Abstract
Rationale
Manipulations of nicotinic cholinergic receptors have been shown to influence both alcohol and nicotine intake. Sazetidine-A [6-(5(((S)-azetidine-2-yl)methoxy)pyridine-3-yl)hex-5-yn-1-ol] is a novel compound that potently and selectively desensitizes α4β2 nicotinic receptors with only modest receptor activation.
Objectives
The goal of the present study was to examine the effects of sazetidine-A on alcohol and nicotine self-administration in alcohol-preferring (P) rats.
Methods
P rats were given the choice of water or alcohol. Once stable baselines were established, the acute (0, 0.1, 0.3, 1, and 3 mg/kg, s.c.) and chronic (3 mg/kg for 10 days) effects of sazetidine-A on alcohol intake were assessed. Naltrexone (2.5 mg/kg) served as a positive control. The effect of sazetidine-A (3 mg/kg) and naltrexone (4 mg/kg) on saccharin (0.2%) preference was also assessed. In addition, the acute effects of sazetidine-A (3 mg/kg) and naltrexone (4 mg/kg) on alcohol intake after alcohol deprivation were evaluated. In another experiment, the effects of sazetidine-A (0, 1, or 3 mg/kg) on IV nicotine self-administration in P and NP rats were assessed.
Results
Sazetidine-A caused a dose-dependent reduction in alcohol intake. Chronic sazetidine-A also effectively reduced alcohol intake until the seventh day of treatment, when partial tolerance appeared to develop. In the post-deprivation study, sazetidine-A significantly reduced alcohol intake and preference. Sazetidine-A at 3 mg/kg significantly reduced nicotine self-administration in both lines.
Conclusions
Sazetidine-A significantly reduced alcohol and nicotine intake in P rats that self-administer higher levels of both drugs. Sazetidine-A may hold promise for the treatment of alcohol and nicotine addiction.
Keywords: Alcoholism, P rats, Nicotinic agonists, Alcohol drinking, Naltrexone, Treatment, Animal model, Saccharin, Nicotine addiction
Introduction
Alcohol dependency and nicotine addiction cause major health, social, and economic problems throughout the world and current treatments for these diseases are far from adequate. Alcohol and nicotine are often co-abused. Approximately, 90% of alcoholics are also addicted to tobacco (Ait-Daoud et al. 2005). Addiction to alcohol and nicotine share some of the same potential neural bases, particularly activation of dopaminergic innervation of the nucleus accumbens (DiChiara and Imperato 1988; Nisell et al. 1994; Tizabi et al. 2007; Volkow et al. 2004; Weiss et al. 1993).
Alcoholism is a complex heterogeneous disease and consequently existing pharmacological tools such as disulfiram (Antabuse®), naltrexone (ReVia®), and acamprosate (Campral®), while efficacious in some (O'Malley et al. 1992; Kenna et al. 2009; Mann et al. 2008), leave many others unaided (Garbutt 2009; Kranzler and van Kirk 2001). Thus, clearly, additional treatment therapies are needed for alcoholism.
With respect to nicotine addiction, currently used treatments result in success for only a minority of people who attempt to quit smoking (Arneric et al. 2007). Although, nicotine replacement therapy improves smoking cessation (Fiore et al. 2008; Levin et al. 1994; Fiore et al. 1994), it is not known whether this is due to nicotinic receptor desensitization or activation of nicotinic receptors. Recently, the α4β2 nicotinic acetylcholine receptor partial agonist varenicline (Chantix®) has been shown to improve smoking cessation rates relative to placebo (Rollema et al. 2007) and bupropion (Jorenby et al. 2006). However, varenicline has been shown to have the potential for significant adverse side effects (Freedman 2007; Morstad et al. 2008). It is possible that at least some of these side effects are related to actions of varenicline at α3β4 and α7 nicotinic receptor subtypes. Using a nicotinic antagonist such as mecamylamine may avoid this problem. Indeed, mecamylamine, has been shown to decrease perception of nicotine levels in tobacco smoke, but it has its own side effects. However, the addition of mecamylamine to nicotine replacement therapy not only did not diminish the effectiveness of nicotine treatment, it actually enhanced its effectiveness (Rose et al. 1998).
An important role for central nicotinic receptors in the reinforcing effects of alcohol has been found. Studies have found that nicotine treatment leads to increased alcohol intake (Lê et al. 2003; Söderpalm et al. 2000); while administration of mecamylamine either systemically (Blomqvist et al. 1996; Lê et al. 2000) or into the ventral tegmental area (VTA) or the nucleus accumbens of rats (Ericson et al. 1998; Söderpalm et al. 2000) markedly reduced alcohol intake and preference. Moreover, voluntary alcohol intake enhances extracellular levels of acetylcholine in the VTA which, upon interacting with VTA nicotinic acetylcholine receptors, stimulates dopamine release in the nucleus accumbens (Larsson et al. 2005). Also, it has been shown that infusion of mecamylamine into the VTA reduced alcohol-induced dopamine release in the nucleus accumbens (Blomqvist et al. 1993; Ericson et al. 1998, 2009; Tizabi et al. 2007). Interestingly, recent studies have demonstrated that administration of varenicline reduces alcohol consumption in rats (Steensland et al. 2007), as well as in heavy-drinking smokers (McKee et al. 2009), suggesting that α4β2 nicotinic receptors may play an important role in alcohol-seeking behaviors. It has been suggested that varenicline reduces the efficacy of acetylcholine activity at nicotinic receptors, leading to a reduction in alcohol intake by decreasing the rewarding properties of alcohol. In fact, recently, it has been shown that varenicline influences alcohol-induced dopamine (DA) activation as well as the interactions between alcohol and nicotine in this respect (Ericson et al. 2009). Thus, it is hypothesized that a compound that can reduce nicotinic receptor activation (for example, through desensitization) without completely blocking the receptor could be useful in helping in improving smoking cessation rates.
Sazetidine-A (Fig. 1), a new compound with selective desensitizing actions on α4β2 nAChRs (and possibly other β2-containing nAChRs) has very high binding affinity for rat α4β2 nAChRs and, more importantly, it is ~10,000-fold and 3,500-fold selective compared to its affinity at rat α3β4 and rat α7 nicotinic receptors, respectively (Xiao et al. 2006; Xiao et al. personal communication). Consistent with its high selectivity for α4β2 receptors in binding assays, sazetidine-A is a much weaker desensitizer of α3β4 and α7 nAChR subtypes (Xiao et al. 2006; Xiao et al., personal communication). Thus, sazetidine-A is a highly selective α4β2 nAChR desensitizer (Xiao et al. 2006). Recently, sazetidine-A has been shown to significantly reduce nicotine self-administration in Sprague–Dawley rats (Levin et al. 2010).
Fig. 1.
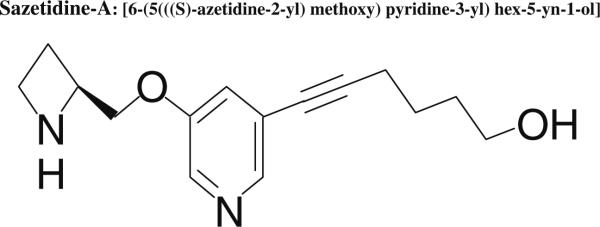
Chemical structure of sazetidine-A, [6-(5(((S)-azetidine-2-yl) methoxy)pyridine-3-yl)hex-5-yn-1-ol]
The goal of this study is to investigate the role of α4β2 nAChRs in alcohol and nicotine intake using a selectively bred alcohol-preferring line of rats (P rats). P rats, in addition to high preference for alcohol (Li et al. 1993; Rezvani et al. 2007, 2009), recently have been shown to have higher rates of nicotine self-administration compared with their alcohol-non-preferring counterpart, NP rats (Lê et al. 2006). It was hypothesized that sazetidine-A, by desensitizing α4β2 nAChRs, would significantly reduce both alcohol and nicotine intake in P rats.
Materials and methods
Animals and housing
Adult male rats obtained from a colony of selectively bred alcohol-preferring (P) and alcohol-non-preferring (NP) lines maintained at University of Indiana School of Medicine, Indianapolis. Rats were housed individually in specialized polycarbonate cages that were fitted with two 100 mL Richter tubes for the recording of water and alcohol (10%, v/v) intake. Animals were kept in a separate room under a constant room temperature of 22±1°C and 12:12 light–dark cycle (7:00 a. m.–7:00 p.m. dark). Animals were fed 5001 Rodent Chow (Lab Diet, Brentwood, MO, USA) and water ad libitum. All procedures were approved by the IACUC at Duke University.
Sazetidine-A effects on alcohol intake
Initiation of alcohol drinking
Alcohol intake was determined using the standard two-bottle choice procedure utilized in our and other laboratories (Murphy et al. 2002; Rezvani et al. 2000). Upon arrival to the lab and after a week of handling and habituation, rats were given free access to water in a graduated Richter tube for 1 day. Next, they were given free access only to a solution of 8% (v/v) alcohol for three consecutive days. During this period, rats became accustomed to drinking from the Richter drinking tube and to the taste and pharmacological effects of alcohol. Thereafter, rats were given free access to water and a solution of 9% alcohol for five consecutive days and then water and a solution of 10% alcohol throughout the study. Water and alcohol intake were indexed by graduated Richter drinking tubes (Rezvani et al. 1993).
Acute sazetidine-A dose–response function
An acute study was first conducted to determine the dose–response for sazetidine-A. After the rats exhibited a stable baseline for alcohol intake over several days, they were injected subcutaneously with 0.1, 0.3 mg/kg of sazetidine-A, the same volume of saline (1 ml/kg) or 2.5 mg/kg naltrexone as a positive control. To expand the dose range, in another set of experiments, rats were injected with 1 or 3 mg/kg sazetidine-A or an equal volume of saline (1 ml/kg). Alcohol and water intake were measured at 1, 2, 4, and 24 h after the drug administration. The preference for alcohol [(alcohol volume)/(alcohol+water volume)×100] was calculated for the same time points as alcohol and water intake (Rezvani et al. 2009). The animal's body weight was recorded immediately before the drug administration and 24 h later. All animals (N=7) received all treatments following a crossover design with random assignment. The interval between injections was at least 3 days.
Chronic sazetidine-A effects on alcohol intake
Sixteen male P were evaluated for alcohol intake, as described above. After establishment of a stable baseline for alcohol and water intake, they were injected with the saline vehicle or 3 mg/kg sazetidine-A (N=8/treatment) for ten consecutive days. Alcohol and water intake were measured at 2, 4, 6, and 24 h after the injection.
Sazetidine-A effects on post-deprivation alcohol consumption
Earlier studies with alcohol-preferring rats in our and other laboratories have shown that these selectively bred animals exhibit rebound increases in their daily alcohol intake when periodically withdrawn from their unlimited access to alcohol (Rezvani et al. 2009). Similar to human conditions, it appears that the “craving” for alcohol is enhanced after a period of alcohol deprivation. This animal model of “relapse” has been used to gauge the potency of anti-craving drugs on suppressing this enhanced craving for alcohol. We used this particular animal model to test the efficacy of sazetidine-A on the post-deprivation model. Three weeks after the termination of the chronic experiment and after re-establishment of a reliable stable baseline for alcohol and water intake, rats were withdrawn from alcohol for four consecutive days while they had free access to food and water. After 4 days of alcohol deprivation and 15 min before the reinstatement of alcohol exposure, rats were injected with 3 mg/kg sazetidine-A, vehicle, or 4 mg/kg naltrexone as a positive control. Alcohol and water intake were measured 1, 2, 4, 6, and 24 h after alcohol reinstatement. All rats went through withdrawal multiple times with treatments in a counter-balanced order. Thus, all animals (N=16) received all treatments following a counter-balanced design with random assignment.
Saccharin study
To determine the selectivity of sazetidine-A effects on alcohol intake, the effects of a high dose of sazetidine-A (3 mg/kg) on preference for saccharin, a palatable rewarding compound, was assessed. For the sake of comparison, the effect of naltrexone on saccharin preference was also determined. Upon the completion of the post-deprivation study, rats were withdrawn from alcohol and were put on two-bottle choice of water and a solution of 0.2% saccharin for ten consecutive days. After the establishment of stable baseline for saccharin intake, rats were administered with the control saline vehicle (N=8), sazetidine-A (3 mg/kg) (N=9), or naltrexone (4 mg/kg) (N=8), and saccharin and water intake were measured at 4, 6, and 24 h after the treatment.
Sazetidine-A effects on nicotine self-administration
A separate group of adult male P and NP rats were used for this study. Before beginning nicotine self-administration, all rats were trained for lever pressing for food reinforcement using dual lever test chambers (Med Associates, Vermont, USA). Using these operant conditioning chambers, rats first were hand-trained to press the levers for food pellet reinforcer. During these tutorial sessions, correct responses were rewarded by the trainer pressing a button, which caused immediate delivery of one 45-mg food pellet and activation of the feedback tone for 0.5 s. Half the animals were reinforced for responding on the right lever, and half for responding on the left. The cue light over the correct lever was illuminated, while the light over the incorrect lever was covered. These tutorial sessions were followed by three daily pellet sessions on a FR-1 schedule. After three training sessions with food reinforcement, rats had catheters and ports (Instech-Solomon, Plymouth Meeting, PA) surgically implanted into the jugular vein to enable them to receive nicotine infusions., The anesthesia was a mixture of ketamine (60 mg/kg) and domitor (15 mg/kg) injected i.p. (Levin et al. 2010). The catheters were connected to the port, which was sutured subcutaneously on their backs for easy access to the tether delivery line. The catheters were flushed daily with a 0.3-ml solution containing 100 U/ml heparinized saline (Baxter Health Corporation, Deerfield, IL) and 8 mg/ml gentamicin (American Pharmaceutical Partners, Shaumburg, IL) as an antibiotic. Following recovery from the surgery, rats were placed in dual lever test chambers for nicotine self-administration. Each chamber was equipped with a tone generator, house light, cue light above each lever, and a stainless steel tether to cover and protect the drug delivery line. A Pentium computer programmed with MED-PC software controlled experimental events and data collection. Each catheter and port was connected to a High Speed Micro-Liter Syringe Pump (MED-Associates, Georgia, VT) with polyethylene tubing for drug delivery. During each session, the rats wore Covance infusion harnesses connected to the stainless steel tethers. The sequence of testing was as follows: three sessions of lever pressing for food reinforcement, and then ten sessions of nicotine reinforcement alone over a period of 2 weeks (five sessions/week). During the nicotine sessions, a lever press on the active side resulted in the activation of the feedback tone for 0.5 s, and the immediate delivery of one 50 μl infusion of nicotine (containing 0.03 mg/kg nicotine base) in less than 1 s. Each infusion was immediately followed by a 1-min timeout in which the cue lights went out, and responses were recorded but not reinforced (Levin et al. 2010).
After the establishment of a reliable baseline for nicotine self-administration, the effects of sazetidine-A (0, 1, and 3 mg/kg) on nicotine self-administration was determined using a repeated measure counter-balanced design. The number of infusions per session (45 min) was assessed with the analysis of variance with dose and repetition phase as factors. Each rat received each dose twice.
Preparation of agents
Solutions of 10% (v/v) alcohol were prepared twice weekly from a solution of 100% ethanol mixed with tap water. Solutions of nicotine bitartrate were prepared weekly in sterilized isotonic saline. The doses of nicotine used were calculated as a function of the nicotine base weight. The pH of the nicotine solution was adjusted to 7.0 using NaOH and then the solution was passed through a 0.22-μm Nalgene filter (Nalgene Nunc International, Rochester, NY) for sterilization. Nicotine solutions were kept refrigerated in the dark between experiments. Solutions of saccharin (0.2% w/v) were prepared with tap water. Sazetidine-A was synthesized according to the method published previously (Xiao et al. 2006). Solutions of sazetidine-A and naltrexone were prepared weekly in sterilized isotonic saline and were injected subcutaneously in a volume of 1 ml/kg, 10 min before testing.
Statistical analysis of data
The data were assessed by the analysis of variance. Drug treatment (sazetidine-A, naltrexone, and saline) was a repeated measures factor. In the nicotine, self-administration study strain (P vs. NP rat) was a between subjects factor. Alcohol intake was calculated as grams per kilogram from volume of 10% (v/v) alcohol consumed. Alcohol preference was calculated as a percent of alcohol consumed over total fluid intake (alcohol+water). Nicotine intake was calculated as number of infusions/session.
Results
Sazetidine-A effects on alcohol intake
Acute dose–response function
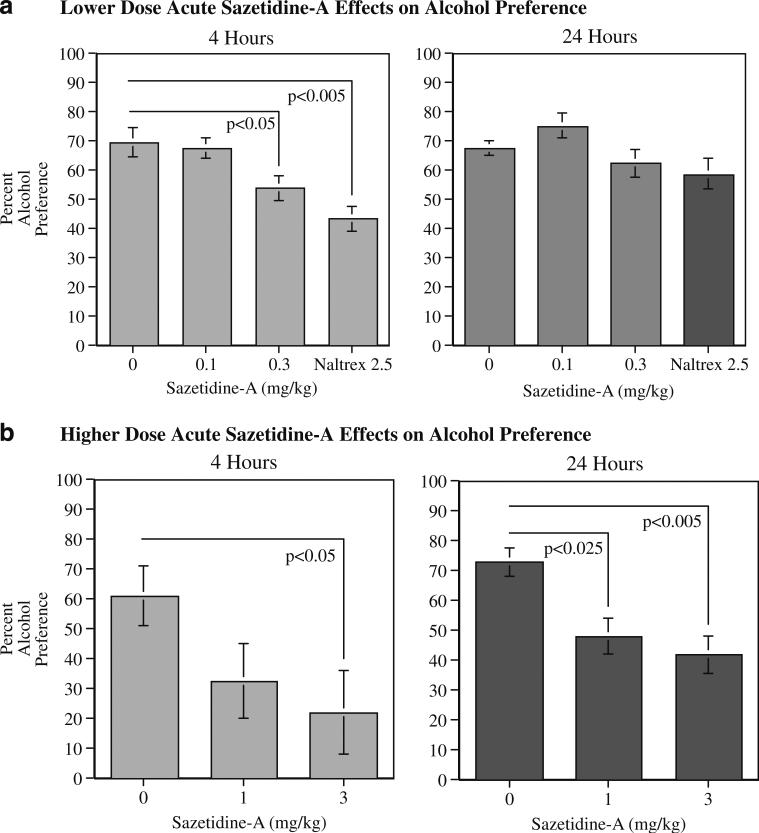
Compared with control vehicle, an acute administration of sazetidine-A significantly and dose-dependently reduced alcohol preference (Fig. 2). The higher dose range of sazetidine-A treatment caused a significant (F(2,12)=5.61, p<0.005) main effect of reducing alcohol preference as measured at the 4 and 24 h time points. Planned comparisons of the treatment effects to control showed that sazetidine-A treatments of 1 mg/kg (p<0.05) and 3 mg/kg (p<0.01) significantly reduced alcohol preference. As shown in Fig. 2b, significant sazetidine-A effects were seen with 3 mg/kg at both the 4 and 24-h post-injection measurements and 1 mg/kg at the 24-h time point (4 h 1 mg/kg NS, 3 mg/kg, p<0.05; 24 h 1 mg/kg, p<0.025, 3 mg/kg p<0.005). The lower doses of sazetidine-A did not show as robust a reduction in alcohol self-administration (Fig. 2a). There was a significant (F(3,18)=5.17, p<0.01) main effect of drug treatment with the lower dose–effect function as well. Neither the 0.1 nor the 0.3-mg/kg sazetidine-A dose caused a significant reduction in alcohol self-administration across the 4 and 24 post-injection, although naltrexone at 2.5 mg/kg did cause a significant (p<0.01) overall reduction in alcohol preference. With the lower dose experiment, there was also a significant (F(3,18)=3.64, p<0.05) interaction of drug treatment×time. The 0.3-mg/kg sazetidine-A dose caused a significant (p<0.05) reduction of alcohol preference at the 4-h post-injection measurement but not at the 24-h time point. The lower 0.1 mg/kg sazetidine-A dose did not cause significant decreases in alcohol preference at either time point. Naltrexone caused a significant reduction in alcohol preference at the 4-h (p<0.005), but not at the 24 h time point.
Fig. 2.
Effect of lower (a) and higher (b) dose acute administration of sazetidine-A on alcohol preference in P rats, 4 and 24 h after injection (mean±SEM), N=7
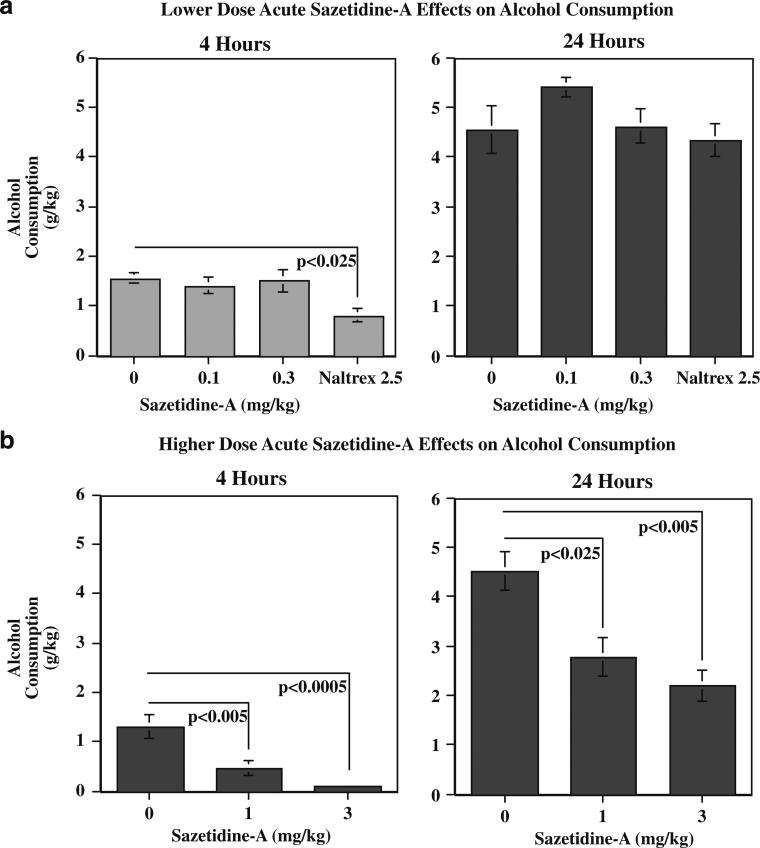
Alcohol intake (grams per kilogram) was similarly affected. Higher-dose sazetidine-A treatment had a significant (F(2,12)=11.14, p<0.005) main effect of reducing alcohol consumption at the 4-h time point. Planned comparisons of the treatment effects to control showed that sazetidine-A treatments of 1 mg/kg (p<0.01) and 3 mg/kg (p<0.001) significantly reduced alcohol consumption over the 4- and 24-h time points. As shown in Fig. 3b, both 1 and 3 mg/kg sazetidine-A doses significantly reduced alcohol consumption at both the 4- and 24-h time points (1 mg/kg 4 h p<0.005, 24 h p<0.025; 3 mg/kg 4 h p<0.0005, 24 h p<0.005). The lower doses of sazetidine-A did not significantly reduce alcohol intake. Naltrexone (2.5 mg/kg) did significantly reduce alcohol intake at the 4-h time point (p<0.025), but not the 24-h tine point (Fig. 3a).
Fig. 3.
Effect of lower (a) and higher (b) dose acute administration of sazetidine-A on alcohol intake (grams per kilogram) in P rats, 4 and 24 h after injection (mean±SEM), N=7
Sazetidine-A did not appear to decrease alcohol consumption merely by sedation or hypodipsia. Water intake was not decreased by any of the treatments. In fact, at the 24-h time point water intake was significantly increased by both 1 mg/kg (36.1±3.4 ml/kg, p<0.025) and 3 ml/kg (39.1±4.7 ml/kg p<0.01) sazetidine compared with control treatment (21.6±4.1 ml) in compensation for the reduced alcohol consumption.
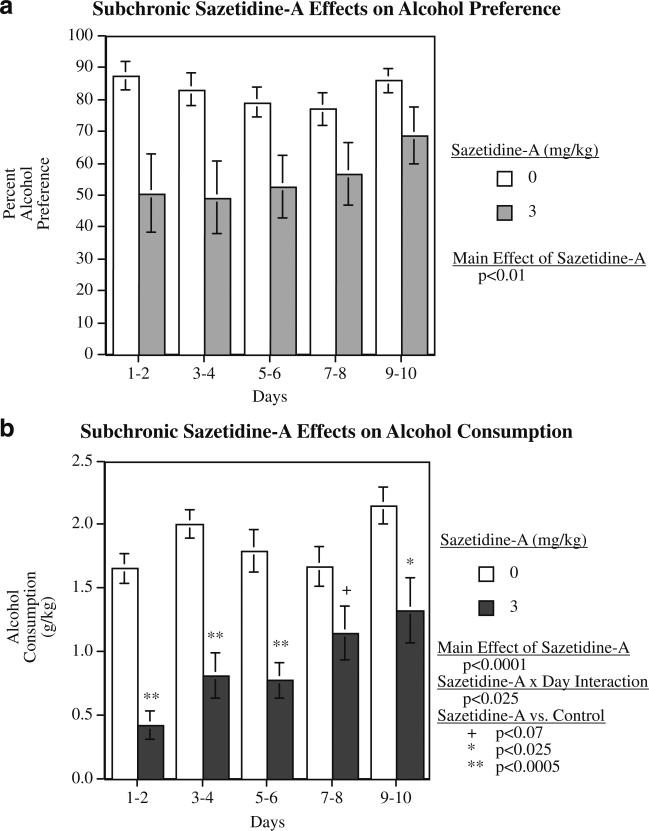
Subchronic sazetidine-A effects on alcohol intake
The effective acute dose of 3 mg/kg of sazetidine-A was tested in the subchronic study with ten consecutive daily injections. This treatment caused a significant decrease in percent alcohol consumption with a significant main effect of sazetidine-A (F(1,14)=9.38, p<0.01). The mean percent alcohol preference over the 10 days of treatment in the sazetidine-A group was 55.6±8.2% compared with 82.5± 3.3% in the vehicle-treated controls. As shown in Fig. 4a, there was a suggestion of an attenuated effect of sazetidine-A over the 10-day period, but the sazetidine-A×days interaction was not significant. After withdrawal of sazetidine-A treatment, there was no longer any detectable effect (control=84.4±3.1% and sazetidine-A=87.4±4.7%).
Fig. 4.
Effect of subchronic administration of sazetidine-A (3 mg/kg) given on ten consecutive days on alcohol preference (a) and alcohol intake (b) at different time points in P rats, 4 h after injection (mean±SEM), N=8/treatment
Alcohol intake (grams per kilogram) was also affected by repeated administration of sazetidine-A. There was a significant (F(1,14)=28.18, p<0.001) main effect of sazetidine-A treatment. At 4-h time point, the controls averaged 1.85±0.10 g/kg of alcohol consumption over the 10-day period, while the rats treated with 3 mg/kg/day of sazetidine-A averaged 0.90±0.15 g/kg over the same time. There was also a significant sazetidine-A×day interaction (F(4,56)=3.12, p<0.025), with effect of sazetidine-A attenuating during the later portion of the 10-day treatment. As shown in Fig. 4b, sazetidine-A over the first 6 days of treatment caused dramatic and very significant (p<0.0005) reductions in alcohol consumption. The reduction in the sazetidine-A group was not quite significant (p<0.07) during sessions 7–8 but was significantly (p<0.026) lower than controls during the final two sessions. After withdrawal from sazetidine-A, alcohol consumption in the sazetidine-A group (2.02±0.21 g/kg/4 h) returned to the control level (1.92±0.08 g/kg/4 h).
As with acute sazetidine-A administration, chronic administration did not decrease water consumption. In fact, water intake was significantly increased from 10.1±1.6 ml/day in controls to 23.4±5.0 ml/day in the sazetidine- (3 mg/kg) treated rats in compensation for the lower amount of alcohol drunk.
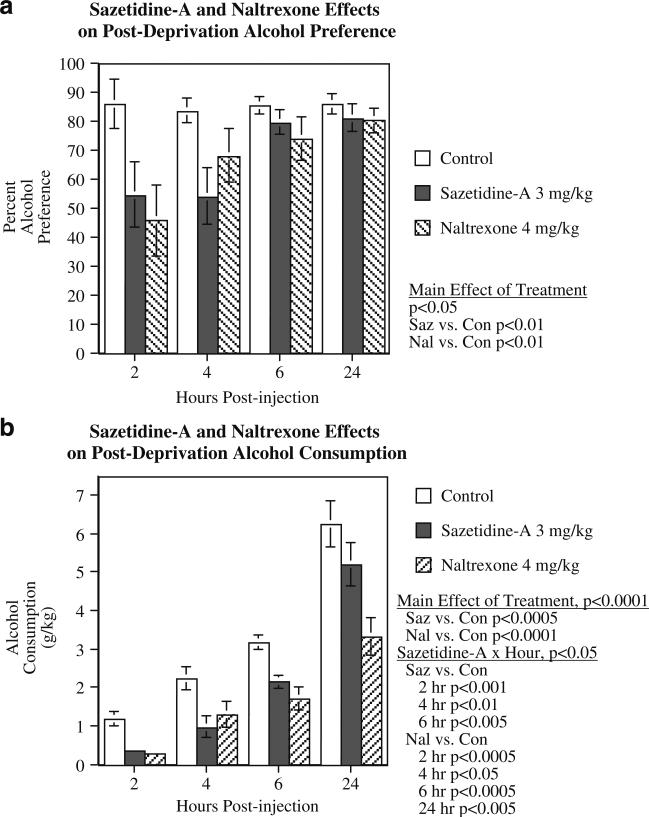
Sazetidine-A effects on post-deprivation alcohol consumption
In the post-deprivation study, there was a significant main effect of treatment (F(2,30)=5.76, p<0.01), with both sazetidine-A (p<0.01) and naltrexone (p<0.01) causing significant decreases in alcohol preference relative to controls (Fig. 5a). The controls averaged 85.1±2.4% ethanol preference, while those treated with sazetidine-A averaged 67.2±5.7%, and those treated with naltrexone averaged 66.2±5.6%. There appeared to be a decrease in effectiveness of the treatments 6 and 24 h after injection, but the interaction of treatment×time was not significant.
Fig. 5.
Effect of sazetidine-A (3 mg/kg) and naltrexone (4 mg/kg) on alcohol preference (a) and alcohol intake (b) after alcohol deprivation (mean±SEM), N=16
Alcohol consumption was also significantly affected by drug treatment during the post- deprivation period (Fig. 5b). There was a significant main effect of treatment (F(2,30)= 20.88, p<0.0001), with both sazetidine-A (p<0.0005) and naltrexone (p<0.0001) causing significant decreases in alcohol consumption relative to controls. There was also a significant treatment×hour interaction (F(6,90)=2.24, p< 0.05). Sazetidine-A treatment caused a significant reduction in alcohol consumption at 2 h (p<0.001), 4 h (p<0.01), and 6 h (p<0.005) post-injection, but not at 24 h post-injection. Naltrexone (4 mg/kg) caused significant reductions in alcohol consumption at 2 h (p<0.0005), 4 h (p<0.05), 6 h (p<0.0005), and 24 h (p<0.005) post-injection. No significant drug effects were seen on water consumption. In the control condition, the rats had consumed 0.2±0.1, 2.7±0.9, 4.1±1.1, and 6.9±2.3 ml/kg of water (mean±SEM) after 2, 4, 6, and 24 h, respectively, while in the 3 mg/kg sazetidine-A condition, they consumed 1.4±0.5, 3.1±1.0, 4.4±1.2, and 6.7±1.6 ml/kg, and in the 4 mg/kg naltrexone condition, they consumed 0.4±0.2, 2.4±0.8, 2.9±2.3, and 5.2±1.3 ml/kg at the same time points.
Sazetidine-A effects on nicotine intake
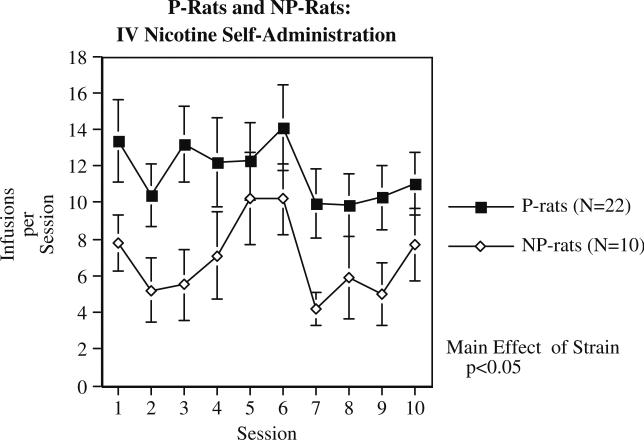
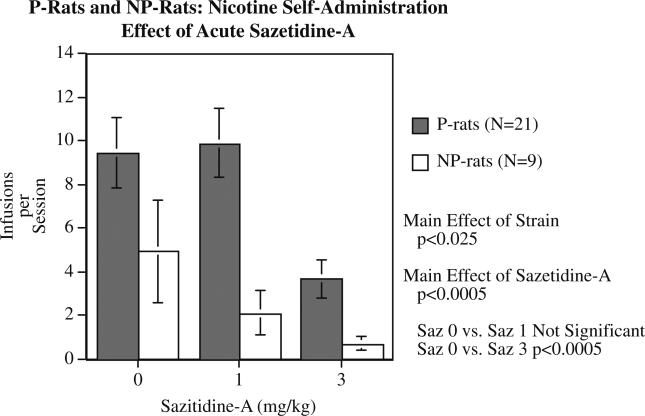
Compared to NP rats, the P rats self-administered significantly (F(1,30)=5.02, p<0.05) more nicotine over ten sessions, with the P rats averaging 11.66±1.33 infusions/session and the NP rats averaging 6.88±1.14 infusions/session (Fig. 6). There were significant effects of strain in all parts of the study. The P rats also self-administered significantly (F(1,30)=8.22, p<0.0.01) greater number of food pellets over three training sessions (P rats=242.0±9.8 and NP rats=196.9±8.8). During the sazetidine-A treatment study, there continued to be a significant main effect of strain (F(1,28)=6.74, p<0.025) with the P rats self-administering more nicotine than the NP rats (Fig. 7). There was also a significant main effect of sazetidine-A (F(2,56)= 10.50, p<0.0005) on nicotine self-administration. Planned comparisons of each sazetidine-A dose to the control treatment showed that the 1 mg/kg dose did not significantly reduce nicotine self-administration, but the 3 mg/kg sazetidine-A dose was effective (p<0.0005) in reducing nicotine self-administration in both P and NP rats. There was not a significant main effect of treatment phase or any significant interactions.
Fig. 6.
IV self-administration of nicotine (0.03 mg/kg/infusion) in alcohol-preferring (P) and alcohol-non-preferring (NP) rats (mean±SEM), N for P rats=22 and for NP rats=10
Fig. 7.
Effect of acute administration of sazetidine-A on nicotine self-administration in P and NP rats (mean±SEM), N for P rats=21 and for NP rats=9
Sazetidine-A effects on saccharin intake and preference
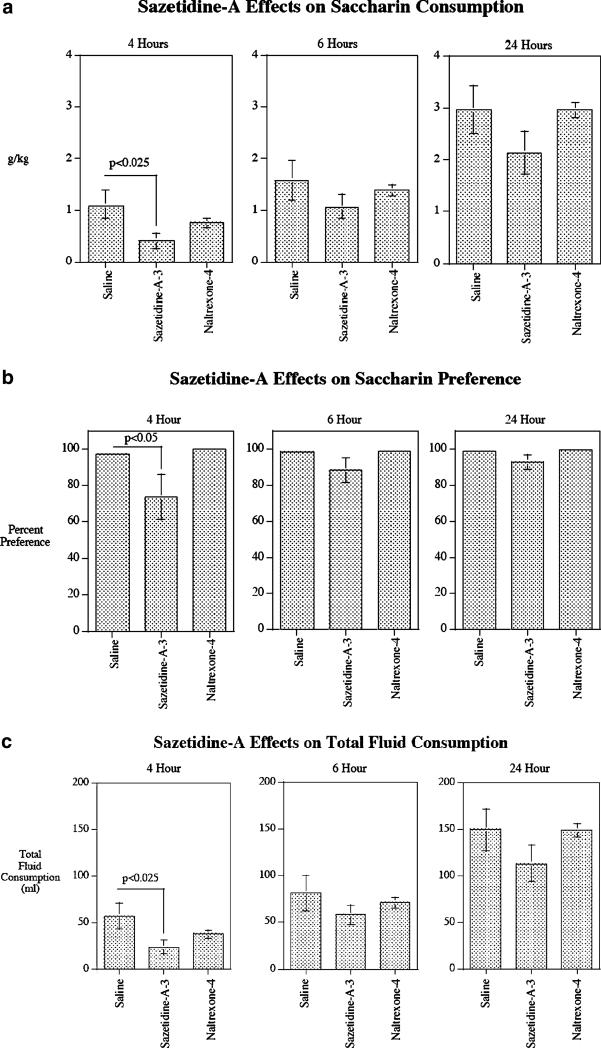
To assess sazetidine-A (3 mg/kg) effects in P rats on another reinforcer in addition to alcohol and nicotine, we tested its effects on preference for saccharin (Fig. 8). The control treatment was injection with the vehicle saline. As in the previous experiments, naltrexone (4 mg/kg) was run for comparison. Sazetidine-A caused a significant (p<0.025) decrease in grams per kilogram of saccharin consumed by 4 h after treatment compared with saline control treatment (control=1.01±0.27 g/kg; Sazetidine-A=0.41±0.15 g/kg). The total fluid consumption was also significantly (p<0.025) lowered by sazetidine-A treatment at the 4-h time point (control=56.3±13.8 ml; sazetidine-A=22.8±7.3 ml). This degree to which saccharin consumption was specifically reduced by sazetidine-A was shown by the percent preference at the 4-h time point in terms of a significant (p<0.05) reduction in percent saccharin vs. water consumption (control=96.9±1.3%; sazetidine-A=73.7±12.2%). No effects of sazetidine-A were seen on saccharin consumption or preference at later time points. No effects of naltrexone were seen at any time points (Fig. 8).
Fig. 8.
Effect of acute administration of sazetidine-A (3 mg/kg) and naltrexone (4 mg/kg) on saccharin intake (grams per kilogram) (a), saccharin preference (b), and total fluid intake (c) at different time points in P rats (mean±SEM), N=9 for sazetidine-A, N=8 for saline control, and N=8 for naltrexone
Discussion
The current study showed that both acute and chronic administration of sazetidine-A, significantly reduced alcohol intake and preference in alcohol-preferring P rats. Even after alcohol post-deprivation, when drinking is significantly enhanced, sazetidine-A significantly reduced the increased alcohol intake. Although the subchronic administration of sazetidine-A significantly reduced alcohol intake and preference initially, partial tolerance developed after the seventh treatment. We and others previously have shown that tolerance also develops with repeated administration of naltrexone and naloxone (Rezvani et al. 2007; Cowen et al. 1999; Overstreet et al. 1999). Presently, it is not clear if the observed partial tolerance is the consequence of the central nicotinic receptor modification or is of pharmacokinetic nature. Experience with naltrexone, which is widely used in the treatment of alcoholism; suggests that partial tolerance development to the suppressant effect of sazetidine-A on alcohol intake in rodents need not be regarded as a barrier to its clinical trial (Table 1).
Table 1.
Effects of subchronic administration of sazetidine-A on alcohol intake (grams per kilogram per day) and alcohol preference
| Days |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Sazetidine-A, 3 mg/kg | ||||||||||
| Alcohol (g/kg/day) | 4.37±.88 | 4.06±.51 | 3.19±.56 | 3.06±.56 | 3.32±.55 | 3.51±.6 | 3.47±.65 | 4.41±.61 | 5.37±.81 | 4.81±.90 |
| Preference (%) | 73±10 | 66±9 | 62±12 | 57±13 | 63±12 | 65±13 | 62±12 | 72±10 | 84±9 | 66±13 |
| Control saline | ||||||||||
| Alcohol (g/kg/day) | 5.62±.32 | 5.97±.41 | 5.77±.17 | 5.21±.33 | 4.43±.50 | 5.69±.65 | 4.99±.53 | 6.06±.43 | 6.26±.41 | 4.96±.98 |
| Preference (%) | 91±3 | 92±2 | 92±2 | 87±4 | 83±4 | 89±3 | 82±4 | 84±7 | 91±2 | 87±3 |
Data represent means±SEM; N=8/group
Regarding the possible sedative effects of sazetidine-A, the significant reduction of percent alcohol preference as a result of an increase in water intake provides assurance that sedation does not account for the sazetidine-A-induced suppressant of alcohol intake. The fact that alcohol intake decreased, and concomitantly water intake increased, suggests that sazetidine does not cause sedation at doses used in these experiments. The brief sazetidine-A-induced reduction in activity seen in the previous studies quickly abated such that there were no detectable effects over a 1-h session. (Cucchiaro et al. 2008; Levin et al. 2010). The mechanism of this short-lasting sedative effect is unclear.
In contrast to the acute effects of sazetidine-A on alcohol intake, which lasted for up to 6 h, its effect on saccharin intake appeared to be transient. Although not conclusive, this observation may suggest the specificity of the compound for nicotine and alcohol. However, further comprehensive studies are needed to study the specificity of this compound on alcohol and nicotine intake. Recently, it was reported that, compared with alcohol-non-preferring (NP) rats, P rats also self-administered greater amounts of nicotine (Lê et al. 2006). We replicated this effect and furthermore showed that sazetidine-A significantly reduced nicotine self-administration in both P and NP rats, extending our earlier findings that sazetidine-A significantly reduced nicotine self-administration in Sprague–Dawley rats (Levin et al. 2010). These findings along with epidemiological and clinical observations suggest that the tendency to use nicotine and alcohol may share a common neuronal mechanism.
Interactions between alcohol and the nicotinic system have been documented for some time. Potthoff et al. (1983) reported that rats implanted subcutaneously with nicotine pellets, consume more alcohol than do controls or animals treated with drugs such as caffeine, phencyclidine, mescaline, or haloperidol. Mecamylamine has been shown to reduce alcohol intake (Farook et al. 2009; Hendrickson et al. 2009), suggesting the involvement of central nicotinic cholinergic receptors in the regulation of alcohol intake. Studies on selectively bred mouse lines also demonstrate a substantial overlap between sensitivity to alcohol and sensitivity to nicotine (Collins 1990). In particular, P rats bred for high alcohol intake are more sensitive to nicotine than the NP rats, bred for low intake, in a drug discrimination procedure (Gordon et al. 1993). Nicotinic receptors in the brain have been implicated in excessive drinking (Li et al. 2007; Söderpalm et al. 2009); thus, nAChR desensitizing treatments may be useful in addiction to both alcohol and nicotine. The VTA and the mesocorticolimbic DA system have been increasingly implicated in reinforcing properties of both nicotine and alcohol (Brody et al. 2009). Central nicotinic receptors in the VTA are involved in mediating the mesolimbic DA, activating and reinforcing effects of alcohol (Ericson et al. 1998; Söderpalm et al. 2009). Both nicotine and alcohol appear to be able to modulate the DA system in general and the mesolimbic pathways in particular through neuronal nicotinic receptors (Blomqvist et al. 1993, 1996; Nisell et al. 1994). Because nicotinic receptors are located on the cell bodies of dopamine cells in the VTA as well as on synaptic endings in the nucleus accumbens, they provide key points of control over the reward circuitry underlying drug reinforcement essential to drug addiction. This localization puts nicotinic receptors at important locations for controlling not only nicotine addiction but also other types of drug addiction such as alcoholism.
Recently, the crucial role of specific nicotinic receptors in the VTA in systemic nicotine self-administration (Pons et al. 2008) and alcohol intake in rats (Kuzmin et al. 2009) has been demonstrated. Several subunits of nAChRs, such as α3, α 5, α7, and β4 have been implicated in alcohol and nicotine effects (Greenbaum et al. 2006; Kamens and Phillips 2008; Rigbi et al. 2008; Schlaepfer et al. 2008). Furthermore, it has been shown that ligands for α3, α6, β2, and β3 subunits influence alcohol-induced DA release in nucleus accumbens and reduce alcohol self-administration in rodents (Löf et al. 2007; Kuzmin et al. 2009). Among all nicotinic receptor subtypes, the α4β2 subtype stands out not only because of its prevalence and distribution in most areas of the brain, but also because it is increased by chronic administration of nicotine in rats and mice and in the brain of smokers. In human smokers, these receptors become virtually saturated even by smoking of a single cigarette (Brody et al. 2006). Therefore, it is likely that most of the α4β2 nicotinic receptors in a smoker's brain are in a state of desensitization during the time the addicted individual is awake and smoking at a typical rate. In fact, as the brain nicotine concentration drops and the increased numbers of desensitized receptors begin to recover function, the resumption of endogenous acetylcholine signaling through even a relatively small percentage of these receptors, may provide the critical neurophysiological cues for the individual to smoke the next cigarette and thus silence those cues by once again desensitizing the receptors. Under this scenario, an addicted individual smokes in part to prevent the negative effects that ensue when the increased number of α4β2 nicotinic receptors returns to functional status (Xiao et al. 2006). It is thus possible to see how a cycle of desensitization/activation/desensitization of strategically located α4β2 nicotinic receptor subtypes could be directly responsible for or at least critically involved in nicotine addiction. Thus, a drug that maintains the α4β2 nicotinic receptors in a desensitized state for a prolonged period would markedly help a motivated individual break the addiction to nicotine.
Although, similar to nicotine itself (Langley and Dickenson 1889; Katz and Thesleff 1957), sazetidine-A has both agonistic and desensitizing effects on α4β2 nicotinic receptors, its desensitizing effects is much more profound (Xiao et al. 2008). Initial activation of α4β2 receptors in cell culture by sazetidine-A lasts less than 1 s, whereas the desensitization which follows activation lasts more than 1 h even with continual washing of the cell (Yasuda et al. 2009). Thus, it is more plausible to suggest that the suppressant effect of sazetidine-A on alcohol and nicotine intake is mostly attributed to its desensitizing effects.
Overall, the current results support the view that nicotinic receptor desensitization, in particular desensitization of α4β2 nicotinic receptor, significantly reduces self-administration of alcohol and nicotine in a rat strain that has high preference for both drugs. Thus, sazetidine-A and similar compounds that desensitize α4β2 nicotinic receptors could be a valuable new approach for the treatment of alcoholism as well as nicotine addiction.
Acknowledgements
The authors thank Alan P. Kozikowski, Sheela K. Chellappan, Krishna Mohan Bajjuri, for synthesizing some of the sazetidine-A samples used in this study. Georgetown University holds patent rights for sazetidine-A, and Drs. Xiao and Kellar are two of the inventors on this patent. This research was supported by an unrestricted grant from Philip Morris USA.
Contributor Information
Amir H. Rezvani, Department of Psychiatry and Behavioral Sciences, Duke University Medical Center, Box 104790, Durham, NC 27710, USA azadi@duke.edu
Susan Slade, Department of Psychiatry and Behavioral Sciences, Duke University Medical Center, Box 104790, Durham, NC 27710, USA.
Cori Wells, Department of Psychiatry and Behavioral Sciences, Duke University Medical Center, Box 104790, Durham, NC 27710, USA.
Ann Petro, Department of Psychiatry and Behavioral Sciences, Duke University Medical Center, Box 104790, Durham, NC 27710, USA.
Lawrence Lumeng, Department of Internal Medicine, University of Indiana, Indianapolis, IN, USA.
Ting-Kai Li, Department of Psychiatry and Behavioral Sciences, Duke University Medical Center, Box 104790, Durham, NC 27710, USA.
Yingxian Xiao, Department of Pharmacology, Georgetown University School of Medicine, Washington, DC, USA.
Milton L. Brown, Department of Drug Discovery Program, Georgetown University School of Medicine, Washington, DC, USA
Mikell A. Paige, Department of Drug Discovery Program, Georgetown University School of Medicine, Washington, DC, USA
Brian E. McDowell, Department of Drug Discovery Program, Georgetown University School of Medicine, Washington, DC, USA
Jed E. Rose, Department of Psychiatry and Behavioral Sciences, Duke University Medical Center, Box 104790, Durham, NC 27710, USA
Kenneth J. Kellar, Department of Pharmacology, Georgetown University School of Medicine, Washington, DC, USA
Edward D. Levin, Department of Psychiatry and Behavioral Sciences, Duke University Medical Center, Box 104790, Durham, NC 27710, USA
References
- Ait-Daoud N, Wiesbeck GA, Bienkowski P, Li MD, Pfutzer SMV, Lesch OM, Johnson BA. Comorbid alcohol and nicotine dependence: from bimolecular basis to clinical consequences. Alc: Clin Exp Res. 2005;29:1541–1549. doi: 10.1097/01.alc.0000174692.20933.49. [DOI] [PubMed] [Google Scholar]
- Arneric SP, Holladay M, Williams M. Neuronal nicotinic receptors: a perspective on two decades of drug discovery research. Biochem Pharmacol. 2007;74:1092–1101. doi: 10.1016/j.bcp.2007.06.033. [DOI] [PubMed] [Google Scholar]
- Blomqvist O, Engel JA, Nissbrandt H, Soderpalm B. The mesolimbic dopamine-activating properties of ethanol are antagonized by mecamylamine. Eur J Pharmacol. 1993;249:207–213. doi: 10.1016/0014-2999(93)90434-j. [DOI] [PubMed] [Google Scholar]
- Blomqvist O, Ericson M, Johnson DH, Engel JA, Soderpalm B. Voluntary ethanol intake in the rat: effects of nicotinic acetylcholine receptor blockade or subchronic nicotine treatment. Eur J Pharmacol. 1996;314:257–267. doi: 10.1016/s0014-2999(96)00583-3. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Olmstead RE, Farahi J, Scheibal D, Jou J, Allen V, Tiongson E, Chefer SI, Koren AO, Mukhin AG. Cigarette smoking saturates brain alpha 4 beta 2 nicotinic acetylcholine receptors. Arc Gen Psychiat. 2006;63:907–915. doi: 10.1001/archpsyc.63.8.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Allen-Martinez Z, Scheibal D, Abrama AL, Costello MR, Farahi J, Saxena S, Monterosso J, London ED. Ventral striatum dopamine release in response to smoking a regular vs a denicotinized cigarette. Neuropsychopharmacology. 2009;34:282–289. doi: 10.1038/npp.2008.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AC. Interactions of ethanol and nicotine at the receptor level. Recent Dev Alcohol. 1990;8:221–231. [PubMed] [Google Scholar]
- Cowen MS, Rezvani AH, Jarrott B, Lawrence AJ. Ethanol consumption by fawn-hooded rats following abstinence: effects of naltrexone and changes in μ-opioid receptors density. Alc: Clin Exp Res. 1999;23:1008–1014. [PubMed] [Google Scholar]
- Cucchiaro G, Xiao Y, Gonzalez-Sulser A, Kellar KJ. Analgesic effects of sazetidine-A, a new nicotinic cholinergic drug. Anesthesiology. 2008;109:512–519. doi: 10.1097/ALN.0b013e3181834490. [DOI] [PubMed] [Google Scholar]
- DiChiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;8514:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson M, Blomqvist O, Engel JA, Söderpalm B. Voluntary ethanol intake in the rat and the associated accumbal dopamine overflow are blocked by ventral tegmental mecamylamine. Eur J Pharmcol. 1998;358:189–196. doi: 10.1016/s0014-2999(98)00602-5. [DOI] [PubMed] [Google Scholar]
- Ericson M, Löf E, Stomberg R, Söderpalm B. The smoking cessation medication varenicline attenuates alcohol and nicotine interactions in the rat mesolimbic dopamine system. J Pharma Exp Ther. 2009;329:225–230. doi: 10.1124/jpet.108.147058. [DOI] [PubMed] [Google Scholar]
- Farook JM, Lewis B, Gaddis JG, Littleton JM, Barron S. Effects of mecamylamine on alcohol consumption and preference in male C57BL/6J mice. Pharmacology. 2009;83:379–384. doi: 10.1159/000219488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore MC, Smith SS, Jorenby DE, Baker TB. The effectiveness of the nicotine patch for smoking cessation—a meta-analysis. JAMA. 1994;271:1940–1947. [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, Dorfman SF, Froelicher ES, Goldstein MG, Healton CG, Henderson PN, Heyman RB, Koh HK, Kottke TE, Lando HA, Mecklenburg RE, Mermelstein RJ, Mullen PD, Orleans CT, Robinson L, Stitzer ML, Tommasello AC, Villejo L, Wewers ME. Clinical practice guideline: treating tobacco use and dependence: 2008 update. Department of Health and Human Services; 2008. [Google Scholar]
- Freedman R. Exacerbation of schizophrenia by varenicline. Am J Psychiat. 2007;164:169. doi: 10.1176/appi.ajp.2007.07020326. [DOI] [PubMed] [Google Scholar]
- Garbutt JC. The state of pharmacotherapy for the treatment of alcohol dependence. J Subst Abuse Treat. 2009;36:15–23. [PubMed] [Google Scholar]
- Gordon TL, Meehan SM, Schechter MD. P and NP rats respond differently to the discriminative stimulus effects of nicotine. Pharmacol Biochem Behav. 1993;45:305–308. doi: 10.1016/0091-3057(93)90243-m. [DOI] [PubMed] [Google Scholar]
- Greenbaum L, Kanyas K, Karni O, Merbl Y, Olender T, Horowitz A, Yakir A, Lancet D, Ben-Asher E, Lerer B. Why do young women smoke? I. Direct and interactive effects of environment, psychological characteristics and nicotinic cholinergic receptor genes. Mol Psychiatry. 2006;11:312–322. doi: 10.1038/sj.mp.4001774. [DOI] [PubMed] [Google Scholar]
- Hendrickson LM, Zhao-Shea R, Tapper AR. Modulation of ethanol drinking-in-the-dark by mecamylamine and nicotinic acetylcholine receptor agonists in C57BL/6J mice. Psychopharmacology. 2009;204:563–572. doi: 10.1007/s00213-009-1488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, KE KE, Billing CB, Gong J, Reeves KR, Group VPS. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Kamens HM, Phillips TJ. A role for neuronal nicotinic acetylcholine receptors in ethanol-induced stimulation, but not cocaine- or methamphetamine-induced stimulation. Psychopharmacology. 2008;196:377–387. doi: 10.1007/s00213-007-0969-7. [DOI] [PubMed] [Google Scholar]
- Katz B, Thesleff S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957;138:63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenna GA, Lomastro TL, Schiesl A, Leggio L, Swift RM. Review of topiramate: an antiepileptic for the treatment of alcohol dependence. Current Drug Abuse Reviews. 2009;2:135–142. doi: 10.2174/1874473710902020135. [DOI] [PubMed] [Google Scholar]
- Kranzler JH, Van Kirk J. Efficacy of naltrexone and acamprosate for alcoholism treatment: a meta-analysis. Alc Clin Exp Res. 2001;25:1335–1341. [PubMed] [Google Scholar]
- Kuzmin A, Jerlhag E, Liljequist EJ. Effects of subunit selective nACh receptors on operant ethanol self-administration and relapse-like ethanol-drinking behavior. Psychopharmacology. 2009;203:99–108. doi: 10.1007/s00213-008-1375-5. [DOI] [PubMed] [Google Scholar]
- Langley JH, Dickenson WL. On the local paralysis of the peripheral ganglia and on the connexion of different classes of nerve fibers with them. Proc Royal Soc. 1889;46:423–431. [Google Scholar]
- Larsson A, Edström L, Svensson L, Söderpalm B, Engel JA. Voluntary ethanol intake increases extracellular acetylcholine levels in the ventral tegmental area in the rat. Alcohol Alcohol. 2005;40:349–358. doi: 10.1093/alcalc/agh180. [DOI] [PubMed] [Google Scholar]
- Lĕ AD, Corrigall WA, Harding JW, Juzytsch W, Li TK. Involvement of nicotinic receptors in alcohol self-administration. Alcohol Clin Exp Res. 2000;24:155–163. doi: 10.1111/j.1530-0277.2000.tb04585.x. [DOI] [PubMed] [Google Scholar]
- Lĕ AD, Wang A, Harding S, Juzytsch W, Shaham Y. Nicotine increases alcohol self-administration and reinstates alcohol seeking in rats. Psychopharmacology. 2003;168:216–221. doi: 10.1007/s00213-002-1330-9. [DOI] [PubMed] [Google Scholar]
- Lê AD, Li Z, Funk D, Shram M, Li TK, Shaham Y. Increased vulnerability to nicotine self-administration and relapse in alcohol-naive offspring of rats selectively bred for high alcohol intake. J Neurosci. 2006;26:1872–1879. doi: 10.1523/JNEUROSCI.4895-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Westman EC, Stein RM, Carnahan E, Sanchez M, Herman S, Behm FM, Rose JE. Nicotine skin patch treatment increases abstinence, decreases withdrawal symptoms and attenuates rewarding effects of smoking. J Clin Psychopharmacol. 1994;14:41–49. [PubMed] [Google Scholar]
- Levin ED, Rezvani AH, Xiao Y, Slade S, Cauley M, Wells C, Hampton D, Petro A, Rose JE, Brown ML, Paige MA, McDowell BE, Kellar KJ. Sazetidine-A, a selective α4β2 nicotinic receptor desensitizing agent and partial agonist, reduces nicotine self-administration in rats. J Pharmacol Exp Ther. 2010;332:933–939. doi: 10.1124/jpet.109.162073. [DOI] [PubMed] [Google Scholar]
- Li TK, Lumeng L, Doolittle DP. Selective breeding for alcohol preference and associated response. Behv Genet. 1993;23:163–170. doi: 10.1007/BF01067421. [DOI] [PubMed] [Google Scholar]
- Li TK, Volkow ND, Baler RD, Egli M. The biological bases of nicotine and alcohol co-addiction. Biol Psychiatry. 2007;61:1–3. doi: 10.1016/j.biopsych.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Löf E, Olausson P, deBejczy A, Stomberg R, McIntosh JM, Taylor JR, Söderpalm B. Nicotine acetylcholine receptors in the ventral tegmental area mediate the dopamine activating and reinforcing properties of ethanol cues. Psychopharmacology. 2007;195:333–343. doi: 10.1007/s00213-007-0899-4. [DOI] [PubMed] [Google Scholar]
- Mann K, Kiefer F, Spanagel R, Littleton J. Acamprosate: recent findings and future research directions. Alc: Clin Exp Res. 2008;32:1105–1110. doi: 10.1111/j.1530-0277.2008.00690.x. [DOI] [PubMed] [Google Scholar]
- McKee SA, Harrison EL, O'Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, Picciotto MR, Petrakis IL, Estevez N, Balchunas E. Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol Psychiatry. 2009;66:185–190. doi: 10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morstad AE, Kutscher EC, Kennedy WK, Carnahan RM. Hypomania with agitation associated with varenicline use in bipolar II disorder. Ann Pharmacother. 2008;42:288. doi: 10.1345/aph.1K511. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, Lumeng L, Li TK. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav Genet. 2002;312:363–388. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- Nisell M, Nomikos GG, Svensson TH. Systemic nicotine-induced dopamine release in the rat nucleus accumbens is regulated by nicotinic receptors in the ventral tegmental area. Synapse. 1994;16:36–44. doi: 10.1002/syn.890160105. [DOI] [PubMed] [Google Scholar]
- O'Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B. Naltrexone and coping skills therapy for alcohol dependence: a controlled study. Arch Gen Psychiatry. 1992;49:881–887. doi: 10.1001/archpsyc.1992.01820110045007. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Kampov-Polevoy AB, Rezvani AH, Braun C, Bartus RB, Crews F. Suppression of alcohol intake by chronic naloxone treatment in P rats: tolerance development and elevation of opiate receptor binding. Alco: Clin Exp Res. 1999;23:1761–1771. [PubMed] [Google Scholar]
- Pons S, Fattore L, Cossu G, Tolu S, Porcu E, McIntosh JM, Changeux U, Maskos JP, Fratta W. Crucial role of a4 and a6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. J Neurosci. 2008;28:12318–12327. doi: 10.1523/JNEUROSCI.3918-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potthoff AD, Elison G, Nelson L. Ethanol intake increases during continuous administration of amphetamine and nicotine but not several other drugs. Pharmacol Biochem Behav. 1983;18:489–493. doi: 10.1016/0091-3057(83)90269-1. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Grady DR, Pucilowski O. Nimodipine, a Ca2+ channel antagonist, attenuates alcohol preference in alcohol preferring rats. Drugs Dev. 1993;2:143–151. [Google Scholar]
- Rezvani AH, Overstreet DH, Mason GA, Janowsky DS, Hamedi M, Jr C, Ying Y. Combination pharmacotherapy: a mixture of small doses of naltrexone, fluoxetine, and a TRH analog reduces alcohol intake in three strains of alcohol preferring rats. Alcohol Alcohol. 2000;35:76–83. doi: 10.1093/alcalc/35.1.76. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Overstreet DH, Levin ED, Rosenthal DI, Kordik CP, Reitz AB, Vaidya AH. The effects of atypical anxiolytic N-phenyl-2-[1-[3-(2-pyridinylethynyl)benzoyl]-4-piperidine]acet-amide (JNJ-5234801) on alcohol intake in alcohol-preferring P rats: comparison with naltrexone. Alc: Clin Exp Res. 2007;31:57–63. doi: 10.1111/j.1530-0277.2006.00264.x. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Overstreet DH, Vaidya AH, Zhao B, Levin ED. Carisbamate, a novel antiepileptic candidate compound, attenuates alcohol intake in alcohol preferring rats. Alc: Clin Exp Res. 2009;33:1366–1373. doi: 10.1111/j.1530-0277.2009.00966.x. [DOI] [PubMed] [Google Scholar]
- Rigbi A, Kanyas K, Yakir A, Greenbaum L, Pollak Y, Ben-Asher E, Lancet D, Kertzman S, Lerer B. Why do young women smoke? V. Role of direct and interactive effects of nicotinic cholinergic receptor gene variation on neurocognitive function. Genes Brain Behav. 2008;7:164–172. doi: 10.1111/j.1601-183X.2007.00329.x. [DOI] [PubMed] [Google Scholar]
- Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, Lu Y, Mansbach RS, Mather RJ, Rovetti CC, Sands SB, Schaeffer E, Schulz DW, Tingley FD, 3rd, Williams KE. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52:985–994. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC. Nicotine–mecamylamine treatment for smoking cessation: the role of pre-cessation therapy. Exp Clin Psychopharmacology. 1998;6:331–343. doi: 10.1037//1064-1297.6.3.331. [DOI] [PubMed] [Google Scholar]
- Schlaepfer IR, Hoft NR, Collins AC, Corley RP, Hewitt JK, Lessem HCJ, JM McQueen MB, Rhee SH, Ehringer MA. The CHRNA5/A3/B4 gene cluster variability as an important determinant of early alcohol and tobacco initiation in young adults. Biol Psychiatry. 2008;63:1039–1046. doi: 10.1016/j.biopsych.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderpalm B, Ericson M, Olausson P, Blomqvist O, Engel JA. Nicotinic mechanisms involved in the dopamine activating and reinforcing properties of ethanol. Behav Brain Res. 2000;113:85–96. doi: 10.1016/s0166-4328(00)00203-5. [DOI] [PubMed] [Google Scholar]
- Söderpalm B, Lof E, Ericson M. Mechanistic studies of ethanol's interaction with the mesolimbic dopamine reward system. Pharmacopsychiatry. 2009;42(Suppl 1):S87–S94. doi: 10.1055/s-0029-1220690. [DOI] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Holgate J, Richards JK, Barlett SE. Varenicline, an α4β2 nicotinic acetylcholine receptors partial agonist, selectively decreases ethanol consumption and seeking. PNAS. 2007;104:12518–12523. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizabi Y, Bai L, Copeland RL, Jr, Taylor RE. Combined effects of systemic alcohol and nicotine on dopamine release in the nucleus accumbens shell. Alcohol Alcohol. 2007;42:413–416. doi: 10.1093/alcalc/agm057. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry. 2004;9:557–569. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–258. [PubMed] [Google Scholar]
- Xiao Y, Fan H, Musachio JL, Wei ZL, Chellappan SK, Kozikowski AP, Kellar KJ. Sazetidine-A, a novel ligand that desensitizes alpha4beta2 nicotinic acetylcholine receptors without activating them. Mol Pharmacol. 2006;70:1454–1460. doi: 10.1124/mol.106.027318. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Yasuda RP, Sahibzada N, Horton L, DiPietro JR, Iwueze AF, Paige MA, McDowell BE, Brown ML, Wolfe BB, Kellar KJ. Pharmacological properties of sazetidine-A, a selective ligand of α4β2 nicotinic acetylcholine receptors. Proceedings of the 38 Annual Meeting of the Society for Neuroscience; Washington DC. 2008. [Google Scholar]
- Yasuda RP, Xiao Y, Sahibzada N, McDowell BE, Paige MA, Brown ML, Wolfe BB, Kellar KJ. Desensitization and time course of recovery of α4β2 nicotinic receptors after brief exposure to agonists and partial agonists. Proceedings of the 39 Annual Meeting of the Society for Neuroscience; Chicago, IL. 2009. [Google Scholar]