Abstract
After its identification in 1980s, HIV has infected more than 30 million people worldwide. In the era of highly active anti-retroviral therapy, anti-retroviral drug resistance results from insufficient anti-retroviral pressure, which may lead to treatment failure. Preliminary studies support the idea that anti-retroviral drug resistance has evolved largely as a result of low-adherence of patients to therapy and extensive use of anti-retroviral drugs in the developed world; however, a highly heterogeneous horde of viral quasi-species are currently circulating in developing nations. Thus, the prioritizing of strategies adopted in such two worlds should be quite different considering the varying anti-retroviral drug resistance prevalence. In this article, we explore differences in anti-retroviral drug resistance patterns between developed and developing countries, as they represent two distinct ecological niches of HIV from an evolutionary standpoint.
Keywords: HIV infection, Evolution, Drug resistance, Developed world, Developing world
1. Introduction
In community ecology, co-evolutionary processes can be observed in predatory or parasitic interactions in which the emergence of an adaptive feature in an organism results in a selective pressure that would in turn evolve a counter-adaptation in the other organism[1]–[3]. For example, micro-organisms causing sexually transmitted infections (STI) have persisted and co-evolved throughout human generations, changing their infective and transmission properties parallel to the civilization of mankind and its societal configuration[4]–[6]. Humanity has faced many serious health-related threats by means of STIs throughout the last decades, from which the newly-emerged HIV is an archetype. By means of infecting CD4-positive immune cells, HIV targets almost every organ system, further resulting in the weakening of the host's immune capabilities and the development of AIDS. With this weakened immune system, opportunistic infections may easily invade the immune-deficient host during advanced stages of the HIV infection. More than 33 million people were living with HIV infection at the end 2008[7].
Though many believe that HIV may have been circulating through human generations for many years before its documentation in the 1980s, its drug resistance attributes have been very recently identified only after the invention and extensive use of highly active anti-retroviral therapy (HAART). The introduction of HAART not only revolutionized the clinical care of HIV patients, but also led to the increased lifespan of patients, further changing the general epidemiology of the infection. Meanwhile, HIV also altered its infective and virulence features by means of genomic shifts and modifications. The efficacy of current anti-retroviral (ARV) therapeutic options is overwhelmed by the numerous resistance mutations that are increasing every day, further highlighting the needs for inventing new effective ARV medications[8]. In this review, we aim to discuss this evolutionary look at the HIV drug resistance phenomenon as an arms race based on simple evolutionary concepts.
2. Origins and macro-evolution of HIV
Two different types of HIV; HIV-1 and HIV-2, have assaulted humanity. HIV-1, which is responsible for the great majority of the world's infections, encompasses three distinct groups (M, N and O); while the less transmissible HIV-2, largely restricted to West Africa, includes eight groups (A-H). HIV-1 group M comprises 10 different clades that possess various epidemiological features and have affected different geographical realms. For example, subtype B has largely spread in the USA and Americas, while subtype C has affected most of Southern Africa and India[9].
Although HIV-1 group M accounts for approximately 99% of the reported infections worldwide, and thus is the only HIV strain that has reached pandemic levels, the identification of other closely related HIV strains has shed light on the phylogeny and evolutionary pathways through which HIV has radiated and diversified. It is well established that HIV-1 arose from cross-species transmission of simian immunodeficiency virus in Pan troglodytes (SIVcpz) or the common chimpanzee, while HIV-2 is designated to SIV strains (SIVsmm) related to sooty mangabey (Cercocebus atys)[10]. To date, more than 36 primate species have been shown to be infected by various SIV strains mostly restricted to regions in Africa. Evolutionary processes gave rise to several different strains that could also infect human populations; HIV-1 groups have been most likely introduced from primates to humans independently, giving rise to the three major wide-spread groups[10].
To date, a growing body of evidence supports the parsimonious idea that links the origins of HIV-1 group M to the locales where common chimpanzees exist, most remarkably Cameroon. Almost certainly, other HIV-1 groups and HIV-2 subtypes have been introduced within the same continent to the human population, and subsequently, have spread to other continents and infected major-at-risk human populations worldwide. Moreover, such viral strains might have entered human populations at multiple cross sections of time, among which the best estimate for the first introduction of HIV-1 group M into humans is 1931[11].
3. Retroviral diversification
The evolution of any system relies upon three basic processes: reproduction, competition and diversity. The retroviral infection is characterized by numerous shifts and diversification of nucleotides during replication[12],[13]. As an infidel reverse transcriptase (RT), the rate of nucleotide shifts occurring during replication of HIV is six orders of magnitude more than the replication of a mammalian genome, allocating to a heterogeneous crowd of virions within host bodily systems[14]. Such prominent inaccuracy of retroviral RT results from a lack of proofreading in combination with a physically large active site[15]. In addition, recombination or the re-arrangement of viral alleles during reverse transcription provides the means for the selection of recombinants with increased fitness[16]–[18]. The wide dispersion of circulating recombinant forms (CRF) demonstrates the impact of viral gene assortment in diversity of HIV variants with increased fitness worldwide[12],[19],[20].
From an evolutionary viewpoint, retroviruses have gained an advantage from rapid and error-prone replication, which allows swift variations in both intra-host and inter-host community levels. As a matter of fact, selective forces acting on resident viral sub-populations in various anatomic regions of the host bodily systems would lead to HIV genetic compartmentalization soon after the activation of immunologic responses. The emergence of such quasi-species during each infection event is also reflected in the community level; many HIV-1 and HIV-2 variants with various genomic dissimilarities have emerged as a result of diverse selective pressures in both developed and developing worlds[12],[21].
In addition to the many retroviral factors augmenting the diversification of viral quasi-species, several host factors do also account for the inter-individual variations observed in response to infection. For instance, certain major histocompatibility complex (MHC) alleles' polymorphisms (e.g. HLA B27 and HLA B5701), chemokine alleles and several other host genes may be associated with altered disease progression[22],[23]. It is noteworthy that under settings with high selective pressures on the host, such as nations with high prevalence and low life expectancy, selective forces may have been selecting specific alleles that are pivotal in directing the immune responses[2]. Although such scenarios might be evident in case of eminent parasites such as Plasmodium, the extent to which selective forces have altered the general population genetics of certain human communities remains unidentified.
4. History and mechanisms of anti-retroviral drug resistance (ARDR)
ADAR, an important hurdle and an inconvenient consequence of ARV therapy, is associated with the occurrence of treatment failure[24],[25]. It is well established that resistance phenomenon is associated with mutations in HIV proteins that are primarily targeted by ARV agents[26]. With reference to some conservative evaluations, up to 10% of patients who are controlled by HAART show some types of genotypic drug resistance after two years of therapy. In this regard, reports indicate that up to 30% of patients experience viral failure with more than one major resistance mutation after less than six years of commencing HAART[27]. Therefore, the increasing prevalence of ARDR has become a growing concern, especially among developed countries with higher availability of ARV agents. The high prevalence of ARDR in such communities would eventually result in high rates of virological failure among the newly infected patients who have initiated first line therapy[28].
Standard and commonly prescribed ARV medications include drugs that are classified into three main categories: nucleoside reverse transcriptase inhibitors (NRTI), non-nucleoside reverse transcriptase inhibitors (NNRTI) and protease inhibitors (PI). Table 1 presents a brief description on the different drug classes that are used for treatment of HIV infection. Thus, ARDR is defined as a failure of an ARV drug due to the gradually increasing resistance of HIV[29],[30].
Table 1. Some of the anti-retroviral drugs prescribed in the treatment of HIV infection.
| Drugs | Action mechanism |
| Nucleoside reverse transcriptase inhibitors | |
| Zidovudine | Triphosphate derivatives |
| Stavudine | Viral DNA integration |
| Lamivudine, Didanosine, Zalcitabine, Abacavir | Termination of Viral DNA Synthesis |
| Non-nucleoside reverse transcriptase inhibitors | |
| Nevirapine | Viral DNA polymerase inhibition |
| Efavirenz, Delavirdine | For inactivation of HIV-2 (hence some types are intrinsically resistant to NRTIs) |
| Protease inhibitors | |
| Saquinavir, Ritonavir, Indinavir Nelfinavir, Amprenavir, Lopinavir | Deactivate the HIV-1 protease by binding to the active site |
| Fusion inhibitors | |
| Enfuvirtide | Glycoprotein 41–dependent membrane fusion |
The first cases of ARDR were reported about Zidovudine, which was prescribed for persons with late-stage HIV infection as monotherapy. Subsequently, the first transmission of drug resistance was reported about Zidovudine isolates in 1992[31]. Such clinical drug resistance arises from the residual replication of mutant viral quasi-species that have endured incomplete suppressive drug regimens[32]. In the case of most ARV agents, cumulative effects of several mutations are required to result in clinical resistance; however, single mutations could also confer resistance to certain agents such as NNRTIs and NRTIs[33]. For example, there are certain mutations that confer resistance to NNRTIs and specifically found in the binding site of the RT gene (e.g. the Y181C and K103N mutations); thereby, extensive cross-resistance is a frequently observed phenomenon among this class of drugs[34],[35]. Additionally, the M184V mutations may incur complete resistance both to lamivudine and emcitritabine[36]. In contrast, multiple mutations are frequently required to confer resistance to PIs[37].
In addition to the agents' mechanism of action, the applied regimen may extensively influence the possibility of resistance as well. In this regard, it has been well established that mono-therapy with all agents may result in a high level resistance and therefore, combination therapy with three or more fully active agents is highly preferred[38]. It is noteworthy that the replicative as well as transmission properties of mutant and wild type variants are highly different. For example, certain data suggest that some drug resistant variants possess reduced replicative capacity in comparison with wild-type virus and therefore, drug resistant infection might be of some virological benefit to the hosts' immune system[39]–[41].
To date, there is no generally accepted model for the identification of evolutionary pathways, which drive drug resistant assets of HIV. It has been proposed that point mutations have a gradual progression, and recombination plays a remarkable role in the evolution of drug resistance depending on the viral population size and the intensity of selective pressures[42]. Some authors have declared that the presence of high numbers of effective HIV populations and deterministic models could explain the evolution of drug resistance; however, some other authors have emphasized the role of low number HIV populations and stochastic models[43]–[45].
5. Prevalence and spatial distribution of ARDR
Many studies have addressed the prevalence of ARDR to date[46]–[48]. In general, it seems that the prevalence of transmitted HIV drug resistance (TDR) has followed a steady pattern in developed countries[49], recently conducted surveys in western Europe and United States have shown that the trend of TDR has become stable in recent years[50],[51]. Increasing efficacy of HAART and development of new generation ARV agents have been proposed as possible reasons for this trend[52]–[55].
In some previous studies in developed settings, HIV drug resistance was more common among men who have sex with men (MSM), as well as among individuals with a positive history of STIs, mono-therapy, and NNRTI use[30]. Based on another survey, the overall prevalence of ARDR was 13%[56]. In another study, prevalence of resistance to any ARV drug was 7.4% from 2005 to 2010 globally. Based on each class, the prevalence was 4.2%, 2.5% and 1.7% for NRTI, NNRTI and PI respectively[57],[58].
According to the current literature, future therapeutic options might be highly limited due to the transmission of multi-drug resistant variants. Nevertheless, the TDR rate in developed nations worldwide has shown a decline from 11.3% in 2004-2006 to 8.4% in 2007-2010[56]. Such observation has been previously attributed to the extensive use of combination ARV and adherence-promoting interventions[59]–[61].
6. Novel ARV agents: are we on the wrong side of the track?
The need for the development of novel drugs with effective ARV activity has been announced as a global priority[62],[63]. In general, new regimens should possess high efficacy, high tolerability, low cost and low pill burden. Considering the different epidemiology of infections in different geographical realms, other factors such as different patients' adherence and socio-economic conditions should also be taken into account. For example, some medications need to be taken with high-fat food, or some other may require refrigeration. Consequently, multiple factors further highlight the necessity for the use of novel drugs in the two settings. In addition, considerations should be given to the genotypic diversity that has emerged as a consequence of different selective pressures between varying host communities. Most importantly, the sub-optimal regimens might have directed the course of HIV evolution dissimilar to that of the western societies. Nevertheless, in order to most efficiently combat the pandemic in different regions of the world, any attempt toward novel drug discovery should take such various evolutionary pathways into account[64].
Generally speaking, novel anti-HIV drug discovery initiatives have mostly focused on the development of drugs that target new viral elements and possess a more robust genetic barrier to resistance. Conventionally, much attention has been paid to the development of drugs that primarily target the replication cycle and the dissemination of virus within host bodily systems, from which the classical NRTIs and NNRTIs are of note. Nevertheless, more recently developed agents have targeted new viral proteins involved in other functions of the viral replicative cycle[65]. Enfuvitide (T20) was introduced about ten years ago and inhibits the viral fusion that is necessary for the intra-cytoplasmic insertion of the HIV viral core[66],[67]. In addition, new drugs in development (BMS 488043 and PRO 542) act as phase I attachment inhibitors. Consequently, recently conducted clinical attempts show divergence toward using novel agents with almost entirely different mechanisms, instead of converging toward the traditionally established drug classes. Although such achievements are promising, we have no evaluation whether the pace is even sufficient to take over the phenomenon of resistance evolution.
From an evolutionary perspective, novel drug with an entirely different anti-retroviral mechanism has low levels of circulating viral resistance in the population; however, the extent to which slowly occurring recombinational events and point mutations may alter their efficacy through time, has yet to be revealed. For example, the broadly used NNRTIs target the hydrophobic pocket of RT enzyme and therefore, single mutations that affect the biochemical properties of this region may result in the evolution of high-level resistance[68]. Thereby, the agents' mechanism of action and the rate of spontaneously occurring recombination and mutations in their source genes should be addressed prior to conducting any novel drug development initiative program. Even more basically, the unanswered question is: Do we need to consider the possibility and means of resistance evolution prior to conducting clinical trials for novel agents?
7. Different evolutionary pathways and two ecological niches
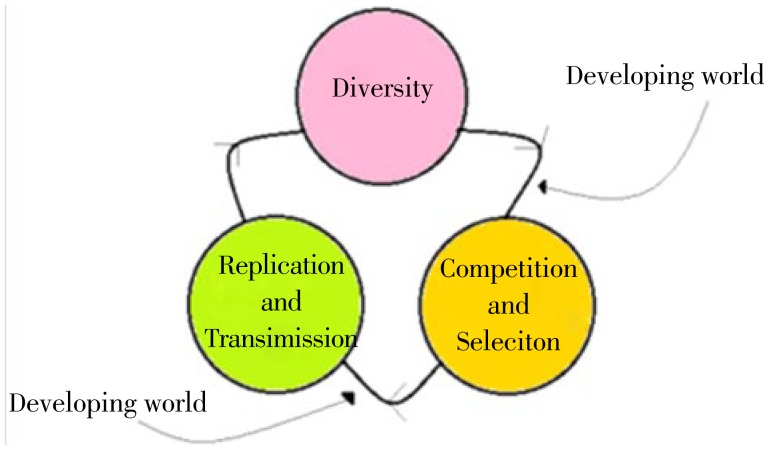
Many developed nations have vowed to scale up access and use of anti-retroviral therapy for all people living with HIV/AIDS (PLWHA); however, resource allocation in the developing world especially among low-income countries remains a challenge. Additionally, numerous studies have shown that patients' level of adherence to the prescribed therapy plays a pivotal role in the evolution of ARDR; thus, efforts have been undertaken to demonstrate the role of adherence-enhancement interventions in reducing the prevalence of ARDR[69]. After all, near-complete adherence might be regarded as a behavioral adaptation necessary for stalling the development of ARDR especially in developing settings. Many other ethnologic, genetic, socioeconomic, demographic and virologic factors may decisively shape the pattern and epidemiology of resistance globally (Table 2). Therefore, HIV as an obligatory parasite is circulating in at least two ecological niches with various environmental competences: the developing and developed worlds (Figure 2).
Table 2. Some of the different factors between developed and developing nations that affect the pace and direction of ARDR.
| Cultural, societal and ethnologic factors | |
| Sexual behaviors | Homosexuality (i.e. MSM*) |
| Marital traditions and norms: monogamy, multi-parity, promiscuity, extra-marital breeding, inbreeding, divorce and separation taboos, etc. | |
| Cultural context: religious beliefs, sexual activity boundaries, societal determinants, etc. accessibility, affordability and acceptance of persistent use of barriers (i.e. condom) | |
| Demographics and community | Population age and sex structure |
| Education, technology and industry | |
| Traditions, addiction (i.e. IDU**), criminality and incarceration, migration | |
| Structure of the health-care system, political factors | |
| Economic factors | |
| Directly health- care system related | Availability and accessibility of antiretroviral therapy, voluntary and counseling centers, surveillance, resource allocation and distribution, increased lifespan and quality of life of patients, etc. |
| Non directly health-care system related | Poverty, occupation and income, etc. |
| Virologic factors | |
| Circulating recombinant forms (CRF), various HIV-1 clades, etc. | |
| Genetic factors | |
| Different MHC*** and immunologic alleles, various immunologic pathways, population genetics, etc. | |
*MSM: Men who have sex with men; **IDU: Injection drug use; **MHC: Major histocompatibility complex
Figure 2. Evolution comprises three principles: diversity, competition and replication.
According to the current literature, if we assume that the prevalence of ARDR resistance in the developed world is higher in comparison to developing world, then two such niches would benefit best from prioritizing different strategies against ARDR. The developed world should primarily invest in strategies that would stall the replication and transmission of drug resistant variants, while the strategies in developing world should focus on methods that would decrease the pace of ARDR selection. The arrows theoretically indicate the stages of implementing preventive strategies.
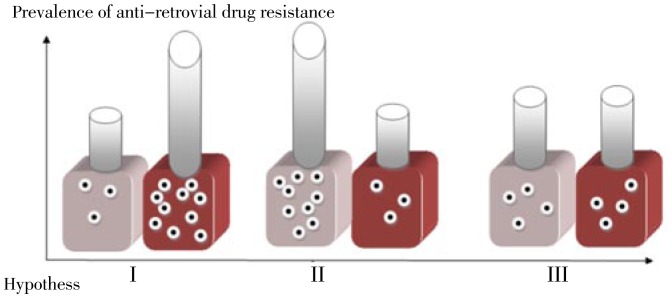
Figure 1. Evolution and prevalence of ARDR in developed and developing world as two different but inter-connected ecological niches.
The red box and the grey box are indicators of the developed and developing worlds respectively, while the number of dots in each represents the relative frequency of ARDR. In hypothesis I, ARDR is more prevalent in the developed world as compared to the developing world, while in hypotheses II and III; ARDR is more prevalent in the developing world or has an almost equal prevalence among the two niches respectively.
In order to further explain this model, three hypotheses may elucidate the current condition of drug resistance among developed and developing countries. The first hypothesis is that, due to the extensive use of HAART with varying level of patients' adherence in the developed world, drug resistance has enormously evolved and diverged from that of the developing world, resulting in the numerous reports indicating that ARDR has evolved and is responsible for treatment failure in many cases. On the other hand, there is a paucity of data in regard to the prevalence of ARDR and TDR in many developed nations. Thereby, an alternative hypothesis is that ARDR has mostly evolved and diverged in developing nations where inadequate drug pressures have led to the massive divergence of drug resistant variants through time. Finally, an ultimate hypothesis also concludes that ARDR has evolved in somewhat equal extents when comparing between the two niches, although the directions and mechanisms could be surprisingly different.
The current available literature lacks of enough evidence to strongly support any of the aforementioned hypotheses. However, the first hypothesis seems to be the most parsimonious and scientifically relevant according to the current data. In fact, the developing world is facing challenges that are distinctly related to the diagnosis and treatments of naive patients. Considering the limited availability of HAART in most low-income nations, it is plausible to conclude that selective forces have not yet narrowed the broad range of potentially ARV resistant quasi-species. Conventionally, such conclusions could be true, at least in the case of low-income nations with prevalent infections or concentrated epidemics. Thereby, a profound population of potentially resistant variants is circulating in such an ecological niche. However, in the case of developed countries, the availability of HAART in addition to the non-adherence of patients has already led to the emergence of highly resistant variants over decades. The numerous reports of drug resistance from developed settings are consistent with such an explanation.
8. The future ahead
If we consider the first hypothesis as the most parsimonious according to the available global reports on the prevalence of ARDR and TDR, the priorities for implementing strategies by which developed and developing nations may combat the evolution of ARDR would be quite different as well. Based on simple evolutionary facts, resource-poor nations would do best to launch interventions that at the very first step restrain or pose limitations toward influential selective pressures that are acting upon viral quasi-species. In this regard, providing the grounds for complete adherence in naive patients should be the first priority at least in settings with low ARDR prevalence[70]. However, consistent with the reports indicating high TDR prevalence, developed nations are dealing with frequently resistant virus that tend to highly disperse throughout host societal structures. Under such circumstances, devotion to prevention strategies, inducing adequate viral suppression and disengaging the transmission routes seem to be the optimal approaches. Taking advantage of novel resistance reporting and genotyping systems in addition to building up accessible and comprehensive databases, both locally and nationally, are also of utmost importance[71],[72]. For both niches, newly invented anti-retroviral agents should be thoughtfully introduced to the host community, which would eventually thwart the emergence of resistantvirus. It is noteworthy that adoption of combinational approaches is necessary to limit the prevalence of drug resistance in settings of both developed and developing countries. Thus, implication of evolutionary and ecological models, as discussed herein, should not restrain the implementation of other prevention strategies or fade their precedence.
Currently, we have not only precisely identified the major determinants that derive the course and direction of human and HIV co-evolution, but also highlighted the vast capacity of viral replication in generating mutations and allele re-assortments that confounds the predictability of any given model. Considering such inequality of evolutionary capacities between humankind and HIV, we may not precisely define how viral genomic capacities may determine the direction of an arms race. Thus, our behavioral and societal adaptations might possess a much more deterministic role in the identification of the direction of ARDR and an arms race. In conclusion, the continued evolution of resistant HIV variants will only be curtailed with constant patient education and support to ensure adequate adherence as well as with the ongoing development of improved and novel drug regimens with enhanced potency and tolerability. Nonetheless, it seems like that in the case of HIV infection, financial investments to induce simple behavioral and psychological changes such as enhancing adherence, could encourage more apparent benefits compared with designing costly and partially effective drugs. Although this evolutionary model underrates the complexity of the situation, it could be useful in prioritizing the prevention strategies that decelerate the evolution of counter-adaptations within a viral community.
Acknowledgments
We would like to thank the electronic library of Tehran University of Medical Sciences for providing us full texts of articles. This article was supported by Tehran University of Medical Sciences (grant no. 55/10848).
Comments
Background
Anti-retroviral drug resistance has been pronounced as an inconvenient consequence of extensive anti-retroviral therapy administered worldwide, which is becoming more prevalent, intricate and troublesome especially for patients in developing settings.
Related reports
Many prior investigations have addressed the varying levels of transmitted drug resistance in both developed and developed countries; although none have basically corroborated the idea that preventive strategies might be varying due to the various concerns and circumstances.
Peer review
This review describes some well-known facts of HIV infection, thus the two ultimate parts explain that how such facts may play role in determining the patterns of HIV evolution under both developed and developing circumstances. I believe the ideas discussed in section 7 need further justification, as the role of factors should be more investigated.
Footnotes
Foundation Projec: Supported by Tehran University of Medical Sciences (grant no. 55/10848).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Gandon S, Buckling A, Decaestecker E, Day T. Host-parasite coevolution and patterns of adaptation across time and space. J Evol Biol. 2008;21(6):1861–1866. doi: 10.1111/j.1420-9101.2008.01598.x. [DOI] [PubMed] [Google Scholar]
- 2.Schmid-Hempel P, Ebert D. On the evolutionary ecology of specific immune defence. Trends Ecol Evol. 2003;18(1):27–32. [Google Scholar]
- 3.Soler JJ, Marta-nez JG, Soler M, Muller AP. Coevolutionary interactions in a host parasite system. Ecol Lett. 2008;4(5):470–476. [Google Scholar]
- 4.Nesse RM, Foxman B. Evolutionary approaches to sexually transmitted infections. Ann N Y Acad Sci. 2011;1230(1):1–3. doi: 10.1111/j.1749-6632.2011.06078.x. [DOI] [PubMed] [Google Scholar]
- 5.Antonovics J, Boots M, Abbate J, Baker C, McFrederick Q, Panjeti V. Biology and evolution of sexual transmission. Ann N Y Acad Sci. 2011;1230(1):12–24. doi: 10.1111/j.1749-6632.2011.06127.x. [DOI] [PubMed] [Google Scholar]
- 6.Nahmias S, Nahmias D. Society, sex, and STIs: human behavior and the evolution of sexually transmitted diseases and their agents. Ann N Y Acad Sci. 2011;1230(1):59–73. doi: 10.1111/j.1749-6632.2011.06079.x. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization . Geneva: World Health Organization; 2009. AIDS epidemic update. [Google Scholar]
- 8.Paydary K, Emamzadeh-Fard S, Khorshid HR, Kamali K, Seyed Alinaghi S, Mohraz M. Safety and efficacy of setarud (IMOD TM) among people living with HIV/AIDS: A review. Rec Pat Antiinfect Drug Discov. 2012;7(1):66–72. doi: 10.2174/157489112799829756. [DOI] [PubMed] [Google Scholar]
- 9.Skar H, Hedskog C, Albert J. HIV-1 evolution in relation to molecular epidemiology and antiretroviral resistance. Ann N Y Acad Sci. 2011;1230(1):108–118. doi: 10.1111/j.1749-6632.2011.06128.x. [DOI] [PubMed] [Google Scholar]
- 10.Sharp PM, Hahn BH. The evolution of HIV-1 and the origin of AIDS. Philos Trans R Soc Lond B Biol Sci. 2010;365(1552):2487–2494. doi: 10.1098/rstb.2010.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tebit DM, Arts EJ. Tracking a century of global expansion and evolution of HIV to drive understanding and to combat disease. Lancet Infect Dis. 2011;11(1):45–56. doi: 10.1016/S1473-3099(10)70186-9. [DOI] [PubMed] [Google Scholar]
- 12.Shi B, Kitchen C, Weiser B, Mayers D, Foley B, Kemal K, et al. et al. Evolution and recombination of genes encoding HIV-1 drug resistance and tropism during antiretroviral therapy. Virology. 2011;404(1):5–20. doi: 10.1016/j.virol.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson VA, Calvez V, Ganthard HF, Paredes R, Pillay D, Shafer R, et al. et al. Update of the drug resistance mutations in HIV-1. HIV Med. 2011;18:156–163. [PMC free article] [PubMed] [Google Scholar]
- 14.Simon Loriere E, Holmes EC. Why do RNA viruses recombine? Nat Rev Microbiol. 2011;9(8):617–626. doi: 10.1038/nrmicro2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abram ME, Ferris AL, Shao W, Alvord WG, Hughes SH. Nature, position, and frequency of mutations made in a single cycle of HIV-1 replication. J Virol. 2010;84(19):9864–9878. doi: 10.1128/JVI.00915-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown RJ, Peters PJ, Caron C, Gonzalez-Perez MP, Stones L, Ankghuambom C, et al. et al. Intercompartmental recombination of HIV-1 contributes to env intrahost diversity and modulates viral tropism and sensitivity to entry inhibitors. J Virol. 2011;85(12):6024–6037. doi: 10.1128/JVI.00131-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lauring AS, Andino R. Quasispecies theory and the behavior of RNA viruses. PLoS Path. 2011;6(7):1001005. doi: 10.1371/journal.ppat.1001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moir S, Chun TW, Fauci AS. Pathogenic mechanisms of HIV disease. Ann Rev Path. 2011;6:223–248. doi: 10.1146/annurev-pathol-011110-130254. [DOI] [PubMed] [Google Scholar]
- 19.Archer J, Pinney JW, Fan J, Simon-Loriere E, Arts EJ, Negroni M, et al. et al. Identifying the important HIV-1 recombination breakpoints. PLoS Comput Biol. 2008;4(9):1000178. doi: 10.1371/journal.pcbi.1000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whitney JB, Lim SY, Wainberg MA. Evolutionary mechanisms of retroviral persistence. AIDS Rev. 2011;13(4):234–239. [PubMed] [Google Scholar]
- 21.van Marle G, Church DL, Nunweiler KD, Cannon K, Wainberg MA, Gill MJ. Higher levels of zidovudine resistant HIV in the colon compared to blood and other gastrointestinal compartments in HIV infection. Retrovirology. 2010;7(1):74. doi: 10.1186/1742-4690-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neogi U, Shet A, Sahoo PN, Bontell I, Ekstrand ML, Banerjea AC, et al. et al. Human APOBEC3G-mediated hypermutation is associated with antiretroviral therapy failure in HIV-1 subtype C-infected individuals. J Int AIDS Soc. 2013;16:18472. doi: 10.7448/IAS.16.1.18472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lever RA, Lever AML. Intracellular defenses against HIV, viral evasion and novel therapeutic approaches. J Formos Med Assoc. 2011;110(6):350–362. doi: 10.1016/S0929-6646(11)60053-3. [DOI] [PubMed] [Google Scholar]
- 24.Skoura L, Metallidis S, Buckton AJ, Mbisa JL, Pilalas D, Papadimitriou E, et al. et al. Molecular and epidemiological characterization of HIV-1 infection networks involving transmitted drug resistance mutations in Northern Greece. J Antimicrob Chemother. 2011;66(12):2831–2837. doi: 10.1093/jac/dkr386. [DOI] [PubMed] [Google Scholar]
- 25.Wang D, Hicks CB, Goswami ND, Tafoya E, Ribeiro RM, Cai F, et al. et al. Evolution of drug-resistant viral populations during interruption of antiretroviral therapy. J Virol. 2011;85(13):6403–6415. doi: 10.1128/JVI.02389-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mousavi SM, Hamkar R, Gouya MM, Safaie A, Zahraei SM, Yazdani Z, et al. et al. Surveillance of HIV drug resistance transmission in Iran: experience gained from a pilot study. Arch Virol. 2010;155(3):329–334. doi: 10.1007/s00705-009-0583-6. [DOI] [PubMed] [Google Scholar]
- 27.Thompson MA, Aberg JA, Cahn P, Montaner JSG, Rizzardini G, Telenti A, et al. et al. Antiretroviral treatment of adult HIV infection. JAMA. 2012;304(3):321–333. doi: 10.1001/jama.2010.1004. [DOI] [PubMed] [Google Scholar]
- 28.Alteri C, Svicher V, Gori C, Arrigo DR, Ciccozzi M, Ceccherini-Silberstein F, et al. et al. Characterization of the patterns of drug-resistance mutations in newly diagnosed HIV-1 infected patients nave to the antiretroviral drugs. BMC Infect Dis. 2009;9(1):111. doi: 10.1186/1471-2334-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization Report of a WHO consultation. Rome: World Health Organization, Department of Communicable Disease Surveillance and Response. 10-11 October 2000. 2001.
- 30.Wheeler WH, Ziebell RA, Zabina H, Pieniazek D, Prejean J, Bodnar UR, et al. et al. Prevalence of transmitted drug resistance associated mutations and HIV-1 subtypes in new HIV-1 diagnoses, US-2006. AIDS. 2010;24(8):1203. doi: 10.1097/QAD.0b013e3283388742. [DOI] [PubMed] [Google Scholar]
- 31.Paredes R, Lalama CM, Ribaudo HJ, Schackman BR, Shikuma C, Giguel F, et al. et al. Pre-existing minority drug-resistant HIV-1 variants, adherence, and risk of antiretroviral treatment failure. J Infect Dis. 2010;201(5):662–671. doi: 10.1086/650543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buendia P, Cadwallader B, DeGruttola V. A phylogenetic and Markov model approach for the reconstruction of mutational pathways of drug resistance. Bioinformatics. 2009;25(19):2522–2529. doi: 10.1093/bioinformatics/btp466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cortez KJ, Maldarelli F. Clinical management of HIV drug resistance. Viruses. 2011;3(4):347–378. doi: 10.3390/v3040347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Briz V, Palladino C, Navarro ML, Jimenez de Ory S. Etravirine based highly active antiretroviral therapy in HIV infected paediatric patients. HIV Med. 2011;12(7):442–446. doi: 10.1111/j.1468-1293.2010.00907.x. [DOI] [PubMed] [Google Scholar]
- 35.Li JZ, Paredes R, Ribaudo HJ, Svarovskaia ES, Metzner KJ, Kozal MJ, et al. et al. Low-frequency HIV-1 drug resistance mutations and risk of NNRTI-based antiretroviral treatment failure a systematic review and pooled analysis. JAMA. 2011;305(13):1327–1335. doi: 10.1001/jama.2011.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang MW, Shafer RW. HIV-1 antiretroviral resistance: scientific principles and clinical applications. Drugs. 2012;72(9):1–25. doi: 10.2165/11633630-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinez-Cajas JL, Wainberg MA, Oliveira M, Asahchop EL, Doualla-Bell F, Lisovsky I, et al. et al. The role of polymorphisms at position 89 in the HIV-1 protease gene in the development of drug resistance to HIV-1 protease inhibitors. J Antimicrob Chemother. 2012;67(4):988–994. doi: 10.1093/jac/dkr582. [DOI] [PubMed] [Google Scholar]
- 38.Kumari G, Singh KR. Anti-HIV drug development: structural features and limitations of present day drugs and future challenges in the successful HIV/AIDS treatment. Curr Pharm Des. 2006;19(10):1767–1783. doi: 10.2174/13816128113199990295. [DOI] [PubMed] [Google Scholar]
- 39.Li JZ, Kuritzkes DR. Clinical implications of HIV-1 minority variants. Clin Infect Dis. 2013;56(11):1667–1674. doi: 10.1093/cid/cit125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abram ME, Hluhanich RM, Goodman DD, Andreatta KN, Margot NA, Ye L, et al. et al. Impact of primary Elvitegravir resistance-associated mutations in HIV-1 integrase on drug susceptibility and viral replication fitness. Antimicrob Agents Chemother. 2013;57(6):2654–2663. doi: 10.1128/AAC.02568-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rouzine IM, Weinberger LS. The quantitative theory of within-host viral evolution. J Stat Mech. 2013;2013(1):01009. [Google Scholar]
- 42.Carvajal-Rodra-guez A, Crandall KA, Posada D. Recombination favors the evolution of drug resistance in HIV-1 during antiretroviral therapy. Infect Genet Evol. 2007;7(4):476–483. doi: 10.1016/j.meegid.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giorgi EE, Korber BT, Perelson AS, Bhattacharya T. Modeling sequence evolution in HIV-1 infection with recombination. J Theor Biol. 2013;21:82–93. doi: 10.1016/j.jtbi.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bershteyn A, Eckhoff PA. A model of HIV drug resistance driven by heterogeneities in host immunity and adherence patterns. BMC Syst Biol. 2013;7(1):11. doi: 10.1186/1752-0509-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiao Y, Miao H, Tang S, Wu H. Modeling antiretroviral drug responses for HIV-1 infected patients using differential equation models. Adv Drug Deliv Rev. 2013 Apr 17; doi: 10.1016/j.addr.2013.04.005. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bontell I, Cuong DD, Agneskog E, Diwan V, Larsson M, Sannerborg A. Transmitted drug resistance and phylogenetic analysis of HIV CRF01_AE in Northern Vietnam. Infect Genet Evol. 2012;12(2):448–452. doi: 10.1016/j.meegid.2011.04.034. [DOI] [PubMed] [Google Scholar]
- 47.Gupta RK, Jordan MR, Sultan BJ, Hill A, Davis DHJ, Gregson J, et al. et al. Global trends in antiretroviral resistance in treatment-naive individuals with HIV after rollout of antiretroviral treatment in resource-limited settings: a global collaborative study and meta-regression analysis. Lancet. 2012;6:1250–1258. doi: 10.1016/S0140-6736(12)61038-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.von Wyl V, Yerly S, Boni J, Shah C, Cellerai C, Klimkait T, et al. et al. Incidence of HIV-1 drug resistance among antiretroviral treatment naive individuals starting modern therapy combinations. Clin Infect Dis. 2012;54(1):131–140. doi: 10.1093/cid/cir728. [DOI] [PubMed] [Google Scholar]
- 49.de Mulder M, Yebra G, Navas A, Martin L, de Jose MI, Navarro ML, et al. et al. Trends in drug resistance prevalence in HIV-1 infected children in Madrid: 1993 to 2010 analysis. Pediatr Infect Dis J. 2012;31(11):213–221. doi: 10.1097/INF.0b013e3182678c7c. [DOI] [PubMed] [Google Scholar]
- 50.Little SJ, Holte S, Routy JP, Daar ES, Markowitz M, Collier AC, et al. et al. Antiretroviral-drug resistance among patients recently infected with HIV. N Engl J Med. 2002;347(6):385–394. doi: 10.1056/NEJMoa013552. [DOI] [PubMed] [Google Scholar]
- 51.Masquelier B, Bhaskaran K, Pillay D, Gifford R, Balestre E, Jargensen LB, et al. et al. Prevalence of transmitted HIV-1 drug resistance and the role of resistance algorithms: data from seroconverters in the CASCADE collaboration from 1987 to 2003. J Acquir Immune Defic Syndr. 2005;40(5):505–511. doi: 10.1097/01.qai.0000186361.42834.61. [DOI] [PubMed] [Google Scholar]
- 52.Bannister WP, Cozzi-Lepri A, Clotet B, Mocroft A, Kjaer J, Reiss P, et al. et al. Transmitted drug resistant HIV-1 and association with virologic and CD4 cell count response to combination antiretroviral therapy in the EuroSIDA Study. J Acquir Immune Defic Syndr. 2008;48(3):324–333. doi: 10.1097/QAI.0b013e31817ae5c0. [DOI] [PubMed] [Google Scholar]
- 53.Vercauteren J, Wensing AMJ, van de Vijver DAMC, Albert J, Balotta C, Hamouda O, et al. et al. Transmission of drug-resistant HIV-1 is stabilizing in Europe. J Infect Dis. 2009;200(10):1503–1508. doi: 10.1086/644505. [DOI] [PubMed] [Google Scholar]
- 54.Oette M, Reuter S, Kaiser R, Lengauer T, Fantkenheuer G, Knechten H, et al. et al. Epidemiology of transmitted drug resistance in chronically HIV-infected patients in Germany. Intervirology. 2012;55(2):154–159. doi: 10.1159/000332015. [DOI] [PubMed] [Google Scholar]
- 55.Karlsson A, Biokman P, Bratt G, Ekvall K, Gisslen M, Sinnerborg A, et al. et al. Low prevalence of transmitted drug resistance in patients newly diagnosed with HIV-1 infection in Sweden 2003-2012. PLoS One. 2012;7(3):33484. doi: 10.1371/journal.pone.0033484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frentz D, Boucher CA, van de Vijver DA. Temporal changes in the epidemiology of transmission of drug-resistant HIV-1 across the world. AIDS Rev. 2012;14(1):17–27. [PubMed] [Google Scholar]
- 57.Avila-Raos S, Garci-a-Morales C, Garrido-Rodraguez D, Ormsby CE, Hernandez-Juan R, Andrade-Villanueva J, et al. et al. National prevalence and trends of HIV transmitted drug resistance in Mexico. PLoS One. 2011;6(11):27812. doi: 10.1371/journal.pone.0027812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soria J, Bull M, Mitchell C, La Rosa A, Dross S, Kraft K, et al. et al. Transmitted HIV resistance to first-line antiretroviral therapy in Lima, Peru. AIDS Res Hum Retroviruses. 2012;28(4):333–338. doi: 10.1089/aid.2011.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yebra G, de Mulder M, Perez-Elias MJ, Perez-Molina JA, Galan JC, et al. et al. Increase of transmitted drug resistance among HIV-infected sub-Saharan Africans residing in Spain in contrast to the native population. PLoS One. 2011;6(10):26757. doi: 10.1371/journal.pone.0026757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Emamzadeh-Fard S, Fard SE, Seyed Alinaghi S, Paydary K. Adherence to anti-retroviral therapy and its determinants in HIV/AIDS patients: A review. Infect Disord Drug Targets. 2012;12(5):346–356. doi: 10.2174/187152612804142251. [DOI] [PubMed] [Google Scholar]
- 61.Jahanbakhsh F, Hattori J, Matsuda M, Ibe S, Monavari SHR, Memarnejadian A, et al. et al. Prevalence of transmitted HIV drug resistance in Iran between 2010 and 2011. PLoS One. 2013;8(4):61864. doi: 10.1371/journal.pone.0061864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Amon JJ. Dangerous medicines: unproven AIDS cures and counterfeit antiretroviral drugs. Global Health. 2008;4:5. doi: 10.1186/1744-8603-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alinaghi SAS, Paydary K, Emamzadeh-Fard S, Mohraz M. Treatment with IMODTM as a novel immune modulator in HIV positive patients. J AIDS Clin Res. 2013;3(180):223–228. [Google Scholar]
- 64.Demeulemeester J, Tintori C, Botta M, Debyser Z, Christ F. Development of an alpha screen-based HIV-1 integrase dimerization assay for discovery of novel allosteric inhibitors. J Biomol Screen. 2012;17(5):618–628. doi: 10.1177/1087057111436343. [DOI] [PubMed] [Google Scholar]
- 65.Demarest JF, Amrine-Madsen H, Irlbeck DM, Kitrinos KM. Virologic failure in first-line human immunodeficiency virus therapy with a CCR5 entry inhibitor, aplaviroc, plus a fixed-dose combination of lamivudine-zidovudine: nucleoside reverse transcriptase inhibitor resistance regardless of envelope tropism. Antimicrob Agents Chemother. 2009;53(3):1116–1123. doi: 10.1128/AAC.01055-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hanna GJ, Lalezari J, Hellinger JA, Wohl DA, Nettles R, Persson A, et al. et al. Antiviral activity, pharmacokinetics, and safety of BMS-488043, a novel oral small-molecule HIV-1 attachment inhibitor, in HIV-1-infected subjects. Antimicrob Agents Chemother. 2011;55(2):722–728. doi: 10.1128/AAC.00759-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou N, Nowicka-Sans B, Zhang S, Fan L, Fang J, Fang H, et al. et al. In vivo patterns of resistance to the HIV attachment inhibitor BMS-488043. Antimicrob Agents Chemother. 2011;55(2):729–737. doi: 10.1128/AAC.01173-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Clavel F, Hance AJ. HIV drug resistance. N Engl J Med. 2004;350(10):1023–1035. doi: 10.1056/NEJMra025195. [DOI] [PubMed] [Google Scholar]
- 69.Paydary K, Esmaeeli S, Seyed Alinaghi S, Rouzrokh P, Emamzadeh-Fard S. Emerging HIV drug resistance in the resource-poor world: challenges and strategies. J AIDS Clin Res. 2013;5:2. [Google Scholar]
- 70.Ji H, Massa N, Tyler S, Liang B, Li Y, Merks H, et al. et al. HIV drug resistance surveillance using pooled pyrosequencing. PLoS One. 2012;5(2):9263. doi: 10.1371/journal.pone.0009263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goldberg DE, Siliciano RF, Jacobs JWR. Outwitting evolution: fighting drug-resistant TB, malaria, and HIV. Cell. 2012;148(6):1271–1283. doi: 10.1016/j.cell.2012.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hamers RL, Sigaloff KCE, Wensing AM, Wallis CL, Kityo C, Siwale M, et al. et al. Patterns of HIV-1 drug resistance after first-line antiretroviral therapy (ART) failure in 6 sub-Saharan African countries: implications for second-line ART strategies. Clin Infect Dis. 2012;54(11):1660–1669. doi: 10.1093/cid/cis254. [DOI] [PubMed] [Google Scholar]