Abstract
Background
Many anaesthetics when given to young animals cause cell death and learning deficits that persist until much later in life. Recent attempts to compare the relative safety or toxicity between different agents have not adequately controlled for the relative dose of anaesthetic given, thereby making direct comparisons difficult.
Methods
Isoflurane or sevoflurane were given at 1 minimum alveolar concentration (MAC) for 4 h to postnatal day 7 (P7) rat pups. Beginning at P75 these animals underwent fear conditioning and at P83 Morris water maze testing to assess working memory, short-term memory and early long-term memory using delays of 1 min, 1 h, and 4 h.
Results
No difference between groups was seen in fear conditioning experiments. Morris water maze learning was equivalent between groups, and no difference was seen in working memory. Sevoflurane-treated animals had a deficit in early long-term memory, and isoflurane-treated animals had a deficit in both short-term and early long-term memory.
Conclusions
Both isoflurane and sevoflurane delivered at 1 MAC for 4 h to immature rats caused a deficit in long-term memory. Isoflurane also caused a deficit in short-term memory. Isoflurane might be more detrimental than sevoflurane in very young animals.
Keywords: animals, isoflurane toxicity, memory drug effects, newborn, sevoflurane toxicity
Editor's key points.
Agent-specific differences in developmental neurotoxicity would be of great interest in minimizing adverse outcomes after general anaesthesia in immature animals.
Equipotent isoflurane and sevoflurane administered to neonatal rats resulted in neurocognitive deficits in young adulthood.
Both agents impaired long-term memory, whereas isoflurane also impaired short-term memory, consistent with agent-specific neurotoxicity profiles.
Every year, millions of children receive general anaesthesia for surgical and diagnostic procedures.1–3 There is strong evidence that anaesthesia kills brain cells in the developing mammalian brain, including that of primates,4–8 and causes long-term neurocognitive dysfunction.4,6–8 This has led to concern over the safety of anaesthesia administration in young children both in the anaesthesia community and among the general public.9
The relative toxicities of commonly used inhaled anaesthetics are not well known. Recent studies have attempted to compare equipotent doses of the inhaled anaesthetics isoflurane and sevoflurane and their effects on neuroapoptosis and neurocognitive outcome in neonatal rodents. A critical prerequisite of any comparative neurotoxicity study is equipotency. In a recent study, isoflurane, sevoflurane, and desflurane caused a similar extent and distribution of neuroapoptosis.10 A second study11 found that desflurane significantly impaired working memory in mice, while isoflurane and sevoflurane did not. Desflurane, isoflurane, and sevoflurane all impaired long-term memory and induced neuroapoptosis in similar anatomic patterns, although desflurane caused greater levels of neuroapoptosis in some regions.11 In both studies,10,11 less sevoflurane was used than isoflurane primarily because of a lack of agreement on how equipotency should be achieved in immature rodents.
Minimum alveolar concentration (MAC) is a well-accepted measure of anaesthetic potency. MAC is defined as the minimum amount of inhaled anaesthetic necessary to keep an animal from responding by gross movement to a painful stimulus.12,13 Anaesthetic depth is in turn expressed as a ratio of alveolar concentration of anaesthetic to MAC.12 MAC in immature rodents is not one constant anaesthetic concentration but rather decreases steadily over time with increasing duration of anaesthesia.11,14 This is unlike MAC in adult rodents which is constant over time.15
The two previously published comparative studies of anaesthetic neurotoxicity in immature rodents10,11 did not achieve equipotency, because both used constant alveolar concentrations of each anaesthetic, thereby making a direct comparison uncertain.16,17 It has been argued that the decrease of MAC over time is an artifact caused by environmental factors associated with anaesthesia in immature rodents, such as hypercarbia and acidosis.18 If so, one would expect MAC to be lowest when these environmental conditions are most pronounced, which in our hands is 1 h after induction of general anaesthesia.19 However, by the time MAC reaches a minimum 4 h after induction of general anaesthesia, hypercarbia, and acidosis have largely resolved. Furthermore, Kodama and colleagues,11 using a different methodology in immature mice, arrived at the same conclusion, that MAC decreases with increasing duration of anaesthesia.
Here, we compare the effects of two inhaled anaesthetics, isoflurane and sevoflurane, at true equipotent MAC levels, using a neurocognitive outcome. We then examined long-term neurocognitive outcome via two neurobehavioural tests: Pavlovian fear conditioning to examine hippocampal and amygdala dependent aspects of memory20 and the Morris water maze to examine spatial working memory, short-term memory, and early long-term memory.21–23
Methods
Animals
All experiments were conducted with approval from the Institutional Animal Care and Use Committee at the University of California, San Francisco. Sprague-Dawley dams were purchased from the Charles River Laboratories (Gilroy, CA, USA). Male pups were randomized at postnatal day 7 (P7) into one of three treatment groups. At P16 animals were housed on a 12 h reverse light/dark cycle with ad libitum access to food and water. All animals were kept in standard housing and maintained an average body weight of 454 g over the testing period (range, 333–592 g).
Anaesthesia
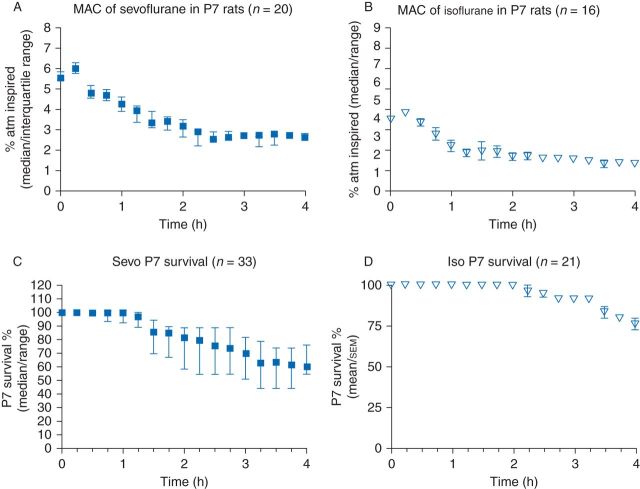
Anaesthesia was conducted as described previously.16,19 Rats in the anaesthetized groups received a 4 h session of either sevoflurane or isoflurane in air and oxygen  . MAC of sevoflurane and isoflurane were determined by tail clamping every 15 min (Fig. 1a and b). 33 pups were anaesthetized with sevoflurane, and 20 survived to enter the behavioural experiments described below (Fig. 1c). Twenty-one pups were anaesthetized with isoflurane, and 16 survived and underwent behavioural testing (Fig. 1d).
. MAC of sevoflurane and isoflurane were determined by tail clamping every 15 min (Fig. 1a and b). 33 pups were anaesthetized with sevoflurane, and 20 survived to enter the behavioural experiments described below (Fig. 1c). Twenty-one pups were anaesthetized with isoflurane, and 16 survived and underwent behavioural testing (Fig. 1d).
Fig 1.
MAC and survival during sevoflurane and isoflurane anaesthesia in rats on P7. Rats were exposed to sevoflurane (n=33) or isoflurane (n=21) on P7. As determined by tail clamping, anaesthetic concentration was adjusted to MAC as described.14,24 MAC of sevoflurane during three exposures (a) and isoflurane during two exposures (b). Survival of P7 rats over 4 h of sevoflurane (c) and isoflurane (d).
During sevoflurane and isoflurane sessions, control rats were concurrently placed in an anaesthesia glove box of the same materials and identical conditions, except they were not exposed to anaesthetic agents, did not undergo tail clamping, and did not have temperature probes inserted. Temperature of the skin over the skull was measured with a laser infrared thermometer immediately upon removal from the dam and entry into the glove box and periodically thereafter to assure that temperature remained normal. When measured in this way P7 rats had a temperature of 34°C which remained constant.
Behavioural tests
All animals were handled for 1 week before testing. They were tested during their dark cycle in a pseudorandom order, and tests were conducted by experimenters blind to treatment groups.
Fear conditioning
Apparatus
From P75–P80, trace fear conditioning tests were conducted in chambers (length, 32 cm; width, 25 cm; and height, 25 cm) constructed of clear acrylic and equipped with a speaker, camera, grid floor (19 stainless steel rods, 4 mm in diameter, and spaced 16 mm in the centre), a stainless steel drop pan, and a shock delivery system (Med Associates, St Albans, VT, USA). Before and after each session, chambers were wiped with a 5% solution of pine-scented cleaner (Pine-Sol; Clorox Company, Oakland, CA, USA). A single 30-watt red bulb lit the room, and a ventilation fan provided background noise (65 dB). Experimenters observed four animals at a time, counterbalanced for group assignment.
Habituation and training
On Day 1, at P75, animals were given 20 min of free exploration in the chamber. On Day 2, animals returned to the same chamber for one long trace fear conditioning session (four trials). Each trial consisted of a tone (16 s, 2 kHz, 100 dB), paired with a shock through the grid floor (2 s, 1 mA) separated by a stimulus-free trace interval (18 s). A 3 min baseline preceded the presentation of the four trials, and each trial was separated by 3 min intervals.
Freezing responses (the absence of all but respiratory movement) were recorded for each rat and used to measure learned fear. Three experimenters, blinded to group assignment, assessed each animal's freezing behaviour for 2 s every 8 s during the entire training period, and a percentage was calculated using the formula 100fn−1, where f is the number of complete 2 s freezing events per rat, and n is the total number of observations per rat.
Testing
On Day 3, rats were tested for context fear acquisition and for tone fear acquisition. For the context test, each rat returned to the chamber for 8 min with the exact same training conditions, excluding the presentation of shock and tone. For the tone test, each rat was transported in a plastic pot (height, 14 cm; diameter, 15.5 cm) to a different context. The chambers were isosceles triangular prisms, constructed of clear acrylic panels (floor, 28×25 cm; 2 sidewalls angled 45° to the floor, 28×22 cm). Before and after each session, chambers were wiped with acetic acid (1%; Fisher Scientific, St Louis, MO, USA). After a 3 min baseline, rats were presented with three 30 s tones (2000 Hz, 100 dB) separated by 1 min intervals. One minute after the last tone, rats were removed from the chamber. The order of the context and tone tests were counterbalanced so that half of each treatment group was tested to the context and then to the tone and vice versa. Freezing behaviour during the testing periods was assessed as described previously.
Morris water maze
Water maze tests were performed after methods previously described by Shih and colleagues24 with a few modifications. Overtraining sessions were administered, and first trial data were used for analysis instead of an average of the three trials.
Working memory
From P83–P101, subjects underwent working memory testing in the Morris water maze. Rats were placed in a circular pool of warm (24°C) opaque water. An escape platform (diameter, 10.3 cm) was submerged 1.27 cm below the water surface, and external cues surrounded the pool to orient the rat as it navigated within the pool. Each day, one session was administered, and one of the eight possible platform locations was used.19,24 A session consisted of a 60 s free swim (performance not scored), during which the rat was allowed to explore the maze, and three subsequent scored trials, with a 1 min interval between the free swim and scored trials. If the rat found the platform during the free swim, it was allowed to remain on the platform for 15 s. If the rat did not locate the platform during the free swim, it was guided to the platform where it remained for 15 s. After the free swim, three trials were administered, in which the rat was released from one of the eight pseudorandomly chosen locations facing the wall of the tank.19,24 The drop location was pseudorandomly varied to incorporate one short, one medium, and one long swim. Each trial continued for 90 s or until the rat had ascended the platform. If the rat did not locate the platform within 90 s, it was guided to the platform. In both cases, the rat was removed after 15 s on the platform. After the three trials, the rat was removed, dried off with a towel, and returned to the home cage. Time to reach the platform (latency), path length, swimming speed, and time-integrated distance to the platform were recorded using EthoVision® video-tracking system (Noldus Instruments, Wageningen, Holland) to analyse 10 samples s−1. Training sessions were administered until group medians for latency were less than 15 s during the first trial (Session 7). Subsequently, eight overtraining sessions were administered utilizing each of the eight escape platform locations.
Short-term and early long-term memory
After rats achieved the predefined performance criterion (locating the hidden platform in under 15 s during the first trial) and completed the eight overtraining sessions, a 1 min interval was administered (Session 16), and then increasing delays were introduced (Sessions 17 and 18). On P100, the delay was increased to 1 h, which tests short-term memory,22 and on P101, the delay was extended to 4 h during which short-term memory is no longer operant and early long-term memory has been established.22 Performance on the first trial swim after the free swim on P99 (1 min delay), P100 (1 h delay), and P101 (4 h delay) was used as a measure of working memory, short-term memory, and early long-term memory, respectively.
Statistical analysis
Fear conditioning data that did not meet parametric assumptions, revealed by D'Agostino and Pearson's normality test, were expressed as medians and interquartile ranges and were analysed using Kruskall–Wallis’ test with Dunn's multiple comparisons test. Here, the effect size is reported as the rank-sum difference. Data that met parametric assumptions were expressed as means and 95% confidence intervals (CIs) and analysed by one-way analysis of variance (anova) with Bonferroni's post hoc correction when groups were compared at a single time-point and repeated measures two-way anova with Bonferroni's post hoc correction when groups were compared over multiple time points. For the anova analyses, the effect size is reported as the difference in means and 95% CI of the difference in means.
Water maze data were analysed using repeated measures two-way anova with Bonferroni's post hoc correction and expressed as means and 95% CIs. In the short-term and early long-term memory tasks, measures of latency, path length, and time-integrated distance to the platform were all highly positively correlated (Spearman's rank correlation coefficient was at least r=0.91, P<0.001 within groups). As the performance measures correlated closely and the swim speeds did not differ between groups, water maze data are only reported as latencies to the hidden platform for clarity and economy.
All comparisons were run as two-tailed tests. P<0.05 was considered statistically significant. All analyses were performed using GraphPad Prism for Mac version 5.0 or 6.0 (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Fear conditioning
Four rats (two control and two sevoflurane-treated rats) fell asleep during the conditioning day, and their data were excluded. All other trials in all experiments were included.
Conditioning
All subjects exhibited near-zero baseline freezing before the tone/shock presentation with no significant differences across groups (Fig. 2a; P = 0.15, Kruskal–Wallis test). Post-shock freezing did not differ significantly across groups over the course of the four tone/shock pairings, and there was no interaction detected between the treatment groups and the tone/shock pairings [Fig. 2b; treatment F(2,49) = 0.15, P = 0.87; shock F(3,147) = 9.9, P < 0.0001; interaction (treatment × shock) F(6,147) = 0.70, P = 0.65, repeated measures two-way anova].
Fig 2.
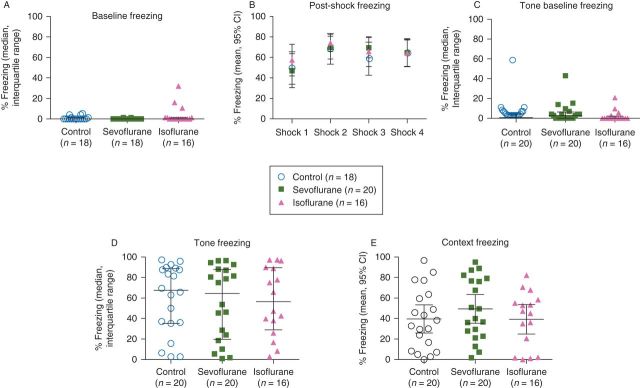
Fear conditioning: freezing was assessed before the first of four tone/shock pairings (a) and immediately after (b). Tone and shock were separated by a stimulus-free 18 s trace interval. Twenty-four hour post-conditioning, freezing was measured before re-exposure to the tone in a novel context (c) and after exposure to the tone (d). Freezing was also assessed in the original context, in the absence of the tone and shock (e).
Tone test
There was no effect of anaesthesia on freezing either before or in response to the tone 24 h post-conditioning (Fig. 2c and d; P=0.25 and 0.93, respectively, Kruskal–Wallis test).
Context test
There was no effect of anaesthesia on freezing in response to the fear conditioning context 24 h post-conditioning (Fig. 2e; treatment F(2,53)=0.76, P=0.47, one-way anova).
Working memory, short-term memory and early long-term memory
After a 60 s unscored swim to learn the new platform location for that session, each rat was given three scored trials. A 1 min interval separated the free swim and the three subsequent trials. Only data from the first scored trial was used for analysis. When rats achieved the predefined performance criterion (median time to find the platform <25 s during the first trial), eight overtraining sessions were administered, followed by three additional sessions (Sessions 16–18) with 1 min, 1 h and 4 h delays.
Training sessions
Throughout the training sessions, there was no difference in latency between any of the treatment groups (treatment F(2,53)=2.57; P=0.086, repeated measures two-way anova) with session number accounting for most of the variance (Session F(14,742)=7.81; P<0.0001; Fig. 3a). There were also no differences in swim speeds between treatment groups (treatment F(2,53)=1.43; P=0.25, repeated measures two-way anova) but a decrease in swim speed across sessions as the animals learned the task (Session F(14,742)=11.77; P<0.0001; Fig. 3b).
Fig 3.
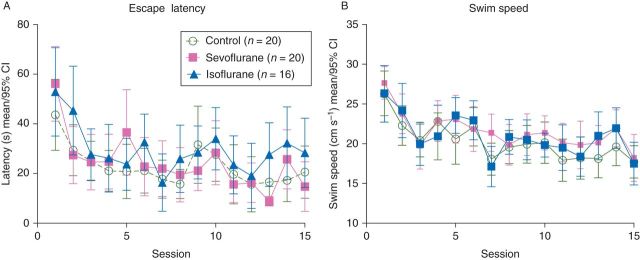
Morris water maze: escape latencies to the hidden platform (a) and swim speeds (b) of the first trial swims across training sessions 1–15. There was no effect of treatment and the only variability was due to session as the animals learned the task.
Memory test
At the end of overtraining the delay sessions were analysed at 1 min (Session 16) testing working memory, 1 h (Session 17) testing short-term memory and 4 h (Session 18) testing early long-term memory; differences emerged between treatment groups (treatment F(2,53) = 7.66; P = 0.0012, repeated measures two-way anova; Fig. 4a). Post hoc analysis revealed differences at both the 1 h delay and the 4 h delay. At 1 h, the isoflurane-treated group performed significantly worse, indicated by a longer latency to the platform, than both the control group and the sevoflurane-treated group [difference in means (control vs isoflurane): 25.8, 95% CI: 5.6–46.0, P<0.01; (sevoflurane vs isoflurane): 28.7, 95% CI: 8.5–48.9, P<0.01, Bonferroni post hoc corrections]. At the 4 h delay, both the isoflurane and sevoflurane-treated animals showed worse performance than the control animals [difference in means (control vs isoflurane): 25.4, 95% CI: 5.2–45.6, P<0.01; (control vs sevoflurane): 21.8, 95% CI: 2. 7–40.8, P<0.05, Bonferroni post hoc corrections].
Fig 4.
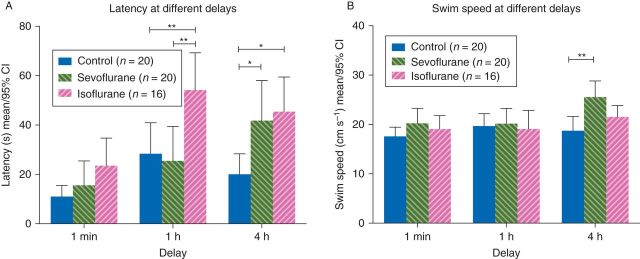
Memory test: at the end of the training sessions the delay between the exploratory swim and trial was 1 min (working memory), 1 h (short-term memory), and then 4 h (early long-term memory). Escape latencies (a) and swim speeds (b) of the first trial swims for each treatment group during the delay sessions shown. The isoflurane-treated group had a longer latency than control or sevoflurane treatment groups to find the platform after a 1 h delay, and both sevoflurane and isoflurane had a longer delay to find the platform after a 4 h delay.
During the delay trials, analysis of swim speeds revealed no difference due to anaesthesia (treatment F(2,53)=3.10; P=0.05, repeated measures two-way anova; Fig. 4b), and no interaction between time and anaesthesia (interaction F(4,106) = 2.15; P = 0.079, repeated measures two-way anova). However, post hoc analysis showed that sevoflurane-treated animals swam faster than control animals in the 4 h time delay [(control vs sevoflurane): 6.8, 95% CI: 2.3–11.4, P<0.01, Bonferroni post hoc corrections]. Despite this faster swim speed the sevoflurane-treated group still took longer to find the platform as noted in Figure 4a.
Taken together, spatial memory dysfunction was apparent in both short-term and early long-term memory in the isoflurane-treated group and in long-term memory in the sevoflurane-treated group.
Discussion
The main findings of this study are that equipotent doses of isoflurane or sevoflurane given to 7-day old rats impaired early long-term memory, but only isoflurane impaired short-term memory. Three months after administration of anaesthetic, both isoflurane and sevoflurane exposed groups took significantly longer to find the hidden platform than their unanaesthetized littermate controls when the delay between memory encoding and retrieval was 4 h. In addition, isoflurane-treated animals were impaired in short-term memory (1 h delay) relative to both the sevoflurane-treated and control groups. All groups performed equally in tests of working memory after a 1 min delay and in fear conditioning experiments.
These results replicate our previous finding that isoflurane impairs spatial short-term memory when the delay between memory acquisition and retrieval was extended from 1 min to 1 h,19 and in addition we now find memory impairment after a 4 h delay. We have also previously reported a deficit following sevoflurane anaesthesia when there is a 1 h delay or after a 4 h delay when compared with animals raised in an enriched environment.24 This differs from results presented here, where we only find a difference after a 4 h delay in sevoflurane-treated animals.
The present study compares equivalent doses of sevoflurane and isoflurane directly. We find slightly worse outcomes after isoflurane exposure than after sevoflurane exposure. This lends some strength to reports that isoflurane does not have an equal neurotoxicity profile to sevoflurane.25 Liang and colleagues found isoflurane to cause a worse injury than sevoflurane, but they found no difference in Morris water maze behaviour relative to control. This is likely due to use of a much lower concentration of anaesthetic and not testing under more challenging conditions by introducing a delay as we did in this study. Furthermore, we have previously reported that a 2 h isoflurane (1 MAC) anaesthetic induced significant cell death in the brains of 7-day-old rats, but did not lead to long-term cognitive dysfunction unlike a 4 h anaesthetic, which caused both brain cell death and long-term behavioural deficits.19 It is possible that a similar effect would be seen with decreasing depth of anaesthesia as well [i.e. that similar cumulative dose integrals (MAC-fraction over time) cause similar cognitive outcomes]. This possibility would require formal testing in future studies. We feel that it is important to point out that we make no mechanistic implications regarding the two outcomes—neuronal cell death and cognitive dysfunction. We view neuronal cell death as a marker of the severity of the insult rather than the cause of the cognitive decline.26 The present study also differs from other studies in that we did not find a deficit in fear conditioning.11,27 This could be due to differences in anaesthetic depth, duration (4 vs 6 h) or species studied (rats vs mice). That said, with one exception28 neither trace nor delay fear conditioning in adulthood is sensitive to the effect of anaesthesia during infancy in our laboratory (current study and see also19,24).
Recent studies attempting to assess the comparative effect of various volatile anaesthetic agents on neurodegeneration and behaviour have been designed in ways that do not allow a clear and direct comparison.10,11 MAC, is the most clinically relevant way to compare different anaesthetic drugs, and has been shown by two different methods to decrease over time in neonatal rodents.11,14 Despite claims to have achieved MAC, neither of the prior comparative studies10,11 anaesthetized subjects using a decreasing anaesthetic concentration, and they are therefore difficult to interpret.
We utilized tail clamping every 15 min throughout the 4 h anaesthetic session to simulate a surgical injury and to achieve a clinically relevant endpoint, MAC. Tail clamping provides a clinically relevant analogue because it causes tissue injury, haemorrhage and scarring, similar to what occurs during a surgical procedure that would warrant the use of anaesthesia, but has been shown to have no effect on the behavioural outcomes we examined.24 More importantly, we compared the cognitive effects of isoflurane and sevoflurane. This direct comparison on an equipotent basis revealed that both sevoflurane and isoflurane impair early long-term memory and that isoflurane impaired short-term memory as well. The two drugs are similar in that both impair memory, but the fact that sevoflurane did not impair short-term memory could mean that isoflurane is somewhat more harmful in rats. Further careful study using a strict definition of MAC will be required to verify these results or to make additional comparisons to other anaesthetic agents. Kodama and colleagues11 compared desflurane, sevoflurane and isoflurane using a set concentration of anaesthetic for 6 h and found sevoflurane and isoflurane to be similar and desflurane to cause more cell damage and worse outcome on one measure of cognitive function using the Y-maze. Despite the difference in anaesthetic strategy, this agrees with our results in that we did not find a difference after the shortest delay (1 min) between memory encoding and retrieval. Both paradigms can be considered working memory tasks. We did not compare sevoflurane and isoflurane to desflurane, which is not commonly used in paediatric anaesthesia, so we cannot say whether it would truly be worse if studies were done on a MAC basis. Nonetheless, this would be important and potentially clinically relevant to consider in future studies.
Limitations
In the water maze, cued trials are used to examine the presence of gross sensory or motor deficits by testing the rats' ability to swim to a visible platform. By excluding cued trials from our study, we risk the remote possibility that the deficits caused by isoflurane might be related to visual disturbances. However, during our previous experiments using cued trials we concluded that the apparent deficit in anaesthesia-treated rats was due to difficulties in learning the rules of the new task, and not to visual deficits.19,28 We also did not conduct probe trials, a common Morris water maze paradigm used to assess retention of the hidden platform location. We previously reported no difference in probe trial performance between isoflurane-treated19,28 or sevoflurane-treated24 and control animals.
The present study is also limited by the absence of data to suggest the mechanism of isoflurane or sevoflurane mediated impairment of cognition in infantile rats. While we have carefully described the extent of neurodegeneration caused by exposure to sevoflurane and isoflurane,19,24 additional studies will need to be performed in order to determine the neural correlate for the behavioural deficits observed. This will be an important step for the field given that nearly all the studies performed so far have shown a correlation between cell death, synapse changes, or stem cell changes, but none has been able to demonstrate a causal relationship between neuropathology and cognitive function. These experiments were performed in male rats only, so it is unknown whether the cellular and behavioural effects observed are limited to males or occur in both sexes.
Finally, and importantly, these data collected in rodents are limited by the inability to extrapolate them to clinical practice. It is possible that if in rats very similar agents cause different cognitive outcomes, the same might be true in humans. This illustrates the danger of switching clinical practice on the basis of animal data, speculation or both. Unless outcome differences between various anaesthetic techniques and agents can be demonstrated in humans, a change in clinical practice could do more harm than good. Such comparisons are urgently needed to guide clinical practice in paediatric anaesthesia.
Conclusions
Early postnatal anaesthetic exposure to isoflurane or sevoflurane at one MAC for 4 h impaired memory function in male rats. Both agents impaired early long-term spatial memory whereas only isoflurane, but not sevoflurane, impaired early short-term spatial memory.
Declaration of interest
None declared.
Funding
UCSF Department of Anesthesia (G.S.); NIH K08 GM06511 (J.W.S.); UCSF Department of Anesthesia Hamilton Award (J.W.S.)
References
- 1.Sun LS, Li G, Dimaggio C, et al. Anesthesia and neurodevelopment in children: time for an answer? Anesthesiology. 2008;109:757–61. doi: 10.1097/ALN.0b013e31818a37fd. [DOI] [PubMed] [Google Scholar]
- 2.Loepke AW, Soriano SG. An assessment of the effects of general anesthetics on developing brain structure and neurocognitive function. Anesth Analg. 2008;106:1681–707. doi: 10.1213/ane.0b013e318167ad77. [DOI] [PubMed] [Google Scholar]
- 3.Anand KJ, Soriano SG. Anesthetic agents and the immature brain: are these toxic or therapeutic? Anesthesiology. 2004;101:527–30. doi: 10.1097/00000542-200408000-00033. [DOI] [PubMed] [Google Scholar]
- 4.Jevtovic-Todorovic V, Hartman RE, Izumi Y, et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–82. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slikker W, Jr, Zou X, Hotchkiss CE, et al. Ketamine-induced neuronal cell death in the perinatal rhesus monkey. Toxicol Sci. 2007;98:145–58. doi: 10.1093/toxsci/kfm084. [DOI] [PubMed] [Google Scholar]
- 6.Fredriksson A, Archer T, Alm H, Gordh T, Eriksson P. Neurofunctional deficits and potentiated apoptosis by neonatal NMDA antagonist administration. Behav Brain Res. 2004;153:367–76. doi: 10.1016/j.bbr.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 7.Fredriksson A, Ponten E, Gordh T, Eriksson P. Neonatal exposure to a combination of N-methyl-d-aspartate and gamma-aminobutyric acid type A receptor anesthetic agents potentiates apoptotic neurodegeneration and persistent behavioral deficits. Anesthesiology. 2007;107:427–36. doi: 10.1097/01.anes.0000278892.62305.9c. [DOI] [PubMed] [Google Scholar]
- 8.Paule MG, Li M, Allen RR, et al. Ketamine anesthesia during the first week of life can cause long-lasting cognitive deficits in rhesus monkeys. Neurotoxicol Teratol. 2011;33:220–30. doi: 10.1016/j.ntt.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mellon RD, Simone AF, Rappaport BA. Use of anesthetic agents in neonates and young children. Anesth Analg. 2007;104:509–20. doi: 10.1213/01.ane.0000255729.96438.b0. [DOI] [PubMed] [Google Scholar]
- 10.Istaphanous GK, Howard J, Nan X, et al. Comparison of the neuroapoptotic properties of equipotent anesthetic concentrations of desflurane, isoflurane, or sevoflurane in neonatal mice. Anesthesiology. 2011;114:578–87. doi: 10.1097/ALN.0b013e3182084a70. [DOI] [PubMed] [Google Scholar]
- 11.Kodama M, Satoh Y, Otsubo Y, et al. Neonatal desflurane exposure induces more robust neuroapoptosis than do isoflurane and sevoflurane and impairs working memory. Anesthesiology. 2011;115:979–91. doi: 10.1097/ALN.0b013e318234228b. [DOI] [PubMed] [Google Scholar]
- 12.Merkel G, Eger EI., II A comparative study of halothane and halopropane anesthesia including method for determining equipotency. Anesthesiology. 1963;24:346–57. doi: 10.1097/00000542-196305000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Eger EI, 2nd, Saidman LJ, Brandstater B. Minimum alveolar anesthetic concentration: a standard of anesthetic potency. Anesthesiology. 1965;26:756–63. doi: 10.1097/00000542-196511000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Stratmann G, Sall JW, Eger EI, 2nd, et al. Increasing the duration of isoflurane anesthesia decreases the minimum alveolar anesthetic concentration in 7-day-old but not in 60-day-old rats. Anesth Analg. 2009;109:801–6. doi: 10.1213/ane.0b013e3181aff364. [DOI] [PubMed] [Google Scholar]
- 15.Eger EI, 2nd, Johnson BH. MAC of I-653 in rats, including a test of the effect of body temperature and anesthetic duration. Anesth Analg. 1987;66:974–6. [PubMed] [Google Scholar]
- 16.Stratmann G, Alvi RS. Can minimum alveolar concentrations in immature rodents be a single number? Anesthesiology. 2011;115:1132–3. doi: 10.1097/ALN.0b013e3182303c66. [DOI] [PubMed] [Google Scholar]
- 17.Stratmann G. Review article: Neurotoxicity of anesthetic drugs in the developing brain. Anesth Analg. 2011;113:1170–9. doi: 10.1213/ANE.0b013e318232066c. [DOI] [PubMed] [Google Scholar]
- 18.Istaphanous GK, McAuliffe JJ, Loepke AW. In reply. Anesthesiology. 2011;115:1133–5. [Google Scholar]
- 19.Stratmann G, May LV, Sall JW, et al. Effect of hypercarbia and isoflurane on brain cell death and neurocognitive dysfunction in 7-day old rats. Anesthesiology. 2009;110:849–61. doi: 10.1097/ALN.0b013e31819c7140. [DOI] [PubMed] [Google Scholar]
- 20.Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–7. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- 21.Izquierdo I, Barros DM, Mello e Souza T, de Souza MM, Izquierdo LA, Medina JH. Mechanisms for memory types differ. Nature. 1998;393:635–6. doi: 10.1038/31371. [DOI] [PubMed] [Google Scholar]
- 22.Izquierdo I, Izquierdo LA, Barros DM, et al. Differential involvement of cortical receptor mechanisms in working, short-term and long-term memory. Behav Pharmacol. 1998;9:421–7. doi: 10.1097/00008877-199809000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Lindner MD, Plone MA, Schallert T, Emerich DF. Blind rats are not profoundly impaired in the reference memory Morris water maze and cannot be clearly discriminated from rats with cognitive deficits in the cued platform task. Brain Res Cogn Brain Res. 1997;5:329–33. doi: 10.1016/s0926-6410(97)00006-2. [DOI] [PubMed] [Google Scholar]
- 24.Shih J, May LD, Gonzalez HE, et al. Delayed environmental enrichment reverses sevoflurane-induced memory impairment in rats. Anesthesiology. 2012;116:586–602. doi: 10.1097/ALN.0b013e318247564d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang G, Ward C, Peng J, Zhao Y, Huang B, Wei H. Isoflurane causes greater neurodegeneration than an equivalent exposure of sevoflurane in the developing brain of neonatal mice. Anesthesiology. 2010;112:1325–34. doi: 10.1097/ALN.0b013e3181d94da5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stratmann G, Sall JW, May LD, Loepke AW, Lee MT. Beyond anesthetic properties: the effects of isoflurane on brain cell death, neurogenesis, and long-term neurocognitive function. Anesth Analg. 2010;110:431–7. doi: 10.1213/ANE.0b013e3181af8015. [DOI] [PubMed] [Google Scholar]
- 27.Satomoto M, Satoh Y, Terui K, et al. Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology. 2009;110:628–37. doi: 10.1097/ALN.0b013e3181974fa2. [DOI] [PubMed] [Google Scholar]
- 28.Stratmann G, Sall JW, May LV, et al. Isoflurane differentially affects neurogenesis and long-term neurocognitive function in 60-day old and 7-day old rats. Anesthesiology. 2009;110:834–48. doi: 10.1097/ALN.0b013e31819c463d. [DOI] [PubMed] [Google Scholar]