Abstract
Background
Ventricular assist devices (VADs) improve survival and quality of life in patients with advanced heart failure, but their use is frequently complicated by infection. There are limited data on the microbiology and epidemiology of these infections.
Methods and Results
150 patients scheduled for VAD implantation were enrolled (2006–2008) at 11 U.S. cardiac centers and followed prospectively up to transplantation, explantation for recovery, death, or for one year. 86 (57%) patients received Heartmate II® devices. Data were collected on potential pre-, intra-, and post-operative risk factors for infection. Clinical, laboratory, and microbiologic data were collected for suspected infections and evaluated by an infectious diseases specialist. 33 (22%) subjects developed 34 VAD-related infections with an incidence rate of 0.10 per 100 person-days (95% CI, 0.073–0.142). The median time to infection was 68 days. The driveline was the most commonly infected site (n=28); 18 (64%) were associated with invasive disease. Staphylococci were the most common pathogen (47%), but Pseudomonas or other Gram-negative bacteria caused 32% of infections. A history of depression and elevated baseline serum creatinine were independent predictors of VAD infection (HRadj=2.8,P=0.007 and HRadj=1.7,P=0.023, respectively). The Heartmate II® was not associated with a decreased risk of infection. VAD infection increased one-year mortality (HRadj=5.6, P<0.0001).
Conclusions
This prospective, multicenter study demonstrates that infection frequently complicates VAD placement and is a continuing problem despite the use of newer, smaller devices. Depression and renal dysfunction may increase the risk of VAD infection. VAD infection is a serious consequence as it adversely affects patient survival.
Keywords: heart-assist device, infection, heart failure, depression, renal
Introduction
Heart failure (HF) is a major cause of morbidity and mortality worldwide. In the United States, approximately 5.7 million people have HF and 292,000 die annually. Advanced HF patients have a very poor prognosis without cardiac transplantation. The donor supply is limited and most are not transplant candidates. Ventricular assist devices (VADs) are an option for those with refractory HF and have been demonstrated to significantly improve survival and quality of life.1, 2 They are being used with increasing frequency to bridge patients to transplantation or transplant candidacy or to support them indefinitely as ‘destination therapy’ (DT).3
Although lifesaving, VADs are often associated with infections. In the Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure trial (REMATCH) where VADs were first evaluated as DT, 42% of patients developed sepsis within one year.4 Infections directly related to a VAD have been estimated to occur in 14–59% of cases.3, 5–7 Numerous pathogens, most commonly staphylococci, cause VAD infections.6 VAD infections often involve the percutaneous driveline, but may also involve the pump pocket, the pump and/or cannula, the pump interior, the sternal wound and the bloodstream.8 Multiple sites are often infected. Systemic infections appear to be associated with higher mortality than local infections.6
Despite the high incidence and potential increased morbidity and mortality associated with VAD infections, the epidemiology has not been well-described. Prior studies have been limited by sample size, retrospective and/or single-center design, and have used variable definitions of device infection. There are a lack of large, multicenter studies with prospectively collected data focused on carefully identifying and characterizing these infections and describing their microbiology, epidemiology, risk factors, and outcomes.6 This study was designed to fill this gap, as these data are needed to better diagnose, further study, treat, and prevent VAD infections.
Methods
Study Design
Eleven U.S. clinical centers participated in this study between January 2006 and 2009. The International Center for Health Outcomes and Innovation Research (InCHOIR) at Columbia University coordinated enrollment and data collection, monitored data quality, and provided statistical support. Study procedures were standardized across sites according to the protocol. Data were entered into a centralized, secure, electronic data capture system. Each site received Institutional Review Board approval, and subjects gave written informed consent and signed a Health Insurance Portability and Accountability Act release.
Study Subjects
Patients 18 years of age and older approved to receive a VAD for either DT or bridge to transplantation (BTT) were eligible to enroll. Subjects were monitored for infection until explantation or up to one year. Subjects who underwent device replacement remained in the study.
Data Collection and Adjudication
Baseline data included demographics, past medical history, details of a physical exam performed within two weeks of VAD implantation, and a comprehensive laboratory assessment performed within 24 hours prior to surgery. Information on infections, their microbiology, and treatment within 30 days prior to surgery was also recorded.
Variables collected regarding the VAD surgery included the type and location of VAD(s), cardiopulmonary bypass time and quantities of infused blood products. Details of the index hospitalization were recorded, including days in the intensive care unit (ICU), as were subsequent hospitalizations and surgeries. Clinical sites reported non-infection adverse events using INTERMACS definitions.9
When a patient was transplanted or explanted, the VAD was inspected for signs of infection, and cultured if applicable. VADs were examined for evidence of infection when autopsies were performed.
A “Clinical Infection Assessment” was conducted any time there was clinical suspicion of infection, fever ≥ 38.3°C after postoperative day 3, or addition of non-prophylactic antibiotics. Assessments included vital signs, routine laboratory testing, examination of the driveline exit site, the pump pocket, and the sternal wound, as well as the results of blood or other applicable cultures. Administered antibiotics were recorded. Within a week of the initiation of the infection assessment, the clinical center answered the question, “Was the clinical impression that a VAD infection was present?” and, if “uncertain” or “yes”, then the infected sites were specified.
An infectious diseases specialist (R.J.G.) reviewed all available data, including source documentation, from all Clinical Infection Assessments. Clinical, microbiologic, and histopathologic data were used to diagnose VAD infections, verify the date of infection and describe which parts of the device were infected with what microorganism(s). Specific pre-defined criteria were used to confirm infections, including a combination of positive cultures (often multiple) from one or more sites, purulent fluid (e.g. neutrophils) in the pump pocket, gross or histopathologic evidence of infection upon device removal or pump pocket exploration, excess and/or abnormal drainage from the driveline exit site, and local signs of infection (erythema, pain, swelling, wound dehiscence, and non-integration of the driveline). Systemic signs of infection (e.g. sepsis, fever, and leukocytosis) as well as antibiotic treatment frequently supported the diagnosis. If there was disagreement between the first specialist and the clinical center, a second infectious diseases specialist (F.D.L) determined the outcome. Details of other healthcare associated infections such as pneumonias, urinary tract infections (UTIs), Clostridium difficile colitis, wound infections, and non-VAD-related bloodstream infections (BSIs) were also evaluated.
Statistics
The Kaplan-Meier product limit estimate10 was used to explore time to first VAD infection. The log-rank test was employed to compare differences among groups. In addition, Cox proportional hazards (PH) regression models11 were used to explore univariate and multivariable associations between potential risk factors and VAD infection. Patients who did not experience VAD infections were censored if they died, withdrew, or were transplanted, explanted for recovery or lost to follow-up. Covariates with p values <0.15 in the univariate model were individually tested in the multivariable model in a manual stepwise procedure to explore potential associations, collinearity, confounding and relevant interactions. Hazard ratios (HRs) with 95% CIs were generated and HRs for continuous variables are expressed per unit change. The hazard function with 95% CIs was plotted to demonstrate the instantaneous risk of infection over the study period.12, 13
The main multivariable model and a Heartmate II® subgroup analysis included different covariates. There were 2 of 150 subjects with missing values in the main multivariable model and 4 of 85 subjects with missing values in the subgroup analysis. A sensitivity analysis was performed using an expectation-maximization algorithm to estimate these missing values, which supported the study findings. Subjects with missing values have been excluded from the figures and tables.
A Cox PH model was also used to investigate the effect of infection on one-year mortality. Other adverse events were examined as time-dependent variables.
A competing risks analysis was performed to calculate the cumulative incidence of the four major VAD surgery outcomes (alive with device, transplanted, explanted and died), stratified by infection status.
P values were not adjusted for multiple comparisons and inflation of the type I error. SAS software, version 9.1.3 (SAS Institute Inc., Cary, NC) was used to perform all analyses.
Results
Characteristics of Study Participants
150 patients with advanced HF approved for VAD implantation were enrolled at eleven cardiac centers (1 to 29 patients per center, supplementary table 1). Study subjects are described in Table 1. The mean age at enrollment was 54 +13 years, 126 (84%) were male, 101 (67%) were white/Caucasian and 32 (21%) were black/African American.
Table 1.
Characteristics of Study Subjects: Demographics and Medical Information*
| Entire Cohort n=150 |
VAD Infection Group n=33 |
No VAD Infection Group n=117 |
Hazard Ratio (95% CI) | P value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, mean (SD) | 57.4 (13.2) | 57.4 (12.0) | 57.4 (13.5) | 1.00 (0.97–1.02) | 0.82 |
| Male | 126 (84) | 26 (79) | 100 (85) | 0.69 (0.30–1.58) | 0.38 |
| Race | |||||
| white | 101 (67.3) | 24 (73) | 77 (66) | 1.50 (0.69–3.22) | 0.30 |
| black | 32 (21.3) | 5 (15) | 27 (23) | ||
| asian | 1 (0.7) | 0 | 1 (0.1) | ||
| american indian or alaska native |
1 (0.7) | 0 | 1 (0.1) | ||
| other | 14 (9.3) | 3 (9) | 11 (9) | ||
| Past Medical History | |||||
| COPD | 21 (14) | 4 (12) | 17 (14) | 0.89 (0.31–2.54) | 0.83 |
| Chronic Renal | 45 (30) | 14 (42) | 31 (27) | 1.95 (0.98–3.98) | 0.06 |
| Dysfunction | |||||
| Depression | 23 (15) | 10 (30) | 13 (11) | 2.62 (1.25–5.51) | 0.01 |
| Diabetes | 54 (36) | 13 (39) | 41 (35) | 1.23 (0.61–2.47) | 0.56 |
| Malignancy | 13 (9) | 3 (9) | 10 (9) | 0.98 (0.30–3.22) | 0.98 |
| Infection Pre- | |||||
| VAD Surgery† | 38 (25) | 6 (18) | 32 (27) | 0.68 (0.28–1.64) | 0.38 |
| Antibiotics Pre- | |||||
| VAD Surgery† | 58 (39) | 13 (41) | 45 (39) | 1.26 (0.62–2.55) | 0.40 |
| Past Cardio/cerebrovascular History | |||||
| Myocardial | |||||
| Infarction | 72 (48) | 17 (52) | 55 (47) | 1.40 (0.70–2.77) | 0.34 |
| Cardiac stents | 41 (27) | 12 (36) | 29 (25) | 1.59 (0.78–3.24) | 0.20 |
| Atrial | |||||
| Fibrillation | 66 (44) | 14 (42) | 52 (44) | 0.92 (0.46–1.84) | 0.82 |
| Ventricular | |||||
| Arrhythmia | 63 (42) | 12 (36) | 51 (44) | 0.78 (0.38–1.59) | 0.50 |
| Stroke | 18 (12) | 5 (15) | 13 (11) | 1.43 (0.55–3.72) | 0.46 |
| Past Surgical and Device-Related History | |||||
| AICD | 120 (80) | 26 (79) | 94 (80) | 0.96 (0.42–2.20) | 0.92 |
| Intraaortic | |||||
| balloon pump | 37 (25) | 8 (24) | 29 (25) | 1.15 (0.52–2.56) | 0.73 |
| Long term | |||||
| access line | 47 (31) | 12 (36) | 35 (30) | 1.41 (0.69–2.87) | 0.34 |
| Permanent | |||||
| Pacemaker | 97 (65) | 18 (54) | 79 (68) | 0.68 (0.35–1.36) | 0.28 |
| CABG | 51 (34) | 11 (33) | 40 (34) | 1.03 (0.50–2.13) | 0.93 |
| Va l v e | |||||
| replacement | 20 (13) | 4 (12) | 16 (14) | 0.72 (0.25–2.04) | 0.53 |
| Prior sternotomy | 64 (43) | 13 (39) | 51 (44) | 0.89 (0.44–1.78) | 0.74 |
Numbers in ( ) are % unless otherwise stated
’Definite’ infections diagnosed and antibiotics administered in the 30 days prior to VAD surgery, excluding perioperative antibioitics for the VAD implant; COPD,chronic obstructive pulmonary disease; AICD,automatic implanted cardioverter defibrillator; CABG,coronary artery bypass graft
Seventy (47%) subjects were listed with the United Network for Organ Sharing for potential cardiac transplantation. Fifty-three percent had ischemic HF. The cohort’s previous cardiac history included: 72 (48%) with myocardial infarction, 51 (34%) with coronary artery bypass graft surgery, 44 (29%) with percutaneous coronary intervention, 66 (44%) with atrial fibrillation, 63 (42%) with ventricular arrhythmia, and 20 (13%) with a prosthetic valve(s). Other co-morbidities such as diabetes (36%) and chronic renal insufficiency (30%) were common. Given the severity of illness in the population, many were receiving circulatory or ventilatory support prior to VAD implantation (e.g. 25% had an intraaortic balloon pump).
Baseline physical exam findings and laboratory values are listed in Table 2. This ill population had evidence of both renal and hepatic dysfunction. Poor nutritional status was common as 51% had serum albumin <3.4 g/dL and 18% were described as cachectic on physical exam. Twenty-five percent were obese.
Table 2.
| Entire Cohort n=150 |
VAD Infection Group n=33 |
No VAD Infection Group n=117 |
Hazard Ratio (95% CI) |
P value | |
|---|---|---|---|---|---|
| Temperature °C | 36.6 (0.6) | 36.4 (0.6) | 36.7 (0.6) | 0.53 (0.29–0.95) | 0.03 |
| Body Mass Index | 28.0 (5.3) | 29.1 (6.2) | 27.7 (5.0) | 1.04 (0.98–1.11) | 0.16 |
| Cachexia (%) | 27 (18) | 6 (19) | 21 (18) | 1.03 (0.42–2.49) | 0.96 |
| Dialysis (%) | 6 (4) | 2 (6) | 4 (3) | 2.48 (0.59–10.4) | 0.22 |
| Mechanical | |||||
| ventilation (%) | 15 (10) | 3 (9) | 12 (10) | 1.62 (0.49–5.37) | 0.43 |
| Baseline Laboratory Values | |||||
| Leukocyte count/mm3 | 8.7 (4.0) | 8.8 (3.0) | 8.7 (4.3) | 1.04 (0.95–1.13) | 0.43 |
| Hematocrit, % | 34.8 (5.8) | 36.2 (6.4) | 34.4 (5.6) | 1.04 (0.98–1.10) | 0.19 |
| Glucose mg/dL | 123 (45) | 118 (40) | 124 (47) | 1.00 (0.99–1.01) | 1.00 |
| Creatinine | |||||
| mg/dL | 1.6 (0.7) | 1.7 (0.7) | 1.5 (0.7) | 1.57 (1.00–2.45) | 0.05 |
| Albumin g/DL | 3.5 (0.6) | 3.4 (0.6) | 3.5 (0.6) | 0.70 (0.38–1.28) | 0.25 |
| Total bilirubin mg/dL | 1.4 (1.0) | 1.7 (1.5) | 1.4 (0.82) | 1.37 (1.02–1.83) | 0.04 |
| Alanine | |||||
| aminotransferase U/L | 75 (144) | 72 (138) | 76 (147) | 1.00 (0.997–1.002) | 0.86 |
| Aspartate | |||||
| aminotransferase U/L |
69 (134) | 66 (132) | 69 (135) | 1.00 (0.997–1.003) | 0.89 |
Values are mean and SD unless otherwise stated
Subjects with missing data are excluded
In the month prior to surgery, 38 (25%) were diagnosed with at least one infection and 58 (39%) received at least one antibiotic. Pre-implant infections included BSIs (n=10, 5 catheter-related), pneumonias (n=8), UTIs (n=15), wound infections (n=3), and C. difficile colitis (n=2). Antibiotics were given to treat these infections, as empiric therapy without a confirmed infection, or as pre-procedure prophylaxis.
VAD surgery
The median duration of VAD support was 244 days. 145 patients underwent univentricular left VAD support. These included: Heartmate II® (n=85), Heartmate I® (n=50), Thoratec® IVAD (n=5), VentrAssist™ (n=3; Ventracor Ltd, Chatswood, NSW), and Novacor (n=2; WorldHeart Inc., Salt Lake City, UT). Five patients received biventricular support: Thoratec paracorporeal BiVAD (n=3), Thoratec HeartMate I®/Abiomed RVAD (n=1) [Abiomed Inc., Danvers, MA], and Thoratec HeartMate II®/Abiomed RVAD (n=1). Heartmate II® and VentrAssist™ are continuous-flow VADs while the others are pulsatile. Overall, 62 (41%) received at least one pulsatile VAD; 59 were implantable and 3 were paracorporeal.
Implantable VADs were placed in the ‘preperitoneal’ space (n=117), abdomen (n=27) or left thorax (n=1) [not reported, n=2]. The median total cardiopulmonary bypass time was 87 minutes (range:10–341). Exogenous blood products were administered to 140 patients while the cell saver alone was utilized in ten. The following blood products were administered (mean units +SD, range): packed red blood cells (4.3+4.6, 0–27), platelets (8.6+10.5, 0–50), fresh frozen plasma (4.7+4.3, 0–22), and cryoprecipitate (4.1+8.2, 0–50). Perioperative antibiotics were administered, but varied considerably within and among the clinical centers.
Adverse Events
Numerous serious adverse events occurred prior to VAD infection or study termination: major bleeding (44%), respiratory failure (21%), neurologic events (16%), renal failure (12%), right heart failure (7%), device malfunction (7%), and hepatic dysfunction (3%). Several subjects had multiple adverse events including repeated episodes of the same event, such as bleeding (supplementary table 2). Two subjects with a HeartMate I® and one with a HeartMate II®, required device replacement for pump failure and thrombosis, respectively, at approximately 10–11 months post-implantation.
Fifty-eight patients (39%) experienced non-VAD-related infections including UTIs (15%), BSIs (13%), pneumonias (13%), wound infections (4%), and C. difficile colitis (6%). Patients often experienced multiple infections (supplementary table 2).
VAD Infections
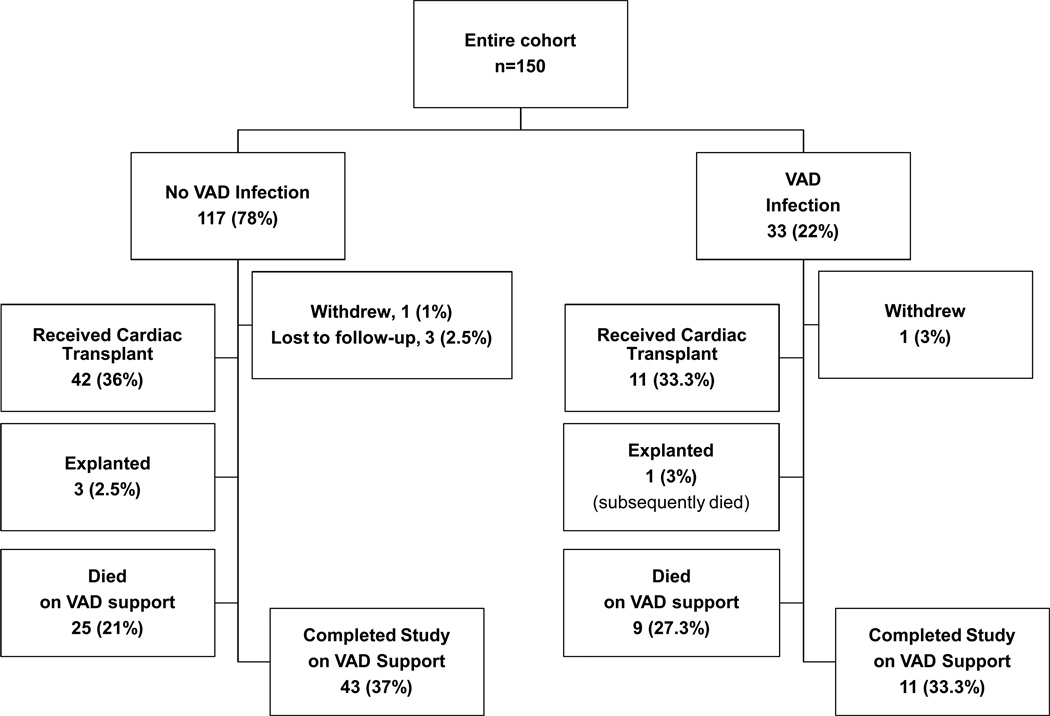
Thirty-three (22%) patients experienced 34 VAD infections (Table 3, Figure 1) with an incidence rate of 0.10 VAD infections per 100 patient-days (95% CI, 0.073–0.142). The median time to first infection was 68 days. The hazard function (figure 2) illustrates that the risk of VAD infection peaked at 18 days post-surgery and was lower and fairly constant after 60 days. At least 28 of the 34 infections involved the VAD driveline (82%); however, most infections also involved other sites such as the pump pocket, pump housing and bloodstream. Almost half of all infections (n=16) were associated with BSIs, some of which were complicated by septic emboli.
Table 3.
Description of 34 VAD-related infections
| Event # |
Causative Organism(s) |
Description of VAD Infection* |
Device Type† |
Days on VAD support prior to infection |
|---|---|---|---|---|
| Gram-Positive | ||||
| 1 |
Staphylococcus epidermidis |
DL, PP, BSI | HM I | 36 |
| 2 | Coagulase-negative staphylococcus |
DL, BSI, PP | HM I | 8 |
| 3 | DL +/− BSI | HM I | 14 | |
| 4 | DL, PP, BSI | HM I | 22 | |
| 5 | DL, PP, BSI, septic emobli |
HM II | 15 | |
| 6 | PP | HM II | 20 | |
| 7 | DL | HM II | 102 | |
| 8 |
Staphylococcus aureus |
DL | HM I | 195 |
| 9 | DL, BSI, mycotic aneurysm with septic emboli and involvement of inflow/outflow tract |
HM I | 310 | |
| 10 | DL | HM II | 34 | |
| 11 | DL | HM II | 285 | |
| 12 | DL, PP, BSI | HM II | 177 | |
| 13 | DL | HM II | 120 | |
| 14 | DL, BSI | HM II | 42 | |
| 15 |
Enterococcus faecalis |
DL, PP, BSI | HM I | 68 |
| 16 | DL, SW, PP, BSI | HM I | 44 | |
| 17 |
E. faecalis and/or CoNS‡ |
PP, BSI +/− DL | HM I | 28 |
| 18 | E. faecium | PP, BSI | Ventrassist | 19 |
| 19 | Viridans streptococci | DL, SW, PP | HM II | 235 |
| Gram-Negative | ||||
| 20 |
Pseudomonas aeruginosa |
DL, PP, BSI, VAD pump housing outflow |
HM I | 16 |
| 21 | DL, SW, PP | HM II | 36 | |
| 22 | DL, BSI | HM II | 174 | |
| 23 |
P. aeruginosa +/− Pflourescens |
DL | HM I | 130 |
| 24 |
P. aeruginosa and Enterobacter aerogenes |
DL, PP | HM I | 206 |
| 25 |
Stenotrophomonas maltophilia |
DL | HM II | 237 |
| 26 | Escherichia coli | PP | HM II | 10 |
| 27 | Enterobacter cloacae | DL | HM II | 251 |
| 28 | Proteus mirabilis | DL | HM II | 242 |
| 29 |
Klebsiella pneumoniae and Morganella morganii |
SW, PP, BSI | HM I | 36 |
| 30 | Enterobacter, K. pneumoniae CoNS |
DL | HM II | 168 |
| 31 |
Yeast (Candida glabrata) |
DL, PP, BSI, eroded graft –->exsanguination |
HM I | 60 |
| 32 | Culture Negative | DL, PP | HM I | 86 |
| 33 | PP | HM II | 82 | |
| 34 | PP, outflow graft, inner device surface |
HM II | 65 |
DL, driveline; PP, pump pocket; SW, sternal wound; BSI, bloodstream infection.
HM I, Heartmate I®; HM II, Heartmate II®
Coagulase-negative staphylococcus (CoNS)
Figure 1.
Patient outcomes.
Figure 2.
Instantaneous risk (hazard) of VAD infection with 95% Cls.
The microbiology of VAD infections varied, but Gram-positive organisms predominated. Coagulase-negative staphylococci were involved in 9 infections. Staphylococcus aureus caused 7 infections. Enterococci or Viridans streptococci caused 5 infections. There were 5 infections with Pseudomonas aeruginosa while assorted enteric Gram-negative pathogens caused several others. Five infections were polymicrobial. One infection was due to yeast (Candida glabrata). This infection resulted in graft erosion, exsanguination, and death. Three infections were culture-negative, each with strong clinical or histopathologic evidence of infection and previous exposure to antibiotics.
Infections occurred at 8 of the 11 centers and did not vary by geographic location (Western, Midwestern, Eastern, Supplemental table 1). The three centers without any infections enrolled 6 or fewer subjects. At centers with infections, 10–40% of patients experienced a VAD infection. There were no major associations between microbiology and clinical center nor were there trends in time to infection by clinical center or microorganism.
Univariate Analysis
In univariate analysis (Table 1), a history of depression and renal insufficiency were associated with VAD infection. Higher baseline serum creatinine and total bilirubin were also associated with VAD infection (Table 2). No demographic characteristics or other past or current medical or surgical conditions increased infection risk, including dialysis, mechanical ventilation, or having an indwelling line or cardiac device. A lower body temperature (recorded within the week prior to surgery) was associated with an increased infection risk; however, given the variation in the time the temperature was taken in relation to VAD surgery, this variable was not included in the multivariable model. No other physical factors such as cachexia or obesity were associated with increased infection risk.
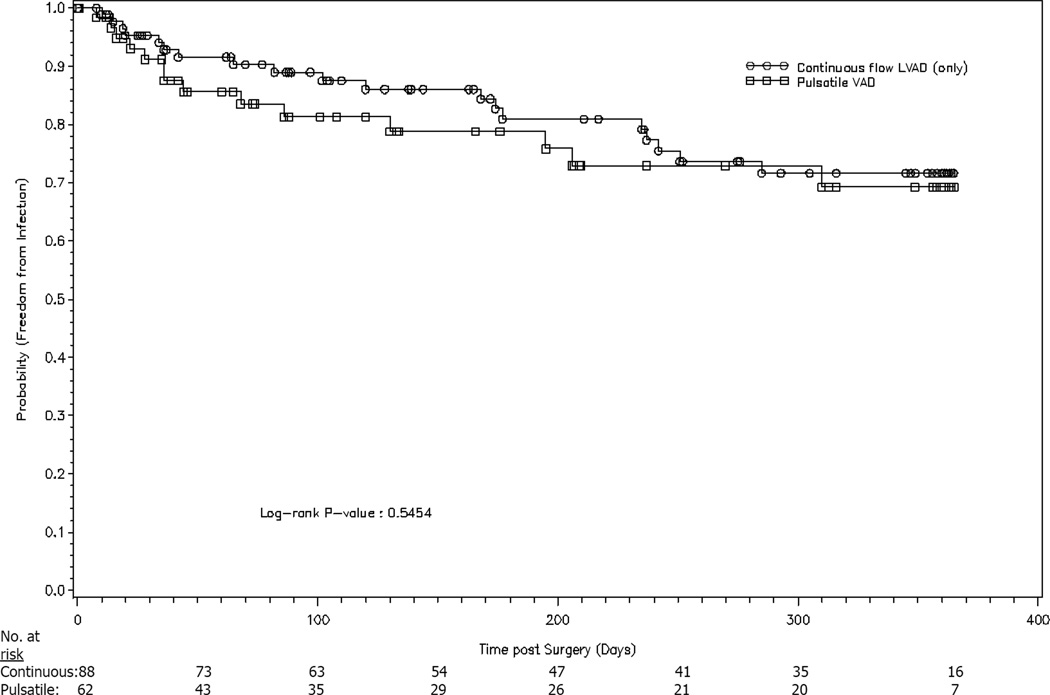
Having an infection or receiving antibiotics within the month prior to VAD implantation also did not affect the risk of infection on VAD support. Furthermore, no surgical factors related to VAD implantation, such as total bypass time, units of blood products received or location of the device, affected the outcome. Notably, there was no difference in infection risk among those who received pulsatile devices vs. those who received continuous-flow support (Figure 3). No patients on biventricular support developed infections.
Figure 3.
Pulsatile vs. continuous-flow VADs and probability of infection.
Neither time in the ICU, nor time in the hospital between admission and the diagnosis of a VAD infection or study termination were associated with an increased risk of VAD infection. Furthermore, no adverse events or non-VAD-related infections on VAD support increased the risk of subsequent VAD infection. Data suggested that the number of bleeding events heightened infection risk (HR=1.14, P=0.06).
Multivariable Analysis
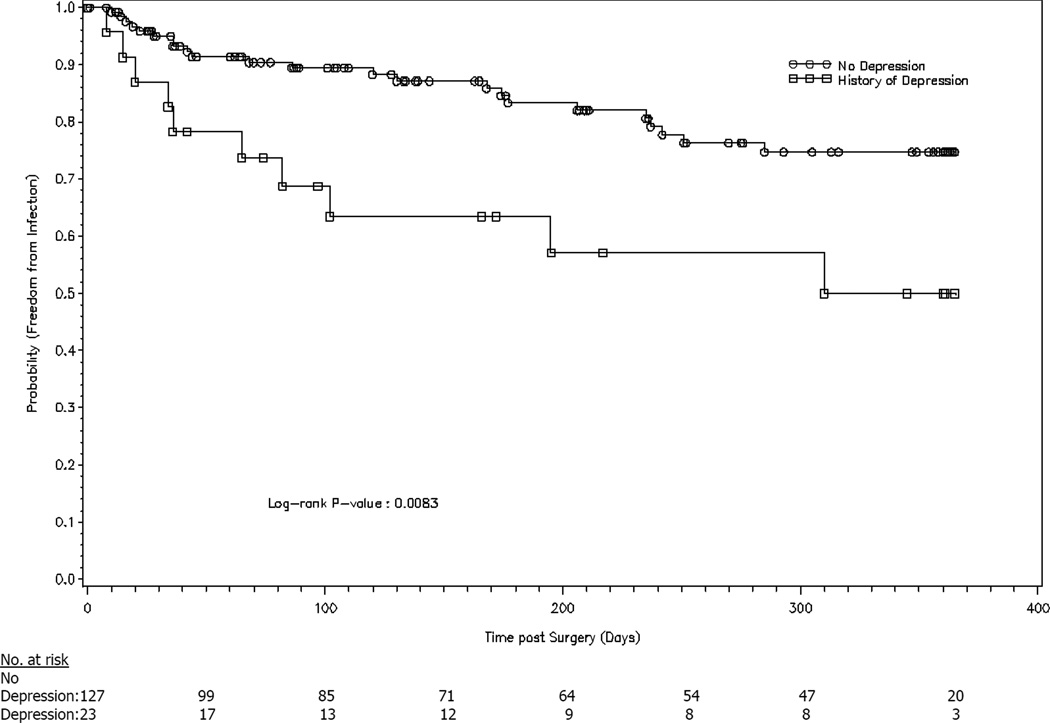
In the multivariable analysis, a history of depression (HRadj=2.8, 95% CI 1.3–6.0, P=0.007) and elevated serum creatinine (HRadj=1.7, 95% CI 1.1–2.7, P=0.025) remained significant predictors of VAD infection (Table 4). Figures 4 and 5 show the probability of infection stratified by depression and serum creatinine, respectively.
Table 4.
Multivariable Cox proportional hazards model describing risk factors for VAD-related infection, n=148*
| Variable | Hazard Ratioadj | 95% CI | P value |
|---|---|---|---|
| History of depression |
2.8 | 1.3–6.0 | 0.007 |
| Serum creatinine (mg/dL) |
1.7 | 1.1–2.7 | 0.025 |
Two subjects with missing data are excluded
Figure 4.
History of depression vs. no depression and probability of infection.
Figure 5.
Baseline serum creatinine <1.8 and >1.8 mg/dL and probability of infection (1.8 is the 75th percentile). Two subjects with missing data are excluded.
DT and BTT patients had the same risk of VAD infection (HRadj=1.04, 95% CI 0.52–2.11, P=0.90) although DT patients were older, had a lower mean albumin, were more likely to have chronic renal dysfunction and were less likely to have an intra-aortic balloon pump. DT and BTT patients are compared in Supplemental table 3.
Heartmate II® patients
Of the 85 patients who received a Heatmate II® device, 15 (18%) developed a VAD infection. In univariate and multivariable sub-group analysis, a history of depression and increased total bilirubin were both statistically significantly associated with VAD infection (HRadj=3.6, 95% CI 1.3–9.8, P=0.014 and HRadj=1.5, 95% CI 1.04–2.10, P=0.029, respectively) [Supplemental table 4]. Serum creatinine was not associated with infection in this analysis.
Transplantation and Survival Outcomes
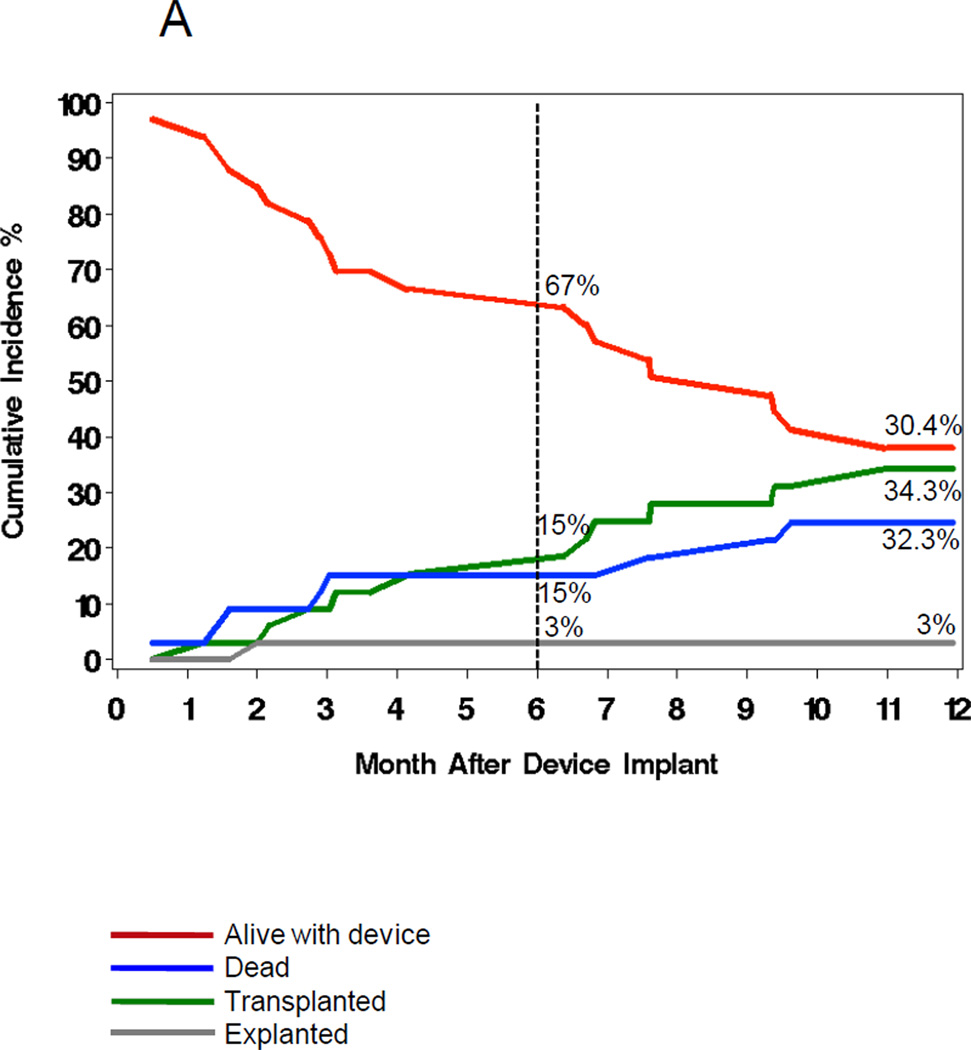
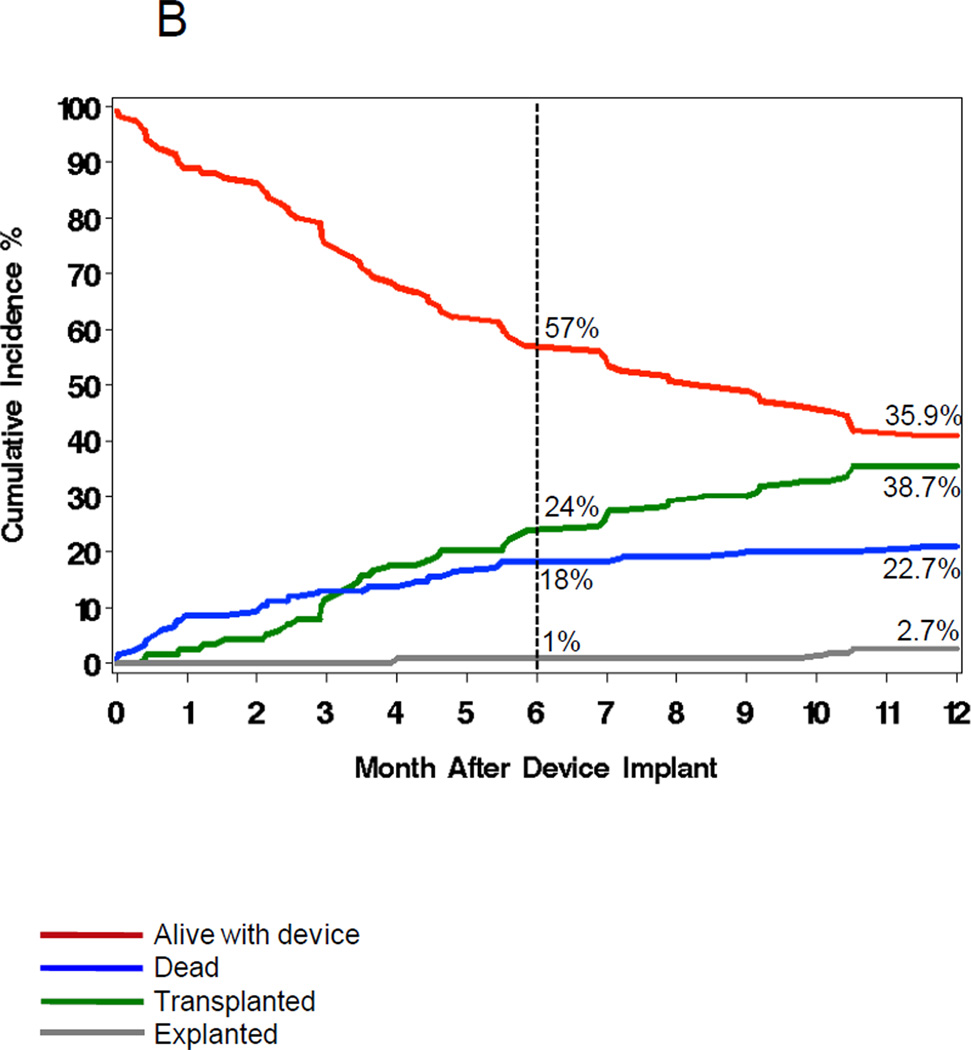
Figures 1 and 6 show the outcomes of patients with and without VAD infection. A similar proportion (33–36%) received a cardiac transplant. Overall, 27% of those with VAD infections died compared to 21% of those without infections. In the multivariable analysis (Table 5), VAD infection increased one-year mortality (HRadj=5.6, 95% CI 2.4–12.8, P<0.0001).
Figure 6.
The cumulative incidence (%) of VAD surgery outcomes (red=alive, green=transplanted, blue=dead, grey=explanted) in patients with (6A) and without (6B) VAD infections.
Table 5.
Multivariable Cox proportional hazards model describing risk factors for death in VAD recipients, n=150*
| Variable | Hazard Ratioadj | 95% CI | P value |
|---|---|---|---|
| VAD infection | 5.6 | 2.4–12.8 | <0.0001 |
| Neurological adverse event |
4.6 | 2.2–9.4 | <0.0001 |
| Bloodstream infection† |
3.0 | 1.2–7.1 | 0.01 |
| Respiratory failure | 2.9 | 1.4–6.2 | 0.005 |
| History of atrial fibrillation |
2.4 | 1.1–5.2 | 0.03 |
| Serum albumin (g/dL) |
0.4 | 0.2–0.7 | 0.004 |
5 subjects with missing data are excluded
Bloodstream infections unrelated to VAD infections
Other adverse events including BSIs unrelated to the VAD, respiratory failure, major neurological events (e.g. strokes and transient ischemic attacks) and a history of atrial fibrillation were independent predictors of mortality. Increased baseline serum albumin was protective. Neither depression nor renal dysfunction affected survival. There was no difference in mortality between those who received pulsatile vs. continuous-flow VADs or among DT vs. BTT patients.
Discussion
This is one of the first large, prospective, multicenter studies to describe the microbiology and epidemiology of VAD infections. Twenty-two percent of 150 VAD recipients experienced VAD infections with a wide-range of pathogens. The study was designed specifically to identify the earliest signs and symptoms of infection and to obtain concomitant, appropriate microbiologic cultures. Each case was carefully reviewed by one to two infectious disease specialists who used criteria they postulated a priori would be convincing evidence of VAD infection. Most previous studies have been either retrospective, single-center, and/or have not used rigorous criteria to diagnose and describe VAD infections.6 Recognizing the lack of uniform criteria, the International Society for Heart and Lung Transplantation Infectious Diseases Working Group proposed definitions for VAD infections in 2011, well after the completion of this study.8 However, this study utilized similar clinical, microbiological, and histopathologic diagnostic requirements. The detailed collection and evaluation of infection-related data in VAD patients with suspected infections is one of the major strengths of this study.
Given the study design, this is likely one of the most accurate and current descriptions of the risk of VAD infection. The Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) is a U.S. national registry that records adverse events on mechanical circulatory support.3 VAD-specific infections involving the pump pocket or driveline as well as components of the device in contact with the bloodstream as well as “sepsis” are reported to INTERMACS (www.uab.edu/intermacs). Although this registry is important, infections are diagnosed and reported without expert infectious disease oversight. “Sepsis” may or may not be associated with the VAD or another non-VAD-related infection. Furthermore, INTERMACS has not reported an overall incidence of VAD-specific infection with supporting microbiologic data; therefore, this study adds valuable information to that accrued by INTERMACS.
The risk of VAD infection peaked at 18 days post-surgery and was lower and fairly constant after 60 days. Our median time to infection was 68 days, considerably later than other studies where most infections occurred approximately one month post-operatively.4, 14, 15 This may be because patients are frequently discharged from the hospital and are remaining on VAD-support for longer. Others have also reported ‘late’ VAD infections, particularly of the driveline.16
It was hypothesized that those with implanted pulsatile devices would be at higher risk for VAD infection than those with continuous-flow devices (almost all were Heartmate II®). Only 14% of subjects in an observational Heartmate II® study had a device-related infection,5 supporting observations by clinicians that infection rates have been declining. A randomized study by Slaughter et al.7 comparing pulsatile to continuous-flow devices found that pulsatile devices had approximately twice the infection rate. Proposed reasons include the fact that implanted pulsatile devices generally have larger surface areas, require more dissection for placement, and have bigger, more rigid drivelines than continuous-flow devices.
If pulsatile devices are associated with increased infection risk, we may not have detected it because the study was underpowered. Also, the study was conducted when Heartmate II® devices were first introduced and clinicians were becoming familiar with their implantation and management.
Nonetheless, having a continuous-flow device did not decrease the infection risk in this study and it is plausible that device type does not affect infection outcomes. The decreased infection rate in continuous-flow devices reported by Slaughter7 may not be generalizable for several reasons: 1) infection was a subgroup analysis, 2) the study only included DT patients, and 3) 33% of patients on pulsatile support required a device exchange compared to 9% of patients on continuous-flow support, which may have increased pulsatile support infection rates. Whether flow is continuous or pulsatile, both devices have a driveline where pathogens can enter and establish infection. In the future, totally implantable devices have the potential to abolish this “Achilles Heel.”16
As expected, the vast majority of VAD infections (82%) involved the driveline although almost two-thirds of these infections involved sites such as the pump pocket and/or bloodstream, including two cases of septic emboli and one case of graft erosion. The driveline serves as an entry point for pathogens and lead to pump pocket and pump housing infections that can become systemic.6 The Heartmate II® study, which followed subjects on continuous-flow support, only reported driveline infections and sepsis.5 In this study, it was unclear if sepsis was associated with a VAD infection and the follow-up time was limited to six months. Our study clearly shows that driveline infections are frequently associated with more extensive involvement of the VAD and concomitant systemic infection.
Staphylococci caused 41% of VAD infections, a finding consistent with past studies.14, 17–20 Staphylococci frequently cause device infections, including VAD infections, because they colonize the skin and nose and can adhere to implanted prosthetic material and form biofilms.6, 21–23 A significant proportion of cases were caused by Pseudomonas spp., which can also form biofilms, as well as other Gram-negative enteric organisms. In choosing perioperative antibiotic prophylaxis or empiric treatment of VAD infections, clinicians should consider the antibiograms of their institution’s Gram-negative pathogens. Although candidal infections are not common, they can be serious.24 The one VAD infection caused by Candida glabrata resulted in graft erosion, exsangination and death. The efficacy of perioperative fluconazole for preventing these infections is unknown.
Many potential risk factors for VAD infection were evaluated in this study. Although several factors were potentially associated with VAD infection in univariate analysis, only serum creatinine and depression were significant in the multivariable model. For every 1 mg/dL increase in creatinine, there was a 70% increase in the risk of VAD infection. This may be because those with renal dysfunction are relatively immunocompromised25 and generally have a higher severity of illness. In the REMATCH study, a history of renal disease significantly increased the risk of sepsis.4 Malani et al26 reported that VAD recipients on hemodialysis were at increased risk for deep surgical site infections. Our study supports these findings.
A notable finding was that patients with a history of depression before VAD surgery had a 3-fold higher risk of subsequent VAD infection. This was statistically significant in the overall multivariable model and the Heartmate II® subgroup analysis.
Depression is commonly associated with HF and has been associated with increased morbidity and mortality.27–33 Studies involving cardiac surgery patients, including cardiac transplant recipients, have also shown a correlation between depressive symptoms and poor outcomes, including wound infections.34–37
VAD recipients are at high risk for depression and other mood disorders; nonetheless, there are limited data.38–40 BTT patients struggle with the uncertainties of donor organ availability and the possibility of dying awaiting transplantation.40 Psychiatric conditions may be even more pronounced in DT patients—they have no hope of transplantation and must indefinitely comply with a complex medical regimen, including the technically difficult task of caring for the device. One case report discussed a DT patient who committed suicide by disconnecting his driveline.41
The effect of depression on VAD complications is even less-well understood. The association we found between a history of depression and infection is plausible. Those who were depressed prior to surgery were likely at higher risk for depression after surgery. Depressed individuals may have poorer compliance with hygiene, device care, rehabilitation, and physician visits.39 These factors may increase the risk of infection in depressed VAD patients.
VAD infection had serious consequences, increasing one-year mortality five to six-fold. This is understandable given that the majority of infections in the study were ultimately invasive/systemic. Infection also adversely affected survival in other studies where subjects experienced systemic infections related to the VAD.4, 18, 42 BSIs unrelated to the VAD, respiratory failure, atrial fibrillation and neurological adverse events were other independent predictors of mortality in this study. These results are consistent with INTERMACS reports where infection, neurological events and respiratory failure were among the five leading causes of death in VAD recipients.3
Sample size was a limitation of this study (22% of 150 infected) and may have restricted our ability to identify additional risks for VAD infection. Potential predictors were specified a priori, but the study was exploratory in nature. Because screening data were not obtained, we could not compare our cohort to the screened and not enrolled population. However, the demographics of our patients and those in the INTERMACS registry are similar, indicating that our population was likely a good representation of the average device recipient.43 Perioperative antibiotic regimens varied among and within institutions, and consequently, we were unable to evaluate the efficacy of different prophylactic antibiotics, including anti-pseudomonal and anti-fungal agents. Furthermore, it was difficult to determine specific predictors of VAD infection, which may be attributed to the overall high severity of illness in this patient population. However, baseline renal dysfunction and a history of depression were identified risk factors. Depression increased risk in both Heartmate I and II® recipients. A further limitation of the study is that depression was not measured by a validated index, but rather by a past history. Regardless, depression is a condition that is frequently overlooked and undertreated. Future research should evaluate this association and identify effective interventions. Efforts to address the mental health of these patients may improve outcomes.
In summary, this study demonstrates that VAD infections remain common and are caused by a wide-variety of microorganisms. Infections adversely affect survival as these infections frequently become invasive or systemic. They also may be occurring later, well after the immediate post-operative period. Although it is generally accepted that continuous-flow devices confer a reduced infection risk, this was not demonstrated in the study. A totally implantable device may help decrease this risk. Lastly, the VAD population suffers numerous adverse events and has a high severity of illness, but depression, baseline elevated creatinine and elevated bilirubin may increase the chance of infection. Further studies of remediable risk factors such as depression and their treatments are warranted.
Supplementary Material
Clinical Summary.
Ventricular assist devices (VADs) significantly improve quality of life and decrease mortality in patients with advanced heart failure, including those awaiting cardiac transplantation. Unfortunately, VAD implantation is frequently complicated by infection. To our knowledge, this is the first and largest prospective, multicenter VAD study focused on carefully characterizing the microbiology, epidemiology, risk factors, and outcomes of VAD infections. Previous studies have either been small, retrospective, single-center, and/or have not used rigorous criteria to diagnose and describe VAD infections. Infectious Diseases specialists were closely involved in this study, which details the microbiology of these infections. The results have important implications for guiding perioperative prophylaxis regimens and empiric therapy. Furthermore, we show that while the risk of VAD infection is highest immediately post-implantation, the risk of infection persists until at least one-year after surgery. The percutaneous VAD driveline likely serves as an entry point for pathogens. While most infections involve the driveline, a substantial proportion also involve multiple sites and/or become invasive. These infections are also associated with increased morbidity and mortality, making prevention crucial. Increased serum creatinine and a history of depression were identified as risk factors for VAD infection. Depression is frequently underdiagnosed and undertreated and has been associated with poor outcomes in other populations. Therefore, we have potentially identified a remediable risk factor for VAD infection that requires further investigation. Given the lack of organ donors and the importance of VAD therapy, we hope this research will lead to better diagnosis, treatment, prevention of VAD infections.
Acknowledgements
We thank Peter Vavagiakis for his technical support, Ben Miko for his review of the manuscript and Qiuhu Shi for performing the competing risks analysis.
Funding Sources: This work was supported in part by the National Heart, Lung, and Blood Institute [NIH Grant #HL077096] for all authors except R.J.G. R.J.G. received funding from the National Institute of Allergy and Infectious Diseases [NIH Grant #K08A1072043].
Disclosures: D.D.A, A.M. and A.C.G. are investigators on the NHLBI-funded Cardiothoracic Surgical Trials Network (CTSN). D.G. is a consultant for Thoratec Corp. F.D.P discloses contract research from the NHLBI and HeartWare for the REVIVE-IT Pilot Study. M.S.S. receives Thoratec Corp. research grant support. R.D. is a consultant to Abiomed Inc. and an advisor to CircuLite, Inc. R.M.A. and W.B. are consultants/on advisory board, in the speakers’ bureau and have received honoraria for meetings/training from Thoratec Corp. W.B. has been an expert witness for Triflow Corp. The other authors have no disclosures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Frazier OH, Rose EA, Oz MC, Dembitsky W, McCarthy P, Radovancevic B, Poirier VL, Dasse KA. Multicenter clinical evaluation of the heartmate vented electric left ventricular assist system in patients awaiting heart transplantation. J Thorac Cardiovasc Surg. 2001;122:1186–1195. doi: 10.1067/mtc.2001.118274. [DOI] [PubMed] [Google Scholar]
- 2.Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, Long JW, Ascheim DD, Tierney AR, Levitan RG, Watson JT, Meier P, Ronan NS, Shapiro PA, Lazar RM, Miller LW, Gupta L, Frazier OH, Desvigne-Nickens P, Oz MC, Poirier VL. Long-term mechanical left ventricular assistance for end-stage heart failure. N Engl J Med. 2001;345:1435–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 3.Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, Miller MA, Ulisney KL, Baldwin JT, Young JB. Second intermacs annual report: More than 1,000 primary left ventricular assist device implants. J Heart Lung Transplant. 2010;29:1–10. doi: 10.1016/j.healun.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holman WL, Park SJ, Long JW, Weinberg A, Gupta L, Tierney AR, Adamson RM, Watson JD, Raines EP, Couper GS, Pagani FD, Burton NA, Miller LW, Naka Y. Infection in permanent circulatory support: Experience from the rematch trial. J Heart Lung Transplant. 2004;23:1359–1365. doi: 10.1016/j.healun.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 5.Miller LW, Pagani FD, Russell SD, John R, Boyle AJ, Aaronson KD, Conte JV, Naka Y, Mancini D, Delgado RM, MacGillivray TE, Farrar DJ, Frazier OH. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357:885–896. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 6.Gordon RJ, Quagliarello B, Lowy FD. Ventricular assist device-related infections. Lancet Infect Dis. 2006;6:426–437. doi: 10.1016/S1473-3099(06)70522-9. [DOI] [PubMed] [Google Scholar]
- 7.Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, Sun B, Tatooles AJ, Delgado RM, 3rd, Long JW, Wozniak TC, Ghumman W, Farrar DJ, Frazier OH. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 8.Hannan MM, Husain S, Mattner F, Danziger-Isakov L, Drew RJ, Corey GR, Schueler S, Holman WL, Lawler LP, Gordon SM, Mahon NG, Herre JM, Gould K, Montoya JG, Padera RF, Kormos RL, Conte JV, Mooney ML. Working formulation for the standardization of definitions of infections in patients using ventricular assist devices. J Heart Lung Transplant. 2011;30:375–384. doi: 10.1016/j.healun.2011.01.717. [DOI] [PubMed] [Google Scholar]
- 9.Interagency registry for mechanical circulatory support. www.uab.edu/intermacs.
- 10.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 1958;53:457–481. [Google Scholar]
- 11.Cox DR. Regression models and life tables (with discussion) J R Stat Soc. 1972;34:187–220. [Google Scholar]
- 12.Blackstone EH, Naftel DC, Turner ME., Jr The decomposition of time-varying hazard into phases, each incorporating a separate stream of concomitant information. J Am Stat Assoc. 1986;81:615–624. [Google Scholar]
- 13.Allison PD. Survival analysis using sas: A practical guide. SAS Institute Inc. 2010 [Google Scholar]
- 14.Simon D, Fischer S, Grossman A, Downer C, Hota B, Heroux A, Trenholme G. Left ventricular assist device-related infection: Treatment and outcome. Clin Infect Dis. 2005;40:1108–1115. doi: 10.1086/428728. [DOI] [PubMed] [Google Scholar]
- 15.Sivaratnam K, Duggan JM. Left ventricular assist device infections: Three case reports and a review of the literature. Asaio J. 2002;48:2–7. doi: 10.1097/00002480-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Zierer A, Melby SJ, Voeller RK, Guthrie TJ, Ewald GA, Shelton K, Pasque MK, Moon MR, Damiano RJ, Jr, Moazami N. Late-onset driveline infections: The achilles' heel of prolonged left ventricular assist device support. Ann Thorac Surg. 2007;84:515–520. doi: 10.1016/j.athoracsur.2007.03.085. [DOI] [PubMed] [Google Scholar]
- 17.Deng MC, Loebe M, El-Banayosy A, Gronda E, Jansen PG, Vigano M, Wieselthaler GM, Reichart B, Vitali E, Pavie A, Mesana T, Loisance DY, Wheeldon DR, Portner PM. Mechanical circulatory support for advanced heart failure: Effect of patient selection on outcome. Circulation. 2001;103:231–237. doi: 10.1161/01.cir.103.2.231. [DOI] [PubMed] [Google Scholar]
- 18.Monkowski DH, Axelrod P, Fekete T, Hollander T, Furukawa S, Samuel R. Infections associated with ventricular assist devices: Epidemiology and effect on prognosis after transplantation. Transpl Infect Dis. 2007;9:114–120. doi: 10.1111/j.1399-3062.2006.00185.x. [DOI] [PubMed] [Google Scholar]
- 19.Sinha P, Chen JM, Flannery M, Scully BE, Oz MC, Edwards NM. Infections during left ventricular assist device support do not affect posttransplant outcomes. Circulation. 2000;102:III194–III199. doi: 10.1161/01.cir.102.suppl_3.iii-194. [DOI] [PubMed] [Google Scholar]
- 20.Kalya AV, Tector AJ, Crouch JD, Downey FX, McDonald ML, Anderson AJ, Bartoszewski CJ, Hosenpud JD. Comparison of novacor and heartmate vented electric left ventricular assist devices in a single institution. J Heart Lung Transplant. 2005;24:1973–1975. doi: 10.1016/j.healun.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Toba FA, Akashi H, Arrecubieta C, Lowy FD. Role of biofilm in staphylococcus aureus and staphylococcus epidermidis ventricular assist device driveline infections. J Thorac Cardiovasc Surg. 2011;141:1259–1264. doi: 10.1016/j.jtcvs.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arrecubieta C, Toba FA, von Bayern M, Akashi H, Deng MC, Naka Y, Lowy FD. Sdrf, a staphylococcus epidermidis surface protein, contributes to the initiation of ventricular assist device driveline-related infections. PLoS Pathog. 2009;5:e1000411. doi: 10.1371/journal.ppat.1000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arrecubieta C, Asai T, Bayern M, Loughman A, Fitzgerald JR, Shelton CE, Baron HM, Dang NC, Deng MC, Naka Y, Foster TJ, Lowy FD. The role of staphylococcus aureus adhesins in the pathogenesis of ventricular assist device-related infections. J Infect Dis. 2006;193:1109–1119. doi: 10.1086/501366. [DOI] [PubMed] [Google Scholar]
- 24.Gordon SM, Schmitt SK, Jacobs M, Smedira NM, Goormastic M, Banbury MK, Yeager M, Serkey J, Hoercher K, McCarthy PM. Nosocomial bloodstream infections in patients with implantable left ventricular assist devices. Ann Thorac Surg. 2001;72:725–730. doi: 10.1016/s0003-4975(01)02888-0. [DOI] [PubMed] [Google Scholar]
- 25.Hauser AB, Stinghen AE, Kato S, Bucharles S, Aita C, Yuzawa Y, Pecoits-Filho R. Characteristics and causes of immune dysfunction related to uremia and dialysis. Perit Dial Int. 2008;28(Suppl 3):S183–S187. [PubMed] [Google Scholar]
- 26.Malani PN, Dyke DB, Pagani FD, Chenoweth CE. Nosocomial infections in left ventricular assist device recipients. Clin Infect Dis. 2002;34:1295–1300. doi: 10.1086/340052. [DOI] [PubMed] [Google Scholar]
- 27.Konstam V, Moser DK, De Jong MJ. Depression and anxiety in heart failure. J Card Fail. 2005;11:455–463. doi: 10.1016/j.cardfail.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 28.Adams J, Kuchibhatla M, Christopher EJ, Alexander JD, Clary GL, Cuffe MS, Califf RM, Krishnan RR, O'Connor CM, Jiang W. Association of depression and survival in patients with chronic heart failure over 12 years. Psychosomatics. 2012;53:339. doi: 10.1016/j.psym.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frasure-Smith N, Lesperance F, Habra M, Talajic M, Khairy P, Dorian P, Roy D. Elevated depression symptoms predict long-term cardiovascular mortality in patients with atrial fibrillation and heart failure. Circulation. 2009;120:134–140. doi: 10.1161/CIRCULATIONAHA.109.851675. 133p following 140. [DOI] [PubMed] [Google Scholar]
- 30.Diez-Quevedo C, Lupon J, Gonzalez B, Urrutia A, Cano L, Cabanes R, Altimir S, Coll R, Pascual T, de Antonio M, Bayes-Genis A. Depression, antidepressants, and long-term mortality in heart failure. Int J Cardiol. 2012 Apr 14; doi: 10.1016/j.ijcard.2012.03.143. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 31.Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48:1527–1537. doi: 10.1016/j.jacc.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 32.Pelle AJ, Gidron YY, Szabo BM, Denollet J. Psychological predictors of prognosis in chronic heart failure. J Card Fail. 2008;14:341–350. doi: 10.1016/j.cardfail.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Jiang W, Kuchibhatla M, Clary GL, Cuffe MS, Christopher EJ, Alexander JD, Califf RM, Krishnan RR, O'Connor CM. Relationship between depressive symptoms and long-term mortality in patients with heart failure. Am Heart J. 2007;154:102–108. doi: 10.1016/j.ahj.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 34.Doering LV, Martinez-Maza O, Vredevoe DL, Cowan MJ. Relation of depression, natural killer cell function, and infections after coronary artery bypass in women. Eur J Cardiovasc Nurs. 2008;7:52–58. doi: 10.1016/j.ejcnurse.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tully PJ, Pedersen SS, Winefield HR, Baker RA, Turnbull DA, Denollet J. Cardiac morbidity risk and depression and anxiety: A disorder, symptom and trait analysis among cardiac surgery patients. Psychol Health Med. 2011;16:333–345. doi: 10.1080/13548506.2011.553960. [DOI] [PubMed] [Google Scholar]
- 36.Owen JE, Bonds CL, Wellisch DK. Psychiatric evaluations of heart transplant candidates: Predicting post-transplant hospitalizations, rejection episodes, and survival. Psychosomatics. 2006;47:213–222. doi: 10.1176/appi.psy.47.3.213. [DOI] [PubMed] [Google Scholar]
- 37.Doering LV, Cross R, Vredevoe D, Martinez-Maza O, Cowan MJ. Infection, depression, and immunity in women after coronary artery bypass: A pilot study of cognitive behavioral therapy. Altern Ther Health Med. 2007;13:18–21. [PubMed] [Google Scholar]
- 38.Casida JM, Parker J. A preliminary investigation of symptom pattern and prevalence before and up to 6 months after implantation of a left ventricular assist device. J Artif Organs. 2012;15:211–214. doi: 10.1007/s10047-011-0622-4. [DOI] [PubMed] [Google Scholar]
- 39.Eshelman AK, Mason S, Nemeh H, Williams C. Lvad destination therapy: Applying what we know about psychiatric evaluation and management from cardiac failure and transplant. Heart Fail Rev. 2009;14:21–28. doi: 10.1007/s10741-007-9075-5. [DOI] [PubMed] [Google Scholar]
- 40.Baba A, Hirata G, Yokoyama F, Kenmoku K, Tsuchiya M, Kyo S, Toyoshima R. Psychiatric problems of heart transplant candidates with left ventricular assist devices. J Artif Organs. 2006;9:203–208. doi: 10.1007/s10047-006-0353-0. [DOI] [PubMed] [Google Scholar]
- 41.Tigges-Limmer K, Schonbrodt M, Roefe D, Arusoglu L, Morshuis M, Gummert JF. Suicide after ventricular assist device implantation. J Heart Lung Transplant. 2010;29:692–694. doi: 10.1016/j.healun.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 42.Minami K, El-Banayosy A, Sezai A, Arusoglu L, Sarnowsky P, Fey O, Koerfer R. Morbidity and outcome after mechanical ventricular support using thoratec, novacor, and heartmate for bridging to heart transplantation. Artif Organs. 2000;24:421–426. doi: 10.1046/j.1525-1594.2000.06621.x. [DOI] [PubMed] [Google Scholar]
- 43. http://www.Uab.Edu/intermacs/images/federal_quarterly_report/intermacs_quarterly_statistical_report_-_2012_q2_2.Pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.