Abstract
Objective
The antimicrobial activity of the ethanol extract of the Auklandia (Saussurea lappa)root plant was investigated to verify its medicinal use in the treatment of microbial infections.
Methods
The antimicrobial activity of the ethanol extract was tested against clinical isolates of some multidrug-resistant bacteria using the agar well diffusion method. Commercial antibiotics were used as positive reference standards to determine the sensitivity of the clinical isolates.
Results
The extracts showed significant inhibitory activity against clinical isolates of methicillin resistant Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumonia, Extended Spectrum Beta-Lactemase, Acinetobacter baumannii. The minimum inhibitory concentration values obtained using the agar dilution test ranged from 2.0 µg/µL-12.0 µg/µL. In the contrary the water extract showed no activity at all against the tested isolates. Furthermore, the results obtained by examining anti-resistant activity of the plant ethanolic extract showed that at higher concentration of the plant extract (12 µg) all tested bacteria isolates were inhibited with variable inhibition zones similar to those obtained when we applied lower extract concentration using the well diffusion assay.
Conclusion
The results demonstrated that the crude ethanolic extract of the Auklandia (Saussurea lappa) root plant has a wide spectrum of activity suggesting that it may be useful in the treatment of infections caused by the above clinical isolates (human pathogens).
Keywords: Auklandia, Saussurea lappa, Ethanol extract, Antimicrobial activity, Anti-resistant activity, Minimum inhibitory concentration (MIC)
1. Introduction
Plant-derived drugs remain an important resource, especially in developing countries, to combat serious diseases. Approximately 60-80% of the world's population still relying on traditional medicines for the treatment of common illnesses[1]–[4]. There are about 60-90% of patients with arthritis who have used complementary and alternative medicine; most used traditional Chinese medicine[5]. The use of medicinal plants as a source for relief from illness can be traced back over five millennia to the written documents of the early civilization in China, India and the Near east, but it is doubtless an art as old as mankind[6].
Although, the potential of higher plants as source for new drugs is still largely unexplored. Over the past decades, there has been increasing interest in the investigation of the natural products from different sources particularly from higher plants for the discovery of new antimicrobial and antioxidant agents, such as tannins, terpenoids, alkaloids, and flavonoids, which have been demonstrated to have in vitro antimicrobial properties[7]–[10]. As this may give a new source of antimicrobial agents, many research groups that are now engaged in medicinal plants research have given much attention to these natural resources.
The activities have been selected because of their great medicinal relevance and emergence of antibiotic resistance. Within the recent years, infections have increased to a great extent and resistance against antibiotics becomes an ever-increasing therapeutic problem[11]. Antibiotics are often used against diseases caused by Klebsiella spp and other human pathogens including methicillin-resistant Staphylococcus aureus(MRSA), Acineto bacterbuamani(A. bacterbuamani), Pseudomonas aeruginosa (P. aeruginosa), Escherichia coli (E. coli) and Extended Spectrum Beta-Lactemase (ESBL), bacteria. However, these pathogens are becoming increasingly antibiotic resistant, so that many are now labeled as multidrug-resistant[12]. Thus, it is an important task for the researcher to find out alternative agent.
Currently, there are few or no antibiotics available or likely to be available soon to treat life-threatening infections caused by some of these bacteria. For example, Acinetobacter baumanii has caused outbreaks of infections in intensive-care units[13]. This is because bacteria resistant to “last-line” antibiotics, such as carbapenems (e.g., meropenem), glycopeptides (e.g., vancomycin), fluoroquinolones (e.g., ciprofloxacin).
Medicinal plant can be very effective as a candidate for treating urinary tract infections and typhoid fever[14]. So that plants predicated antimicrobials provide a vast untapped source for medicines and further exploration of plant antimicrobials needs to occur.
Thus, herbal medicine can be the future promise candidate that provides adequate solution for effective treatment of diseases caused by bacteria. The efficacy of herbal Auklandia (Costus) (Saussurea lappa) (S. lappa) root extract over the multidrug resistant bacteria isolates has been investigated in the present study. Apart from antimicrobial activities, S. lappa root extracts are also exploited for therapeutic purpose to cure several disorders. The crude extract of the S. lappa root was found to inhibit the severity of diarrhea induced by some of the investigated bacteria strains[15],[16]. It is speculated that the extract was able to inhibit electrolyteperme ability in the intestine due to castoroiland/or through the inhibition of prostaglandins release.
The main aim of this study was to investigate the antimicrobial activity of S. lappa root plant(Figure 1). The herb is well known by many traditional healers to be used as a traditional remedy for many diseases. Such uses are for diarrhea and dysentery-like disorders, or for abdominal pain and tenesmus. The current investigation was based on the evaluation of such traditional herb on its antibacterial activity against human pathogenic multidrug resistant bacteria.
Figure 1. The Auklandia-(Costus also known as S. lappa) root.

2. Materials and methods
2.1. Plant materials
One thousand gram of Costus (S. lappa) root was purchased from Alseeb, General Herbal Market, Muscat, Oman. The plant root was identified at the Pharmacognosy Department, Faculty of Pharmacy, Sana'a University. Part of the identification of the investigated plants was done by Dr. A. Wadieh, Department of Botany, Naser College, in Lahj Governorate, University of Aden, Republic of Yemen. Voucher specimens were deposited at the Pharmacognosy Department, Faculty of Medicine, University of Science and Technology. Sana'a, Republic of Yemen.
2.2. Extraction of plant material
2.2.1. Ethanol extract
The method of extraction using ethanol was followed as stated by Randhir et al.(2004). Two hundred and fifty grams of ground samples were soaked in 400 mL of 99.9% ethanol and homogenized in an electric blender for approximately 5 min and incubated at room temperature for 4 d. The mixture was then filtered twice using vacuum and then the solvent (ethanol) was allowed to evaporate at room temperature and then re-extracted with 250 mL of 99.9% ethanol by using a shaking water-bath at 70 °C for 6 h. The mixture was then filtered and ethanol was allowed to evaporate at room temperature. The residue (extract) was collected and kept at 4 °C.
2.2.2. Water extract
To obtain a water extract of S. lappa root, ethanol extraction protocol (as above) was followed with water replacing ethanol.
2.2.3. Test organisms
Common pathogenic bacteria were used to test the antimicrobial activity of the extract. They included methicillin-resistant S. aureus (MRSA), multi-drug resistant P. aeruginosa, E. coli, K. pneumonia, ESBL, A. baumannii which were routinely isolated, identified Rxn[17] and tested for antibiotic susceptibility in the microbiology diagnostic laboratory, Sultan Qaboos University Hospital, Muscat, Oman.
2.2.4. Preparation of bacteria isolates
According to Kirby-Bauer[18] the organisms should be in the log phase of growth in order for the results to be valid.Therefore, fresh cultures (3 to 4 h cultures) were used. All Gram-negative bacteria were sub-cultured on CLED media and incubated at 37 °C for 24 h. All Gram-positive bacteria were sub-cultured on blood agar and incubated at 37 °C for 24 h. Each of the bacterial isolate used in this study was preserved in 2 mL of human blood at -80 °C till ready to use.
2.3. Bacterial susceptibility testing
2.3.1. Well diffusion method
The antimicrobial activity of the extract was determined by the well diffusion method according to the Clinical Laboratory Standards Institute. Mueller-Hinton agar media were inoculated with 24 h cultures of bacterial suspensions (0.5 McFarland). Holes were made in the agar media by using 5 mm cork borer. Each hole was filled with 50, 100, 150, 200, 250 and 300 µL of 40 mg/mL extracts. Media were incubatedin refrigerator for one hour for proper diffusion then incubated at 37 °C for 24 h. Sterile distilled water, instead of the extract, was used as negative control.The antibacterial activity was assessed by measuring the inhibition zone diameter (mm) around the well and the mean of triplicate result was taken.
2.3.2. Determination of minimum inhibitory concentration and minimum bactericidal concentration
Prior to test, the bacterial strains were activated by incubating them at 35 ± 2°C for 24 hours in brain heart infusion broth (BHI, Difco, UK). The minimum inhibitory concentration (MIC) of the extract was determined for each of the test organisms in triplicates. The MIC of the control which consisted of standard antibiotics Colistine (10 µg) and Vancomycine (30 µg) was determined using the test organisms. To test tubes, 0.5 mL of varying concentrations (2, 4, 6, 8, 10 and 12 mg/mL) of the extracts and the control antibiotics, 2 mL of nutrient broth and a loop-full of the test organism previously diluted to 0.5 McFarland turbidity standard were added. A tube containing nutrient broth only, was inoculated with the test organisms to serve as bacterial growth control. After tubes were incubated at 37 °C for 24 h they were examined for the lowest concentration of the extract or the antibiotic control showing no growth indicated by lack of turbidity. All experiments were made in triplicate. The minimum bactericidal concentration (MBC) was also determined by sub-culturing the various concentrations of the extract and the antibiotic control onto a fresh Mueller Hinton and incubated for 18-24 at 37 °C. The highest dilution that yielded no single bacterial colony on the solid medium was taken as MBC.
2.3.3. Examining of anti-resistant activity of the S. lappa root
This is a developed assay to evaluate the efficacy of the plant extract against the bacteria resistant activity. To 0.5 mL of 4 mg/mL concentration of the extract, 2 mL of nutrient broth (as stated above) was added and then a loop-full of the previously diluted “0.5 McFarland turbidity standard for (bacterial isolates)” test organism was introduced to the tubes. Tubes containing bacterial cultures were then incubated at 37 °C for 24 h. Subsequently, bacteria were sub-cultured to a fresh drug free solid medium and incubated further for 24 h. Furthermore, the bacteria isolates weresub-cultured on CLED media and incubated further at 37 °C for another 24 h. Finally, a well diffusion assay was performed as stated above to investigate whether there is resistant activity to the plant extract. Sterile distilled water and a specific antibiotic [Colistine (10 µg) and Vancomycine (30 µg)] added to each bacteria isolate were used as negative and positive controls, respectively.
3. Results
3.1. Bacterial susceptibility testing
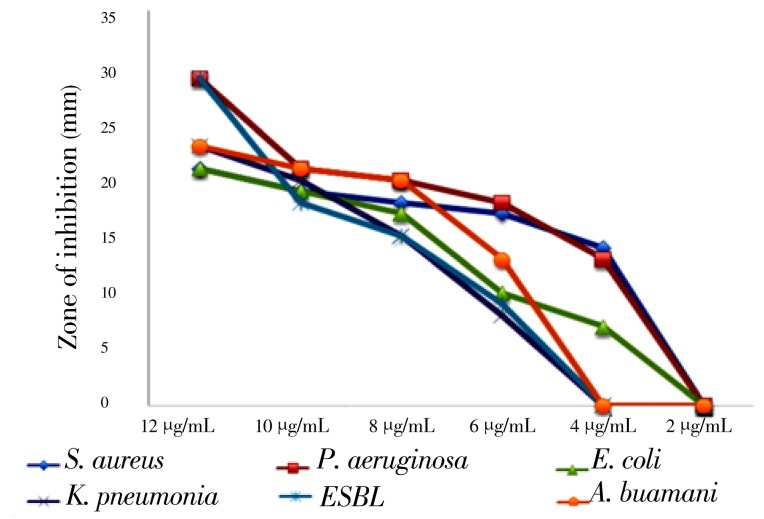
The data pertaining to the antimicrobial activity of the S. lappa root ethanol extract and MIC and MBC are presented in Figure 2 and Table 1, respectively.
Figure 2. Dose dependent antibacterial activity of the ethanolic extract of S. lappa root against human bacteria isolates through agar diffusion method (zone of inhibition in mm). Six different human isolates were tested for their inhibition by the stated ethanolic plant extract.
Table 1. MIC and MBC of the ethanolic extract of the S. lappa root.
| Organisms | *MIC (µg/mL) | *MBC (µg/mL) |
| S. aureus (MRSA) (ATCC 6538) | 2000 | 6000 |
| P. aeruginosa(ATCC 27853) | 2000 | 4000 |
| E. coli | 2000 | 8000 |
| K. pneumonia | 6000 | 6000 |
| ESBL | 6000 | 8000 |
| A. buamannii | 6000 | 6000 |
* = Mean of three determinations
The ethanol extract exhibited a concentration-dependent zone of inhibition with the lowest zone of inhibition corresponding with the lowest extract concentration and the largest zone of inhibition corresponding to highest concentration (Figure 2). The zone of inhibition was found to be within a range of 8 - 29 mm. However, the water extract showed no activity at all against the tested bacteria (data not shown).
3.2. Determination of MIC and MBC
The ethanol extract was bacteriostatic to S. aureus (MRSA) at 2000 µg/mL but three times this concentration (6000 µg/mL) was bactericidal. Similarly, 2000 µg/mL of the extract was bacteriostatic to P. aeruginosa while 4000 µg/mL concentration was bactericidal. A concentration of 2000 µg/mL of the extract was bactericidal to K. pneumoniae, A. baumannii (Table 1).
3.3. Examination of anti-resistant activity
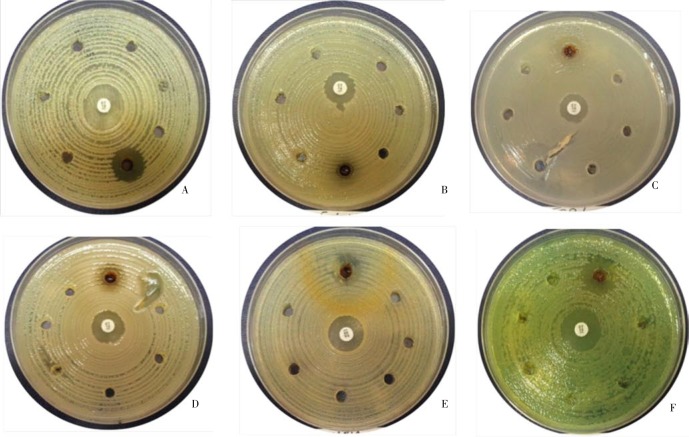
The anti-resistance activity testing showed that at higher concentration of the plant extract (12 µg) all tested bacteria strains were inhibited with variable inhibition zones and similar to that obtained when we applied lower extract concentration using the well diffusion assay (Figure 3).
Figure 3. Anti-resistance activity of S. lappa root plant. The bacteria isolates develop some resistance to all the extract dilutions with the exception with the initial concentration of 12 µg (300 µL).
(a) A. baumannii (18 mm); (b) E. coli - (5 mm), (c) ESBL (19 mm), (d) K. pneumonia (7 mm), (e) S. aureus (MRSA) +(15 mm), (f) P. aeruginosa (17 mm).
4. Discussion
Plant substances continue to serve as viable source of drugs for the world population and several plant-based drugs are in extensive clinical use. For the past few decades, enormous number of plants has been widely used for the treatment of various diseases due to their antibacterial activity. Therefore, screening of medically important plants for their antimicrobial activity is an important aspect as an alternative potential for therapeutic use. The first step towards this goal is the in vitro antibacterial activity assay.
Although manyreports areavailable on the antiviral, antibacterial, antifungal, anthelmintic and anti-inflammatory properties of plants[19] not many reports are available on the exploitation of such plants' properties for developing commercial formulations forapplicationsincropnor human protection.
The initial concentration of 12 µg of plant extract in the agar well is justified by the fact that the complex mixture of molecules should be in different amounts, therefore, the highest the initial concentration most probably is the detection of any biological activity. Sequentially, serial dilution of the initial concentration allowed us to determine the MIC exhibiting antimicrobial activity.
Our data shows that ethanolic extract of S. lappa root has an excellent anti-bacterial activity against human bacteria isolates including MRSA, ESBL and A. baumannii. As low as 4 µg/mL of the extract can produce an inhibition zone of 14 mm by agar diffusion methods, 2000 µg exhibited bacteriostatic while 6000 µg can be bactericidal. The MRSA has become a major nosocomial pathogen in community hospitals, long-term-care facilities, and tertiary care hospitals[20]. Community associated MRSA (CA-MRSA) strain has also been reported in populations lacking risk factors for exposure to the health care settings[21]. The anti-MRSA activity of S. lappa root promises an excellent alternative therapy of challenging MRSA infections. Extended-spectrum β-lactamases (ESBLs) have been repeatedly reported globally, not only in the nosocomial, but also in the community setting with treatment failure being the main obstacle[22]. Our data has shown that 6000 µg/mL can inhibit ESBL producing bacteriaand 8000 µg/mL is bactericidal, also promises break of vicious cycle of emergence of antibiotic resistant bacteria, every time a new generation of antibiotic developed. The bacteriostatic and bactericidal activity of S. lappa root against multi-drug resistant organism such as P. aeruginosa and A. baumannii showed by our data further supports the promising anti-bacterial activity of this plant. The relatively low MIC and MBC of the MRSA as compared to the ESBL and A. baumannii is that our data that we have shown may indicate compositional and structural difference between gram positive and gram negative bacteria. The results showed that ethanolic extract gave maximum zone of inhibition at a concentration of 12 µg against P. aeruginosa (29 mm), ESBL (29 mm), K. pneumonia (23 mm), A.baumannii (23 mm), E. coli (21 mm) and S. aureus (MRSA) (21mm). On the contrary, we observed that the water extract showed no role in such activity.
MIC is defined as the lowest concentration of an extract that completely inhibits the growth of the microorganism in 24 h. The MIC and MBC of the S. lappa root was observed with the ethanolic extract, which came out to be in a range between 2000 to 8000 µg/mL for the concentration of 12000 µg/mL.
Although the results of the MIC and MBC haveshowed that the highest MIC and MBC values of ESBL is an indication that either the plant extractis less effective on some gram positive bacteria or that the organism has the potential of developing antibiotic resistance, while the low MIC and MBC values for other bacteria is an indication of the efficacy of the plant extract. This is confirmed indirectly by the resistant induction assay. Although the assay confirmed that the bacteria isolate developstotal resistance to all the extract dilutions still being inhibited to some extent by the initial concentration of 12 µg.
The results of the present investigation clearly indicate that the antibacterial activity vary with the concentration of the plant material used. Thus, the study as certains the value of S. lappa root as antibiotic due to that it may possess a new source of antimicrobial agents with possibly novel mechanisms of action. Systematic screening of such molecules, i.e., antibacterial agents, may result in the discovery of novel active compounds.
Acknowledgments
The authors would like to thank the college of Medicine and Health Science, Sultan Qaboos University, for providing fund [Micro/Immu - Immu; 2013/Int/07], and facilities to perform this study.
Comments
Background
This is a good study and considered for publication in the journal in which the authors evaluated the antimicrobial activity of S. lappa root using bacterial pathogenic strains. This is a good study in which the authors work in the pathogens, which are antibiotics resistance. The results demonstrated aqueous and ethanolic extract of S. lappa root highly effective on six bacterial stains including two Staphylococcus spp. The final results are interesting and the extracts are having the potential compounds for its activity. The study has very good scope to further continue this work with more advanced tools to prove the plant's antibacterial activity.
Research frontiers
The study was carried out to investigate the antimicrobial activity of S. lappa root against six multiresistant human pathogens. Among different extracts, with an effective ethanolic extract that showed maximum antimicrobial activity. The paper has information on the following research frontiers: Extraction of crude antimicrobial agent(s) from the S. lappa root; Antibacterial potential of the above extracts; Evaluation of the antiresistant ability of the extract against multiresistant human pathogen. The main cutting edge in the field of research in this paper is the first report of antimicrobial activity in S. lappa root against against clinical isolates of methicillin resistant S. aureus (MRSA), P. aeruginosa, E. coli, K. pneumonia, ESBL, A. baumannii.
Related reports
Authors have taken note of earlier studies of other genus plant [such as Achyranthes by Balakrishnan et al., 2003 in A. bidentata and many others in A. aspera by Gupta et al., 2004 and Parmar et al., 2012] to carry out the experiments in the species. The main cutting edge in the field of research in this paper is the first report of antimicrobial activity in S. lappa root.
Innovations and breakthroughs
It has been demonstrated successfully that the S. lappa root was significant in inhibiting clinically important and highly multiresistant human pathogens. The study showed that the S. lappa root have some potential and promising phytochemicals, which are responsible for the activity against multiresistant human pathogen particularly, MRSA and P. aeruginosa pathogens.
Applications
The present investigation revealed the S. lappa root plays a major role in the defense mechanism against Human infections caused by multi resistant pathogens. It is important to study this plant in detail as the plant carry endemic status. In the present scenario of microbial strains developing resistance against many drugs, it has been important field of research to find out new sources. It may act as substitute for many antibiotics currently used now a adys a and because its endemic status may contain novel phytoconstitutent. This study may lead to identification of potent drugs from plant sources.
Peer review
This is an adequate pilot study in which the authors have evaluated the antimicrobial activity of S. lappa root against bacterial pathogenic strains. The results have demonstrated ethanolic root extract of S. lappa to be highly effective on six human bacterial stains. The paper has concentrated on the isolation, characterization and evaluation of antibacterial potentials of S. lappa root extracts. It also concentrated on some preliminary studies such as phytochemcial screening potential of the extracts. Currently, the bacterial and fungal pathogens are resistance to the antibiotics. The treatment for the pathogens is complicated one. Due to this reason, identifying noval compounds for the treatment of human infection is more important and vital. Finally this is a well-demonstrated study to be considered for publication in the journal. Because, it has very good scope to further continue this work with more advanced tools to prove such plant potentials.
Footnotes
Foundation Project: Supported by the College of Medicine and Health Science, Sultan Qaboos University with the fund [Micro/Immu - Immu; 2013/Int/07].
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Tekeli Y, Zengin G, Aktumsek A, Sezgin M, Torlak E. Antibacterial activities of extracts from twelve Centaurea species from Turkey. Arch Biol Sci Belgrade. 2011;63(3):685–690. [Google Scholar]
- 2.World Health Organization . Geneva: WHO; 2007. WHO Country Cooperation Strategy 2006-2011: Supplement on traditional medicine [R] [Google Scholar]
- 3.Dev S. Impact of natural products in modern drug development. Indian J Exp Biol. 2010;48(3):191–198. [PubMed] [Google Scholar]
- 4.Schuster D, Wolber G. Identification of bioactive natural products by pharmacophore-based virtual screening. Curr Pharm Des. 2010;16(15):1666–81. doi: 10.2174/138161210791164072. [DOI] [PubMed] [Google Scholar]
- 5.Zhao J, Zha Q, Jiang M, Cao H, Lu A. Expert consensus on the treatment of rheumatoid arthritis with Chinese patent medicines. J Alt Com Med. 2013;19(2):111–118. doi: 10.1089/acm.2011.0370. [DOI] [PubMed] [Google Scholar]
- 6.Lohitha P, Ravi KV, Mohan BKR, Nataraj K, Alankritha RP, Madhavi N, et al. et al. Phytochemical screening and in vitro antimicrobial activity of Butea monosperma bark ethanolic and aqueous extract. Int J Pharm Sci Res. 2010;1(10):150–155. [Google Scholar]
- 7.González-Lamothe R, Mitchell G, Gattuso M, Moussa S, Malouin DF, Bouarab K. Plant antimicrobial agents and their effects on plant and human pathogens. Int J Mol Sci. 2009;10(8):3400–3419. doi: 10.3390/ijms10083400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fessenden RJ, Fessenden JS. Organic chemistry. 6th ed. California: Brooks/Cole Publishing Inc. Pacific Grove; 1998. pp. p.134–141. [Google Scholar]
- 9.Mothana RAA, Abdo Salah AA, Hasson S, Althawab FMN, Alaghbari SAZ, Lindequist U. Antimicrobial, antioxidant and cytotoxic activities and phytochemical screening of some Yemeni medicinal plants. Evid Based Complement Alternat Med. 2010;7(3):323–330. doi: 10.1093/ecam/nen004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Habbal OA, Al-Jabri AA, El-Hag AG. Antimicrobial properties of Lawsoniainermis(henna): a review. Aust J Med Her. 2007;19:114–125. [Google Scholar]
- 11.Shihabudeen MS, Priscilla HH, Thirumurugan DK. Antimicrobial activity and phytochemical analysis of selected Indian folk medicinal plants. Int J Pharma Sci Res. 2010;1(10):430–434. [Google Scholar]
- 12.Sharmeen R, Hossain N, Rahman M, Foysal J, Miah F. In-vitro antibacterial activity of herbal aqueous extract against multi-drug resistant Klebsiella spp. isolated from human clinical samples. Int Cur Pharm J. 2012;1(6):133–137. [Google Scholar]
- 13.Camp C, Tatum OL. A Review of Acinetobacter baumannii as a highly successful pathogen in times of war. Lab Med. 2010;(41):649–657. [Google Scholar]
- 14.Tambekar DH, Dahikar SB. Exploring antibacterial potential of some ayurvedic preparations to control bacterial enteric infections. J Chem Pharm Res. 2010;2(5):494–501. [Google Scholar]
- 15.Irshad S, Mahmood M, Perveen F. In-vitro anti-bacterial activities of three medicinal plants using agar well diffusion method. Res J Bio. 2012;2(1):1–8. [Google Scholar]
- 16.Randhir R, Lin YT, Shetty K. Phenolic, their antioxidant and antimicrobial activity in dark germinated fenugreek sprouts in response to peptide and phytochemical elicitors. Asian Pac J Clin Nutr. 2004;13:295–307. [PubMed] [Google Scholar]
- 17.Holt JG, Krieg RN, Sneath PHA, Staley JT, Williams ST. Bergey's manual of determinative bacteriology. 9th ed. Baltimore: Williams and Wilkins; 1994. [Google Scholar]
- 18.Hudzicki J. Kirby-Bauer disk diffusion susceptibility test protocol. Am Soc Microbiol. 2009 [Google Scholar]
- 19.Kakkar A, Dubey PK, Dubey S, Khare P, Bias N, Netam R. Studies on synergistic antimicrobial potential of aloe-wheatgrass extract combination. Asian J Pharm Life Sci. 2012;2(2):291–294. [Google Scholar]
- 20.Kakhandki LS, Peerapur BV. Study of nasal carriage of MRSA among the clinical staff and health care workers of a teaching hospital of Karnataka, India. Al Ameen J Med Sci. 2012;5(4):367–370. [Google Scholar]
- 21.David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol. 2010;23:616–687. doi: 10.1128/CMR.00081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trecarichi EM, Cauda R, Tumbarello M. Detecting risk and predicting patient mortality in patients with extended-spectrum β-lactamase-producing Enterobacteriaceae bloodstream infections. Future Microbiol. 2012;7:1173–1189. doi: 10.2217/fmb.12.100. [DOI] [PubMed] [Google Scholar]