Highlights
► High dietary vitamin D was able to prevent premalignant lesions caused by AOM/DSS. ► Increasing vitamin D intake raised serum 25-D3 levels reaching a plateau ≥1000 IU/kg. ► Serum 25-D3 levels over 30 ng/ml are needed to prevent tumorigenesis.
Keywords: Vitamin D, Colorectal cancer, Dysplasia, Chemoprevention, cyp24a1, cyp27b1
Abstract
Colorectal cancer (CRC) is one of the leading causes of cancer morbidity and mortality in Western countries. One of the risk factors for colorectal tumorigenesis is vitamin D insufficiency.
The aim of this study was to establish whether increasing dietary vitamin D intake can prevent or delay development of chemically induced preneoplastic lesions in the colon of mice.
We fed six weeks old female C57BL/6 J mice (n = 28) with increasing vitamin D3 concentrations (100, 400, 1000, 2500, 5000 IU/kg diet). To induce dysplasia, a preneoplastic lesion, we injected mice with the carcinogen azoxymethane (10 mg/kg) intraperitoneally, followed by three cycles of 2% dextran sodium sulfate salt, a tumor promoter, in the drinking water.
To test our hypothesis that high vitamin D intake prevents formation of preneoplastic lesions, we have investigated the effect of increasing dietary vitamin D on development of premalignant colorectal lesions, serum 25-hydroxyvitamin D3 (25-D3) levels, and expression of renal vitamin D system genes.
Dietary vitamin D concentration correlated inversely with dysplasia score (Spearman's correlation coefficient, ρ: −0.579, p = 0.002) and positively with serum 25-D3 levels (ρ: 0.752, p = 0.001). Increasing dietary vitamin D concentration beyond 1000 IU/kg led to no further increase in circulating 25-D3 levels, while the dysplasia score leveled out at ≥2500 IU/kg vitamin D. High dietary vitamin D intake led to increased renal mRNA expression of the vitamin D catabolizing enzyme cyp24a1 (ρ: 0.518, p = 0.005) and decreased expression of the vitamin D activating enzyme cyp27b1 (ρ: −0.452, p = 0.016), protecting the body from toxic serum levels of the active vitamin D metabolite 1,25-dihydroxyvitamin D3 (1,25-D3).
Our data showed that increasing dietary vitamin D intake is able to prevent chemically induced preneoplastic lesions. The maximum impact was achieved when the mice consumed more than 2500 IU vitamin D/kg diet.
This article is part of a Special Issue entitled ‘Vitamin D Workshop’.
1. Introduction
Colorectal cancer is one of the most common cancers, therefore, its primary and secondary prevention is of extreme importance [1]. There is evidence that vitamin D is an effective chemopreventive agent against colorectal cancer. While human interventional studies [2–6] are less conclusive regarding tumor protective effects of vitamin D, animal studies [7–12] are more clear-cut.
High dietary vitamin D intake as well as treatment with 1,25-dihydroxyvitamin D3 (1,25-D3) or its analogues led to fewer or smaller tumors or even prevented formation of colonic tumors in several rodent models. This effect was observed irrespective of whether the tumor was caused by nutrition (Western-style diet, [7]), genetic risk factors (Apcmin/+ mice [8]), or was chemically induced with azoxymethane (AOM)/dextran sulfate sodium salt (DSS) [9,10], 1,2-dimethylhydrazine [11], or dexamethasone [12]. Using the active vitamin D metabolite, 1,25-D3, might cause hypercalcemia, therefore it is less useful in prevention strategies.
In the present study we investigated the effect of increased dietary vitamin D intake on progress of colorectal lesions in a mouse model of chemically induced colonic tumorigenesis. We aimed to determine the lowest vitamin D concentration that is able to prevent or delay the development of premalignant lesions. Our results showed that increasing dietary vitamin D intake reduced significantly the dysplasia score in mice treated with AOM and DSS and this reduction correlated significantly with serum 25-hydroxyvitamin D3 (25-D3) levels.
2. Materials and methods
2.1. Animals
Six weeks old female1 C57BL/6 J mice (Charles River, Sulzfeld, Germany) were housed in controlled environment in the animal facility of the Institute of Pathophysiology and Allergy Research at the Medical University of Vienna with a 12 h light–dark cycle. Living conditions and experiments were conducted according to the European Union Regulations on Care and Use of Laboratory Animals. The study was approved by the Ethics Committee of the Medical University of Vienna (Nr. 66.009/0245-II/36/2010).
Upon arrival, the animals were separated into five diet groups (six mice per group) receiving AIN-93G diet containing 100, 400, 1000, 2500 or 5000 IU vitamin D3/kg diet (LASvendi, Soest, Germany).
2.2. Model of chemically induced tumorigenesis
After 2.5 weeks of acclimatization to the diet, 8.5 weeks old mice were injected once with 10 mg/kg AOM (Sigma Aldrich, St. Louis, MO, USA) intraperitoneally (day 1) to induce tumorigenesis. We treated the mice for three 4-day cycles (days: 5–8, 26–29, and 47–50) with 2% DSS (MP Biomedicals, Solon, OH, USA) dissolved in the drinking water as tumor promoter. On day 64, mice were anaesthetized, blood was collected, centrifuged and the serum was stored at −20 °C until analysis. Mice were killed by cervical dislocation, kidneys were removed and immediately shock frozen in liquid nitrogen. The colon was removed, washed with ice-cold PBS and 4% formalin and rolled into a plane spiral (so-called Swiss roll [13]). The Swiss rolls were fixed in 4% buffered formalin, dehydrated, and paraffin embedded.
2.3. Serum parameters
Serum 25-D3 was determined using a chemiluminescence assay (IDS, iSYS 25(OH)D; Immunodiagnostic systems Ltd, Boldon, UK) on an IDS-iSYS multi-discipline automated analyser. Within-day coefficients of variation were 5.5–12.1% and inter-day coefficients of variation were 8.9–16.9%, respectively.
Calcium was measured using the COBAS 8000 Modular Analyser (F. Hoffmann-La Roche AG, Basel, Switzerland) according to the manufacturer's instructions.
2.4. RNA extraction and reverse transcription (RT)
Total renal RNA was isolated with TRIzol reagent (Invitrogen, Grand Island, NY, USA) according to the manufacturer's instructions. Integrity of the RNA was assessed on agarose gels by staining with Gel Red (Biotium, Hayward, CA, USA). 2 μg of total RNA were reverse transcribed using RevertAid H Minus Reverse Transcriptase and Random Hexamer Primers (Fermentas, Ontario, Canada) as described before [14].
Quantitative real time RT-PCR (qRT-PCR) was performed using Power SYBR Green Master Mix (Applied Biosystems, Carlsbad, CA, USA) on the ABI StepOnePlus Real-Time PCR System (Applied Biosystems) in duplicates. PCR conditions were 10 min 95 °C, 40 cycles of 15 s 95 °C and 60 °C 60 s. Relative expression of the target genes was normalized to the expression of the housekeeping gene: eukaryotic translation elongation factor 1 β 2 (eef1β2), set relative to the calibrator, Mouse Colon Total RNA (Clontech, Mountain View, CA, USA), and calculated with the ΔΔCt method. Primer sequences are stated in Table 1.
Table 1.
Primer pairs used in the qRT-PCR, oligonucleotide sequence 5′ → 3′.
| Primer | Forward | Reverse |
|---|---|---|
| cyp24a1 | GAGTCCATGAGGCTTACCC | GTGTATTCACCCAGAACGG |
| cyp27b1 | GTGTTGAGATTGTACCCTGTG | GGGAGACTAGCGTATCTTGG |
| eef1β2 | AGAGCTACATTGAGGGGTACG | GACTTGATGTGATTATACCAACGTAG |
| vdr | GGATCTGTGGAGTGTGTGGAGACC | CTTCATCATGCCAATGTCCAC |
2.5. Histological examination of colon sections
Sections (4 μm) of paraffin-embedded colons were stained with Mayer's Hematoxylin Solution and Eosin (Sigma Aldrich), and images of whole sections were acquired using TissueFAXS 2.04 (TissueGnostics GmbH, Vienna, Austria). A pathologist identified the dysplastic regions in the colon sections. The size of the areas with low grade and high grade dysplasia were determined with the HistoQuest software 3.03 (TissueGnostics GmbH), and calculated as percentage of the examined epithelial area. Dysplasia score was calculated based on the method established by Riddell et al. [15]. In short, low grade dysplasia was scored 2, high grade dysplasia was scored 3. These scores were multiplied by the percentage of the affected region of the examined epithelial area. Since the use of Swiss roles enabled us to examine the cross-section of the entire colon, and to distinguish between reactive changes and dysplasia with a high degree of accuracy, we excluded the category “indefinite dysplasia” from our analysis. Detection of Ki67 positivity was performed on sections of paraffin-embedded colons as previously described [16]. Rabbit anti Ki67 (1:200, Novus Biologicals, Littelton, CO, USA) was used as the primary antibody and X-Cell Plus HRP (Menarini, Florence, Italy) was used as the detection system. Whole slide images were scanned using a Scanscope® scanner (Aperio, Vista, CA, USA). The Positive Pixel Count Algorithm of the ImageScope software (Aperio) was used to quantify the positive signal.
2.6. Statistical analysis
We calculated Spearman's correlation coefficient (ρ) with two-tailed significance levels and determined the medians, because data were not normally distributed and the number of animals per group was too small for group comparison analysis. Statistical analysis was performed using the SPSS statistics package, version 18.0, graphs were made using GraphPadPrism 5.0.
3. Results
3.1. Effect of dietary vitamin D intake on AOM/DSS-mediated tumorigenesis
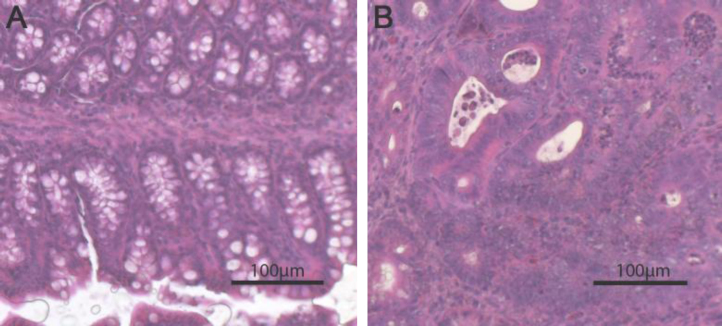
Mice fed diets with low vitamin D concentrations (≤1000 IU/kg) showed more and further progressed dysplastic regions in the colon compared with mice fed high amounts of vitamin D (Figs. 1A and 2 and Supplementary Fig. 1).
Fig. 1.
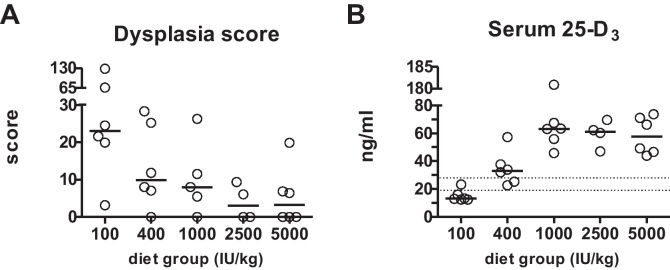
High dietary vitamin D decreases dysplasia score and increases serum 25-D3 levels. (A) Effect of diets containing increasing amounts of vitamin D on dysplasia score. Score was calculated by adding percentage of low dysplasia region multiplied by 2 and percentage of high dysplasia regions multiplied by 3. Circles represent colonic dysplasia scores of individual mice, horizontal lines represent median. (B) Effect of vitamin D content of the diet on serum 25-D3 levels. Horizontal lines represent median. Discontinous lines are the borders of vitamin D deficiency (<20 ng/ml), and sufficiency (≥30 ng/ml).
Fig. 2.
High dietary vitamin D prevents preneoplastic changes in colon of mice. Hematoxylin–Eosin staining of (A) normal colon of a mouse fed with a diet containing 5000 IU/kg and (B) colon with high grade dysplasia from a mouse fed 100 IU vitamin D/kg diet.
In order to understand the impact of dietary vitamin D intake and serum 25-D3 levels on AOM/DSS-induced tumorigenesis, we determined the size of low and high grade dysplasia, and calculated the dysplasia score. Due to the low sample size we were not able to perform group comparisons, however we assessed the strength of the association among vitamin D intake or serum 25-D3 levels, and the size of dysplasia or the dysplasia score by calculating Spearman‘s correlation coefficient. Closer the value of this coefficient is to 1, the stronger the association is. Positive values indicate a direct association, negative values an inverse correlation.
In our study the average size of low grade dysplasia correlated inversely with dietary vitamin D concentration (ρ: −0.593, p = 0.001) and serum 25-D3 levels (ρ: −0.666, p = 0.001, Table 2). Regions of high grade dysplasia were only found in mice fed with vitamin D concentrations ≤1000 IU/kg. Dysplasia score calculation revealed a clear decrease of dysplastic lesions in colons of mice fed vitamin D concentrations ≥2500 IU/kg compared with mice fed a diet containing 100, 400 or 1000 IU vitamin D/kg (Fig. 1A). The dysplasia score showed negative correlation with dietary vitamin D (ρ: −0.579, p = 0.002) and serum 25-D3 levels (ρ: −0.618, p = 0.001, Table 2).
Table 2.
Summary of Spearman's correlation analysis.
| cyp24a1 | cyp27b1 | 25-D3 | Low grade dysplasia | Dysplasia score | ||
|---|---|---|---|---|---|---|
| Diet group | Correlation coefficient | 0.518b | −0.452a | 0.752b | −0.593b | −0.579b |
| Sig. (2-tailed) | 0.005 | 0.016 | 0.001 | 0.001 | 0.002 | |
| N | 28 | 28 | 28 | 27 | 27 | |
| Serum 25-D3 | Correlation coefficient | 0.313 | −0.419a | 1.0000 | −0.666b | −0.618b |
| Sig. (2-tailed) | 0.105 | 0.026 | 28 | 0.001 | 0.001 | |
| N | 28 | 28 | 27 | 27 | ||
Correlation is significant at the 0.05 level (2-tailed).
Correlation is significant at the 0.01 level (2-tailed).
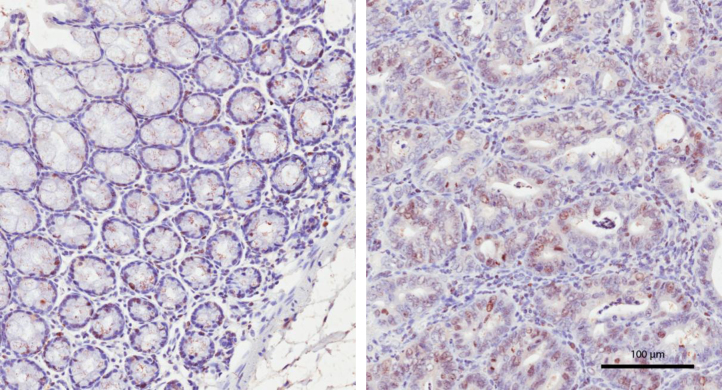
The expression of the proliferation marker Ki67 in the whole colon section was lower in mice fed with 100 IU/kg vitamin D compared with other diet groups. However, a marked increase in Ki67 expression was observed in all regions with high grade dysplasia when compared with non-dysplasic regions of the same animal (Fig. 3).
Fig. 3.
Ki67 protein expression is increased in dysplastic region compared with normal mucosa. Immunohistochemistry of a colon from a mouse fed with 100 IU/kg vitamin D. Proliferation marker Ki67 was highly expressed in dysplastic region (right panel) compared with the adjacent normal mucosa (left panel) of the same mouse.
3.2. Effect of vitamin D intake on serum parameters in mice treated with AOM/DSS
Serum 25-D3 concentration increased with increasing vitamin D intake, reaching a plateau from 1000 IU vitamin D/kg diet onwards (Fig. 1B). The reference values for serum 25-D3 in humans define vitamin D deficiency as <20 ng/ml, levels between 21 and 29 ng/ml as vitamin D insufficiency and >30 ng/ml as sufficiency [17]. According to these values, 83.3% of the mice receiving 100 IU vitamin D/kg diet were vitamin D deficient. One animal in this group (16.7%) and two in the 400 IU/kg group (33.4%) were vitamin D insufficient. All the other animals could be considered to have adequate 25-D3 levels.
Serum calcium concentrations remained relatively stable and did not correlate with serum 25-D3 levels (data not shown). Systemic levels of the active 1,25-D3 are regulated by the kidneys; therefore, we measured gene expression of the vitamin D activating enzyme 25-hydroxyvitamin D3 1α-hydroxylase (cyp27b1), the vitamin D degrading enzyme 1,25-hydroxyvitamin D3 24-hydroxylase (cyp24a1), and the vitamin D receptor (vdr) by qRT-PCR. High dietary vitamin D intake increased cyp24a1 (ρ: 0.518, p = 0.005) and inhibited cyp27b1 (ρ: −0.452, p = 0.016) gene expression. Furthermore, cyp27b1 expression correlated negatively with serum 25-D3 levels (ρ: −0.419, p = 0.026, Fig. 4, Table 2). The expression of vdr was not influenced either by diet or serum 25-D3 levels.
Fig. 4.
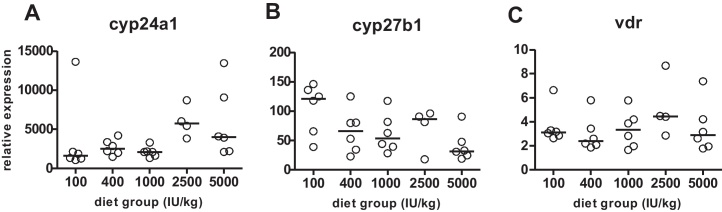
High dietary vitamin D increases cyp24a1, decreases cyp27b1 and does not affect vdr expression. Effect of increasing vitamin D concentration in the diet on renal cyp24a1 (A), cyp27b1 (B) and vdr (C) expression. Circles represent relative expression of individual mice, horizontal lines represent median.
4. Discussion
The aim of this study was to find the appropriate (lowest) dietary vitamin D level that would prevent formation of premalignant lesions in a mouse model of chemically induced colonic tumorigenesis. To our knowledge this is the first study investigating the effect of dose-dependency of vitamin D intake on preventing AOM/DSS-induced colonic tumorigenesis in mice. We could show unequivocally that increasing vitamin D intake was able to delay formation of chemically induced low grade preneoplastic lesions and even prevent development of high grade dysplasia in the colon. We observed a very clear inverse correlation between dysplasia score and higher vitamin D intake as well as serum 25-D3 levels. Our data support the hypothesis that increasing vitamin D in the diet has chemopreventive effects on chemically induced precursors of colorectal tumors in mice. This effect seems to plateau at an intake of 2500 IU vitamin D/kg diet or higher.
The effect of vitamin D, its metabolites and vitamin D analogues on colonic tumorigenesis in mice was investigated in several studies, however, the results are contradictory. Majority of the studies that were not able to show any preventing or delaying effect of vitamin D and its analogues on colorectal tumorigenesis used vitamin D-sufficient control groups. In these studies, control diets contained 1000 IU/kg vitamin D [18,19], a level that is probably already chemopreventive. Our study, similar to the data presented by Fleet et al. [20], shows that 1000 IU vitamin D/kg diet provides 25-D3 serum levels substantially higher than 30 ng/ml. Nevertheless there were reports for the effectiveness of vitamin D even compared with standard control diet, e.g. the study by Fichera et al. [9] showing that the 1,25-D3 analogue Ro26-2198 given for 28 days reduced colonic dysplasia in mice even compared with controls receiving standard AIN-76A chow containing 1000 IU vitamin D/kg diet.
In most of the studies demonstrating that high vitamin D intake reduced colorectal tumorigenesis, the control diets contained ≤500 IU vitamin D/kg diet [7,8,11]. In the study of Xu et al., Apcmin/+ mice were fed a vitamin D deficient diet and then received either 0.33 μg/kg 1,25-D3, the same amount of the analogue QW, or the analogue BTW intraperitoneally three times a week. The number of aberrant crypt foci (ACF, a tumor precursor) as well as crypt multiplicity were reduced in each of the three groups compared with Apcmin/+ mice injected with vehicle only [8]. The formation of colonic tumors caused by the so-called new Western-style diet (NWD, mimicking dietary habits of Western populations, e.g. increased fat content, decreased vitamin D and calcium) could be prevented by supplementing the NWD with vitamin D (2300 IU/kg diet instead of 110 IU/kg) and calcium (7000 mg/kg [7]). Similar results were observed in a study by Mokady, who compared 1,2-dimethylhydrazine (DMH)-caused colonic tumorigenesis in rats fed either a stress diet (more calories, 500 IU/kg vitamin D, less calcium, less phosphorus), or a stress diet supplemented with 2000 IU/kg vitamin D. All DMH-treated rats developed colonic tumors, however, multiplicity of adenocarcinomas was reduced significantly in the rats fed the vitamin D supplemented stress diet compared with rats fed the stress diet alone [11].
The vitamin D concentration used in most laboratory rodent chow (1000 IU/kg, corresponding to 500 IU/day intake in a 2000 kcal human diet) lies between the estimated average requirement (400 IU/day) and the recommended dietary allowance (600 IU/day) of the Institute of Medicine [17,21]). In our study we examined the effect of both lower (100 and 400 IU/kg) and higher (2500 and 5000 IU/kg) vitamin D intake on colon tumorigenesis. It is now accepted that the tumor preventive effect of high vitamin D intake is hindered by low calcium intake. In our study the diet contained 0.51% calcium, which should be enough to further the tumor preventive effect of vitamin D.
Fleet et al. [20] observed a steep curvilinear rise in serum 25-D3 levels in mice fed with increasing doses of vitamin D in the diet. Interestingly, in our study serum 25-D3 levels increased with increasing vitamin D intake from 100 to 1000 IU/kg, but then reached a plateau. Further increasing vitamin D intake to 2500 or even 5000 IU had no significant effect on serum 25-D3 levels. This observation might be due to the effect of AOM and DSS on the liver of the mice. Both substances are highly toxic and were shown to impair liver function in rodents [22,23]. The liver is the main organ responsible for the synthesis of 25-D3 [24], therefore any damage may lead to lower circulating 25-D3 levels [25]. We presume that the plateau seen in serum 25-D3 levels in mice receiving >1000 IU/kg vitamin D is a consequence of the incapability of the liver to cope with higher vitamin D intakes, due to the damages caused by AOM and DSS.
Enhancing vitamin D concentrations in the diet led to increased renal cyp24a1 and decreased cyp27b1 gene expression. These changes are likely to result in a lower conversion rate of 25-D3 to the hormonally active 1,25-D3 and in an enhanced degradation of both 25-D3 and 1,25-D3 [20]. Due to this negative feedback mechanism, high concentrations of serum 1,25-D3 are prevented.2 Changes in cyp24a1 and cyp27b1 expression and activity were described also by Akeno et al., although in that study the vitamin D-deficient diet contained less calcium and phosphorus than the sufficient diet [12], which might have also affected renal gene expression. Dietary vitamin D had not affected vdr gene expression, confirming data from other groups [12,20].
We could show that dietary vitamin D intake higher than 2500 IU/kg diet delayed formation of premalignant lesions, such as colonic dysplasia, in mice. Based on our data we conclude that serum 25-D3 levels higher than 65 ng/ml would be necessary to delay or prevent colorectal tumorigenesis in a chemically induced colon cancer model in mice. Whether this value can be translated to humans is not clear as yet, but it suggests that for cancer prevention the desirable serum 25-D3 levels are in the upper “normal” reference range. Our study underlines the significance of vitamin D as a promising chemopreventive agent.
Acknowledgement
This work has been supported by the Austrian Science Fund, project nr. P22200-B11.
Footnotes
Male mice were much more sensitive to AOM, more than half of them died shortly after AOM treatment.
Serum volume was insufficient to measure 1,25-D3 levels as well.
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jsbmb.2012.09.003.
Contributor Information
Doris Maria Hummel, Email: doris.hummel@meduniwien.ac.at.
Ursula Thiem, Email: ursula.thiem@meduniwien.ac.at.
Julia Höbaus, Email: julia.hoebaus@meduniwien.ac.at.
Ildiko Mesteri, Email: ildiko.mesteri@meduniwien.ac.at.
Lukas Gober, Email: a0603006@unet.univie.ac.at.
Caroline Stremnitzer, Email: caroline.stremnitzer@meduniwien.ac.at.
João Graça, Email: joao.graca@astrazeneca.com.
Barbara Obermayer-Pietsch, Email: barbara.obermayer@medunigraz.at.
Enikö Kallay, Email: enikoe.kallay@meduniwien.ac.at.
Appendix A. Supplementary data
The following is the supplementary data to this article:
Supplementary Fig. 1. Increasing amounts of dietary vitamin D lead to reduced dysplasia. Hematoxylin-Eosin staining of representative colon sections of mice fed with either 100, 400, 1000, 2500, or 5000 IU/kg. Bars equal 50 μm.
References
- 1.Jemal A., Bray F., Center M.M., Ferlay J., Ward E., Forman D. Global cancer statistics. CA: A Cancer Journal for Clinicians. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Jenab M., Bueno-de-Mesquita H.B., Ferrari P., van Duijnhoven F.J., Norat T., Pischon T., Jansen E.H., Slimani N., Byrnes G., Rinaldi S., Tjønneland A., Olsen A., Overvad K., Boutron-Ruault M.C., Clavel-Chapelon F., Morois S., Kaaks R., Linseisen J., Boeing H., Bergmann M.M., Trichopoulou A., Misirli G., Trichopoulos D., Berrino F., Vineis P., Panico S., Palli D., Tumino R., Ros M.M., van Gils C.H., Peeters P.H., Brustad M., Lund E., Tormo M.J., Ardanaz E., Rodríguez L., Sánchez M.J., Dorronsoro M., Gonzalez C.A., Hallmans G., Palmqvist R., Roddam A., Key T.J., Khaw K.T., Autier P., Hainaut P., Riboli E. Association between pre-diagnostic circulating vitamin D concentration and risk of colorectal cancer in European populations: a nested case–control study. BMJ. 2010;340:b5500. doi: 10.1136/bmj.b5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee J.E. Circulating levels of vitamin D, vitamin D receptor polymorphisms, and colorectal adenoma: a meta-analysis. Nutrition Research Practical. 2011;5(5):464–470. doi: 10.4162/nrp.2011.5.5.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei M.Y., Garland C.F., Gorham E.D., Mohr S.B., Giovannucci E. Vitamin D and prevention of colorectal adenoma: a meta-analysis. Cancer Epidemiology, Biomarkers and Prevention. 2008;17(11):2958–2969. doi: 10.1158/1055-9965.EPI-08-0402. [DOI] [PubMed] [Google Scholar]
- 5.Feskanich D., Ma J., Fuchs C.S., Kirkner G.J., Hankinson S.E., Hollis B.W., Giovannucci E.L. Plasma vitamin D metabolites and risk of colorectal cancer in women. Cancer Epidemiology, Biomarkers and Prevention. 2004;13(9):1502–1508. [PubMed] [Google Scholar]
- 6.Wactawski-Wende J., Kotchen J.M., Anderson G.L., Assaf A.R., Brunner R.L., O'Sullivan M.J., Margolis K.L., Ockene J.K., Phillips L., Pottern L., Prentice R.L., Robbins J., Rohan T.E., Sarto G.E., Sharma S., Stefanick M.L., Van Horn L., Wallace R.B., Whitlock E., Bassford T., Beresford S.A., Black H.R., Bonds D.E., Brzyski R.G., Caan B., Chlebowski R.T., Cochrane B., Garland C., Gass M., Hays J., Heiss G., Hendrix S.L., Howard B.V., Hsia J., Hubbell F.A., Jackson R.D., Johnson K.C., Judd H., Kooperberg C.L., Kuller L.H., LaCroix A.Z., Lane D.S., Langer R.D., Lasser N.L., Lewis C.E., Limacher M.C., Manson J.E., Investigators W.s.H.I. Calcium plus vitamin D supplementation and the risk of colorectal cancer. New England Journal of Medicine. 2006;354(7):684–696. doi: 10.1056/NEJMoa055222. [DOI] [PubMed] [Google Scholar]
- 7.Newmark H.L., Yang K., Kurihara N., Fan K., Augenlicht L.H., Lipkin M. Western-style diet-induced colonic tumors and their modulation by calcium and vitamin D in C57Bl/6 mice: a preclinical model for human sporadic colon cancer. Carcinogenesis. 2009;30(1):88–92. doi: 10.1093/carcin/bgn229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu H., Posner G.H., Stevenson M., Campbell F.C. Apc(MIN) modulation of vitamin D secosteroid growth control. Carcinogenesis. 2010;31(8):1434–1441. doi: 10.1093/carcin/bgq098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fichera A., Little N., Dougherty U., Mustafi R., Cerda S., Li Y.C., Delgado J., Arora A., Campbell L.K., Joseph L., Hart J., Noffsinger A., Bissonnette M. A vitamin D analogue inhibits colonic carcinogenesis in the AOM/DSS model. Journal of Surgical Research. 2007;142(2):239–245. doi: 10.1016/j.jss.2007.02.038. [DOI] [PubMed] [Google Scholar]
- 10.Murillo G., Nagpal V., Tiwari N., Benya R.V., Mehta R.G. Actions of vitamin D are mediated by the TLR4 pathway in inflammation-induced colon cancer. Journal of Steroid Biochemistry and Molecular Biology. 2010;121(1–2):403–407. doi: 10.1016/j.jsbmb.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mokady E., Schwartz B., Shany S., Lamprecht S.A. A protective role of dietary vitamin D3 in rat colon carcinogenesis. Nutrition and Cancer. 2000;38(1):65–73. doi: 10.1207/S15327914NC381_10. [DOI] [PubMed] [Google Scholar]
- 12.Akeno N., Matsunuma A., Maeda T., Kawane T., Horiuchi N. Regulation of vitamin D-1alpha-hydroxylase and -24-hydroxylase expression by dexamethasone in mouse kidney. Journal of Endocrinology. 2000;164(3):339–348. doi: 10.1677/joe.0.1640339. [DOI] [PubMed] [Google Scholar]
- 13.Moolenbeek C., Ruitenberg E.J. The Swiss roll: a simple technique for histological studies of the rodent intestine. Laboratory Animals. 1981;15(1):57–59. doi: 10.1258/002367781780958577. [DOI] [PubMed] [Google Scholar]
- 14.Nittke T., Kallay E., Manhardt T., Cross H.S. Parallel elevation of colonic 1,25-dihydroxyvitamin D3 levels and apoptosis in female mice on a calcium-deficient diet. Anticancer Research. 2009;29(9):3727–3732. [PubMed] [Google Scholar]
- 15.Riddell R.H., Goldman H., Ransohoff D.F., Appelman H.D., Fenoglio C.M., Haggitt R.C., Ahren C., Correa P., Hamilton S.R., Morson B.C. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Human Pathology. 1983;14(11):931–968. doi: 10.1016/s0046-8177(83)80175-0. [DOI] [PubMed] [Google Scholar]
- 16.Price S.A., Davies D., Rowlinson R., Copley C.G., Roche A., Falkenberg F.W., Riccardi D., Betton G.R. Characterization of renal papillary antigen 1 (RPA-1), a biomarker of renal papillary necrosis. Toxicologic Pathology. 2010;38(3):346–358. doi: 10.1177/0192623310362246. [DOI] [PubMed] [Google Scholar]
- 17.Holick M.F., Binkley N.C., Bischoff-Ferrari H.A., Gordon C.M., Hanley D.A., Heaney R.P., Murad M.H., Weaver C.M., Society E. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. Journal of Clinical Endocrinology and Metabolism. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 18.Huerta S., Irwin R.W., Heber D., Go V.L., Koeffler H.P., Uskokovic M.R., Harris D.M. 1alpha,25-(OH)(2)-D(3) and its synthetic analogue decrease tumor load in the Apc(min) mouse. Cancer Research. 2002;62(3):741–746. [PubMed] [Google Scholar]
- 19.Irving A.A., Halberg R.B., Albrecht D.M., Plum L.A., Krentz K.J., Clipson L., Drinkwater N., Amos-Landgraf J.M., Dove W.F., DeLuca H.F. Supplementation by vitamin D compounds does not affect colonic tumor development in vitamin D sufficient murine models. Archives of Biochemistry and Biophysics. 2011;515(1–2):64–71. doi: 10.1016/j.abb.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleet J.C., Gliniak C., Zhang Z., Xue Y., Smith K.B., McCreedy R., Adedokun S.A. Serum metabolite profiles and target tissue gene expression define the effect of cholecalciferol intake on calcium metabolism in rats and mice. Journal of Nutrition. 2008;138(6):1114–1120. doi: 10.1093/jn/138.6.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ross A.C., Manson J.E., Abrams S.A., Aloia J.F., Brannon P.M., Clinton S.K., Durazo-Arvizu R.A., Gallagher J.C., Gallo R.L., Jones G., Kovacs C.S., Mayne S.T., Rosen C.J., Shapses S.A. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. Journal of Clinical Endocrinology and Metabolism. 2011;96(1):53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masubuchi Y., Horie T. Endotoxin-mediated disturbance of hepatic cytochrome P450 function and development of endotoxin tolerance in the rat model of dextran sulfate sodium-induced experimental colitis. Drug Metabolism and Disposition: The Biological Fate of Chemicals. 2004;32(4):437–441. doi: 10.1124/dmd.32.4.437. [DOI] [PubMed] [Google Scholar]
- 23.Matkowskyj K.A., Marrero J.A., Carroll R.E., Danilkovich A.V., Green R.M., Benya R.V. Azoxymethane-induced fulminant hepatic failure in C57BL/6J mice: characterization of a new animal model. American Journal of Physiology. 1999;277(2 (Pt. 1)):G455–G462. doi: 10.1152/ajpgi.1999.277.2.G455. [DOI] [PubMed] [Google Scholar]
- 24.Ponchon G., Kennan A.L., DeLuca H.F. Activation of vitamin D by the liver. Journal of Clinical Investigation. 1969;48(11):2032–2037. doi: 10.1172/JCI106168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Putz-Bankuti C., Pilz S., Stojakovic T., Scharnagl H., Pieber T.R., Trauner M., Obermayer-Pietsch B., Stauber R.E. Association of 25-hydroxyvitamin D levels with liver dysfunction and mortality in chronic liver disease. Liver International. 2012;32(5):845–851. doi: 10.1111/j.1478-3231.2011.02735.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. Increasing amounts of dietary vitamin D lead to reduced dysplasia. Hematoxylin-Eosin staining of representative colon sections of mice fed with either 100, 400, 1000, 2500, or 5000 IU/kg. Bars equal 50 μm.