Abstract
Objective
To assess the magnitude and antimicrobial susceptibility patterns of Streptococcus pneumoniae isolates from various clinical specimens.
Methods
A record based on retrospective study was conducted at Gondar University Teaching Hospital from September 2007 to January 2012. All patients who visited Gondar University Hospital and provided clinical specimens (body fluids, discharge, swab and blood) for routine bacteriological culturing and antimicrobial susceptibility testing were taken for analysis. Clinical specimens were processed for bacterial culture according to the standard procedures. Antimicrobial susceptibility test for isolated organisms was done using agar disk diffusion method. The data were entered and analyzed using SPSS software version 16 package.
Results
One hundred and fifty three Streptococcus pneumoniae were isolated from patients who visited Gondar University Teaching Hospital bacteriology laboratory for culture. Majority of the pneumococcal isolates were from inpatients [111(72.5%)], and 74(48.4%) were from body fluids. Out of the total isolates, 93(61%) were found to be resistant to at least one antibiotic used for susceptibility testing. Forty eight (43.2%) of the isolates were multi-drug resistant (resistant to two or more drugs). The resistance rate noted for both ciprofloxacin 17(11.1%) and ceftriaxone 15(9.8%) were alarming.
Conclusions
High proportions of the isolates tend to be increasingly resistant to the commonly prescribed drugs. The recommended drug of choice like ciprofloxacin and ceftriaxone were found to be less susceptible in the study area. Based on the findings, we therefore recommend that antimicrobial agents should be inspected for acceptable activity before they are prescribed and administered empirically. Further study with a better design and survey of antimicrobial susceptibility at large scale shoule be performed to draw advanced information.
Keywords: Streptococcus pneumoniae, Antimicrobial agents, Susceptibility patterns, Gondar Ethiopia
1. Introduction
Streptococcus pneumoniae (S. pneumoniae) remains a common pathogen and leading cause of morbidity and mortality[1]. Transmission of S. pneumoniae occurs as the result of direct person-to-person contact via respiratory droplets and by autoinoculation in persons carrying the bacteria in their upper respiratory tract[2]. This organism continues to be common causes of mild to severe and life threatening diseases including pneumonia, bacteremia and meningitis, and it is also a frequent causes of upper respiratory tract infections like otitis media and sinusitis[3].
S. pneumoniae was known to be completely susceptible to penicillin and other beta-lactam antibiotics. However, since 1980s, a dramatic increase in antibiotic resistance among S. pneumoniae has been observed in many parts of the world[4]–[6].
In recent times, pneumococcal strains with high level of resistance to penicillin have emerged, and these organisms have shown resistance to other antibiotics such as tetracycline, erythromycin, chloramphenicol, sulfonamide, ciprofloxacillin and clindamycin. An important increase in the prevalence of penicillin resistant S. pneumoniae isolates occurred in the early 1990s in USA[7]–[9]. Such an increase had previously been observed in other parts of the world[10]. Moreover, isolates of S. pneumoniae that are resistant to tetracycline, chloramphenicol, trimethoprim-sulfamethoxazole, erythromycin alone or in combination were being recovered more and more often in the USA[11]. This problem has also been recognized in the Asia-Pacific region[12], Europe[13] and South Africa[12] but data from the rest of African countries including Ethiopia is scarce.
In Ethiopia, there is lack of literatures on streptococcal drug resistance. However, few previous studies conducted showed the presence of resistance to commonly prescribed antibiotics except to ciprofloxacin[14]. Thus, it is imperative to inform clinicians and other health professionals, policy makers and programers about the distribution of S. pneumoniae and to produce a systematized data on drug susceptibility profile of this isolates in a regular base. As a result, an optimized empirical treatment policy would be designed. Therefore, this study would provide up-to-date data information on the magnitude and pattern of antimicrobial resistance of S. pneumoniae to the commonly prescribed antibiotics in the settings.
2. Materials and methods
2.1. Study design, period and settings
A record based on retrospective study was conducted at Gondar University Teaching Hospital, Gondar, Ethiopia from September 2007 to January 2012. Gondar town is located 737 km away from Addis Ababa and it has a projected population of 248 784. The hospital is serving more than 5 million of people from Gondar town and the adjacent regions.
2.2. Sampling technique and sample size
All patients who visited Gondar University Teaching Hospital Laboratory and gave clinical specimens for bacteriological culturing and antimicrobial susceptibility testing were included in the study. All clinical specimens of the patients who gave for bacteriological culturing and antimicrobial susceptibility testing from September 2007 to January 2012 were included in the study.
2.3. Data collection procedure
Data on socio-demographic variables (age and sex), types of clinical specimens, sender of clinical specimen and microbiological data were abstracted from patients' records in the microbiology laboratory registration book by using a pre-prepared data abstraction format.
2.4. Specimen collection and processing
Specimens for the recovery of S. pneumoniae from nasopharyngeal, throat and wound swabs, ear and eye discharges, cerebrospinal fluid, pleural, peritoneal and ascetic fluids, pus, urine, sputum, and blood were collected aseptically with sterile cotton plagued applicator sticks and sterile test tubes as per the routine clinical management of the patients. Specimens were immediately delivered to the bacteriology section for culture and bacteriological analysis. Specimens were cultured by the streak plate methods using wire loop into blood and chocolate agars (Oxoid Basing-stoke, UK) for cultural isolation of S. pneumoniae from those specimens and were incubated overnight at 37 °C. The chocolate agar plates were incubated in a candle jar so as to create a reduced oxygen tension (5%-10% additional CO2 tension). The media were observed for growth for up to 48 h and plates with characteristic growth of bacterial colonies were selected. Gram positivity was checked by Gram's staining procedure. Blood agar plates with alpha hemolytic colonies were isolated and tested for Optochin sensitivity so as to differentiate Pneumococci from other alpha hemolytic Streptococci. The alpha hemolytic isolates with characteristic colonies of S. pneumoniae which were optochin sensitive were presumptively taken as being S. pneumoniae. The disk diffusion test was performed to test susceptibility of the S. pneumoniae following the method of Bauer et al. A McFarland 0.5 standardized suspension of the bacteria in 0.85% sterile saline was then prepared and swabbed over the entire surface of chocolate agar plate (Oxoid Basking-stoke, UK) with a sterile cotton swab. A ring of disks (Mast Diagnostic, UK) containing single concentration of each antimicrobial agent was then placed onto the inoculated surface. Following overnight incubation at 37 °C, clear zones produced by antimicrobial inhibition of bacterial growth were measured in millimeters using straight-line ruler. The zone of diameter was interpreted using an interpretive chart for zone sizes as sensitive, intermediate and resistant as per the standard protocol[15]. The following antimicrobial agents and concentrations were used: penicillin (10 IU), ampicillin (30 µg), tetracycline (30 µg), chloramphenicol (30 µg), erythromycin (15 µg), co-trimoxazole (25 µg), gentamicin (10 µg), ciprofloxacin (5 µg), and ceftriaxone (30 µg). Reference strains, E. coli ATCC25922 and S. aureus ATCC25923 were used as controls.
2.5. Data processing and analysis
Data were entered and analyzed using SPSS statistical software version 16 package. Descriptive statistics were used to present the results of this study.
2.6. Ethical consideration
The study was conducted after obtaining institutional ethical clearance from research and publication office of University of Gondar. Permission was taken from Gondar University Teaching Hospital administrators and Bacteriology section. In addition, all information that was necessary for the management and further investigation was given to the responsible health professionals to create awareness.
3. Results
During the 6-year period from September 2007 to January 2012, a total of 153 S. pneumoniae isolates had been recovered from patients who visited Gondar University Teaching Hospital bacteriology laboratory for specimens cultured. Of the 153 isolates, 29(19%) were from male children less than 5 years of age and 26(17%) were from female children less than 5 years of age (Table 1).
Table 1. Distribution of S. pneumoniae isolates with age, sex, and sender of clinical specimen at Gondar University Teaching Hospital, Northwest Ethiopia from September 2007 to January 2012[n(%)].
| Characteristic | Frequency |
|||
| Male | Female | Total | ||
| Age | ≤5 | 29(19.0) | 26(17.0) | 55(36.0) |
| 6-15 | 21(13.7) | 17(11.1) | 38(24.8) | |
| 16-34 | 23(15.0) | 17(11.1) | 40(26.2) | |
| 35-55 | 11(7.2) | 6(3.9) | 17(11.1) | |
| >55 | 1(0.7) | 2(1.3) | 3(1.9) | |
| Sources | OPD | 18(11.8) | 24(15.7) | 42(27.5) |
| Ward | 59(38.4) | 52(34.0) | 111(72.5) | |
| Total | 77(50.3) | 76(49.7) | 153(100.0) | |
n: individual number; OPD: Out Patient Department.
Of the 153 pneumococcal isolates, 74(48.4%) were from body fluids followed by 23(15%) from wound discharge, 19(12.4%) from eye discharge, 17(11.1%) from swabs, 8(5.2%) from ear discharge, 7(4.6%) from pus, and 4(2.6%) were from urine. Majority of the pneumococcal isolates were from ward [111(72.5%)], 59(38.4%) of which were from males and 52(34.0%) were from females (Table 2).
Table 2. Isolates of S. pneumoniae from specimens of outpatients and inpatients at Gondar University Teaching Hospital, Northwest Ethiopia from September 2007 to January 2012 [n(%)].
| Specimens | OPD | Ward | Total |
| Swabs | 10(6.50) | 7(4.60) | 17(11.10) |
| Body fluids | 10(6.50) | 64(41.80) | 74(48.40) |
| Ear discharge | 5(3.30) | 3(2.00) | 8(5.20) |
| Wound discharge | 7(4.57) | 16(10.50) | 23(15.00) |
| Eye discharge | 1(0.70) | 6(3.90) | 7(4.60) |
| Pus | 5(3.30) | 14(9.20) | 19(12.40) |
| Urine | 3(2.00) | 1(0.70) | 4(2.60) |
| Others | 1(0.70) | 0(0.00) | 1(0.70) |
| Total | 42(27.50) | 111(72.50) | 153(100) |
OPD: Out Patient Department; n: number of isolates.
A total of 93(61%) were found to be resistant to at least one antibiotic tested. Resistance to two or more antimicrobials (multiple drug resistance) was observed in 66(43.2%) of the isolates. In detail, 80(11.8%), 13(8.5%), 8(5.2%), 13(8.5%) and 14(9.2%) of the isolates were found to be resistant to two, three, four, five, six or more antibiotics, respectively. The resistance rate noted for both ciprofloxacin 17(11.1%) and ceftriaxone 15(9.8%) were alarming. The rate of resistance for ampicillin, penicillin G, tetracycline, co-trimoxazole, chloramphenicol, erythromycin, and gentamicin were 44(29.0%), 48(31.3%), 48(31.3%), 49(32.0%), 44(29.0%), 31(20.3%), and 43(28.1%), respectively. One isolate from 1.5 year old male infant cerebrospinal fluid sample was found to be resistant to all antibiotics tested. Table 3 depicts the susceptibility pattern and the rate of MDR among the isolates.
Table 3. In vitro antimicrobial susceptibility patterns of S. pneumoniae isolate at Gondar University Teaching Hospital, Northwest Ethiopia from September 2007 to January 2012 [n(%)].
| Antibiotics | Susceptible isolate | Resistant (Intermediate+ Complete) isolate |
| Ampicillin | 109(71.0) | 44(29.0) |
| Penicillin G | 105(68.7) | 48(31.3) |
| Tetracycline | 105(68.7) | 48(31.3) |
| Co-trimoxazole | 104(67.9) | 49(32.0) |
| Chloramphenicol | 109(71.0) | 44(29.0) |
| Erythromycin | 122(79.7) | 31(20.3) |
| Gentamicin | 110(71.9) | 43(28.1) |
| Ciprofloxacin | 136(88.9) | 17(11.1) |
| Ceftriaxone | 138(91.2) | 15(9.8) |
n: individual number.
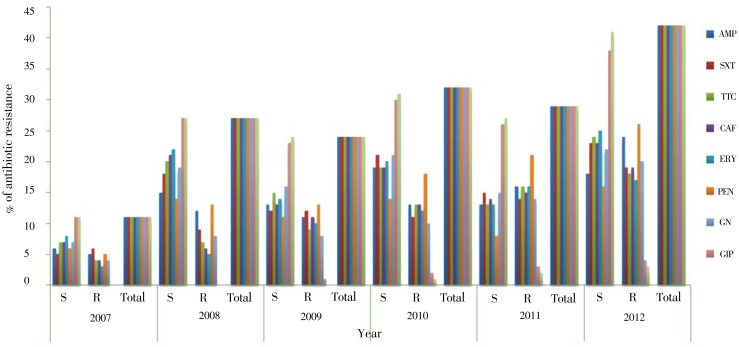
Figure 1 shows the trends of antimicrobial susceptibility patterns of S. pneumoniae isolates. They tend to be increasingly resistant to the commonly prescribed drugs as well as the recommended drugs of choice like ciprofloxacin and ceftriaxone which were found to be decreased susceptible in each year of the study area.
Figure 1. Trends of antimicrobial susceptibility patterns of S. pneumoniae isolates in each year at Gondar University Teaching Hospital, Northwest Ethiopia from September 2007 to January 2012.
AMP: Ampicillin; SXT: Sulfamethoxazole (Co- trimoxazole); TTC: Tetracycline; CAF: Chloramphenicol; ERY: Erythromycin; PEN: Penicillin G; GN: Gentamicin; CIP: Ciprofloxacin; CEF: Ceftriaxone.
4. Discussion
The successful management of patients suffering from bacterial illness relies on the identification of the types of organisms that causes the diseases and the selection of an effective antibiotic against organisms in question[2]. Thus, findings in this study could provide information on the selection of antimicrobial agents for the treatment of S. pneumoniae in northwest Ethiopia.
All penicillin resistant isolates in this study were also resistant to one or more of the commonly used antimicrobials which is consistent to the findings of researches conducted in Malaysia, Ethiopia, and USA[14],[16]. The rate of penicillin resistant S. pneumoniae found in this study (31.3%) is in line with the study conducted in USA (29.3%)[16]. However, the rate of penicillin resistant S. pneumoniae in our study (31.3%) is greater than the rate of penicillin resistant S. pneumoniae at Kota Bharu Malaysia (3%), USA (21.2%), Addis Ababa Ethiopia (17%), and Span 25.9%[16],[17] but lower than studies conducted in Hawassa, Ethiopia (64.2%), Kuwait (63%), Nigeria (83%) and 48.6% Israel[14],[18],[19]. The reasons might be due to the difference in study design, study period, methodology used and types as well as size of samples included in the study.
A study conducted in Italy forwarded an increase of pneumococcal resistance to erythromycin from 6% to 31.7%, which is in agreement to the increase of resistance to erythromycin from the previous study showed 11.5% to 20.3% in the present study area, respectively[18]. However, the present study is inconsistent with studies conducted in USA, which showed (29.3%) pneumococcal resistance to erythromycin[15], Kuwait (42%)[18], Nigeria (56.6%)[20], Bangladesh (74%)[21] and Sweden (26%)[22]. The difference might due to study design and methodology difference, and majority of the studies used dilution techniques.
Different studies illustrated that growing of the third generation antimicrobial resistance, and this reference reduced the use of antimicrobial drugs that show insufficient sensitivity against S. pneumoniae to prevent resistance and associated treatment failure. The present findings of this study showed that 11.1% of the isolates were resistant to ciprofloxacin which is inconsistence with studies conducted in Nigeria (20%)[20] and the previous study done in Gondar, Ethiopia (16%)[23]. However, our result stands in opposition to another study conducted in Gondar, Ethiopia which depicted no (0%) resistance to ciprofloxacin[24]. This might be due to an ever increasing empirical treatment of meningitis and other infections by this antimicrobial agent.
A study conducted in Israel showed that there was an increasing rate of ceftriaxone resistance which rose from 0% to 10% of intermediate resistance in 1998-1999[25]. Results of our study revealed that 9.8% of resistance to ceftriaxone is in line with study conducted in Dhaka, Bangladesh (8%)[21]and Israel (8.6%)[19]. However, inconsistence with the study done in Kuwait showed 15%[18]. This might due to the difference in the methodology and study design used in the study period.
The emergence of multiple drug-resistances has complicated the empirical treatment of pneumococcal infections. Several surveillance programs in various countries indicated that the proportion of drug-resistant S. pneumoniae isolates continues to increase worldwide[26]. In the present study, 43.2% of the isolates showed resistance to two or more antibiotic (Multidrug resistance was defined as resistance to ≥2 antimicrobial agents) which is higher than that in the study conducted in Gondar, Ethiopia (24%)[24], USA (30%)[16], Nigeria 12.7%[20], Spain 14.8%[17]and Kenya 7.6%[27]. However, it is lower than the study conducted in Hawassa Ethiopia showed 64.2%[14]. There is an exceedingly high rate of resistance of microorganisms to different antibiotics commonly prescribed[28]. This reasoning is also based on the fact that empirical treatments, overuses and indiscriminate use of antibiotics in developing countries and exhaustively uses in developed countries.
This study showed that substantial amount of commonly used drugs have demonstrated a decrease in activity on pneumococcal isolates as shown by an increase in the proportion of intermediate susceptibility and resistance to one or more of these drugs. High proportions of the isolates tend to be increasingly resistant to the commonly prescribed drugs in the area. This condition seeks greater emphasis more than ever before and appropriate measures enacted immediately. We recommended that performing of antimicrobial susceptibility tests before they are prescribed and administered empirically. Moreover, the recommended drug of choice like ciprofloxacin and ceftriaxone are found to have decreased susceptibility in S. pneumoniae isolates, and further study with a better design and survey of antimicrobial susceptibility at large scale should be done to draw advanced information.
Isolation of S. pneumoniae was merely by conventional culture methods (colony characteristics) and biochemical tests. However, inconsistence availability of the biochemical test sometimes may not differentiate the species and thus the findings are reported as Streptococcus species. This may have an impact on the findings of our study. In addition, the number and types of antibiotics used for susceptibility testing vary from time to time, and susceptibility test was done with disc diffusion methods. These may have a direct effect on our findings.
Acknowledgments
This work was supported by Research and Community Services of University of Gondar with Grant No. RPO/55/43/2008. The authors are indebted to the University of Gondar Teaching Hospital Laboratory, Bacteriology section staff for their support and facilities during this the study.
Comments
Background
Antimicrobial resistance among S. pneumoniae continues to be a serious healthcare concern. Since 1980s, a dramatic increase in antibiotic resistance among S. pneumoniae has been observed in many parts of the world, especially for the frequently utilized beta-lactams, macrolides and fluoroquinolones. Consequently, there is a need to survey national and local changes in antimicrobial susceptibility patterns in order to monitor the increase in resistance, provide better therapy for patients, and maintain the effectiveness of available drugs.
Research frontiers
This study demonstrated an increase in the proportion of intermediate susceptibility and resistance to one or more of commonly used drugs. High proportions of the isolates tend to be increasingly resistant to the commonly prescribed drugs in the study area.
Related reports
Trends in antibacterial resistance among S. pneumoniae isolates which showed only penicillin and erythromycin resistance among pneumococcal isolates has remained high (Stephen et al., 2008). In this study, the recommended drugs of choice like ciprofloxacin and ceftriaxone were found to have decreased susceptibility in the study area. This may be due to the fact that empirical treatments, overuses and indiscriminate use of antibiotics in developing countries and exhaustively uses in developed countries.
Innovations and breakthroughs
In this study, 43.2% of the isolates showed resistance to two or more antibiotic (Multidrug resistance was defined as resistance to ≥2 antimicrobial agents) which is higher than studies conducted in Gondar, Ethiopia (24%) as well as many other underdeveloped countries.
Applications
It may be significant to know the distribution of S. pneumoniae and to produce a systematic data on drug susceptibility profile of these isolates in a regular base. As a result, an optimized empirical treatment policy would be designed. Therefore, this study would provide up-to-date data information on the magnitude and pattern of antimicrobial resistance of S. pneumoniae to the commonly prescribed antibiotics in the settings.
Peer review
The authors have made an attempt to assess the magnitude and antimicrobial susceptibility patterns of S. pneumoniae isolates from various clinical specimens. The manuscript is well written and provides significant contribution and importance in the concerned area.
Footnotes
Foundation Project: This work was supported by Research and Community Services of University of Gondar with Grant No. RPO/55/43/2008.
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Ying QH, Zhang JJ, Yang L, Ding JL, Song XH, Du W, et al. et al. Antimicrobial resistance, serotypes and genotypes of Streptococcus pneumoniae isolates associated with ocular infection in China. Afr J Microbiol Res. 2012;6(1):160–164. [Google Scholar]
- 2.Patrick RM, Kent R. Medical Microbiology. 4th ed. St Louis, USA: Mosby Inc; 2002. pp. p.232–234. [Google Scholar]
- 3.Rijal B, Tandukar S, Adhikari R, Tuladhar NR, Sharma PR, Pokharel BM, et al. et al. Pattern and serotyping of Streptococcus pneumoniae isolated from Kanti Children Hospital in Nepal. Kathmandu Univ Med J. 2010;8(2):164–168. doi: 10.3126/kumj.v8i2.3551. [DOI] [PubMed] [Google Scholar]
- 4.Sabrina JM, Steinbakk M, Aboud S, Mkopi N, Kasubi M, Blomberg B, et al. et al. Penicillin resistance and serotype distribution of Streptococcus pneumoniae in nasopharyngeal carrier children under 5 years of age in Dar es Salaam, Tanzania. J Med Microbiol. 2012;61(7):952–959. doi: 10.1099/jmm.0.042598-0. [DOI] [PubMed] [Google Scholar]
- 5.So Hyun K. Changing trends in antimicrobial resistance and serotypes of Streptococcus pneumoniae isolates in Asian countries: an Asian Network for Surveillance of Resistant Pathogens (ANSORP) study. Antimicrob Agents Chemother. 2012;56(3):1418–1426. doi: 10.1128/AAC.05658-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sara H, Takele L, Gabremaskal H, Wondu A, Vicky C, Zhaoxia Z, et al. et al. The decline penicillin resistance after cessation of mass antibiotic distribution for trachoma. Clin Infect Dis. 2010;51(5):571–574. doi: 10.1086/655697. [DOI] [PubMed] [Google Scholar]
- 7.Gertz RE., Jr Increased penicillin non-susceptibility of nonvaccine-serotype invasive pneumococci other than serotypes 19A and 6A in post-7-valent conjugate vaccine era. J Infect Dis. 2010;201:770–775. doi: 10.1086/650496. [DOI] [PubMed] [Google Scholar]
- 8.Abubakar MS, Fatihu MY, Ibrahim NDG, Oladele SB, Abubakar MB. Camel pneumonia in Nigeria: Epidemiology and bacterial flora in normal and diseased lung. Afr J Microb Res. 2010;9:2479–2483. [Google Scholar]
- 9.Biek D, Critchley IA, Riccobene TA, Thye DA. Ceftaroline fosamil: a novel broad-spectrum cephalosporin with expanded anti- Gram-positive activity. J Antimicrob Chemother. 2010;65(4):9–16. doi: 10.1093/jac/dkq251. [DOI] [PubMed] [Google Scholar]
- 10.Reddy EA, Shaw AV, Crump JA. Community-acquired bloodstream infections in Africa: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:417–432. doi: 10.1016/S1473-3099(10)70072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marchesz D, Ardito F, Fudda G, Fontana R, Lo Casscio G, Nicoletti G, et al. et al. The sentinel project; An update on the prevalence antimicrobial resistance in community acquired respiratory S. pneumoniae and Haemophilus species in Italy. Int J Antimicrob Agents. 2005;26:8–12. doi: 10.1016/j.ijantimicag.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 12.Ashley EA, Lubell Y, White NJ, Turner P. Antimicrobial susceptibility of bacterial isolates from community acquired infections in Sub-Saharan Africa and Asian low and middle income countries. Trop Med Int Health. 2011;16(9):1167–1179. doi: 10.1111/j.1365-3156.2011.02822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laval CB, Pimenta FC. Serotypes of carriage and invasive isolates of Streptococcus pneumoniae in Brazilian children in the era of pneumococcal vaccine. Clin Microbiol Infect. 2006;12(1):50–55. doi: 10.1111/j.1469-0691.2005.01304.x. [DOI] [PubMed] [Google Scholar]
- 14.Derese D, Eskindir L, Araya G. Streptococcus pneumoniae and antimicrobial resistance Hawassa University Referral Hospital. J Med Lab Diagn. 2011;2(3):27–30. [Google Scholar]
- 15.Clinical and Laboratory Standards Institute . Performance standards for antimicrobial susceptibility testing. 21st informational supplement M100-S21. Pennsylvania: Clinical and Laboratory Standards Institute; 2011. [Google Scholar]
- 16.Jenkins SG, Brown SD, Farrell DJ. Trends in antibacterial resistance among Streptococcus pneumonia isolated in the USA: update from PROTEKT US Years 1-4. Ann Clin Microbiol Antimicrob. 2008;7(1):1–11. doi: 10.1186/1476-0711-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vila-Corcoles A, Bejarano-Romero F, Salsench E, Ochoa-Gondar O, de Diego C, Gomez-Bertomeu F, et al. et al. Drug-resistance in Streptococcus pneumoniae isolates among Spanish middle aged and older adults with community-acquired pneumonia. BMC Infect Dis. 2009;9(36) doi: 10.1186/1471-2334-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mokaddas EM, Rotimi VO, Albert MJ. Increasing prevalence of antimicrobial resistance in Streptococcus pneumoniae in Kuwait: Implications for therapy. Microbial Drug Resist. 2007;13(4):227–233. doi: 10.1089/mdr.2007.774. [DOI] [PubMed] [Google Scholar]
- 19.Waijburd-Zinman O, Bilausky E, Tirosh N, Samra Z, Amir J. Penicillin and ceftriaxone susceptibility of S. pneumoniae isolated from CSF of children with meningitis hospitalized in a tertiary hospital in Israel. Isr Med Assoc J. 2010;12(4):225–228. [PubMed] [Google Scholar]
- 20.Akanbi HAA, Taiwo SS, Babatunde SK, Onile BA, Abdulraheem IS. Antibiotic susceptibility pattern of Streptococcus pneumoniae in Ilorin, Nigeria. Afr J Clin Exp Microbiol. 2004;5(2):173–176. [Google Scholar]
- 21.Shahriar M, Madak M, Haque A, Kabir S, Saha MR. Current status of antimicrobial sensitivity pattern of Streptococcus pneumonia strains collected from clinical sources in Dhaka, Bangladesh. Stamford J Pharm Sci. 2010;3(1):59–62. [Google Scholar]
- 22.Hogberg L, Fkdahl K, Sjostrom K, Olsson-Liljequist B, Walder M, Melander E, et al. et al. Penicillin-resistance pneumococci in Sweden 1997-2003: Increase multi-resistance despite stable prevalence and decrease antibiotic use. Microbial Drug Resist. 2006;12(1):16–22. doi: 10.1089/mdr.2006.12.16. [DOI] [PubMed] [Google Scholar]
- 23.Sebhat E, Yenew K, Andargachew M. Increased resistance of Streptococcus pneumoniae isolates to antimicrobial drugs at a Referral Hospital in North West Ethiopia. Trop Doct. 2008;38:110–112. doi: 10.1258/td.2007.006190. [DOI] [PubMed] [Google Scholar]
- 24.Andargachew M, Belay T, Afework K. Bacterial isolates from CSF and their antimicrobial susceptibility pattern in Gondar University Hospital. Ethiop J Health Dev. 2005;19(2):160–164. [Google Scholar]
- 25.Greenberg D, Dagan R, Muallem M, Porat N. Antibiotic-resistance invasive pediatric S. pneumoniae clones in Israel. J Clin Microbiol. 2003;41(12):5541–5545. doi: 10.1128/JCM.41.12.5541-5545.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoa NQ, Trung NV, Larsson M, Eriksson B, Phuc HD, Nguyen TK Chuc, et al. et al. Decreased Streptococcus pneumoniae susceptibility to oral antibiotics among children in rural Vietnam: a community study. BMC Infect Dis. 2010;10:85. doi: 10.1186/1471-2334-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kariuki S, Muyodi J, Miraza B, Muatu W, Daniels JJD. Antimicrobial susceptibility in community acquired-bacterial pneumonia in adults. East Afr Med J. 2003;8(4):213–217. doi: 10.4314/eamj.v80i4.8645. [DOI] [PubMed] [Google Scholar]
- 28.Gebrehiwot A, Lakew W, Moges F, Anagaw B, Yismaw G, Unakal C, et al. et al. Bacterial profile and drug susceptibility pattern of neonatal sepsis in Gondar University Hospital, Gondar Northwest Ethiopia. Der Pharmacia Lettre. 2012;4(6):1811–1816. [Google Scholar]