Abstract
Introduction or background
The adult lung is a complex organ whose large surface area interfaces extensively with both the environment and circulatory system. Yet, in spite of the high potential for exposure to environmental or systemic harm, epithelial cell turnover in adult lung is comparatively slow. Moreover, loss of lung function with advancing age is becoming an increasingly costly healthcare problem. Cell-based therapies stimulating endogenous stem/progenitor cells or supplying exogenous ones have therefore become a prime translational goal. Alternatively when lung repair becomes impossible, replacement with tissue-engineered lung is an attractive emerging alternative using a decellularized matrix or bioengineered scaffold.
Sources of data
Endogenous and exogenous stem cells for lung therapy are being characterized by defining developmental lineages, surface marker expression, functions within the lung and responses to injury and disease. Seeding decellularized lung tissue or bioengineered matrices with various stem and progenitor cells is an approach that has already been used to replace bronchus and trachea in human patients and awaits further development for whole lung tissue.
Areas of agreement
Cellular therapies have clear potential for respiratory disease. However, given the surface size and complexity of lung structure, the probability of a single cellular population sufficing to regenerate the entire organ, as in the bone marrow, remains low. Hence, lung regenerative medicine is currently focused around three aims: (i) to identify and stimulate resident cell populations that respond to injury or disease, (ii) to transplant exogenous cells which can ameliorate disease and (iii) to repopulate decellularized or bioengineered lung matrix creating a new implantable organ.
Areas of controversy
Lack of consensus on specific lineage markers for lung stem and progenitor cells in development and disease constrains transferability of research between laboratories and sources of cellular therapy. Furthermore, effectiveness of individual cellular therapies to correct gas exchange and provide other critical lung functions remains unproven. Finally, feasibility of autologous whole organ replacement has not been confirmed as a durable therapy.
Growing points
Cellular therapies for lung regeneration would be enhanced by better lineage tracing within the lung, the ability to direct differentiation of exogenous stem or progenitor cells, and the development of functional assays for cellular viability and regenerative properties. Whether endogenous or exogeneous cells will ultimately play a greater therapeutic role remains to be seen. Reducing the need for lung replacement via endogenous cell-mediated repair is a key goal. Thereafter, improving the potential of donor lungs in transplant recipients is a further area where cell-based therapies may be beneficial. Ultimately, lung replacement with autologous tissue-engineered lungs is another goal for cell-based therapy.
Areas timely for developing research
Defining ‘lung stem or progenitor cell’ populations in both animal models and human tissue may help. Additionally, standardizing assays for assessing the potential of endogenous or exogenous cells within the lung is important. Understanding cell–matrix interactions in real time and with biomechanical insight will be central for lung engineering.
Cautionary note
Communicating the real potential for cell-based lung therapy needs to remain realistic, given the keen expectations of patients with end-stage lung disease.
Keywords: endogenous lung stem cells, exogenous stem cells, lung regeneration and repair, regenerative medicine, stem cell therapies
What are cell-based therapies?
Cell-based therapies encompass strategies where cells, whether endogenous (residing within the organ) or exogenous (isolated from outside the organ) are manipulated to ameliorate disease progression or repair, regenerate or replace a diseased organ.
The lung is a target for new cellular therapies since unlike organs such as the liver, it has more limited native regenerative capacity beyond childhood. However, the complex architecture and diversity of cell types and niches within the lung imposes significant challenges. This review focuses on the optimum sources and biological uses of cells for lung repair and regeneration.
Cellular therapeutic strategies for the lung: challenges and possibilities
Adult human lung has 23 airway generations from trachea to alveolar ducts. These terminate in millions of alveoli. Each airway region has distinct structure and function, with characteristic tissue and cell types (>40 cell lineages identified thus far). Larger conducting airways are lined by ciliated columnar epithelium, goblet cells, basal cells, brush cells, serous cells and pulmonary neuroendocrine cells. More distally, respiratory bronchioles are lined by Clara cells and a few ciliated cells while alveoli are lined by type I and II pneumocytes.
The large number of lung cell lineages means disease afflicts multiple cell types and in variable number depending on anatomical location. Given the complex topography and regionalized cell composition of lung tissue, it is currently challenging to imagine a single stem cell type as an endogenous ‘catch all’ therapeutic. Multiple niches may support different populations and their progenitors. At present, each niche and its role in homeostasis, injury and repair are not fully characterized.
The diversity of lung diseases requires further consideration when choosing cell therapy to be used. For example, in acute alveolar injury, where closure of breaches in the alveolar surface is urgent, targeting and supporting the healing potential of endogenous progenitors make sense. Thus, disease states resulting from apoptosis and damage to epithelia such as acute inhalational injury or blast injury could be managed through increased proliferation and repair by endogenous cells as well as transplantation of cells endogenous to the lung that can quickly repopulate the damaged area, without having to be ‘reprogrammed’. However, for other disease states resulting from developmental deficiencies or chronic depletion of endogenous stem cell pools such as old age and chronic obstructive pulmonary disease (COPD), exogenous stem cell therapies may represent the better approach.1 Progressive diseases such as pulmonary fibrosis, which affects the regenerative capacity of alveolar epithelium and results in its replacement with fibrotic tissue, might benefit from exogenous cellular therapies that may not only repopulate various cellular niches, but may aid in slowing the progression of tissue destruction (Table 1).
Table 1.
Lung diseases potentially treatable with cellular therapies
| Lung disease | Pathology | Affected regions | Therapeutic target |
|---|---|---|---|
| Adult RDS | Inflammation, hypoxemia and impaired gas exchange | Alveolar epithelium and capillary endothelium | Regeneration of epithelia and endothelium |
| Asthma | Inflammation, bronchospasam and airflow obstruction | Airway epithelium, myofibroblasts and airway smooth muscle | Reduce inflammatory milieu inhibit airway wall remodeling, inhibit smooth muscle hypertrophy and hyperplasia |
| Bronchiolitis obliterans | Inflammation and fibrosis of bronchioles | Airway epithelium | Regeneration of epithelia |
| Bronchopulmonary dysplasia | Necrotizing bronchiolitis and alveolar septal injury | Alveolar epithelium, interstitial fibroblasts and capillary endothelium | Reduce inflammatory milieu, regeneration of alveolar septa and epithelium |
| Congenital lung hypoplasia | Incomplete development of lung resulting in reduced number or size of bronchopulmonary segments or alveoli | Alveolar epithelium, interstitial fibroblasts and capillary endothelium | Generate alveolar septa and 3D alveolar structure |
| Cystic fibrosis | CFTR mutation resulting in decreased mucociliary clearance and inflammation | Airway epithelium | Delivery of functional CFTR |
| Neonatal RDS | Insufficient surfactant production in the lungs | Alveolar epithelium and capillary endothelium | Generation of surfactant production and regenerate epithelia and endothelia |
| Pulmonary emphysema (COPD) | Loss of alveolar integrity and reduction of ventilation | Alveolar epithelium, interstitial fibroblasts and capillary endothelium | Generate alveolar septa and 3D alveolar structure |
| Pulmonary fibrosis | Inflammation and fibrosis of alveolar tissue | Alveolar epithelium, interstitial fibroblasts and endothelium | Reduce inflammation, reduce alveolar epithelia loss and inhibit fibroblast proliferation |
| Sarcoidosis | Inflammation accompanied by granuloma formation | Epithelium | Reduction of inflammation and regeneration of epithelia |
RDS, respiratory distress syndrome; CFTR, cystic fibrosis transmembrane conductance regulator.
What is a stem/progenitor cell?
Cellular therapy aims to restore diseased tissue to a ‘normal’ state. However, what type of stem or progenitor cells should be used?
Stem cells are primitive, undifferentiated cells that can self-renew, while giving rise to descendants with distinct characteristics of differentiated daughter cell lineages. Stem cells appear critical for normal development, growth and adult tissue homeostasis. With age, depleted and/or dysfunctional adult stem cells may underlie inadequate tissue regeneration following injury, degenerative disease as well as aging per se.2 This decline may be due to decrease in chromosomal telomere length with age.3 Conversely, lung stem cells that proliferate out of control are hypothesized to play a role in cancer.4
Progenitor cells are committed to a lineage and differentiate into a specific cell or cell types. Unlike stem cells, progenitor cells are thought to have a more limited capacity for self-renewal and represent an ‘intermediate’ between stem cells and terminally differentiated ones. Progenitors are thought to occupy niches within an organ and repopulate tissue during growth, injury or disease: failure in these roles may underpin various disorders.5
Endogenous progenitors and their niches in the lung
Stem or progenitor cell ‘niches’ describe special microenvironments thought to help maintain undifferentiated stem or progenitor cells, while also specifying fate. Localization and properties of such niches and the cells within them are presently a major research focus. The nature and location of stem and progenitor cells within the lung remain controversial and has, for the most part, been scrutinized in murine rather than the importantly different human lung.
At least five putative niches have been postulated in adult mouse lung (Fig. 1).6
Airway submucosal glands (SMGs) occur in the trachea. Transplantation of decellularized trachea into the nude mice showed reconstitution of surface tracheal epithelia from the remaining gland duct and acinar cells found in airway SMGs.
Keratin-14 (K14)-expressing tracheal basal cells are considered a second nidus of stem/progenitors. When Clara cells are injured with naphthalene in mice, K14-expressing basal cells can repopulate the denuded airway epithelia, including columnar secretory and ciliated cells, which is indicative of a progenitor-progeny response to injury.7 Recently, human trachea has been bioengineered from the two main autologous cell types in the trachea, mesenchymal stem cell-derived cartilage-like cells and respiratory epithelial cells.8 Earlier still, tissue-engineered bronchus has been transplanted successfully to replace a tubercular, stenotic human main stem bronchus using stem/progenitor cells and decellularized matrix.9
Variant Clara cells (Clarav) are believed also to mediate cell renewal in the distal airways. Clarav cells are located either within neuroendocrine bodies (NEBs) or at bronchoalveolar duct junctions.10 Clarav cells associated with NEBs are in close contact with sensory nerve fibers and may also have an endocrine function. These cells are long lived and reside in a specialized niche that allows for their differentiation and proliferation following naphthalene injury in mice.11 Naphthalene, which ablates Clara cells, has no effect on Clarav cells, which survive to repopulate the distal airways. The population of Clarav cells located at the bronchoalveolar duct junction (BADJ) has also been designated as bronchoalveolar stem cells (BASCs). BASCs characteristically express both surfactant protein C, as well as the marker termed Clara cell 10-kDa secretory protein (CC10). Like their NEB counterparts, they are highly resistant to injury and can proliferate and repopulate the distal parts of the airways following injury.
Alveolar epithelial type II cells (AECII) are responsible for generating fully differentiated type I pneumocytes (AECI) in the alveolus. AECII produce surfactant proteins and are relatively resistant to apoptosis, suggesting that this population of cells can re-epithelialize damaged alveoli.12 Expression of telomerase, a stem/progenitor cell marker, is elevated within these cells following acute oxygen injury.13
Fig. 1.
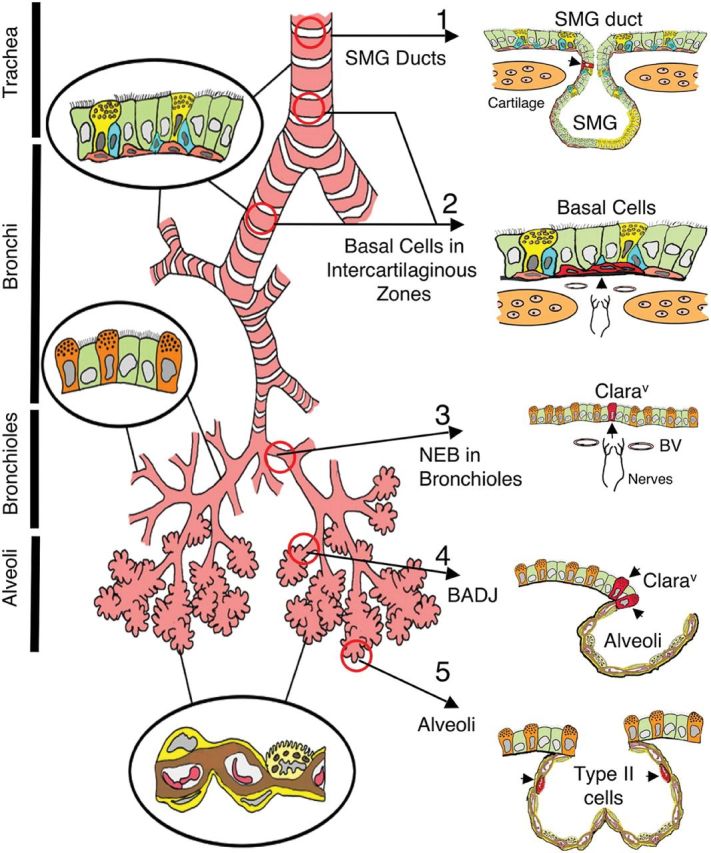
Illustration of putative stem cell niches in the adult mouse lung. Epithelia of the adult mouse lung can be divided into four major, biologically distinct trophic units (trachea, bronchi, bronchioles and alveoli), each of which encompasses unique types of airway epithelial cells (epithelia relevant to each unit are shown inside circles). Five potential stem cell niches for these various trophic units are shown on the right, with locations of candidate stem cells marked by arrowheads (cells are in red). Stem cells and niches include the following: 1, an unknown cell type in the SMG ducts of the proximal trachea; 2, basal cells in the intercartilaginous zones of the lower trachea and bronchi (these structures may also be associated with innervated NEBs; 3, Clarav associated with NEBs in bronchioles; 4, Clara cell associated with BADJ and 5, alveolar type II cells of the alveoli. Reprinted with permission from the American Thoracic Society. Copyright © American Thoracic Society. From Liu and Engelhardt6. Official Journal of the American Thoracic Society. Diane Gern, Publisher.
Multipotent cells can give rise to some but not all cell lineages. Isolation, purification and maintenance of a population termed multipotent lung stem cells (MLSCs) from the lung were recently reported. This cell population was shown to generate endothelial, Clara, AECI and AECII cells as well as to differentiate into mesenchymal cells in vitro.14 Furthermore, while these cells showed minimal engraftment in elastase-injured lungs, transplanting them significantly improved survival when compared with non-treated mice. These data suggest that MLSCs are a potential target for future research and translational strategies in human lung disease.
Another candidate population of lung epithelial stem/progenitor cells recently identified in the adult mouse was resolved from endogenous lung mesenchymal progenitor cells consistent with the existence of distinct lung epithelial and mesenchymal lineages.15 When cultured in a system designed to mimick the normal 3D microenvironment of the lung, this epithelial stem/progenitor population gave rise to epithelial colonies comprising airway, alveolar or mixed lung epithelial cell lineages. Importantly, mixed lineage colonies were able to self-renew and give rise to all epithelial lineages, suggesting that an epithelial stem/progenitor cell hierarchy exists in the adult mouse lung.
Finally, a recent controversial study has claimed the existence of multipotent stem cells within the human lung. Like previously identified stem/progenitor cells in the mouse lung, these cells were described as multipotent, clonogenic and self-renewing. Isolated on the basis of the well-known stem cell associated marker c-kit, these cells were shown to differentiate into cell phenotypes characteristic of their organ of origin.16 Following transplantation into the damaged mouse lung, this candidate human lung stem cell population was reported to form bronchioles, alveoli and pulmonary vessels. While the initial prospect for lung regeneration described in this study is exciting, significant reservations have been raised about the experimental designs employed,17 and caution should be exercised before more rigorous experiments have been performed to replicate and validate the study.
On balance, multiple kinds of lung stem and progenitor cell sources will likely be needed for complete lung tissue regeneration. This highlights the importance of establishing the potential and regenerative capacity of endogenous stem and progenitor cells distributed along the proximal–distal axis of the lung; the properties of the regional microenvironmental niches in which they reside and which specify their fate and how they are affected in different lung diseases.18 The importance of endogenous progenitor cells during lung injury and disease cannot be overlooked if treatment strategies aimed at reinvigorating the regenerative potential of these cells is to be effective. While much effort has been directed at inhibiting the proliferation of aberrant cells in cancer, studies focused on rejuvenating non-proliferating cells remain at an early but promising stage. Recent studies using gene transfer vectors have shown the potential for targeting specific cell populations in the lung. For example, rhinoviral infection of cultured tracheal epithelium showed that basal cells were selectively infected.19 Thus targeting of distinct stem cell populations with vectors that induce directed expression could provide a strategy for ‘awakening’ resident stem cells.
Exogenous stem and progenitor cell candidates for cell therapies
Hematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs): stem cells originating within the bone marrow have garnered much attention in the stem cell field as these progenitors are not just a phenomenon of early development, but continue to function throughout life. HSCs and MSCs are thought to travel through the blood stream, alight at sites of disease or injury and participate in the repair. Furthermore, HSCs and MSCs are considered good candidates for use in cell-based regenerative therapies as they have been well characterized as safe and have shown healing potential both spontaneously and when genetically manipulated. However, their ability to efficiently and fully differentiate into lung epithelia appears doubtful.20,21
A certain amount of lung repair and regeneration is attributed to circulating progenitor cells. For example, the introduction of these cells into diseased tissue has aided in the amelioration of injury and the augmentation of endogenous injury responses.22–24 This suggests that these cells may be acting ‘pharmacologically’ by modulating the immune or inflammatory homeostatic milieu within the lung. A logical target for this ameliorating, protective and/or activating effect may be the endogenous progenitor populations previously described, which could therefore respond to exogenous stem cell administration by effecting the repair of damage. The potential for the use of such progenitor cells is exciting when considering cases such as COPD and emphysema, in which patients have experienced the destruction of much normal respiratory tissue.
The role that circulating progenitors, HSCs and MSCs, may play in lung repair has until recently been largely controversial as many lung diseases are attributed to tissue specific mesenchymal abnormalities. As a caveat to this potential benefit of circulating progenitors, it must be noted that circulating fibrocytes have been quite recently identified as a potential major contributor to the progression of pulmonary fibrosis.25 Thus, the potential of these cells to facilitate rather than inhibit fibrosis must be considered when balancing the need for therapy against possibly harmful side effects. These conflicting roles for circulating progenitor cells, of either mesenchymal or hematopoietic origin, thus requires further study and characterization in order to determine which specific populations contribute beneficial versus deleterious, outcomes following administration.
Induced pluripotent stem cells (iPS cells) and embryonic stem cells (ES cells) have shown promise as therapeutic agents in other organs; however, full exploration of their potential in lung disease is yet to be tested.26 Researchers have been successful in generating upper airway epithelial cells as well as cells that express epithelial type II cell markers from ES cells. More recently, AECII-like cell lines were established from human ES cell lines and shown to differentiate in vitro into AECI cells. Furthermore, when transplanted intratracheally into mice following bleomycin injury, which induces pulmonary fibrosis, these cells expressed phenotypic markers consistent with AECII and AECI cells.27 But such lung cell-directed differentiation is a rare event and the functionality of these cells in vivo has yet to be elucidated.28 Human ES cells have not been fully investigated in the lung largely due to ethical considerations and governmental regulations. Attempts to use iPS cells instead of the more controversial ES cells have also raised concerns after tumorgenicity was observed in mouse models.29 Refinement of the iPS procedure to inactivate or remove the oncogenes used to drive de-differentiation of these lines is underway, and the potential for these cells as a therapeutic source remains optimistic.
Fetal-associated stem cells: amniotic fluid, cord blood, Wharton's jelly, placenta and amnion-derived stem cells are also attractive candidates for use in lung repair. Their use is not constrained by the ethical considerations and potential hazards associated with embryonic stem cells, and they have been shown to express lung epithelial markers in vivo.30 Human amniotic fluid stem cells (hAFSCs) can be derived from discarded amniocentesis specimens and thus circumvent the ethically charged arguments about ES cells. Similarly, placental and umbilical cord-derived stem cells can be collected at birth and stored for potential future use. These fetal-associated cells are characterized as mostly mesenchymal cells and have shown immunomodulatory and regenerative effects in various lung injury models.
hAFSCs are pluripotent, giving rise to all three germ layers in mouse chimeras as well as in vitro under carefully controlled conditions.31 hAFSCs have also shown the ability to differentiate into various tissue types, such as the embryonic kidney or lung, when placed into the correct microenvironment, and have not been shown to produce teratomas as ES cells do.32 It has been shown that hAFSCs can incorporate into mouse embryonic lung and express human lung epithelial cell markers. Following lung injury in the nude mice, it has also been shown that after intravenous injection, they become trapped in the lung and remain at sites of injury. hAFSCs persist in the lung after injury, but decrease over time and their safety, efficacy and translational potential in lung repair remain to be fully elucidated.
Engineering whole lung regeneration
When the prospect of repairing the lung is out of the question, researchers have begun investigating the potential for whole organ regeneration. This type of regeneration is based on the theory that scaffolding, whether obtained from decellularized donor lungs or bioengineered materials, can be seeded with various cell types and cultured to make an entire functional organ, such as the lung. Recently, a whole lung decellularization method and subsequent tissue engineering using neonatal lung epithelia has been reported.33 It has long been known that seeded epithelial cells can repopulate alveolar structures within denuded lung matrix. Yet, rat lung tissue that had been decellularized and then recellularized was recently found to be capable of supporting short-term perfusion and gas exchange when transplanted back into a rat. In another study, seeding of endothelium and epithelium was successfully accomplished on a decellularized rat lung. Following orthotopic transplantation, the re-engineered lung was likewise capable of short-term gas exchange as shown through blood gas analysis.34 Bioengineered scaffolds on the other hand have been utilized to create artificial large airways of the lung, such as the trachea and bronchus. However, the fine architecture of alveoli and terminal bronchioles remains a challenge for tissue engineers.
In sum, while the decellularized lung matrix seems to be the most promising scaffold for whole lung regeneration, artificial biomatrix constructs will likely serve for the larger single airways.
Production and delivery of cellular therapies
There are a number of practical considerations which must be addressed when devising cellular therapies for lung regeneration and repair. Robust cell separation protocols for the isolation and scalable production of highly enriched stem and progenitor cell targets capable of repairing and regenerating affected lung cell lineages still need to be devised. Ex vivo cell culture systems able to expand stem cells without compromising their regenerative potential need to be developed. Also, since stem cell fate is dictated by extrinsic signals provided by their tissue microenvironment, preconditioning strategies and routes and mode of delivery will need to be optimized to maximize their engraftment and harness their regenerative capacity.
The main delivery choices for cell therapy in the lung are down the airway or through the pulmonary arterial circulation. Systemic intravenous injection of therapeutic cells has been used in numerous studies as the lung acts as a cellular strainer and thus transiently traps cells that are too big to continue on through the pulmonary circulation. An important caveat is that once entrapped in the lung microvasculature, regenerative cells may not be able to efficiently cross the endothelial barrier to home and lodge at the site of epithelial injury. An alternative approach is the aerosolization or discrete placement of cells within the lung, either as a bolus of cells or embedded within a dissolving matrix. As with intravenous delivery, cells may become trapped in the alveoli and may not be able to migrate easily to mesenchymal or endothelial sites of injury. Thus, while both intravenous and intratracheal routes offer relatively efficient initial deposition of cells, in the absence of ongoing tissue injury, long-term uptake, retention and differentiation are relatively inefficient in vivo. Thus, using these approaches to achieve significant replacement of damaged lung or airway remains quite impractical. Finally, by both routes, the bulk of exogenous cells are cleared either into the local lymphatic tissue or via the circulation to the liver and spleen.
Cellular therapy versus whole lung engineering
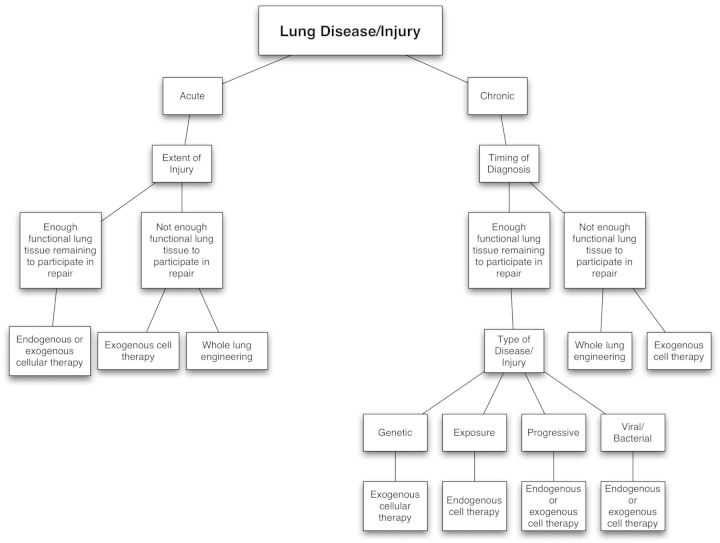
The decision to use the approach of cellular therapy versus whole lung engineering when approaching specific lung diseases is rather complex. The timing of diagnosis coupled with the extent of the injury or disease is essential when a clinician is tasked with determining an appropriate course of treatment (Fig. 2). Cellular therapies are advantageous when minimally invasive procedures are favored. Additionally, cellular therapies are more attractive than whole lung engineering when the repair of injury can be achieved by utilizing remaining lung tissue or the patient has sufficient remaining lung function to withstand prolonged therapy that may require the growth of cells and secretion of specific therapeutic factors by the transplanted cells.
Fig. 2.
Cellular therapy decision tree.
Whole lung engineering with a patient's own scaffolding, or a donor scaffold, with self-progenitor cells is likely to be less immunogenic for the patient than transplanting non-self lungs. Furthermore, patient survival rates could be improved if exact donor matches prove to be unnecessary, since transplantation rates would only then be limited by donor lung scaffold availability and organ re-engineering time.
Areas of agreement
Lung researchers have expended considerable effort in attempting to come to a consensus regarding the potential for cellular therapies within the lung. It is fair to say that researchers and clinicians alike agree that current clinical strategies are simply not effective when dealing with progressive lung diseases. Thus, cellular therapies have been agreed upon as an important new frontier in lung research and disease treatment. Furthermore, it has been agreed that various cell types will contribute to the population and repopulation of the respiratory architecture.
Areas of controversy
Attempts at characterizing individual progenitor populations, their designation as ‘stem cells’ versus ‘progenitor cells’, the identification of ‘niches’, the potential of engraftment of exogenous populations and their efficacy is where many lung researchers disagree. The existence of stem cells as they are classically defined has recently been called into question, with some scholars asserting that stem cells do not exist independent of their niches, and are in fact driven to behave as stem cells only when they are within their niches. This calls into question the identification of the endogenous stem cells within the lung discussed herein as being cells that are induced to act only in response to injury, disease or the need to repopulate surfaces, rather than cells that can be characterized by the intrinsic properties of ‘stemness.’ In addition to these technical and biological questions, a layer of ethical controversy can be added when one considers the possibility of exogenous stem cells differentiating into unwanted tissue. Finally, there has been much disagreement over the definition of ‘engraftment’ when relating to transplantation of endogenous and exogenous stem cells.
Future challenges
The first hurdle that must be overcome in the area of endogenous stem/progenitor cells within the lung is the identification of consensus markers that can be used to reliably identify these cells. Second, robust functional assays are needed that test the ability of these cells to either self-renew or proliferate and differentiate. Finally, in vivo assays are needed to measure how the functional ability of endogenous stem cells is impaired following injury and in the diseased lung, and how endogenous and engrafted stem cells are recruited to repair the lung. While those in the field recognize the exciting potential of cellular therapies for lung diseases, much further work is needed to characterize the optimal cells and develop safe and efficient strategies to harness their therapeutic potential before they are ready for the clinic.
References
- 1.Rock JR, Tandell SH, Hogan BL. Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis Models Mech. 2010;3:545–56. doi: 10.1242/dmm.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee J, Reddy R, Barsky L, et al. Lung alveolar integrity is compromised by telomere shortening in telomerase-null mice. Am J Physiol Lung Cell Mol Physiol. 2009;296:L57–70. doi: 10.1152/ajplung.90411.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warburton D, Perin L, DeFilippo R, et al. Stem/progenitor cells in lung development, injury repair, and regeneration. Proc Am Thorac Soc. 2008;5:703–6. doi: 10.1513/pats.200801-012AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giangreco A, Groot KR, Janes SM. Lung cancer and lung stem cells: strange bedfellows? Am J Respir Crit Care Med. 2007;175:547–53. doi: 10.1164/rccm.200607-984PP. [DOI] [PubMed] [Google Scholar]
- 5.Weiss DJ, Bertoncell I, Borok Z, et al. Stem cells and cell therapies in lung biology and disease. Proc Am Thorac Soc. 2011;8:223–72. doi: 10.1513/pats.201012-071DW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu X, Engelhardt JF. The glandular stem/progenitor cell niche in airway development and repair. Proc Am Thorac Soc. 2008;5:682–8. doi: 10.1513/pats.200801-003AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong KU, Reynolds SD, Watkins S, et al. In vivo differentiation potential of tracheal basal cells: evidence for multipotent and unipotent subpopulations. Am J Physiol Lung Cell Mol Physiol. 2004;286:L643–9. doi: 10.1152/ajplung.00155.2003. [DOI] [PubMed] [Google Scholar]
- 8.Bader A, Macchiarini P. Moving towards in situ tracheal regeneration: the bionic tissue engineered transplantation approach. J Cell Mol Med. 2010;14:187–89. doi: 10.1111/j.1582-4934.2010.01073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jungebluth P, Alici E, Baiguera S, et al. Tracheobronchial transplantation with a stem-cell-seeded bioartificial nanocomposite: a proof-of-concept study. Lancet. 2011 doi: 10.1016/S0140-6736(11)61715-7. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 10.Giangreco A, Shen H, Reynolds SD, et al. Molecular phenotype of airway side population cells. Am J Physiol Lung Cell Mol Physiol. 2004;286:L624–30. doi: 10.1152/ajplung.00149.2003. [DOI] [PubMed] [Google Scholar]
- 11.Hong KU, Reynolds SD, Giangreco A, et al. Clara cell secretory protein-expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am J Respir Cell Mol Biol. 2001;24:671–81. doi: 10.1165/ajrcmb.24.6.4498. [DOI] [PubMed] [Google Scholar]
- 12.Lee J, Reddy R, Barsky L, et al. Contribution of proliferation and DNA damage repair to alveolar epithelial type 2 cell recovery from hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2006;290:L685–94. doi: 10.1152/ajplung.00020.2005. [DOI] [PubMed] [Google Scholar]
- 13.Driscoll B, Buckley S, Bui KC, et al. Telomerase in alveolar epithelial development and repair. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1191–8. doi: 10.1152/ajplung.2000.279.6.L1191. [DOI] [PubMed] [Google Scholar]
- 14.Hegab AE, Kubo H, Fujino N, et al. Isolation and characterizaiton of murine multipotent lung stem cells. Stem Cells Dev. 2010;19:523–36. doi: 10.1089/scd.2009.0287. [DOI] [PubMed] [Google Scholar]
- 15.McQualter JL, Yuen K, Williams B, et al. Evidence of an epithelial stem/progenitor cell hierarchy in the adult mouse lung. Proc Natl Acad Sci USA. 2010;107:1414–9. doi: 10.1073/pnas.0909207107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kajstura J, Rota M, Hall SR, et al. Evidence for human lung stem cells. N Engl J Med. 2011;364:1795–806. doi: 10.1056/NEJMoa1101324. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Gellar GB. Lung stem cells: looking beyond the hype. Nat Med. 2011;17:788. doi: 10.1038/nm0711-788. [DOI] [PubMed] [Google Scholar]
- 18.Perl AK, Wert SE, Nagy A, et al. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc Natl Acad Sci USA. 2002;99:10482–7. doi: 10.1073/pnas.152238499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jakiela B, Crockman-Schneider R, Amineva S, et al. Basal cells of differentiated bronchial epithelium are more susceptible to rhinovirus infection. Am J Respir Cell Mol Biol. 2008;38:517–523. doi: 10.1165/rcmb.2007-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mattsson J, Jansson M, Wernerson A, et al. Lung epithelial cells and type II pneumocytes of donor origin after allogeneic hematopoietic stem cell transplantation. Transplantation. 2004;78:154–7. doi: 10.1097/01.tp.0000132326.08628.74. [DOI] [PubMed] [Google Scholar]
- 21.Kotton DN, Fabian AJ, Mulligan RC. Failure of bone marrow to reconstitute lung epithelium. Am J Respir Cell Mol Biol. 2005;33:328–34. doi: 10.1165/rcmb.2005-0175RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prockop DJ. Stemness does not explain the repair of many tissues by mesenchymal stem/multipotent stromal cells (MSCs) Clin Pharmacol Ther. 2007;82:241–3. doi: 10.1038/sj.clpt.6100313. [DOI] [PubMed] [Google Scholar]
- 23.Gupta N, Su X, Popov B, et al. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179:1855–63. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- 24.Serikov VB, Mikhaylov VM, Krasnodembskay AD, et al. Bone marrow-derived cells participate in stromal remodeling of the lung following acute bacterial pneumonia in mice. Lung. 2008;186:179–90. doi: 10.1007/s00408-008-9078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersson-Sjöland A, de Alba CG, Nihlberg K, et al. Fibrocytes are a potential source of lung fibroblasts in idiopathic pulmonary fibrosis. Int J Biochem Cell Biol. 2008;40:2129–40. doi: 10.1016/j.biocel.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 26.Rippon HJ, Ali NN, Polak JM, et al. Initial observations on the effect of medium composition on the differentiation of murine embryonic stem cells to alveolar type II cells. Cloning Stem Cells. 2004;6:49–56. doi: 10.1089/1536230041372328. [DOI] [PubMed] [Google Scholar]
- 27.Wang D, Morales JE, Calame DG, et al. Transplantation of human embryonic stem cell-derived alveolar epithelial type II cells abrogates acute lung injury in mice. Mol Ther. 2010;18:625–34. doi: 10.1038/mt.2009.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coraux C, Nawrocki-Raby B, Hinnrasky J, et al. Embryonic stem cells generate airway epithelial tissue. Am J Respir Cell Mol Biol. 2005;32:87–92. doi: 10.1165/rcmb.2004-0079RC. [DOI] [PubMed] [Google Scholar]
- 29.Pei D, Xu J, Zhuang Q, et al. Induced pluripotent stem cell technology in regenerative medicine and biology. Adv Biochem Eng Biotechnol. 2010;123:127–41. doi: 10.1007/10_2010_72. [DOI] [PubMed] [Google Scholar]
- 30.Carraro G, Perin L, Sedrakyan S, et al. Human amniotic fluid stem cells can integrate and differentiate into epithelial lung lineages. Stem Cells. 2008;26:2902–11. doi: 10.1634/stemcells.2008-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Coppi P, Bartsch G, Jr, Siddiqui MM, et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;1:100–6. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- 32.Perin L, Giuliani S, Jin D, et al. Renal differentiation of amniotic fluid stem cells. Cell Prolif. 2007;40:936–48. doi: 10.1111/j.1365-2184.2007.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peterson TH, Calle EA, Zhao L, et al. Tissue-engineered lungs for in vivo implantation. Science. 2010 doi: 10.1126/science.1189345. doi:10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ott HC, Clippinger B, Conrad C, et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16:927–33. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]