Abstract
In all primates, the cortical control of hand and arm movements is initiated and controlled by a network of cortical regions including primary motor cortex (M1), premotor cortex (PM), and posterior parietal cortex (PPC). These interconnected regions are influenced by inputs from especially visual and somatosensory cortical areas, and prefrontal cortex. Here we discuss recent evidence showing M1, PM, and PPC can be subdivided into a number of functional zones or domains, including several that participate in guiding and controlling hand and arm movements. Functional zones can be defined by the movement sequences evoked by microstimulation within them, and functional zones related to the same type of movement in all three cortical regions are interconnected. The inactivation of a functional zone in each of the regions has a different impact on motor behavior. Finally, there is considerable plasticity within the networks so that behavioral recoveries can occur after damage to functional zones within a network.
Introduction
Three regions of cortex have long been known to be involved in the production and guidance of motor behavior; primary motor cortex (M1), premotor cortex (PM), and posterior parietal cortex (PPC) (Fig. 1). Primary motor cortex has a somatotopic organization that can be revealed by electrical stimulation with surface electrodes or penetrating microelectrodes. Thus, medial stimulation sites evoke leg and foot movements, more lateral sites evoke arm and hand movements, and the most lateral sites evoke face, mouth and tongue movements (e.g., 1; see 2 for review. Modern maps or representations of the motor organization of M1 generally are based on short trains of electrical pulses at low levels of current delivered with penetrating microelectrodes with tips close to layer 5 pyramidal neurons (e.g., 3 such that only a barely detectable, brief movement is evoked and that often involves only a few muscles and a single body part 2. When higher levels of current, over longer periods of time are used to stimulate, more complex movements are evoked, but a motor map can be constructed based on the first movement that occurs in a sequence 4.
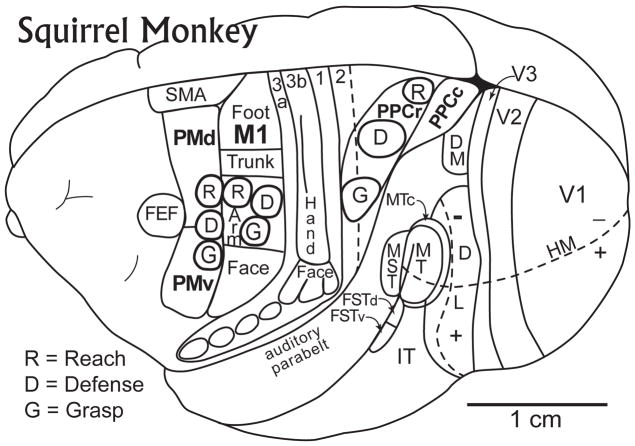
Figure 1.
Motor areas of cortex on a dorsolateral view of a Squirrel monkey brain. Primary motor cortex (M1) represents foot and leg, trunk, hand and forelimb, and face movements in a sequence of mediolateral segments. Other motor representations in frontal cortex include the dorsal premotor area, PMD, the ventral premotor area, PMV, the supplementary motor area, SMA, and the frontal eye field, FEF. Within M1, PMV, and PMD, different complex movements can be evoked by electrically stimulating neurons within small “functional zones”. The approximate locations of three such zones, for grasping (G), defensive (D), and reaching (R) movements are shown in M1 and premotor cortex (PMV and PMD). Matching functional zones (G, D, and R) for these and other motor behaviors are found in posterior parietal cortex, with the grasping zone extending into area 2. Somatosensory areas (3a, 3b, 1 and 2, as well as several visual areas, are shown for reference. Based on Gharbawie et al. (2011a).
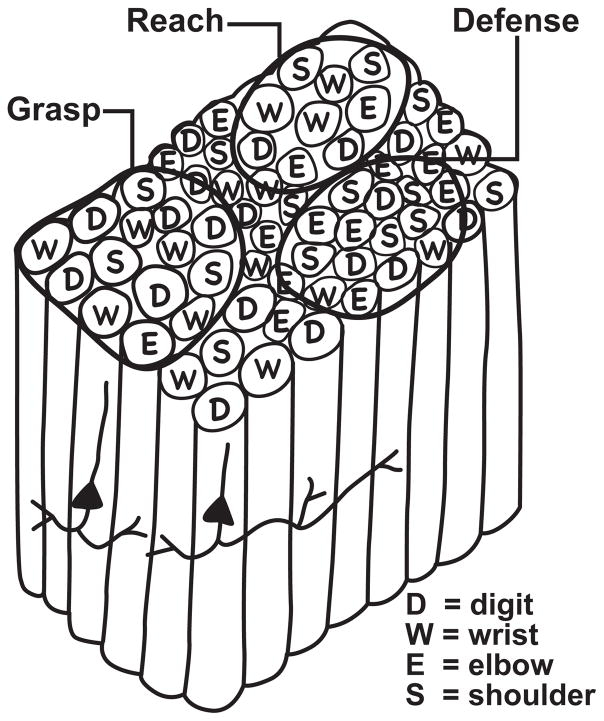
The threshold or first movement maps of M1 have been puzzling to a certain extent, in that they are only crudely somatotopic, thereby differing considerably from the more precise somatosensory, visual, and auditory maps in early sensory areas. Thus, in the forearm-hand region of M1 of various primates, sites where movements of specific digits are evoked are often next to sites where wrist, elbow, and shoulder movements are evoked. Moreover, similar digit, wrist, elbow, or shoulder movements can be evoked from several separate sites in M1 5–11. Gould et al. (1986) initially described M1, not as a single representation, but as a mosaic of cortical stimulation sites, corresponding to narrow vertical columns of neurons for different movements, with multiple sites or columns for each type of movement (Fig. 2). The movement of a single digit or a few digits, for example, could be evoked from several sites distributed in the hand-forearm region of M1. Sites for digit movements would be next to sites for different hand-forearm movements, depending on location within M1. With this type of organization, short lateral connections 12,13 could excite or inhibit adjoining columns for different movements to produce complex sequences of digit, hand, and arm movements that would differ according to where in M1 the movement sequence was initiated. This type of interpretation of M1 function, originally based on threshold stimulation maps of M1, is consistent with more recent results produced when sites in M1 were stimulated for longer times (0.5 sec.) with trains of electrical pulses at higher levels of current 14–16. Under these conditions, longer (approximately 0.5 sec.) sequences of movements were evoked from M1, including those reasonably described as grasping or manipulation by the hand, reaching, hand-to-mouth, or defensive movements of the arm and/or face. These findings are more fully discussed in a subsequent section of this review.
Figure 2.
The proposed functional organization of the hand-forearm segment of M1 in monkeys and other primates. Microstimulation studies have shown that electrical stimulation of a small number of adjacent pyramidal neurons in layer 5 with short bursts of near-threshold pulses evokes simple movements of digits (D), the wrist (W), the elbow (E) or the shoulder (S) from different sites in M1, and that sites for each of these body parts are scattered and repeated within the hand-forelimb segment of M1. Microstimulation with longer bursts of electrical pulses (0.5 sec.) at higher current levels evokes more complex patterns of movements, such as that for reaching (R), grasping (G) or defense of the head and body (D) from larger functional zones (see Figure 1) that would contain a number of motor columns of different types. The complex movement patterns may depend on the short lateral neuronal interconnections within a functional zone that are excitatory or inhibitory. Two pyramidal neurons with intrinsic connections are illustrated for example. Longer lateral connections between functional zones may excite mainly inhibitory neurons, suppressing a tendency for a different complex movement sequence.
Premotor areas, although smaller, with more compressed representations, also contain mosaics of intermingled movement sites. All primates appear to have dorsal and ventral premotor areas, PMD and PMV 7, and these dorsal and ventral premotor areas have functionally distinct subdivisions in some primates 17–19. PMD and PMV have been the most studied of the premotor areas, and they are the most relevant premotor fields for this review because they have been most studied within the context of parietal-frontal sensorimotor networks 20. At threshold levels of current, short trains of microstimulation in PMD and PMV evoke simple, discrete movements similar to those evoked from M1, although typically at higher levels of current 5,19,21. The two premotor fields differ from each other somewhat somatotopically, with PMD representing hindlimb, forelimb and face movements in a dorsoventral sequence, and PMV mainly representing forelimb, facial, and oral movements in more of a dorsorostral to ventrorostral sequence. However, as in M1, neither of these premotor areas has a precise somatotopy, and different types of movements are typically evoked from nearby stimulation sites. Thus, these fields appear to contain patchworks of narrow motor columns, as in M1. Moreover, both PMV and PMD appear to have larger functional zones of cortex for more complex movements, much as in M1. 14,15,22.
The supplementary motor area (SMA) represents body movements from hindlimb to face in a caudorostral cortical sequence 1,5,7,23. Movements elicited with microstimulation from SMA at near threshold levels of current are often restricted to a single muscle or joint, much as in M1. Again, each subregion for a major body part in SMA appears to contain a collection of smaller columns of neurons representing different movements during brief threshold activations. Although movements can be evoked from the cingulate motor areas 7,11 the overall somatotopy of motor maps in these fields have been largely inferred from the distributions of labeled cells in these fields after injections of tracers into various levels of the spinal cord 24. In addition, there are still uncertainties about how cingulate cortex is divided into motor areas and how these areas represent the movements of body parts (Wu et al., 2000). Finally, stimulation of the frontal eye field produces saccadic eye movements in all studied primates 7,25,26, while smooth pursuit eye movements have been evoked from a caudal subregion of FEF 27.
The other cortical region of major concern in this review is posterior parietal cortex (PPC). This cortex has been repeatedly implicated in sensorimotor functions by having dense connections with frontal motor areas together with visual and somatosensory inputs that could be used for guiding motor functions 15,28–32. Many microelectrode recording studies of single neurons have revealed response properties that are consistent with the concept that several functionally distinct subdivisions of PPC are yet functionally similar in that they are involved in the sensory guidance and initiation of various motor behaviors (e.g., 33,34. In particular, there have been many studies of the cortex of the intraparietal sulcus of macaque monkeys, and this cortex has been divided into an anterior intraparietal region, AIP, thought to be particularly involved in grasping behavior 35, a lateral intraparietal region, LIP, with a major role in producing eye movements 36, and a ventral intraparietal region, VIP, thought to be involved in visually and multisensory guided motor behavior via a representation of external space 37. More specifically, VIP appears to be involved in defensive behaviors as microstimulation in VIP produces movements of the face and arm that have been described as defensive movements 38. More caudally in the intraparietal cortex, investigators have described a parietal reach region, PRR, where neurons respond during reaching behavior and the intention to reach 34. Other parts of PPC also contain neurons with response properties and connections that implicate then in sensorimotor behavior. However, the focus here is on regions with direct connections to motor and premotor cortex where microstimulation evokes a complex sequence of movements.
Long-train microstimulation evokes complex behaviors from motor (M1) and premotor cortex (PMC)
Simple movements are evoked from microstimulation sites in M1 when stimulation consists of brief (~ 60 msec.) trains of ~ 0.2 msec. pulses of current at levels low enough to evoke just noticeable movements. However, longer (0.5 sec.) trains of pulses at higher levels of current are long enough to be compatible with longer sequences of movements, and they are capable of evoking complex movements from motor and premotor cortex. These complex movements were noticed by early investigators, such that when Leyton and Sherrington (1917) charted their maps of primary motor cortex in chimpanzees they found that evoked movements were quite complex, and often involved a sequence of consecutive movements. To obtain the somatotopic maps similar to those common today, Leyton and Sherrington (1917) based the maps on the first movements of any evoked sequence. More recently, Graziano and co-workers have re-introduced the use of longer, more powerful trains of electrical pulses while stimulating motor and premotor cortex in macaque monkeys, and described the more complex movements that resulted 14,39. Using 500 msec. trains of electrical pulses, approximating the time needed for a complex movement, Graziano and co-workers found separate regions of M1 of macaque monkeys where hand-to-mouth, defensive, reaching, manipulation or grasping, or climbing movements of forelimbs were evoked. For hand-to-mouth movements, wherever the hand was located, it moved to the mouth and the mouth opened when the cortex was stimulated. Defensive movements, mainly contralateral to the stimulated cortex, included an eye blink and squint, a facial grimace, and a movement of the arm next to the head as if to block a blow. In evoked reaching, the shoulder flexed and the arm straightened so that the contralateral hand extended, while the fingers opened. In manipulation or grasping, the hand grasped as in making a fist, or made a precision grip, or turned the palm toward the face with fingers spread as if to reveal a grasped object. Climbing movements were those that involved both arm and leg movements, typically bilateral. As the monkeys were constrained in a monkey chair, it was not clear if these movements represented climbing or perhaps leaping. Other types of complex movements were also observed.
Our experiments involving long-train microstimulation of motor and premotor cortex in primates produced similar results. We stimulated frontal cortex in anesthetized prosimian galagos, owl monkeys, squirrel monkeys and macaque monkeys 15,16,40. In all these primates we were able to evoke different complex movements from small zones in M1, and often, similar complex movements from small zones in PMD or PMV. In order to limit the duration of our stimulation sessions, we usually focused on demonstrating and delimiting the parts of frontal cortex where reaching, defensive, or grasping movements could be evoked from M1 and premotor cortex. When the locations of these patches were compared to the typical first movements or threshold movement maps of M1 and premotor cortex, it was clear that complex movements involving the hand or arm were evoked from the hand-forelimb portions of the maps (Fig. 1), while those that included face movements included the face portions. The patch-like functional zones of cortex for different complex, movements revealed by long train electrical stimulation suggest an interpretation of the functional significance of the mosaic nature of the motor representations in the threshold maps. We propose that each functional zone of M1 or premotor cortex contains a mixture of different columns of neurons that together are devoted to initiating the components of a more complex movement. Thus, there would be shoulder, elbow, wrist, and digit movement columns within the reaching module, and other combinations of these columns within other modules for other complex movements (grasping, defense, climbing). We define a functional zone as the region of cortex where microstimulation evokes a particular class of complex movement, although slight variations of that movement may occur as different sites within the zone are stimulated.
The selective projections of functional zones in posterior parietal cortex to matching functional zones in premotor and motor cortex
Here we describe functional zones in PPC that we characterized by long-train microstimulation, and the connections of these zones with frontal cortex. Microstimulation has not been traditionally used to explore the organization of PPC. An exception has been the LIP region of PPC in macaque monkeys. Using moderately long trains of electrical stimulation via penetrating microelectrodes, a number of investigators have elicited saccadic eye movements from LIP 41–44. A major target of LIP in the frontal lobe is the frontal eye field, FEF 36,45 where saccadic eye movements are also evoked by electrical stimulation. Thus, the FEF is directly activated by a functionally matched zone in PPC.
Consistent with the stimulation and anatomical results that demonstrate the relationship of a functional eye movement zone (LIP) in PPC to a matching eye-movement zone in frontal cortex (FEF), we have been able to demonstrate that a number of types of functional zones exist in PPC and frontal cortex of a range of primate species, and that functional zones of the same type are directly interconnected. Our most extensive work has been with prosimian galagos, a small primate that has the advantage of having few cortical fissures so that all of motor cortex is exposed on the brain surface. Long-train microstimulation in galagos revealed a series of different zones where aggressive face movements, defensive face movements, grasping, hand-to-mouth movements, forelimb defensive movements, reaching movements and bilateral forelimb and hindlimb movements (climbing) were evoked in a lateromedial sequence across PPC 32,46. M1 and PMC were not studied as extensively, but matching functional zones for most of these PPC zones were found in frontal cortex. As expected, each functional zone in PPC projected to matching a pair of matching functional zones, one in PMC and one in M1 40. Additionally, optical imaging of frontal cortex while different functional zones in PPC were electrically stimulated demonstrated that only matching functional zones in PMC and M1 were highly activated 47. When only short trains of electrical pulses were applied to the same functional zones in PPC cortex, matching zones in PMC and M1 were still activated, but less intensively and for a shorter period, and no movements or only the initial movements were evoked. Such results suggest that the motor behavior evoked by stimulating PPC was mediated by these activating connections to PMC and M1.
We were able to demonstrate the functional relationship of cortical zones to each other directly by applying muscimol, a GABA agonist that blocks neural activity reversibly, to functional zones in frontal and PPC 48. After injections in a functional zone in M1, movements could not be evoked from that zone, or from matching functional zones in PMC or PPC, although other classes of movements could be evoked from other functional zones. Blocking a functional zone in PMC or PPC produced only minor changes in the movements produced by stimulating matching functional zones in M1. Thus, the outputs of M1 were essential to the behavior, and the effects of PPC and PMC stimulation appeared to be largely mediated via their connections with M1.
While M1, PMV, PMD, and PPC are clearly the key nodes in the cortical networks for specific types of common, adaptive complex movements, connections with other structures are undoubtedly important, but not as critical. PPC is activated by various sensory inputs, especially higher order visual inputs from dorsal stream visual areas 20, as well as somatosensory inputs from area 2, the parietal ventral area, S2 and other fields 15,16. Under certain conditions, these sensory inputs likely activate functional zones in PPC to the extent that they compete with each other and initiate one type of action while suppressing others 20. Other connections are with cingulate motor areas and the supplementary motor area, which also have direct connections with M1 and PMC. PMC also has inputs from prefrontal cortex, which likely further influences the selection of a mode of response. Additionally, there are connections with motor nuclei of the thalamus, the basal ganglia, and essential outputs to pools of motor neurons in the brainstem and spinal cord (see, 22,49,50. A more comprehensive theory of motor cortex function will need to include the roles of these and other structures in the selection, initiation, and production of sequences of motor behavior.
Behavioral deficits and recoveries after damage to frontal motor areas and PPC
Our present understandings of the parallel networks for specific classes of motor behavior involving M1, PMC, and PPC suggest that lesions involving different functional zones will lead to different functional deficits. However, the results of a number of recent studies of cortical plasticity suggest that partial and even extensive recoveries may occur, and these recoveries may be promoted by behavioral and other therapies. Large lesions of frontal cortex, such as those produced by a major stroke, can result in extensive and persistent difficulties in the initiation and control of movements of mainly the contralateral half of the body, although some limited recovery is possible 51. Given the organization of motor cortex and the frontal-parietal networks, we should also consider the immediate and long-term consequences of more restricted damage to nodes in the networks, such as those caused by ischemic infarcts and focal neural loss. While no one yet has determined the effects of small cortical lesions of the functional zones that have been identified and defined by long-trains of microstimulation, the short-term blocking effects of muscimol suggest that impairments should be greatest after M1 lesions, and less after PMC and PPC lesions 48. This conclusion is consistent with the differences in the behavioral effects of small, focal lesions of motor cortex and PPC in monkeys.
The behavioral effects of small lesions of part of the forelimb representation in M1 have been studied in squirrel monkeys by Nudo and co-workers 52–54. In these studies, after the hand representation in M1 had been defined with short trains of electrical microstimulation, blood vessels were cauterized so that an ischemic lesion destroyed about one-third of the hand representation. Skilled use of the contralateral hand in food retrieval tasks was impaired, and did not spontaneously recover fully after weeks of time, as the monkeys compensated by using proximal arm and postural adjustments. A remapping of remaining forelimb cortex with microstimulation revealed a further loss of sites in M1 for digit movements as evoked movements at these sites were replaced by shoulder movements. Thus, the spontaneous reorganization of motor cortex that occurs after focal lesions may not always be adaptive. In addition, very early training on reaching tasks after focal lesions of motor cortex may extend the damage to adjoining cortex 55. However, training on hand use tasks in squirrel monkeys with small lesions of the hand region of M1 led to behavioral improvements and an increased representation of digits in the remaining hand cortex 53. In addition, the organization of PMV changed so that the territory of the hand representation enlarged 56, and injections of tracers in PMV revealed an increase in connections from the hand representations in areas 1 and 2 of somatosensory cortex to PMV 57. These and other studies (e.g., 58 suggest that the motor maps in M1 and other motor areas reorganize after partial lesions of M1, and that hand use can influence the reorganization process and the formation of new connections that promote behavioral recoveries. As the motor maps in M1 and PMC change so that sites formerly representing one type of movement come to represent another type of movement, the terminal axon arbors of preserved corticospinal neurons likely expand to reach new motor neuron targets after a cortical lesion removes competing axon arbors 8,11. Over time, the new synapses would be activity dependent, and preserved and shaped by use.
Less is known about the effects of focal lesions of PPC on manual abilities. However, Padberg et al. (2010) trained macaque monkeys on several hand use tasks and tested them on these tasks after a restricted lesion of a hand representation region 59 of area 5 of PPC. The lesions were just caudal to the grasp node in area 2 of macaque monkeys (Fig 1), which likely extends into area 5 15. While the lesions produced immediate deficits in hand use, the deficits rapidly disappeared over several days. Thus, recoveries were more extensive and more rapid after PPC lesions than M1 lesions.
Conclusions
These results, and the features of organization of frontal and parietal motor areas, suggest ways of interpreting the functional impacts and behavioral recoveries that occur after restricted lesions of motor and motor-related cortex. First, lesions of functional zones in parietal-frontal networks for specific motor actions can be expected to impair those motor functions, while having less or little impact on others, or perhaps even releasing them from competitive suppression. For example, hand-to-mouth movements might be selectively impaired by a lesion of the hand-to-mouth zone in M1 without impairing grasping or reaching movements, since these would depend on intact functional zones of M1. Second, impairments would be greatest and longest lasting for comparable lesions of M1 than for PMC, and the least and shortest for PPC. This may be due to connectional plasticity that provides alternative ways of accessing the appropriate and critical modules in M1. Third, partial or nearly complete recoveries of lost motor abilities are possible because of use-dependent plasticity and alterations in the competition for synaptic space that reform connection patterns and the functional organization of remaining motor representations. Such changes are widespread and are distributed across motor and somatosensory areas, and especially within the partially damaged motor representations. The effectiveness of partially spared pathways can be greatly enhanced over time 60 as they effectively activate more and more neurons directly and indirectly via the formation of new connections. Fourth, behavior therapies currently provide the most effective way to promote brain reorganization and behavioral recoveries. However, the use of pharmacological treatments that promote the growth of new connections and synapses has great promise (e.g.,) 61.
Acknowledgments
Funding: National Institutes of Health, USA NS16446 and EY2686 to J.H.K.
Footnotes
Conflicts of interest: none
References
- 1.Woolsey CN. In: Biological and Biochemical Bases of Behavior. Harlow HF, Woolsey CN, editors. U of Wisconsin Press; 1958. pp. 63–81. [Google Scholar]
- 2.Schieber MH. Constraints on somatotopic organization in the primary motor cortex. J Neurophysiol. 2001;86:2125–2143. doi: 10.1152/jn.2001.86.5.2125. [DOI] [PubMed] [Google Scholar]
- 3.Asanuma H, Rosen I. Topographic organization of cortical efferent zones projecting to distal forelimb muscles in the monkey. Exp Brain Res. 1972;14:243–256. doi: 10.1007/BF00816161. [DOI] [PubMed] [Google Scholar]
- 4.Leyton ASF, Sherrington CS. Observations on the excitable cortex of the chimpanzee, orangutan, and gorilla. Quart J Exp Physiol. 1917;11:137–222. [Google Scholar]
- 5.Gould HJ, Cusick CG, Pons TP, Kaas JH. The relationship of corpus callosum connections to electrical stimulation maps of motor, supplementary motor, and the frontal eye fields in owl monkeys. J Comp Neurol. 1986;247:297–325. doi: 10.1002/cne.902470303. [DOI] [PubMed] [Google Scholar]
- 6.Donoghue JP, Leibovic S, Sanes JN. Organization of the forelimb area in squirrel monkey motor cortex: representation of digit, wrist, and elbow muscles. Exp Brain Res. 1992;89:1–19. doi: 10.1007/BF00228996. [DOI] [PubMed] [Google Scholar]
- 7.Wu CW, Bichot NP, Kaas JH. Converging evidence from microstimulation, architecture, and connections for multiple motor areas in the frontal and cingulate cortex of prosimian primates. J Comp Neurol. 2000;423:140–177. doi: 10.1002/1096-9861(20000717)423:1<140::aid-cne12>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 8.Qi HX, Stepniewska I, Kaas JH. Reorganization of primary motor cortex in adult macaque monkeys with long--standing amputations. J Neurophysiol. 2000;84:2133–2147. doi: 10.1152/jn.2000.84.4.2133. [DOI] [PubMed] [Google Scholar]
- 9.Kwan HC, MacKay WA, Murphy JT, Wong YC. Spatial organization of precentral cortex in awake primates. II. Motor outputs. J Neurophysiol. 1987;41:1126–1131. doi: 10.1152/jn.1978.41.5.1120. [DOI] [PubMed] [Google Scholar]
- 10.Sato KC, Tanji J. Digit--muscle responses evoked from multiple intracortical foci in monkey precentral motor cortex. J Neurophysiol. 1989;62:959–970. doi: 10.1152/jn.1989.62.4.959. [DOI] [PubMed] [Google Scholar]
- 11.Wu CW, Kaas JH. Reorganization in primary motor cortex of primates with long---standing therapeutic amputations. J Neurosci. 1999;19:7679–7697. doi: 10.1523/JNEUROSCI.19-17-07679.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huntley GW, Jones EG. Relationship of intrinsic connections to forelimb movement representations in monkey motor cortex: a correlative anatomic and physiological study. J Neurophysiol. 1991;66:390–413. doi: 10.1152/jn.1991.66.2.390. [DOI] [PubMed] [Google Scholar]
- 13.Keller A. Intrinsic synaptic organization of the motor cortex. Cereb Cortex. 1993;3:430–431. doi: 10.1093/cercor/3.5.430. [DOI] [PubMed] [Google Scholar]
- 14.Graziano MSA, Taylor CSR, Moore T. Complex movements evoked by microstimulation of precentral cortex. Neuron. 2002;34:841–851. doi: 10.1016/s0896-6273(02)00698-0. [DOI] [PubMed] [Google Scholar]
- 15.Gharbawie OA, Stepniewska I, Qi HX, Kaas JH. Multiple parietal--frontal pathways mediate grasping in macaque monkeys. J Neurosci. 2011;31:11660–11677. doi: 10.1523/JNEUROSCI.1777-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gharbawie OA, Stepniewska I, Kaas JH. Cortical connections of functional zones in posterior parietal cortex and frontal cortex motor regions in New World monkeys. Cereb Cortex. 2011;21:1981–2002. doi: 10.1093/cercor/bhq260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matelli M, Luppino G, Rizzolatti G. Patterns of cytochrome oxidase activity in the frontal agranular cortex of macaque monkey. Behav Brain Res. 1985;18:125–137. doi: 10.1016/0166-4328(85)90068-3. [DOI] [PubMed] [Google Scholar]
- 18.Belmalih A, et al. A multiarchitectonic approach for the definition of functionally distinct areas and domains in the monkey frontal cortex. J Anat. 2007;211:199–211. doi: 10.1111/j.1469-7580.2007.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Preuss TM, Stepniewska I, Kaas JH. Movement representation in the dorsal and ventral premotor areas of owl monkeys: a microstimulation study. J Comp Neurol. 1996;371:649–676. doi: 10.1002/(SICI)1096-9861(19960805)371:4<649::AID-CNE12>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 20.Kaas JH, Gharbawie OA, Stepniewska I. The organization and evolution of dorsal stream multisensory motor pathways in primates. Front Neuroanat. 2011;5:1–7. doi: 10.3389/fnana.2011.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Godschalk M, Mitz AR, van Duin B, van der Brug H. Somatotopy of monkey premotor cortex examined with microstimulation. Neurosci Res. 1995;23:269–279. doi: 10.1016/0168-0102(95)00950-7. [DOI] [PubMed] [Google Scholar]
- 22.Gharbawie OA, Stepniewska I, Burish MJ, Kaas JH. Thalamocortical connections of functional zones in posterior parietal cortex and frontal cortex motor regions in New World monkeys. Cereb Cortex. 2010;20:2391–2410. doi: 10.1093/cercor/bhp308. bhp308 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitz AR, Wise SP. The somatotopic organization of the supplementary motor area: intracortical microstimulation mapping. J Neurosci. 1987;7:1010–1021. doi: 10.1523/JNEUROSCI.07-04-01010.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He SQ, Dum RP, Strick PL. Topographic organization of corticospinal projections from the frontal lobe motor areas of the medial surface of the hemisphere. J Neurosci. 1995;15:3284–3306. doi: 10.1523/JNEUROSCI.15-05-03284.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huerta MF, Krubitzer LA, Kaas JH. Frontal eye field as defined by intracortical microstimulation in squirrel monkeys, owl monkeys, and macaque monkeys. II. Cortical connections. J Comp Neurol. 1987;265:332–361. doi: 10.1002/cne.902650304. [DOI] [PubMed] [Google Scholar]
- 26.Schall JD. Neural basis of saccade target selection. Rev Neurosci. 1995;6:63–85. doi: 10.1515/revneuro.1995.6.1.63. [DOI] [PubMed] [Google Scholar]
- 27.Tian J, Lynch JC. Functional defined smooth and saccadic eye movement subregions in frontal eye field of Cebus monkeys. J Neurophysiol. 1996;76:2740–2753. doi: 10.1152/jn.1996.76.4.2740. [DOI] [PubMed] [Google Scholar]
- 28.Fogassi L, Luppino G. Motor functions of the parietal lobe. Curr Opin Neurobiol. 2005;15:626–631. doi: 10.1016/j.conb.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 29.Luppino G, Muvata A, Govoni P, Matelli M. Largely segregated parietofrontal connections linking rostral intraparietal cortex (areas AIP and VIP) and the ventral premotor cortex (areas F5 and F4) Exp Brain Res. 1999;128:181–187. doi: 10.1007/s002210050833. [DOI] [PubMed] [Google Scholar]
- 30.Jennerod M, Arbib MA, Rizzolatti G, Sakata H. Grasping objects: the cortical mechanisms of visuomotor transformation. Trends Neurosci. 1995;18:314–320. [PubMed] [Google Scholar]
- 31.Borra E, et al. Cortical connections of the macaque anterior intraparietal (AIP) area. Cereb Cortex. 2008;18:1094–1111. doi: 10.1093/cercor/bhm146. [DOI] [PubMed] [Google Scholar]
- 32.Stepniewska I, Cerkevich CM, Fang PC, Kaas JH. Organization of posterior parietal cortex in galagos: II. Ipsilateral cortical connections of physiologically identified zones within anterior sensorimotor region. J Comp Neurol. 2009;517:783–807. doi: 10.1002/cne.22190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andersen RA, Buneo CA. Intentional maps in posterior parietal cortex. Ann Rev Neurosci. 2002;25:189–220. doi: 10.1146/annurev.neuro.25.112701.142922. [DOI] [PubMed] [Google Scholar]
- 34.Andersen RA, Cui H. Intention, action planning, and decision making in parietal--frontal circuits. Neuron. 2009;63:568–583. doi: 10.1016/j.neuron.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 35.Sakata H, Taira M, Murata A, Mine S. Neural mechanisms of visual guidance of hand action in the parietal cortex of the monkey. Cereb Cortex. 1995;5:429–438. doi: 10.1093/cercor/5.5.429. [DOI] [PubMed] [Google Scholar]
- 36.Andersen RA, Brotchie PR, Mazzoni P. Evidence for the lateral intraparietal area as the parietal eye field. Curr Opin Neurobiol. 1992;21:840–846. doi: 10.1016/0959-4388(92)90143-9. [DOI] [PubMed] [Google Scholar]
- 37.Schlack A, Sterbing--D’Angelo SJ, Hartung K, Hoffman KP, Bremmer F. Multisensory space representations in the macaque ventral intraparietal area. J Neurosci. 2005;25:4616–4625. doi: 10.1523/JNEUROSCI.0455-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cooke DF, Taylor CR, Moore T, Graziano MSA. Complex movements evoked by microstimulation of the ventral intraparietal area. Proc Natl Acad Sci U S A. 2003;100:6163–6168. doi: 10.1073/pnas.1031751100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Graziano MSA, Atlalo TNS, Cooke PF. Arm movements evoked by electrical stimulation in the motor cortex of monkeys. J Neurophysiol. 2005;94:4209–4223. doi: 10.1152/jn.01303.2004. [DOI] [PubMed] [Google Scholar]
- 40.Stepniewska I, Fang PC, Kaas JH. Organization of posterior parietal cortex in galagos: I. Functional zones identified by microstimulation. J Comp Neurol. 2009;517:765–782. doi: 10.1002/cne.22181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kurylo DD, Skavenski AA. Eye movements elicited by electrical stimulation of area PG in the monkey. J Neurophysiol. 1991;65:1243–1253. doi: 10.1152/jn.1991.65.6.1243. [DOI] [PubMed] [Google Scholar]
- 42.Thier P, Andersen RA. Electrical microstimulation distinguishes distinct saccade--related areas in the posterior parietal cortex. J Neurophysiol. 1998;80:1713–1735. doi: 10.1152/jn.1998.80.4.1713. [DOI] [PubMed] [Google Scholar]
- 43.Shibutani H, Sakata H, Hyvarinen J. Saccade and blinking evoked by microstimulation of the posterior parietal association cortex of the monkey. Exp Brain Res. 1984;55:1–8. doi: 10.1007/BF00240493. [DOI] [PubMed] [Google Scholar]
- 44.Constantin AG, Wang H, Martinez-Trujillo JC, Crawford JD. Frames of reference for gaze saccades evoked during stimulation of lateral intraparietal cortex. J Neurophysiol. 2007;98:696–709. doi: 10.1152/jn.00206.2007. [DOI] [PubMed] [Google Scholar]
- 45.Cavada C, Goldman--Rakic PS. Posterior parietal cortex in rhesus monkeys II. Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. J Comp Neurol. 1989;287:422–445. doi: 10.1002/cne.902870403. [DOI] [PubMed] [Google Scholar]
- 46.Stepniewska I, Fang PC, Kaas JH. Microstimulation reveals specialized subregions for different complex movements in posterior parietal cortex of prosimian galagos. Proc Natl Acad Sci USA. 2005;102:4878–4883. doi: 10.1073/pnas.0501048102. 15772167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stepniewska I, et al. Optical imaging in galagos reveals parietal--frontal circuits underlying motor behavior. Proc Natl Acad Sci USA. 2011;108:E725–732. doi: 10.1073/pnas.1109925108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stepniewska I, Gharbawie O, Burish MJ, Kaas JH. 6th FENS Mtg Abstr; Geneva. 2008. [Google Scholar]
- 49.Parent A, Hazrati L--N. Functional anatomy of the basal ganglia. I. The cortico--basal ganglia--thalamo--cortical loop. Brain Res. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- 50.Galea MP, Darian--Smith C. Multiple corticospinal neuron populations in the macaque monkey are specified by their unique cortical origins, spinal terminations, and connections. Cereb Cortex. 1994;4:166–194. doi: 10.1093/cercor/4.2.166. [DOI] [PubMed] [Google Scholar]
- 51.Eisner--Janowiez I, et al. Early and late changes in the distal forelimb representation of the supplementary motor area after injury to frontal motor areas in the Squirrel Monkey. J Neurophysiol. 2008;100:1498–1512. doi: 10.1152/jn.90447.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nudo RL, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. J Neurophysiol. 1996;75:2144–2149. doi: 10.1152/jn.1996.75.5.2144. [DOI] [PubMed] [Google Scholar]
- 53.Nudo RJ, Wise BM, SiFuentes F, Milliken GW. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science. 1996;272:1791–1794. doi: 10.1126/science.272.5269.1791. [DOI] [PubMed] [Google Scholar]
- 54.Friel KM, Nudo RJ. Recovery of motor function after cortical injury in primates. Compensatory movement patterns used during rehabilitative training. Somatosens Mot Res. 1998;15:173–189. doi: 10.1080/08990229870745. [DOI] [PubMed] [Google Scholar]
- 55.Risedal A, Zeng J, Johnansson BB. Early training may exacerbate brain damage after focal brain ischemia in the rat. J Cereb Blood Flow Metab. 1999;19:997–1003. doi: 10.1097/00004647-199909000-00007. [DOI] [PubMed] [Google Scholar]
- 56.Frost SB, Barbay S, Friel KM, Plautz EJ, Nudo RJ. Reorganization of remote cortical regions after ischemic brain injury: a potential substrate for stroke recovery. J Neurophysiol. 2003;89:3205–3214. doi: 10.1152/jn.01143.2002. [DOI] [PubMed] [Google Scholar]
- 57.Dancause N, et al. Extensive cortical rewiring after brain injury. J Neurosci. 2005;25:10167– 10179. doi: 10.1523/JNEUROSCI.3256-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaas JH. In: Movement and Action Links to Intelligent Behavior, From Theory to Practice. Stockman IJ, editor. Elsevier Academic Press; 2004. pp. 75–91. [Google Scholar]
- 59.Seelke AMH, et al. Topographic maps within Brodmann’s area 5 of macaque monkeys. Cereb Cortex. 2011 In press. [Google Scholar]
- 60.Qi HX, Chen LM, Kaas JH. Reorganization of somatosensory cortical areas 3b and 1 after unilateral section of dorsal columns of the spinal cord in squirrel monkeys. J Neurosci. 2011;31:13662–13675. doi: 10.1523/JNEUROSCI.2366-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bowes C, Massey JM, Burish M, Cerkevich C, Kaas JH. Chondroitinase ABC promotes selective reactivation of somatosensory cortex in squirrel monkeys after a cervical dorsal column lesion. Proc Natl Acad Sci U S A. 2012;109(7):2595–2600. doi: 10.1073/pnas.1121604109. [DOI] [PMC free article] [PubMed] [Google Scholar]