Abstract
Dysphagia is very common in patients with Parkinson’s disease (PD) and often leads to aspiration pneumonia, the most common cause of death in PD. Unfortunately, current therapies are largely ineffective for dysphagia. As pharyngeal sensation normally triggers the swallowing reflex, we examined pharyngeal sensory nerves in PD for Lewy pathology. Sensory nerves supplying the pharynx were excised from autopsied pharynges obtained from patients with clinically diagnosed and neuropathologically confirmed PD (n = 10) and healthy age-matched controls (n = 4). We examined: the glossopharyngeal nerve (IX); the pharyngeal sensory branch of the vagus nerve (PSB-X); and the internal superior laryngeal nerve (ISLN) innervating the laryngopharynx. Immunohistochemistry for phosphorylated α-synuclein was used to detect potential Lewy pathology. Axonal α-synuclein aggregates in the pharyngeal sensory nerves were identified in all of the PD subjects but not in the controls. The density of α-synuclein-positive lesions was significantly greater in PD subjects with documented dysphagia compared to those without dysphagia. In addition, α-synuclein-immunoreactive nerve fibers in the ISLN were much more abundant than those in the IX and PSBX. These findings suggest that pharyngeal sensory nerves are directly affected by the pathologic process of PD. This anatomic pathology may decrease pharyngeal sensation impairing swallowing and airway protective reflexes, thereby contributing to dysphagia and aspiration.
Keywords: Alpha-synuclein aggregates, Dysphagia, Glossopharyngeal nerve, Immunohistochemistry, Internal superior laryngeal nerve, Lewy neurites, Nerve degeneration, Parkinson disease, Peripheral nervous system, Pharyngeal sensory nerves, Pharynx, Swallowing, Vagus nerve
INTRODUCTION
Parkinson disease (PD) is a multiple system neurodegenerative disorder characterized by a large number of motor and non-motor features. In addition to the hallmark symptoms (resting tremor, bradykinesia, rigidity, and postural instability), PD patients frequently exhibit a number of secondary motor symptoms such as dysphagia, dysarthria, sialorrhoea, and non-motor symptoms including autonomic dysfunction and sensory abnormalities (1). Among these, impaired swallowing and voice/speech represent a large clinical problem in PD as approximately 90% of patients with PD develop these disorders (2, 3). It is estimated that there are about 8 million or more people in the world each year that have or will have swallowing and speech disorders (3). Unfortunately, treatment outcomes for these disorders have been disappointing. Although anti-PD drugs and deep brain stimulation have significant therapeutic effects on limb motor functions, their effects on swallowing in PD are less impressive, and in some cases, adverse (3–5). Importantly, 25% to 50% of PD patients experience tracheal penetration/aspiration (6–8). Aspiration pneumonia is the leading cause of death among PD patients with dysphagia (9–11). As dysphagia is a challenging clinical issue, an urgent need exists to understand its pathophysiological mechanism for the development of effective therapies.
Despite the extremely high incidence of dysphagia in PD, the exact mechanism of this deficit remains unknown. Oropharyngeal swallowing involves a complex integration of motor and sensory modalities, many of which may be compromised in PD. Therefore, oropharyngeal dysphagia and associated aspiration can be caused by dysfunction of the motor and/or sensory nervous system controlling the upper aerodigestive tract. It is well known that the sensory branches of the glossopharyngeal nerve (cranial nerve IX) and vagus nerve (cranial nerve X) are major contributors to sensory innervation of the pharynx (12) and play important roles in reflex initiation and modulation of patterned motor behavior (13). We hypothesized that oropharyngeal dysphagia may be associated, at least in part, with sensory dysfunction as a result of PD-induced nerve damage. An important aspect of understanding oropharyngeal dysphagia and aspiration is to determine whether the sensory nervous system of upper aerodigestive tract is affected in PD as peripheral sensory mediation of the pharynx and larynx is crucial for triggering swallowing and upper airway protective reflexes.
The goal of this study was to determine PD-related degenerative changes in the peripheral sensory nerves innervating the pharynx. Specifically, autopsied cranial nerve IX and pharyngeal sensory branch of the cranial X nerve (PSB-X) supplying the oropharynx and internal superior laryngeal nerve (ISLN) innervating the larynx and laryngopharynx from patients with PD and healthy age-matched controls were examined to detect Lewy pathology using immunohistochemistry for phosphorylated α-synuclein.
MATERIALS AND METHODS
Human Subjects
Sensory nerves supplying the pharyngnx were obtained at autopsy from 10 deceased human subjects with clinically diagnosed and neuropathologically confirmed PD and 4 age-matched healthy controls. The PD pharynges and tongues were provided by the Brain and Body Donation Program at Banner Sun Health Research Institute (BSHRI), which is part of Banner Health, a regional non-profit health care provider centered in metropolitan Phoenix, Ariz (14). BSHRI and the Mayo Clinic Arizona are the principal institutional members of the Arizona Parkinson’s Disease Consortium, which conducts a longitudinal clinicopathological study of PD and normal aging subjects with annual examinations from entry until death and autopsy. The healthy control pharynges without known systemic neuromuscular disorders were obtained from BSHRI (n = 2) and the Department of Pathology at Hackensack University Medical Center (n = 2), Hakensack, NJ.
Clinical and Neuropathologic Evaluations
The clinical features of the 10 patients with PD and 4 controls are summarized in Table 1. For each PD case, detailed clinical and neuropathologic data were provided by the Arizona PD Consortium. Subjects received standardized neuropathological examinations performed by experienced movement disorder specialists (Charles H. Adler, John N. Caviness, Holly A. Shill, Johan E. Samanta), who rated the clinical severity of PD using Hoehn and Yahr (H&Y) scale (15) and the Unified Parkinson’s Disease Rating Scale (UPDRS) (16). Specific clinicopathologic diagnostic criteria for PD were used (17). Dysphagia was evaluated subjectively using item 7 of the UPDRS part II scale (i.e., swallowing score [0–4]: 0 [normal], 1 [rare choking], 2 [occasional choking], 3 [requires soft food], and 4 [requires nasogastric tube or percutaneous endoscopic gastrostomy feeding]). Gross and microscopic neuropathologic assessments were made by a neuropathologist (Thomas G. Beach), who provided a detailed report on the findings for each patient with PD.
TABLE 1.
Demographic and Clinical Data
| Case No. | Gender | Age at Death, y |
Age at PD Onset, y |
PD Duration, y |
H&Y Stages |
Motor UPDRS |
Cause of Death |
UPDRS Months Before Death |
PMI (h) |
Dysphagia |
|---|---|---|---|---|---|---|---|---|---|---|
| PD 1 | M | 75 | 55 | 20 | 2 | 17 | es-PD | 21 | 69 | Yes |
| PD 2 | M | 73 | 62 | 11 | 3 | 18 | es-PD | 26 | 26 | No |
| PD 3 | M | 78 | 59 | 19 | 4 | 51 | CAD | 8 | 76 | Yes |
| PD 4 | F | 84 | 64 | 20 | 3 | 29 | CPF | 10 | 48 | No |
| PD 5 | M | 80 | 69 | 11 | 4 | 53 | c-PD | 11 | 26 | No |
| PD 6 | M | 81 | 70 | 11 | 4 | 43 | es-PD | 19 | 58 | Yes |
| PD 7 | F | 79 | 68 | 11 | 4 | 47 | Pneu | 9 | 16 | No |
| PD 8 | M | 75 | 45 | 30 | 4 | 66 | es-PD | 1 | 36 | Yes |
| PD 9 | M | 80 | 63 | 17 | 5 | 40 | CPF | 5 | 23 | No |
| PD 10 | M | 79 | 56 | 23 | 2 | 28 | MNP | 9 | 34 | Yes |
| Mean (range) | 8 (73–84) | 61 (45–70) | 17 (11–30) | 3.5 (2–5) | 39 (17–66) | 12 (1–26) | 41 (16–76) | |||
| Control 1 | F | 74 | — | — | — | — | MSF | — | 24 | No |
| Control 2 | M | 80 | — | — | — | — | PC | — | 73 | No |
| Control 3 | M | 70 | — | — | — | — | stroke | — | 26 | No |
| Control 4 | F | 70 | — | — | — | — | CPF | — | 30 | No |
| Mean (range) | 73.5 (70–80) | 38 (24–73) |
CAD, coronary artery disease; c-PD, complications of PD; CPF, cardiopulmonary failure; es-PD, end stage of PD; F, female; H&Y, Hoehn-Yahr clinical rating scale (score range, 1–5); M, male; MNP, malignant neoplasm of prostate; MSF, multisystem failure; PC, pancreatic cancer; PD, Parkinson disease; PMI, postmortem interval; Pneu, pneumonia; UPDRS, Unified Parkinson’s Disease Rating Scale.
Nerve Sampling and Sectioning
Sensory nerves supplying the pharynx were excised from autopsied pharynges obtained from patients with PD and healthy control subjects. The mean postmortem interval between death and completed tissue preparation was 41 hours (range, 16–76 hours) for PD specimens and 38 hours (range, 24–73 hours) for controls (Table 1); this postmortem interval does not hamper reliable histochemical analysis of autopsied tissues when the body has been stored in a refrigerated area (18, 19).
The sensory nerves examined included cranial nerve IX, PSB-X and ISLN as they provide sensory innervation of the pharynx (12, 13, 20). The cranial nerve IX is divided into a pharyngeal branch to innervate the mucosa of the lateral and posterior pharyngeal walls and a lingual branch to supply the dorsal mucosa of the posterior tongue. The PSB-X and superior laryngeal nerve (SLN) are branches derived from cranial nerve X. The SLN gives off an internal sensory branch (ISLN) and an external motor branch which innervates the cricothyroid muscle. The PSB-X innervates the mucosa of the lateral and posterior pharyngeal walls, whereas the ISLN supplies the mucosa of the laryngopharynx and larynx. Overall, the oropharynx receives its sensory innervation mainly from the IX and PSB-X, whereas the laryngopharynx and larynx receive their sensory nerve supply predominantly from the ISLN (12).
For each nerve studied, a 1-cm-long nerve segment on each side was sampled as shown in Figure 1. Specifically, the IX was sampled before it gives off its pharyngeal and lingual branches. The PSB-X was sampled immediately after it is given off from the pharyngeal branch of the X nerve. The ISLN was sampled before its entrance into the laryngopharynx and larynx on the thyrohyoid membrane. The removed nerve samples on the left side were used in this study, whereas those on the right side were prepared for other purposes. The nerve segments used for this study were fixed with 10% neutral buffered formalin overnight and embedded in paraffin. For each nerve sample, serial sections were cut (4–µm thick) longitudinally for immunostaining.
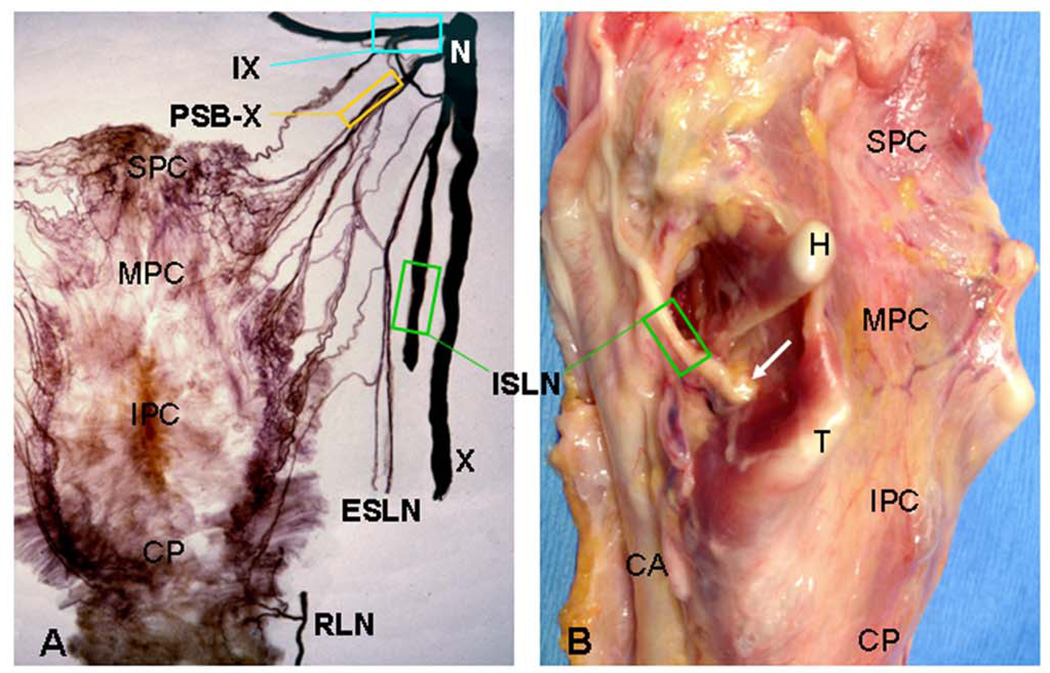
Figure 1.
Images from adult human pharynges showing sampling sites of the pharyngeal sensory nerves. A Posterior view of a normal human pharynx processed with Sihler’s stain, a whole mount nerve staining technique. (Adapted and reproduced in part with permission from Mu and Sanders [20].) B Posterolateral view of a fresh pharynx and larynx from a patient with PD. The sampling site for each nerve studied is outlined (enclosed region). The arrow indicates the entry point of the internal superior laryngeal nerve (ISLN) into the pharynx and larynx through the cricothyroid membrane. CA, carotid artery; CP, cricopharyngeus; H, greater cornu of hyoid bone; IPC, inferior pharyngeal constrictor; IX, glossopharyngeal nerve; MPC, middle pharyngeal constrictor; N, nodose ganglion of X nerve; PSB-X, pharyngeal sensory branch of the X nerve; RLN, recurrent laryngeal nerve; SPC, superior pharyngeal constrictor; T, superior cornu of thyroid cartilage; X, vagus nerve.
Immunohistochemistry
Nerve sections were deparaffinized and stained with an immunohistochemical method for phosphorylated α-synuclein (psyn) as described (21–24). Briefly, the nerve sections were pretreated with 1:100 proteinase K (Enzo Life Sciences, Farmingdale, NY) diluted in 0.1 mol/L PBS at 37°C for 20 minutes and washed 3 times in PBS. The pretreated sections were immersed for 30 minutes in 1% H2O2 in 0.1 mol/L PBS with 0.3% Triton X-100 (PBS-TX) at pH 7.4 and washed 3 times in PBS-TX. The sections were then incubated at room temperature overnight in anti-psyn monoclonal antibody (psyn no. 64; Wako, Richmond, VA) at 1:1000 dilution in PBS-TX and washed 3 times in PBS-TX. The sections were then incubated with a secondary biotinylated antibody (anti-mouse immunoglobulin G diluted 1:1000 in PBS-TX; Vectastain, Vector Laboratories, Burlingame, CA) for 2 hours at room temperature and washed 3 times in PBS-TX. The incubated sections were treated for 30 minutes with avidin-biotin complex (Vector), with A and B components of the kit both at 1:1000 dilution, and followed by 2 washes in PBS-TX and a last wash in 0.05 mol/L Tris buffer at pH 7.6. The sections were then treated with 3,3’-diaminobenzidine (Sigma, St. Louis, MO) (5 mg/100 ml) with added saturated nickel ammonium sulfate (2 mL/100 mL) and H2O2 (5 µL/100 mL of 1% H2O2) for 30 minutes in the dark and washed 3 times in 0.05 mol/L Tris buffer at pH 7.6. Finally, the sections were cleared with xylene and coverslipped. Control sections were stained as described above except that incubation with the primary antibody was omitted.
Quantification
Stained nerve sections were examined under a Zeiss photomicroscope (Axioplan; Carl Zeiss, Oberkochen, Germany) and photographed with a Sony digital camera (model DXC-5500) attached to the photomicroscope and connected to a personal computer with image analysis software. All sections were assessed by a single investigator (Jingming Chen) without knowledge of subject identity or diagnosis.
For a given nerve sample from either nerve IX, PSB-X, or ISLN, 3 nerve sections at different spatial levels stained for psyn were chosen to count Lewy neurites (LNs), each of which was counted separately. For each section, 3 microscopic fields with higher density of LNs were identified and the LNs were counted at 200x magnification (field diameter, 1 mm) with a computerized image analysis system (SigmaScan; Jandel Scientific, San Rafael, CA). The density of the LNs in the 3 fields per section and the 3 selected sections for each nerve sample was averaged. The severity of α-synuclein-positive lesions in each of the nerve samples was rated according to the mean density of the LNs and assessed semiquantitatively following an arbitrary grading system as described (21): −, no lesions; +, 1–20 lesions/field (mild); ++, 21–50 lesions/field (moderate); +++, >50 lesions/field (severe). Data on the density of lesions and semiquantitative assessment rates from the nerve samples collected from the same patient and experimental condition were averaged.
Statistical Analysis
This study was to examine the differentials of the severity of α-synuclein-positive lesions in nerve samples from PD patients with or without dysphagia for three nerve-types: 1) ISLN; 2) IX; and 3) PSB-X.
All continuous variables were expressed as mean (SD) or median (interquartile range) depending on whether or not there was evidence that sample data did not come from the normal distribution. Categorical variables were expressed as frequencies (percentages). Demographic and patient characteristics included: PD duration, Hoehn-Yahr (H&Y) stages (score range: 1–5), motor Unified Parkinson’s disease rating scale (UPDRS), and indication of dysphagia (yes or no).
The primary endpoint, severity of α-synuclein-positive lesions, was derived from the number of α-synuclein-positive lesions using the following categorization: i) none: 0 α-synuclein-positive lesions; ii) mild: 1–20 lesions; iii) moderate: 21–50 lesions; iv) severe: >50 lesions. Ordinal logistic regression model was used to examine the association between the ordered severity and explanatory variables.
To evaluate any potential significant relationships with severity of α-synuclein-positive lesions, a multivariable model was fitted and estimates of ordinal logistic regression for this model were obtained using generalized estimating equations (GEE) method. The GEE analysis was conducted using PROC GENMOD in SAS with link function, cumulative logit (clogit) and independent correlation structure to capture the within-patient dependencies attributed to repeated measurement over nerve-types. The focus of the study is on the evidence of significant associations hence the independent correlation was considered sufficient for our purposes. The results of this analysis were presented as odds ratios (OR), 95% confidence interval (CI) and p-value. Any p <0.05 was considered statistically significant. All data analysis was performed using SAS version 9.2 (SAS Institute Inc, Cary, NC).
RESULTS
Demographic and Clinical Data
Demographics and relevant clinical information for PD patients and control subjects obtained from standardized research neurological testing, medical records and autopsy reports are summarized in Table 1. For all PD patients, clinical diagnosis was neuropathologically confirmed. All PD patients were white and 7 PD cases had developed dementia.
PD group consisted of 8 men and 2 women, whose average age was 78 years (range, 73–84 years) and whose average disease duration was 17 years (range, 11–30 years). The mean age at onset of PD was 61 years (range, 45–70 years). PD severity ranged between 2 and 5 (mean, 3.5) on the Hoehn and Yahr scale (0 to 5, with 0 being the least and 5 the most severe): 2 patients were stage 2, 2 patients were stage 3, 5 patients were stage 4, and 1 patient was stage 5. The mean score for the motor UPDRS (UPDRS: Part III) was 39 points (range, 17–66). The mean time since last neuro/movement examination (UPDRS months before death) was 12 months (range, 1–26 months), and the mean time since last dose of anti-PD medication was 6 hours (range, 2–12 hours).
On the basis of the UPDRS Part II scale, dysphagia occurred in 5 of the 10 PD patients (Table 1). In the 5 dysphagic patients, the swallowing score was rated as 1 in 2 cases (PD 1 and 6) and 2 in 3 cases (PD 3, 8, and 10). The 4 age-matched control subjects included 2 men and 2 women with a mean age of 73.5 years (range, 70–80 years). The cause of death for each of PD patients and control subjects was given in Table 1.
Neuropathologic Findings in PD Brains
In this study, all autopsied brains of the PD patients met neuropathologic criteria for PD. Microscopic examinations revealed moderate to marked depletion or loss of pigmented neurons in the substantia nigra and locus ceruleus and there were several Lewy bodies in each region (data not shown). Immunohistochemical staining for psyn showed frequent immunoreactive Lewy bodies (LBs) and LNs in the olfactory bulb, brainstem, amygdala, transentorhinal area, and cingulate gyrus, with variable densities in the 3 neocortical regions examined (temporal, frontal, and parietal). The major spinal cord subdivisions were examined in 7 cases; 6 of these had positive Lewy-type synucleinopathy in the spinal cord. Using the Unified Staging System for Lewy Body Disorders (22), 6 cases were classified as “neocortical stage” and 4 were “brainstem and limbic stage” (Table 2). Seven of the PD cases also had dementia; of these, 5 cases also met consensus neuropathologic criteria for Alzheimer’s disease (25). One other case had dementia on the basis of progression of PD without concurrent Alzheimer’s disease.
TABLE 2.
The Unified Lewy Stage and Summary Score of Lewy Body (LB) Density for Ten Standardized Brain Regions in Subjects with PD (n = 10)
| Case No. | Unified LB Stage | Summary Score of LB Densitya |
|---|---|---|
| PD 1 | IV. Neocortical | 35 |
| PD 2 | IV. Neocortical | 37 |
| PD 3 | III. Brainstem/Limbic | 29 |
| PD 4 | III. Brainstem/Limbic | 26 |
| PD 5 | IV. Neocortical | 37 |
| PD 6 | IV. Neocortical | 30 |
| PD 7 | IV. Neocortical | 36 |
| PD 8 | IV. Neocortical | 35 |
| PD 9 | III. Brainstem/Limbic | 15b |
| PD 10 | III. Brainstem/Limbic | 26 |
The maximum summary score is 40.
For this case, only seven regions were examined and therefore the score is not strictly comparable to the others.
Phosphorylated α-Synuclein Aggregates in the Pharyngeal Sensory Nerves
Anti-psyn immunohistochemistry showed axonal synucleinopathy lesions in the pharyngeal sensory nerves in PD (Fig. 2). In contrast, Lewy pathology was not observed in the control samples (data not shown).
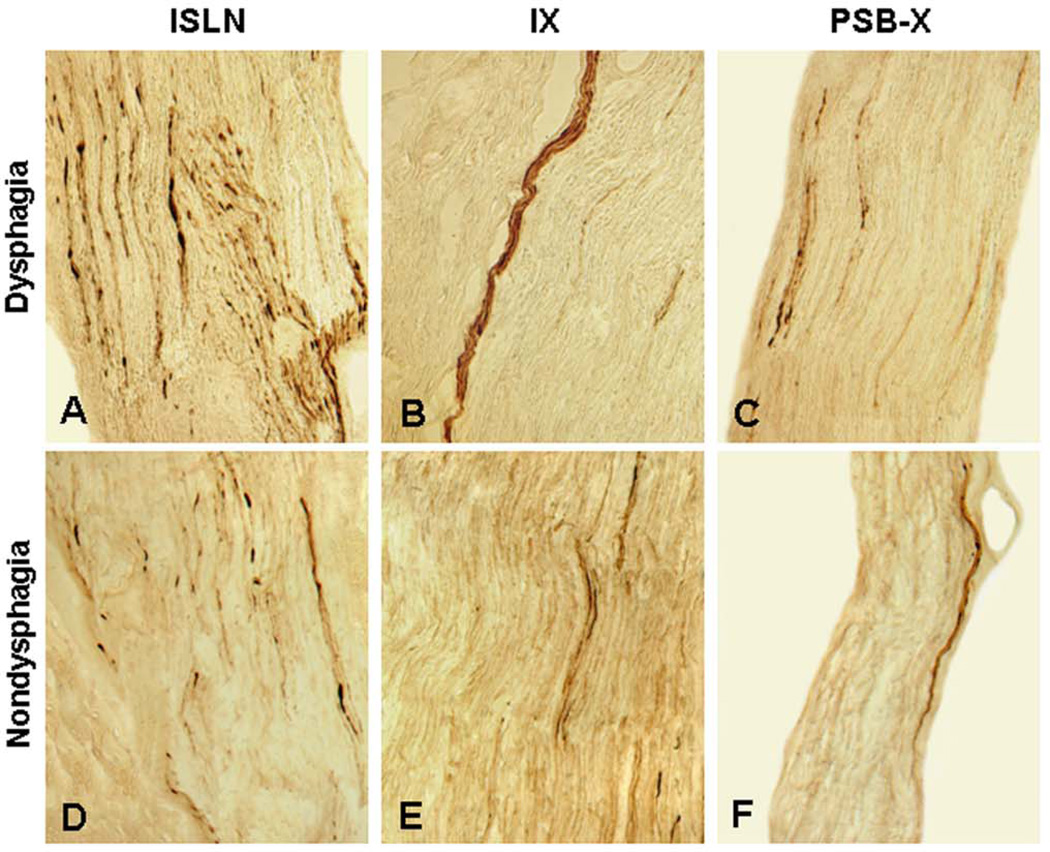
Figure 2.
Photomicrographs of the longitudinal sections of the pharyngeal sensory nerves from PD patients with dysphagia A–C and nondysphagia D–F immunostained with monoclonal antiphosphorylated α-synuclein antibody (psyn#64), showing phosphorylated α-synuclein-immunoreactive neurites (threads and dots). A–C Stained sections of the ISLN A, IX B, and PSB-X C from a PD patient with dysphagia (PD 6, 81-year-old man with disease duration of 11 years, Hoehn & Yahr 4, and motor UPDRS 43). Note that α-synuclein aggregates (darkly stained threads and dots) were more abundant in the ISLN (lesion severity: severe, +++) than in the IX (lesion severity: moderate, ++) and PSB-X (lesion severity: moderate, ++). D–F Stained sections of the ISLN, IX, and PSB-X from a PD subject without dysphagia (PD 5, 80-year-old man with disease duration of 11 years, Hoehn & Yahr 4, and motor UPDRS 53). Note that the severity of axonal α-synuclein aggregates in the ISLN (lesion severity: moderate, ++), IX (lesion severity: mild, +), and PSB-X (lesion severity: mild, +) in this case without dysphagia was lower than that observed in the PD subject with dysphagia. Original magnification: A–F 200x.
In this series, the most frequently affected sensory nerve in PD was the ISLN, followed by the IX and PSB-X. Specifically, phosphorylated α-synuclein aggregates were identified in the ISLN for 10 cases (10/10), in the IX for 8 cases (8/10), and in the PSB-X for 7 cases (7/10) (Table 3).
TABLE 3.
Frequency and Severity of α-Synuclein-Positive Lesions in Pharyngeal Sensory Nerves in Patients With PD
| ISLN |
IX |
PSB-X |
||||
|---|---|---|---|---|---|---|
| Case No. | LNs (range) | Severity of Lesions |
LNs (range) | Severity of Lesions |
LNs (range) | Severity of Lesions |
| PD 1 | 25 (18–31) | ++ | 13 (9–17) | + | 8 (3–14) | + |
| PD 2 | 9 (6–13) | + | 4 (2–7) | + | 3 (1–7) | + |
| PD 3 | 15 (10–22) | + | 10 (6–14) | + | 4 (2–6) | + |
| PD 4 | 11 (7–15) | + | 0 0 | − | 0 0 | − |
| PD 5 | 30 (20–39) | ++ | 18 (11–25) | + | 10 (5–16) | + |
| PD 6 | 63 (56–68) | +++ | 25 (19–32) | ++ | 22 (12–34) | ++ |
| PD 7 | 8 (4–13) | + | 0 0 | − | 0 0 | − |
| PD 8 | 22 (13–32) | ++ | 9 (5–14) | + | 6 (1–10) | + |
| PD 9 | 13 (8–17) | + | 4 (2–7) | + | 0 0 | − |
| PD 10 | 52 (43–59) | +++ | 23 (9–34) | ++ | 7 (4–11) | + |
| Mean (range) | 25 (8–63) | 11 (0–25) | 6 (0–22) | |||
The severity of α-synuclein-positive lesions in the pharyngeal sensory nerves was rated on the basis of the mean density of the Lewy neurites (LNs) and assessed semiquantitatively using an arbitrary grading system: −, no lesions; +, 1–20 lesions/field (mild); ++, 21–50 lesions/field (moderate); +++, >50 lesions/field (severe). ISLN, internal superior laryngeal nerve; IX, glossopharyngeal nerve; PSB-X, pharyngeal sensory branch of the X nerve.
The average densities of α-synuclein-positive lesions were higher in the ISLN than in the IX and the PSB-X. Specifically, the relative frequency of α-synuclein aggregates in PD samples was ranked in the following order: ISLN (mean, 25 LNs) > IX (mean, 11 LNs) > PSB-X (mean, 6 LNs) (Table 3).
Antipsyn-immunoreactive axons commonly appeared as threads, dots, or bundles of immunoreactive axons in the affected sensory nerves examined (Fig. 2). Overall, axonal α-synuclein aggregates in the ISLN (Fig. 2A, D) were much more abundant than those in the IX (Fig. 2B, E) and PSB-X (Fig. 2C, F). Variable lesion severity in the sensory nerves examined was presented in Figure 2 and summarized in Table 3. The severity of α-synuclein lesions in each of the nerve samples for each patient with PD was assessed on the basis of the mean density of antipsyn-immunoreactive axons. According to the scoring scheme used in this study, the density or severity of α-synuclein-positive lesions for the ISLN was rated as mild (+) in 5 cases, moderate (++) in 3 cases, and severe (+++) in 2 cases. The lesion severity in the IX was absent (−) in 2 cases, mild in 6 cases, and moderate in 2 cases. The PSB-X presented absence of lesions in 3 cases, mild lesions in 6 cases, and moderate lesions in 1 case. ISLN was moderately to severely affected in 5 cases (5/10), whereas IX was moderately affected in 2 cases (2/10) and PSB-X was moderately affected in 1 case (1/10).
The differences in the distribution of frequency and severity of α-synuclein-positive lesions in the ISLN, IX, and PSB-X nerves in PD patients with and without dysphagia are summarized in Table 4. In this series, PD patients with dysphagia had a higher density of α-synuclein-positive lesions in the nerves examined, especially in the ISLN, as compared with those without dysphagia (Fig. 2; Tables 3, 4). For example, in the 5 PD patients with dysphagia (PD 1, 3, 6, 8, and 10) the ISLN exhibited mild lesions in 1 case (PD 3), moderate lesions in 2 cases (PD 1 and 8), and severe lesions in 2 cases (PD 6 and 10). In contrast, in the 5 PD patients without dysphagia (PD 2, 4, 5, 7, and 9), the ISLN exhibited mild lesions in 4 cases (PD 2, 4, 7, and 9) and moderate lesions in 1 case (PD 5).
TABLE 4.
Differences in the Distribution of Frequency and Severity of α-Synuclein-Positive Lesions in the ISLN, IX, and PSB-X Nerves in PD Patients With Dysphagia Versus Those Without
| Absence of Dysphagia |
Presence of Dysphagia |
|||||
|---|---|---|---|---|---|---|
| α-Synuclein Lesions | ISLN | IX | PSB-X | ISLN | IX | PSB-X |
| Frequency | ||||||
| Median (IQR) | 11(9–13) | 4(0–4) | 0(0–3) | 25(22–63) | 13(10–23) | 7(6–8) |
| Range | (8–30) | (0–18) | (0–10) | (15–63) | (9–25) | (4–22) |
| Severity | ||||||
| None | − | 2(40%) | 3(60%) | − | − | − |
| Mild | 4(80%) | 3(60%) | 2(40%) | 1(20%) | 3(60%) | 4(80%) |
| Moderate | 1(20%) | − | − | 2(40%) | 2(40%) | 1(20%) |
| Severe | − | − | − | 2(40%) | − | − |
IQR, interquartile range: 25th –75th percentile.
Multivariable analysis of α-synuclein-positive lesions in the nerves examined in PD patients with and without dysphagia is presented in Table 5. First of all, we examined the relationships between severity of α-synuclein-positive lesions and both presence of dysphagia and nerve type without adjusting for patient characteristics. The overall GEE analysis indicated that there was a significant association with both dysphagia (p = 0.0064) and nerve type (p = 0.0017). Compared to patients without dysphagia, patients with dysphagia were associated with an increased risk of severe α-synuclein-positive lesions (OR = 64.5; 95% CI: 3.2, 1291; p = 0.0064). Compared to IX nerve, the ISLN was associated with an increased risk of α-synuclein-positive lesions (OR = 21.3; 95% CI: 3.1, 149; p = 0.0020). Compared to PSB-X nerve, the ISLN was associated with an increased risk of α-synuclein-positive lesions (OR = 50.3; 95% CI: 5.9, 430; p = 0.0003). There was no evidence of different risk between the IX and PSB-X nerves (OR = 2.4; 95% CI: 0.8, 7; p = 0.1336). These results suggest the severity of α-synuclein-positive lesions categorization has discriminative ability between the absence and presence of dysphagia and between the ISLN nerve and the other two nerves IX and PSB-X. Next, we evaluated the association between the severity of α-synucleinpositive lesions and dysphagia and nerve type, while additionally taking into account for PD duration, H&Y stages, and motor UPDRS. This GEE analysis confirmed significance of outcomes observed in previous analysis (note that in most cases the estimated probabilities in this adjusted model were larger and the confidence intervals were wider). There was a significant effect of dysphagia (p = 0.0095) and nerve type (p = 0.0038) on the severity of α-synuclein-positive lesions. There was a significant association with PD duration (p = 0.0315), but GEE analysis failed to show any significant associations with the other covariates: H&Y stages (p = 0.5725) and motor UPDRS (p = 0.6623). In contrast with absence of dysphagia, presence of dysphagia was associated with an increased risk of severe α-synuclein-positive lesions (OR = 502.5; 95% CI: 4.6, 55102; p = 0.0095). In contrast with the IX nerve, the ISLN nerve was more likely to be associated with increased risk of severe α-synuclein-positive lesions (OR = 45.8; 95% CI: 2.9, 717; p = 0.0064). In contrast with the PSB-X nerve, the ISLN nerve was more likely to be associated with increased risk of severe α-synuclein-positive lesions (OR = 137.8; 95% CI: 7.5, 2535; p = 0.0009). This study failed to show significantly different risk of severe α-synuclein-positive lesions between the IX and PSB-X nerves (OR = 3.0; 95% CI: 0.8, 12; p = 0.1187). Thus, accounting for the patient characteristics, the results indicated again a significant association between the severity of α-synuclein-positive lesions and the presence of dysphagia and nerve type.
TABLE 5.
Multivariable Analysis of α-Synuclein-Positive Lesions in the ISLN, IX, and PSB-X Nerves in PD Patients With/Without Dysphagia
| Two-Factor Unadjusteda Risk |
Two-Factor Adjustedb Risk |
|||||
|---|---|---|---|---|---|---|
| Variable | Odds Ratio | 95% CI | P Value | Odds Ratio | 95% CI | P Value |
| Absence/presence of dysphagia | ||||||
| Ref category: absence | 64.5 | (3.2, 1291) | 0.0064 | 502.5 | (4.6, 55102) | 0.0095 |
| Nerve type | ||||||
| ISLN vs. IX | 21.3 | (3.1, 149) | 0.0020 | 45.8 | (2.9, 717) | 0.0064 |
| ISLN vs. PSB-X | 50.3 | (5.9, 430) | 0.0003 | 137.8 | (7.5, 2535) | 0.0009 |
| IX vs. PSB-X | 2.4 | (0.8, 7) | 0.1336 | 3.0 | (0.8, 12) | 0.1187 |
The two factors dysphagia and nerve type were not adjusted for patient characteristics.
The two factors dysphagia and nerve type were adjusted for PD duration, H&Y stages, and motor UPDRS.
DISCUSSION
To our knowledge, this is the first demonstration of Lewy-type pathology in the pharyngeal sensory nerves in patients with PD. There are several key findings. First of all, peripheral sensory nerves in the pharynx were affected in PD as indicated by the presence of phosphorylated α-synuclein aggregates. Second, severity of α-synuclein-positive lesions varied with different sensory nerves in PD. In the PD pharynx, the average density of α-synuclein aggregates was higher in the ISLN than that in the IX and PSB-X. Finally, PD patients with dysphagia had a higher density of phosphorylated α-synuclein lesions in the ISLN as compared with those without dysphagia. We noted a significant positive correlation between the severity of nerve lesions and dysphagia. These findings support our hypothesis that sensory nerve damage in the pharynx is one of the risk factors disturbing swallowing in PD. The data presented here suggest that the sensory nerve damage in the PD pharynx could decrease pharyngeal sensation impairing swallowing and airway protective reflexes, thereby contributing to oropharyngeal dysphagia and aspiration.
The pathophysiological mechanism of oropharyngeal dysphagia remains to be determined even though this disorder in PD is well recognized. Oropharyngeal dysphagia appears to be caused by multiple factors. Lewy pathology found in the peripheral motor and sensory nerves innervating the pharynx is part of the neural basis contributing to pharyngeal dysphagia in PD. Our recent studies have demonstrated that pharyngeal motor nerves and their innervating muscles are affected in PD (21, 26). In the PD pharyngeal muscles, disease-related changes include fiber denervation, grouped fiber atrophy, fiber-type grouping, and fast-to-slow myosin heavy chain transformation (26). Phosphorylated α-synuclein aggregates have been revealed in the PD pharyngeal motor nerves (21). Evidently, the changes in the pharyngeal muscles are most likely caused by degenerated motor nerve fibers. PD-induced neuromuscular alterations in the pharynx could reduce muscle strength and pharyngeal peristalsis/constrictor function, resulting in oropharyngeal dysphagia.
In addition to motor impairment, sensory disturbances are part of the clinical picture of PD. Peripheral sensory nerve involvement in PD has been documented by morphometric and functional assessments and α-synuclein immunohistochemistry of cutaneous sensory nerve endings (27–31). However, no studies have been performed to determine PD-induced alterations in the peripheral sensory nervous system in the upper aerodigestive tract. Swallowing is a complex motor sequence by which a bolus is transported from the mouth to the stomach in three phases: oral, pharyngeal, and esophageal (32, 33). Sensory feedback is critical for swallowing and the laryngeal cough reflex. When the bolus reaches the fauces and stimulates faucial sensory receptors, the involuntary pharyngeal phase of swallowing begins (33, 34). The important role of the pharyngeal sensory nerves in the swallowing reflex has been confirmed by both the electrical nerve stimulation and elimination of sensitivity of the pharynx. Electrical stimulation of the ISLN, IX and pharyngeal branch of the X nerve initiates pharyngeal swallowing (35–37). The ISLN is the most important sensory nerve for the initiation of reflex swallowing from the larynx and laryngopharynx. It is well known that electrical stimulation of the ISLN can readily elicit reflex swallowing (35–39) and laryngeal closure (40) in humans and other mammals. Some investigators reported that stimulation of the IX nerve is less effective for eliciting swallowing reflex than ISLN stimulation (36, 37). However, recent studies demonstrated that electrical stimulation of the Ph-IX, not the lingual branch of the IX, is also effective for initiating swallowing (41, 42). In addition, simultaneous electrical stimulation of both the ISLN and IX nerves could increase the rate of swallowing (41–44), suggesting that spatiotemporal summation of afferent signals from both pharyngeal sensory nerves could increase motoneuronal activity in the medullary swallowing center, thus enhancing reflex swallowing. However, the pharyngeal branch of the X nerve has been reported to play a minor role for the pharyngeal swallowing (45). Animal experiments showed that transection of the pharyngeal and laryngeal sensory nerves leads to dysphagia. For example, unilateral transection of the ISLN altered the sequence of pharyngeal muscle activity, whereas bilateral ISLN transection resulted in dysphagia and aspiration (46). Recent studies (41) showed that after bilateral transection of the pharyngeal branch of the IX nerve, mechanical pharyngeal stimulation failed to elicit reflex swallowing. These findings indicate that pharyngeal sensory nerves play an important role in initiation of pharyngeal swallowing.
Neurogenic dysphagia results from sensorimotor impairment of the oral and pharyngeal phases of swallowing due to neurologic disorders including PD. Electrophysiological assessments showed that patients with PD had prolonged triggering of the swallowing reflex (47). As the ISLN mediates most sensation of the mucosa in the larynx and the laryngopharynx, it plays a critical role in the protection of the airway by initiating reflex glottic closure during swallowing and coughing (48). Pharyngolaryngeal sensory impairment could decrease sensitivity of the upper airway. Aspiration is also related to decreased sensation and diminished cough reflex (11, 49). These observations suggest that dysphagia and aspiration in PD patients are associated with decreased sensation in the pharyngolaryngeal area. Sensory deficits in the pharynx and larynx can be caused by sensory nerve damage as demonstrated in the present study. The data from this report and our recent studies (21, 26) suggest that oropharyngeal dysphagia in PD is most likely associated with degeneration of both the peripheral sensory and motor nerves innervating the pharynx. The conventionally proposed mechanism of dysphagia by a reduction in basal ganglia dopaminergic activity appears to be incomplete.
The histological hallmark for diagnosis of PD is the presence of aggregates of phosphorylated α-synuclein called Lewy bodies and Lewy neurites. Various neuronal systems are likely involved in PD, thus resulting in multiple neuromediator dysfunctions that account for the complex patterns of functional deficits (1). In PD, phosphorylated α-synuclein histopathology have been identified not only in the brain, but also in multiple organs including the dorsal horn spinal cord (23), the peripheral autonomic nervous system, including the enteric nervous system of the alimentary tract (23, 50, 51), cardiac plexus (52–56), and skin (28–31, 57, 58). Lewy pathology has been also revealed in the peripheral mixed nerves such as sciatic nerve (23) and cervical X nerve (21, 23, 24, 59, 60). Recent studies from our group demonstrate that both the motor (21, 26) and sensory (present study) pharyngeal nerves are affected in PD. All of these findings indicate that neurodegeneration in PD occurs both in the central and peripheral nervous systems. However, it remains unknown about whether axonal degeneration of the pharyngeal motor and sensory nerves progresses from peripheral to central versus from central to peripheral.
We distinguished dementia with Lewy bodies (DLB) from PD unambiguously using accepted international clinical diagnostic criteria (third revision) published by the Dementia with Lewy Bodies Consortium (61). In this scheme, PD is diagnosed if parkinsonism presents at least one year prior to the making of a clinical diagnosis of dementia. Both PD and DLB commonly progress, by the time of autopsy, to a neocortical stage wherein Lewy bodies and associated fiber pathology are common throughout the neocortex. Although DLB subjects are more likely to be neocortical stage than PD subjects, the neocortical stage is also common in PD (22). In this group, the neocortical stage was more common than is typical for a larger autopsy group but this is likely just due to chance in this small sample size. The pharyngeal phosphorylated alpha-synuclein nerve pathology that we describe here and in our previous publications (21, 26) may not be restricted to PD as subjects with DLB also have widespread phosphorylated alpha-synuclein pathology in the peripheral nervous system (23). Further studies with larger sample size are needed to determine the differences between PD and DLB in the severity and distribution of the peripheral nerve pathology.
Clinico-pathologic-neurobiological correlation studies would be ideal for elucidating the pathophysiological basis of specific clinical symptoms of PD such as dysphagia. In this regard, the present study revealed a significant positive correlation between dysphagia and the severity of α-synuclein-positive lesions in the pharyngeal sensory nerves. We also found that the severity of nerve damage had a positive correlation with PD duration, but not with disease severity as indicated by H&Y stage and UPDRS. We failed to reveal a positive correlation between the Lewy body pathology density scores in the brain and the severity of α-synuclein lesions in the pharyngeal sensory nerves. However, further clinico-neuropathologic correlation studies with larger sample size are needed to clarify the relationship between neuropathologic observations and clinical findings. The severity of the peripheral nerve damage might correlate better with the degree of dysphagia rather than the pathological burden in the brain. Elucidating and characterizing the peripheral nervous system lesions in the pharynx, larynx, and other related structures will provide better insight into the pathogenesis of neurodegeneration in PD and related upper airway disorders.
ACKNOWLEDGMENTS
This research is supported by National Institutes of Health Grant 5 R01 DC004728 from the National Institute on Deafness and Other Communication Disorders (to Dr. Mu). The Brain and Body Donation Program is supported by the National Institute of Neurological Disorders and Stroke (U24 NS072026 National Brain and Tissue Resource for Parkinson’s Disease and Related Disorders), the National Institute on Aging (P30 AG19610 Arizona Alzheimer’s Disease Core Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05–901 and 1001 to the Arizona Parkinson's Disease Consortium) and the Michael J. Fox Foundation for Parkinson’s Research.
The authors thank the Banner Sun Health Research Institute Brain and Body Donation Program and the Arizona Parkinson’s Disease Consortium for the provision of whole-mount tongue-pharynx-larnyx specimens and associated clinical and neuropathologic data from PD subjects.
The authors also thank the anonymous reviewers for their constructive comments on the manuscript.
REFERENCES
- 1.Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79:368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- 2.Ramig LO, Fox C, Sapir S. Speech Treatment for Parkinson’s disease. Expert Rev Neurother. 2008;8:297–309. doi: 10.1586/14737175.8.2.297. [DOI] [PubMed] [Google Scholar]
- 3.Sapir S, Ramig L, Fox C. Speech and swallowing disorders in Parkinson disease. Curr Opin Otolaryngol Head Neck Surg. 2008;16:205–210. doi: 10.1097/MOO.0b013e3282febd3a. [DOI] [PubMed] [Google Scholar]
- 4.Hunter PC, Crameri J, Austin S, et al. Response of parkinsonian swallowing dysfunction to dopaminergic stimulation. J Neurol Neurosurg Psychiatry. 1997;63:579–583. doi: 10.1136/jnnp.63.5.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duff J, Sime E. Surgical interventions in the treatment of Parkinson’s disease (PD) and essential tremor (ET): medial pallidotomy in PD and chronic deep brain stimulation (DBS) in PD and ET. Axon. 1997;18:85–89. [PubMed] [Google Scholar]
- 6.Robbins JA, Logemann JA, Kirshner HS. Swallowing and speech production in Parkinson's disease. Ann Neurol. 1986;19:283–287. doi: 10.1002/ana.410190310. [DOI] [PubMed] [Google Scholar]
- 7.Stroudley J, Walsh M. Radiological assessment of dysphagia in Parkinson’s disease. Br J Radiol. 1991;64:890–893. doi: 10.1259/0007-1285-64-766-890. [DOI] [PubMed] [Google Scholar]
- 8.Bird MR, Woodward MC, Gibson EM, et al. Asymptomatic swallowing disorders in elderly patients with Parkinson's disease: a description of findings on clinical examination and videofluoroscopy in sixteen patients. Age Aging. 1994;23:251–254. doi: 10.1093/ageing/23.3.251. [DOI] [PubMed] [Google Scholar]
- 9.Wermuth L, Stenager EN, Stenager E, et al. Mortality in patients with Parkinson’s disease. Acta Neurol Scand. 1995;92:55–58. doi: 10.1111/j.1600-0404.1995.tb00466.x. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez HH, LaPane KL. Predictors of mortality among nursing home residents with a diagnosis of Parkinson’s disease. Med Sci Monit. 2002;8:241–246. [PubMed] [Google Scholar]
- 11.Ebihara S, Saito H, Kanda A, et al. Impaired efficacy of cough in patients with Parkinson's disease. Chest. 2003;124:1009–1015. doi: 10.1378/chest.124.3.1009. [DOI] [PubMed] [Google Scholar]
- 12.Mu L, Sanders I. Sensory nerve supply of the human oro- and laryngopharynx: A preliminary study. Anat Rec. 2000;258:406–420. doi: 10.1002/(SICI)1097-0185(20000401)258:4<406::AID-AR9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 13.Williams PL, Bannister LH, Berry MM, et al. Gray’s Anatomy. 38th ed. New York: Churchill Livingstone; 1995. [Google Scholar]
- 14.Beach TG, Sue LI, Walker DG, et al. The Sun Health Research Institute Brain Donation Program: description and experience: 1987–2007. Cell Tissue Bank. 2008;9:229–245. doi: 10.1007/s10561-008-9067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 16.Fahn S, Elton R, Committee DU. Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, editors. Recent Developments in Parkinson’s Disease. New York, NY: MacMillan; 1987. pp. 153–163. [Google Scholar]
- 17.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56:33–39. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- 18.Eriksson PO, Eriksson A, Ringqvist M, et al. The reliability of histochemical fiber typing of human necropsy muscles. Histochemistry. 1980;65:193–205. doi: 10.1007/BF00493169. [DOI] [PubMed] [Google Scholar]
- 19.Eriksson PO, Thornell LE. Histochemical and morphological muscle-fiber characteristics of the human masseter, the medial pterygoid and the temporal muscles. Arch Oral Biol. 1983;28:781–795. doi: 10.1016/0003-9969(83)90034-1. [DOI] [PubMed] [Google Scholar]
- 20.Mu L, Sanders I. Neuromuscular specializations within human pharyngeal constrictor muscles. Ann Otol Rhinol Laryngol. 2007;116:604–617. doi: 10.1177/000348940711600809. [DOI] [PubMed] [Google Scholar]
- 21.Mu L, Sobotka S, Chen J, et al. Alpha-synuclein pathology and axonal degeneration of the peripheral motor nerves innervating pharyngeal muscles in Parkinson disease. J Neuropathol Exp Neurol. 2013;72:119–129. doi: 10.1097/NEN.0b013e3182801cde. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beach TG, Adler CH, Lue L, et al. Unified staging system for Lewy body disorders: Correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol. 2009;117:613–634. doi: 10.1007/s00401-009-0538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beach TG, Adler CH, Sue LI, et al. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010;119:689–702. doi: 10.1007/s00401-010-0664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Del Tredici K, Hawkes CH, Ghebremedhin E, et al. Lewy pathology in the submandibular gland of individuals with incidental Lewy body disease and sporadic Parkinson’s disease. Acta Neuropathol. 2010;119:703–713. doi: 10.1007/s00401-010-0665-2. [DOI] [PubMed] [Google Scholar]
- 25.The National Institute on Aging and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. Neurobiol Aging. 1997;18:S1–S2. [PubMed] [Google Scholar]
- 26.Mu L, Sobotka S, Chen J, et al. Altered pharyngeal muscles in Parkinson disease. J Neuropathol Exp Neurol. 2012;71:520–530. doi: 10.1097/NEN.0b013e318258381b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toth C, Breithaupt K, Ge S, et al. Levodopa, methylmalonic acid, and neuropathy in idiopathic Parkinson disease. Ann Neurol. 2010;68:28–36. doi: 10.1002/ana.22021. [DOI] [PubMed] [Google Scholar]
- 28.Dabby R, Djaldetti R, Shahmurov M, et al. Skin biopsy for assessment of autonomic denervation in Parkinson’s disease. J Neural Transm. 2006;113:1169–1176. doi: 10.1007/s00702-005-0431-0. [DOI] [PubMed] [Google Scholar]
- 29.Ikemura M, Saito Y, Sengoku R, et al. Lewy body pathology involves cutaneous nerves. J Neuropathol Exp Neurol. 2008;67:945–953. doi: 10.1097/NEN.0b013e318186de48. [DOI] [PubMed] [Google Scholar]
- 30.Nolano M, Provitera V, Estraneo A, et al. Sensory deficit in Parkinson's disease: evidence of a cutaneous denervation. Brain. 2008;131:1903–1911. doi: 10.1093/brain/awn102. [DOI] [PubMed] [Google Scholar]
- 31.Shishido T, Ikemura M, Obi T, et al. α-Synuclein accumulation in skin nerve fibers revealed by skin biopsy in pure autonomic failure. Neurology. 2010;74:608–610. doi: 10.1212/WNL.0b013e3181cff6d5. [DOI] [PubMed] [Google Scholar]
- 32.Logemann JA. Evaluation and treatment of swallowing disorders. San Diego: College-Hill Press; 1983. [Google Scholar]
- 33.Dodds WJ. The physiology of swallowing. Dysphagia. 1989;3:171–178. doi: 10.1007/BF02407219. [DOI] [PubMed] [Google Scholar]
- 34.Perlman AL. The neurology of swallowing. Sem Speech Lang Hear. 1991;12:171–184. [Google Scholar]
- 35.Doty RW. Influence of stimulus pattern on reflex deglutition. Am J Physiol. 1951;166:142–158. doi: 10.1152/ajplegacy.1951.166.1.142. [DOI] [PubMed] [Google Scholar]
- 36.Doty RW. Neural organization of deglutition. In: Code CF, editor. Handbook of Physiology. Section 6, Alimentary canal. Volume 4. Washington, D.C: Am Physiol Soc; 1968. pp. 861–902. [Google Scholar]
- 37.Sinclair WJ. Role of the pharyngeal plexus in initiation of swallowing. Am J Physiol. 1971;221:1260–1263. doi: 10.1152/ajplegacy.1971.221.5.1260. [DOI] [PubMed] [Google Scholar]
- 38.Doty RW, Bosma JF. An electromyographic analysis of reflex deglutition. J Neurophysiol. 1956;19:44–60. doi: 10.1152/jn.1956.19.1.44. [DOI] [PubMed] [Google Scholar]
- 39.Storey AT. Laryngeal initiation of swallowing. Exp Neurol. 1968;20:359–365. doi: 10.1016/0014-4886(68)90079-4. [DOI] [PubMed] [Google Scholar]
- 40.Murakami Y, Kirchner JA. Mechanical and physiological properties of reflex laryngeal closure. Ann Otol Rhinol Laryngol. 1972;81:59–71. doi: 10.1177/000348947208100106. [DOI] [PubMed] [Google Scholar]
- 41.Kitagawa J, Shingai T, Takahashi Y, et al. Pharyngeal branch of the glossopharyngeal nerve plays a major role in reflex swallowing from the pharynx. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1342–R1347. doi: 10.1152/ajpregu.00556.2001. [DOI] [PubMed] [Google Scholar]
- 42.Kitagawa J, Nakagawa K, Hasegawa M, et al. Facilitation of reflex swallowing from the pharynx and larynx. J Oral Sci. 2009;51:167–171. doi: 10.2334/josnusd.51.167. [DOI] [PubMed] [Google Scholar]
- 43.Weerasuriya A, Bieger D, Hockman CH. Interaction between primary afferent nerves in the elicitation of reflex swallowing. Am J Physiol. 1980;239:R407–R414. doi: 10.1152/ajpregu.1980.239.5.R407. [DOI] [PubMed] [Google Scholar]
- 44.Ootani S, Umezaki T, Shin T, et al. Convergence of afferents from the SLN and GPN in cat medullary swallowing neurons. Brain Res Bull. 1995;37:397–404. doi: 10.1016/0361-9230(95)00018-6. [DOI] [PubMed] [Google Scholar]
- 45.Sinclair WJ. Initiation of reflex swallowing from the naso- and oropharynx. Am J Physiol. 1970;218:956–960. doi: 10.1152/ajplegacy.1970.218.4.956. [DOI] [PubMed] [Google Scholar]
- 46.Venker-van Haagen AJ, Van den Brom WE, Hellebrekers LJ. Effect of superior laryngeal nerve transection on pharyngeal muscle contraction timing and sequence of activity during eating and stimulation of the nucleus solitarius in dogs. Brain Res Bull. 1999;49:393–400. doi: 10.1016/s0361-9230(99)00067-2. [DOI] [PubMed] [Google Scholar]
- 47.Ertekin C, Tarlaci S, Aydogdu I, et al. Electrophysiological evaluation of pharyngeal phase of swallowing in patients with Parkinson's disease. Mov Disord. 2002;17:942–949. doi: 10.1002/mds.10240. [DOI] [PubMed] [Google Scholar]
- 48.Stedman H, Bradley R, Mistretta C, et al. Chemosensitive responses from the cat epiglottis. Chem Senses. 1980;5:233–245. [Google Scholar]
- 49.Merati AL, Heman-Ackah YD, Abaza M, et al. Common movement disorders affecting the larynx: a report from the neurolaryngology committee of the AAO-HNS. Otolaryngol Head Neck Surg. 2005;133:654–665. doi: 10.1016/j.otohns.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 50.Wakabayashi K, Takahashi H, Ohama E, et al. Parkinson’s disease: an immunohistochemical study of Lewy body-containing neurons in the enteric nervous system. Acta Neuropathol. 1990;79:581–583. doi: 10.1007/BF00294234. [DOI] [PubMed] [Google Scholar]
- 51.Braak H, de Vos RAI, Bohl J, et al. Gastric α-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett. 2006;396:67–72. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 52.Amino T, Orimo S, Itoh Y, et al. Profound cardiac sympathetic denervation occurs in Parkinson disease. Brain Pathol. 2005;15:29–34. doi: 10.1111/j.1750-3639.2005.tb00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Orimo S, Amino T, Itoh Y, et al. Cardiac sympathetic denervation precedes neuronal loss in the sympathetic ganglia in Lewy body disease. Acta Neuropathol. 2005;109:583–588. doi: 10.1007/s00401-005-0995-7. [DOI] [PubMed] [Google Scholar]
- 54.Orimo S, Takahashi A, Uchihara T, et al. Degeneration of cardiac sympathetic nerve begins in the early disease process of Parkinson’s disease. Brain Pathol. 2007;17:24–30. doi: 10.1111/j.1750-3639.2006.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Orimo S, Uchihara T, Nakamura A, et al. Axonal α-synuclein aggregates herald centripetal degeneration of cardiac sympathetic nerve in Parkinson’s disease. Brain. 2008;131:642–650. doi: 10.1093/brain/awm302. [DOI] [PubMed] [Google Scholar]
- 56.Fujishiro H, Frigerio R, Burnett M, et al. Cardiac sympathetic denervation correlates with clinical and pathologic stages of Parkinson’s disease. Mov Disord. 2008;23:1085–1092. doi: 10.1002/mds.21989. [DOI] [PubMed] [Google Scholar]
- 57.Michell AW, Luheshi LM, Barker RA. Skin and platelet a-synuclein as peripheral biomarkers of Parkinson’s disease. Neurosci Lett. 2005;381:294–298. doi: 10.1016/j.neulet.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 58.Miki Y, Tomiyama M, Ueno T, et al. Clinical availability of skin biopsy in the diagnosis of Parkinson's disease. Neurosci Lett. 2010;469:357–359. doi: 10.1016/j.neulet.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 59.Del Tredici K, Braak H. A not entirely benign procedure: progression of Parkinson's disease. Acta Neuropathol. 2008;115:379–384. doi: 10.1007/s00401-008-0355-5. [DOI] [PubMed] [Google Scholar]
- 60.Bloch A, Probst A, Bissig H, et al. α-Synuclein pathology of the spinal and peripheral autonomic nervous system in neurologically unimpaired elderly subjects. Neuropathol Appl Neurobiol. 2006;32:284–295. doi: 10.1111/j.1365-2990.2006.00727.x. [DOI] [PubMed] [Google Scholar]
- 61.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]