Introduction
The global epidemic of obesity continues to escalate despite the substantial rise in medical, governmental and public awareness of weight gain. Obesity is a multifactorial disorder of behaviour, genetics and environment. Many obese individuals suffer from several comorbidities, including type 2 diabetes mellitus, cardiovascular disease and cancer. Recently, the World Health Organization predicted that the global population in 2015 will comprise approximately 2.3 billion overweight adults and more than 700 million obese adults.1 The UK government predicts that according to current trends, 60% of all males and half of all females will be obese by 2050.2 As a result, the treatment of an expanding obese population carries a heavy burden on healthcare resources aimed at weight loss therapies, but also the associated comorbidities that also require additional costs.
Although a fundamental need for disease prevention is universally acknowledged, the increased prevalence of obesity now mandates the widespread application of disease treatments and interventions. Numerous medical treatments have been developed to promote weight loss although the majority have not demonstrated long term efficacy or safety, particularly for the morbidly obese population.3 Surgical strategies for weight loss have been in place for over half a century and are being considered with increased favour due to consistent success in providing sustained weight loss for the complex morbidly obese population. These operations that were initially titled as bariatric surgery have also demonstrated considerable benefits to cardiovascular outcomes, cancer risk and the systemic metabolism such that they can resolve type 2 diabetes mellitus in approximately 75% of morbidly obese patients so that now they are also termed as metabolic procedures.4–7 As a result, national and international healthcare associations such as the UK's National Institute for Health and Clinical Excellence (NICE), National Institutes of Health in the USA and the International Federation for the Surgery of Obesity advise surgery for patients with a body mass index (BMI) greater than 40 kg/m2, or a BMI >35 kg/m2 in addition to an associated comorbidity such as diabetes or hypertension which could be improved by weight loss.8 These international guidelines are continually reassessed to consider increasing evidence relating to performing these operations at lower weight categories,9 aimed at treating metabolic dysfunction in addition to excess weight. Currently, NICE also stipulates that: (1) all appropriate non-surgical measures have been tried but have failed to achieve or maintain adequate, clinically beneficial weight loss for at least 6 months; (2) the person has been receiving or will receive intensive management in a specialist obesity service; (3) the person is generally fit for anaesthesia and surgery; (4) the person commits to the need for long term follow-up; and (5) bariatric surgery is also recommended as a firstline option (instead of lifestyle interventions or drug treatment) for adults with a BMI of >50 kg/m2 in whom surgical intervention is considered appropriate.8
Annually, over 344 000 procedures are performed worldwide: 220 000 of these take place in the USA/Canada and 6000 are performed in the UK, with over 90% being performed laparoscopically.10 Of the multitude of surgical operations available, three are most widely accepted by the majority of bariatric/metabolic surgeons. These include: (1) Roux-en-Y gastric bypass; (2) adjustable gastric band; and (3) sleeve gastrectomy (a newer operation that can be performed as a standalone procedure or combined simultaneously, or prior to, a duodenal switch procedure). Each procedure offers distinctive effects on the resolution of obesity but also carries healthcare costs that reflect both the expected operative expenses on obese patients (with their unique geometry and increased thrombotic risk) and also the long term follow-up of these cases. Although some units report a 1 year mortality of 4.6%,11 a meta-analysis of 361 studies on 85 048 patients undergoing a wide spectrum of bariatric procedures revealed a perioperative (≤30 days) mortality of 0.28% and a 2 year postoperative mortality of 0.35%,12 which is further corroborated by a recent multicentre prospective study focusing on the perioperative safety of bariatric surgery.13
It has recently been demonstrated that only 2.5–33% of patients eligible for surgery receive bariatric procedures.2 The question therefore arises whether healthcare providers such as the National Health Service or private insurers can afford such a treatment strategy in the current climate of rigorous financial accountability and societal responsibility.
The financial cost of obesity
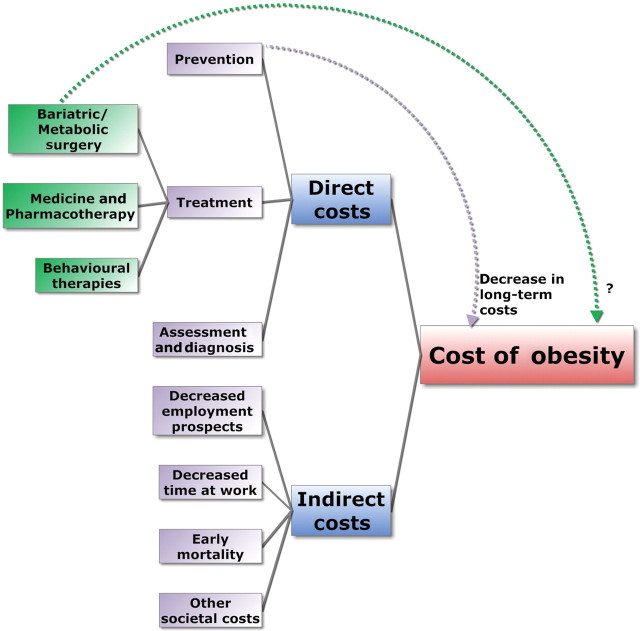
The cost of obesity can be evaluated through its direct and indirect effects on expenditure (figure 1). Direct costs include those of disease prevention, assessment, diagnosis and treatment. These include the cost of treating comorbidities such as diabetes, heart disease, hypertension, metabolic syndrome, sleep apnoea, cancer and joint degeneration. Indirect costs include lower economic output as a result of early mortality, decreased employment prospects and decreased time at work (due to illness). Furthermore, there are the added indirect expenses of larger public seating, reinforced wheelchairs and hospital beds, and even broader airplane seats.
Figure 1.
The cost of obesity.
In the UK, the direct costs of obesity have been estimated at £4.3 billion2 compared with £2.6 billion calculated from 1998 at the National Audit Office. The broader costs across 15 European Union (EU) states have been calculated at £28 billion,14 and for 25 EU states as £34 billion per year. The addition of overweight individuals to this calculation results in an overall cost of £68 billion pounds in the EU as a result of both the obese and overweight population.15 In the USA, the direct healthcare costs of obesity have been quoted as approximately £48 billion (US$75 billion) based on the 1998 Medical Expenditure Panel Survey model. This cost focused on the direct costs of obesity and is considered an underestimate as it does not include the indirect cost of obesity or the overheads associated with the pre-obese overweight population.16
It has been estimated that the direct cost of obesity can be as high as 2.6% of all healthcare costs in the EU17 (and up to 7% worldwide),18 corresponding to 0.9% of total gross domestic product in the EU and up to 1.2–1.4% of total gross domestic product in the USA.17
The direct costs of obesity represent the ‘tip of the iceberg’ as the indirect costs of obesity also carry a considerable burden. In England alone, the National Audit Office has estimated that national obesity has resulted in 18 million sick days and 31 000 deaths each year. This corresponds to approximately 40 000 lost years of working life associated with a decreased lifespan of approximately 9 years in the obese population.14
Direct and indirect costs of bariatric surgery
Offering patients bariatric surgery requires the establishment of specialist bariatric centres with inhouse multidisciplinary expertise. This includes metabolic physicians, gastroenterologists, endocrinologists and radiologists with an interest in obesity, dieticians, psychiatrists and psychologists, respiratory physicians with an interest in sleep apnoea, specialist nurses, bariatric surgeons and anaesthetists. These tertiary clinical units integrate services with primary and secondary care to achieve a cohesive treatment system for obese patients.
Many of the clinical facilities for bariatric patients can be offered through established hospital facilities and protocols; however, obese patients have some unique needs that can incur extra healthcare costs. These include specialist equipment such as longer operating instruments, and large hospital and operating beds. Furthermore, there are the added costs of potential reoperations and revisional surgery (including the problems of surgery for loose skin).
Different health systems have different costing pathways. The start-up costs for setting up a hypothetical bariatric practice in the US utilising Medicare reimbursement for 300 cases has been quoted to be as much as £285 000 ($444 592). The total reimbursement for such a practice would be £330 500 ($516 158), resulting in £45 500 pay for surgical expertise and subsequent practice investment.19 This is one example of financial expense in a private bariatric service and does not take into account variation of operations or clinical practice. For example, the gastric bypass procedure is considered more invasive than an adjustable gastric band in view of the requirement for operative enterotomies and anastomoses. Conversely, however, the gastric band has been demonstrated as more expensive in view of the larger number of necessary postoperative follow-ups to adjust band tightness.20
In the UK, funding bodies (the outgoing primary care trusts and likely the forthcoming NHS commissioning board and General Practice Consortia) pay for each patient to undergo surgery. Although they adhere to NICE guidelines, in view of funding considerations, they focus on more stringent criteria such as NORCOM (North Derbyshire County primary care trusts, South Yorkshire and Bassetlaw Commissioning Consortium), often only supporting surgery if the BMI is >45 with comorbidities, or >50 alone. The overall treatment cost paid to each hospital trust is approximately £5000–£10 000. The operative costs in the USA and UK have decreased by approximately 25%.10 21 This may have resulted from a switch in open operative practice towards laparoscopic surgery so that there are lower overall hospital costs (through decreased patient stay and complications) although the cost of disposable instruments is now conversely higher than open surgery. There is also a significant variation in disposable costs between different laparoscopic techniques.
Several studies have listed the cost comparisons of performing bariatric surgery versus the cost of treating patients medically (table 1). Although many of these studies were cohort studies without randomisation, the majority demonstrate a clear trend in decreasing the direct costs of clinical utilisation and medication expenditure. The financial recuperation of the initial payment for surgery would occur within 1–4 years in health systems within the USA, Canada and the UK. After this time, both the cumulative cost and annual cost of postsurgical patients is lower than the treatment costs for controls. One economic model predicted that if 25% of eligible UK patients were to undergo bariatric surgery, then the national cost saving could be as high as £1295 million. The studies assessing a surgical reversal of indirect costs of obesity such as a decrease in sick days and increased working potential did not universally demonstrate a significant improvement in costs but offered a positive trend in employment related attributes.
Table 1.
Cost comparison of patients undergoing bariatric procedures compared with controls
| Author/year/country/type of cost | Procedure | Economic data | No of surgical subjects | No of control subjects | Time period (years) | Cost comparison (surgical patients vs controls) |
|---|---|---|---|---|---|---|
| Näslund22 1991, Sweden (indirect cost) | RYGB, AGB, VBG | Questionnaire data | 79 | 54 | 5 | Higher employment, more working hours, fewer sick days and a higher income. Less medical care and higher quality of life |
| Martin23 1995, USA (direct cost) | RYGB | Cost per pound (lb) of weight loss analysis | 201 | 161 (very low calorie diet) | 6 | £500/lb vs £1000/lb |
| Narbro24 1999, Sweden (indirect cost) SOS Study | RYGB, AGB, VBG | Sick leave and disability pension analysis | 369 | 371 | 4 | Days of sickness tended to be lower in the surgical group (p=0.07) |
| Narbro25 2002, Sweden (direct cost) SOS Study | RYGB, AGB, VBG | Medication cost comparison | 510 | 455 | 6 | £80 vs £66 combined medication cost for diseases (per year). Although the relative drop in medication costs for the surgical candidates was much larger than controls. |
| Agren26 2002, Sweden (direct cost) SOS Study | RYGB, AGB, VBG | Cost comparison | 481 | 481 | 6 | There were no significant differences between the groups in number of hospital days or hospitalisation costs. |
| Agren27 2002, Sweden (direct cost) SOS Study | RYGB, AGB, VBG | Medication cost comparison | 279 (≥15% weight loss) | 410 (<5% weight loss) | 6 | £42 vs £87 combined medication cost for diabetes and cardiovascular disease (per year). |
| Gallagher28 2003, USA (direct cost) | RYGB | Cost comparison | 25 | 25 preoperative patients | 1 | £1800 vs £7000 (per year) |
| Sampalis29 2004, Canada (direct cost) | RYGB, VBG | Insurance utilisation costs | 1035 | 5746 | 5 | £12 vs £15.6 million per 1000 patients (cumulative) |
| Cost recovery from surgery at 3.5 years | ||||||
| Cremieux30 2008, USA (direct cost) | RYGB, AGB, VBG | Insurance utilisation costs | 3651 | 3651 | 4 | Cost recovery from surgery at 2–4 years |
| Makary31 2010, USA (direct cost) | RYGB, AGB, VBG, BPD | Insurance utilisation costs for diabetic medications | 2235 (type 2 diabetic patients) | 2235 (type 2 diabetic patients) preoperative patients | 3 | £2800 vs £4100 (per year). |
| Klein32 2010, USA (direct cost) | RYGB, AGB, VBG | Insurance utilisation costs | 808 (type 2 diabetic patients) | 808 (type 2 diabetic patients) | 3 | Cost recovery from surgery at 2.16 years |
| Office of Health Economics2 2010, UK (direct cost) | RYGB, AGB, SG | Economic model | Estimated 11 000–140 000 eligible for surgery | Estimated 11 000–140 000 (not receiving surgery) | 3 | If 5% undergo surgery: saving for first 3 years = £382 million |
| If 25% undergo surgery: saving for first 3 years = £1295 million | ||||||
| Cost recovery from surgery at 1 year | ||||||
| Finkelstein33 2011, USA (direct cost) | AGB | Insurance utilisation costs | >7000 | >7000 | 4 | £12 500 cost in the 90 days before and after the procedure |
| Cost recovery from surgery at just >2 years (diabetics) | ||||||
| Cost recovery from surgery at 4 years (non-diabetics) |
All costs converted to UK pounds in January 2011.
AGB, adjustable gastric banding; RYGB, Roux-en-Y gastric bypass; VBG, vertical banded gastroplasty.
Cost effectiveness of bariatric surgery
The analysis of pure financial cost advantage in relation to patient outcome does not always reflect the goal of healthcare strategies, which are ultimately aimed to preserve and improve the quality of life (QoL). There is now increasing evidence that bariatric surgery offers profound benefits not only to patient outcomes, but also QoL.34 35 Economic techniques that assess cost value in terms of particular outcomes are known as cost effectiveness analyses. A subgroup of these known as cost utility analyses consider QoL in their methodology. The outcome of a cost utility analysis is measured in terms of cost per quality adjusted life year (QALY). Traditionally, there has been a general consensus that the decisions to accept treatment modalities depend on whether the intervention offers a threshold of £20 000–£30 000/QALY36 (or $50 000 in the USA). Several studies using deterministic decision models have provided evidence that bariatric operations offer a cost per QALYS of between £2000 and £22 500 (table 2). There have been calls to increase the threshold range for national costs per QALY although the majority of bariatric economic models already offer a cost utility well within nationally agreed guidelines.37 Although surgery offers a consistent cost effectiveness, this has not yet been based on the highest levels of evidence set in the context of large scale randomised trials. As a result, the improved QoL and cost utility reported for these operations may have been derived from the selected subgroup of patients who have personally favoured or been offered surgery. Clearly there is an increased need for larger scale, better designed robust trials to formulate economic cost effectiveness models. These should include enhanced evidence regarding the cost effectiveness of these procedures on decreasing the risk of obesity associated comorbidities such as those for diabetes, cardiovascular disease, sleep apnoea and even cancer.4–7 38 39
Table 2.
Economic analysis of bariatric procedures
| Author/year/country | Procedure | Economic data | No of surgical subjects | No of control subjects | Time period (years) | Cost utility (cost per QALY) |
|---|---|---|---|---|---|---|
| Van Gemert40 1999, The Netherlands | VBG | Direct treatment costs | 21 | 21 | 2 | £3000 per QALY |
| Craig and Tseng41 2002, USA | RYGB | Deterministic decision model based on healthcare cost | NA | NA | NA | £3200–£10 200 per QALY (Women), £6500–£22 500 per QALY (Men) |
| Clegg42 43 2003, UK | RYGB, AGB, VBG, BPD | Deterministic decision model based on healthcare cost | NA | NA | NA | £11 000 per QALY |
| Jensen44 2004, USA | RYGB | Deterministic decision model based on healthcare cost | NA | NA | NA | £4500 per QALY |
| Salem45 2008, USA | RYGB, AGB | Deterministic decision model based on healthcare cost | NA | NA | Lifetime | Overall <£16 000 per QALY |
| £5600 AGB vs £9200 RYGB per QALY (women), £7300 AGB vs £11 600 RYGB per QALY (men) | ||||||
| Ikramuddin46 2009, USA | RYGB | Stochastic decision model CORE Diabetes Model using Monte Carlo simulation | 204 | 204 | 35 | £13 800 per QALY |
| £76 500 per QALY over short term (10 years) | ||||||
| Picot47 2009, UK | RYGB, AGB, VBG, BPD | Deterministic decision model based on healthcare cost | NA | NA | 20 | £2000 to £4000 per QALY |
| Campbell48 2010, USA (data based on European cohort) | RYGB, AGB | Stochastic decision model (Markov) based on healthcare cost | 51 | 51 | 5 | Overall <£16 000 per QALY |
| £3380 per QALY AGB vs £3500 per QALY RYGB |
All costs converted to UK pounds in January 2011.
AGB, adjustable gastric banding; BPD, biliopancreatic diversion; NA, not available; QALY, quality adjusted life year; RYGB, Roux-en-Y Gastric Bypass; VBG, vertical banded gastroplasty.
Limitations of current studies
Although many of the current economic models offer some important information regarding bariatric operations, they also carry several limitations. These derive from the absence of experimental evidence, the lack of like-with-like comparisons and the high level of heterogeneity and variation in current studies. Future studies will require increased precision to enhance the robustness of current models in order to decrease the uncertainty of current economic predictions. Calculating the surgical risk and costs of bariatric surgery are not dissimilar to that performed for screening in vascular disease or the risk of cerebrovascular accident/death after carotid surgery.49 50 In such cases, prospect economic models should define cost per QALY in relation to an acceptable postoperative mortality or morbidity.
Furthermore, there is an ongoing argument by obesity specialists that the existing economic analyses of obesity and bariatric surgery represent an example of a tendency to ‘medicalise’ society and its problems and challenges. Obesity is considered in an unquestioning way to be a disease that requires assessment, diagnosis and treatment, and that this conceptualisation of obesity does not give sufficient ‘front end’ analysis to the nature of obesity, related causal pathways and potential solutions. Future economic models require an increased depth of analysis to consider obesity in its broadest sense.
Conclusion
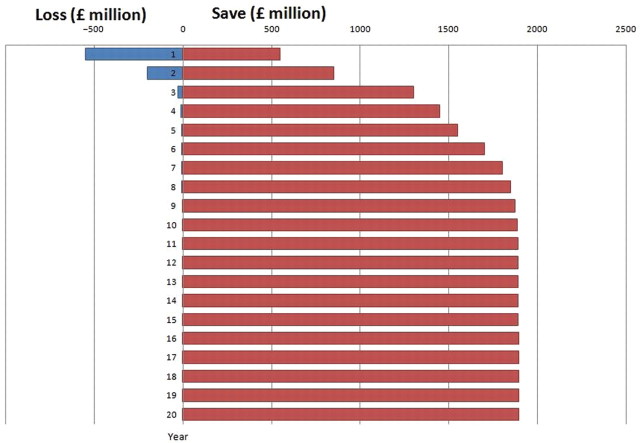
The progressing avalanche of global obesity will continue to challenge healthcare systems despite the worldwide necessity to reduce healthcare costs. Bariatric surgery is a successful intervention in the arsenal of obesity treatments, offering successful weight loss, decreased healthcare risks and in some cases bionic effects.38 Based on longitudinal trials and case series, bariatric surgery demonstrates powerful cost savings through direct cost comparisons and cost effectiveness studies. There is however a clear requirement to formulate economic models based on the highest levels of evidence, including well designed multicentre randomised control trials. Future studies should also incorporate high precision cost–benefit analyses to equip policy makers and clinicians with advanced decision making guidance that should increasingly incorporate patient focused outcomes such as patient reported outcome measures. The future for the management of obesity will continually require a strong preventive element; nevertheless, bariatric surgery can increasingly offer successful disease resolution and cost–benefit for an increasing proportion of obese, overweight and metabolically disordered patients. This will most likely increase in view of the continual evolution of operative techniques that currently include single incision laparoscopic surgery, endoscopic bypass procedures (such as the EndoBarrier system), transoral gastroplasty (such as the TOGA System) and the newer intragastric balloons. The cost effectiveness of bariatric surgery can result in an increased number of cases with commensurate cost savings (figure 2). The length of time for which bariatric procedures can be favoured will however depend on our ability to develop safer, cheaper and more efficacious antiobesity alternatives.
Figure 2.
Hypothetical projection of the cost–benefit of bariatric surgery to UK society over 20 years.
Footnotes
Funding: The authors are grateful for support from the Wellcome Trust and the NIHR Biomedical Research Centre Funding Scheme.
Competing interests: None.
Provenance and peer review: Commissioned; externally peer reviewed.
References
- 1.World Health Organization. Obesity and overweight—Fact Sheet No 311, 2006. http://www.who.int/mediacentre/factsheets/fs311/en/ (accessed 12 February 2011).
- 2.Office of Health Economics. Shedding the pounds: obesity management, NICE guidance and bariatric surgery in England. London: Office of Health Economics, 2010. [Google Scholar]
- 3.Knowler WC, Fowler SE, Hamman RF, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009;374:1677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashrafian H, Athanasiou T, Li JV, et al. Diabetes resolution and hyperinsulinaemia after metabolic Roux-en-Y gastric bypass. Obes Rev 2010. Epub ahead of print 29 September 2010. doi: 10.1111/j.1467-789X.2010.00802.x. [DOI] [PubMed] [Google Scholar]
- 5.Ashrafian H, Bueter M, Ahmed K, et al. Metabolic surgery: an evolution through bariatric animal models. Obes Rev 2010;11:907–20. [DOI] [PubMed] [Google Scholar]
- 6.Ashrafian H, le Roux CW, Darzi A, et al. Effects of bariatric surgery on cardiovascular function. Circulation 2008;118:2091–102. [DOI] [PubMed] [Google Scholar]
- 7.Ashrafian H, Ahmed K, Rowland SP, et al. Metabolic surgery and cancer: protective effects of bariatric procedures. Cancer 2010. Epub ahead of print 29 November 2010, PMID: 21117217. [DOI] [PubMed] [Google Scholar]
- 8.National Institute for Health and Clinical Excellence. Obesity: NICE Clinical Guideline 43. London: NICE, 2006. [Google Scholar]
- 9.Shah SS, Todkar JS, Shah PS, et al. Diabetes remission and reduced cardiovascular risk after gastric bypass in Asian Indians with body mass index <35 kg/m2. Surg Obes Relat Dis 2010;6:332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2008. Obes Surg 2009;19:1605–11. [DOI] [PubMed] [Google Scholar]
- 11.Flum DR, Salem L, Elrod JA, et al. Early mortality among Medicare beneficiaries undergoing bariatric surgical procedures. JAMA 2005;294:1903–8. [DOI] [PubMed] [Google Scholar]
- 12.Buchwald H, Estok R, Fahrbach K, et al. Trends in mortality in bariatric surgery: a systematic review and meta-analysis. Surgery 2007;142:621–32. [DOI] [PubMed] [Google Scholar]
- 13.Flum DR, Belle SH, King WC, et al. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med 2009;361:445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fry J, Finley W. The prevalence and costs of obesity in the EU. Proc Nutr Soc 2005;64:359–62. [DOI] [PubMed] [Google Scholar]
- 15.Commission of the European Communities. Impact Assessment Report: A Strategy for Europe on Nutrition, Overweight and Obesity Related Health Issues. Brussels: Commission of the European Communities, 2007. [Google Scholar]
- 16.Finkelstein EA, Fiebelkorn IC, Wang G. State-level estimates of annual medical expenditures attributable to obesity. Obes Res 2004;12:18–24. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. The challenge of obesity in the WHO European Region and the strategies for response. Copenhagen: WHO Regional Office for Europe, 2007. [Google Scholar]
- 18.World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 2000;894:78–99. [PubMed] [Google Scholar]
- 19.Madan AK, Powelson JE, Tichansky DS. Cost analysis of laparoscopic gastric bypass practice using current Medicare reimbursement and practice costs. Surg Obes Relat Dis 2008;4:131–6. [DOI] [PubMed] [Google Scholar]
- 20.Livingston EH. Hospital costs associated with bariatric procedures in the United States. Am J Surg 2005;190:816–20. [DOI] [PubMed] [Google Scholar]
- 21.Angus LD, Cottam DR, Gorecki PJ, et al. DRG, costs and reimbursement following Roux-en-Y gastric bypass: an economic appraisal. Obes Surg 2003;13:591–5. [DOI] [PubMed] [Google Scholar]
- 22.Näslund I I, Ågren G. Social and economic effects of bariatric surgery. Obes Surg 1991;1:137–40. [DOI] [PubMed] [Google Scholar]
- 23.Martin LF, Tan TL, Horn JR, et al. Comparison of the costs associated with medical and surgical treatment of obesity. Surgery 1995;118:599–606. [DOI] [PubMed] [Google Scholar]
- 24.Narbro K, Agren G, Jonsson E, et al. Sick leave and disability pension before and after treatment for obesity: a report from the Swedish Obese Subjects (SOS) study. Int J Obes Relat Metab Disord 1999;23:619–24. [DOI] [PubMed] [Google Scholar]
- 25.Narbro K, Agren G, Jonsson E, et al. Pharmaceutical costs in obese individuals: comparison with a randomly selected population sample and long-term changes after conventional and surgical treatment: the SOS intervention study. Arch Intern Med 2002;162:2061–9. [DOI] [PubMed] [Google Scholar]
- 26.Agren G, Narbro K, Jonsson E, et al. Cost of in-patient care over 7 years among surgically and conventionally treated obese patients. Obes Res 2002;10:1276–83. [DOI] [PubMed] [Google Scholar]
- 27.Agren G, Narbro K, Näslund I, et al. Long-term effects of weight loss on pharmaceutical costs in obese subjects. A report from the SOS intervention study. Int J Obes Relat Metab Disord 2002;26:184–92. [DOI] [PubMed] [Google Scholar]
- 28.Gallagher SF, Banasiak M, Gonzalvo JP, et al. The impact of bariatric surgery on the Veterans Administration healthcare system: a cost analysis. Obes Surg 2003;13:245–8. [DOI] [PubMed] [Google Scholar]
- 29.Sampalis JS, Liberman M, Auger S, et al. The impact of weight reduction surgery on health-care costs in morbidly obese patients. Obes Surg 2004;14:939–47. [DOI] [PubMed] [Google Scholar]
- 30.Cremieux PY, Buchwald H, Shikora SA, et al. A study on the economic impact of bariatric surgery. Am J Manag Care 2008;14:589–96. [PubMed] [Google Scholar]
- 31.Makary MA, Clarke JM, Shore AD, et al. Medication utilization and annual health care costs in patients with type 2 diabetes mellitus before and after bariatric surgery. Arch Surg 2010;145:726–31. [DOI] [PubMed] [Google Scholar]
- 32.Klein S, Ghosh A, Cremieux PY, et al. Economic impact of the clinical benefits of bariatric surgery in diabetes patients with BMI ≥35 kg/m(2). Obesity (Silver Spring) 2010 Epub ahead of print 9 September 2010, PMID: 20829800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finkelstein EA, Allaire BT, Burgess SM, et al. Financial implications of coverage for laparoscopic adjustable gastric banding. Surg Obes Relat Dis 2010. Epub ahead of print 29 October 2010, PMID 21195677. [DOI] [PubMed] [Google Scholar]
- 34.Livingston EH, Fink AS. Quality of life: cost and future of bariatric surgery. Arch Surg 2003;138:383–8. [DOI] [PubMed] [Google Scholar]
- 35.Cawley J, Prinz T, Beane S. Health insurance claims data as a means of assessing reduction in co-morbidities 6 months after bariatric surgery. Obes Surg 2006;16:852–8. [DOI] [PubMed] [Google Scholar]
- 36.National Institute for Health and Clinical Excellence. Guide to the methods of technology appraisal. London: NICE, 2008. [PubMed] [Google Scholar]
- 37.Towse A. Should NICE's threshold range for cost per QALY be raised? Yes. BMJ 2009;338:b181. [DOI] [PubMed] [Google Scholar]
- 38.Ashrafian H, Darzi A, Athanasiou T. Autobionics: a new paradigm in regenerative medicine and surgery. Regen Med 2010;5:279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ashrafian H, le Roux CW. Metabolic surgery and gut hormones—a review of bariatric entero-humoral modulation. Physiol Behav 2009;97:620–31. [DOI] [PubMed] [Google Scholar]
- 40.van Gemert WG, Adang EM, Kop M, et al. A prospective cost-effectiveness analysis of vertical banded gastroplasty for the treatment of morbid obesity. Obes Surg 1999;9:484–91. [DOI] [PubMed] [Google Scholar]
- 41.Craig BM, Tseng DS. Cost-effectiveness of gastric bypass for severe obesity. Am J Med 2002;113:491–8. [DOI] [PubMed] [Google Scholar]
- 42.Clegg A, Colquitt J, Sidhu M, et al. Clinical and cost effectiveness of surgery for morbid obesity: a systematic review and economic evaluation. Int J Obes Relat Metab Disord 2003;27:1167–77. [DOI] [PubMed] [Google Scholar]
- 43.Colquitt J, Clegg A, Sidhu M, et al. Surgery for morbid obesity. Cochrane Database Syst Rev 2003;4:CD003641. [DOI] [PubMed] [Google Scholar]
- 44.Jensen C, Flum DR. The costs of nonsurgical and surgical weight loss interventions: is an ounce of prevention really worth a pound of cure? Surg Obes Relat Dis 2005;1:353–7. [DOI] [PubMed] [Google Scholar]
- 45.Salem L, Devlin A, Sullivan SD, et al. Cost-effectiveness analysis of laparoscopic gastric bypass, adjustable gastric banding, and nonoperative weight loss interventions. Surg Obes Relat Dis 2008;4:26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ikramuddin S, Klingman D, Swan T, et al. Cost-effectiveness of Roux-en-Y gastric bypass in type 2 diabetes patients. Am J Manag Care 2009;15:607–15. [PubMed] [Google Scholar]
- 47.Picot J, Jones J, Colquitt JL, et al. The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health Technol Assess 2009;13:1–190, 215–357, iii–iv. [DOI] [PubMed] [Google Scholar]
- 48.Campbell J, McGarry LA, Shikora SA, et al. Cost-effectiveness of laparoscopic gastric banding and bypass for morbid obesity. Am J Manag Care 2010;16:e174–87. [PubMed] [Google Scholar]
- 49.Guilhaume C, Saragoussi D, Cochran J, et al. Modeling stroke management: a qualitative review of cost-effectiveness analyses. Eur J Health Econ 2010;11:419–26. [DOI] [PubMed] [Google Scholar]
- 50.Ross Naylor A. Known knowns, known unknowns and unknown unknowns: a 2010 update on carotid artery disease. Surgeon 2010;8:79–86. [DOI] [PubMed] [Google Scholar]