend the use of low dose of ZA (at 4 mg/6 months) in all ER positive premenopausal women whose treatment regimens includes LHRH agonist, or those who develop complete ovarian suppression following adjuvant chemotherapy. At this dose level of ZA, the associated bone loss will be effectively prevented in the treated patients, which will be the ideal approach to maintain their bone health. Furthermore ZA at this dose can effectively interrupt the cross talk between DTC and the estrogen poor bone microenvironment, a step that has been reported to potentially improve DFS in EBC. Importantly, the ABCSG-12 which is the only study that included a pure premenopausal population (median age 45 years) has recently reported in a preplanned subgroup analyses based on age (≤40 years or >40 years), that ZA significantly improved DFS by 34% in women over 40 years of age (n=1,390; HR=0.66; P=0.013), while it did not improve the DFS in women who were 40 years of age or younger (n=413) (53). The authors have attributed this to the assumption that women over 40 years of age may achieve more complete ovarian suppression. While this statement is certainly valid for women treated by adjuvant chemotherapy, it cannot be applied to the population included in the ABCSG 12 (less than 10% received chemotherapy only during the neoadjuvant phase). Furthermore, the results in women ≤40 years of age were concluded from a total of 77 DFS events at 84 months, which looks as insufficient evidence to preclude ZA benefit in these women. As the anti-tumor effects of adjuvant BPs might be exclusively observed in patients with estrogen depletion and accelerated bone loss, or in other words in those patients with a susceptible soil, then we confidently assume that it is the menopausal status rather than age that will determine the benefit of adjuvant BPs in young women. Taken together, the biological concept that one size does not fit all, seems to be very true when it comes to the role of BPs in premenopausal women with EBC.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Roodman GD. Cell biology of the osteoclast. Exp Hematol 1999;27:1229-41 [DOI] [PubMed] [Google Scholar]
- 2.Guise TA, Mundy GR. Cancer and bone. Endocr Rev 1998;19:18-54 [DOI] [PubMed] [Google Scholar]
- 3.Guise TA, Mohammad KS, Clines G, et al. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin Cancer Res 2006;12:6213s-6s [DOI] [PubMed] [Google Scholar]
- 4.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2002;2:584-93 [DOI] [PubMed] [Google Scholar]
- 5.Dougall WC. RANKL signaling in bone physiology and cancer. Curr Opin Support Palliat Care 2007;1:317-22 [DOI] [PubMed] [Google Scholar]
- 6.Azim HA, Kamal NS, Azim HA., Jr Bone metastasis in breast cancer: the story of RANK-ligand. J Egypt Natl Canc Inst 2012;24:107-14 [DOI] [PubMed] [Google Scholar]
- 7.Aapro M, Abrahamsson PA, Body JJ, et al. Guidance on the use of bisphosphonates in solid tumours: recommendations of an international expert panel. Ann Oncol 2008;19:420-32 [DOI] [PubMed] [Google Scholar]
- 8.Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev 2001;27:165-76 [DOI] [PubMed] [Google Scholar]
- 9.Pavlakis N, Schmidt R, Stockler M.Bisphosphonates for breast cancer. Cochrane Database Syst Rev 2005:CD003474. [DOI] [PubMed] [Google Scholar]
- 10.Rosen LS, Gordon DH, Dugan W, Jr, et al. Zoledronic acid is superior to pamidronate for the treatment of bone metastases in breast carcinoma patients with at least one osteolytic lesion. Cancer 2004;100:36-43 [DOI] [PubMed] [Google Scholar]
- 11.Fleisch H.Development of bisphosphonates. Breast Cancer Res 2002;4:30-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rogers MJ, Gordon S, Benford HL, et al. Cellular and molecular mechanisms of action of bisphosphonates. Cancer 2000;88:2961-78 [DOI] [PubMed] [Google Scholar]
- 13.Brown JE, Ellis SP, Lester JE, et al. Prolonged efficacy of a single dose of the bisphosphonate zoledronic acid. Clin Cancer Res 2007;13:5406-10 [DOI] [PubMed] [Google Scholar]
- 14.Viereck V, Emons G, Lauck V, et al. Bisphosphonates pamidronate and zoledronic acid stimulate osteoprotegerin production by primary human osteoblasts. Biochem Biophys Res Commun 2002;291:680-6 [DOI] [PubMed] [Google Scholar]
- 15.Daubiné F, Le Gall C, Gasser J, et al. Antitumor effects of clinical dosing regimens of bisphosphonates in experimental breast cancer bone metastasis. J Natl Cancer Inst 2007;99:322-30 [DOI] [PubMed] [Google Scholar]
- 16.Clezardin P.Potential anticancer properties of bisphosphonates: insights from preclinical studies. Anticancer Agents Med Chem 2012;12:102-13 [DOI] [PubMed] [Google Scholar]
- 17.Sasaki A, Boyce BF, Story B, et al. Bisphosphonate risedronate reduces metastatic human breast cancer burden in bone in nude mice. Cancer Res 1995;55:3551-7 [PubMed] [Google Scholar]
- 18.Hiraga T, Williams PJ, Mundy GR, et al. The bisphosphonate ibandronate promotes apoptosis in MDA-MB-231 human breast cancer cells in bone metastases. Cancer Res 2001;61:4418-24 [PubMed] [Google Scholar]
- 19.Michigami T, Hiraga T, Williams PJ, et al. The effect of the bisphosphonate ibandronate on breast cancer metastasis to visceral organs. Breast Cancer Res Treat 2002;75:249-58 [DOI] [PubMed] [Google Scholar]
- 20.Benford HL, Frith JC, Auriola S, et al. Farnesol and geranylgeraniol prevent activation of caspases by aminobisphosphonates: biochemical evidence for two distinct pharmacological classes of bisphosphonate drugs. Mol Pharmacol 1999;56:131-40 [DOI] [PubMed] [Google Scholar]
- 21.Luckman SP, Hughes DE, Coxon FP, et al. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res 1998;13:581-9 [DOI] [PubMed] [Google Scholar]
- 22.Winter MC, Holen I, Coleman RE. Exploring the anti-tumour activity of bisphosphonates in early breast cancer. Cancer Treat Rev 2008;34:453-75 [DOI] [PubMed] [Google Scholar]
- 23.Stresing V, Fournier PG, Bellahcène A, et al. Nitrogen-containing bisphosphonates can inhibit angiogenesis in vivo without the involvement of farnesyl pyrophosphate synthase. Bone 2011;48:259-66 [DOI] [PubMed] [Google Scholar]
- 24.Rogers TL, Holen I. Tumour macrophages as potential targets of bisphosphonates. J Transl Med 2011;9:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nussbaumer O, Gruenbacher G, Gander H, et al. DC-like cell-dependent activation of human natural killer cells by the bisphosphonate zoledronic acid is regulated by γδ T lymphocytes. Blood 2011;118:2743-51 [DOI] [PubMed] [Google Scholar]
- 26.Dieli F, Gebbia N, Poccia F, et al. Induction of gammadelta T-lymphocyte effector functions by bisphosphonate zoledronic acid in cancer patients in vivo. Blood 2003;102:2310-1 [DOI] [PubMed] [Google Scholar]
- 27.Benzaïd I, Mönkkönen H, Stresing V, et al. High phosphoantigen levels in bisphosphonate-treated human breast tumors promote Vgamma9Vdelta2 T-cell chemotaxis and cytotoxicity in vivo. Cancer Res 2011;71:4562-72 [DOI] [PubMed] [Google Scholar]
- 28.Fournier PG, Stresing V, Ebetino FH, et al. How do bisphosphonates inhibit bone metastasis in vivo? Neoplasia 2010;12:571-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Braun S, Vogl FD, Naume B, et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med 2005;353:793-802 [DOI] [PubMed] [Google Scholar]
- 30.Li L, Neaves WB. Normal stem cells and cancer stem cells: the niche matters. Cancer Res 2006;66:4553-7 [DOI] [PubMed] [Google Scholar]
- 31.Shiozawa Y, Havens AM, Pienta KJ, et al. The bone marrow niche: habitat to hematopoietic and mesenchymal stem cells, and unwitting host to molecular parasites. Leukemia 2008;22:941-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meads MB, Hazlehurst LA, Dalton WS. The bone marrow microenvironment as a tumor sanctuary and contributor to drug resistance. Clin Cancer Res 2008;14:2519-26 [DOI] [PubMed] [Google Scholar]
- 33.Aguirre-Ghiso JA. The problem of cancer dormancy: understanding the basic mechanisms and identifying therapeutic opportunities. Cell Cycle 2006;5:1740-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gnant M, Hadji P.Prevention of bone metastases and management of bone health in early breast cancer. Breast Cancer Res 2010;12:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim MY, Oskarsson T, Acharyya S, et al. Tumor self-seeding by circulating cancer cells. Cell 2009;139:1315-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aft R, Naughton M, Trinkaus K, Effect of zoledronic acid on disseminated tumour cells in women with locally advanced breast cancer: an open label, randomised, phase 2 trial. Lancet Oncol 2010;11:421-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rack B, Jückstock J, Genss EM, et al. Effect of zoledronate on persisting isolated tumour cells in patients with early breast cancer. Anticancer Res 2010;30:1807-13 [PubMed] [Google Scholar]
- 38.Solomayer EF, Gebauer G, Hirnle P, et al. Influence of zoledronic acid on disseminated tumor cells in primary breast cancer patients. Ann Oncol 2012;23:2271-7 [DOI] [PubMed] [Google Scholar]
- 39.Hoffmann O, Aktas B, Goldnau C, et al. Effect of ibandronate on disseminated tumor cells in the bone marrow of patients with primary breast cancer: a pilot study. Anticancer Res 2011;31:3623-8 [PubMed] [Google Scholar]
- 40.Clarke BL, Khosla S. Physiology of bone loss. Radiol Clin North Am 2010;48:483-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Azim H, Azim HA., Jr Targeting RANKL in breast cancer: bone metastasis and beyond. Expert Rev Anticancer Ther 2013;13:195-201 [DOI] [PubMed] [Google Scholar]
- 42.Orr W, Varani J, Gondex MK, et al. Chemotactic responses of tumor cells to products of resorbing bone. Science 1979;203:176-9 [DOI] [PubMed] [Google Scholar]
- 43.Lipton A, Chapman JA, Demers L, et al. Elevated bone turnover predicts for bone metastasis in postmenopausal breast cancer: results of NCIC CTG MA.14. J Clin Oncol 2011;29:3605-10 [DOI] [PubMed] [Google Scholar]
- 44.Goss PE, Ingle JN, Pritchard KI, et al. Exemestane versus anastrozole in postmenopausal women with early breast cancer: NCIC CTG MA.27--a randomized controlled phase III trial. J Clin Oncol 2013;31:1398-404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shepherd LE, Chapman JA, Ali SM, et al. Effect of osteoporosis in postmenopausal breast cancer patients randomized to adjuvant exemestane or anastrozole: NCIC CTG MA.27. J Clin Oncol 2012;30: abstr 501.
- 46.Holen I, Wang N, Reeves KJ, et al. Zoledronic acid specifically inhibits development of bone metastases in the post-menopausal setting-evidence from an in vivo breast cancer model. Cancer Research 2012; 72:PD07-08 [Google Scholar]
- 47.Winter MC, Coleman RE. Bisphosphonates in the adjuvant treatment of breast cancer. Clin Oncol (R Coll Radiol) 2013;25:135-45 [DOI] [PubMed] [Google Scholar]
- 48.Powles T, Paterson A, McCloskey E, et al. Reduction in bone relapse and improved survival with oral clodronate for adjuvant treatment of operable breast cancer [ISRCTN83688026] Breast Cancer Res 2006;8:R13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diel IJ, Jaschke A, Solomayer EF, et al. Adjuvant oral clodronate improves the overall survival of primary breast cancer patients with micrometastases to the bone marrow: a long-term follow-up. Ann Oncol 2008;19:2007-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saarto T, Vehmanen L, Virkkunen P, et al. Ten-year follow-up of a randomized controlled trial of adjuvant clodronate treatment in node-positive breast cancer patients. Acta Oncol 2004;43:650-6 [DOI] [PubMed] [Google Scholar]
- 51.Ha TC, Li H. Meta-analysis of clodronate and breast cancer survival. Br J Cancer 2007;96:1796-801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gnant M, Mlineritsch B, Schippinger W, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med 2009;360:679-91 [DOI] [PubMed] [Google Scholar]
- 53.Gnant M, Mlineritsch B, Luschin-Ebengreuth G, et al. Long-Term Follow-Up in ABCSG-12: Significantly Improved Overall Survival with Adjuvant Zoledronic Acid in Premenopausal Patients with Endocrine-Receptor-Positive Early Breast Cancer. Cancer Res 2011;71:S1-2 [Google Scholar]
- 54.de Boer R, Bundred N, Eidtmann H.Long-Term Survival Outcomes among Postmenopausal Women with Hormone Receptor-Positive Early Breast Cancer Receiving Adjuvant Letrozole and Zoledronic Acid: 5-Year Follow-Up of ZO-FAST. Cancer Res 2012;71:S1-3 [Google Scholar]
- 55.Coleman RE, Marshall H, Cameron D, et al. Breast-cancer adjuvant therapy with zoledronic acid. N Engl J Med 2011;365:1396-405 [DOI] [PubMed] [Google Scholar]
- 56.Paterson AH, Anderson SJ, Lembersky BC, et al. Oral clodronate for adjuvant treatment of operable breast cancer (National Surgical Adjuvant Breast and Bowel Project protocol B-34): a multicentre, placebo-controlled, randomised trial. Lancet Oncol 2012;13:734-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mobus V, Diel IJ, Harbeck H. GAIN Study: A Phase III Trial To Compare ETC vs. EC-TX and Ibandronate vs. Observation in Patients with Node-Positive Primary Breast Cancer-1st Interim Efficacy Analysis. Cancer Res 2012;71:S2-4 [Google Scholar]
- 58.Brufsky AM, Harker WG, Beck JT, et al. Final 5-year results of Z-FAST trial: adjuvant zoledronic acid maintains bone mass in postmenopausal breast cancer patients receiving letrozole. Cancer 2012;118:1192-201 [DOI] [PubMed] [Google Scholar]
- 59.Llombart A, Frassoldati A, Paija O, et al. Immediate Administration of Zoledronic Acid Reduces Aromatase Inhibitor-Associated Bone Loss in Postmenopausal Women With Early Breast Cancer: 12-month analysis of the E-ZO-FAST trial. Clin Breast Cancer 2012;12:40-8 [DOI] [PubMed] [Google Scholar]
- 60.Vidal L, Ben-Aharon I, Rizel S, et al. Bisphosphonates in the adjuvant setting of breast cancer therapy: Effect on survival--A systematic review and meta-analysis. J Clin Oncol 2012;30: abstr 548. [DOI] [PMC free article] [PubMed]
- 61.Steinman RA, Brufsky AM, Oesterreich S. Zoledronic acid effectiveness against breast cancer metastases-a role for estrogen in the microenvironment? Breast Cancer Res 2012;14:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu CC, Howard GA. Bone-cell changes in estrogen-induced bone-mass increase in mice: dissociation of osteoclasts from bone surfaces. Anat Rec 1991;229:240-50 [DOI] [PubMed] [Google Scholar]
- 63.Martini G, Gennari L, Merlotti D, et al. Serum OPG and RANKL levels before and after intravenous bisphosphonate treatment in Paget’s disease of bone. Bone 2007;40:457-63 [DOI] [PubMed] [Google Scholar]
- 64.Perifanis V, Vyzantiadis T, Tziomalos K, et al. Effect of zoledronic acid on markers of bone turnover and mineral density in osteoporotic patients with beta-thalassaemia. Ann Hematol 2007;86:23-30 [DOI] [PubMed] [Google Scholar]
- 65.Hofbauer LC, Schoppet M, Schüller P, et al. Effects of oral contraceptives on circulating osteoprotegerin and soluble RANK ligand serum levels in healthy young women. Clin Endocrinol (Oxf) 2004;60:214-9 [DOI] [PubMed] [Google Scholar]
- 66.Sheridan JP, Marsters SA, Pitti RM, et al. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science 1997;277:818-21 [DOI] [PubMed] [Google Scholar]
- 67.Neville-Webbe HL, Cross NA, Eaton CL, et al. Osteoprotegerin (OPG) produced by bone marrow stromal cells protects breast cancer cells from TRAIL-induced apoptosis. Breast Cancer Res Treat 2004;86:269-79 [DOI] [PubMed] [Google Scholar]
- 68.Reid P, Holen I.Pathophysiological roles of osteoprotegerin (OPG). Eur J Cell Biol 2009;88:1-17 [DOI] [PubMed] [Google Scholar]
- 69.Eaton CL, Wells JM, Holen I, et al. Serum osteoprotegerin (OPG) levels are associated with disease progression and response to androgen ablation in patients with prostate cancer. Prostate 2004;59:304-10 [DOI] [PubMed] [Google Scholar]
- 70.Jung K, Lein M, Ringsdorf M, et al. Diagnostic and prognostic validity of serum bone turnover markers in metastatic renal cell carcinoma. J Urol 2006;176:1326-31 [DOI] [PubMed] [Google Scholar]
- 71.MacFarlane M, Merrison W, Dinsdale D, et al. Active caspases and cleaved cytokeratins are sequestered into cytoplasmic inclusions in TRAIL-induced apoptosis. J Cell Biol 2000;148:1239-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Winter MC, Coleman RE. Bisphosphonates in the adjuvant treatment of breast cancer. Clin Oncol (R Coll Radiol) 2013;25:135-45 [DOI] [PubMed] [Google Scholar]