Abstract
The steroid hormone vitamin D is historically recognized for its relevance to bone health and calcium homeostasis. Recent years have witnessed a shift in focus to non-skeletal benefits of vitamin D; in this latter context, an accruing body of literature attests to a relevance of vitamin D to reproductive physiology. This article reviews the existing data about the diverse and previously underappreciated roles for vitamin D in reproductive health. A large body of available literature suggests that vitamin D deficiency may be detrimental to reproductive biology. However, given that our appreciation of vitamin D's role in reproductive physiology is almost entirely shaped by ‘associative’ studies and that data based on prospective interventional trials are limited, these concepts remain predominantly conjectural. Exact mechanisms whereby vitamin D may participate in the regulation of reproductive physiology remain far from clear. This review underscores a need for appropriately designed intervention trials to address the existing knowledge gaps and to delineate the specific roles of vitamin D signaling in reproductive biology.
Keywords: female tract, sperm quality, oocyte quality, environmental, effects
Introduction
A role for vitamin D in bone health was first reported in the 1920's when McCollum and colleagues created a rat model of diet-induced rickets (Mellanby, 1919). Disease manifestations were found to be prevented by a fat-soluble agent that was discovered in oxidized cod liver oil, and was subsequently named vitamin D (Mellanby, 1919; Rajakumar, 2003). Although the importance of this vitamin in calcium-phosphate homeostasis and for bone health was established early on, an understanding of the molecular biology of vitamin D was thwarted until the late 1960's when DeLuca and colleagues (Lund and DeLuca, 1966) generated radio-labeled vitamin D, a critical step in allowing the recognition of nuclear localization of vitamin D in a variety of tissues (Rajakumar, 2003; Christakos et al., 2007). A succession of strides in vitamin D research has since furthered our understandings of the myriad roles that vitamin D plays in biological responses. Non-skeletal effects of vitamin D have been the focus of much interest in the past decade and an accruing body of literature is supportive of a relevance of vitamin D for a variety of organ systems beyond the skeleton (Reichel et al., 1989; Walters, 1992). This review focuses on data that are based on non-human animal models as well as clinical studies and identify a relevance of an individual's vitamin D status to reproductive biology.
Physiology
Metabolism
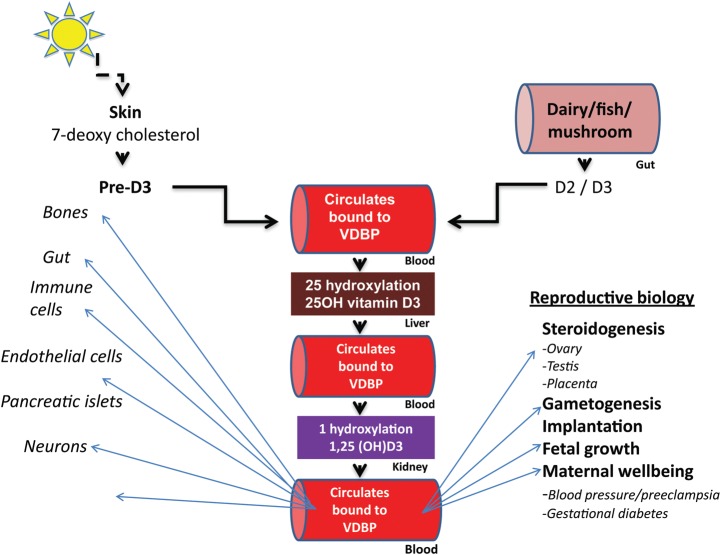
In humans, the predominant source of vitamin D is endogenous cutaneous synthesis, whereas dietary sources contribute to <20% of the circulating levels of vitamin D (Holick et al., 1987; Lips, 2006). Endogenous synthesis of vitamin D (cholecalciferol or D3) occurs after photolytic conversion of 7-dehydrocholesterol, located in the dermal fibroblasts and epidermal keratinocytes, by the ultraviolet B component of sunlight (wavelength between 290 nm and 315 nm; Ashwell et al., 2010; Fig. 1).
Figure 1.
An overview of vitamin D metabolism and salient actions. VDBP, vitamin D binding protein; D2, ergocalciferol; D3, cholecalciferol.
Nutritional forms of vitamin D consist of D3 (cholecalciferol), which is found in foods such as fatty fish (i.e. sardine, salmon and mackerel), eggs and calf liver, and D2 (ergocalciferol) which is manufactured through the ultraviolet irradiation of ergosterol from yeast and fungi (i.e. mushrooms; Vieth, 1999; Moore et al., 2004). Both forms of dietary vitamin D are inactive and are efficiently absorbed by the gut. The dietary vitamin D (D3 and D2) is nearly identical to the skin-derived form of this seco steroid; in this review, the term vitamin D is implied to reflect either of the two variants (D3 and D2).
The circulating vitamin D prohormone (i.e. dietary or endogenously synthesized) is further metabolized by a set of cytochrome P450 enzymes; the hepatic 25-hydroxylase (CYP27A1), converts the prohormone to an intermediary metabolite, 25-hydroxy vitamin D (25(OH)D), whereas 1-α hydroxylase (CYP27B1), primarily from the kidneys, generates the metabolically active form, 1,25-di-hydroxy vitamin D (1,25(OH)2D; Schuster, 2011). The two circulating metabolites (i.e. 25(OH)D and 1,25(OH)2D) demonstrate distinct kinetics and physiological potency. 25(OH)D has a half-life of 2–3 weeks, and its circulating levels are recognized to reflect bodily stores of the vitamin. 1,25(OH)2D in contrast, has a much shorter half-life of 4–6 h and represents the most biologically active variant of vitamin D (Vieth, 1999). An overview of metabolism and salient actions of vitamin D are summarized in Fig. 1.
Renal 1-α hydroxylase (CYP27B1) is recognized as the principal determinant and the rate-limiting enzyme in the generation of the active 1,25(OH)2D metabolite. Recently, the expression of CYP27B1 has been described in a variety of non-renal tissues, suggesting the existence of local mechanisms by which the metabolically active form of the vitamin D is generated (Zehnder et al., 2001; Fischer et al., 2009). The activity of CYP27B1 is directly regulated by the parathyroid hormone (PTH; Lund and DeLuca, 1966; Schuster, 2011). More recently, the activity of CYP27B1 has been shown to be inhibited by fibroblast growth factor 23 (FGF-23), a component of the previously unrecognized hormonal bone–parathyroid–kidney axis that is itself modulated by PTH, 1,25(OH)2D and serum phosphorus levels (Bai et al., 2004; Martin et al., 2012).
Mechanism of action
Vitamin D belongs to the family of steroid hormones. The cellular effects of vitamin D and its metabolites are mediated primarily through the cognate intranuclear vitamin D receptor (VDR; Christakos et al., 2007), a ligand-activated transcription factor that belongs to the nuclear hormone receptor super-family. VDR is expressed in a variety of tissues other than the skeleton, including the intestines, parathyroid glands, immune cells, and more recently, the hypothalamic–pituitary axis and the reproductive tract (Christakos et al., 2007; Mizwicki and Norman, 2009). The presence of VDR in the ovary, uterus, placenta and the testis suggests a regulatory role for vitamin D in reproductive physiology (Weisman et al., 1979; Dokoh et al., 1983; Stumpf et al., 1987a; Johnson et al., 1996). The VDR binds 1,25(OH)2D with an affinity in the range of 0.1–1 nM compared with >100 µM for the lesser active 25(OH)D metabolite (Wecksler and Norman, 1980a,b; Wecksler et al., 1980). The receptor-ligand binding initiates a cascade of events that include receptor phosphorylation and nuclear translocation; recruitment and heterodimerization with the 9-cis retinoid receptor (RXR) then ensues (Christakos et al., 1996). The VDR/RXR heterodimer complexes with the steroid receptor co-activators, VDR-interacting protein and co-regulatory proteins, before binding to the vitamin D responsive elements within the promoter region of target genes, thereby allows the transcription regulation of tissue-specific genes (Christakos et al., 1996).
Although the effect of active 1,25(OH)2D on target cells primarily reflects genomic activity, more recent data suggest an additional non-genomic signaling mechanism via membrane associated rapid response steroid-binding receptor (MARRS, also known as Erp57/Grp58); such a mechanism is suggested in a variety of tissues including the intestine, bone, parathyroid gland, liver, monocytes and the pancreatic beta cells (Chatterjee, 2001; Erben et al., 2002; Christakos et al., 2007; Yu et al., 2012). The function of the non-genomic signaling pathway, however, is less understood. Signaling via the VDR has additionally been linked to CYP19 (aromatase) gene expression, functionally linking vitamin D with the family of reproductive steroid hormones (Kinuta et al., 2000; for review Christakos et al., 2003).
Pandemic of vitamin D deficiency
The data collected by the National Health and Nutrition Examination Surveys within North America document a 4-fold increase in the prevalence of vitamin D deficiency over the past 10–15 years with as much as 36% of the USA population being affected (Nesby-O'Dell et al., 2002; Looker et al., 2008). Embedded within these data are the alarming findings that populations with the greatest physiological needs for vitamin D, such as pregnant women, neonates, children and adolescents are also at highest risk for vitamin D deficiency (McCullough, 2007; Alemzadeh et al., 2008; Kovacs, 2008).
Suboptimal dietary vitamin D intake, increasing environmental pollution, a shift in lifestyle with consequent reduced sun exposure, a concomitant increased use of sunscreen arising from concerns about the carcinogenic potential of sunlight are all recognized as contributors to the near-pandemic of vitamin D insufficiency (Diehl and Chiu, 2010). An inverse relationship between serum 25(OH)D levels and body mass index (BMI) is also well described (Bell et al., 1988; Liel et al., 1988). Although a ‘cause and effect’ paradigm to this relationship is unclear, obesity is recognized as an independent risk factor for hypovitaminosis D (Wortsman et al., 2000); sequestration of the fat soluble vitamin within the adipose tissue is proposed as a mechanism to explain the lower circulating levels of 25(OH)D observed in the overweight and the obese (Liel et al., 1988; Wortsman et al., 2000).
While increasing the prevalence of obesity may partly explain the escalating trends in vitamin D insufficiency (Nesby-O'Dell et al., 2002; Looker et al., 2008), the latter in turn may itself be contributory to the burgeoning pandemic of obesity (Foss, 2009). Secondary hyperparathyroidism that occurs consequent to hypovitaminosis Dis suggested to stimulate 1-α hydroxylase activity, contributing to a compensatory elevation in 1,25(OH)2D levels (Arunabh et al., 2003). Recent in vitro experiments suggest that 1,25(OH)2D causes an increase in the intra-adipocyte calcium ion concentration, which in turn can stimulate lipogenesis and inhibit lipolysis (Shi et al., 2001; Zemel, 2002). Obesity is hypothesized to be linked to abnormal as well as reduced leptin receptor signaling (for review, Gautron and Elmquist, 2011). Interestingly, studies in transgenic leptin deficient and leptin receptor knockout mice suggest that leptin and its cognate receptor may also regulate renal CYP27b1 and 1,25 (OH)2D synthesis (Matsunuma and Horiuchi, 2007).
Vitamin D and reproduction
The importance of vitamin D for the reproductive biology is supported by several rodent studies; an appreciation that vitamin D signaling may be relevant for reproductive health in humans, however, is relatively recent (Table I). The goal of this review is to summarize a relatively underappreciated body of literature that highlights the diverse physiological roles for vitamin D and its metabolites in reproductive physiology.
Table I.
Vitamin D and reproduction.
| Parameter | Experimental models | Human studies |
|---|---|---|
| Folliculogenesis | + | ± |
| Spermatogenesis | + | + |
| Steroidogenesis | + | − |
| Implantation | + | + |
| Relevance in pregnancy | + | + |
| Relevance for progeny | + | + |
A comparison between experimental models and human studies with regard to recognized target effects of vitamin D on specified reproductive parameters. +Evidence for involvement; −no evidence for involvement; ±contradictory evidence.
Lessons from animal models
The majority of the experimental data defining a role for 1,25 (OH)2D, the active metabolite of vitamin D, in reproduction are either derived from diet-induced vitamin D-deficient rodent models or from transgenic Vdr or Cyp27b1 null mice (Halloran and DeLuca, 1979; Johnson and DeLuca, 2001; Sun et al., 2010; Dicken et al., 2012). The available evidence makes a strong case for the importance of vitamin D for procreative success, while underscoring the need for additional studies to allow a better understanding of the mechanistic relevance of vitamin D in reproduction.
Vitamin D and male reproductive physiology: animal models
The expression of Vdr on sperm and throughout the male testes, the presence and activity of Cyp27b1 in the testes and the evidence of local synthesis of 1,25(OH)2D within the Sertoli and Leydig cells all attest to a relevance of vitamin D in male reproduction (Stumpf et al., 1987b; Osmundsen et al., 1989; Kwiecinski et al., 1989a). Perturbations in male behavior, spermatogenesis and fertility are described in the context of vitamin D deficiency (Kinuta et al., 2000). Inseminating wild-type females with sperm collected from diet-induced vitamin D deficient male rats resulted in 65% fewer sperm deposited in the genital tract, and 73% fewer pregnancies in vitamin D sufficient female rats (Osmundsen et al., 1989; Kwiecinski et al., 1989a). Likewise, oligoasthenospermia, hypergonadotrophic hypogonadism and altered tissue expression of aromatase activity are described in the transgenic Vdras well as in the Cyp27b1 null mice; these data suggest a regulatory role for vitamin D signaling in gonadal function. Of interest,the reproductive and gonadal phenotypes of transgenic estrogen receptor alpha and aromatase null mice are similar to the Vdrnull male mutant (Eddy et al., 1996; Mahato et al., 2000; Couse et al., 2001). These latter observations may suggest that theimpaired spermatogenesis and subfertility that are apparent in Vdrnull male mice may partly be mediated through defects in estrogen signaling, a hypothesis that merits detailed assessment. Others, however, maintain that hypocalcemia and hypophosphatemia resulting from vitamin D deficiency are the primary mechanisms that underlie the observed defects in spermatogenesis. Vitamin D deficiency related hypocalcemia is hypothesized to compromise capacitation and acrosome reactions required for fertilization(Breitbart, 2002). Consistent with this hypothesis high calcium-phosphorous diets rescue male fertility in Vdrtransgenic null mice and in male rats with diet-induced vitamin D deficiency (Uhland et al., 1992; Kinuta et al., 2000; Johnson and DeLuca, 2001).
Vitamin D and female reproductive physiology: animal model
Similar to the observations invitamin D deficient males, several lines of evidence suggest that vitamin D deficiency disrupts female reproductive physiology(Halloran and DeLuca, 1980; Kwiecinksi et al., 1989b; Panda et al., 200; Dicken et al., 2012). Nuclear localization of 1,25(OH)2D and VDR are described in a variety of female reproductive organs including the hypothalamus, pituitary gland, uterus, oviduct, ovary, mammary gland and the placenta. Diet-induced vitamin D deficiency in female rats results in severely compromised fertility: a 45–70% reduction in the probability of becoming pregnant, 67–100% reduction in the number of viable pups and 0–33% probability of rearing normal sized and healthy litters are described in experimental models of vitamin D deficiency (Halloran and DeLuca, 1980; Kwiecinksi et al., 1989b).
While the exact mechanisms are far from understood, disrupted estrogen signaling is hypothesized to contribute to impaired reproductive physiology in vitamin D deficient females (Yoshizawa et al., 1997; Panda et al., 2001).Similar to the males, female Vdrnull mice also exhibit hypergonadotropic hypogonadism accompanied by reduced aromatase activity (Kinuta et al., 2000). While hypergonadotropic hypogonadism is common to aromatase deficient as well as the transgenic Vdror Cyp27b1 nullfemale mice, the ovarian phenotypes for these animal models are quite different. Ovarian morphology in the aromatase null females is characterized by large hemorrhagic cysts, suggestive of an ovulatory defect (Britt et al., 2000, 2001). In contrast, in the Vdras well as in Cyp27b1 transgenic null females, the ovaries are devoid of corpora lutea, exhibit hypertrophied interstitial cells, and early follicular development appears to be arrested (Panda et al, 2001; Dicken et al., 2012). Hypergonadotropic hypogonadism is evident in the Vdr transgenic null females, suggesting gonadotrophin resistant (Kinuta et al., 2000). The transgenic Cyp27b1 null females in contrast are eugonadotropic and exhibit a robust ovarian response to exogenous gonadotrophins (Dicken et al., 2012). While these data identify that neither ovarian failure nor gonadotrophin resistance accounts for the abnormal reproductive phenotype of the Cyp27b1 females, some degree of hypothalamic–pituitary axis dysfunction is, however, suggested by the follicular response to exogenous gonadotrophins that is described in the Cyp27b1 null mice (Dicken et al., 2012). Contrary to Dicken et al., however, Sun et al., observed reduced numbers of oocytes retrieved from the oviducts in the Cyp27b1 null mice compared with WT controls following ovulation induction in 24–25-old mice (Sun et al., 2010). Differences in the age of experimental animals may account for the disparity in observations reported in the latter two studies; Dicken et al., focused on the young adult animals, whereas Sun et al., superovulated females who most likely were developmentally peri-pubertal. Thus, it is possible that the pool of gonadotrophin responsive oocytes is reduced in the Cyp27b1 null peri-pubertal animals compared with the young adult females (Sun et al., 2010; Dicken et al., 2012).
In a recent study by Malloy et al., the authors provide novel implications of Vdr-mediated signaling for ovarian function. An up-regulation of the luciferase activity was observed in HeLA cells (cervical cancer cell line) that were transfected with wild-type (WT) VDR cDNA expressing vector and anti-Mullerian hormone (AMH) reporter plasmid; these observations suggest that the promoter for AMH has a putative domain for the vitamin D response element (Malloy et al., 2009). AMH, a member of the transforming growth factor (TGF–β) family, is produced by the ovarian granulosa cells and is recognized to regulate follicular recruitment and selection, as well as inhibit the aromatase activity (Durlinger et al., 2002; Visser et al., 2006). Given that vitamin D signaling can directly modulate ovarian AMH expression, it is plausible that vitamin D deficiency in females may disrupt ovarian physiology via altering AMH signaling.
In contrast to the males, the ability of calcium to rescue female reproductive physiology and fertility in vitamin D deficient animal models is far from consistent; different studies have reported complete, partial or no rescue (Johnson and DeLuca, 2002; Sun et al., 2010; Dicken et al., 2012). While Johnson et al. have suggested that calcium supplementation in the VDR null mice rescued female fertility, the magnitude of effect in the described model is difficult to interpret because the control group (wild type litter mates) was fed a high calcium diet and both groups had equally small litter sizes (5.29 ± 2.1 versus 3.5 ± 1.3; n= 4–7; Johnson and DeLuca, 2001).
VDR is expressed in the uterine endometrial cells as well as in the immune cells residing within the uterine endometrium (Evans et al., 2006). In addition to uterine hypoplasia, impaired bone formation and skeletal compromise are also evident in the Vdr null mice (i.e. phenotypes consistent with hypoestrogenism). The uteri of the Vdr null mice are responsive to estrogen priming suggesting the uterine defect in the Vdr null is in part a consequence of hypogonadism (Zarnani et al., 2010). However, neither estradiol priming nor calcium supplementation suppressed gonadotrophins nor rescued the reproductive phenotype in Vdr null females.
Heterogeneity in the phenotypes of females with vitamin D deficiency caused by Vdr and Cyp27b1 gene deletion suggest that our understanding of the regulatory role of vitamin D is not complete. In addition to abnormal reproductive phenotypes, Vdr null mice exhibit early aging that is characterized by cognitive dysfunction and alopecia; these latter features are not apparent in the Cyp27b1 transgenic null mice, nor in comparably aged, diet-induced vitamin D deficient rats (Lester et al., 1982). It is possible that the disruption of the Vdr gene and the resultant inability to form VDR/RXR heterodimers results in physiological effects that are distinct from those specific to vitamin D deficiency alone (Keisala et al., 2009).
A recent study using the Cyp27b1 transgenic null females compared with WT littermates demonstrated that mice fed regular mouse chow (deficient of active vitamin D) for 3 months post-weaning exhibited irregular estrous cycles, reduced fertility and decreased pup survival that appeared to be rescued by calcium–phosphate diet supplementation (Sun et al., 2010). However, this study was limited because the authors did not study females weaned onto a truly vitamin D-deficient diet; instead the females were weaned onto a regular chow diet which is fortified with the vitamin D prohormone. The significance of this latter point is that when circulating 25(OH)D levels are high, as occurs in the Cyp27b1 and Vdr null experimental models (Panda et al., 2004), the 25(OH)D itself can activate Vdr (Rowling et al., 2007). Thus, it is likely that calcium supplementation rescued the reproductive phenotype of these females because high circulating levels of 25(OH)D allowed the recovery of VDR signaling and consequently a less dramatic phenotype. Consistent with this hypothesis, Dicken et al., demonstrated that the estrous cycle irregularity was not rescued in Cyp27b1 null females weaned onto a diet devoid of vitamin D but supplemented with calcium (Dicken et al., 2012).
Vitamin D, pregnancy, parturition and lactation: animal models
The importance of vitamin D in embryo implantation is also suggested. HOXA 10 is a transcription factor that is recognized as being critical for embryo-endometrial cross talk which initiates the process of implantation. Endometrial expression of HOXA10 is up-regulated by vitamin D, thereby raising a possibility that vitamin D signaling contributes to successful embryo implantation and to immune tolerance (Curtis Hewitt et al., 2002; Du et al., 2005).
Alterations in maternal feeding behavior, milk production and lactation are described in vitamin D deficient and hypocalcemic post-partum females (dams). Reduced maternal feeding and milk production are evident in experimental models of vitamin D deficiency (Brommage and DeLuca, 1984b). Dams with diet-induced vitamin D deficiency, exhibit anorexia, abnormal maternal behavior and reduced milk production (Brommage and DeLuca, 1984a; Brommage et al., 1984). Poor maternal nutrition and reduced milk production are hypothesized to contribute to failure in the pups to thrive and survive under conditions of maternal vitamin D deficiency (Brommage and DeLuca, 1984b). Despite the above-summarized evidence linking maternal vitamin D deficiency to poor outcomes in the offspring, it is difficult to ascertain if the poor neonatal outcomes reflect impaired vitamin D signaling, calcium–phosphate dysregulation, or if the sequelae were consequent to maternal malnutrition and/or reduced maternal capacity to lactate.
As currently understood, while reproductive compromise is clearly evident in the animal models of vitamin D deficiency, the exact downstream mechanisms whereby reproductive physiology is affected is minimally understood.
Vitamin D and reproduction: relevance in humans
As discussed in the preceding section, ample solid evidence exists supporting a role for vitamin D in the regulation of reproductive physiology in the non-human models. In contrast, literature describing a role for vitamin D in the reproductive biology of humans is, however, sparse and almost entirely correlative.
Vitamin D and male reproductive physiology: human data
Calcium is recognized as a critical intracellular signal in male physiology with defined roles during spermatogenesis, sperm motility, hyperactivation and acrosome reaction (Yoshida et al., 2008). VDR is detected in the human testis, prostate and in human spermatozoa (Corbett et al., 2006). More recently, vitamin D metabolizing enzymes are described in the human testis, the ejaculatory tract, mature spermatozoa and in Leydig cells (Blomberg et al., 2012; Foresta et al., 2010). CYP24A1 is a vitamin D-metabolizing enzyme that acts as a check on vitamin D signaling; 24-hydroxylation is generally the first step in the catabolism of active metabolites of vitamin D. CYP24A1 is induced by 1,25(OH)2D, and offers a feedback mechanism to avoid vitamin D toxicity and to titrate cellular responsiveness to vitamin D. CYP24A1 expression has recently been described in the human sperm annulus (Blomberg et al., 2012). In a study comparing the expression of CYP24A1 in the ejaculated sperm from 77 subfertile and 50 healthy young men, the authors observed significantly reduced CYP24A1-expressing spermatozoa in the subfertile men compared with the healthy group (1 versus 25%, P< 0.001). CYP24A1 expression was further observed to positively correlate with sperm concentration, motility and morphology (P< 0.004 for each parameter). The authors further noted that the presence of >3% CYP24A1-positive spermatozoa distinguished the healthy men from the subfertile men with a sensitivity of 66.0%, a specificity of 77.9% and a positive predictive value of 98.3% (Blomberg et al., 2012). To understand if vitamin D signaling was causative to the observed associations, these authors further conducted in vitro functional studies on sperm from an additional 40 men (22 young, 18 subfertile); exposure to 1,25(OH)2D increased intracellular calcium concentration and improved motility in the sperm from healthy young subjects, but failed to impact on sperm from the subfertile men (Blomberg et al., 2012). A recent review by the same group of investigators offers a comprehensive coverage of the non-genomic effect of vitamin D in human spermatozoa (Bloomberg et al., 2012b).In a cross-sectional study of 307 men, Ramlau-Hansen et al., however, failed to identify any clinical relevance of vitamin D deficiency on sperm parameters (Ramlau-Hansen et al., 2011).
While the aforementioned data imply a role for vitamin D and VDRsignaling in spermatogenesis, sperm maturation and testicular endocrine function, additional studies are required to clearly define the importance of VDR signaling in male gamete and gonadal physiology.
Vitamin D and female reproductive physiology: human data
Several recent studies explore the role of vitamin D and its metabolites in overall health and in specific reproductive disorders of women. VDR mRNA and protein are detected in the human endometrium, myometrium, ovarian, cervical and breast tissues (Friedrich et al., 2003; Vienonen et al., 2004). Vitamin D deficiency is hypothesized to contribute to the pathophysiology of a spectrum of gynecological disorders, of which polycystic ovary syndrome (PCOS) appears to be most well studied.
A small number ofobservational studies identify an inverse association between serum 25(OH)D levels with insulin resistance, features of hyperandrogenism and circulating androgens in women with PCOS (Panidis et al., 2005; Hahn et al., 2006; Mahmoudi et al., 2010; Ngo et al., 2011; Wehr and Pilz). In an observational study, Thys-Jacobs et al. describednormalization of menstrual cyclicity in 7 out of 9 oligomenorrheic women with PCOS who underwent supplementation with vitamin D and calcium over a 6 month period; the authors implied therapeutic efficacy of vitamin D for women with PCOS (Thys-Jacobs et al., 1999). Others report dietary supplementation with vitamin D or an analog improves insulin sensitivity (Kotsa et al., 2009; Selimoglu et al., 2010), circulating testosterone (Selimoglu et al., 2010) and parameters of ovarian folliculogenesis and ovulation (Rashidi et al., 2009).In a pilot study of 12 overweight and vitamin D deficient women with PCOS, we observed a significant lowering in circulating androgens (total testosterone and androstenedione) following three month intervention with vitamin D and calcium (Pal et al., 2012).
Insulin resistance, obesity, inflammation and dyslipidemia, i.e. metabolic phenomenon that are commonly encountered in PCOS, are well described in the setting of vitamin D insufficiency (Botella-Carretero et al., 2007; Holick, 2007; Foss, 2009);vitamin D insufficiency is thus theorized to underlie the pathophysiology of PCOS. However, because the majority of women with PCOS are either overweight or obese it is difficult to determine if vitamin D deficiency independent of excess body mass contributes to the pathogenesis of PCOS.
A limited number of studies relate vitamin D deficiency to premenstrual syndrome, uterine fibroids, dysmenorrhea and more recently to early menarche (Bertone-Johnson et al., 2010; Sharan et al., 2011; Villamor et al., 2011; Lasco et al., 2012). Compared with placeboa significant improvement in severity of dysmenorrhea was observed over a two month period following a single dose of 300 000 IU vitamin D; this latter study is one of the few instances where a cause-effect relationship between low vitamin D status and the studied outcome is supported by the randomized controlled study design (Lasco et al., 2012).
A variety of reproductive tract tissues express VDR and messenger RNA for 1CYP27B1. Expression of VDR is reported in normal and neoplastic cervical (Friedrich et al., 2003), ovarian and breast tissue (Friedrich, 2000). While the role of vitamin D signaling incervical and ovarian cancer isunclear, a significant body of epidemiological data suggest vitamin D deficiency is a risk for non-inherited forms of breast cancer (Shao et al., 2012). Interestingly endometriosis has the distinction of being one of the few disorders identified as resulting from arelative excess of vitamin D (Somigliana et al., 2007). A recent study on proteomic analysis of serum collected from patients with endometriosisdemonstrated a significantly higher expression of vitamin D-binding protein (VDBP) in sera of patients with endometriosis (Faserl et al., 2011). Others have similarly demonstrated a increased plasma and peritoneal fluid VDBP levels in patients with endometriosis compared with controls (Ferrero et al., 2005).An over-expression of VDR and vitamin D metabolizing enzymes is also described in the peritoneal lesions and in endometrial tissue of women with endometriosis compared with healthy controls (Agic et al., 2007). The authors suggest that activity of immune cells and cytokines that are thought to play pathogenic roles in the development and maintenance of endometriosis may be modulated by the higher than normal peritoneal levels of vitamin D and its metabolites (Agic et al., 2007). While these data imply that VDBP and vitamin D signaling may play a role in the pathogenesis of endometriosis, the underlying mechanisms are minimally investigated. It is notable that existing data describing abnormalities in vitamin D metabolism and levels in disorders of reproductive tract are almost entirely associative; a direct cause and effect of the observed relationship requires substantiation.
Beyond the suggested associations with specific metabolicdisorders a roleforvitamin D is also implied for procreative success in women. Serum 25(OH)D levels are reported to predict ovarian response in women undergoing ovulation induction with clomiphene citrate (CC). Low levels of 25(OH)D and vitamin D deficiency (<25 nmol/l or <10 ng/ml) were found to be associated with lower rates of follicledevelopment and pregnancy after ovarian stimulation with 50 mg CC (Ott et al., 2012); of note, the threshold taken to define vitamin D insufficiency in this latter study is consistent with a state of severe vitamin D deficiency (Hollis et al., 2011). Implications of vitamin D status for reproductive success have also been suggested in women undergoing in vitro fertilization (IVF). In a prospective study of 84 women undergoing IVF, Ozkan et al.assessed 25(OH)D levels in the ovarian follicular fluid and determined an association between vitamin D status and cycle outcome. Follicular fluid levels of 25(OH)D were observed to be significantly higher in women achieving clinical pregnancy following fresh embryo transfer compared with those with failed outcome (34.42 ± 15.58 versus 25.62 ± 10.53 ng/ml, P= 0.013). Highest implantation rates were observed in women with a follicular fluid level of 25(OH)D in the highest tertile (43.01 ± 10.65 ng/ml) compared with those with follicular fluid 25(OH)D levels in the lower tertiles (P= 0.041). While these data relate vitamin D status with reproductive success in otherwise healthy, albeit infertile, women undergoing IVF (Ozkan et al., 2010), others fail to confirm these associations (Aleyasin et al., 2011). Additionally another group of investigators reported adverse impact of higher 25(OH)D levels on embryo quality (Anifandis et al., 2010). Additional studies are needed to explore these associations better and to study the direct effects of vitamin D and its metabolites on the endometrium and implantation.
Vitamin D and pregnancy: human data
Maternal calcium metabolism undergoes dramatic modulation so as to maintain the skeletal mineralization needs of the developing fetus (Abrams, 2007). The gravid woman's physiology adapts to the escalating fetal requirements with advancing gestation; increasing gastrointestinal calcium absorption is evident early in pregnancy, and maximized in the last trimester (Cross et al., 1995; Ritchie et al., 1998). The increasing fetal demand for calcium is further met by the increased production of 1,25(OH)2D via the maternal kidneys and placenta (Ritchie et al., 1998). Compared with pre-pregnancy levels, there is a 2-fold increase in both the total plasma levels of 1,25(OH)2D and VDBP in the first and second trimesters. In the third trimester, however, a continued rise in 1,25(OH)2D occurs without a concomitant increase in VDBP, resulting in increased levels of free (unbound) 1,25(OH)2D (Urrutia and Thorp, 2012). Placental hormones including parathyroid hormone-related protein (PTHrP) and placental lactogen may be particularly relevant in modulating vitamin D homeostasis during pregnancy (Hosking, 1996; Kovacs, 2012). PTHrP, placental lactogen, as well as prolactin stimulate renal CYP27B1 activity, contributing to the increased circulating levels of 1,25(OH)2D in pregnancy (Hosking, 1996; Kovacs, 2012).The significance of vitamin D for maternal and fetal wellbeing is thus suggested by the aforementioned phenomena that ensure an increased availability of the active form of vitamin D to the mother and the fetus during pregnancy, particularly in the third trimester (Urrutia and Thorp, 2012).
Several pregnancy-related disorders that have been hypothesized to relate to maternal vitamin D deficiency includ pre-eclampsia, gestational diabetes mellitus (GDM) and a risk for delivery by Cesarean section (Fischer et al., 2007; Merewood et al., 2009; Shand et al., 2010; Soheilykhah et al., 2010). Pre-eclampsia is one of the most common obstetric complications, and a significant contributor to maternal and fetal morbidity and mortality. While the etiology is not entirely clear, abnormal placentation, poor placental perfusions, endothelial dysfunction and oxidative stress are recognized mechanisms underlying pre-eclampsia. The presence of vitamin D and its receptors in the placenta as well as the ability of vitamin D to modulate immune, inflammatory and vascular responses has suggested a causative role of maternal vitamin D deficiency in the pathogenesis of pre-eclampsia (Bodnar et al., 2007a). Higher maternal vitamin D levels are associated with a lower incidence of pre-eclampsia and with lower blood pressure readings (Shand et al., 2010; Ringrose et al., 2011). Others have explored the relationship between maternal D levels earlier in pregnancy with the likelihood of developing pre-eclampsia during the course of pregnancy. A Norwegian study of >23 000 nulliparous pregnant women found that supplemental vitamin D intake protects against the development of pre-eclampsia (Haugen et al., 2009). The effects of maternal vitamin D supplementation on lowering blood pressures in the third trimester are reported in a randomized control trial of vitamin D supplementation versus control (Marya et al., 1987). In this study, 400 gravid women were randomly selected to either receive calcium (375 mg/day) and vitamin D (1200 IU/day) at 20–24 weeks onward or non-supplemented throughout the pregnancy. At 32 and 36 weeks of pregnancy, the systolic and diastolic blood pressure of the vitamin D supplementation group was significantly lower than the non-supplemented group. However, the incidence of pre-eclampsia in the supplemented group (6%) was not significantly different from the non-supplemented group (9%; Marya et al., 1987). The existing literature relating maternal vitamin D status with the risk of pre-eclampsia is equivocal. In a case–control study, Yu et al. (2012) quantified maternal serum vitamin D level in the first trimester and explored its relevance for risk of developing early (<34 weeks) or late (≥34 weeks) pre-eclampsia. The authors failed to identify any relationship between maternal serum vitamin D levels with biochemical (pregnancy-associated plasma protein) or biophysical (uterine artery pulsatility index and mean arterial pressure) markers of impaired placental perfusion or function (Yu et al., 2012).
Although a relationship between vitamin D deficiency and risk for type 2 diabetes mellitus is well described in non-pregnant populations (Chiu et al., 2004), the association of vitamin D with GDM, however, has shown mixed results. In one case–control study by Zhang et al., women with vitamin D deficiency early in pregnancy had a 2.66-fold (confidence interval (CI): 1.01–7.02) increased chance of developing GDM compared with women with normal levels (Zhang et al., 2008). Two additional case–control studies, however, failed to identify any relationship between maternal vitamin D levels and subsequent maternal risk for GDM (Baker et al., 2012; Makgoba et al., 2011).
Maternal rickets and concomitant pelvic deformities are a recognized contributor to inefficient labor and difficulties with parturition. A recent study of 253 women identified a 4-fold increase in likelihood for delivery by Cesarean section in women with low vitamin D (<37.5 nmol/l) at the time of delivery compared with those with higher (>37.5 nmol/l) vitamin D levels (Merewood et al., 2009). The increased risk of Cesarean section (emergent or elective) was attributed to negative effects of low vitamin D levels on uterine musculature and contractibility. However, the relationship between vitamin D and the route of delivery is not consistent. Moreover, others researchers fail to observe any effect of maternal vitamin D status (assessed early or mid-pregnancy or at the time of delivery) on the route of delivery (Dror et al., 2011; Savvidou et al., 2012).
Maternal vitamin D deficiency has been linked to a predisposition to a spectrum of infectious etiologies including periodontal disease (Boggess et al., 2011), bacterial vaginosis (BV; Bodner et al., 2009; Hensel et al., 2011) and HIV morbidity and mortality associated with opportunistic illnesses (French et al., 2011; Mehta et al, 2011). In a case–control study of 117 pregnant women diagnosed with moderate-to-severe periodontal disease at <26 weeks of gestation compared with 118 periodontally healthy controls, Boggess et al. identified a 2-fold increase in the prevalence of vitamin D insufficiency (serum 25(OH)D < 75 nmol/l [i.e. <30 ng/ml]) in women with periodontal disease (Boggess et al., 2011). Cause-specific mortality attributable to HIV, disease progression and disease-related anemia were significantly higher in a cohort of HIV-positive gravid Tanzanian women with low vitamin D levels (serum 25(OH)D < 32 ng/ml) compared with those with normal vitamin D levels (Findelstein et al., 2011; Mehta et al., 2011). In a randomized, double-blind, placebo-controlled trial by Mehta et al., 884 Tanzanian HIV-infected gravid women received either vitamin supplementation (including vitamin D) or placebo. Women with low 25(OH)D levels (<32 ng/ml) had significantly higher upper respiratory infections [odds ratio (OR): 1.27 (1.04–1.54)], and thrush [OR: 2.74 (1.29–5.83)] diagnosed during the first 2 years of follow-up (Mehta et al., 2011). Additionally, women with low vitamin D status were at higher risk for HIV-related wasting (Mehta et al., 2011). The relationship between BV and vitamin D status has been explored in a study of >3500 women (pregnant and non-pregnant; Hensel et al., 2011); vitamin D deficiency (serum 25(OH)D < 30 ng/ml) was identified as an independent determinant of risk for BV (OR: 2.9, 95% CI: 1.1–7.3) in the pregnant populations. Similarly, Bodnar et al. showed in a prospective cohort study of 469 pregnancy women in the first trimester that the mean serum 25(OH)D concentration was lower among BV cases (29.5 nmol/l) compared with women with normal vaginal flora (40.1 nmol/l; Bodner et al., 2009). Approximately 57% of women with a low serum 25(OH)D level (<20 nmol/l) had BV compared with 23% of women with normal serum 25(OH)D levels (>80 nmol/l; Bodner et al., 2009). These two studies clearly show an association between vitamin D deficiency and BV in pregnant women.
As evident, the existing literature relating maternal status of vitamin D with pregnancy-related outcomes is almost exclusively ‘associative’. While the data suggest that insufficient maternal levels of vitamin D may increase the likelihood of common obstetric conditions such as pre-eclampsia, GDM and infectious entities such as BV, these concepts require additional investigation. If maternal vitamin D deficiency modulates maternal risk for the afore-discussed morbidities, aggressive vitamin D supplementation in pregnancy would offer a simple, inexpensive and safe strategy that could hold the potential to positively impact maternal and neonatal health. Additional studies are needed to bridge gaps in knowledge regarding mechanism and the ability to rescue the pathophysiology with dietary vitamin D supplementation.
Maternal Vitamin D status and implications for fetal development and neonatal well-being: human data
Maternal and cord blood levels of 25(OH)D have been shown to closely correlate across populations (Bassir et al., 2001; Wang et al., 2010; Dror et al., 2011; Hossain et al., 2011). Given the fetal dependence on maternal stores of 25(OH)D freely crossing the placental barrier, maternal vitamin D deficiency is likely to affect the fetus and the health of the newborn.
Maternal vitamin D status may hold long-term implications for the health of the progeny. Recently, a prospective cohort study of 424 pregnant women demonstrated that maternal vitamin D insufficiency may affect fetal femoral bone development as early as 19 weeks of gestation (Mahon et al., 2010); lower maternal 25(OH)D concentrations relate to greater femoral bone metaphyseal cross-sectional area and higher femoral splaying index in the fetuses. Additionally a longitudinal cohort study, Javaid et al., observed that lower maternal 25(OH)D serum levels correlated with a lower whole-body and lumbar spine bone mineral content in children at the age of 9 years (Javaid et al., 2006).
Small randomized clinical trials and observational studies also suggest a relationship between higher maternal vitamin D levels in the third trimester of pregnancy with neonatal birthweight (Ertl et al., 2012). A reduced risk for small for gestational age (weight less than 10 percentile, Gairdner standards) babies was observed in 59 women randomized to vitamin D supplementation (1000 IU D3 daily) initiated in the third trimester, compared with 67 gravid women given placebo. Nearly twice as many infants in the control group were small for gestational age compared with those women receiving vitamin D supplementation (29 versus 15%; Brooke et al., 1980). Higher birthweights were similarly observed in another randomized control study of vitamin D supplementation (a bolus each of 600 000 IU administered at gestational months 7 and 8, plus 1200 IU D2/day during the third trimester) versus placebo (Marya et al., 1987). More recently, Hollis et al. (2011) conducted the largest randomized trial of nearly 500 pregnant women who were randomized to supplementation with 400, 2000 or 4000 IU of vitamin D. Although a significant improvement in circulating 25(OH)D levels was seen across all groups, the investigators did not observe any differences in birthweights across the dose categories (Hollis et al., 2011).
Higher maternal serum levels of 25(OH)D have been related to a reduced likelihood of reactive airway disease in the children. Litonjua (2012) observed a 40% increased risk of asthma in children at ages of 3–5 years whose mothers had low levels of 25(OH)D in pregnancy. Although childhood eczema, respiratory infections, neurocognitive parameters and autism have all been related to maternal deficiency, a cause and effect relationship is not clear (Gale et al., 2008; Grant and Soles, 2009; Morales et al., 2012; Whitehouse et al., 2012). Collectively, the existing data imply that maternal vitamin D status may have long-lasting health implications for their progeny, and negative connotations of maternal vitamin D deficiency may extend well into the years beyond infancy. These concepts, yet again, underscore the need for appropriately designed future studies.
Vitamin D and lactation: human data
Human breast milk is a poor source of vitamin D (Hollis and Wagner, 2011; Holick et al., 2012). Therefore, maternal vitamin D sufficiency is thus particularly relevant in exclusively breastfed infants. Daily intake of 600 IU of vitamin D as per current recommendations (ACOG Committee Opinion, 2011; IOM, 2011) is suggested to be inadequate for either prevention or for the correction of documented vitamin D deficiency during the periods of pregnancy and lactation (Hollis and Wagner, 2004; Bodnar et al., 2007b). Additional supplementation with 1000 IU of vitamin D beyond the dose inclusive in the commonly utilized prenatal vitamins (400 IU) has been suggested to ensure that serum levels of 25(OH)D are maintained >30 ng/ml (Endocrine Society Clinical Guidelines, 2011; Holick et al., 2011). To satisfy the requirements of an infant who is exclusively breastfed, the mother requires 4000–6000 IU/day to transfer enough vitamin D into her milk if she chooses not to give a vitamin D supplement to the infant (Hollis and Wagner, 2004; Endocrine Society Clinical Guidelines, 2011).
Recommended daily vitamin D intake
Despite circulating 25(OH)D level being recognized to reliably reflect an individual's vitamin D status, a consensus regarding the optimal serum level for health maintenance is, however, lacking (Aloia, 2011). The revised guidelines issued by the Institute of Medicine (IOM) identify serum 25(OH)D threshold of 20 ng/ml (50 nmol/l), at and above which, adverse skeletal sequelae may be avoided (IOM, 2011; ACOG, 2011; Table II). The Endocrine Society of North America, however, maintains a higher 25(OH)D threshold of 30 ng/ml to differentiate between states of vitamin D sufficiency (>30 ng/ml) and insufficiency (≤30 ng/ml ≥20 ng/ml). Of particular concern is whether the IOM specified RDA for vitamin D is sufficient to maintain 25(OH)D levels >30 ng/ml in the at-risk populations such as those already deficient in vitamin D or during pregnancy and lactation (Lee et al., 2007; IOM, 2011; Holick et al., 2012). The Endocrine Society Clinical Practice Guidelines recommend higher daily allowance for vitamin D during pregnancy and lactation than proposed by the IOM (Table III).
Table II.
Recommended daily allowance and tolerable upper limit for vitamin D as per guidelines issued by the IOM.
| Age (years) | RDA vitamin D (IU/day) | Upper limit vitamin D (IU/day) |
|---|---|---|
| 1–3 | 600 | 2500 |
| 4–8 | 600 | 3000 |
| 9–18 | 600 | 4000 |
| 19–50 | 600 | 4000 |
| 51–70 | 600 | 4000 |
| 71+ | 800 | 4000 |
| Pregnancy | 600 | 4000 |
| Lactation | 600 | 4000 |
Source: IOM (2011).
Table III.
Recommended daily allowance and tolerable upper limit for vitamin D in pregnancy and lactation as per the Endocrine Society Practice Guidelines.
| Age | Daily requirement (IU/day) | Upper limit vitamin D (IU/day) |
|---|---|---|
| Pregnancy | ||
| 14–18 | 600–1000 | 4000 |
| 19–30 | 1500–2000 | 10000 |
| 31–50 | 1500–2000 | 10000 |
| Lactation | ||
| 14–18 | 600–1000 | 4000 |
| 19–30 | 1500–2000 | 10000 |
| 31–50 | 1500–2000 | 10000 |
Source: Holick et al. (2011).
Conclusions
In the recent years emerging data have suggested that vitamin D is not only critical for the maintenance of bone health and for calcium and phosphate homeostasis, but also imposes multisystem regulatory effects that modulate overall wellbeing and health (Holick et al., 2007). Herein, we have reviewed the existing literature that supports a plausible role for vitamin D in reproductive physiology. Summarizing the available data, we have attempted to convey the similarities as well as differences in data collected from studies carried out in rats, transgenic mice as well as in clinical settings. It is, however, noteworthy that transgenic models of vitamin D deficiency (Vdr and cyp27b1 null) are not defined by a critical circulating level of vitamin D or its metabolites. Thus, the direct extrapolation of existing data from transgenic mice to humans must be treated with caution. Nevertheless, a growing body of literature suggests that an individual's vitamin D status may adversely impact reproductive functions. However, the dearth of prospective interventional studies and studies that define the mechanisms whereby vitamin D affects reproductive physiology underscores the dire need for appropriately designed intervention trials. Given its recognized safety, accessibility and ease of administration vitamin D supplementation could prove to be a cost effective strategy to improve public health.
Author's' roles
All authors contributed to the different components of the review paper. All authors have drafted and/or critically read and revised the manuscript for important intellectual content and have approved the final version of the manuscript for submission.
Funding
N.P.'s effort was supported by the R21 HD066355 and the Albert Einstein College of Medicine Department of Obstetrics and Gynecology and Women's Health.
Conflict of interest
None declared.
References
- Abrams SA. In utero physiology: role in nutrient delivery and fetal development for calcium, phosphorus, and vitamin D. Am J Clin Nutr. 2007;85:604S–607S. doi: 10.1093/ajcn/85.2.604S. [DOI] [PubMed] [Google Scholar]
- ACOG Committee Opinion. Vitamin D: screening and supplementation during pregnancy. Obstet Gynecol. 2011;118:197–198. doi: 10.1097/AOG.0b013e318227f06b. [DOI] [PubMed] [Google Scholar]
- Agic A, Xu H, Altgassen C, Noack F, Wolfler MM, Diedrich K, Friedrich M, et al. Relative expression of 1,25-dihydroxyvitamin D3 receptor, vitamin D 1 alpha-hydroxylase, vitamin D 24-hydroxylase, and vitamin D 25-hydroxylase in endometriosis and gynecologic cancers. Reprod Sci. 2007;14:486–497. doi: 10.1177/1933719107304565. [DOI] [PubMed] [Google Scholar]
- Alemzadeh R, Kichler J, Babar G, Calhoun M. Hypovitaminosis D in obese children and adolescents: relationship with adiposity, insulin sensitivity, ethnicity, and season. Metabolism. 2008;57:183–191. doi: 10.1016/j.metabol.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Aleyasin A, Hosseini MA, Mahdavi A, Safdarian L, Fallahi P, Mohajeri MR, et al. Predictive value of the level of vitamin D in follicular fluid on the outcome of assisted reproductive technology. Eur J Obstet Gynecol Reprod Biol. 2011;159:132–137. doi: 10.1016/j.ejogrb.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Aloia JF. Clinical review: the 2011 report on dietary reference intake for vitamin D: where do we go from here? J Clin Endocrinol Metab. 2011;10:2987–2996. doi: 10.1210/jc.2011-0090. [DOI] [PubMed] [Google Scholar]
- Anifandis GM, Dafopoulos K, Messini CI, Chalvatzas N, Liakos N, Pournaras S, et al. Prognostic value of follicular fluid 25-OH vitamin D and glucose levels in the IVF outcome. Reprod Biol Endocrinol. 2010;8:91. doi: 10.1186/1477-7827-8-91. Reprod Biol Endocrinol 2010 Jul 28; 8:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arunabh S, Pollack S, Yeh J, Aloia JF. Body fat content and 25-hydroxyvitamin D levels in healthy women. J Clin Endocrinol Metab. 2003;88:157–161. doi: 10.1210/jc.2002-020978. [DOI] [PubMed] [Google Scholar]
- Ashwell M, Stone EM, Stolte H, Cashman KD, Macdonald H, Lanham-New S, Hiom S, Webb A, Fraser D. UK Food Standards Agency Workshop Report: an investigation of the relative contributions of diet and sunlight to vitamin D status. Br J Nutr. 2010;104:603–611. doi: 10.1017/S0007114510002138. [DOI] [PubMed] [Google Scholar]
- Bai X, Miao D, Li J, Goltzman D, Karaplis AC. Transgenic mice overexpressing human fibroblast growth factor 23 (R176Q) delineate a putative role for parathyroid hormone in renal phosphate wasting disorders. Endocrinology. 2004;145:5269–5279. doi: 10.1210/en.2004-0233. [DOI] [PubMed] [Google Scholar]
- Baker Am, Haeri S, Camargo CA, Jr, Stuebe AM, Boggess KA. First trimester maternal vitamin D status and risk for gestational diabetes mellitus: a nested case–control study. Diabetes Metab Res Rev. 2012;28:164–168. doi: 10.1002/dmrr.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassir M, Laborie S, Lapillonne A, Claris O, Chappuis MC, Salle BL. Vitamin D deficiency in Iranian mothers and their neonates: a pilot study. Acta Paediatr. 2001;90:577–579. [PubMed] [Google Scholar]
- Bell NH, Epstein S, Greene A, Shary J, Oexmann MJ, Shaw S. Evidence for alteration of the vitamin D-endocrine system in obese subjects. J Clin Invest. 1988;76:370–373. doi: 10.1172/JCI111971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertone-Johnson ER, Chocano-Bedoya PO, Zagarins SE, Micka AE, Ronnenberg AG. Dietary vitamin D intake, 25-hydroxyvitamin D3 levels and premenstrual syndrome in a college-aged population. J Steroid Biochem Mol Biol. 2010;121:434–437. doi: 10.1016/j.jsbmb.2010.03.076. [DOI] [PubMed] [Google Scholar]
- Blomberg Jensen M, Jørgensen A, Nielsen JE, Bjerrum PJ, Skalkam M, Petersen JH, Egeberg DL, Bangsbøll S, Andersen AN, Skakkebaek NE, et al. Expression of the vitamin D metabolizing enzyme CYP24A1 at the annulus of human spermatozoa may serve as a novel marker of semen quality. Int J Androl. 2012 doi: 10.1111/j.1365-2605.2012.01256.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, Roberts JM. Maternal vitamin D deficiency increases the risk of preeclampsia. J Clin Endocrinol Metab. 2007a;92:3517–3522. doi: 10.1210/jc.2007-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar LM, Simhan HN, Powers RW, Frank MP, Cooperstein E, Roberts JM. High prevalence of vitamin D insufficiency in black and white pregnant women residing in the northern United States and their neonates. J Nutr. 2007b;137:447–452. doi: 10.1093/jn/137.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodner LM, Krohn MA, Sihan HN. Maternal vitamin D deficiency is associated with bacterial vaginosis in the first trimester of pregnancy. J Nutr. 2009;139:1157–1161. doi: 10.3945/jn.108.103168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggess K, Espinola J, Moss K, Bck J, Offenbacher S, Carmargo C. Vitamin D status and peridontal disease among pregnant women. J Periodontol. 2011;82:195–200. doi: 10.1902/jop.2010.100384. [DOI] [PubMed] [Google Scholar]
- Botella-Carretero JI, Alvarez-Blasco F, Villafruela JJ, Balsa JA, Vázquez C, Escobar-Morreale HF. Vitamin D deficiency is associated with the metabolic syndrome in morbid obesity. Clin Nutr. 2007;26:573–580. doi: 10.1016/j.clnu.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Breitbart H. Intracellular calcium regulation in sperm capacitation and acrosomal reaction. Mol Cell Endocrinol. 2002;187:139–144. doi: 10.1016/s0303-7207(01)00704-3. [DOI] [PubMed] [Google Scholar]
- Britt KL, Drummond AE, Cox VA, Dyson M, Wreford NG, Jones ME, et al. An age-related ovarian phenotype in mice with targeted disruption of the Cyp 19 (aromatase) gene. Endocrinology. 2000;141:2614–2623. doi: 10.1210/endo.141.7.7578. [DOI] [PubMed] [Google Scholar]
- Britt KL, Drummond AE, Dyson M, Wreford NG, Jones ME, Simpson ER, et al. The ovarian phenotype of the aromatase knockout (ArKO) mouse. J Steroid Biochem Mol Biol. 2001;79:181–185. doi: 10.1016/s0960-0760(01)00158-3. [DOI] [PubMed] [Google Scholar]
- Brommage R, DeLuca HF. Vitamin D-deficient rats produce reduced quantities of a nutritionally adequate milk. Am J Physiol. 1984a;246:E221–E226. doi: 10.1152/ajpendo.1984.246.3.E221. [DOI] [PubMed] [Google Scholar]
- Brommage R, DeLuca HF. A maternal defect is responsible for growth failure in vitamin D-deficient rat pups. Am J Physiol. 1984b;246:E216–E220. doi: 10.1152/ajpendo.1984.246.3.E216. [DOI] [PubMed] [Google Scholar]
- Brommage R, Jarnagin K, DeLuca HF. 1,25-Dihydroxyvitamin D3 normalizes maternal food consumption and pup growth in rats. Am J Physiol. 1984;246:E227–E231. doi: 10.1152/ajpendo.1984.246.3.E227. [DOI] [PubMed] [Google Scholar]
- Brooke OG, Brown IR, Bone CD, Carter ND, Cleeve HJ, Maxwell JD, Robinson VP, Winder SM. Vitamin D supplementation in pregnant Asian women: effects on calcium status and fetal growth. Br Med J. 1980;280:751–754. doi: 10.1136/bmj.280.6216.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee M. Vitamin D and genomic stability. Mutat Res. 2001;475:69–87. doi: 10.1016/s0027-5107(01)00080-x. [DOI] [PubMed] [Google Scholar]
- Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79:820–825. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- Christakos S, Raval-Pandya M, Wernyj RP, Yang W. Genomic mechanisms involved in the pleiotropic actions of 1,25-dihydroxyvitamin D3. Biochem J. 1996;316:361–371. doi: 10.1042/bj3160361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakos S, Barletta F, Huening M, Dhawan P, Liu Y, Porta A, Peng X. Vitamin D target proteins: function and regulation. J Cell Biochem. 2003;88:238–244. doi: 10.1002/jcb.10349. [DOI] [PubMed] [Google Scholar]
- Christakos S, Dhawan P, Benn B, Porta A, Hediger M, Oh GT, Jeung EB, Zhong Y, Ajibade D, Dhawan K, Joshi S. Vitamin D: molecular mechanism of action. Ann N Y Acad Sci. 2007;1116:340–348. doi: 10.1196/annals.1402.070. [DOI] [PubMed] [Google Scholar]
- Corbett ST, Hill O, Nangia AK. Vitamin D receptor found in human sperm. Urology. 2006;68:1345–1349. doi: 10.1016/j.urology.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Couse JE, Mahato D, Eddy EM, Korach KS. Molecular mechanism of estrogen action in the male: insights from the estrogen receptor null mice. Reprod Fertil Dev. 2001;13:211–219. doi: 10.1071/rd00128. [DOI] [PubMed] [Google Scholar]
- Cross NA, Hillman LS, Allen SH, Krause GF, Vieira NE. Calcium homeostasis and bone metabolism during pregnancy, lactation, and postweaning: a longitudinal study. Am J Clin Nutr. 1995;61:514–523. doi: 10.1093/ajcn/61.3.514. [DOI] [PubMed] [Google Scholar]
- Curtis Hewitt S, Goulding EH, Eddy EM, Korach KS. Studies using the estrogen receptor alpha knockout uterus demonstrate that implantation but not decidualization-associated signaling is estrogen dependent. Biol Reprod. 2002;67:1268–1277. doi: 10.1095/biolreprod67.4.1268. [DOI] [PubMed] [Google Scholar]
- Dicken CL, Israel D, Davis JB, Sun Y, Shu J, Hardin J, Neal-Perry G. Peripubertal vitamin D3 deficiency delays puberty and disrupts the estrous cycle in adult female mice. Biol Reprod, 2012 doi: 10.1095/biolreprod.111.096511. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl JW, Chiu MW. Effects of ambient sunlight and photoprotection on vitamin D status. Dermatol Ther. 2010;23:48–60. doi: 10.1111/j.1529-8019.2009.01290.x. [DOI] [PubMed] [Google Scholar]
- Dokoh S, Donaldson CA, Marion SL, Pike JW, Haussler MR. The ovary: a target organ for 1,25-dihydroxyvitamin D3. Endocrinology. 1983;112:200–206. doi: 10.1210/endo-112-1-200. [DOI] [PubMed] [Google Scholar]
- Dror DK, King JC, Durand DJ, Allen LH. Association of modifiable and nonmodifiable factors with vitamin D status in pregnant women and neonates in Oakland, CA. J Am Diet Assoc. 2011;111:111–116. doi: 10.1016/j.jada.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Du H, Daftary GS, Lalwani SI, Taylor HS. Direct regulation of HOXA10 by 1,25-(OH)2D3 in human myelomonocytic cells and human endometrial stromal cells. Mol Endocrinol. 2005;19:2222–2233. doi: 10.1210/me.2004-0336. [DOI] [PubMed] [Google Scholar]
- Durlinger AL, Visser JA, Themmen AP. Regulation of ovarian function: the role of anti-Mullerian hormone. Reproduction. 2002;124:601–609. doi: 10.1530/rep.0.1240601. [DOI] [PubMed] [Google Scholar]
- Eddy EM, Washburn TF, Bunch DO, Goulding EH, Gladen BC, Lubahn DB, Korach KS. Targeted disruption of the estrogen receptor gene in male mice causes alteration of spermatogenesis and infertility. Endocrinology. 1996;137:4796–4805. doi: 10.1210/endo.137.11.8895349. [DOI] [PubMed] [Google Scholar]
- Endocrine Society Clinical Guidelines. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society Clinical Practice Guidelines. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- Erben RG, Soegiarto DW, Weber K, Zeitz U, Lieberherr M, Gniadecki R, Möller G, Adamski J, Balling R. Deletion of deoxyribonucleic acid binding domain of the vitamin D receptor abrogates genomic and nongenomic functions of vitamin D. Mol Endocrinol. 2002;16:1524–1537. doi: 10.1210/mend.16.7.0866. [DOI] [PubMed] [Google Scholar]
- Ertl R, Yu Ck, Samaha R, Akolekar R, Nicolaides K. Maternal serum vitamin D at 11–13 weeks in pregnancies delivering small for gestational age neonates. Fetal Diagn Ther. 2012;31:103–108. doi: 10.1159/000333810. [DOI] [PubMed] [Google Scholar]
- Evans KN, Nguyen L, Chan J, Innes BA, Bulmer JN, Kilby MD, et al. Effects of 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 on cytokine production by human decidual cells. Biol Reprod. 2006;75:816–822. doi: 10.1095/biolreprod.106.054056. [DOI] [PubMed] [Google Scholar]
- Faserl K, Golderer G, Kremser L, Lindner H, Sarg B, Wildt L, et al. Polymorphism in vitamin D-binding protein as a genetic risk factor in the pathogenesis of endometriosis. J Clin Endocrinol Metab. 2011;96:E233–E241. doi: 10.1210/jc.2010-1532. [DOI] [PubMed] [Google Scholar]
- Ferrero S, Gillott DJ, Anserini P, Remorgida V, Price KM, Ragni N, Grudzinskas JG. Vitamin D binding protein in endometriosis. J Soc Gynecol Investig. 2005;12:272–277. doi: 10.1016/j.jsgi.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Findelstein JL, Mehta S, Duggan CP, Spiegelman S, Aboud S, Kupka R, Msamanga GI, Fawzi WW. Predictors of anaemia and iron deficiency in HIV-infected pregnant women in Tanzania: a potential role for vitamin D and parasitic infections. Public Health Nutr. 2011;15:928–937. doi: 10.1017/S1368980011002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer D, Schroer A, Lüdders D, Cordes T, Bücker B, Reichrath J, Friedrich M. Metabolism of vitamin D3 in the placental tissue of normal and preeclampsia complicated pregnancies and premature births. Clin Exp Obstet Gynecol. 2007;34:80–84. [PubMed] [Google Scholar]
- Fischer D, Thome M, Becker S, Cordes T, Diedrich K, Friedrich M, Thill M. Expression of 25-hydroxyvitamin D3-24-hydroxylase in benign and malignant ovarian cell lines and tissue. Anticancer Res. 2009;29:3635–3639. [PubMed] [Google Scholar]
- Foresta C, Selice R, Di Mambro A, Strapazzon G. Testiculopathy and vitamin D insufficiency. Lancet. 2010;376:1301. doi: 10.1016/S0140-6736(10)61916-2. [DOI] [PubMed] [Google Scholar]
- Foss YJ. Vitamin D deficiency is the cause of common obesity. Med Hypotheses. 2009;72:314–321. doi: 10.1016/j.mehy.2008.10.005. [DOI] [PubMed] [Google Scholar]
- French AL, Adeyemi OM, Agniel DM, Evans CT, Yin MT, Anastos K. The association of HIV status with bacterial vaginosis and vitamin D in the United States. J Women's Health. 2011;20:1497–1503. doi: 10.1089/jwh.2010.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich M. Vitamin D and breast cancer: new approaches for hormonal therapy of breast cancer. Clin Exp Obstet Gynecol. 2000;27:77–82. [PubMed] [Google Scholar]
- Friedrich M, Rafi L, Mitschele T, Tilgen W, Schmidt W, Reichrath J. Analysis of the vitamin D system in cervical carcinomas, breast cancer and ovarian cancer. Recent Results Cancer Res. 2003;164:239–246. doi: 10.1007/978-3-642-55580-0_17. [DOI] [PubMed] [Google Scholar]
- Gale CR, Robinson SM, Harvy NC, Javaid MK, Jiang B, Martyn CN, Godfrey KM, Cooper C Princess Anne Hospital Study Group. Maternal vitamin D status during pregnancy and child outcomes. Eur J Clin Nutr. 2008;62:68–77. doi: 10.1038/sj.ejcn.1602680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautron L, Elmquist JK. Sixteen years and counting: an update on leptin in energy balance. J Clin Invest. 2011;121:2087–2093. doi: 10.1172/JCI45888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant WB, Soles CM. Epidemiologic evidence supporting the role of maternal vitamin D deficiency as a risk factor for the development of infantile autism. Dermatoendocrinology. 2009;1:223–228. doi: 10.4161/derm.1.4.9500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn S, Haselhorst U, Tan S, Quadbeck B, Schmidt M, Roesler S, Kimmig R, Mann K, Janssen OE. Low serum 25-hydroxyvitamin D concentrations are associated with insulin resistance and obesity in women with polycystic ovary syndrome. Exp Clin Endocrinol Diabetes. 2006;114:577–583. doi: 10.1055/s-2006-948308. [DOI] [PubMed] [Google Scholar]
- Halloran BP, DeLuca HF. Vitamin D deficiency and reproduction in rats. Science. 1979;204:73–74. doi: 10.1126/science.432628. [DOI] [PubMed] [Google Scholar]
- Halloran BP, DeLuca HF. Effect of vitamin D deficiency on fertility and reproductive capacity in the female rat. J Nutr. 1980;110:1573–1580. doi: 10.1093/jn/110.8.1573. [DOI] [PubMed] [Google Scholar]
- Haugen M, Brantsaeter AL, Trogstad L, Alexander J, Roth C, Magnus P, Meltzer HM. Vitamin D supplementation and reduced risk of preeclampsia in nulliparous women. Epidemiology. 2009;20:720–726. doi: 10.1097/EDE.0b013e3181a70f08. [DOI] [PubMed] [Google Scholar]
- Hensel KJ, Randis TM, Gelber SE, Ratner AJ. Pregnancy-specific association of vitamin D deficiency and bacterial vaginosis. Am J Obstet Gynecol. 2011;204:41.e1–41.e9. doi: 10.1016/j.ajog.2010.08.013. [DOI] [PubMed] [Google Scholar]
- Holick MF. Vitamin D deficiency: review. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- Holick MF, Smith E, Pincus S. Skin as the site of vitamin D synthesis and target tissue for 1,25-dihydroxyvitamin D3. Use of calcitriol (1,25-dihydroxyvitamin D3) for treatment of psoriasis. Arch Dermatol. 1987;123:1677–1683. [PubMed] [Google Scholar]
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011;7:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Guidelines for preventing and treating vitamin D deficiency and insufficiency revisited. J Clin Endocrinol Metab. 2012;97:1153–1158. doi: 10.1210/jc.2011-2601. [DOI] [PubMed] [Google Scholar]
- Hollis BW, Wagner CL. Vitamin D requirements during lactation: high-dose maternal supplementation as therapy to prevent hypovitaminosis D for both the mother and the nursing infant. Am J Clin Nutr. 2004;80:1752S–1758S. doi: 10.1093/ajcn/80.6.1752S. [DOI] [PubMed] [Google Scholar]
- Hollis BW, Wagner CL. The vitamin D requirement during human lactation: the facts and IOM's ‘utter’ failure. Public Health Nutr. 2011;14:748–749. doi: 10.1017/S1368980011000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis BW, Honson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res. 2011;26:2341–2357. doi: 10.1002/jbmr.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosking DJ. Calcium homeostasis in pregnancy. Clin Endocrinol. 1996;45:1–6. [PubMed] [Google Scholar]
- Hossain N, Khanani R, Hussain-Kanani F, Shah T, Arif S, Pal L. High prevalence of vitamin D deficiency in Pakistani mothers and their newborns. Int J Gynaecol Obstet. 2011;12:229–233. doi: 10.1016/j.ijgo.2010.09.017. [DOI] [PubMed] [Google Scholar]
- IOM (Institute of Medicine) Dietary Reference Intakes for Calcium and Vitamin D. Committee to Review Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academies Press, Institute of Medicine; 2011. [Google Scholar]
- Javaid MK, Crozier SR, Harvey NC, Gale CR, Dennison EM, Boucher BJ. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet. 2006;367:36–43. doi: 10.1016/S0140-6736(06)67922-1. [DOI] [PubMed] [Google Scholar]
- Johnson LE, DeLuca HF. Vitamin D receptor null mutant mice fed high levels of calcium are fertile. J Nutr. 2001;131:1787–1791. doi: 10.1093/jn/131.6.1787. [DOI] [PubMed] [Google Scholar]
- Johnson LE, DeLuca HF. Reproductive defects are corrected in vitamin D-deficient female rats fed a high calcium, phosphorus and lactose diet. J Nutr. 2002;132:2270–2273. doi: 10.1093/jn/132.8.2270. [DOI] [PubMed] [Google Scholar]
- Johnson JA, Grande JP, Roche PC, Kumar R. Immunohistochemical detection and distribution of the 1,25-dihydroxyvitamin D3 receptor in rat reproductive tissues. Histochem Cell Biol. 1996;105:7–15. doi: 10.1007/BF01450873. [DOI] [PubMed] [Google Scholar]
- Keisala T, Minasyan A, Lou YR, Zou J, Kalueff AV, Pyykkö I, Tuohimaa P. Premature aging in vitamin D receptor mutant mice. J Steroid Biochem Mol Biol. 2009;115:91–97. doi: 10.1016/j.jsbmb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Kinuta K, Tanaka H, Moriwake T, Aya K, Kato S, Seino Y. Vitamin D is an important factor in estrogen biosynthesis of both female and male gonads. Endocrinology. 2000;141:1317–1324. doi: 10.1210/endo.141.4.7403. [DOI] [PubMed] [Google Scholar]
- Kotsa K, Yavropoulou MP, Anastasiou O, Yovos JG. Role of vitamin D treatment in glucose metabolism in polycystic ovary syndrome. Fertil Steril. 2009;92:1053–1058. doi: 10.1016/j.fertnstert.2008.07.1757. [DOI] [PubMed] [Google Scholar]
- Kovacs CS. Vitamin D in pregnancy and lactation: maternal, fetal, and neonatal outcomes from human and animal studies. Am J Clin Nutr. 2008;88:520S–528S. doi: 10.1093/ajcn/88.2.520S. [DOI] [PubMed] [Google Scholar]
- Kovacs CS. The role of vitamin D in pregnancy and lactation: insights from animal models and clinical studies. Annu Rev Nutr. 2012 doi: 10.1146/annurev-nutr-071811-150742. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Kwiecinski GG, Petrie GI, DeLuca HF. Vitamin D is necessary for reproductive functions of the male rat. J Nutr. 1989a;119:741–744. doi: 10.1093/jn/119.5.741. [DOI] [PubMed] [Google Scholar]
- Kwiecinksi GG, Petrie GI, DeLuca HF. 1,25-Dihydroxyvitamin D3 restores fertility of vitamin D-deficient female rats. Am J Physiol. 1989b;256:E483–E487. doi: 10.1152/ajpendo.1989.256.4.E483. [DOI] [PubMed] [Google Scholar]
- Lasco A, Catalano A, Benvenga S. Improvement of primary dysmenorrhea caused by a single oral dose of vitamin D: results of a randomized, double-blind, placebo-controlled study. Arch Intern Med. 2012;172:366–367. doi: 10.1001/archinternmed.2011.715. [DOI] [PubMed] [Google Scholar]
- Lee JM, Smith JR, Philipp BL, Chen TC, Mathieu J, Holick MF. Vitamin D deficiency in a healthy group of mothers and newborn infants. Clin Pediatr (Phila) 2007;46:42–44. doi: 10.1177/0009922806289311. [DOI] [PubMed] [Google Scholar]
- Lester GE, VanderWiel CJ, Gray TK, Talmage RV. Vitamin D deficiency in rats with normal serum calcium concentrations. Proc Natl Acad Sci. 1982;79:4791–4794. doi: 10.1073/pnas.79.15.4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liel Y, Ulmer E, Shary J, Hollis BW, Bell NH. Low circulating vitamin D in obesity. Calcif Tissue Int. 1988;43:199–201. doi: 10.1007/BF02555135. [DOI] [PubMed] [Google Scholar]
- Lips P. Vitamin D physiology. Prog Biophys Mol Biol. 2006;92:4–8. doi: 10.1016/j.pbiomolbio.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Litonjua AA. Vitamin D deficiency as a risk factor for childhood allergic disease and asthma. Curr Opin Allergy Clin Immunol. 2012;12:179–185. doi: 10.1097/ACI.0b013e3283507927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looker AC, Pfeiffer CM, Lacher DA, Schleicher RL, Picciano MF, Yetley EA. Serum 25-hydroxyvitamin D status of the US population: 1988–1994 compared with 2000–2004. Am J Clin Nutr. 2008;88:1519–1527. doi: 10.3945/ajcn.2008.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund J, DeLuca HF. Biologically active metabolite of vitamin D3 from bone, liver, and blood serum. J Lipid Res. 1966;6:739–744. [PubMed] [Google Scholar]
- Mahato D, Goulding EH, Korach KS, Eddy EM. Spermatogenic cells do not require estrogen receptor-alpha for development or function. Endocrinology. 2000;141:1273–1276. doi: 10.1210/endo.141.3.7439. [DOI] [PubMed] [Google Scholar]
- Mahmoudi T, Gourabi H, Ashrafi M, Yazdi RS, Ezabadi Z. Calciotropic hormones, insulin resistance, and the polycystic ovary syndrome. Fertil Steril. 2010;93:1208–1214. doi: 10.1016/j.fertnstert.2008.11.031. [DOI] [PubMed] [Google Scholar]
- Mahon P, Harvey N, Crozier S, Inskip H, Robinson S, Arden N, Swaminathan R, Cooper C, Godfrey K SWS Study Group. Low maternal vitamin D status and fetal bone development: cohort study. J Bone Miner Res. 2010;25:14–19. doi: 10.1359/jbmr.090701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makgoba M, Nelson SM, Savvidou M, Messow CM, Nicolaides K, Satter N. First-trimester circulating 25-hydroxyvitamin D levels and development of gestational diabetes mellitus. Diabetes Care. 2011;34:1091–1093. doi: 10.2337/dc10-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloy PJ, Peng L, Wang J, Feldman D. Interaction of the vitamin D receptor with a vitamin D response element in the Mullerian-inhibiting substance (MIS) promoter: regulation of MIS expression by calcitriol in prostate cancer cells. Endocrinology. 2009;150:1580–1587. doi: 10.1210/en.2008-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, David V, Quarles LD. Regulation and function of the FGF23/Klotho endocrine pathways. Physiol Rev. 2012;92:131–155. doi: 10.1152/physrev.00002.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marya RK, Rathee S, Manrow M. Effect of calcium and vitamin D supplementation on toxaemia of pregnancy. Gynecol Obstet Invest. 1987;24:38–42. doi: 10.1159/000298772. [DOI] [PubMed] [Google Scholar]
- Matsunuma A, Horiuchi N. Leptin attenuates gene expression for renal 25-hydroxyvitamin D3-1alpha-hydroxylase in mice via the long form of the leptin receptor. Arch Biochem Biophys. 2007;463:118–127. doi: 10.1016/j.abb.2007.02.031. [DOI] [PubMed] [Google Scholar]
- McCullough ML. Vitamin D deficiency in pregnancy: bringing the issues to light. J Nutr. 2007;137:305–306. doi: 10.1093/jn/137.2.305. [DOI] [PubMed] [Google Scholar]
- Mehta S, Mugusi F, Spiegelman D, Villamor E, Finkelstein JL, Hertzmark E, Giovannucci EL, Msamanga GI, Fawzi WW. Vitamin D status and its association with morbidity including wasting and opportunistic illnesses in HIV-infected women in Tanzania. AIDS Patient Care STDs. 2011;25:579–585. doi: 10.1089/apc.2011.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellanby E. An experimental investigation of rickets. Lancet. 1919;1:407–412. doi: 10.1111/j.1753-4887.1976.tb05815.x. [DOI] [PubMed] [Google Scholar]
- Merewood A, Mehta SD, Chen TC, Bauchner H, Holick MF. Association between severe vitamin D deficiency and primary caesarean section. J Clin Endocrinol Metab. 2009;94:940–945. doi: 10.1210/jc.2008-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizwicki MT, Norman AW. The vitamin D sterol-vitamin D receptor ensemble model offers unique insights into both genomic and rapid-response signaling. Sci Signal. 2009;2:re4. doi: 10.1126/scisignal.275re4. [DOI] [PubMed] [Google Scholar]
- Moore C, Murphy MM, Keast DR, Holick MF. Vitamin D intake in the United States. J Am Diet Assoc. 2004;104:980–983. doi: 10.1016/j.jada.2004.03.028. [DOI] [PubMed] [Google Scholar]
- Morales E, Romieu I, Guerra S, Ballester F, Rebagliato M, Vioque J, Tardón A, Rodriguez Delhi C, Arranz L, Torrent M, Espada M, Basterrechea M, Sunyer J INMA Project. Maternal vitamin D status in pregnancy and risk of lower tract infections, wheeze and asthma in offspring. Epidemiology. 2012;23:64–71. doi: 10.1097/EDE.0b013e31823a44d3. [DOI] [PubMed] [Google Scholar]
- Nesby-O'Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, Allen C, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2002;76:187–192. doi: 10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- Ngo DT, Chan WP, Rajendran S, Heresztyn T, Amarasekera A, Sverdlov AL, O'Loughlin PD, Morris HA, Chirkov YY, Norman RJ, Horowitz JD. Determinants of insulin responsiveness in young women: impact of polycystic ovarian syndrome, nitric oxide, and vitamin D. Nitric Oxide. 2011;25:326–330. doi: 10.1016/j.niox.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Osmundsen BC, Huang HF, Anderson MB, Christakos S, Walters MR. Multiple sites of action of the vitamin D endocrine system: FSH stimulation of testis 1,25-dihydroxyvitamin D3 receptors. J Steroid Biochem. 1989;34:339–343. doi: 10.1016/0022-4731(89)90105-2. [DOI] [PubMed] [Google Scholar]
- Ott J, Wattar L, Kurz C, Seemann R, Huber JC, Mayerhofer K, Vytiska-Binstorfer E. Parameters for calcium metabolism in women with polycystic ovary syndrome who undergo clomiphene citrate stimulation: a prospective cohort study. Eur J Endocrinol. 2012 doi: 10.1530/EJE-11-1070. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Ozkan S, Jindal S, Greenseid K, Shu J, Zeitlian G, Hickmon C, Pal L. Replete vitamin D stores predict reproductive success following in vitro fertilization. Fertil Steril. 2010;94:1314–1319. doi: 10.1016/j.fertnstert.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal L, Berry A, Coraluzzi L, Kustan E, Danton C, Shaw J, Taylor H. Therapeutic implications of vitamin D and calcium in overweight women with polycystic ovary syndrome. Gynecol Endocrinol. 2012 doi: 10.3109/09513590.2012.696753. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda DK, Miao D, Tremblay ML, Sirois J, Farookhi R, Hendy GN, Goltzman D. Targeted ablation of the 25-hydroxyvitamin D 1alpha -hydroxylase enzyme: evidence for skeletal, reproductive, and immune dysfunction. Proc Natl Acad Sci USA. 2001;98:7498–7503. doi: 10.1073/pnas.131029498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda DK, Miao D, Bolivar I, Li J, Huo R, Hendy GN, Goltzman D. Inactivation of the 25-hydroxyvitamin D 1alpha-hydroxylase and vitamin D receptor demonstrates independent and interdependent effects of calcium and vitamin D on skeletal and mineral homeostasis. J Biol Chem. 2004;279:16754–16766. doi: 10.1074/jbc.M310271200. [DOI] [PubMed] [Google Scholar]
- Panidis D, Balaris C, Farmakiotis D, Rousso D, Kourtis A, Balaris V, Katsikis I, Zournatzi V, Diamanti-Kandarakis E. Serum parathyroid hormone concentrations are increased in women with polycystic ovary syndrome. Clin Chem. 2005;51:1691–1697. doi: 10.1373/clinchem.2005.052761. [DOI] [PubMed] [Google Scholar]
- Rajakumar K. Vitamin D, cod-liver oil, sunlight, and rickets: a historical perspective. Pediatrics. 2003;112:e132–e135. doi: 10.1542/peds.112.2.e132. [DOI] [PubMed] [Google Scholar]
- Ramlau-Hansen CH, Moeller UK, Bonde JP, Olsen J, Thulstrup AM. Are serum levels of vitamin D associated with semen quality? Results from a cross-sectional study in young healthy men. Fertil Steril. 2011;95:1000–1004. doi: 10.1016/j.fertnstert.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Rashidi B, Haghollahi F, Shariat M, Zayerii F. The effects of calcium-vitamin D and metformin on polycystic ovary syndrome: a pilot study. Taiwan J Obstet Gynecol. 2009;48:142–147. doi: 10.1016/S1028-4559(09)60275-8. [DOI] [PubMed] [Google Scholar]
- Reichel H, Koeffler HP, Norman AW. The role of vitamin D endocrine system in health and disease. N Engl J Med. 1989;320:980–991. doi: 10.1056/NEJM198904133201506. [DOI] [PubMed] [Google Scholar]
- Ringrose JS, Paul-Jenssen AM, Wilson M, Blanco L, Ward H, Wilson TN. Vitamin D and hypertension in pregnancy. Clin Invest Med. 2011;34:E147–E154. doi: 10.25011/cim.v34i3.15187. [DOI] [PubMed] [Google Scholar]
- Ritchie LD, Fung EB, Halloran BP, Turnlund JR, Van Loan MD, Cann CE, King JC. A longitudinal study of calcium homeostasis during human pregnancy and lactation and after resumption of menses. Am J Clin Nutr. 1998;67:693–701. doi: 10.1093/ajcn/67.4.693. [DOI] [PubMed] [Google Scholar]
- Rowling MJ, Gliniak C, Welsh J, Fleet JC. High dietary vitamin D prevents hypocalcemia and osteomalacia in CYP27B1 knockout mice. J Nutr. 2007;137:2608–2615. doi: 10.1093/jn/137.12.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savvidou M, Makgoba M, Castro P, Akolekar R, Nicolaies K. First trimester maternal serum vitamin D and mode of delivery. Br J Nutr. 2012;24:1–4. doi: 10.1017/S0007114512000207. [DOI] [PubMed] [Google Scholar]
- Schuster I. Cytochromes P450 are essential players in the vitamin D signaling system. Biochim Biophys Acta. 2011;1814:186–199. doi: 10.1016/j.bbapap.2010.06.022. [DOI] [PubMed] [Google Scholar]
- Selimoglu H, Duran C, Kiyici S, Ersoy C, Guclu M, Ozkaya G, Tuncel E, Erturk E, Imamoglu S. The effect of vitamin D replacement therapy on insulin resistance and androgen levels in women with polycystic ovary syndrome. J Endocrinol Invest. 2010;33:234–238. doi: 10.1007/BF03345785. [DOI] [PubMed] [Google Scholar]
- Shand AW, Nassar N, Von Dadelszen P, Innis SM, Green TJ. Maternal vitamin D status in pregnancy and adverse pregnancy outcomes in a group at high risk for pre-eclampsia. BJOG. 2010;117:1593–1598. doi: 10.1111/j.1471-0528.2010.02742.x. [DOI] [PubMed] [Google Scholar]
- Shao T, Klein P, Grossbard ML. Vitamin D and breast cancer. Oncologist. 2012;17:36–45. doi: 10.1634/theoncologist.2011-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharan C, Halder SK, Thota C, Jaleel T, Nair S, Al-Hendy A. Vitamin D inhibits proliferation of human uterine leiomyoma cells via catechol-O-methyltransferase. Fertil Steril. 2011;95:247–253. doi: 10.1016/j.fertnstert.2010.07.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Norman AW, Okamura WH, Sen A, Zemel MB. 1,25-Dihydroxyvitamin D3 modulates human adipocyte metabolism via nongenomic action. FASEB J. 2001;15:2751–2753. doi: 10.1096/fj.01-0584fje. [DOI] [PubMed] [Google Scholar]
- Soheilykhah S, Mojibian M, Rashidi M, Rahimi-Saghand S, Jafari F. Maternal vitamin D status in gestational diabetes mellitus. Nutr Clin Pract. 2010;25:524–527. doi: 10.1177/0884533610379851. [DOI] [PubMed] [Google Scholar]
- Somigliana E, Panina-Bordignon P, Murone S, Di Lucia P, Vercellini P, Vigano P. Vitamin D reserve is higher in women with endometriosis. Hum Reprod. 2007;22:2273–2278. doi: 10.1093/humrep/dem142. [DOI] [PubMed] [Google Scholar]
- Stumpf WE, Sar M, O'Brien LP. Vitamin D sites of action in the pituitary studied by combined autoradiography-immunohistochemistry. Histochemistry. 1987a;88:11–16. doi: 10.1007/BF00490160. [DOI] [PubMed] [Google Scholar]
- Stumpf WE, Sar M, Chen K, Morin J, DeLuca HF. Sertoli cells in the testis and epithelium of the ductuli efferentes are targets for 3H 1,25 (OH)2 vitamin D3. An autoradiographic study. Cell Tissue Res. 1987b;247:453–455. doi: 10.1007/BF00218327. [DOI] [PubMed] [Google Scholar]
- Sun W, Xie H, Ji J, Zhou X, Goltzman D, Miao D. Defective female reproductive function in 1,25(OH)2D-deficient mice results from indirect effect mediated by extracellular calcium and/or phosphorus. Am J Physiol Endocrinol Metab. 2010;299:E928–E935. doi: 10.1152/ajpendo.00378.2010. [DOI] [PubMed] [Google Scholar]
- Thys-Jacobs S, Donovan D, Papadopoulos A, Sarrel P, Bilezikian JP. Vitamin and calcium dysregulation in the polycystic ovarian syndrome. Steroids. 1999;64:430–435. doi: 10.1016/s0039-128x(99)00012-4. [DOI] [PubMed] [Google Scholar]
- Uhland AM, Kwiecinski GG, DeLuca HF. Normalization of serum calcium restores fertility in vitamin D-deficient male rats. J Nutr. 1992;122:1338–1344. doi: 10.1093/jn/122.6.1338. [DOI] [PubMed] [Google Scholar]
- Urrutia RP, Thorp JM. Vitamin D in pregnancy: current concepts. Curr Opin Obstet Gynecol. 2012;24:57–64. doi: 10.1097/GCO.0b013e3283505ab3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vienonen A, Miettinen S, Bläuer M, Martikainen PM, Tomás E, Heinonen PK, et al. Expression of nuclear receptors and cofactors in human endometrium and myometrium. J Soc Gynecol Investig. 2004;11:104–112. doi: 10.1016/j.jsgi.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr. 1999;69:842–856. doi: 10.1093/ajcn/69.5.842. [DOI] [PubMed] [Google Scholar]
- Villamor E, Marin C, Mora-Plazas M, Baylin A. Vitamin D deficiency and age at menarche: a prospective study. Am J Clin Nutr. 2011;94:1020–1025. doi: 10.3945/ajcn.111.018168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser JA, de Jong FH, Laven JS, Themmen AP. Anti-Mullerian hormone: a new marker for ovarian function. Reproduction. 2006;131:1–9. doi: 10.1530/rep.1.00529. [DOI] [PubMed] [Google Scholar]
- Walters MR. Newly identified actions of the vitamin D endocrine system. Endocr Rev. 1992;13:719–763. doi: 10.1210/edrv-13-4-719. [DOI] [PubMed] [Google Scholar]
- Wang J, Yang F, Mao M, Liu DH, Yang HM, Yang SF. High prevalence of vitamin D and calcium deficiency among pregnant women and their newborns in Chengdu, China. World J Pediatr. 2010;6:265–267. doi: 10.1007/s12519-010-0224-x. [DOI] [PubMed] [Google Scholar]
- Wecksler WR, Norman AW. A kinetic and equilibrium binding study of 1 alpha,25-dihydroxyvitamin D3 with its cytosol receptor from chick intestinal mucosa. J Biol Chem. 1980a;255:3571–3574. [PubMed] [Google Scholar]
- Wecksler WR, Norman AW. Measurement of kinetic rate constants for the binding of 1 alpha, 25-dihydroxyvitamin D3 to its chick intestinal mucosa receptor using a hydroxyapatite batch assay. Methods Enzymol. 1980b;67:488–494. doi: 10.1016/s0076-6879(80)67060-8. [DOI] [PubMed] [Google Scholar]
- Wecksler WR, Ross FP, Mason RS, Norman AW. Biochemical properties of the 1 alpha, 25-dihydroxyvitamin D3 cytosol receptors from human and chicken intestinal mucosa. J Clin Endocrinol Metab. 1980;50:152–157. doi: 10.1210/jcem-50-1-152. [DOI] [PubMed] [Google Scholar]
- Wehr E, Pilz S, Schweighofer N, Giuliani A, Kopera D, Pieber TR, Obermayer-Pietsch B. Association of hypovitaminosis D with metabolic disturbances in polycystic ovary syndrome. Eur J Endocrinol. 2009;161:575–582. doi: 10.1530/EJE-09-0432. [DOI] [PubMed] [Google Scholar]
- Weisman Y, Harell A, Edelstein S, David M, Spirer Z, Golander A. 1 alpha, 25-Dihydroxyvitamin D3 and 24,25-dihydroxyvitamin D3 in vitro synthesis by human decidua and placenta. Nature. 1979;281:317–319. doi: 10.1038/281317a0. [DOI] [PubMed] [Google Scholar]
- Whitehouse A, Holt B, Serralha M, Holt P, Kusel M, Hart P. Maternal serum vitamin D levels during pregnancy and offspring neurocognitive development. Pediatrics. 2012;129:485–493. doi: 10.1542/peds.2011-2644. [DOI] [PubMed] [Google Scholar]
- Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Kawano N, Yoshida K. Control of sperm motility and fertility: diverse factors and common mechanisms. Cell Mol Life Sci. 2008;65:3446–3457. doi: 10.1007/s00018-008-8230-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa T, Handa Y, Uematsu Y, Takeda S, Sekine K, Yoshihara Y, Kawakami T, Arioka K, Sato H, Uchiyama Y, et al. Mice lacking the Vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet. 1997;16:391–396. doi: 10.1038/ng0897-391. [DOI] [PubMed] [Google Scholar]
- Yu CKH, Ertl R, Skyfta E, Akolekar R, Nicolaides KH. Maternal serum vitamin D levels at 11–13 weeks of gestation and preeclampsia. J Hum Hypertens. 2012 doi: 10.1038/jhh.2012.1. Epub ahead of press. [DOI] [PubMed] [Google Scholar]
- Zarnani AH, Shahbazi M, Salek-Moghaddam A, Zareie M, Tavakoli M, Ghasemi J, Rezania S, Moravej A, Torkabadi E, Rabbani H, et al. Vitamin D(3) receptor is expressed in the endometrium of cycling mice throughout the estrous cycle. Fertil Steril. 2010;93:2738–2743. doi: 10.1016/j.fertnstert.2009.09.045. [DOI] [PubMed] [Google Scholar]
- Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, Hewison M. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J Clin Endocrinol Metab. 2001;86:888–894. doi: 10.1210/jcem.86.2.7220. [DOI] [PubMed] [Google Scholar]
- Zemel MB. Regulation of adiposity and obesity risk by dietary calcium: mechanisms and implications. J Am Coll Nutr. 2002;21:146S–151S. doi: 10.1080/07315724.2002.10719212. [DOI] [PubMed] [Google Scholar]
- Zhang C, Qui C, Hu FB, David Rm, Van Dam Rm, Bralley A, Williams MA. Maternal plasma 25-hydroxyvitamin D concentrations and the risk for gestational diabetes mellitus. PLos One. 2008;3:e3753. doi: 10.1371/journal.pone.0003753. [DOI] [PMC free article] [PubMed] [Google Scholar]