Abstract
Objective
To evaluate the ameliorative role of grapefruit juice on the cytogenetic and testicular damage induced by the antiarrythmic drug amiodarone in albino rats.
Methods
Animals were divided into four groups. Group I was considered as control. Group II was given grapefruit juice at a dose level of 27 mL/kg body weight. Group III was orally administered amiodarone (18 mg/kg body weight) daily for 5 weeks. Animals were sacrificed after 5 weeks of treatment. Bone marrow was collected from the femurs for analysis of chromosomal aberrations and mitotic indices. Testes were removed and stained with H&E for histological examination. Sperms were collected from epidedymis for detection of sperm head abnormalities. Comet assay was used to detect DNA damage.
Results
Amiodarone treatment caused a significant increase in the percentage of chromosomal aberrations, decreased the mitotic index and increased DNA damage. The testis showed many histopathological alterations, inhibition of spermatogenesis and morphometric changes. The number of sperm head abnormalities was increased. Treating animals with amiodarone and grapefruit juice caused a reduction in chromosomal aberrations, mitotic index, DNA damage and testicular alterations caused by amiodarone.
Conclusions
The results of this study indicated that grapefruit juice ameliorates the cytotoxicty and testicular alterations induced by amiodarone in albino rats and this is may be due to the potent antioxidant effects of its components.
Keywords: Amiodarone, Grapefruit, Chromosome, Comet assay, Testis
1. Introduction
Amiodarone is a member of antiarrhythmic drugs which is used in a wide variety of cardiac arrythmias resistant to other treatments. It showed beta-blocker like and potassium channel blocker like actions, increasing the refractory period via sodium and potassium channel effect, besides slowing intera-cardiac conduction of the cardiac action potential via sodium channel effect[1]. On the other hand, the drug has many extracardiac side effects, including corneal microdeposits, photosensitivity, and hypo- or hyper-thyroidism[2]. It was reported that 15%-40% of patients taking amiodrane develop an asymptomatic hepatic dysfunction, which is dose dependent and reversible on stopping treatment[3]. Amiodarone may accumulate within many organs including the testis and was associated with epididymitis[4],[5]. It induced apoptosis in H9c2 cardiac cells and chromosomal aberrations in rats[6],[7].
Plants and plant derived pure chemicals are now used for treatment of many diseases. Grapefruit is a subtropical citrus and is an excellent source of many nutrients and phytochemicals, able to contribute to a healthy diet[8]. The pink and red hues contain the beneficial antioxidant lycopene[9]. Grapefruit seed extract has been claimed to have strong antimicrobial properties[10],[11] and cholesterol reduction ability[12]. There is an evidence of anticancer properties of grapefruit. Miller et al. reported that naringin and naringenin, two flavonoids found in high concentrations in grapefruit, may be able to inhibit the development of oral carcinogenesis[13]. Naringin, abioflovnoid predominant in grape fruit and other citrus fruits, has been found to scavenge free radicals that reduce induced damage such as reduction of aberrant cells and chromosomal aberrations[14]. Consumption of grapefruit juice was found to be beneficial for human health, including protection against the DNA damage[15]. The present work aims to study the ameliorative effect of grapefruit juice on amiodarone-induced cytogenetic and testicular alterations in albino rats.
2. Materials and methods
2.1. Rats and treatments
Two hundred healthy adult male albino rats (Rattus norvegicus), approximately 3 months old and weighing (130±5) g, were used. The animals were kept in individual special rodents cages in the laboratory under constant condition of temperature (25±3) °C with a reveres natural dark-light cycle 12/12 h. Animals were maintained on a standard rodent diet, obtained from Egyptian company of Oils and Soap Kafr-Elzayat Egypt, manufactured especially for laboratory purposes. The diet composed of 20% casein, 15% corn oil, 55% corn starch, 5% salt mixture and 5% vitaminized starch. Water was available ad libitum. Maintenance of animals and experimental procedures were approved by the animal ethical committee in accordance with the guide for care and use of laboratory animals of Menoufia University, Egypt.
Animals were divided into four groups. The rats in Group served as controls. The rats in Group II were orally given grapefruit juice. Juice of grapefruit (Citrus Paradise) was prepared by squeezing the fresh fruit. The rats were regularly orally administrated with grapefruit juice at a dose level of 27 mL/kg body weight[16]. The rats in Group III were treated with amiodarone. Tablets of amiodarone were ground and dissolved in distilled water. It is used at a dose level of 18 mg/kg body weight modified according to therapeutic dose of human, and each animal was orally given 0.5 mL containing the desired dose daily for 5 weeks. The rats in Group IV were given amiodarone (18 mg/kg body weight) followed by grapefruit juice (27 mL/kg body weight) daily for 5 weeks.
2.2. Chromosomal preparation
Animals were sacrificed after 5 weeks of treatment. Bone marrow cell preparations for analysis of chromosomal aberrations and mitotic indices were conducted by colchicines-hypotonic technique. After completion of the treatment period, five animals from each group were intraperitoneally injected, 2 h before sacrifice, with 0.5 mL colchicine (3 mg/kg body weight), to increase the number of metaphase spreads. Bone marrow cells were collected from the femurs in isotonic NaCl solution and bone marrow smears were prepared[17]. For each group, slides were stained with Giemsa and mounted in DPX. For each animal, 50 metaphase spreads were scored for chromosomal aberrations. The mitotic index was obtained by counting the number of mitotic cells in 1 000 cells/animal.
2.3. Comet assay
This technique is widely used for detection of single stranded breaks[18]. Eukaryotic cells are embedded in agarose gel on a microscopic slide, lysed by detergents and high salt at pH 10, and then electrophoresed for damage display which shows increased migration of the DNA from the nucleus towards the anode. This technique permits the detection and an evalution of single-stranded DNA breaks. Low-melting temperature agarose and ultra pure agarose, Triton X-100, sodium sarcosinate, ethylene-diamine-tetra acetic acid disodium salt (Na2-EDTA), Ttizma base and ethidium bromide were obtained from Sigma chemical company. Phosphate buffered saline (PBS) without calcium and magnesium, RBMI 1640 medium (Gibco), Ficoll separating solution and trypan-blue were used in comet assay. Examination was done with a fluorescent microscope (Olympus BX 41, Japan) equipped with an excitation filter of 510 nm and barrier filter of 590 nm. The migration was evaluated by observing and measuring the nuclear DNA, and 500 spots of DNA were examined and classified into three types: (i) normal spots with round shape; (ii) damaged spots in which the length of the migrated fragments was less than or equal to the diameter of the basal nuclear DNA; and (iii) strongly damaged spots where the length of the comet was greater than the diameter of the basal nuclear DNA.
2.4. Histological studies
For light microscopic studies, animals were dissected and their testes were removed and fixed in 10% neutral formalin for 24 h, washed in running tap water for 24 h and transferred to 70% ethyl alcohol. Tissues were dehydrated in ascending series of ethyl alcohol, cleared in xylene and embedded in wax. Sections of 5 µm thickness were cut using rotary microtome and mounted on clean slides without using any adhesive medium. Sections were stained with Ehrlich's haematoxylin, counterstained with eosin and photographed.
2.5. Sperm head abnormalities test
The sperm suspension was obtained from animals by cutting the caudal epidedymis of a testis in few drops of mammalian saline. The sperm suspension was spread on clean glass slides. Sperm smears were dried in air and incubated at oven at 50 °C overnight. The sperms were fixed in methyl alcohol and stained with haematoxylin and eosin. A total of 1 000 sperms were examined for each animal directly under microscope to detect the morphological abnormalities in head region.
2.6. Statistical analysis
In the present work, the results are represented in Tables as mean number ± standard error. The significance of difference between the treated and normal groups was calculated using chi-square and student's t-test.
3. Results
3.1. Chromosomal aberrations and mitotic index
Both structural and numerical aberrations were resulted from the treatment with amiodarone. Structural aberrations including chromatid deletion, chromatid fragment, chromosomal ring, centromeric attenuation of chromosomes, centric fusion of chromosomes, end to end association, chromatid gaps and chromatide breaks were observed (Table 1). Numerical aberrations such as monosomy, trisomy, tetrasomy and polyploidy were also recorded (Table 2). A significant increase in these aberrations was observed after the treatment with amiodarone when compared with the control animals, while the animals given grapefruit juice showed a decrease in these aberrations. The animals treated with amiodarone showed a decrease in the mitotic index when compared with control group and increased after grapefruit juice administration.
Table 1. Average of structural chromosomal abnormalities observed in bone marrow cells of male rats treated with amiodarone and grapefruit juice (mean±SE).
| Animal group | Deletion | Chromatid fragment | Centromeric attenuation | End to end association | Centric fusion | Chromosomal ring | Gaps | Breaks | Total |
| Normal | 10.00±0.44 | 4.20±0.37 | 0.60±0.24 | 0.60±0.24 | 0.00±0.00 | 16.00±0.70 | 0.00±0.00 | 0.00±0.00 | 31.40±1.99 |
| Grapefruit juice | 9.60±2.27 | 16.00±1.12 | 0.00±0.00 | 1.20±0.49 | 1.20±0.97 | 16.20±3.30 | 0.60±0.24 | 0.00±0.00 | 44.80±8.39 |
| Amiodarone | 36.60±3.80 | 16.00±1.18 | 2.40±0.74 | 1.80±0.73 | 2.60±0.67 | 30.80±2.47 | 2.80±0.37 | 1.60±0.40 | 94.60±10.36* |
| Amiodarone+grape- fruit juice | 17.00±2.55 | 13.80±1.62 | 1.40±0.24 | 1.40±0.40 | 2.40±0.24 | 11.00±1.00 | 0.00±0.00 | 0.20±0.20 | 47.20±6.25 |
*Significant at P<0.05.
Table 2. Average of numerical chromosomal abnormalities observed in bonemarrow cells of male rats treated with amiodarone and grapefruit juice (mean±SE).
| Animal group | Monosomy | Trisomy | Tetrasomy | Polyploidy | Total |
| Control | 4.0±0.3 | 0.0±0.0 | 0.0±0.0 | 0.4±0.2 | 4.4±0.6 |
| Grapefruit juice | 6.0±1.1 | 0.6±0.2 | 0.4±0.2 | 1.0±0.3 | 8.0±2.0 |
| Amiodarone | 13.0±1.0 | 8.2±1.9 | 1.2±0.3 | 1.4±0.5 | 23.8±3.9* |
| Amiodarone+ grapefruit juice | 7.6±1.1 | 0.0±0.0 | 0.2±0.2 | 0.2±0.2 | 8.0±1.5 |
*P<0.05 compared with contral.
3.2. Detection of DNA damage by comet assay
Detection of DNA damage by comet assay in blood lymphocytes cells showed a significant increase in DNA fragmentation in the animals treated with amiodarone appeared as damaged and strongly damaged spots, while the animals given grapefruit juice showed an improvement in DNA by decreasing the number of damaged spots (Figure 1, Table 3).
Figure 1. Photomicrograph showing single strand breaks of DNA of rat lymphocytes.

A: Normal DNA spots (no migration); B: Damaged DNA spots (migration towards the anode); C: Strongly damaged DNA spots (more migration towards the anode).
Table 3. Mean value of DNA damage detected by comet assay in rat lymphocytes treated with amiodarone and grapefruit juice (mean±SE).
| Animal group | Normal cells | Damaged cells | Strongly damaged cells | Total damaged cells |
| Control | 97±0.83 | 3±0.83 | 0±0.00 | 3±0.83 |
| Grapefruit juice | 95±1.09 | 5±1.09 | 0±0.00 | 5±1.09* |
| Amiodarone | 80±2.32*# | 18±1.64*# | 2±0.70# | 20±2.32*# |
| Amiodarone+grapefruit juice | 89±1.34*#° | 10±0.83*#° | 1±0.54 | 11±1.34*#° |
*Statistically significant in comparison with control; #Statistically significant in comparison with grapefruit; °Statistically significant in comparison with treatement with cordarone.
3.3. Histological observations
Sections in testis of control rats showed typical structure of normal seminiferous tubules, spermatogenic cells, intertubular connective tissue and spermatozoa (Figure 2). The animals administrated grapefruit juice for 5 weeks showed the same histological structure as those served as controls. Sections of testis of animals given amiodarone for 5 weeks showed a histological difference when compared with the control ones. The outlines of the seminiferous tubules appeared irregular and sometimes were with thick basement and irregular membranes.
Figure 2. Section in testis of a control rat showing normal seminiferous tubules (H&E×400).
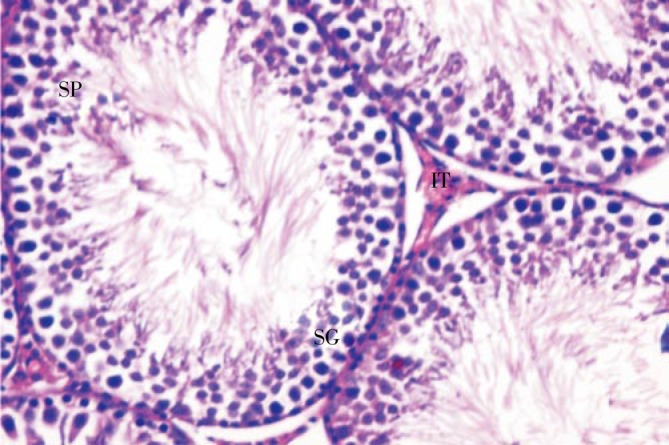
SG: spermatogonia; SP: spermatozoa; IT: intertubular connective tissue.
The interstitial tissue was degenerated and intertubular hemorraghe was observed (Figure 3A). The spermatogenic cells decreased in number, degenerated and appeared with pyknotic nuclei (Figure 3B).
Figure 3. Sections in testes of rats treated with amiodarone for 5 weeks (×400).
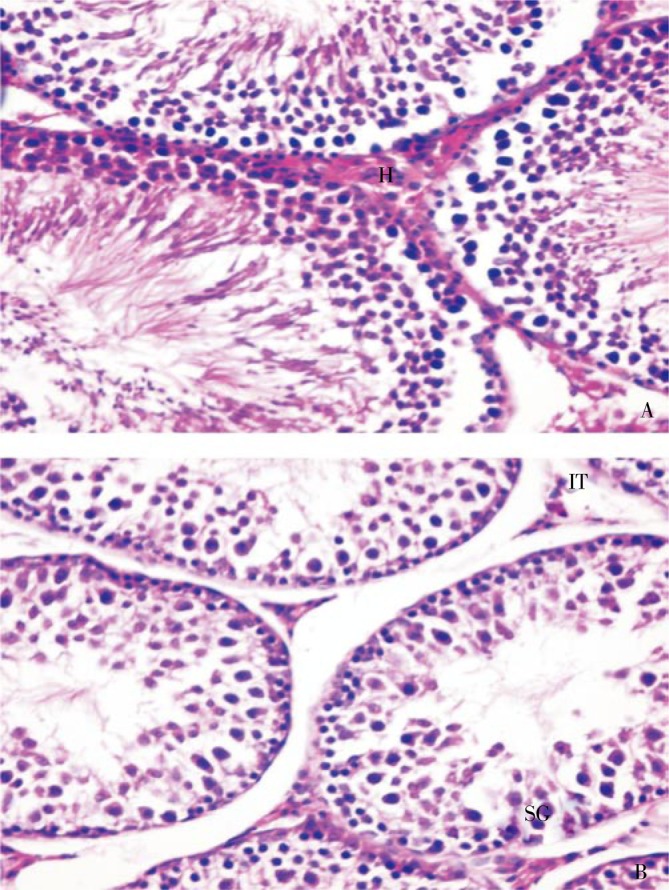
A: Irregular semineferous tubules and intertubular hemorraghe (H); B: Degenerated interstitial tissue (IT) and degenerated spermatogonia (SG) with pyknotic nuclei and loss of spermatozoa.
Few sperms were present and scattered randomly in the tubules. Sections of animals given amiodarone with grapefruit juice showed improvement in the histopathological alterations appeared in animals given amiodarone only. The spermatogenic layers increased with appearance of somewhat normal cells with increase of sperms bundles (Figure 4).
Figure 4. Section in testis of a rat given amiodarone and grapefruit juice showing an improvement in histological appearance and seminiferous tubules showing different stages of spermatogic cells (×400).
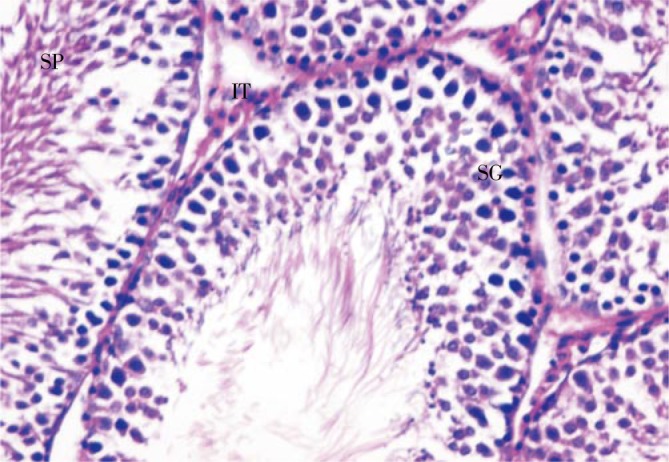
SG: spermatogonia; IT: interstitial tissue; SP: sperms.
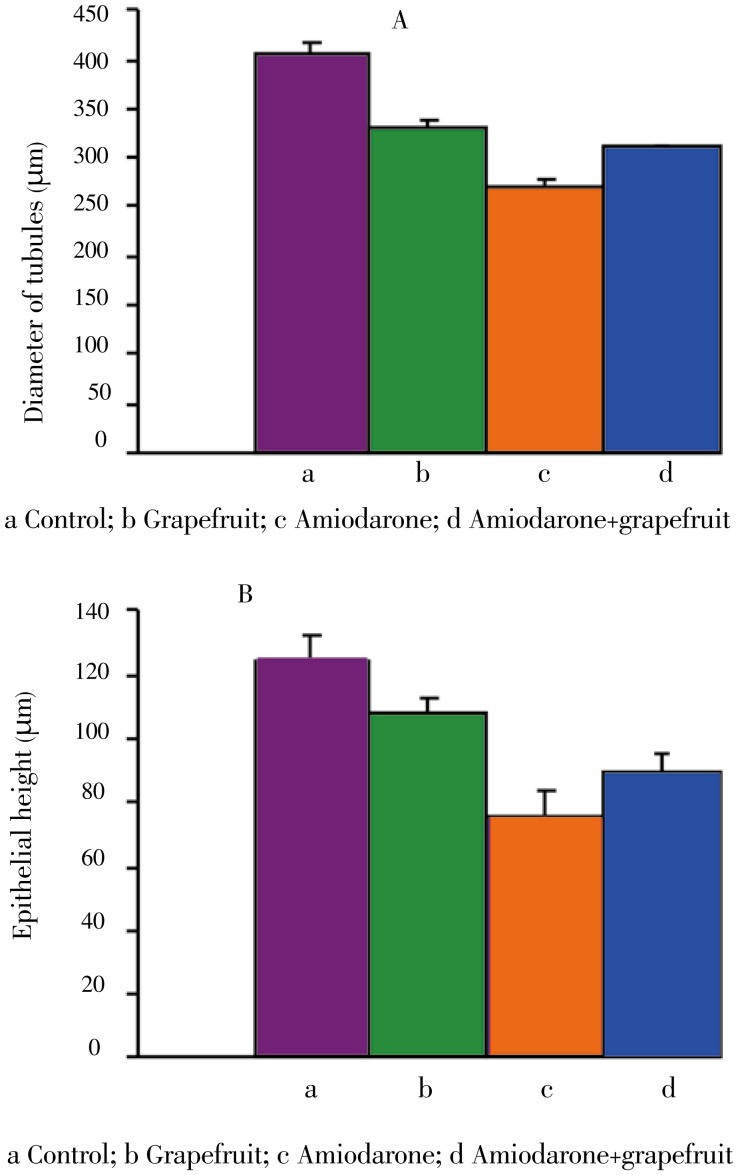
3.4. Morphometric results
Treating animals with amiodarone caused atrophy of the seminiferous tubules. A decrease in diameter of seminiferous tubules and germ cell height of seminiferous tubules was recorded in comparison with normal ones (Figure 5). Animals treated with amiodarone and grapefruit juice showed marked improvement in the mean tubular diameter and germ cell height in comparison with amiodarone treated animals.
Figure 5. Changes of seminiferous tubules in different animal groups.
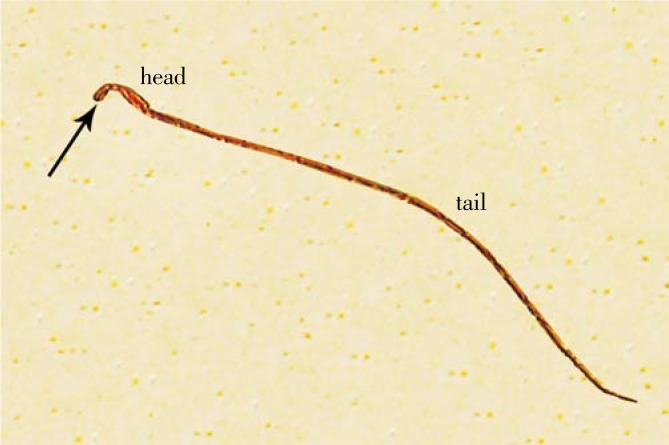
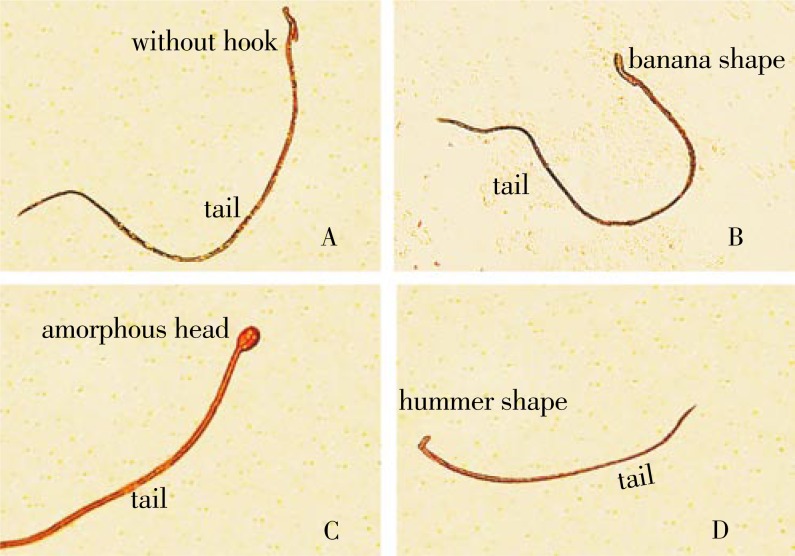
3.5. Sperm head abnormalities
Figure 6 shows a sperm with normal head and tail. Figure 7 shows sperm head abnormalities (without hook, banana type, amorphous head and hummer shape). The rats treated with amiodarone caused a significant increase in the mean number of sperm head abnormalities. On the other hand, the animals given amiodarone and grapefruit juice showed a significant decrease in these abnormalities (Table 4).
Figure 6. Sperm with normal head, tail and hook (arrow) (×1 000).
Figure 7. Sperms with abnormal heads (×1 000).
A: Without hook; B: Banana type; C: Amorphous head; D: Hummer shape.
Table 4. Effect of amiodarone and/or grapefruit juice on sperm head abnormalities.
| Animal group | Normal | Without hook | Banana shape | Amorphous head | Hammer shape |
| Control | 994.40±0.50 | 1.20±0.37 | 1.20±0.20 | 0.00±0.00 | 0.00±0.00 |
| Grapefruit juice | 992.20±1.39 | 2.60±0.81 | 1.80±0.37 | 0.60±0.24 | 0.00±0.00 |
| Amiodarone | 972.60±5.78 | 16.40±2.71* | 5.80±1.20 | 1.80±0.37 | 1.20±0.37 |
| Amiodarone+grapefruit juice | 985.80±1.85 | 9.80±0.66 | 4.40±0.74 | 1.20±0.20 | 0.00±0.00 |
*P<0.05.
4. Disscussion
The present study showed that treating rats with amiodarone caused an increase in the mean number of structural chromosomal aberrations including chromatide deletion, fragments, chromosomal ring, centromeric attenuation of chromosomes, centric fusion, end to end association of chromosomes, chromatid gaps and breaks. Numerical aberrations (monoploidy, triploidy, tetraploidy and polyploidy) were also increased. On the other hand, mitotic index decreased by the treatment with this drug. Similarly, Almeida et al. reported that amiodarone caused an increase in chromosomal aberrations and abnormal metaphase in bone marrow cells of Wistar-Kyoto rats[7]. Telez et al. showed that the antihypertensive drug atenolol was found to induce chromosome loss[19].
DNA damage was recorded after treatment with amiodarone. Isomoto et al. reported that amiodarone induced DNA fragmentation (apoptosis) in culture H9c2 cells[6]. Choi et al. demonstrated that L-132 human lung cell line treated with amiodarone exhibited several features of apoptosis and increase of mRNA levels of bax and caspase-3[20]. Amiodarone was found to cause testicular damage, inhibit spermatogenesis and increase sperm head abnormalities. Dobs et al. reported that atrophic testes were more commonly observed in amiodarone-treated men[21]. They added that amiodarone-treated men had higher serum follicle-stimulating hormone and luteinizing hormone concentrations compared with control subjects. Ward et al. reported a case of epididymitis associated with amiodarone treatment and a high concentration of desethylamiodarone in the semen[5]. Khan et al. reported that patients taking beta-blockers experience sexual dysfunction[22]. El-Sayed et al. reported that atenolol, metoprolol and propranolol have a toxic effect on male fertility and induced significant decrease in percent of progressive motility of sperm besides increase in sperm head and tail abnormalities and cause a significant decrease in the level of testosterone hormone[23].
It was suggested that free radicals are produced during the metabolism of amiodarone and involved in the mechanism of the drug's side effects. Vereckei et al. verified that amiodarone generated free radicals in vitro by chemiluminometric method and caused a significant increase of NADPH and Fe3+ induced lipid peroxidation in the liver microsomal fraction[24]. On the other hand, oxidative stress was not involved in the pathogenesis of amiodarone toxicity[25],[26]. The potential mechanisms of amiodarone toxicity include direct and indirect cytotoxicity. In this concern, Agoston et al. reported that amiodarone treatment increased lysosomal phospholipidosis in liver of male Fischer 344 rats[27]. The role played by oxidative stress in amiodarone-induced mitochondrial toxicity was investigated by Serviddio et al[28]. They reported that amiodarone increased mitochondrial H2O2 synthesis, which in turn induced cardiolipin peroxidation. Moreover, amiodarone inhibited Complex I activity and uncoupled oxidative phosphorylation, leading to a reduction in the hepatic ATP content. One or more of these mechanisms may be responsible for amiodarone toxicity recorded in the present work.
Treatments involving antioxidants have been used successfully to decrease oxidative stress related injuries in different organs. Administrating rats with grapefruit juice ameliorated the cytogenetic and testicular abnormalities induced by amiodarone. The protective effects of grapefruit were studied by many investigators. Attia reported that naringin, a grapefruit flavonone, showed anti-mutagenic effects against lomefloxacin-induced genomic instability in vivo in mouse bone marrow cells and decreased chromosomal aberration[29]. Miyata et al. stated that rats allowed free access to grapefruit juice for 5 days prior to AFB1 administration resulted in clearly reduced DNA damage in liver[30]. Alvarez-González et al. reported that the consumption of grapefruit juice has been associated with various activities potentially beneficial for human health, including protection against the DNA damage produced by dounoubicin[15]. Alvarez-González et al. found that grapefruit juice inhibited the rate of micronucleated polychromatic erythrocytes (MNPE) and sister chromatid exchanges induced by the antineoplastic alkylating agent ifosfamide[31]. Liu et al. reported that quercetin has protective effect against lead-induced hepatic apoptosis[32]. Miyata et al. studied the influence of grapefruit juice intake on 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine-induced colon DNA damage and found that grapefruit juice suppresses PhIP-induced colon DNA damage by a mechanism independent of PhIP absorption in the intestine[33]. Jagetia et al. reported that naringin, a bioflovnoid predominant in grape fruit and other citrus fruits, has been found to scavenge free radicals; and it reduced radiation induced damage such as reduction of aberrant cells and chromosomal aberrations like acentric fragments, chromatid and chromosome breaks, centric rings, dicentrics and exchange[14].
The antioxidant activity of grapefruit components was studied. Girennavar et al. isolated two bioactive compounds from grapefruit juice and grapefruit peel oil[34]. Structures of the compounds were identified as bergaptol and geranylcoumarin. Bergaptol showed a very good radical scavenging activity at all the tested concentrations. Furthermore, these compounds were found to be potent inhibitors of debenzylation activity of CYP3A4 enzyme. Muthukumaran et al. investigated the protective effect of quercetin (QN) against nicotine-induced prooxidant and antioxidant imbalance in circulation, lung, liver and kidney of experimental rats[35]. The extent of DNA damage (evaluated by comet assay) was significantly increased in circulatory blood of nicotine-treated rats, which was effectively brought down by QN treatment. The data suggested that QN exerts its protective effect by modulating the extent of lipid peroxidation and augmenting antioxidant defense system and thus protects the DNA in experimental animals. Thus, it is concluded from the present work that the ameliorative effect of grapefruit juice against amiodarone toxicity may be attributed to its antioxidant activity.
Acknowledgments
Authors are thankful to Faculty of Science, Menoufia University, Egypt, for providing laboratory facilities and instruments to carry out this work.
Comments
Background
Amiodarone is a member of antiarrhythmic drugs which is used in a wide variety of cardiac arrythmias resistant to other treatments. The drug has many extracardiac side effects, including corneal microdeposits, photosensitivity, and hypo- or hyper-thyroidism. As recorded, 15%-40% of patients taking amiodrane develop an asymptomatic hepatic dysfunction, which is dose dependent and reversible on stopping treatment. Amiodarone may accumulate within many organs including the testis.
Research frontiers
The present work aims to study the ameliorative effect of grapefruit juice on amiodarone-induced cytogenetic and testicular alterations in albino rats.
Related reports
Grapefruit may be able to inhibit the development of oral carcinogenesis. Naringin, abioflovnoid predominant in grape fruit and other citrus fruits, has been found to scavenge free radicals that reduce induced damage such as reduction of aberrant cells and chromosomal aberrations. Consumption of grapefruit juice was found to be beneficial for human health, including protection against the DNA damage.
Innovations and breakthroughs
Data in this present study showed that treating rats with amiodarone caused an increase in the mean number of structural chromosomal aberrations including chromatide deletion, fragments, chromosomal ring, centromeric attenuation of chromosomes, centric fusion, end to end association of chromosomes, chromatid gaps and breaks. Numerical aberrations (monoploidy, triploidy, tetraploidy and polyploidy) were also increased. On the other hand, mitotic index decreased by the treatment with this drug.
Applications
It may be significant to know the effect of treatment with grapefruit juice on the cytogenetic damage induced by the antiarrythmic drug amiodarone in albino rats.
Peer review
This is a very good study in which the authors evaluated the ameliorative role of grapefruit juice on the cytogenetic damage induced by the antiarrythmic drug amiodarone in albino rats. The results are interesting and suggested that the ameliorative effect of grapefruit juice against amiodarone toxicity may be attributed to its antioxidant activity.
Footnotes
Foundation Project: Supported by CQAP, Faculty of Science, Menoufia University, Grant # CP4-062-MEN.
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Nuttall SL, Routledge HC, Kendall MJ. A comparison of the beta1-selectivity of three beta1-selective beta-blockers. J Clin Pharm Ther. 2003;28(3):179–186. doi: 10.1046/j.1365-2710.2003.00477.x. [DOI] [PubMed] [Google Scholar]
- 2.Batcher EL, Tang XC, Singh BN, Singh SN, Reda DJ, Hershman JM. Thyroid function abnormalities during amiodarone therapy for persistent atrial fibrillation. Am J Med. 2007;120(10):880–885. doi: 10.1016/j.amjmed.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 3.Mattar W, Juliar B, Gradus-Pizio I, Kwo PY. Amiodarone hepatotoxicity in the context of the metabolic syndrome and right-sided heart failure. J Gastrointestin Liver Dis. 2009;18(4):419–423. [PubMed] [Google Scholar]
- 4.Adams PC, Holt P, Holt DW. Amiodarone in testis and semen. Lancet. 1985;11:341. doi: 10.1016/s0140-6736(85)91111-0. [DOI] [PubMed] [Google Scholar]
- 5.Ward MJ, Routledge PA, Hutchings A, Morris IM. Association of seminal desethylamiodarone concentration and epididymitis with amiodarone treatment. Brit Med J. 1988;296:19–20. doi: 10.1136/bmj.296.6614.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isomoto S, Kawakami A, Arakaki T, Yamashita S, Yano K, Ono K. Effects of antiarrhythmic drugs on apoptotic pathways in H9C2 cardiac cells. J Pharmacol Sci. 2006;101(4):318–324. doi: 10.1254/jphs.fp0050951. [DOI] [PubMed] [Google Scholar]
- 7.Almeida MR, De Oliveira Lima E, Da Silva VJ, Campos MG, Antunes LM, Salma AK, et al. et al. Genotoxic studies in hypertensive and normotensive rats treated with amiodarone. Mutat Res. 2008;657(2):155–159. doi: 10.1016/j.mrgentox.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Kiani J, Imam SZ. Medicinal importance of grapefruit juice and its interaction with various drugs. Nutr J. 2007;6:33. doi: 10.1186/1475-2891-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cassileth B. Lycopene. Oncol. 2010;24(3):296. [PubMed] [Google Scholar]
- 10.Takeoka GR, Dao LT, Wong RY, Harden LA. Identification of benzalkonium chloride in commercial grapefruit seed extracts. J Agric Food Chem. 2005;53(19):7630–7636. doi: 10.1021/jf0514064. [DOI] [PubMed] [Google Scholar]
- 11.Ganzera M, Aberham A, Stuppner H. Development and validation of an HPLC/UV/MS method for simultaneous determination of 18 preservatives in grapefruit seed extract. J Agric Food Chem. 2006;54(11):3768–3772. doi: 10.1021/jf060543d. [DOI] [PubMed] [Google Scholar]
- 12.Platt R. Current concepts in optimum nutrition for cardiovascular disease. Prev Cardiol. 2000;3(2):83–87. doi: 10.1111/j.1520-037x.2000.80364.x. [DOI] [PubMed] [Google Scholar]
- 13.Miller EG, Peacock JJ, Bourland TC, Taylor SE, Wright JM, Patil BS, et al. et al. Inhibition of oral carcinogenesis by citrus flavonoids. Nutr Cancer. 2008;60(1):69–74. doi: 10.1080/01635580701616163. [DOI] [PubMed] [Google Scholar]
- 14.Jagetia GC, Venkatesha VA, Reddy TK. Naringin, a citrus flavonone, protects against radiatin-induced chromosome damage in mouse bone marrow. Mutagenesis. 2003;18(4):337–343. doi: 10.1093/mutage/geg001. [DOI] [PubMed] [Google Scholar]
- 15.Alvarez-Gonalez E, Madrigal-Bujaidar L, Martino-Roaro L, Espinosa-Aguirre JJ. Antigenotoxic and antioxidant effect of grape fruit juice in mice treated with daunoubicin. Toxicol Lett. 2004;152(3):203–211. doi: 10.1016/j.toxlet.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 16.El-Lakkany NM, Seif el-Din SH, Badawy AA, Ebeid FA. Effect of artemether alone and in combination with grape fruit juice on hepatic drug-metabolising enzymes and biochemical aspects in experimental Schistosoma mansoni. Int J Parasitol. 2004;34:1405–1412. doi: 10.1016/j.ijpara.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Mantena SK, Unnikrishnan MK, Uma Devi P. Radioprotective effect of sulfasalazine on mouse bone marrow chromosomes. Mutagenesis. 2008;23(4):285–292. doi: 10.1093/mutage/gen005. [DOI] [PubMed] [Google Scholar]
- 18.Cemeli E, Anderson D. Mechanistic investigation of ROS-induced DNA damage by oestrogenic compounds in lymphocytes and sperm using the comet assay. Int J Mol Sci. 2011;12(5):2783–2796. doi: 10.3390/ijms12052783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Télez M, Ortiz-Lastra E, Gonzalez AJ, Flores P, Huerta I, Ramírez JM, et al. et al. Assessment of the genotoxicity of atenolol in human peripheral blood lymphocytes: correlation between chromosomal fragility and content of micronuclei. Mutat Res. 2010;695(1–2):46–54. doi: 10.1016/j.mrgentox.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 20.Choi IS, Kim BS, Cho KS, Park JC, Jang MH, Shin MC, et al. et al. Amiodarone induces apoptosis in L-132 human lung epithelial cell line. Toxicol Lett. 2002;132(1):47–55. doi: 10.1016/s0378-4274(02)00065-6. [DOI] [PubMed] [Google Scholar]
- 21.Dobs AS, Sarma PS, Guarnieri T, Griffith L. Testicular dysfunction with amiodarone use. J Am Coll Cardiol. 1991;18(5):1328–1332. doi: 10.1016/0735-1097(91)90557-p. [DOI] [PubMed] [Google Scholar]
- 22.Khan UA, Aslam M, Saeed SA. Effect of beta adrenergic antagonist on the production of testosterone by rat's Leydig cells. J Ayub Med Coll Abbottabad. 2004;16(4):26–28. [PubMed] [Google Scholar]
- 23.El-Sayed MG, El-Sayed MT, Elazab AS, Hafeiz MH, El-Komy AA, Hassan E. Effects of some beta-adrenergic blockers on male fertility parameters in rats. Dtsch Tierarztl Wochenschr. 1998;105(1):10–12. [PubMed] [Google Scholar]
- 24.Vereckei A, Blazovics A, Gyorgy I, Feher E, Toth M, Szenasi G, et al. et al. The role of free radicals in the pathogenesis of amiodarone toxicity. J Cardiovasc Electrophysiol. 1993;4(2):161–177. doi: 10.1111/j.1540-8167.1993.tb01220.x. [DOI] [PubMed] [Google Scholar]
- 25.Sarma JS, Pei H, Venkataraman K. Role of oxidative stress in amiodarone-induced toxicity. J Cardiovasc Pharmacol Ther. 1997;2:53–60. doi: 10.1177/107424849700200107. [DOI] [PubMed] [Google Scholar]
- 26.Bolt MW, Racz WJ, Brien JF, Massey TE. Effects of vitamin E on cytotoxicity of amiodarone and desethylamiodarone in isolated hamster lung cells. Toxicol. 2001;166:109–118. doi: 10.1016/s0300-483x(01)00451-6. [DOI] [PubMed] [Google Scholar]
- 27.Agoston M, Orsi F, Fehér E, Hagymási K, Orosz Z, Blázovics A, et al. et al. Silymarin and vitamin E reduce amiodarone-induced lysosomal phospholipidosis in rats. Toxicol. 2003;190(3):231–241. doi: 10.1016/s0300-483x(03)00188-4. [DOI] [PubMed] [Google Scholar]
- 28.Serviddio G, Bellanti F, Giudetti AM, Gnoni GV, Capitanio N, Tamborra R, et al. et al. Mitochondrial oxidative stress and respiratory chain dysfunction account for liver toxicity during amiodarone but not dronedarone administration. Free Radic Biol Med. 2011;51(12):2234–2242. doi: 10.1016/j.freeradbiomed.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Attia SM. Abatement by naringin of lomefloxacin-induced genomic instability in mice. Mutagenesis. 2008;23(6):515–521. doi: 10.1093/mutage/gen045. [DOI] [PubMed] [Google Scholar]
- 30.Miyata M, Takano HQ, Guo L, Nagata K, Yamazoe Y. Grapefruit juice intake does not enhance but rather protects against aflatoxin B1-induced liver DNA damage through a reduction in hepatic CYP3A activity. Carcinogenesis. 2004;25(2):203–209. doi: 10.1093/carcin/bgg194. [DOI] [PubMed] [Google Scholar]
- 31.Alvarez-González I, Madrigal-Bujaidar E, Sánchez-García VY. Inhibitory effect of grapefruit juice on the genotoxic damage induced by ifosfamide in mouse. Plant Food Hum Nutr. 2010;65:367–373. doi: 10.1007/s11130-010-0193-1. [DOI] [PubMed] [Google Scholar]
- 32.Liu CM, Zheng YL, Lu J, Zhang ZF, Fan SH, Wu DM, et al. et al. Quercetin protects rat liver against lead-induced oxidative stress and apoptosis. Environ Toxicol Pharmacol. 2010;29(2):158–166. doi: 10.1016/j.etap.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Miyata M, Takano H, Takahashi K, Sasaki YF, Yamazoe Y. Suppression of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine-induced DNA damage in rat colon after grapefruit juice intake. Cancer Lett. 2002;183(1):17–22. doi: 10.1016/s0304-3835(02)00109-x. [DOI] [PubMed] [Google Scholar]
- 34.Girennavar B, Jayaprakasha GK, Jadegoud Y, Nagana Gowda GA, Patil BS. Radical scavenging and cytochrome P450 3A4 inhibitory activity of bergaptol and geranylcoumarin from grapefruit. Bioorg Med Chem. 2007;15(11):3684–3691. doi: 10.1016/j.bmc.2007.03.047. [DOI] [PubMed] [Google Scholar]
- 35.Muthukumaran S, Sudheer AR, Nalini N, Menon VP. Effect of quercetin on nicotine-induced biochemical changes and DNA damage in rat peripheral blood lymphocytes. Redox Rep. 2008;13(5):217–224. doi: 10.1179/135100008X308948. [DOI] [PubMed] [Google Scholar]