Abstract
Objective
To determine the chemical composition, total phenolic and total flavonoid contents of the crude extracts from leaves and stems of a Lebanese plant Euphorbia macroclada schyzoceras (E. macroclada), and to evaluate their antioxidant potential using DPPH, H2O2, and chelating of ferrous ions tests.
Methods
Quantification of the total phenolic and total flavonoid contents of the crude extracts from leaves and stems and the antioxidant activities were evaluated using spectrophotometric analyses. The chemical composition has been estimated using different techniques such as IR, LC/MS and NMR.
Results
Ethanolic extract from leaves of E. macroclada was better than aqueous extract and showed higher content in total phenolic and total flavonoid than found in the stems. On the other hand, using DPPH and H2O2 tests, this extract from leaves showed higher antioxidant capacity than aqueous extract. However, using the chelating of ferrous ions test, the antioxidant activity of the aqueous extract of both stems and leaves was stronger than that of ethanolic once. The chemical composition of the whole plant showed the presence of some aromatic compounds and fatty acids.
Conclusions
Both ethanolic and water extracts from both parts of this plant are effective and have good antioxidant power. So, this plant can be used in the prevention of a number of diseases related to oxidative stress.
Keywords: Euphorbia macroclada schyzoceras, Total phenolic, Total flavonoid, Antioxidant potential, Chemical composition
1. Introduction
Throughout years, the plant kingdom has been subjected to an extensive chemical investigation. Thousands of samples have been screened for substances of medicinal values or for suitable precursors of therapeutically active compounds. Other plants have been studied chemically from the viewpoints of biosynthesis of active constituents. WHO estimated that 11% of drugs are obtained exclusively from plants, 10% of plants species are tested for different biological activities, and out of all discovered drugs, 252 drugs play important role in saving human life from fatal diseases.
Widespread in nature, and to be found in most classes of natural compounds, phenols are important constituents of some medicinal plants and food industry. They are utilized as coloring agents, flavoring, aromatizens and antioxidants. These natural compounds are found ubiquitously in fruits, nuts, seeds, flowers, vegetables, barks and herbs[1].
We all know that pesticides, tobacco smoke, ionizing radiation and pollution are a good source for free radical existence. Free radicals are chemical materials that have unpaired outer shell electron, that is extremely reactive capable of damaging transient chemical materials. Free radicals can also exist naturally intracellular in a cell: small cytoplasmic molecules, membrane enzymes, peroxisomes, cytoplasmic proteins, mitochondrial electron transport systems and microcosmic electron transport systems. Antioxidants are genuine phenolic substances that deactivate free radical electrons through three mechanisms: It either deactivates free radicals by hydrogen donation, or transfer antioxidants electron to inhibit or decrease activity of metals and carbonyl, or it does both previous mechanism at the same time[2]. Oxidative stress is involved in the pathology of cancer, arteriosclerosis, malaria and rheumatoid arthritis, and could play a role in neurodegenerative diseases and ageing processes[3],[4].
Since the discovery of antioxidant, many researches have been done to discover the importance of this substance, the benefits and the risks. Nowadays, the daily intake of natural antioxidant is closely related with the reduction of cardiovascular diseases, cancers, diabetes and those associated with aging.
Euphorbia macroclada schyzoceras (E. macroclada) belongs to the family of Euphorbiacae. It is widely found in various Lebanese regions. In recent study, it was found that leaves and stems of this plant contain different amounts of phenol, flavonoid, saponin, alkaloid, coumarin, terpenoid and tannin[5]. In this same study, the leaves and stems of this plant showed high antioxidant potential.
Our present study is aimed, for the first time, to quantify the total phenolic and total flavonoid contents of the crude extract from the stems and leaves of a Lebanese plant, E. macroclada, and to evaluate their antioxidant capacity using three in vitro known tests, the DPPH, H2O2, and chelating of ferrous ions. Furthermore, spectrophotometric analyses were employed for the determination of total phenolic and total flavonoid concentrations and for the antioxidant activity.
2. Materials and methods
2.1. Plant collection
Fresh plant was gathered from different regions in Lebanon on spring season between March and May in 2011 and the biological authentication was carried out by Professor George Tohme, president of C.N.R.S of Lebanon. Stems and leaves of this plant were left on air at room temperature for two weeks to be very well dried. After that, they were crushed up and ground to get homogeneous fine powder by a grinder and then kept in a dark place at room temperature till use in different studies.
2.2. Apparatus and chemicals
All the chemicals used were of analytical grade. Absolute ethanol, methanol, n-hexane and sodium hydroxide, ethyl acetate, dichloromethane were purchased from BDH England. Aluminum chloride and FeSO4•7H2O, silica gel was purchased from Merck Germany. Sodium carbonate and hydrogen peroxide were purchased from Unichem India. Ascorbic acid, gallic acid, rutin, Folin-Ciocalteau reagent, EDTA, Ferrozine and DPPH were purchased from sigma Aldrich, USA. PBS was purchased from Gibco, UK. 1H NMR and 13C NMR spectra were recorded on an Avance III (300) spectrometer at 300 Mhz, ESI-MS spectra were recorded on the Shimadzu Series.
2.3. Preparation of crude extracts
A total of 10 g of powdered leaves and stems of E. macroclada were putted into a flask with 500 mL of ethanol, and the mixture has been extracted by agitation for 5 h at 25 °C. Then, a maceration of the extracts was done overnight for 24 h. The ethanolic layer containing the extract was taken. The extraction was repeated on the remaining amount of the precipitate using 150 mL of ethanol and all extracts were filtered by using a 0.45 Millipore filter paper. Two fractions of extracts were mixed together and concentrated using a rotary evaporator at 40 °C under reduced pressure. Extracts were stored at -20 °C till their usage in different tests. The extracts resolved in ethanol and distilled water[6].
The aqueous extract has been prepared using the same steps of ethanolic extraction except the temperature of extraction should be 60 °C.
2.4. Determination of total phenolic content
The Folin–Ciocalteau reagent method has been used for the estimation of total phenolic extracts quantities according to Lister and Wilson[7]. Five concentrations of all crude extracts of the plant have been prepared and then 100 µL have been taken from each concentration and mixed with 0.5 mL of Folin–Ciocalteau reagent (1/10 dilution) and 1.5 mL of Na2CO3 20 g/mL. The blend was incubated in the dark at room temperature for 15 min. The absorbance of blue-colored solution of all samples was measured at 765 nm using a Gene Quant 1300 UV-Vis spectrophotometers. The results were expressed in milligram of gallic acid equivalent per gram of dry weight of plant powders.
2.5. Determination of total flavonoid content
The aluminum chloride method was used according to Quettier-deleu et al. for determination of total flavonoid content of all crude extracts of E. macroclada[8]. A total of 1 mL of various concentrations of all extracts was mixed with 1 mL of 2% methanolic aluminum chloride solution. After an incubation period at room temperature in the dark for 15 min, the absorbance of all samples was determined at 430 nm using a Gene Quant 1300 UV-Vis spectrophotometers. The results were expressed in mg/g of rutin equivalent and methanol was used as blank.
2.6. Antioxidant activity
2.6.1. DPPH radical scavenging activity
The method of Chew et al. has been used for the scavenging ability of DPPH antioxidant test[9]. A total of 1 mL of different concentrations of diluted extracts of each plant parts in ethanol was added to 1 mL of DPPH (0.15 mmol/L in ethanol) and at the same time, a control consisting on 1 mL DPPH with 1 mL ethanol was prepared. The reaction mixtures were mixed very well by hand and then incubated in the dark at room temperature for 30 min and the absorbance was measured at 517 nm by a Gene Quant 1300 UV-Vis spectrophotometer. The ascorbic acid was used as a positive control and the ethanol was used as blank. The DPPH scavenging ability of plant extracts was calculated using the following equation:
%Scavenging activity=Abs control−Abs sample/(Abs control)×100
Where, the Abs control is the absorbance of DPPH+ethanol; Abs sample is the absorbance of DPPH radical+sample. Also, three controls have been prepared.
2.6.2. Scavenging activity of H2O2
The H2O2 scavenging of crude extracts of E. macroclada was determined according to Ruch et al. method[10]. A solution of H2O2 (40 mmol/L) was prepared in PBS (pH 7.4) and concentration was determined spectrophotometrically at 230 nm. Different concentrations of extracts from stems and leaves of both plants in distilled water were added to H2O2 (0.6 mL, 40 mmol/L) and the absorbance of H2O2 at 230 nm was determined after 10 min against a blank solution containing the plants extracts without H2O2.ascorbic acid used as standard reference. The percentage scavenging of H2O2 was calculated using the following equation:
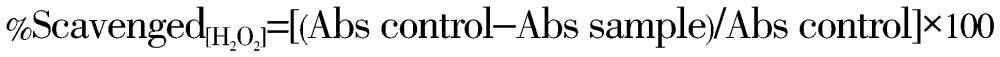 |
Where, the Abs control is the absorbance of DPPH+ethanol; Abs sample is the absorbance of DPPH radical+sample.
2.6.3. Ferrous ion chelating activity
The method of Dinis et al. has been used to estimate the chelating effect on ferrous ions with some modifications[11]. A total of 0.5 mL of various concentrations of all extracts of E. macroclada was mixed with 0.5 mL of FeSO4 (0.12 mmol/L), and with 0.5 mL of ferrozine (0.6 mmol/L). The mixtures were allowed to stand for 10 min at room temperature. After incubation, the absorbance was measured by a spectrophotometer at 562 nm. Ultra-pure water of sample solution was used as a control without extracts. Ultra-pure water instead of ferrozine solution was used as a blank. EDTA was used as reference standard. All measurements were performed in triplicate. The ferrozine solution (3-[2-Pyridyl]-5,6-diphenyl-1,2,4-triazine-4,4′-disulfonic acid Na-salt) (0.6 mmol/L) was prepared in ultra-pure water and stored in the dark at room temperature. The ability of the sample to chelate ferrous ion was calculated relative to the control (consisting of iron and ferrozine only) using the following formula:
Ferrous ion-chelating ability(%)=[(Abs control−Abs sample)/Abs control]×100
Where, the Abs control is the absorbance of DPPH+ethanol; Abs sample is the absorbance of DPPH radical+sample.
2.7. Chemical analysis by IR, LC-MS and NMR
E. macroclada (25 g) were ground into powder and extracted in 80% methanol (500 mL) at reflux temperature for 8 h after 24 h of maceration. The extract was filtered through a Büchner funnel under reduced pressure. After, the extract was concentrated to 100 mL under reduced pressure at 40 ˚C. The concentrated aqueous extract was washed three times with n-hexane with the same volume and then aqueous phase was again extracted three times with ethyl acetate in the same volume. The solvent was then removed under reduced pressure at 40 ˚C. The sticky residue was loaded onto a silica gel column and eluted with methanol/dichloromethane (1:1, 300 mL). The solvent was removed to give a mixture of flavonoids, which is analyzed in LC-MS and NMR instrument to determine their structure[12].
2.8. Mass spectrometry ESI ionization analysis
Analysis occurs on LC-PDA with (150.0×2.0) mmol/L i.d. (Shimadzu, Japan) C18 column, operating on a flow of 0.2 mL/min. MS analysis was performed with ESI ionization in negative mode with a quadruple analyzer.
Gradient of mobile phase: (A) ACN; (B) water+0.06% acetic acid. Elution: 5 min 80% B, decreasing B to 70% in 25 min, then to 45% in 25 min, and stayed constant at 45% for 10 min.
2.9. Statistical analysis
All analyses were carried out in triplicates. The results of scavenger activity, total phenolic and total flavonoid contents were performed from the averages of all samples reading mean±SD using Excel 2003 and SPSS 16 on student one test.
3. Results
The total phenolic and total flavonoid contents in leaves and stems of E. macroclada have been evaluated. The quantitative determination of TPC is expressed as milligram gallic acid equivalents per gram dry weight of sample. As shown in Table 1, both leaves and stems contain high amounts of TPC and TFC. It is clearly appearing that EtOH fraction of both leaves and stems of this plant contains the higher amount of polar phytochemicals, where another fraction has less amounts. The aqueous fraction from leaves has higher amounts of TPC and TFC than that of stems. At the same time, the EtOH fraction from leaves has higher amounts of TPC and TFC than that of stems (Table 1).
Table 1. TPC and TFC in the leaves and stems of E. macroclada.
| Extracts | Leaves (%) |
Stems (%) |
||
| Aqueous | EtOH | Aqueous | EtOH | |
| TPC (mg GA/g of DW) | 3.24±0.07 | 4.04±0.04 | 1.80±0.06 | 2.50±0.06 |
| TFC (mg R/g of DW) | 1.40±0.04 | 2.40±0.05 | 1.10±0.05 | 1.30±0.04 |
Values are the average of triplicate experiments and values expressed as mean±SD.
Results showed that both leaves and stems of E. macroclada have exerted high antioxidant power at different concentrations. The DPPH test demonstrated that 2.5 mg/mL of the aqueous and EtOH extracts from leaves of E. macroclada have significantly increased the percentage of scavenger activity by 67% and 78%, respectively as shown in Table 2. On the other hand, both extracts from the stems of E. macroclada have exerted a scavenger activity by 56% and 50% respectively (Table 2).
Table 2. DPPH scavenging assay of leaf and stem extracts of E. macroclada.
| Concentration (mg/mL) | Leaves (%) |
Stems (%) |
||
| Aqueous | EtOH | Aqueous | EtOH | |
| 0.5 | 43.00±0.04 | 36.00±0.05 | 16.00±0.06 | 2.00±0.04 |
| 1.0 | 44.00±0.04 | 55.00±0.04 | 34.00±0.03 | 13.00±0.03 |
| 1.5 | 48.00±0.03 | 71.00±0.04 | 41.00±0.02 | 27.00±0.02 |
| 2.0 | 54.00±0.03 | 75.00±0.02 | 51.00±0.03 | 40.00±0.04 |
| 2.5 | 67.00±0.04 | 78.00±0.03 | 56.00±0.04 | 50.00±0.06 |
Values are the average of triplicate experiments and values expressed as mean±SD.
On the other side, results obtained by using H2O2 test demonstrated that the aqueous and EtOH crude extracts from this plant were capable of scavenging H2O2 in a concentration dependent manner. The percentage of scavenger activity of leaves of E. macroclada (1.6 mg/mL) was higher than that of stems as shown in Table 3. This percentage was 89% for the aqueous crude extract and 93% for the EtOH crude extract.
Table 3. H2O2 free radical scavenging assay of leaf and stem extracts of E. macroclada.
| Concentration mg/mL | Leaves (%) |
Stems (%) |
||
| Aqueous | EtOH | Aqueous | EtOH | |
| 0.3 | 44.00±0.02 | 12.00±0.01 | 5.00±0.04 | 9.00±0.03 |
| 0.6 | 52.00±0.06 | 50.00±0.06 | 13.00±0.03 | 16.00±0.02 |
| 1.0 | 67.00±0.02 | 72.00±0.02 | 25.00±0.02 | 32.00±0.03 |
| 1.3 | 76.00±0.02 | 87.00±0.03 | 42.00±0.02 | 38.00±0.03 |
| 1.6 | 89.00±0.03 | 93.00±0.02 | 54.00±0.02 | 60.00±0.03 |
Values are the average of triplicate experiments and values expressed as mean±SD.
Table 4 shows the metal chelating effect of two crude extracts from E. macroclada leaf and stem. Ferrozine can quantitatively form complexes with Fe2+. In the presence of samples possessing chelating activity, the formation of complexes is decreased. Therefore, measurement of the rate of color reduction helps to estimate the chelating activity of the samples. As shown in Table 4, chelating capacity of the crude extracts increases with the increase in concentration. Among the two parts of this plant, leaves show higher chelating activity compared to the stems.
Table 4. Iron chelating scavenging assay of leaf and stem extracts of E. macroclada.
| Concentration (mg/mL) | Leaves (%) |
Stems (%) |
||
| Aqueous | EtOH | Aqueous | EtOH | |
| 0.10 | 13.00±0.02 | 37.00±0.04 | 3.00±0.02 | 28.00±0.05 |
| 0.25 | 21.00±0.03 | 53.00±0.04 | 5.00±0.01 | 28.00±0.05 |
| 0.50 | 32.00±0.05 | 63.00±0.06 | 34.00±0.02 | 54.00±0.04 |
| 0.75 | 41.00±0.04 | 66.00±0.05 | 36.00±0.02 | 68.00±0.08 |
| 1.00 | 84.00±0.02 | 79.00±0.05 | 70.00±0.02 | 74.00±0.06 |
Values are the average of triplicate experiments and values expressed as mean±SD.
Identification of a flavone derived compound 1:
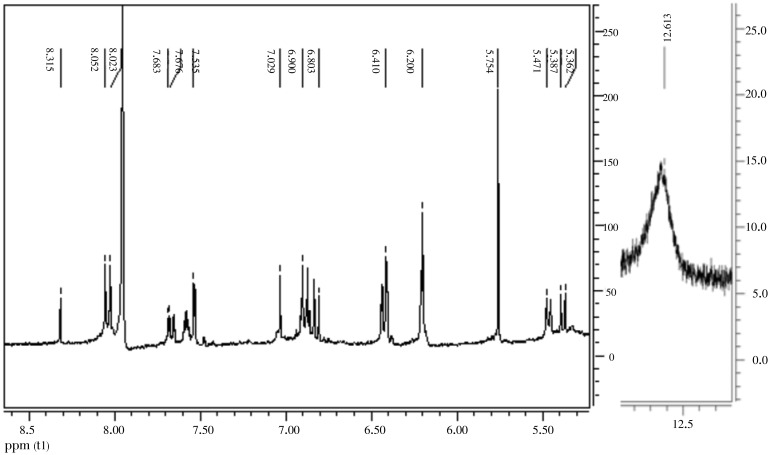
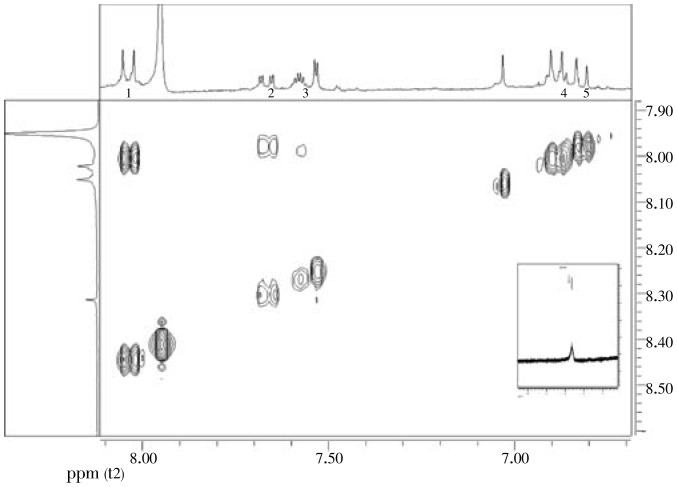
An earlier analysis by IR and NMR showed a mixture of aromatic compounds, among it we can differentiate a flavonoid skeleton. Its IR spectrum exhibited absorption bands (broad) at 3 442 cm−1 (hydroxyl), and at 1 644 cm−1 (carbonyl) compatible with a flavonoid skeleton. 1H NMR spectrum (Figure 1 and Figure 2) of this minor compound attributes a singlet signal at δ12.5 (s) for achelated hydroxyl group at carbon atom C5. Two multiplets at δ8.14 (m, H-2′/H-6′) and 7.57 (m, H-3′/H-4′/H-5′) are appropriate for a mono-substituted benzene ring. Two singlets are at δ6.88 (s, H-6) and 6.84 mg/L (s, H-3″). Carbonyl group at the carbon atom C4 was confirmed by 13C NMR at 178 or 177 mg/L, and as it was showed in MS-ESI, the ions at m/z 367, 339 and 311 were observed, which is attributed to the loss of CO and 2CO respectively. Detailed analysis of 2D NMR spectral data of 6 revealed and affirmed the correlation between these protons for each compound (Figure 2)[20]–[22].
Figure 1. 1H NMR spectrum of the methanol extract showed a signal at δ 12.5 (s) for a chelated hydroxyl group.
Figure 2. NMR 1H-1H COSY and 1H NMR spectrum of extract in DMSO-d6.
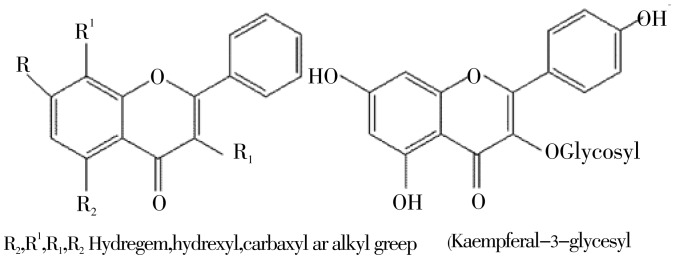
This NMR spectral data (Proton and COSY) combined with the infrared suggested the presence of a flavone derive (Figure 3).
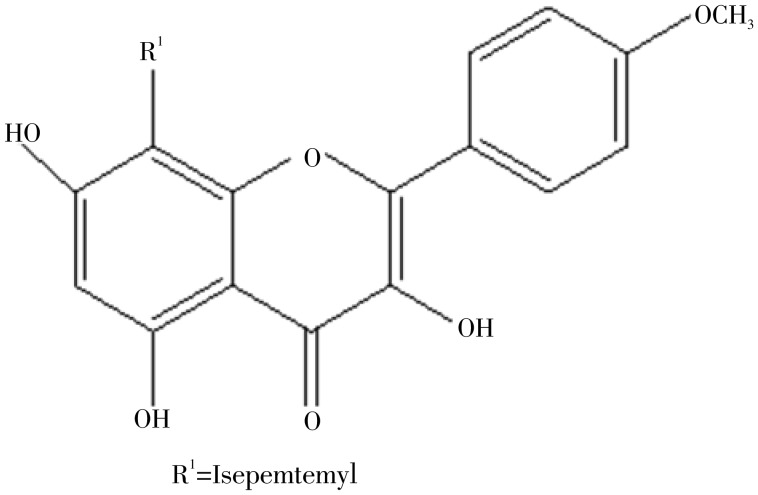
Figure 3. Suggested structure of flavonoid 1 and 2.
Identification of a second aromatic compound 2:
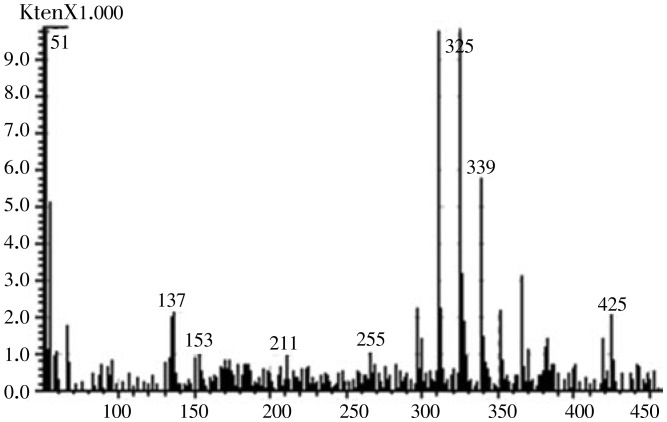
The 1H NMR of flavonoid 2 obtained in traces showed typical signals of substitution for the standard kaempferol flavonoid. The A ring of flavonoid 2 (Figure 4) showed two doublets (J 2.1 Hz) at δH 6.2 and 6.4 mg/L attributed to hydrogen atoms H-6 and H-8, respectively. A correlation between these 2 protons was confirmed by COSY 2D-NMR. The presence of AA′BB′ system in the B ring (4′-hydroxylated) of the flavonoid was revealed by 1H NMR spectra through doublet signals corresponding to H-2′/H-6′ at δH 8.05 (1H, J 8.9 Hz), and H-3′/H-5′ at δH 6.85 (1H, J 8.9 Hz). The COSY spectra showed cross peaks indicating direct 2D-shift-correlations between these hydrogen signals. Singlet signal at δH 12.5 was attributed to the hydroxyl group at carbon atom C-5, and the presence of a carbonyl group at carbon atom C-4 was confirmed by 13C NMR at 177 mg/L. Comparative analysis of 1H (1D and 2D 1H-1H COSY) NMR spectra of 2 revealed the additional presence of signals which allowed the identification of the glycoside moiety represented by two doublets (axial-axial couplings) signals corresponding to anomeric hydrogens H-1g at δH 5.4 and 5.3 mg/L, these two signals indicate the presence of a glucosidic linkage in compound 2 along with unresolved signals for other sugar protons between δH 3-4 mg/L. The Mass Spectrometry showed [M+H]-ion at m/z 447 (Figure 5), suggesting this as a kaempferol glycoside (Figure 4). Further investigations about all the other signals in NMR are underway.
Figure 4. Suggested structure of flavonoid 3.
Figure 5. Electrospray ionization mass spectrometry (ESI-MS) in negative mode (axis X is the ion intensity and Y axis corresponds to the m/z ratio).
Another structure can be proposed by MS spectrum (Figure 5) and attributed to an anhydroicaritin derived: The fragmentation pathway of [M−H]−ion of compound 3 was shown in the negative mode. Firstly, the [M−H]−ion produced a prominention at m/z 367 which generate a moderate [M−H−CH3]•−ion at m/z 352. In the further fragmentation, the ion at m/z 352 yielded the specific ions at m/z 297 corresponding to [M−H−C4H7]− caused by the cleavage of isopentenyl[23].
4. Discussion
It has been proposed that the health beneficial effects of polyphenols could result from either their antioxidant functions and/or independently from these properties e.g. by acting as modulators of cellular signaling processes[13],[14]. Recent studies showed that Lebanese plants contain high amount of different phytochemical products mainly phenol, flavonoid that have various biological properties such as antioxidant[15],[16]. The obtained results from the evaluation of the antioxidant activity of the two parts of E. macroclada showed that it possesses a high antioxidant power that may be benefit to the prevention from the diseases related to oxidative stress.
On the other hand and in order to confirm the antioxidant power of this plant, H2O2 test was used. H2O2 is an important reactive oxygen species because of its ability to penetrate biological membranes. However, it may be toxic if converted to hydroxyl radical in the cell[17]. Scavenging of H2O2 by the plant extracts may be attributed to their phenolics, which donate electron to H2O2, thus reducing it to water.
Antioxidants inhibit interaction between metal and lipid through formation of insoluble metal complexes with ferrous ion. Another test has been used with the aim of evaluating the antioxidant capacity of the two studied parts of E. macroclada, the metal iron chelating test. The iron-chelating capacity test measures the ability of antioxidants to compete with ferrozine in chelating ferrous ion. Ferrozine can quantitatively form complexes with Fe2+. However, in the presence of chelating agents, the complex formation is disrupted with the result that the red color of the complex is decreased. Measurement of color reduction, therefore, allows the estimation of chelating activity of the coexisting chelator. In our obtained results, the leaves of the studied plants exerted higher capacity to scavenge the metal and by consequence they have better antioxidant power. These results may be due to the high content of polyphenol in leaves then in stems.
In the present study, the antioxidant activity of the plant extracts was evaluated by three in vitro methods. The antioxidant activity of phenolic compounds is mainly due to their redox properties which make them act as reducing agents, hydrogen donors, and singlet oxygen quenchers including metallic chelating potential. Our obtained results are in agreement with other studies that demonstrated the relationship between the content in phenolic, flavonoids compounds and the antioxidant activity[18],[19].
Acknowledgments
We thank Professor Ali Hachem and Dr Ali Khalaf (Lebanese University, Faculty of Sciences-I) for supplying materials. We also thank Mr. Mohamad El Hajj and Mr. Mohamad Zein for NMR analysis. This research was funded by Research Platform for Environmental Science, Lebanese University.
Comments
Background
The present article responds to a very important need, namely the search for effective, non-toxic, natural compounds with antioxidant activities. In nature there is a wide variety of naturally occurring antioxidants. Some of these compounds are present in a long list of plants. The authorsdiscussed the biological activity of a Lebanese plant: E. macroclada schyzoceras.
Research frontiers
SThe phenolic compounds and the flavonoids have been suggested to play preventive role in the development of cancer and heart diseases. The extract of Euphorbia has been shown to improve high content in phenolic and flavonoids. And to possess high degree of biological activities. Thus, the Euphorbia can be considered as a potential source of antioxidant compounds.
Related reports
The research are good. we do encourage such research and it's the first of it's kind presented.
Innovations and breakthroughs
The analysis of the crude extracts showed high content of phenolic compounds which are responsible for the found of important biological activity. Three different tests: DPPH, H2O2-test, and the ferrous chelation test, proved that the crude extracts of Euphorbia have the property of a high free radical scavenging potential.
Applications
Based on the obtained results, the Euphorbia macroclada can be used as supplementary materials. It can contribute to manufacture some medicines and to treat a number of diseases such as cardiovascular, cancers, diabetes and those associated with aging. It must be remembered that the daily intake of natural antioxidant is closely related with the reduction of the previously cited diseases.
Peer review
According to the great importance and promising research activity in the plant kingdom and because of the very interesting results obtained in this paper, this manuscript should be accepted for publication.
Footnotes
Foundation Project: Supported by Research Platform for Environmental Science, Lebanese University. The grant is provided by the Lebanese University under the number ER019/UL/EDST.
Conflict of interest statement: We declare that we have no conflict of interest.
Conflict of interest: We declare that we don't have any conflict of interests.
References
- 1.Andersen O, Markham K, editors. Flavonoids: chemistry, biochemistry and applications. Boca Ratn: CRC Press; 2006. p. 219. [Google Scholar]
- 2.Huang D, Ou B, Prior RL. The chemistry behind antioxidant capacity assay. J Agri Food Chem. 2005;53(6):1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- 3.Rammal H, Bouayed J, Younos C, Soulimani R. The impact of high anxiety levels on the oxidative status of mouse peripheral blood lymphocytes, granulocytes and monocytes. Eur J Pharm. 2008a;589(1–3):173–175. doi: 10.1016/j.ejphar.2008.06.053. [DOI] [PubMed] [Google Scholar]
- 4.Rammal H, Bouayed J, Younos C, Soulimani R. Evidence that oxidative stress is linked to anxiety-related behaviour in mice. Brain Behav Immun. 2008;22(8):1156–1159. doi: 10.1016/j.bbi.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Farhan H, Rammal H, Hijazi A, Hamad H, Badran B. Phytochemical screening and extraction of polyphenol from stems and leaves of a Lebanese Euphorbia macrolada schyzoceras Boiss. Ann Biol Res. 2012a;3(1):149–156. [Google Scholar]
- 6.Harborne JB. Phytochemical methods. London: Chapman and Hall Ltd; 1973. pp. 49–188. [Google Scholar]
- 7.Lister E, Wilson P. Measurement of total phenolics and ABTS assay for antioxidant activity. New Zealand: Crop Research Institute Lincoln; 2001. [Google Scholar]
- 8.Quettier-Deleu C, Gressier B, Vasseur J, Dine T, Brunet C, Luyckx M, et al. et al. Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour. J Ethnopharmacol. 2000;72(1–2):35–42. doi: 10.1016/s0378-8741(00)00196-3. [DOI] [PubMed] [Google Scholar]
- 9.Chew YL, Goh JK, Lim YY. Assessment of in vitro antioxidant capacity and polyphenolic composition of selected medicinal herbs from Leguminosae family in Peninsular Malaysia. Food Chem. 2009;119:373–378. [Google Scholar]
- 10.Ruch RJ, Cheng SJ, Klaunig JE. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 1989;10:1003–1008. doi: 10.1093/carcin/10.6.1003. [DOI] [PubMed] [Google Scholar]
- 11.Dinis TCP, Madeira VMC, Almeida LM. Action of phenolic derivatives (acetoaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Bioph. 1994;315(1):161–169. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- 12.Lee JH, Lee SJ, Hae Kyung Kim SP, Jeong WY, Choi JY, Sung NJ, et al. et al. Characterisation of flavonoids in Orostachys japonicus Berger using HPLC MS/MS: Contribution to the overall antioxidant effect. Food Chem. 2011;124:1627–1633. [Google Scholar]
- 13.Bouayed J. Polyphenols: a potential new strategy for the prevention and treatment of anxiety and depression. Curr Nutr Food Sci. 2010;6(1):13–18. [Google Scholar]
- 14.Rammal H, Bouayed J, Hijazi A, Ezzedine M, Soulimani R. Scavenger capacity of Momordica charantia for reactive oxygen species. J Nat Prod. 2012;5:54–59. [Google Scholar]
- 15.Farhan H, Rammal H, Hijazi A, Badran B. Preliminary phytochemical screening and extraction of polyphenol from stems and leaves of a Lebanese plant Malva parviflora L. Int J Curr Pharm Res. 2012;4(1):55–59. [Google Scholar]
- 16.Farhan H, Malli F, Rammal H, Hijazi A, Bassal A, Ajouz N, et al. et al. Phytochemical screening and antioxidant activity of Lebanese Eryngium creticum L. Asian Pac J Trop Biomed. 2012;2(1):1–4. [Google Scholar]
- 17.Gulcin I, Oktay M, Kirecci E, Kufrevioglu OI. Screening of antioxidant and antimicrobial activities of anise (Pimpinella anisum L.) seed extracts. Food Chem. 2003;83(3):371–382. [Google Scholar]
- 18.Dipankar C, Murugan S. In vitro antioxidant and cytotoxic activity of leaves and stem extracts of Ecbolium linneanum. Int J Pharm Bio Sci. 2012;3(3):112–120. [Google Scholar]
- 19.Subhashree B, Anurupa S, Moumita D, Pranabes N, Gouriprosad D. Phytochemical evaluation and in vitro study of antioxidant potency of Amorphophallus campanulatus, Alocasia indica and Colocasia esculenta: a comparative analysis. Int J Pharm Bio Sci. 2012;3(3):170–180. [Google Scholar]
- 20.Aaby K, Ekeberg D, Skrede G. Characterization of phenolic compounds in strawberry (Fragaria ananassa) fruits by different HPLC detectors and contribution of individual compounds to total antioxidant capacity. J Agr Food Chem. 2007;55:4395–4406. doi: 10.1021/jf0702592. [DOI] [PubMed] [Google Scholar]
- 21.Vega MRG, Esteves-Souza A, Vieira IJC, Mathias L, Braz-Filho R, Echevarri A. Flavonoids from Annonadioica leaves and their effects in Ehrlich carcinoma cells, DNA-topoisomerase I and II. J Braz Chem Soc. 2007;18(18):1554–1559. [Google Scholar]
- 22.Cavalcante MGB, Silva RM, Bandeira PN, dos Santos HS, Pesso ODL, Braz-Filho R, et al. et al. Furano flavones and other chemical constituents of Lonchocarpus obtusos. J Braz Chem Soc. 2010;23(2):301–305. [Google Scholar]
- 23.Zhao HY, Sun JH, Fan MX, Fan L, Zhou L, Li Z, et al. et al. Analysis of phenolic compounds in Epimedium plants using liquid chromatography coupled with electrospray ionization mass spectrometry. J Chromatogr A. 2008;1190(1–2):157–181. doi: 10.1016/j.chroma.2008.02.109. [DOI] [PubMed] [Google Scholar]